Human amniotic membrane-derived mesenchymal stem cells (AMMSCs) or adipose tissue-derived mesenchymal stem cells (ADMSCs) were intravenously transplanted into rats once a month. AMMSC and ADMSC transplantation improved cognitive and physical functions of naturally aging rats and extended life span, indicating that repeated transplantation of AMMSCs and ADMSCs elongate both health span and life span, which could be a starting point for antiaging or rejuvenation effects of allogeneic or autologous stem cells with minimum immune rejection.
Keywords: Amniotic membrane-derived mesenchymal stem cell, Life span, Adipose tissue-derived mesenchymal stem cell, Health span, Cognitive function, Physical activity
Abstract
Aging brings about the progressive decline in cognitive function and physical activity, along with losses of stem cell population and function. Although transplantation of muscle-derived stem/progenitor cells extended the health span and life span of progeria mice, such effects in normal animals were not confirmed. Human amniotic membrane-derived mesenchymal stem cells (AMMSCs) or adipose tissue-derived mesenchymal stem cells (ADMSCs) (1 × 106 cells per rat) were intravenously transplanted to 10-month-old male F344 rats once a month throughout their lives. Transplantation of AMMSCs and ADMSCs improved cognitive and physical functions of naturally aging rats, extending life span by 23.4% and 31.3%, respectively. The stem cell therapy increased the concentration of acetylcholine and recovered neurotrophic factors in the brain and muscles, leading to restoration of microtubule-associated protein 2, cholinergic and dopaminergic nervous systems, microvessels, muscle mass, and antioxidative capacity. The results indicate that repeated transplantation of AMMSCs and ADMSCs elongate both health span and life span, which could be a starting point for antiaging or rejuvenation effects of allogeneic or autologous stem cells with minimum immune rejection.
Significance
This study demonstrates that repeated treatment with stem cells in normal animals has antiaging potential, extending health span and life span. Because antiaging and prolonged life span are issues currently of interest, these results are significant for readers and investigators.
Introduction
Aging is characterized by the loss of regenerative capacity of cells and tissues, leading to the shrinkage of body mass and increased susceptibility to stresses [1]. Progressive deterioration of cholinergic and dopaminergic systems is observed during aging [2], affecting both cognitive function and physical activity [3]. Molecular oxidative damage is the main causal factor underlying senescence-associated loss in physiological functions [4]. Oxidative stress causes exhaustion of the stem cell population [5]. When new cells are not able to replace the ones that died, tissue integrity and functions decline [6]. Therefore, it has been suggested that exhaustion of stem cells may be a major cause of aging in humans [7] and that the proliferative potential of stem cells is related to life span [8].
During aging, the concentration of neurotrophins in the brain and muscles decreases, leading to reduced neurogenesis and accelerated muscular atrophy, followed by the impairments of cognitive function and physical activity [9]. Growth and neurotrophic factors are involved not only in the development and protection of cholinergic, dopaminergic, and motor neurons [10, 11], but also in myogenesis, muscular innervation, and angiogenesis [12–14]. Various stem cells exert neuroprotective effect and enhance functional recovery of animals by secreting neurotrophic factors. In Alzheimer’s disease (AD) model animals, neural stem cells (NSCs) improved cognitive deficit by enhancing hippocampal synaptic density mediated by brain-derived neurotrophic factor (BDNF) [15], and NSCs overexpressing nerve growth factor (NGF) markedly restored learning and memory functions [16]. BDNF and glial cell-derived neurotrophic factor (GDNF) also protected dopaminergic system and motor behavior [2, 10]. Therefore, it is believed that growth and neurotrophic factors may be key molecules for the structural and functional integrities of the body.
In our recent studies, transplantation of human NSCs overexpressing choline acetyltransferase (ChAT) gene improved the learning and memory functions of AD model and aging animals by increasing acetylcholine (ACh) concentration [17–19]. Notably, brain and reproductive organs including placenta contain high levels of ChAT, and amniotic membrane epithelial stem cells (AMESCs) and amniotic membrane-derived mesenchymal stem cells (AMMSCs) express ChAT and secrete ACh [20–22]. Indeed, transplantation of AMESCs and AMMSCs ameliorated spatial memory deficit in AD model animals [22, 23]. Therefore, it was expected that transplantation of AMMSCs can contribute to the recovery of cognitive function of aged animals.
Adipose tissue-derived mesenchymal stem cells (ADMSCs) have been proposed as a promising therapy of various neurodegenerative diseases such as AD and stroke [24–26]. Interestingly, ADMSCs not only exerted preventive and therapeutic effects on the memory deficit of AD mice [27] but also improved the cognitive and physical functions of aging mice by increasing brain concentrations of ACh, BDNF, and NGF [28]. Notably, it was demonstrated that muscle-derived stem/progenitor cells (MSPCs) extended the health spans and life spans of progeria mice by restoring microvessels and muscle fibers mediated by secreted factor(s) [29]. In our recent study [30], it was confirmed that ADMSCs secrete a very high concentration of vascular endothelial growth factor (VEGF), contributing to prolongation of life span in an amyotrophic lateral sclerosis mouse model. Such beneficial effects of AMMSCs and ADMSCs on the cognitive function and physical activity led us to investigate whether repeated treatment with the stem cells exert antiaging potentials in normal animals, leading to a life span extension.
Materials and Methods
Ethics Statement
All human tissues were obtained with the approval of the Korea University Medical Center Institutional Review Board (Seoul, Korea). Protocols and procedures of animal experiments complied with the Institutional Animal Care and Use Committee of Laboratory Animal Research Center at Chungbuk National University (Cheongju, Korea).
Animals
Young (7-week-old) and aged (10-month-old) male F344 rats were obtained from Daehan-Biolink (Eumseong, Korea, http://dbl.co.kr). The 10-month-old rats were divided into 3 groups: the vehicle-treated aged group (n = 20), the AMMSC-transplanted group (n = 20), and the ADMSC-transplanted group (n = 30). The 7-week-old rats were used as a young control group. The rats were housed in a room with a constant temperature (22 ± 2°C), relative humidity of 55 ± 10%, and a 12-hour light/dark cycle. The rats were fed standard rodent chow and purified water ad libitum.
Preparation and Transplantation of AMMSCs and ADMSCs
Human AMMSCs and ADMSCs were prepared under good manufacturing practice conditions (Biostar, Seoul, Korea, http://www.k-stemcell.co.kr). For AMMSCs, human placenta was obtained after vaginal delivery from a woman with informed consent. The amnion was mechanically detached from the placenta. After washing with sterile saline, the amnion tissues were cut with scissors and digested with collagenase type I (1 mg/ml), in a shaking incubator at 37°C for 30 minutes. The digested tissues were filtered through 100-μm cell strainers and centrifuged at 850g for 4 minutes. The pellet was resuspended in α-minimum essential medium-based medium (Gibco, Grand Island, NY, http://www.invitrogen.com) containing 10% fetal bovine serum (FBS; Gibco). The cells were used for the experiments at passage 3. For ADMSCs, human abdominal subcutaneous fat tissues were obtained by simple liposuction from a 53-year-old female donor after obtaining an informed consent [31]. The adipose tissues were digested with collagenase I, filtered through a 100-mm nylon sieve, and centrifuged at 470g for 5 minutes. The pellet was resuspended in Dulbecco’s modified Eagle’s medium (Invitrogen, Grand Island, NY, http://www.invitrogen.com) containing 0.2 mM ascorbic acid and 10% FBS. The cell suspension was recentrifuged at 470g for 5 minutes, and the cell pellet was collected. After overnight culture, nonadherent cells were removed by washing with phosphate-buffered saline (PBS). The cell medium was changed to keratinocyte-serum-free keratinocyte medium (SFM; Invitrogen) containing 0.2 mM ascorbic acid, 0.09 mM calcium, 5 ng/ml recombinant epidermal growth factor, and 5% FBS. The cells were maintained for 4–5 days until confluent (passage 0). When the cells reached 90% confluence, they were subculture expanded in keratinocyte-SFM medium until passage 3. FBS from cultured stem cells was completely removed by several washes with PBS and was verified by testing the albumin level below the measurement limit using a bovine albumin enzyme-linked immunosorbent assay quantification kit (Bethyl Laboratories, Montgomery, TX, http://www.bethyl.com). AMMSCs and ADMSCs were dissolved in an appropriate volume of saline (1 × 106 cells per 100 μl per rat) and intravenously transplanted to rats via the tail veins once a month throughout their lives.
Measurement of Physical Activity
Spontaneous locomotor activity was evaluated using a video tracking system (Smart v2.5; Panlab Technology, Barcelona, Spain, http://www.panlab.com) connected to a closed-circuit television monitor 1 week after transplantation of AMMSCs or ADMSCs every month [19, 28]. The rats were placed in a quiet chamber, and the times of each movement type (i.e., resting, slow-moving, and fast-moving) were recorded for 5 minutes following a 15-second adaptation time, and the ratio was analyzed.
Motor coordination and balance were evaluated using a rotarod test system (Panlab Technology) 1 week after transplantation of AMMSCs or ADMSCs every month. Rats were placed on the rotating rod at a constant speed of 12 rpm, and the time to a fall off the rod was recorded.
In order to analyze physical stamina, all rats were allowed to swim for 5 minutes for adaptation to a swimming pool with a constant water temperature of 25 ± 0.5°C for 4 days before the measurement. All rats were subjected to a weight-loaded forced-swimming exercise 1 week after transplantation of AMMSCs or ADMSCs every month. The rats were loaded with a lead ring weighing 5% of their body weight to the tail and were then placed in the swimming pool [32]. The test was performed by forcing rats to swim until exhaustion, which was determined by observing loss of coordinated movements and failure to return to the water surface within 7 seconds. This 7-second cutoff time was used as a criterion of the maximum swimming capacity of the rats.
Measurement of Cognitive Functions
Passive avoidance performance was assessed by shuttle box (ENV-010MD; Med Associates, St. Albans, VT, http://www.med-associates.com) to evaluate memory acquisition and retention when the survival rate of rats was 70% (21 months old). Electric shock (1 mA for 2 seconds) was delivered when rats entered the dark compartment from the light room through a guillotine door. Seven consecutive trials, once a day, were performed. The latency time of remaining in a room with the light on following electric shock in a dark compartment was recorded. The endpoint was set to 300 seconds, denoting full acquisition of memory [17–19, 28].
Morris water-maze performance was assessed in a round water bath (180 cm in diameter) filled with water (27 cm in depth) maintained at 22 ± 2°C to evaluate spatial memory. The bath was divided into four quadrants, and a hidden escape platform (10 cm in diameter, 25 cm in height) was submerged in the center of one quadrant, 2 cm below the surface of water. The rats were subjected to 7 trials once a day to find the platform hidden by white Styrofoam granules (5 cm in diameter) on the surface of the water, based on several cues external to the maze. Escape latency (time needed to escape onto the platform during trials) was recorded. The endpoint was set to 300 seconds [19, 28].
Analysis of ACh Concentration in CSF
The rats were sacrificed 24 hours after the final cognitive function tests. Cerebrospinal fluid (CSF) and gastrocnemius muscles were collected, and ACh concentrations in CSF and muscle homogenate were measured with an Amplex Red acetylcholine/acetylcholinesterase assay kit (Molecular Probes, Eugene, OR, http://probes.invitrogen.com) according to the manufacturer’s instructions [17–19].
Immunohistochemistry in Brain Sections
The brains of rats were perfusion-fixed with 10% paraformaldehyde solution and postfixed in the same fixation solution for 2 days, followed by cryoprotection in 30% sucrose for 3 days. Coronal cryosections of 30-μm thickness were prepared and processed for double immunostaining for human mitochondria (hMito, for human cells), neurofilament-high molecular weight protein (NF-H, for neurons), microtubule-associated protein 2 (MAP2, for neurons), or choline acetyltransferase (ChAT, for cholinergic neurons) using antibodies specific for hMito (1:200; mouse monoclonal; Chemicon, Temecula, CA, http://www.chemicon.com), NF-H (1:200; rabbit polyclonal; Chemicon), MAP2 (1:200; rabbit polyclonal; Santa Cruz Biotechnology Inc., Santa Cruz, CA, http://www.scbt.com), or ChAT (1:200; rabbit polyclonal; Chemicon). In addition, to identify proliferating cells, sections were also immunostained with antibodies of nestin (1:200; mouse polyclonal; Chemicon) or Ki-67 (1:200; rabbit polyclonal; Chemicon). Brain sections were incubated with primary antibodies overnight at 4°C and with secondary antibodies conjugated with Alexa Fluor 488 or 594 (1:500; rabbit polyclonal; Molecular Probes) for 2 hours at room temperature. All samples were examined immediately after staining and photographed with a laser-scanning confocal microscope (LSM710; Carl Zeiss, Oberkochen, Germany, http://www.zeiss.com) [17–19, 28].
RT-PCR Analysis in Brain Tissues
Total RNA was extracted from substantia nigra and hippocampus of rats using TRIzol (Invitrogen) for reverse transcriptase-polymerase chain reaction (RT-PCR) analysis. Complementary DNA templates were obtained from 1 μg of total RNA primed with oligo(dT) primers using 40 U of Moloney murine leukemia virus reverse transcriptase (Promega, Madison, WI, http://www.promega.com) followed by 40 PCR cycles, and RT-PCR products were separated on a 1.2% agarose gel containing ethidium bromide [17–19]. The primers used for RT-PCR described in supplemental online Table 1 (Bioneer, Daejeon, Korea, http://www.bioneer.co.kr).
Western Blot Analysis in Brain Tissues
Whole brain and gastrocnemius muscles of rats were homogenized in RIPA buffer (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com). Separately, hepatic mitochondria were isolated using a mitochondria isolation kit (Pierce, Rockford, IL, http://www.piercenet.com). Proteins were quantified using a BCA protein assay kit (Pierce). Proteins were denatured by heating for 5 minutes at 95°C in 0.5 M Tris-HCl buffer (pH 6.8) containing 10% SDS and 10% ammonium persulfate, separated by electrophoresis on 7.5% or 10% SDS-polyacrylamide gels, depending on protein size, and transferred to a polyvinylidene difluoride membrane in 25 mM Tris buffer containing 15% methanol, 1% SDS, and 192 mM glycine. After blocking for 2 hours with 5% skim milk in Tris-buffered saline-Tween (TBS-T) (pH 7.6), the membrane was incubated with antibodies specific for MAP2 (1:500; rabbit polyclonal; Santa Cruz Biotechnology), NGF (1:500; rabbit polyclonal; Santa Cruz Biotechnology), BDNF (1:500; rabbit polyclonal; Santa Cruz Biotechnology), VEGF (1:500; rabbit polyclonal; Santa Cruz Biotechnology), GDNF (1:500; rabbit polyclonal; Santa Cruz Biotechnology), insulin-like growth factor-1 (IGF-1) (1:500; rabbit polyclonal; Santa Cruz Biotechnology), or mitochondrial catalase (mCAT) (1:500; rabbit polyclonal; Abcam, Cambridge, MA, http://www.abcam.com) overnight at 4°C. After washing with TBS-T, the membrane was incubated with a secondary goat anti-rabbit IgG conjugated with horseradish peroxidase (1:2,000; Santa Cruz Biotechnology) for 2 hours at room temperature. The membrane was then developed using an enhanced chemiluminescence solution (Pierce) [19, 28].
Identification of Microvessels
Microvessel density was verified by immunohistochemical staining for von Willebrand factor (vWF) to identify endothelial cells of blood vessels [33]. The brain and gastrocnemius muscles were fixed in 10% neutral formalin, and paraffin-embedded sections were pretreated with citrate buffer (pH 6.0), blocked with serum, and incubated with primary antibody specific for vWF (1:200; rabbit polyclonal; Chemicon) overnight at 4°C. The sections were incubated with biotinylated secondary antibody for 1 hour at room temperature, followed by an avidin-biotin complex kit (Vector Laboratories, Burlingame, CA, http://www.vectorlabs.com), and developed with diaminobenzidine (Sigma-Aldrich). In order to clarify whether transplanted stem cells form new microvessels, double immunostaining for hMito and vWF was performed as described above.
Assay for Antioxidative Effects
To assess oxidative tissue injury, an aliquot (500 μl) of tissue homogenates was acidified by adding 500 μl of SDS (8.1% solution) and 1 ml of 20% acetic acid (adjusted to pH 3.5). After adding 500 μl of 2-thiobarbituric acid (TBA) (0.75% solution), the mixture was boiled in a glass tube capped for 30 minutes at 95°C. Samples were cooled on ice and centrifuged at 13,000g for 10 minutes, and absorbance of the supernatant was read at 532 nm for the quantification of TBA-reactive substances (TBARSs) [32].
Data and Statistical Analysis
The data are presented as means ± SEM. Statistical significance between the groups was determined by one-way analysis of variance followed by post hoc Tukey’s multiple-comparison tests using the SAS program (version 6.12; SAS Institute, Inc., Cary, NC, http://www.sas.com). p values of <.05 were considered to be statistically significant.
Results
Improvement of Physical Activity and Cognitive Function
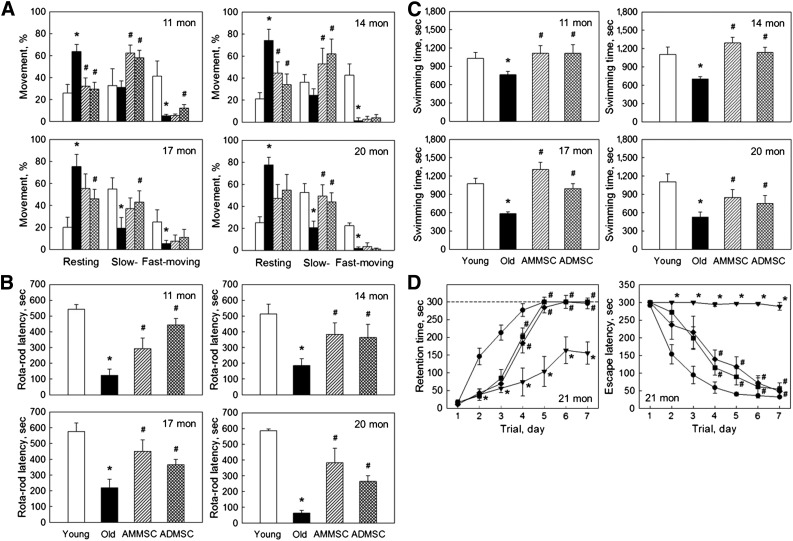
Aged rats (11–20 months old) showed decreased locomotor activity (Fig. 1A). The resting time of aged rats markedly increased to 65%–75%, in comparison with young rats exhibiting 70%–80% moving (slow- and fast-moving) activity. Transplantation of AMMSCs or ADMSCs significantly improved the activity of aged rats, showing increased slow-moving time, up to 20 months of age when the activity testing was finished because only 30% control (vehicle-treated) rats survived, compared with 70% and 100% survival of AMMSC- and ADMSC-treated animals, respectively. The latency time in rotarod performance of aged rats markedly decreased to 10%–40% of that of young rats, exhibiting more severe impairment of the performance with the increase in age (Fig. 1B). However, repeated transplantation of AMMSCs or ADMSCs greatly improved the motor coordination of aged rats. Maximum swimming time of aged rats also decreased, reaching a half level of young rats at 20 months of age (Fig. 1C). Monthly transplantation of AMMSCs or ADMSCs markedly increased the stamina of aged rats, although the effects were relatively low in superaged animals (20 months). The 21-month-old rats displayed severe deficits in learning and memory functions (Fig. 1D); the aged rats showed a greatly delayed increase in retention time and long escape latency during repeated trials in passive avoidance and Morris water-maze performances, respectively, whereas full memory acquisition was obtained on the fifth trials in young animals. Notably, repeated transplantation of AMMSCs or ADMSCs significantly improved the cognitive function in both performances. In comparison, there were no remarkable differences between the effects of AMMSCs and ADMSCs on physical activity and cognitive function.
Figure 1.
Stem cell transplantation recovers physical activity and cognitive function. (A–C): Locomotor activity (resting, slow-moving, and fast-moving times) (A), rotarod latency (B), and maximum swimming time (C) of rats at 11–20 months of age. (D): Retention time and escape latency in passive avoidance (left) and water-maze (right) performances at 21 months of age. White bar, young rats (7 weeks old); black bar, aged rats; striped bar, aged rats transplanted with AMMSCs (106 cells); checked bar, aged rats transplanted with ADMSCs (106 cells). ∗, significantly different from young rats (p < .05). #, significantly different from aged rats (p < .05). Abbreviations: ADMSC, adipose tissue-derived mesenchymal stem cell; AMMSC, amniotic membrane-derived mesenchymal stem cell; mon, months; sec, seconds.
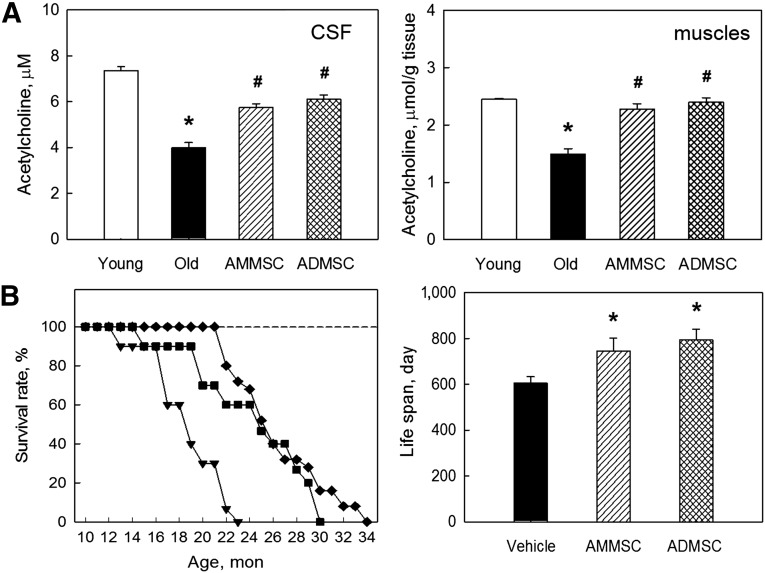
Recovery of ACh Concentration
We analyzed brain and muscular ACh levels to see a relationship with cognitive or motor functions. The ACh concentrations in CSF and muscles of aged (21-month-old) rats were much lower than those of young (7-week-old) animals (Fig. 2A). Such a decreased ACh level in CSF was significantly restored following transplantation of AMMSCs or ADMSCs. Decreased ACh concentration in the muscles of aged rats was also nearly fully recovered by transplantation of the stem cells, although there was no notable difference between the effects of AMMSCs and ADMSCs.
Figure 2.
Stem cell transplantation recovers acetylcholine concentration and extends life spans of aged rats. (A): Acetylcholine concentration in CSF and muscles. (B): Survival rate (left) and mean life span (right). White bar, young rats (7 weeks old); triangle symbol and black bar, aged rats; square symbol and striped bar, aged rats transplanted with AMMSCs (106 cells); diamond symbol and checked bar, aged rats transplanted with ADMSCs (106 cells). ∗, significantly different from young rats (p < .05). #, significantly different from aged rats (p < .05). Abbreviations: ADMSC, adipose tissue-derived mesenchymal stem cells; AMMSC, amniotic membrane-derived mesenchymal stem cells; CSF, cerebrospinal fluid; mon, months.
Extension of Life Span
All F344 rats treated with vehicle (saline) alone died by 23 months of age, displaying 50% mortality at 18.5 months (Fig. 2B). There were 4 cases of tumors observed during the experiment or at autopsy: 1 of 20 rats (5%) in the vehicle-treated aged group and the AMMSC-transplanted group, respectively, and 2 of 30 rats (6.7%) in the ADMSC-transplanted group. All the data of tumor-bearing animals were excluded to avoid the tumor-related alterations in the normal aging including life span. Monthly transplantation of AMMSCs and ADMSCs extended the survival time, leading to 60% and 72% survival rates at 23 months, respectively. Collectively, the mean life span of the rats (604.6 days) was extended to 746.0 days (23.4% increase) and 793.8 days (31.3% increase) by treatment with AMMSCs and ADMSCs, respectively.
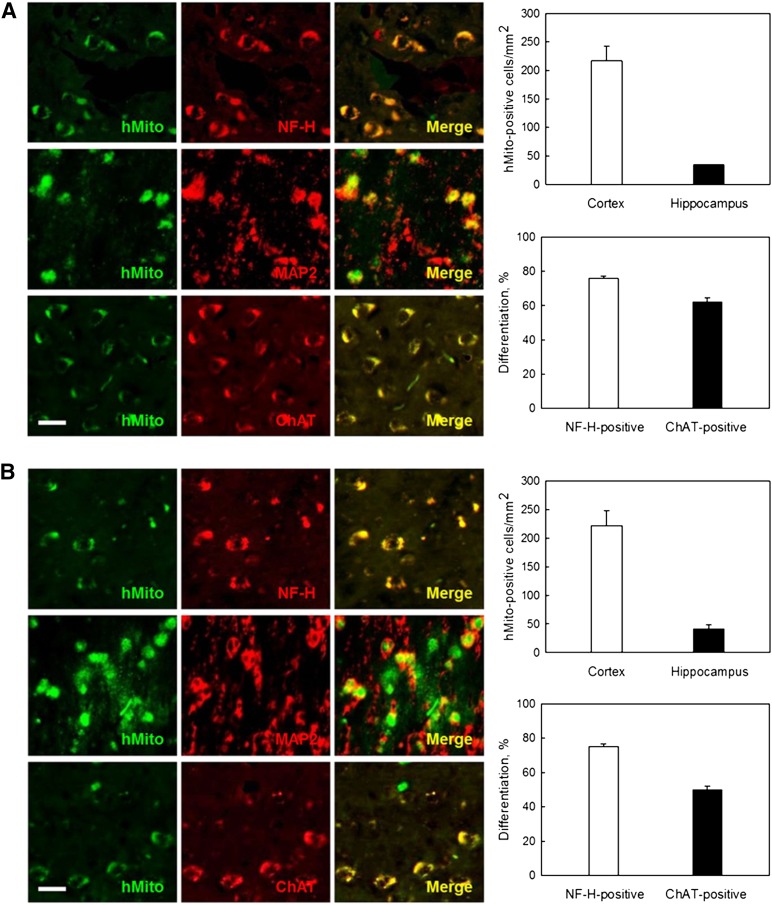
Distribution and Differentiation of AMMSCs and ADMSCs
In analyzing the fate of transplanted cells, hMito immunoreactivity was detected predominantly in the cortex, although a part of cells were found in the hippocampus, after 12 times monthly transplantation of AMMSCs or ADMSCs (106 cells per rat) into 10-month-old rats (Fig. 3A, 3B). The transplanted cells were found to differentiate into NF-H- and MAP2-positive neurons and especially into ChAT-positive cholinergic neurons, exhibiting differentiation rates of 76.0% and 62.1% in AMMSCs and 75.2% and 54.0% in ADMSCs, respectively.
Figure 3.
Transplanted stem cells differentiate into neurons, producing ChAT protein. (A, B): Distribution and differentiation of transplanted human (hMito-positive) amniotic membrane-derived mesenchymal stem cells (A) and adipose tissue-derived mesenchymal stem cells (B) into neurons (NF-H- and MAP2-positive) and cholinergic neurons (ChAT-positive). Scale bar = 20 μm. Abbreviations: ChAT, choline acetyltransferase; hMito, human mitochondria; MAP2, microtubule-associated protein 2; NF-H, neurofilament-high molecular weight protein.
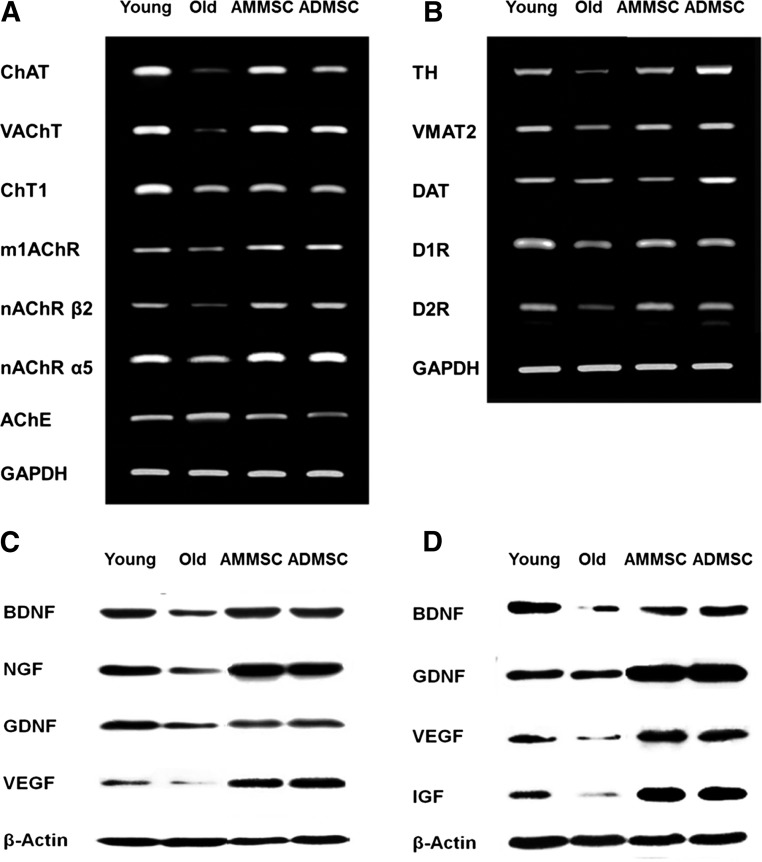
Restoration of Cholinergic and Dopaminergic Nervous Systems
As for the integrity of host nervous systems, the expression of functional genes associated with cholinergic system was markedly changed with aging; genes for ACh synthesis, secretion, and reception, including ChAT, high-affinity choline transporter 1 (ChT1), vesicular ACh transporter (VAChT), muscarinic 1 ACh receptor (m1AChR), nicotinic ACh receptor (nAChR) β2, and nAChR α5 decreased, whereas the gene for acetylcholinesterase, an ACh-degrading enzyme, increased in aged rats (Fig. 4A). Such alterations in the gene expression of cholinergic nerve markers were markedly restored after transplantation of AMMSCs or ADMSCs. The decreased expression of tyrosine hydroxylase (TH), vesicular monoamine transporter 2 (VMAT2), dopamine transporter (DAT), dopamine 1 receptor (D1R), and dopamine 2 receptor (D2R) associated with dopamine synthesis, transportation, and reception in aged rats was also remarkably recovered by transplantation of AMMSCs or ADMSCs (Fig. 4B). Although ADMSCs seemed to be a little bit more effective than AMMSCs in the recovery of cholinergic nerve markers, their relative activities on both nervous systems were not distinguished because of the different effects on each marker.
Figure 4.
Transplanted stem cells restore cholinergic and dopaminergic systems, and increase growth and neurotrophic factors. (A, B): Polymerase chain reaction analysis of cholinergic (A) and dopaminergic (B) nervous system markers in the rat brain. (C, D): Western blot analysis of neurotrophic factors in the brain (C) and muscles (D). Abbreviations: AChE, acetylcholinesterase; ADMSC, adipose tissue-derived mesenchymal stem cells; AMMSC, amniotic membrane-derived mesenchymal stem cells; BDNF, brain-derived neurotrophic factor; ChAT, choline acetyltransferase; ChT1, choline transporter 1; DAT, dopamine transporter; D1R, dopamine 1 receptor; D2R, dopamine 2 receptor; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GDNF, glial cell-derived neurotrophic factor; IGF, insulin-like growth factor; m1AChR, muscarinic 1 acetylcholine receptor; nAChR α5, nicotinic acetylcholine receptor α5; nAChR β2, nicotinic acetylcholine receptor β2; NGF, nerve growth factor; TH, tyrosine hydroxylase; VAChT, vesicular acetylcholine transporter; VEGF, vascular endothelial growth factor; VMAT2, vesicular monoamine transporter 2.
Restoration of Neurotrophic Factors
In order to elucidate functional molecules for neuroprotection and regeneration, we analyzed diverse neurotrophic factors. Concentrations of BDNF, NGF, GDNF, and VEGF markedly decreased in aged rat brain compared with the levels in young animals (Fig. 4C). These neurotrophic factors related to cholinergic and dopaminergic/motor functions were upregulated by transplantation of AMMSCs or ADMSCs. Especially, VEGF possessing an angiogenic potential greatly increased to levels higher than in young rats. Muscular neurotrophic factors such as BDNF, GDNF, VEGF, and IGF-1 decreased in aged rats were also restored by transplantation of AMMSCs or ADMSCs (Fig. 4D). Notably, GDNF and IGF-1 related to motor neuron development and muscular innervation and myogenesis [34, 35], respectively, were upregulated to levels higher than in young rats. In comparison, it was found that AMMSCs and ADMSCs exerted a similar potential on the recovery of neurotrophic factors.
Neovascularization and Antioxidative Capacity
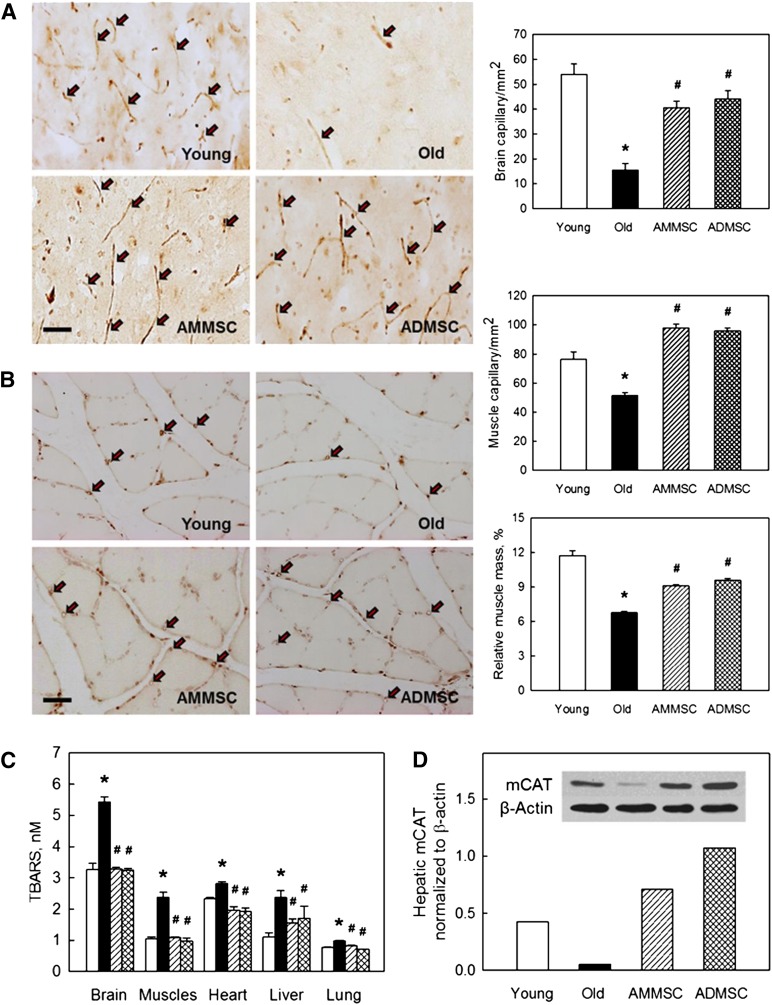
Because capillary density decreases during aging [33], microvessel density was analyzed. In aged rats, the vWF-positive vessel density in the brain was reduced to 28.7% of that of young rats (Fig. 5A). Interestingly, transplantation of AMMSCs or ADMSCs significantly increased the number of blood vessels comparable to that of young rats, indicative of increased angiogenesis. The gastrocnemius muscles of aged rats also showed a significantly decreased vessel density, along with a markedly small muscle mass (Fig. 5B). However, treatment with AMMSCs or ADMSCs restored the number of vWF-positive cells and the muscle weight, to similar levels. Blood flow may affect antioxidative capacity and related tissue damage, and in turn oxidative stress inhibits angiogenesis [36]. The concentrations of TBARS, byproducts of lipid peroxidation, significantly increased in the brain, muscles, heart, liver, and lungs of aged rats with a low vessel density. However, the tissue injury was nearly fully attenuated by transplantation of AMMSCs and ADMSCs (Fig. 5E). In addition, the protein of hepatic mCAT reduced in aged rats was restored to levels higher than young animals following treatment with the stem cells.
Figure 5.
Transplanted stem cells increase microvessel density and muscle mass and enhance antioxidative capacity. (A, B): Density of microvessels (von Willebrand factor-positive) in the brain (A) and muscles (B), and gastrocnemius muscle mass (B). Scale bar = 50 μm. (C): Concentrations of TBARS. (D): Hepatic mCAT. ∗, significantly different from young rats (p < .05). #, significantly different from aged rats (p < .05). Abbreviations: ADMSC, adipose tissue-derived mesenchymal stem cells; AMMSC, amniotic membrane-derived mesenchymal stem cells; mCAT, mitochondrial catalase; TBARS, 2-thiobarbituric acid-reactive substances.
Host Neuroregeneration
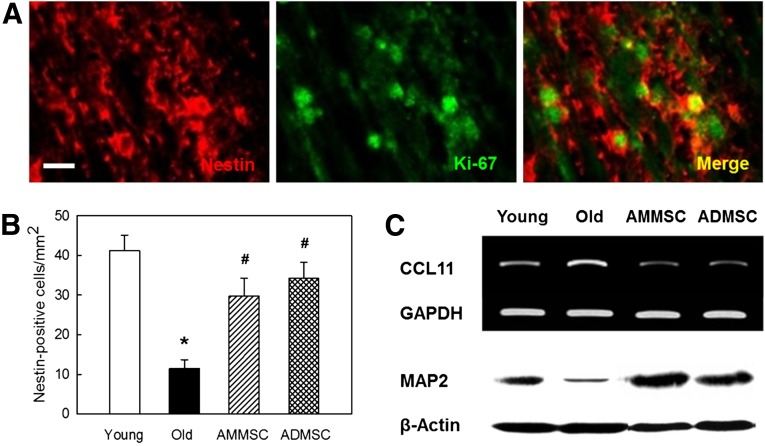
In analysis to confirm host cell regeneration, a high portion of host (nestin-positive) stem cells exhibited immunoreactivity to Ki-67, a proliferating cell marker (Fig. 6A). The number of host stem cells in aged rats was markedly reduced to 27.8% of young rats (Fig. 6B). Transplantation of AMMSCs or ADMSCs increased the number of host stem cells to the level comparable to young rats, indicative of resumption of neurogenesis. Interestingly, expression of CCL11 (eotaxin), a chemokine suppressing neurogenesis, increased during aging [37], in parallel with the marked decrease in the content of MAP2, a neuronal skeletal protein (Fig. 6C). The changes in CCL11 expression and MAP2 production were fully reversed following transplantation of AMMSCs or ADMSCs.
Figure 6.
Stem cell transplantation increases host stem cell proliferation, suppresses CCL11 expression, and restores neuronal integrity. (A): Double immunostaining for host nestin (stem cell marker) and Ki-67 (proliferating cell marker) in the brain of rats transplanted with ADMSCs. Scale bar = 25 μm. (B): Host stem cell counts. White bar, young rats; black bar, aged rats; striped bar, aged rats transplanted with AMMSCs; checked bar, aged rats transplanted with ADMSCs. ∗, significantly different from young rats (p < .05). #, significantly different from aged rats (p < .05). (C): Polymerase chain reaction and Western blot analyses of CCL11 and MAP2, respectively, in the brain. Abbreviations: ADMSC, adipose tissue-derived mesenchymal stem cells; AMMSC, amniotic membrane-derived mesenchymal stem cells; MAP2, microtubule-associated protein 2; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Discussion
Universal characteristics of aging are the decreases in physical activity and cognitive function, which may be due to the loss of regenerative capacity, determining the longevity [1]. In the present study, we demonstrated that repeated intravenous transplantation of AMMSCs and ADMSCs improved the cognitive function and physical activity, leading to an extension of the life spans of rats. In fact, there are several reports on the life span elongation of mammals. Calorie restriction delayed the onset of age-related diseases such as cancer, diabetes, cardiovascular diseases, and brain atrophy in rhesus monkeys and reduced their mortality (50% vs. 80% survival for control feeding and dietary restriction, respectively) [38]. Rapamycin also extended the life span of mice by 9%–14% via target of rapamycin signaling with similar underlying mechanisms to calorie restriction, including reduced oxidative stress [39, 40]. Interestingly, it was reported that calorie restriction enhanced skeletal muscle stem cell function, implying that metabolic factors related to mitochondrial activity play a critical role for regulating stem cell number and function [41]. In addition, MSPCs extended the health spans and life spans of progeria mice [29]. Notably, we used tumor-free F344 rats to avoid a possible effect of stem cells on tumor-related change in longevity and showed antiaging activities of AMMSCs and ADMSCs, leading to 23.4%–31.3% increases in life span in a normal aging model.
Transplanted cells were found in the brain, predominantly in the cortex and hippocampus, and differentiated into ChAT-positive neurons. The increased permeability of the blood-brain barrier with aging, which might be mediated by oxidative damage, has been confirmed [42]. Thus, it is assumed that intravenously transplanted cells migrated into the aged brain and that the production of ChAT protein contributed the ACh concentration in CSF, as was also confirmed in AD model and aging animals [17–19, 28]. Muscarinic and nicotinic receptors are widely distributed in the hippocampus and cortex and play key roles in spatial and working memories [43, 44]. ChAT is also one of the well-known markers of motor neurons [45]. Repeated transplantation of AMMSCs or ADMSCs markedly recovered the decreased levels of MAP2 and cholinergic and dopaminergic nervous system markers in aged rats, indicative of a structural restoration of brain integrity, although the MAP2 level might be a total amount added by the transplanted stem cells following differentiation into MAP2-positive neurons. The improvements of cognitive and motor functions by stem cell transplantation might be due to the increased ACh levels in the brain and muscles originated not only from the transplanted stem cells but also from restored host cholinergic neurons. In addition, the restored dopaminergic nervous system might lead to additional improvement of physical activity.
As functional molecules for neuroprotection and regeneration, neurotrophic factors were upregulated by transplantation of AMMSCs or ADMSCs. It is believed that BDNF, NGF, and GDNF played an important role for restoration of cholinergic system and cognitive function [11, 15, 16], and BDNF and GDNF played an important role for recovery of the dopaminergic system and motor function [2, 10]. Especially, VEGF related to angiogenesis might have increased the microvessels in the brain and muscles, as shown in the progeria mice treated with MSPCs [13, 29]. Because aging led to a decrease in capillary density [33], neovascularization and increased muscle fibers mediated by factor(s) secreted from MSPCs were suggested to play a key role in the extension of health span in progeria mice [29]. The increase in muscle mass might have come from the myogenic and angiogenic roles of IGF [35, 46], which may be supported by angiogenesis and muscle adaptation via VEGF [13, 35, 47] and BDNF [48], leading to the enhanced stamina in forced swimming in the present study.
It has been postulated that mitochondrial oxidative stress provides a strong correlation with overall mammalian longevity and that increased resistance to oxidative stress should exert antiaging effects and lead to increased life span [4]. In support, overexpression of mCAT markedly extended murine life spans [49, 50]. In the present study, AMMSCs and ADMSCs nearly fully suppressed lipid peroxidation in multiple organs and especially restored hepatic mCAT, reduced in aged rats, to levels much higher than in young animals. Previous studies demonstrated that ADMSCs exhibited an antioxidative effect by increasing activities of antioxidant enzymes [51] and that VEGF enhanced antioxidative capacity by upregulating antioxidant enzymes [52]. Therefore, the decreased tissue injury may be in part mediated by antioxidative activity of VEGF and IGF released from stem cells [13, 35].
It was proposed that the increased blood supply may promote regeneration of tissues by overcoming systemic or local negative regulators of cell proliferation during the aging process [29, 37]. The CCL11-suppressing activity of transplanted AMMSCs and ADMSCs might lead to the increase in Ki-67-positive host stem cells under proliferation, in parallel with the marked increase in the content of MAP2, a neuronal skeletal protein. Therefore, it is suggested that the restoration of cholinergic and dopaminergic systems in aging rat brain resulted from the increased neuroregeneration, in addition to the neuroprotective action of neurotrophic factors [11, 53].
The marked protection by AMMSCs and ADMSCs against oxidative stress in multiple organs during aging might contribute to the elongated life span, as suggested in the effects of rapamycin feeding [40] and caloric restriction [38]. In addition, AMMSCs and ADMSCs extended the life span and health span, probably via neurotrophic factors improving degenerative changes and tissue vascularization, as demonstrated in progeria mice transplanted with MSPCs [29]. We assessed the antiaging potentials of AMMSCs and ADMSCs based on their different properties such as the expression of beneficial molecules including ChAT and VEGF [21, 22, 30]. Although ADMSCs was a little bit superior to AMMSCs in the life span-extending capacity (31.3% vs. 23.4%), it was impossible to elucidate key molecule(s) in the present study, because there were a variety of factors affected by stem cell treatment, in addition to the complex facets of aging. In addition to the previous reports, it should be emphasized that stem cell transplantation can restore impaired central nervous system by eliminating negative regulators of host cell proliferation.
Conclusion
Repeated intravenous treatment of stem cells extended health span and life span by recovering neurotrophic factors in the brain and muscles, leading to restoration of MAP2, cholinergic and dopaminergic nervous systems, microvessels, muscle mass, and antioxidative capacity.
Supplementary Material
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT, and Future Planning (2014R 1A 2A 1A 11052232). We thank Eun-Jung Kim, Christine Sungmin Kim, Alice Chang, Solar Sora Kim, and Diana Kim (Dr. Kim Laboratory, University of British Columbia Hospital, Vancouver, BC, Canada) for their continuous support during in vitro experiments.
Author Contributions
D.K., J.K., D.P., and K.S.K.: performance of animal experiments, assembly of histological samples; E.-K.C.: collection and/or assembly of data; K.S.: performance of animal experiments; H.L., I.S.S., and S.K.K.: preparation and characterization of stem cells; J.C.R. and Y.-B.K.: conception and design, data analysis and interpretation, manuscript writing.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120:437–447. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 2.Lapchak PA, Miller PJ, Jiao S. Glial cell line-derived neurotrophic factor induces the dopaminergic and cholinergic phenotype and increases locomotor activity in aged Fischer 344 rats. Neuroscience. 1997;77:745–752. doi: 10.1016/s0306-4522(96)00492-7. [DOI] [PubMed] [Google Scholar]
- 3.Volkow ND, Gur RC, Wang GJ, et al. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry. 1998;155:344–349. doi: 10.1176/ajp.155.3.344. [DOI] [PubMed] [Google Scholar]
- 4.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 5.Ito K, Hirao A, Arai F, et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12:446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- 6.Rossi DJ, Jamieson CH, Weissman IL. Stems cells and the pathways to aging and cancer. Cell. 2008;132:681–696. doi: 10.1016/j.cell.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 7.Sharpless NE, DePinho RA. How stem cells age and why this makes us grow old. Nat Rev Mol Cell Biol. 2007;8:703–713. doi: 10.1038/nrm2241. [DOI] [PubMed] [Google Scholar]
- 8.Van Zant G. Stem cells and genetics in the study of development, aging, and longevity. Results Probl Cell Differ. 2000;29:203–235. doi: 10.1007/978-3-540-48003-7_11. [DOI] [PubMed] [Google Scholar]
- 9.Rosenzweig ES, Barnes CA. Impact of aging on hippocampal function: Plasticity, network dynamics, and cognition. Prog Neurobiol. 2003;69:143–179. doi: 10.1016/s0301-0082(02)00126-0. [DOI] [PubMed] [Google Scholar]
- 10.Boger HA, Mannangatti P, Samuvel DJ, et al. Effects of brain-derived neurotrophic factor on dopaminergic function and motor behavior during aging. Genes Brain Behav. 2011;10:186–198. doi: 10.1111/j.1601-183X.2010.00654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sofroniew MV, Howe CL, Mobley WC. Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci. 2001;24:1217–1281. doi: 10.1146/annurev.neuro.24.1.1217. [DOI] [PubMed] [Google Scholar]
- 12.Clow C, Jasmin BJ. Brain-derived neurotrophic factor regulates satellite cell differentiation and skeltal muscle regeneration. Mol Biol Cell. 2010;21:2182–2190. doi: 10.1091/mbc.E10-02-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrara N. Molecular and biological properties of vascular endothelial growth factor. J Mol Med (Berl) 1999;77:527–543. doi: 10.1007/s001099900019. [DOI] [PubMed] [Google Scholar]
- 14.Lu B, Je HS. Neurotrophic regulation of the development and function of the neuromuscular synapses. J Neurocytol. 2003;32:931–941. doi: 10.1023/B:NEUR.0000020633.93430.db. [DOI] [PubMed] [Google Scholar]
- 15.Blurton-Jones M, Kitazawa M, Martinez-Coria H, et al. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci USA. 2009;106:13594–13599. doi: 10.1073/pnas.0901402106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee HJ, Lim IJ, Park SW, et al. Human neural stem cells genetically modified to express human nerve growth factor (NGF) gene restore cognition in the mouse with ibotenic acid-induced cognitive dysfunction. Cell Transplant. 2012;21:2487–2496. doi: 10.3727/096368912X638964. [DOI] [PubMed] [Google Scholar]
- 17.Park D, Joo SS, Kim TK, et al. Human neural stem cells overexpressing choline acetyltransferase restore cognitive function of kainic acid-induced learning and memory deficit animals. Cell Transplant. 2012;21:365–371. doi: 10.3727/096368911X586765. [DOI] [PubMed] [Google Scholar]
- 18.Park D, Lee HJ, Joo SS, et al. Human neural stem cells over-expressing choline acetyltransferase restore cognition in rat model of cognitive dysfunction. Exp Neurol. 2012;234:521–526. doi: 10.1016/j.expneurol.2011.12.040. [DOI] [PubMed] [Google Scholar]
- 19.Park D, Yang YH, Bae DK, et al. Improvement of cognitive function and physical activity of aging mice by human neural stem cells over-expressing choline acetyltransferase. Neurobiol Aging. 2013;34:2639–2646. doi: 10.1016/j.neurobiolaging.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 20.Roskoski R, Jr, Lim CT, Roskoski LM. Human brain and placental choline acetyltransferase: Purification and properties. Biochemistry. 1975;14:5105–5110. doi: 10.1021/bi00694a013. [DOI] [PubMed] [Google Scholar]
- 21.Sakuragawa N, Misawa H, Ohsugi K, et al. Evidence for active acetylcholine metabolism in human amniotic epithelial cells: Applicable to intracerebral allografting for neurologic disease. Neurosci Lett. 1997;232:53–56. doi: 10.1016/s0304-3940(97)00570-3. [DOI] [PubMed] [Google Scholar]
- 22.Xue SR, Chen CF, Dong WL, et al. Intracerebroventricular transplantation of human amniotic epithelial cells ameliorates spatial memory deficit in the doubly transgenic mice coexpressing APPswe and PS1ΔE9-deleted genes. Chin Med J (Engl) 2011;124:2642–2648. [PubMed] [Google Scholar]
- 23.Kim KS, Kim HS, Park JM, et al. Long-term immunomodulatory effect of amniotic stem cells in an Alzheimer’s disease model. Neurobiol Aging. 2013;34:2408–2420. doi: 10.1016/j.neurobiolaging.2013.03.029. [DOI] [PubMed] [Google Scholar]
- 24.Yeh DC, Chan TM, Harn HJ, et al. Adipose tissue-derived stem cells in neural regenerative medicine. Cell Transplant. 2015;24:487–492. doi: 10.3727/096368915X686940. [DOI] [PubMed] [Google Scholar]
- 25.Chan TM, Chen JY, Ho LI, et al. ADSC therapy in neurodegenerative disorders. Cell Transplant. 2014;23:549–557. doi: 10.3727/096368914X678445. [DOI] [PubMed] [Google Scholar]
- 26.Chan TM, Harn HJ, Lin HP, et al. The use of ADSCs as a treatment for chronic stroke. Cell Transplant. 2014;23:541–547. doi: 10.3727/096368914X678409. [DOI] [PubMed] [Google Scholar]
- 27.Kim S, Chang KA, Kim J, et al. The preventive and therapeutic effects of intravenous human adipose-derived stem cells in Alzheimer’s disease mice. PLoS One. 2012;7:e45757. doi: 10.1371/journal.pone.0045757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park D, Yang G, Bae DK, et al. Human adipose tissue-derived mesenchymal stem cells improve cognitive function and physical activity in ageing mice. J Neurosci Res. 2013;91:660–670. doi: 10.1002/jnr.23182. [DOI] [PubMed] [Google Scholar]
- 29.Lavasani M, Robinson AR, Lu A, et al. Muscle-derived stem/progenitor cell dysfunction limits healthspan and lifespan in a murine progeria model. Nat Commun. 2012;3:608. doi: 10.1038/ncomms1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim KS, Lee HJ, An J, et al. Transplantation of human adipose tissue-derived stem cells delays clinical onset and prolongs life span in ALS mouse model. Cell Transplant. 2014;23:1585–1597. doi: 10.3727/096368913X673450. [DOI] [PubMed] [Google Scholar]
- 31.Ra JC, Shin IS, Kim SH, et al. Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev. 2011;20:1297–1308. doi: 10.1089/scd.2010.0466. [DOI] [PubMed] [Google Scholar]
- 32.Shin S, Yeon S, Park D, et al. Silk amino acids improve physical stamina and male reproductive function of mice. Biol Pharm Bull. 2010;33:273–278. doi: 10.1248/bpb.33.273. [DOI] [PubMed] [Google Scholar]
- 33.Jucker M, Meier-Ruge W. Effects of brovincamine on stereological capillary parameters in adult and old Fischer-344 rats. Microvasc Res. 1989;37:298–307. doi: 10.1016/0026-2862(89)90048-4. [DOI] [PubMed] [Google Scholar]
- 34.Haase G, Dessaud E, Garcès A, et al. GDNF acts through PEA3 to regulate cell body positioning and muscle innervation of specific motor neuron pools. Neuron. 2002;35:893–905. doi: 10.1016/s0896-6273(02)00864-4. [DOI] [PubMed] [Google Scholar]
- 35.Sadat S, Gehmert S, Song YH, et al. The cardioprotective effect of mesenchymal stem cells is mediated by IGF-I and VEGF. Biochem Biophys Res Commun. 2007;363:674–679. doi: 10.1016/j.bbrc.2007.09.058. [DOI] [PubMed] [Google Scholar]
- 36.Perveen S, Patel H, Arif A, et al. Role of EC-SOD overexpression in preserving pulmonary angiogenesis inhibited by oxidative stress. PLoS One. 2012;7:e51945. doi: 10.1371/journal.pone.0051945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Villeda SA, Luo J, Mosher KI, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Colman RJ, Anderson RM, Johnson SC, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cox LS, Mattison JA. Increasing longevity through caloric restriction or rapamycin feeding in mammals: Common mechanisms for common outcomes? Aging Cell. 2009;8:607–613. doi: 10.1111/j.1474-9726.2009.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harrison DE, Strong R, Sharp ZD, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cerletti M, Jang YC, Finley LW, et al. Short-term calorie restriction enhances skeletal muscle stem cell function. Cell Stem Cell. 2012;10:515–519. doi: 10.1016/j.stem.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Popescu BO, Toescu EC, Popescu LM, et al. Blood-brain barrier alterations in ageing and dementia. J Neurol Sci. 2009;283:99–106. doi: 10.1016/j.jns.2009.02.321. [DOI] [PubMed] [Google Scholar]
- 43.Langmead CJ, Watson J, Reavill C. Muscarinic acetylcholine receptors as CNS drug targets. Pharmacol Ther. 2008;117:232–243. doi: 10.1016/j.pharmthera.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 44.Wess J. Muscarinic acetylcholine receptor knockout mice: Novel phenotypes and clinical implications. Annu Rev Pharmacol Toxicol. 2004;44:423–450. doi: 10.1146/annurev.pharmtox.44.101802.121622. [DOI] [PubMed] [Google Scholar]
- 45.Li Y, Chigurupati S, Holloway HW, et al. Exendin-4 ameliorates motor neuron degeneration in cellular and animal models of amyotrophic lateral sclerosis. PLoS One. 2012;7:e32008. doi: 10.1371/journal.pone.0032008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sonntag WE, Lynch C, Thornton P, et al. The effects of growth hormone and IGF-1 deficiency on cerebrovascular and brain ageing. J Anat. 2000;197:575–585. doi: 10.1046/j.1469-7580.2000.19740575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenstein JM, Mani N, Silverman WF, et al. Patterns of brain angiogenesis after vascular endothelial growth factor administration in vitro and in vivo. Proc Natl Acad Sci USA. 1998;95:7086–7091. doi: 10.1073/pnas.95.12.7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sakuma K, Yamaguchi A. The recent understanding of the neurotrophin’s role in skeletal muscle adaptation. J Biomed Biotechnol. 2011;2011:201696. doi: 10.1155/2011/201696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schriner SE, Linford NJ, Martin GM, et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- 50.Wanagat J, Dai DF, Rabinovitch P. Mitochondrial oxidative stress and mammalian healthspan. Mech Ageing Dev. 2010;131:527–535. doi: 10.1016/j.mad.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim WS, Park BS, Kim HK, et al. Evidence supporting antioxidant action of adipose-derived stem cells: Protection of human dermal fibroblasts from oxidative stress. J Dermatol Sci. 2008;49:133–142. doi: 10.1016/j.jdermsci.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 52.Abid MR, Schoots IG, Spokes KC, et al. Vascular endothelial growth factor-mediated induction of manganese superoxide dismutase occurs through redox-dependent regulation of forkhead and IkappaB/NF-kappaB. J Biol Chem. 2004;279:44030–44038. doi: 10.1074/jbc.M408285200. [DOI] [PubMed] [Google Scholar]
- 53.Schäbitz WR, Sommer C, Zoder W, et al. Intravenous brain-derived neurotrophic factor reduces infarct size and counterregulates Bax and Bcl-2 expression after temporary focal cerebral ischemia. Stroke. 2000;31:2212–2217. doi: 10.1161/01.str.31.9.2212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.