Abstract
Background
To investigate the association of repeat expansion size in 10 common degenerative hereditary ataxia genes with essential tremor. These genes were spinocerebellar ataxia (SCA)-1 (ATXN1), SCA-2 (ATXN2), SCA-3 (ATXN3), SCA-6 (CACNA1A), SCA-7 (ATXN7), SCA-8 (ATXN8OS), SCA-10 (ATXN10), SCA-12 (PPP2R2B), SCA-17 (TBP) and dentatorubral-pallidolysian atrophy (DRPLA) (ATN1).
Methods
Genetic analysis of repeat size in 10 degenerative hereditary ataxia loci was performed in 323 essential tremor patients and 299 controls enrolled at Columbia University. To test for differences in the allele distribution between patients and controls, a CLUMP analysis was performed.
Results
None of the essential tremor patients had a repeat expansion in the intermediate or pathogenic range. Significant differences in the distribution of repeats in the ‘normal’ range for SCA2 and SCA8 (both p ≤0.02) were observed between essential tremor patients and controls.
Conclusions
Our study suggests that pathogenic repeat expansions in SCA loci are not associated with essential tremor.
Keywords: Essential Tremor, Spinocerebellar ataxia loci, CAG repeat expansions, genetics
Introduction
Essential tremor (ET), a disease whose hallmark feature is kinetic tremor (i.e., tremor during voluntary movements), is the most common cause of abnormal tremor in humans[1].Prevalence increases with age, reaching ~20% during the 9 th – 10th decade of life[1].There are >30 prevalence studies from around the world, with an estimated 7 million affected individuals in the US alone (i.e., approximately 2.2% of the population). The disease is usually progressive and there is no cure.
Family studies and twin studies[2] provide strong evidence for a genetic contribution to ET, with heritability estimates ranging from 45 – 90% in twin studies[2]. Previously, we and others reported that ET aggregates in families, with many families containing multiple members with ET.
Patients with ET often have cerebellar signs, including intention tremor and mild gait ataxia, and recent postmortem studies are revealing degenerative changes in the cerebellum, including but not limited to Purkinje cell loss in some studies[3].Hence, there is clinical-pathological overlap with the degenerative hereditary ataxias, including the spinocerebellar ataxias (SCAs). The autosomal dominant SCAs are neurodegenerative disorders that are clinically and genetically heterogeneous and neuropathologically characterized by degenerative changes in the cerebellum[4]. Currently, 31 SCA types are known (SCA1-36), with a prevalence (in Europe) of 3 per 100,000, with SCA1, SCA2, SCA3 and SCA6 the most common SCAs worldwide. Different types of mutations have been described at each SCA locus, including point mutations (SCA13 and 27), frameshift (SCA11), deletions (SCA15) and duplications (SCA20), CAG coding repeat expansions (SCA1-3, 6, 7, 17) and noncoding triplet, quintuplet or hexaplet repeat expansions (SCA8, 19, 12, 31, 36).
There is prominent genetic anticipation in the SCAs that involve triplet repeat expansions. The presence of genetic anticipation has been suggested in some ET families as well [5].
In the current study we investigated an association of repeat expansion size in 10 common hereditary degenerative ataxia (esp., SCA) genes: SCA-1 (ATXN1), SCA-2 (ATXN2), SCA-3 (ATXN3), SCA-6 (CACNA1A), SCA-7 (ATXN7), SCA-8 (ATXN8OS), SCA-10 (ATXN10), SCA-12 (PPP2R2B), SCA-17 (TBP) and DRPLA (ATN1) in a clinical-epidemiological study of ET at Columbia University.
Patients and Methods
As described, ET patients (n = 323) were enrolled in an clinical-epidemiological study at the Neurological Institute of New York, Columbia University, New York (2000 – 2007)[6]. Controls (n = 299) were ascertained from the same set of zip codes as ET patients, and were recruited using random-digit telephone dialing, and frequency-matched on age (5-year strata), gender and race categories. Each control was initially screened for tremor using a screening questionnaire and later underwent the same detailed videotaped neurological examination as the patients to ensure that they did not have ET. All participants underwent a demographic and medical history questionnaire, a family history questionnaire (any first- or second-degree relative with nonspecific tremor, ET or Parkinson’s disease [PD]), and a videotaped neurological examination. Self-reported information on race and ethnic group was obtained. Beginning in 2002, self-reported information on Ashkenazi Jewish (AJ) ancestry was also collected. Data on age of onset of tremor, which we have shown to be reliable, were by self-report. On the basis of previous data on the distribution of age of onset in ET, early age of onset was designated as ≤40 years of age.
After review of the history and videotaped examinations, the diagnosis of ET was then reconfirmed by a senior neurologist specializing in movement disorders (E.D.L) using published research criteria for possible, probable or definite ET, which all required moderate amplitude or greater kinetic tremor on several tasks. Definite ET required both a moderate or greater amplitude postural tremor and moderate or greater amplitude kinetic tremor on 4 or more tasks, in the setting of no other tremor etiology. The presence of bradykinesia or any other sign of parkinsonism (except isolated rest tremor) was an exclusionary criterion for ET. No patients or controls had a history of amyotrophic lateral sclerosis (ALS) or evidence of ALS on neurological examination.
The study was approved by the Institutional Review Board at Columbia University and signed informed consent was obtained from all enrollees.
Genotyping/SCA repeat Analysis
PCR was performed using fluorescently-labeled primers flanking the respective repeats in SCA-1 (ATXN1), SCA-2 (ATXN2), SCA-3 (ATXN3), SCA-6 (CACNA1A), SCA-7 (ATXN7), SCA-8 (ATXN8OS), SCA-10 (ATXN10), SCA-12 (PPP2R2B), SCA-17 (TBP) and DRPLA (ATN1) (primers and PCR conditions are available upon request). Following amplification, the PCR products were separated and allele sizes determined on an ABI3730xl genetic analyzer. Data were captured using Peak Scanner software v1.0 (Applied Biosystems, Inc.) and allele size was defined based on comparison with the molecular weight standard Gene Scan 500 LIZ (Applied Biosystems, Inc.). The following positive controls, with repeat sizes determined by sequencing, were also used to accurately determine repeat size. For SCA2, a 22 repeat (19CAG+3CAA) gene scan and genemer control DNA was used (Gene Link Inc, Cat#40-2038-01). For other loci, the following controls with known CAG repeat size were obtained from the Coriell Institute for Medical Research (Camden, NJ): SCA1 (NA06926, 29/52 CAG repeats; NA13537, 32/60 CAG repeats), SCA2 (NA14982, 24/42 CAG repeats), SCA7 (NA03561, 8/62 CAG repeats). For SCA3 and SCA6 patient samples with known repeat sizes were used as positive controls (SCA3, 29/32 CAG repeats and SCA6, 11/13 repeats).
Statistical Analysis
Allele frequencies were calculated from observed genotypes. CLUMP analysis [7], used for association testing when markers produce sparse contingency tables, was used to test differences in allele distribution between ET patients and controls. The stratified analyses involved a separate clump analysis. In stratified clump analyses, matching was accounted for by variable (e.g. ethnicity or gender). In the stratified analyses, based on age at ET onset (≤ 40 years vs. >40 years), ET cases (≤ 40 years or >40 years) were compared to all controls.
Results
Demographic and Clinical Characteristics of Subjects
A total of 622 subjects (323 ET patients and 299 controls), were analyzed for repeat expansion size in ten gene loci (Table 1). The mean age at tremor onset in ET patients was 44.2 years (± 21.9) and 29.4% of ET patients had a family history of ET. There were statistically significant differences in ethnicity and AJ ancestry between ET patients and controls. There were more ET patients with AJ ancestry (38.1%) than controls (19.1%) (p<0.0001) (Table 1). Significant differences were also observed for non-Hispanic white (p=0.0017) and non-Hispanic black ethnicity (p=0.0386) between ET patients and controls (Table 1).
Table 1.
Demographic and Clinical Characteristic of Genotyped Subjects
| ET Cases (N=323) |
Controls (N=299) |
Statistical test |
p- value |
|
|---|---|---|---|---|
| % Male (n) | 48.3 (156) | 41.8 (125) | Χ2=2.39 | 0.1221 |
| Mean age at tremor onset (years) (SD) | 44.2 (21.9) | NA | NA | NA |
| % with family history of ET (n) | 29.4 (95) | NA | NA | NA |
| % Ashkenazi Jewish ancestry (n)* | 38.7 (125) | 19.7 (59) | Χ2=25.91 | <.0001 |
| % Age at onset ≤ 40 yr (n)** | 39.6 (128) | NA | NA | NA |
| % Age at onset > 40 yr (n)** | 56.0 (181) | NA | NA | NA |
| % Non-hispanic White (n)*** | 93.8 (303) | 86.0(257) | Χ2=9.82 | 0.0017 |
| % Non-hispanic Black (n)*** | 2.2(7) | 5.7(17) | Χ2=4.28 | 0.0386 |
| % Hispanic (n)*** | 2.5(8) | 4.0(12) | Χ2=0.74 | 0.3897 |
| % Non-hispanic White-AJ (n)* | 38.1(123) | 19.1(57) | Χ2=26.39 | <.0001 |
Age at onset data not available for 14 ET cases;
Ethnicity data not available on 5 ET cases and 13 controls;
NA, Not applicable.
SCA Repeat Expansions in the Intermediate and Pathogenic Range
Overall, in the ten SCA loci analyzed, we did not identify any ET patients with repeat expansions in the intermediate or pathogenic range. These ranges were as follows: intermediate = 39–41, pathogenic = 41–83 CAG repeats (SCA-1); intermediate = 32–34, pathogenic = 34–77 CAG repeats (SCA-2); intermediate = 40–62, pathogenic = 62–86 CAG repeats (SCA-3); intermediate = 18–21, pathogenic = 21–30 CAG repeats (SCA-6); intermediate = 18–38, pathogenic = 38->200 CAG repeats (SCA-7); intermediate = 91–107, pathogenic = 107–250 CTG repeats (SCA-8); intermediate = 22–550, pathogenic = 550–4500 ATTCT repeats (SCA-10); intermediate = 28–66, pathogenic = 66–78 CAG repeats (SCA-12); intermediate = 43–45, pathogenic = 45–63 CAG repeats (SCA-17); and intermediate = 36–49, pathogenic = 49–88 CAG repeats (DRPLA).
SCA Repeat Size in the Normal Range
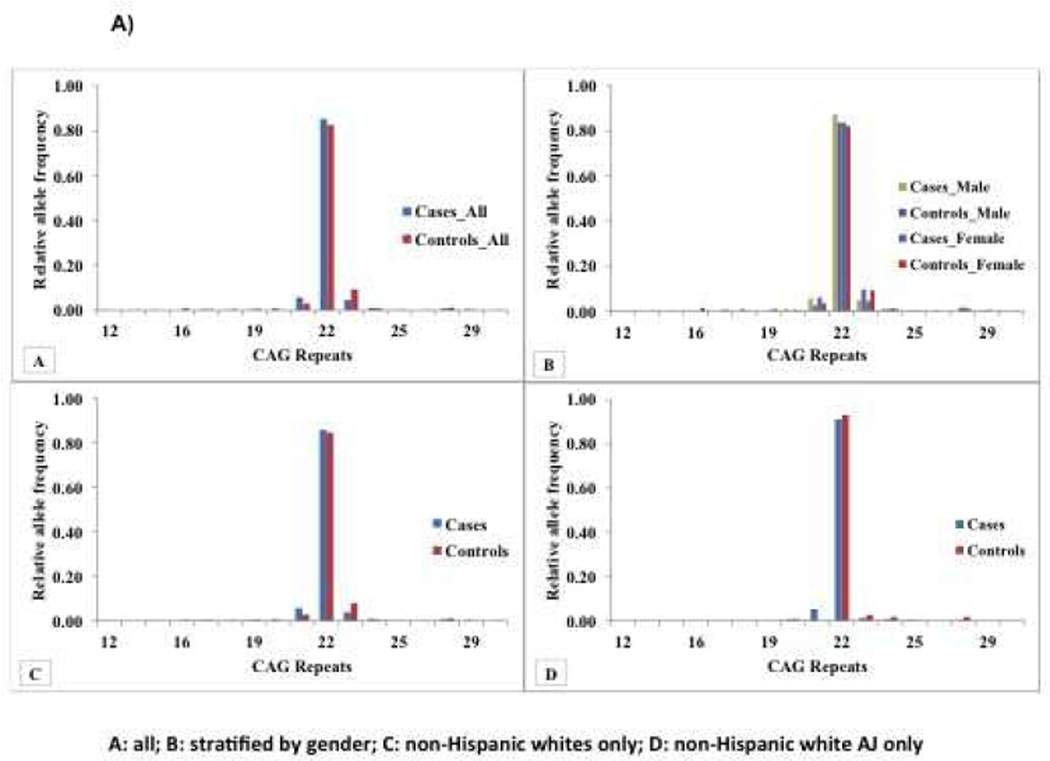
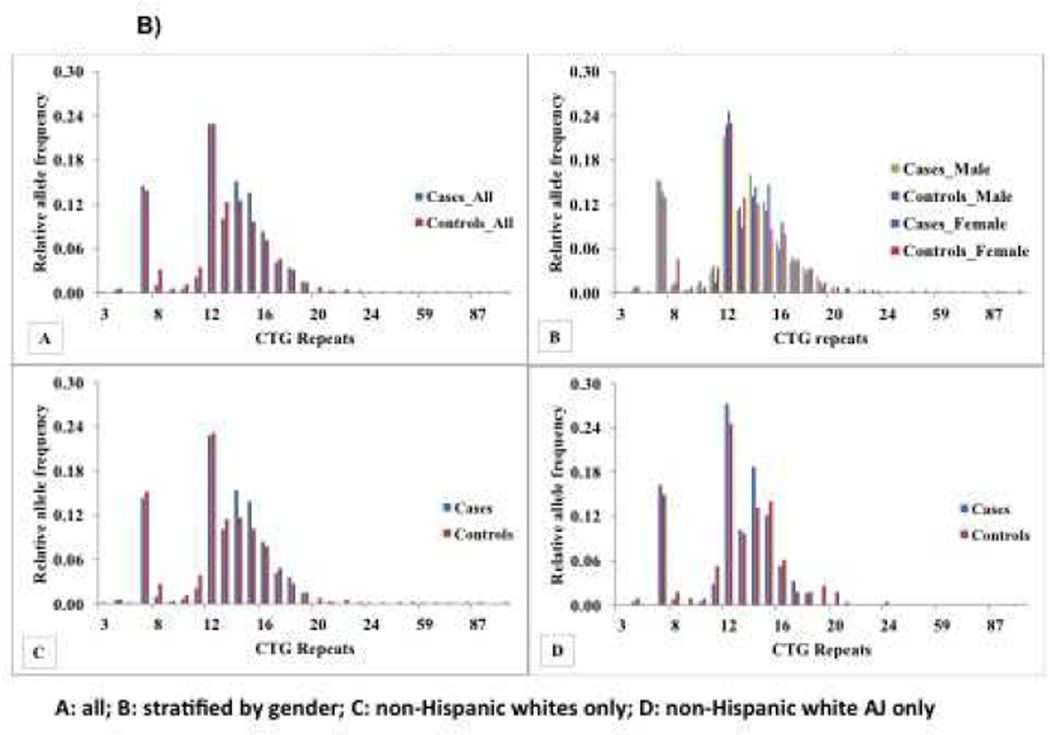
Significant differences in the distribution of repeats in the ‘normal’ range were observed between ET patients and controls for the SCA-2 (Figure 1A) and SCA-8 loci (Figure 1B). Overall there was an increased frequency of specific SCA2 alleles, in ET patients compared to controls (21 CAG repeats), and in controls compared to ET patients (23 CAG repeats) (p=0.004). We also stratified the analysis by clinical and demographic features, including age at ET onset (≤40 years vs. > 40 years), gender, ethnicity (non-Hispanic black, non-Hispanic white, Hispanic) and ancestry (Non-Hispanic white AJ vs. others). There was no association between age at onset of ET and repeat size. A significant difference in the 21 CAG repeat was still observed in ET patients compared to controls when stratified by gender and in non-Hispanic white patients and controls. There was a trend towards significance in non-Hispanic white AJ patients compared to controls (13 ET patients [5.3%] versus 0 controls [0%]), but the difference using the clump statistic was not significant.
Figure 1. Allele Frequency Distribution of Repeats in SCA2 and SCA8 in ET patients and controls.
Figure 1A) Relative Allele Frequency Distribution of SCA2 Repeats in ET patients and Controls: A) All patients and controls (see Table 1 for demographic and clinical characteristics), B) Stratified by gender, 156 male ET patients vs. 125 male controls (mean age at tremor onset in ET cases=43.93 ± 21.88; 150 non-Hispanic white ET patients vs. 110 non-Hispanic white controls; 2 non-Hispanic black ET patients vs. 5 non- Hispanic black controls; 2 Hispanic ET patients vs. 5 Hispanic controls; 51 non-Hispanic white-AJ ET patients vs. 23 non-Hispanic white-AJ controls) and 167 female ET patients vs. 174 female controls mean age at tremor onset in ET patients=44.53 ± 21.92; 153 non-Hispanic white ET patients vs. 147 non-Hispanic white controls; 5 non-Hispanic black ET patients vs. 12 non-Hispanic black controls; 6 Hispanic ET patients vs. 7 Hispanic controls; 72 non-Hispanic white-AJ ET patients vs. 34 non-Hispanic white-AJ controls). C) Stratified by non-Hispanic white ethnicity, 303 ET patients vs. 257 controls (Mean age at tremor onset in ET patients= 44.06±22.07; 150 male ET cases vs. 110 male controls). D) Stratified by non-Hispanic white AJ ancestry, 123 ET cases vs. 57 controls (Mean age at tremor onset in ET patients= 47.38±21.88; 51 male ET cases vs. 23 male controls.
Figure 1B) Relative Allele Frequency Distribution of Repeats in SCA8 in ET patients and controls: A) All patients and controls (see Table 1 for demographic and clinical characteristics), B) Stratified by gender, 156 male ET patients vs. 125 male controls, Stratified by non-Hispanic white ethnicity, 303 ET patients vs. 257 controls and D) Stratified by non-Hispanic white AJ ancestry, 123 ET cases vs. 57 controls. The demographic and clinical characteristics of ET patients and controls within each strata is described in the legend for Figure 1A.
Similar differences for SCA-8 (p=0.02) were observed in the overall sample and in stratified analyses (female ET patients vs. controls) (Figure 1B).
Discussion
We performed an analysis of repeat expansion size in ten common degenerative hereditary ataxia loci in a large case-control study of ET at Columbia University. Our results suggest that repeat expansions in the pathogenic or intermediate range in these 10 are not a cause of (or associated with) ET in the 323 ET patients included in this study. Interestingly, we observed significant differences in the distribution of repeats in the ‘normal’ range between ET patients and controls for the SCA-2 and SCA-8 loci. An association of the SCA-2 CAG repeat or SCA-8 CTG repeat has been described in patients with other neurodegenerative disease including L-dopa-responsive Parkinson’s disease (PD) and in Amyotrophic Lateral Sclerosis (ALS); however the CAG repeat expansions are typically in the ‘intermediate’ or ‘low’ pathological range [8, 9]. The significant difference in the distribution of repeats in the normal range for SCA2 and SCA8 in the current study may indicate in a subset of samples, the presence of a haplotype (founder effect), and that rare variants/mutations located on the haplotype are associated with disease (ET). Although, beyond the scope of the current study, further studies including targeted resequencing of SCA2 and SCA8 in ET patients, may identify rare variants that are associated with disease. An association of rare variants in SCA2 has recently been reported in amyotrophic lateral sclerosis (ALS) [10], and non-ataxic phenotypes of SCA8 mimicking ALS and Parkinson’s disease has also been described. Two studies have previously evaluated SCA-2 and SCA-3 repeat expansions or SCA-12 CAG repeat expansions in Asian and Italian ET patients[11, 12]. While pathogenic SCA-12 CAG repeat expansions were not associated with ET in 30 Italian patients[11], the study of SCA-2 and SCA-3 in 177 ET Asian patients[12] identified 1 ET patient with an SCA-3 CAG repeat in the intermediate range (n=59; heterozygote); none of the 177 ET patients had SCA-2 CAG repeat expansions in the intermediate or pathogenic range.
In conclusion, our study together with previous reports in the literature that have evaluated SCA loci in ET does not provide evidence for disease association with repeat expansion. Evaluation of non-triplet SCA loci in risk for ET in future studies may be warranted.
Conclusions
Our study suggests that repeat expansions in the pathogenic or intermediate range in 10 common degenerative hereditary ataxia genes: SCA-1 (ATXN1), SCA-2 (ATXN2), SCA-3 (ATXN3), SCA-6 (CACNA1A), SCA-7 (ATXN7), SCA-8 (ATXN8OS), SCA-10 (ATXN10), SCA-12 (PPP2R2B), SCA-17 (TBP) and DRPLA (ATN1) are not associated with ET.
Highlights.
ET shares clinical-pathological features with degenerative hereditary ataxias
Analyzed repeat size in 10 degenerative hereditary ataxia loci in 323 ET cases and 299 controls.
Analysis included overall allele distribution, and repeat expansions in the intermediate and pathogenic range.
Our study suggests that pathogenic repeat expansions in SCA loci are not associated with essential tremor.
Acknowledgements
Dr. Louis has received support from the National Institutes of Health: NINDS #R01 NS042859 (PI), NINDS #R01 NS39422 (PI), NINDS #T32 NS07153-24 (PI), NINDS #R01 NS073872 (PI), NINDS #R21 NS077094 (CoI), and NINDS #R01 NS36630 (coI), as well as the Parkinson's Disease Foundation (PI), the Arlene Bronstein Essential Tremor Research Fund (Columbia University), and the Claire O'Neil Essential Tremor Research Fund (Columbia University).
Dr. Clark has received support from NIH: R21NS050487 (PI), R01NS060113 (PI), R01NS0738072 (CoPI), P50AG008702 (CoI), P50 NS038370 (CoI), as well as the Parkinson’s Disease foundation (PI) and the Michael J Fox foundation (CoI).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
LN Clark, Email: lc654@cumc.columbia.edu.
X Ye, Email: xy2177@cumc.columbia.edu.
X Liu, Email: xl2269@cumc.columbia.edu.
K Mirzozoda, Email: km2825@columbia.edu.
ED Louis, Email: elan.louis@yale.edu.
References
- 1.Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord. 2010;25:534–541. doi: 10.1002/mds.22838. [DOI] [PubMed] [Google Scholar]
- 2.Tanner CM, Goldman SM, Lyons KE, Aston DA, Tetrud JW, Welsh MD, et al. Essential tremor in twins: an assessment of genetic vs environmental determinants of etiology. Neurology. 2001;57:1389–1391. doi: 10.1212/wnl.57.8.1389. [DOI] [PubMed] [Google Scholar]
- 3.Louis ED. Essential tremor: from bedside to bench and back to bedside. Current opinion in neurology. 2014;27:461–467. doi: 10.1097/WCO.0000000000000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schols L, Bauer P, Schmidt T, Schulte T, Riess O. Autosomal dominant cerebellar ataxias: clinical features, genetics, and pathogenesis. The Lancet Neurology. 2004;3:291–304. doi: 10.1016/S1474-4422(04)00737-9. [DOI] [PubMed] [Google Scholar]
- 5.Jankovic J, Beach J, Pandolfo M, Patel PI. Familial essential tremor in 4 kindreds. Prospects for genetic mapping. Arch Neurol. 1997;54:289–294. doi: 10.1001/archneur.1997.00550150047015. [DOI] [PubMed] [Google Scholar]
- 6.Clark LN, Park N, Kisselev S, Rios E, Lee JH, Louis ED. Replication of the LINGO1 gene association with essential tremor in a North American population. European Journal of Human Genetics. 2010;18:838–843. doi: 10.1038/ejhg.2010.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sham PC, Curtis D. Monte Carlo tests for associations between disease and alleles at highly polymorphic loci. Annals of human genetics. 1995;59:97–105. doi: 10.1111/j.1469-1809.1995.tb01608.x. [DOI] [PubMed] [Google Scholar]
- 8.Charles P, Camuzat A, Benammar N, Sellal F, Destee A, Bonnet AM, et al. Are interrupted SCA2 CAG repeat expansions responsible for parkinsonism? Neurology. 2007;69:1970–1975. doi: 10.1212/01.wnl.0000269323.21969.db. [DOI] [PubMed] [Google Scholar]
- 9.Tazen S, Figueroa K, Kwan JY, Goldman J, Hunt A, Sampson J, et al. Amyotrophic lateral sclerosis and spinocerebellar ataxia type 2 in a family with full CAG repeat expansions of ATXN2. JAMA neurology. 2013;70:1302–1304. doi: 10.1001/jamaneurol.2013.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cady J, Allred P, Bali T, Pestronk A, Goate A, Miller TM, Mitra RD, Ravits J, Harms MB, Baloh RH. Amyotrophic lateral sclerosis onset is influenced by the burden of rare variants in known amyotrophic lateral sclerosis genes. Ann Neurol. 2015;77:100–113. doi: 10.1002/ana.24306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicoletti G, Annesi G, Carrideo S, Tomaino C, Di Costanzo A, Zappia M, et al. Familial essential tremor is not associated with SCA-12 mutation in southern Italy. Movement Disorders. 2002;17:837–838. doi: 10.1002/mds.10191. [DOI] [PubMed] [Google Scholar]
- 12.Tan EK, Tong J, Pavanni R, Wong MC, Zhao Y. Genetic analysis of SCA 2 and 3 repeat expansions in essential tremor and atypical Parkinsonism. Mov Disord. 2007;22:1971–1974. doi: 10.1002/mds.21699. [DOI] [PubMed] [Google Scholar]