The biological characteristics of haploidentical mesenchymal stem cells (MSCs) from fetal sources were analyzed and compared with maternal decidua MSCs. The results suggest that assessing the prevalence of fetomaternal contamination within placental MSCs is necessary to increase robustness and limit side effects in their clinical use. The results also show evidence positioning fetoplacental cells in the forefront of the quest for superior cell types for applications in regenerative medicine.
Keywords: Mesenchymal stem cells, Umbilical cord, Chorion, Decidua, Angiogenic potential, Immunosuppression, Fetal, Maternal, Placental cells
Abstract
Mesenchymal stem cells (MSCs) of placental origin have become increasingly translational owing to their abundance and accessibility. MSCs of different origin share several features but also present biological differences that might point to distinct clinical properties. Hence, mixing fetal and maternal cells from the same placenta can lead to contradicting results. We analyzed the biological characteristics of haploidentical MSCs isolated from fetal sources, including the umbilical cord (UC-MSCs) and chorion (Ch-MSCs), compared with maternal decidua MSCs (Dc-MSCs). All MSCs were analyzed for general stem cell properties. In addition, immunosuppressive capacity was assessed by the inhibition of T-cell proliferation, and angiogenic potential was evaluated in a Matrigel transplantation assay. The comparison between haploidentical MSCs displayed several distinct features, including (a) marked differences in the expression of CD56, (b) a higher proliferative capacity for Dc-MSCs and UC-MSCs than for Ch-MSCs, (c) a diversity of mesodermal differentiation potential in favor of fetal MSCs, (d) a higher capacity for Ch-MSCs to inhibit T-cell proliferation, and (e) superior angiogenic potential of Ch-MSCs evidenced by a higher capability to form tubular vessel-like structures and an enhanced release of hepatocyte growth factor and vascular endothelial growth factor under hypoxic conditions. Our results suggest that assessing the prevalence of fetomaternal contamination within placental MSCs is necessary to increase robustness and limit side effects in their clinical use. Finally, our work presents evidence positioning fetoplacental cells and notably Ch-MSCs in the forefront of the quest for cell types that are superior for applications in regenerative medicine.
Significance
This study analyzed the biological characteristics of mesenchymal stem cells (MSCs) isolated from fetal and maternal placental origins. The findings can be summarized as follows: (a) important differences were found in the expression of CD56, (b) a different mesodermal differentiation potential was found in favor of fetal MSCs, (c) a higher immunosuppressive capacity for chorion MSCs was noted, and (d) superior angiogenic potential of Ch-MSCs was observed. These results suggest that assessing the prevalence of fetomaternal contamination within placental MSCs is necessary to increase robustness and limit side effects in their clinical use. The evidence should allow clinicians to view fetoplacental cells, notably Ch-MSCs, favorably as candidates for use in regenerative medicine.
Introduction
Mesenchymal stem cells (MSCs) are located within the stroma of the bone marrow and other organs, including the placenta. Efforts to track the identity of tissue-resident MSCs have suggested they are related to a perivascular niche, especially evident in highly vascularized tissues such as the placenta [1, 2]. Although the bone marrow (BM) represents the main source of MSCs, access to this requires an invasive procedure. Also, the frequency of progenitors, capacity of proliferation, and differentiation potential of such cells decrease greatly with donor age [3, 4]. Therefore, other stromal cells from postnatal tissues have gained interest for their potential application in cellular therapy and tissue engineering [5]. The placenta plays an essential role for the support of fetal development and, hence, offers an important reservoir for progenitor and stem cells [6]. From a practical viewpoint, placental tissues present with several advantages for clinical usage compared with other MSC sources. These include their uncomplicated harvest protocol, avoiding any procedure on the donors, a young cellular age of the donors, and, finally, the absence of ethical concerns [7, 8].
Placental MSCs (P-MSCs) have been obtained from multiple areas, including both maternal and fetal origins and umbilical cord blood (UCB) [9, 10], amniotic fluids [11, 12], the amniotic membrane [13], Wharton’s jelly [14], the chorionic membrane [15, 16], chorionic villi [17, 18], and the decidua [19, 20]. The most common placental source for MSCs used in clinical trials is the umbilical cord, more precisely, the UCB [5]. Clinical reports have shown that UCB-MSC transplantation in patients with decompensated liver cirrhosis is safe and improves the patient's quality of life [21]. However, it has also been shown that MSCs are present in the UCB at rather low frequencies, a limitation for their use in largescale applications [22]. In contrast, other fractions of the placenta, such as the chorion and decidua, have shown a higher abundance of MSCs, possibly with major therapeutic potential [4, 23]. Moreover, several preclinical models have evidenced the potential of fetoplacental or maternal MSCs in neuronal regeneration [24, 25], differentiation to glucagon-secreting cells [26], and angiogenic [27, 28] and cardiomyogenic differentiation [29]. Furthermore, several clinical reports have presented evidence regarding the clinical safety and efficacy of fetoplacental or maternal MSCs in conditions such as Crohn's disease [30], idiopathic pulmonary fibrosis [31], and critical limb ischemia [27, 32]. Amniotic membrane-derived cells have also been used in different experimental models, such as spinal cord injury [33]; however, they have a limitation considering their low abundance and low proliferation rate after a certain number of passages [15].
The stem cell niche of origin represents an important factor to be considered when evaluating the biological differences between stem cell sources. Typical MSC properties such as their immunomodulatory, differentiation, and paracrine activities can be highly influenced by microenvironmental changes [34–36]. However, a detailed comparison addressing the properties of MSCs from different placental niches and origins is still missing. This comparison could contribute to the orientation of cell-based trials by matching the most potent source and its properties with a specific clinical indication. However, despite efforts directed at assessing the performance of stem cells derived from different sources, discrepancies persist. One possible reason for the conflicting results in the current data is donor-associated variability. Donor variation in relation to MSC growth, differentiation, and clinical benefit has been a bottleneck in the standardization of therapeutic protocols. Some of these differences can be reduced by working with demographically matched cohorts [37]. However, one study concluded that, irrespective of age, gender, and source of isolation, the cells from all donors showed similar osteogenic potential [38]. Also, in a previously published study, we showed that demographically matched donors presented with different MSC performance that did not cluster according to age range [39].
Although the ideal situation would be to study haploidentical MSCs, this has been difficult to achieve in many cases owing to the difficulty in obtaining a matched pair of samples from the same human donor. However, placental tissue offers the rare opportunity for comparing the biological characteristics of haploidentical MSCs isolated from the same fetal and maternal placental tissue.
A systematic review of the data in this area revealed that only 18% of published works had investigated the fetal or maternal origin of the cells used in their experiments [40]. Maternal cell contamination was still present in 30% of the studies using chorion MSCs (Ch-MSCs). Thus, future studies should focus on the comparative biology of fetal vs. maternal MSCs to conclusively determine their relative functional features. Therefore, we aimed to compare the biological characteristics of haploidentical MSCs isolated from the fetal and maternal parts of placental tissues. Because of the close physical proximity of the chorion with the maternal decidua, it is necessary to define sensitive methods and isolation standards that limit or exclude bidirectional contamination [6]. This complication is not relevant for the isolation of UC-MSCs owing to the absence of such intimate physical contact.
Considering these factors, we performed a comparative analysis of MSCs of fetal origin, including umbilical cord MSCs (UC-MSCs) and Ch-MSCs, and cells from maternal origin, such as decidua MSCs (Dc-MSCs). All cells were analyzed and compared in terms of proliferation, ability to form fibroblast colony-forming units, mesodermal differentiation, surface marker expression, immunosuppression capacity, and, finally, their angiogenic potential, both in vitro and in vivo, using a Matrigel plug assay (BD Biosciences, San Jose, CA, http://www.bdbiosciences.com). Additionally, the cells were isolated from amniotic membranes; however, because of their poor proliferation rates [15], the ex vivo expansion did not yield sufficient cell numbers to be included in the present study (data not shown).
Using haploidentical P-MSCs, our experimental design facilitated a robust comparison among the different sources. However, the maternal and fetal cells were from two different genetic backgrounds and cellular ages. This denotes a limitation of the present study compared with other studies performed using different cells from a single donor [41].
Materials and Methods
Isolation and In Vitro Expansion of Human Cells
Cell Isolation and Culture
The P-MSCs were isolated from full-term human placentas of male newborns collected from cesarean deliveries after informed consent from the maternal donors, and ethical revision and approval from the ethics committees of both the Clínica Dávila Hospital and the “Servicio de Salud Metropolitano Oriente” in Chile. Different parts of the placenta, such as the umbilical cord, chorion villi, and decidua, were separated and dissected into small fragments. Decidual tissues were obtained from the basal plate, and chorionic tissues were dissected from the chorionic plates (details are described in the supplemental online data). UC-MSCs were obtained by explant method and Ch- and Dc-MSCs by enzymatic digestion. BM-MSCs were isolated from three donors by Ficoll density gradient centrifugation and cultured in the same conditions as for P-MSCs. Human umbilical vein endothelial cells (HUVECs) were cultured in endothelial growth medium (EGM-2; Lonza, Walkersville, MD, http://www.lonza.com). All protocols can be found in the supplemental online data.
Characterization of MSCs
P-MSCs and BM-MSCs were characterized by their plastic adherence, fibroblast-like morphology, and proliferation potential using the WST-1 assay. Their immunophenotypic profile was analyzed by flow cytometry. They were also characterized by fibroblast colony-forming unit frequency (CFU-F) and the capacity to differentiate into adipocytes, chondrocytes, and osteoblasts. All experiments were performed when the cells were in passage (P) 4–6. All detailed protocols can be found in the supplemental online data.
Determination of Fetal Cell Proportion in Culture
To evaluate the purity and contamination between the fetal and maternal fractions in P-MSCs from male newborns, absolute quantification of the chromosome Y marker (DYS14) sequence located within the testis-specific protein Y (TSPY) gene located on the human Y chromosome was performed. DNA extraction was performed using the QIAamp DNA mini kit (Qiagen, Valencia, CA, http://www.qiagen.com) and expression of DYS14 marker by real-time quantitative reverse transcription-polymerase chain reaction using the 2× Brilliant III Master Mix (Agilent Technologies, Palo Alto, CA, http://www.agilent.com), the probe 5′-6FAM/TGAGAAATC-ZEN-CCCTACCC-3′ and primer sets (5′-GCCTCAGAATCATACACCCTCT-3′; 5′-GAAAGCGACGAGCAACAGGGA-3′).
T-Cell Proliferation Assays
To evaluate the capacity of P-MSCs and BM-MSCs to suppress the proliferation of T cell in vitro, human peripheral blood mononuclear cells (PBMCs) of healthy donors were isolated (n = 4) by Ficoll density-gradient centrifugation at 400g for 30 minutes. PBMCs were stained with carboxyfluorescein succinimidyl ester (CFSE; Life Technologies, Carlsbad, CA, http://www.lifetechnologies.com), according to the manufacturer's protocol, and cocultured with MSCs in 96-well plates at a 10:1 ratio in Roswell Park Memorial Institute medium supplemented with 10% fetal bovine serum, 1% l-glutamine, 1% nonessential amino acids (minimal essential medium), 100 mM sodium pyruvate, 25 µM β-mercaptoethanol (Gibco, Grand Island, NY, http://www.lifetechnologies.com), and 15 µg/ml phytohemagglutinin (PHA). After 72 hours, PBMCs were harvested and stained with anti-human CD45 and anti-CD3 antibodies in cytometer buffer for 20 minutes at 4°C in the dark. The cells were analyzed on a FACS Canto II Flow cytometer (BD Biosciences), and proliferation was calculated by the decrease in CFSE fluorescence.
In Vitro Tube Formation Assay
The capacity of MSCs to form tube-like structures in vitro was evaluated by plating the cells in EGM in 24-well plates (6 × 104 cells per well), coated with 250 µl of Standard Matrigel matrix (catalog no. 354234; BD Biosciences), according to the manufacturer’s instructions. To determine the angiogenic potential of MSC-conditioned media (CM), the cells were seeded in 6-well plates and incubated under hypoxic or normoxic conditions for 48 hours. Subsequently, HUVECs were plated with the MSC-CM, EGM (positive control), or Dulbecco’s modified Eagle’s medium (DMEM; negative control) on 24-well plates (3 × 104 per well), coated with 250 µl per well of Matrigel matrix growth factor reduced (GFR) (catalog no. 354235; BD Biosciences). In both assays, the evaluation time of tube structure formation was 5 hours after initiation of the culture, and the tube structure was examined with a phase-contrast microscope. Quantification of angiogenesis potential was evaluated in five images per condition using WimTube software (Wimasis GmbH, Munich, Germany, http://www.wimasis.com).
Measurements of Angiogenic Factors
To compare the secretion levels of angiogenic factors among the different MSC sources, 5 × 104 cells were plated in serum-free medium in 6-well plates. After 48 hours of incubation, the conditioned medium was collected, and the secreted levels of vascular endothelial growth factor (VEGF), basic fibroblast growth factor (FGF), and hepatocyte growth factor (HGF) were measured using the DuoSet ELISA Development System (R&D Systems, Minneapolis, MN, http://www.rndsystems.com).
Matrigel Plug Assay
To compare the angiogenic potential of placenta-derived stem cells and BM-MSCs, the Matrigel plug assay was performed in 8-week-old NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice (Jackson Laboratories, Bar Harbor, ME, http://www.jax.org). The Universidad de los Andes institutional ethical committee for animal experimentation reviewed and approved all animal procedures. The mice were randomly divided into six groups: DMEM, HUVEC, UC-MSCs, Ch-MSCs, Dc-MSCs, and BM-MSCs. DMEM (with 50 ng/ml VEGF) or 3 × 106 cells (DMEM, 50 ng/ml VEGF) were mixed with GFR Matrigel (BD Biosciences) and implanted subcutaneously into both flanks. After 14 days, the Matrigel plugs were harvested, and the hemoglobin content was determined using Drabkin’s reagent, as described previously [42]. The number of blood vessels was counted using ImageJ software (NIH, Bethesda, MD, http://www.imagej.nih.gov/ij).
Statistical Analysis
All experiments were performed in biological and experimental triplicate, and the data are expressed as the mean ± SEM. The parameters were compared using one-way analysis of variance followed by Bonferroni’s post-test. Student’s unpaired t test was used to compare differences between 2 groups. A probability value (p) of < .05 was considered statistically significant.
Results
Fetal and Maternal P-MSCs Showed Similar Morphology and Migration Capacity but Different Clonogenic and Proliferation Potential In Vitro
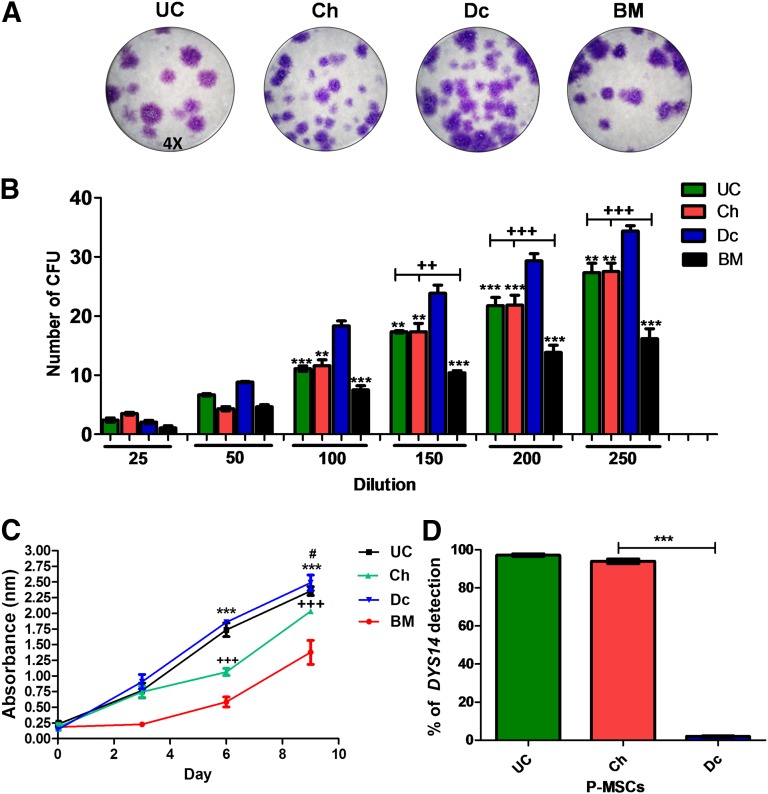
UC-MSCs, Ch-MSCs, and Dc-MSCs were obtained from four different placentas. All cell types presented similar fibroblast-like morphology and plastic adherence in culture (supplemental online Fig. 1), as previously reported [23, 43]. MSCs were characterized both phenotypically and functionally, and the purity with regard to maternal or fetal contamination was quantified using the DYS14 marker sequence. BM-MSCs of a male donor were used to establish the calibration curve. The high proportion of cells from fetal origin in UC-MSCs and Ch-MSCs was corroborated by their high levels of DYS14 (97.2% ± 2.1% and 91.7% ± 6,2%, respectively). Dc-MSCs exhibited a low percentage of fetal cells, with DYS14 levels of only 2% ± 1% (Fig. 1D). Scratch wound assays were performed to compare the migration ability of P-MSCs in vitro. The images taken at 24 hours showed complete wound closure, with similar numbers of migrating cells counted in all P-MSC and BM-MSC experiments (supplemental online Fig. 3). To evaluate the CFU-F potential of P-MSCs and BM-MSCs, the cells were serially diluted. At a dilution starting from 100 cells and greater, Dc-MSCs showed a higher CFU-F compared with UC- and Ch-MSCs. However, all P-MSCs showed a significantly greater CFU-F compared with BM-MSCs starting at 150 cells of dilution (Fig. 1B). Also, the progenitor frequency was calculated for each sample as the value of 1/(CFU-F) from the limiting dilution analysis. The highest progenitor frequency calculated was obtained for Dc-MSCs and ranged from 1/1,082 with a 95% confidence interval of 1/902–1/1,298 (supplemental online Fig. 2A, 2B). UC- and Ch-MSCs had an approximately similar CFU-F of 1/1,516 and 1/1,532. However, the only significant difference was obtained among all the different P-MSCs and BM-MSCs, scoring a frequency of 1/2,470 (supplemental online Fig. 2C). Finally, the proliferation assay showed a significant threefold increase of P-MSCs compared with the BM-MSCs (Fig. 1C; p < .0001 at days 6 and 9). Moreover, at day 6, Dc-MSCs and UC-MSCs showed a threefold greater proliferation compared with Ch-MSCs (p < .0001).
Figure 1.
Fetal and maternal P-MSCs showed different clonogenic proliferation potentials. Representative images of the CFU stained with crystal violet after 20 days in culture (×4) (A) and quantified from 6 different dilutions (B). ∗, p < .05; ∗∗, p < .01; ∗∗∗, p < .001 for Dc-MSCs vs. Ch-MSCs, UC-MSCs, and BM-MSCs, respectively. ++, p < .05; +++, p < .001. (C): Cell proliferation was quantified for 9 days using WST-1 assay, and the obtained absorbance at 450 nm is presented at the different time points. ∗∗∗, p < .001, Dc-MSCs and UC-MSCs vs. Ch-MSCs and BM-MSCs at 6 days and Dc-MSCs vs. BM-MSCs at 9 days; +++, p < .001, Ch-MSCs vs. BM-MSCs at 6 and 9 days; #, p < .05, Dc-MSCs vs. Ch-MSCs at 9 days. (D): The absolute quantification of DYS14 by real-time reverse transcription quantitative polymerase chain reaction (specific marker of Y chromosome) was used to identify fetal and maternal P-MSC fractions and the level of purity of each MSC source. ∗∗∗, p < .001. All data are presented as the mean ± SEM (n = 3) of a minimum of 4 donors. Abbreviations: BM, bone marrow; CFU, colony-forming unit frequency; Ch, chorion; Dc, decidua; DYS14, TSPY1 (testis-specific protein Y-linked 1); MSCs, mesenchymal stem cells; P-MSCs, placental MSCs; UC, umbilical cord.
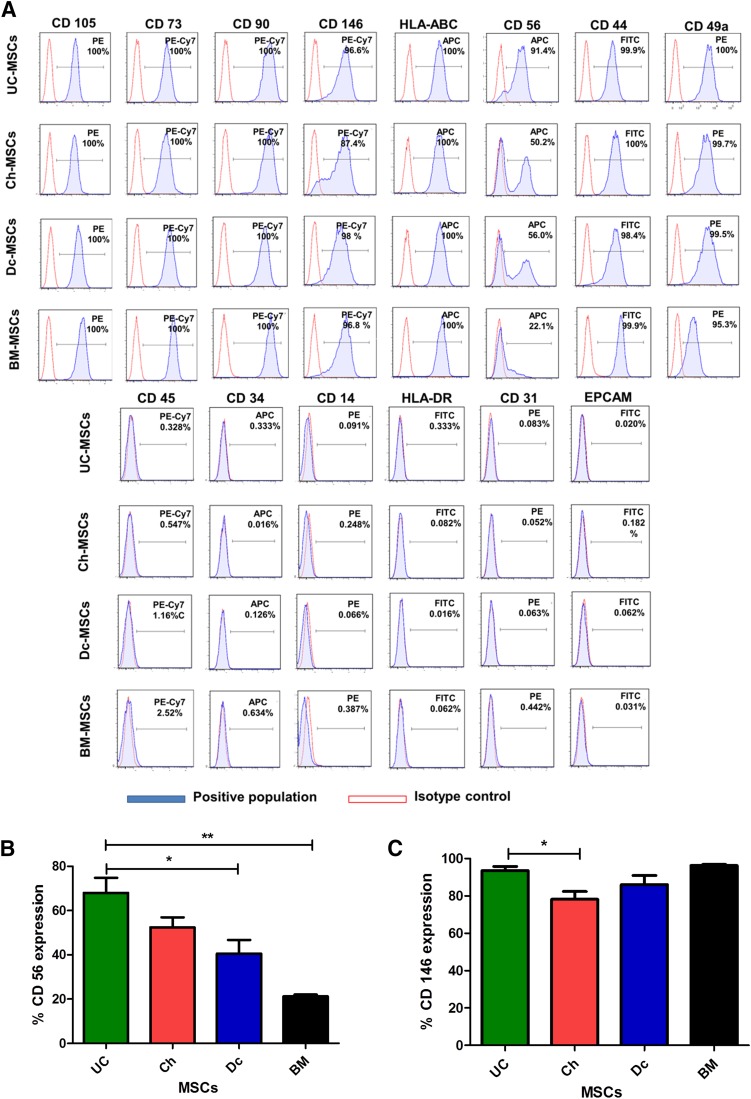
Fetal and Maternal P-MSCs Expressed Common MSC Markers but Exhibited Differences in Expression of CD56 and Mesodermal Differentiation Potential
As shown in Figure 2A, P-MSCs expressed high levels of all the common MSC markers (CD73, CD105, CD90, CD49a, and human leukocyte antigen [HLA]-ABC) and were negative for CD45, CD34, CD14, epithelial cell adhesion molecule, CD31, and HLA-DR. Additionally, other markers were evaluated, such as CD56 and CD146. Among all of these, CD56 was the sole surface marker that was differentially expressed between the fetal and maternal MSCs. UC- and Ch-MSCs displayed higher percentages of CD56+ cells (68.02% ± 19% and 52.4% ± 13%, respectively), and a significantly lower percentage of CD56+ cells were detected in Dc-MSCs and BM-MSCs (40.56% ± 15% and 21.2% ± 0.9%, respectively; Fig. 2B). Moreover, CD146 was found to be differentially expressed between UC-MSCs and Ch-MSCs (p < .05; Fig. 2C).
Figure 2.
Fetal and maternal P-MSCs expressed all the common MSC markers but presented differences in the expression of CD56 and CD146. (A): MSCs were stained with labeled monoclonal antibodies against known MSC surface markers (blue) and their respective isotypes (red); the cells were analyzed by flow cytometry. All MSCs were positive for CD73, CD90, CD105, CD49a, and HLA-ABC and negative for CD14, CD34, CD31, CD45, and HLA-DR. (B): Major phonotypical differences were observed with CD56, with UC- and Ch-MSCs showing a high percentage of expression compared with the intermediate and low expression for Dc-MSCs and BM-MSCs, respectively (∗, p < .05; ∗∗, p < .01). (C): The expression of CD146 was significantly different only between the UC- and Ch-MSCs (∗, p < .05). All data are presented as the mean ± SEM (n = 3) of a minimum of 4 donors. Abbreviations: BM, bone marrow; Ch, chorion; Dc, decidua; EPCAM, epithelial cell adhesion molecule; MSCs, mesenchymal stem cells; UC, umbilical cord.
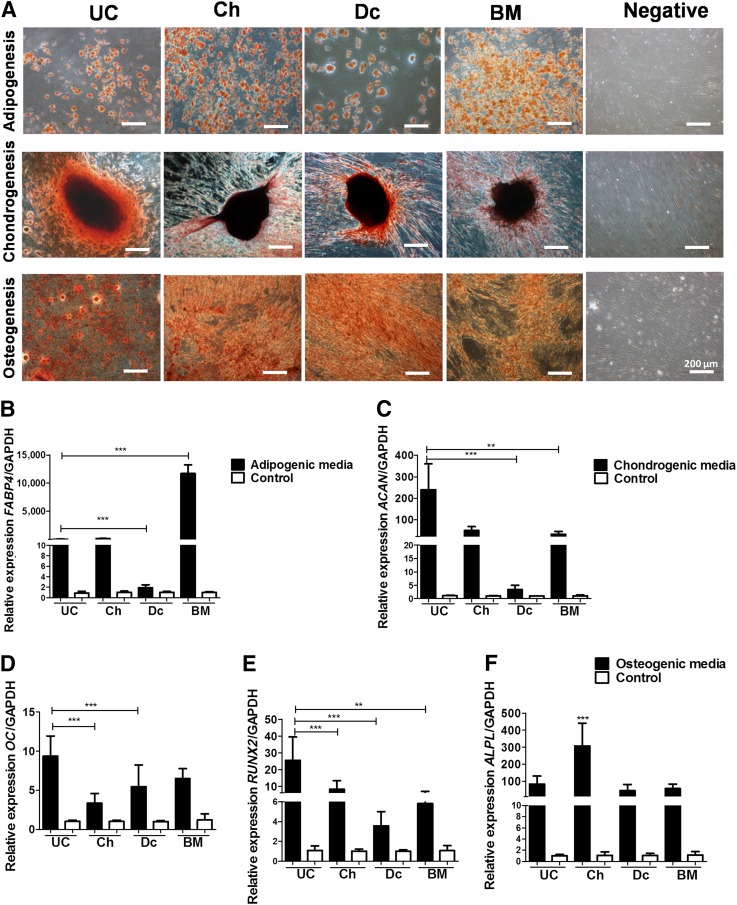
P-MSC differentiation to mesodermal tissues was induced using defined culture conditions. Our results showed that all MSC sources were able to differentiate into adipogenic, osteogenic, and chondrogenic lineages, with variable efficiencies (Fig. 3). The quantification of fatty acid binding protein 4 (FABP4) mRNA expression levels, and Oil Red O staining revealed that BM-MSCs exhibited significantly higher adipogenic differentiation potential (threefold more than P-MSCs; p < .001). Dc-MSCs had the lowest expression and staining levels. The UC- and Ch-MSCs had threefold higher adipogenic differentiation than that of Dc-MSCs (p < .0001; Fig. 3A, 3B). With respect to the chondrogenic potential, UC-MSCs presented with the highest relative expression of aggrecan (ACAN) among all tested cells with threefold higher relative expression compared with Dc-MSCs and twofold higher compared with Ch- and BM-MSCs (p < .01; Fig. 3C). The osteogenic capacity was evaluated by measuring the expression of three genes: RUNX2, OC, and ALPL. According to the relative expression of runt-related transcription factor 2 (RUNX2) and osteocalcin (OC), UC-MSCs showed a threefold higher expression than Ch- and Dc-MSCs (p < .001; Fig. 3D, 3E). In contrast, the relative expression of alkaline phosphatase (ALPL) showed a significant difference only in Ch-MSCs compared with the other MSCs (p < .001; Fig. 3F).
Figure 3.
Fetal and maternal P-MSCs displayed different mesodermal differentiation potential. Fetal MSCs showed significantly higher tridifferentiation potential compared with that of maternal MSCs. (A): Representative images of MSC differentiation after specific inductions and staining: adipocytes (Oil Red O), osteocytes (alizarin red), and chondrocytes (safranin O). Scale bars = 200 µm. (B–F): The relative expression levels of several genes involved in mesoderm differentiation were measured by real-time quantitative reverse transcription-polymerase chain reaction: FABP4 (adipogenesis; ∗∗∗, p < .001 (B); ACAN (chondrogenesis; ∗∗∗, p < .001; ∗∗, p < .05); and RUNX2 (D), OC (E), and ALPL (osteogenesis) (F) (∗∗, p < .01; ∗∗∗, p < .001). All data are presented as mean ± SEM (n = 3) of a minimum of 4 donors. Abbreviations: ACAN, aggrecan; ALPL, alkaline phosphatase; FABP4, fatty acid binding protein 4; GADPH, glyceraldehyde-3-phosphate dehydrogenase; MSCs, mesenchymal stem cells; OC, osteocalcin; P-MSCs, placental MSCs; RUNX2, runt-related transcription factor 2.
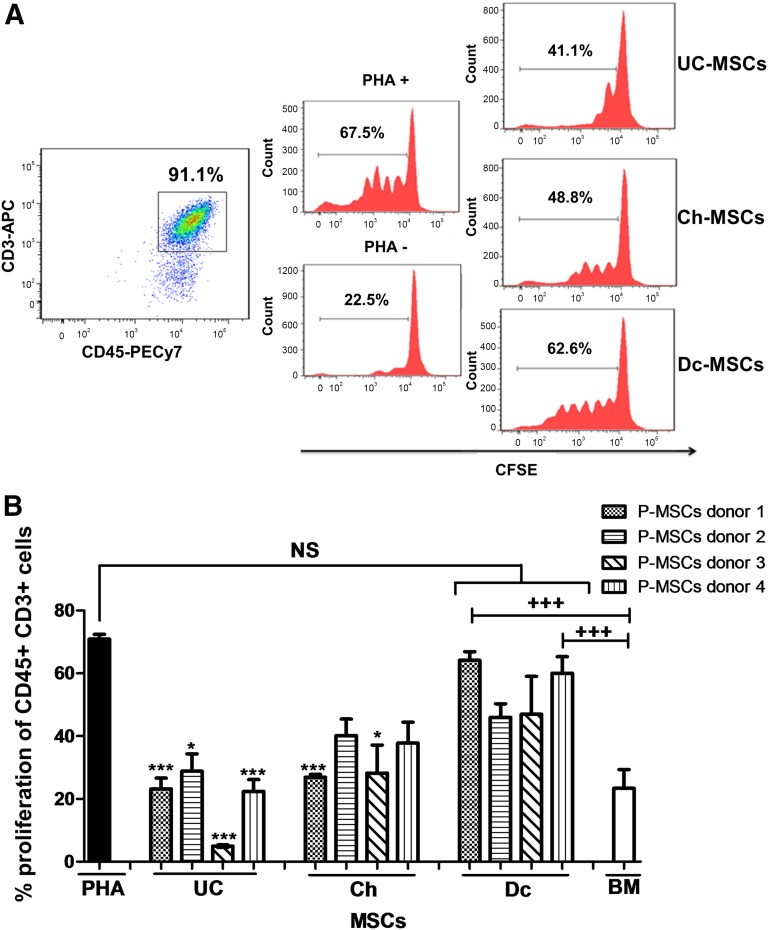
Fetal MSCs Presented Higher Immunosuppressive Properties Evaluated by Inhibition of T-Cell Proliferation In Vitro
The immunomodulatory properties of P-MSCs were assessed by evaluating their effect on the proliferative response of PBMCs to PHA in vitro. T-cell proliferation was calculated as the percentage of loss of CFSE staining (Fig. 4A). The analysis showed separate results obtained from four different donors of full-term placentas. All four UC- and Ch-MSCs exhibited important suppression of T-cell proliferation in vitro. The percentage of inhibition was 22.26% ± 12.52% and 34.26% ± 13.37% for UC- and Ch-MSCs with respect to PHA-induced proliferation in the absence of MSCs. These effects were comparable to those observed with BM-MSCs. In contrast, Dc-MSCs failed to display suppressive properties, with no difference seen compared with the baseline proliferative response to PHA. Moreover, a significant difference was obtained when comparing each of the UC- or Ch-MSCs with their matching Dc-MSCs from the same donor (Fig. 4B).
Figure 4.
Fetal MSCs presented with higher immunosuppressive capacity to inhibit T-cell proliferation in vitro. (A): Allogenic PHA-activated PBMCs labeled with CFSE were cocultured in the presence or absence of MSCs. Representative flow cytometry panels of stained T cells with CFSE and coculture with MSCs at a 1:10 ratio (MSCs/T cells) are shown. T-cell proliferation was evaluated by the reduction in CFSE intensity at 72 hours after culture. (B): The proliferation percentage of CD45+CD3+ cells demonstrated a higher immunosuppressive capacity for UC- and Ch-MSCs than for Dc-MSCs and the control condition (without MSCs) (∗, p < .05; ∗∗∗, p < .001 for UC-MSCs and Ch-MSCs compared with Dc-MSCs in the same donor; +++, p < .001). All data are presented as mean ± SEM (n = 3) of a minimum of 4 donors. Abbreviations: APC, allophycocyanin; BM, bone marrow; CFSE, carboxyfluorescein diacetate succinimidyl ester; Ch, chorion; Dc, decidua; MSCs, mesenchymal stem cells; NS, not significant; P-MSCs, placental MSCs; PBMCs, peripheral blood mononuclear cells; PHA, phytohemagglutinin; UC, umbilical cord.
Ch-MSCs and Their Conditioned Media Showed a Higher Potential to Form Tube-Like Structures in Matrigel Through Enhanced Release of HGF and VEGF Under Hypoxic Conditions
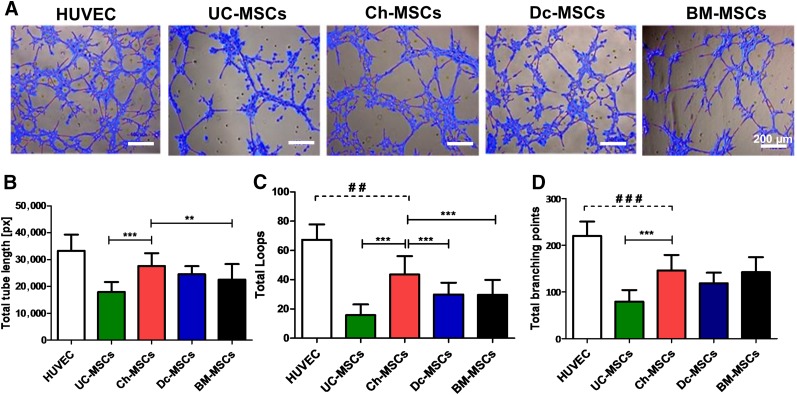
The capacity of maternal and fetal MSCs to participate directly in the formation of tubular structures, in contrast to those (indirect) effects mediated by the secretion of angiogenic factors within the placental tissue, has not been properly assessed. The ability of P-MSCs to form tubular networks in semisolid medium was first tested in an in vitro angiogenesis assay. P-MSCs and BM-MSCs were resuspended in EGM and seeded on a Matrigel culture system. As shown in Figure 5A, image analysis of the tube formation, evaluated after 5 hours of seeding, demonstrated a more extensive network of capillary-like structures in Ch-MSC conditions compared with UC-, Dc-, and BM-MSC conditions. Quantitative analysis of the total tube length (Fig. 5B), total loops (Fig. 5C), and total branching points (Fig. 5D) in the Matrigel assay showed that Ch-MSCs exert a significant angiogenic effect compared with UC-MSCs (threefold; p < .001). In addition, Ch-MSCs showed a significant increase in total tube length (p < .01) and total loops (p < .001) compared with BM-MSCs.
Figure 5.
Ch-MSCs show a higher potential to form tube-like structures in Matrigel coating cultures. MSCs (6 × 104 cells per well) were cultured in a Matrigel supplemented with angiogenic factors, and tube-like formation was assessed at 5 hours after culture. (A): Representative images of MSCs cultured over the Matrigel and analyzed using WimTube software. Scale bars = 200 µm. The quantitative analysis compared the different cell sources with the following parameters: total branching points (B), total loops (C), and total tube length (D). ∗∗, p < .01; ∗∗∗, p < .001; ##, p < .01; ###, p < .001. All data are presented as mean ± SEM (n = 3) of a minimum of 4 donors. Abbreviations: BM, bone marrow; Ch, chorion; Dc, decidua; HUVEC, human umbilical vein endothelial cell; MSCs, mesenchymal stem cells; px, pixels; UC, umbilical cord.
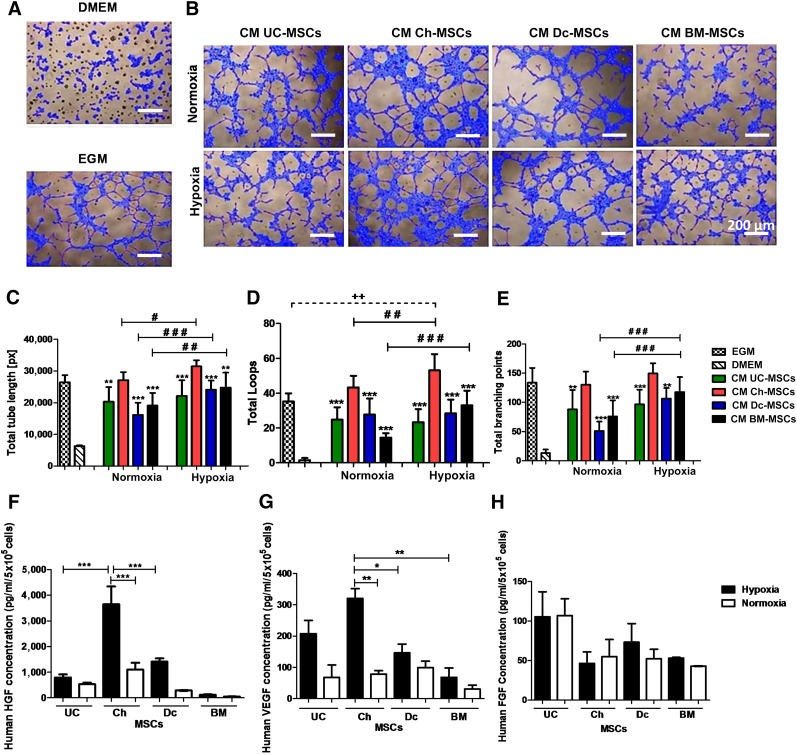
In a similar assay, we separately tested the angiogenic factors present in CM harvested from P-MSCs and BM-MSCs. In order to mimic an ischemic condition in vitro, the effect of CM collected under hypoxic conditions was also evaluated on the tube-like formation of HUVECs. The tubular structure formation analyzed at 5 hours showed significantly higher angiogenic capacity for the Ch-MSC CM than for the CM of P-MSCs or BM-MSCs in normoxia (Fig. 6A) and also higher loop numbers (43.3 ± 6.6) than in Dc-MSC CM (27.7 ± 9.1; Fig. 6D). A longer tube length (27,112 ± 2,530.3 pixels [px]) developed with Ch-MSC CM than that in Dc-MSC CM (16,157 ± 3,821 [px]; Fig. 6C). Also, the total branching points were increased (130.2 ± 22) with Ch-MSC CM compared with that in CM from Dc-MSCs (51 ± 15.4; Fig. 6D). This capacity was markedly enhanced when CM harvested under hypoxic conditions was tested, showing higher loop numbers (53.1% ± 9.3%) for Ch-MSC CM compared with those in Dc-MSC CM (28.3% ± 7.9%) or BM-MSC CM (33% ± 8.4%).
Figure 6.
Ch-MSC CM showed superior potential to induce tube-like structures through enhanced release of HGF and VEGF under hypoxic conditions. (A): The negative and positive controls are represented by DMEM (basal media) and EGM, respectively. (B): The CM was obtained after incubation of MSCs under normoxic or hypoxic conditions for 48 hours. Subsequently, HUVECs (3 × 104 per well) were incubated with the CM and cultured in a growth factor-reduced Matrigel. The formation of tubular structures was analyzed at 5 hours. Quantitative analysis was performed of the angiogenic potential using WimTube software considering the following parameters: total tube length (C), total loops (D), and total branching points (E). ∗∗, p < .01; ∗∗∗, p < .001 for CM Ch-MSCs compared with CM of other MSC sources under hypoxic and normoxic conditions. ++, p < .01; ###, p < .001; ##, p < .01; # p < .05. ELISA assay was used to determine HGF (∗∗∗, p < .001) (F), VEGF (∗, p < .05; ∗∗, p < .01; ∗∗∗, p < .001), and FGF (H) protein levels. All data are presented as mean ± SEM (n = 3) of a minimum of 4 donors. Abbreviations: Ch, chorion; CM, conditioned media; DMEM, Dulbecco's modified Eagle's medium; EGM, endothelial growth medium; FGF, fibroblast growth factor; HGF, hepatocyte growth factor; HUVEC, human umbilical vein endothelial cells; MSCs, mesenchymal stem cells; px, pixels; VEGF, vascular endothelial growth factor.
To further understand these angiogenic properties of MSC-CM, we quantified the secreted levels of HGF, VEGF, and FGF (Fig. 6F–6H) in each case. Under hypoxic conditions, a major increase in VEGF secretion was noted in the different cell types (Fig. 6G). Although all tested cells increased secreted factors under hypoxia, Ch-MSC CM showed significant increments of VEGF in CM (twofold in hypoxic conditions vs. normoxic conditions). These levels were higher compared with maternal or BM-MSCs (one- and twofold increase, respectively; Fig. 6G). In contrast, Ch-MSC CM showed significant increases in HGF levels under low oxygen conditions compared with UC-MSCs, DC-MSCs, and BM-MSCs (threefold; Fig. 6F). FGF secretion remained unchanged under low and high oxygen conditions, and no difference was noted among the different cell types (Fig. 6H).
Angiogenic Potential of Ch-MSCs In Vivo Outmatched All Other Placental and BM Sources
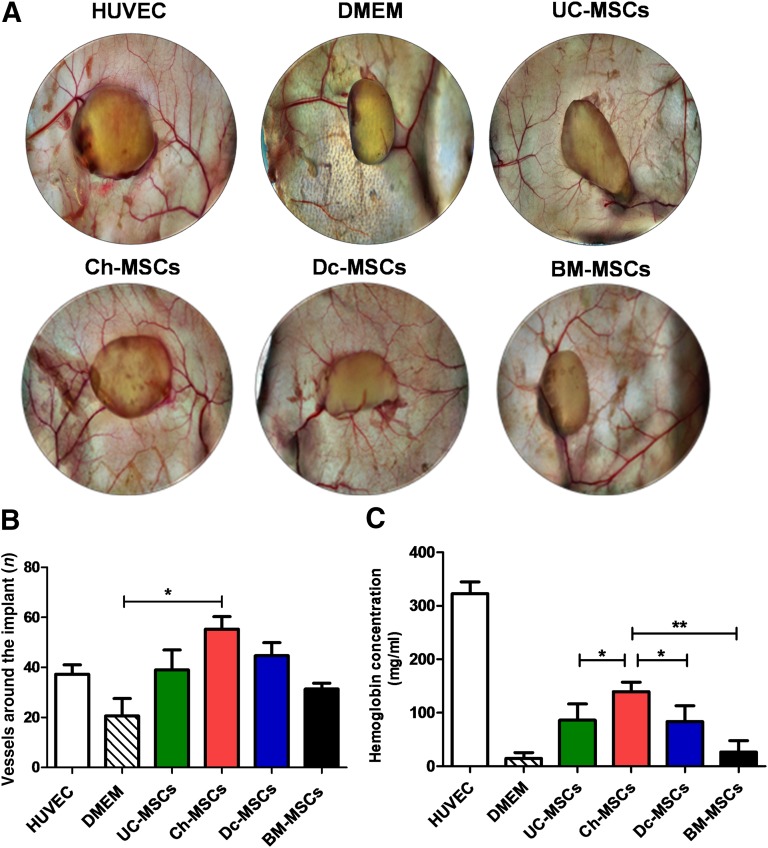
To comparatively evaluate the angiogenic potential of P-MSCs and BM-MSCs, a Matrigel plug assay was implanted in NSG mice. After 14 days, the plugs were collected and photographs taken. As shown in Figure 7A, all plugs generated vessels around and inside the implants. However, image analysis reflected a significant difference in the number of vessels converging toward the implants for the Ch-MSC group compared with the control (p < .05; Fig. 7B). Additionally, the implants were extracted and analyzed for their hemoglobin content (Fig. 7C). Similar to the results observed in vitro, the plugs containing Ch-MSCs showed the highest hemoglobin (Hb) concentration (139.4 ± 43.7 mg/ml) compared UC-MSCs (86.2 ± 30.4 mg/ml; p < .05), Dc-MSCs (83.5 ± 29.5 mg/ml; p < .05), and BM-MSCs (14.5 ± 11.1 mg/ml), indicating a higher level of vascularization. All plugs containing cells showed higher Hb content, with the exception of the BM-MSCs. Altogether; our data demonstrate that Ch-MSCs have more angiogenic potential than the other P-MSCs and BM-MSCs.
Figure 7.
Ch-MSCs outmatched all other placental sources for their angiogenic potential in vivo. To comparatively determine the angiogenic potential of placenta-derived stem cells and BM-MSCs, a Matrigel plug assay was performed in NSG mice. The mice were randomly divided into six groups (and six plugs per group): DMEM (as negative control), HUVEC (as positive control), UC-MSCs, Ch-MSCs, Dc-MSCs, and BM-MSCs. The different cells (3 × 106 cells) were mixed with factor-reduced Matrigel and implanted subcutaneously. At 14 days after transplantation, the implants were harvested (A), and images of the plugs were taken (B). The hemoglobin content (mg/ml) was quantified using Drabkin’s reagent in different conditions.∗, p < .05; ∗∗, p < .01. (C): Quantification of the vessels around the implant was performed using ImageJ software (∗, p < .05). All data are presented as mean ± SEM (n = 3) of a minimum of 4 donors. Abbreviations: BM, bone marrow; Ch, chorion; Dc, decidua; DMEM, Dulbecco's modified Eagle's medium; HUVEC, human umbilical vein endothelial cell; MSCs, mesenchymal stem cells; NSG, NOD SCID-γ; UC, umbilical cord.
Discussion
Human placental tissues have been postulated as a promising source for cell therapy because of their abundance and accessibility. However, comparative studies have shown that different cell sources can display different characteristics, such as colony frequency, proliferative capability, and differentiation potential [44–47]. Therefore, extrapolation of properties from a well-characterized source and niche to other MSCs types has been considered out of context.
In the present report, an absolute quantification of the DYS14 marker, studying tissues only from male newborns, was used to determine the maternal and/or fetal contamination or overgrowth of cultures. Additionally, Wulf et al. [48] reported a mixed population of fetal and maternal MSCs up to P2, followed by an overgrowth of maternal cells after P3. To avoid this source of variability, all contamination testing was performed at P5 and later. This quantification method showed a 2% ± 1% proportion of fetal cells within the decidua-derived MSCs and more than 94% ± 3.5% in chorion. Parolini et al. proposed that maternal contamination in chorionic cells must not exceed 1% [6]; however, no threshold has yet been determined in published studies for the contamination of decidual cells. This low level of contamination varies largely with the method of isolation. It also might result from maternal blood contamination during the isolation process and the bidirectional cellular exchange between the mother and fetus [49–51]. It has only been assumed that the fetal origin has a biological and, therefore, a translational advantage over the adult maternal cells. These assumptions remain to be proved in head-to-head comparative studies. However, in the absence of evidence comparing diverse P-MSCs, Dc-MSCs have been preferred in certain allogenic applications (clinicaltrials.gov identifiers NCT00919958 and NCT01679990; http://www.clinicaltrials.gov). In the case of personal cryobanking for future autologous application, it is still confusing whether it would be preferable to preserve the maternal or fetal cells. Consistent with the supposition that cells from different sources can have different attributes, mixing fetal and maternal cells from the same tissues can lead to confusing results.
In the present report, the comparison between haploidentical MSCs obtained from the umbilical cord, chorion, and decidua displayed several distinct features. First, although all cells shared a similar immunophenotypic profile, important differences in the percentage of a CD56+ population were observed. Second, a higher proliferation capacity was obtained for Dc-MSCs and UC-MSCs than for Ch-MSCs. Third, the P-MSC populations displayed different mesodermal differentiation potential in vitro. Fourth, fetal MSCs showed a higher capacity to inhibit T-cell proliferation compared with maternal cells. Finally, Ch-MSCs exhibited superior angiogenic potential.
It has been shown that CD56+ MSCs exhibit increased clonogenic and differentiation potentials compared with cells from the same sample that do not express CD56 [52]. We have shown for first time that Ch-MSCs and Dc-MSCs have a significantly different proportion of a CD56+ subpopulation compared with UC-MSCs. Previous reports have demonstrated the expression of CD56+ subpopulation in amniotic membrane and chorion MSCs [53]. CD146 has been involved in the osteogenic differentiation capacity of MSCs, in which high expression levels of CD146 defines a population enriched in osteogenic potential [54]. Our results have shown that the osteogenic potential of BM-MSCs did not surpass that of P-MSCs, and CD146 expression was similar between BM-MSCs and P-MSCs. However, we found that UC-MSCs presented with a higher CD146 expression profile that correlated with higher relative expression of some genes involved in osteogenic differentiation compared with the other placental cells. The osteogenic and adipogenic potential evaluated in our work are in agreement with previous reports demonstrating that BM-MSCs have higher capacity for adipogenic lineage and less osteogenic differentiation potential than UC-MSCs [55]. Another study also showed a lower adipogenic differentiation capacity of P-MSCs obtained from a tissue mixture containing cells isolated from amnion, chorion, and decidua basalis [56]. Chang et al. [55] showed that leptin, which is synthesized and secreted by the placenta, blocked the accumulation of lipid in adipocytes and had positive effects on osteoblasts differentiation and bone mineralization [57]. In summary, we have shown that the different fetal MSCs populations showed a higher adipogenic, osteogenic, and chondrogenic potential than did the maternal MSCs.
Several investigations have described the immunosuppressive properties of BM- and P-MSCs, an important feature in the context of inflammatory-mediated disorders [58–62]. Furthermore, the placenta is involved in maintaining fetal tolerance and contains cells that display immunomodulatory properties [6]. However, it is still unclear whether both fetal and maternal MSCs share similar levels of immunosuppressive capacities compared with each other. The study of the immunomodulatory properties of the different P-MSCs has pointed to the important suppression of T-cell proliferation in vitro exhibited by UC- and Ch- MSCs. The percentage of inhibition was 22.26% ± 12.52% and 34.26% ± 13.37% for UC- and Ch-MSCs, respectively. These percentages were comparable to those observed with BM-MSCs. In contrast, Dc-MSCs isolated from four different donors failed to display any immunosuppressive properties. Other studies have addressed the immunomodulatory difference of fetoplacental MSCs compared with BM-MSCs or adipose MSCs. Although one group demonstrated the superiority of P-MSCs over the other MSC sources, including BM [63], another study showed that BM-MSCs have higher immunomodulatory capacity than Ch-MSCs [64]. In both cases, a mix of cells from both maternal and fetal origins was used instead of a single pure MSC origin. The present study is the first time in which such an important difference has been exposed between haploidentical MSCs from fetal and maternal origins. In contrast with our results, other reports [65, 66] have shown that maternal MSCs constitutively express indoleamine 2,3-dioxygenase (IDO), display low immunogenicity, and exhibit immunosuppressive effects. Another report [67] also showed that MSCs from placenta decidua basalis can inhibit the secretion of interferon-γ by PBMCs. Similar to other comparative studies, some studies did not properly address the composition and purity of their sources. The in vitro inhibition of T-cell proliferation is also dose dependent on the MSC/PBMC ratio and time and conditions of coculture. Furthermore, no relative comparison of the inhibition levels was present in relation to other sources of MSCs. Finally, donor variability can also account for the discrepancies in the results. However, a recent published study showed that Ch-MSCs expressed significantly more CD200 (negative regulator of immune cells) than did Dc-MSCs. Hence, they proposed that fetal MSCs exhibit more immunosuppressive qualities by showing an increase in the tolerance of transplanted skin in an allograft animal model implanted with Ch-MSCs. Also, they showed a reversion of the regulatory capacity when the cells were pretreated with anti-CD200 [68]. In conformity with these lines of evidence, the direct comparison of Dc-MSCs with its haploidentical UC- or Ch-MSCs resulted in a significant difference in favor of the fetal cells in all four tested donors included in our report.
Furthermore, we have demonstrated that Ch-MSCs have more capacity than Dc-MSCs and UC-MSCs to contribute to angiogenesis in both a direct and an indirect manner. This was evidenced by the higher potential to form tube-like structures and secrete HGF and VEGF. In the early stage of placental development, MSCs can also differentiate into perivascular cells, which are considered predecessors of capillary endothelial cells. However, placental microvascular endothelial cells exhibit remarkable changes in their angiogenic status throughout pregnancy, passing from a high angiogenic activity status—associated with rapid placental expansion during early pregnancy—to an angiostatic condition associated with placental growth arrest when the pregnancy approaches term [69]. All the MSCs used in the present study were isolated from full-term placentas; thus, the negative regulation of angiogenesis in the maternal surface parallels the special requirements to end the pregnancy. This might explain the difference in the angiogenic potential observed in Dc-MSCs compared with Ch-MSCs.
Moreover, although recent studies have demonstrated that fetal and maternal MSCs have different HGF expression [68], a comparison of the angiogenic potency of UC-MSCs, Ch-MSCs, and Dc-MSCs is still missing. Another study showed that a CD349-negative fraction of Ch-MSCs has a significant effect on new vessel formation in ischemic tissue after vascular occlusion in mice [70].
Conclusion
The present report has described several significant differences between MSCs derived from different placental compartments. We found that UC-MSCs and Ch-MSCs have a greater capacity to suppress activated T-cell proliferation in vitro than do MSCs from a maternal source. Additionally, Ch-MSCs present with a more robust angiogenic potential both in vitro and in vivo compared with other sources and, therefore, present an advantage for angiogenic therapies. The evidence we have presented should encourage efforts directed toward separating fetal and maternal cells when isolating haploidentical P-MSCs. The assessment of the prevalence of maternal contamination in the case of a specific clinical application seems necessary. Finally, our work presents evidence that underscores some of the advantages of using fetoplacental cells in regenerative medicine, tissue engineering, and gene therapy.
Supplementary Material
Acknowledgments
We acknowledge the technical expertise and assistance of Macarena Ocaña and Claudia Rubí in the animal experiments. We are thankful to Paulina Maffud, Cristian Vasquez, and Rafael Armijo for excellent technical assistance and to Victor Soto for the English revision of the manuscript. This work was supported by a grant from the Corporación de Fomento de la Producción (Código 11IEI-9766).
Author Contributions
P.L.G.: conception/design, collection and assembly of data, data analysis and interpretation, manuscript writing; C.C. and J.C.: collection and assembly of data, data analysis and interpretation; F.A.-M.: collection and assembly of data, data analysis and interpretation, manuscript review; F.E.F.: conception/design, manuscript revision, final approval of manuscript; J.B.: provision of study material or patients; L.S.-A.: experimental design, data analysis and interpretation, manuscript review; M.K.: conception/design, data analysis and interpretation, manuscript writing.
Disclosure of Potential Conflicts of Interest
P.L.G., J.C., F.A.-M., and L.S.-A. have research stipends from Cells for Cells. C.C. is a compensated employee of Cells for Cells. M.K. is CSO of Cells for Cells and has a patent filed. The other authors indicated no potential conflicts of interest.
References
- 1.Corselli M, Chen CW, Crisan M, et al. Perivascular ancestors of adult multipotent stem cells. Arterioscler Thromb Vasc Biol. 2010;30:1104–1109. doi: 10.1161/ATVBAHA.109.191643. [DOI] [PubMed] [Google Scholar]
- 2.Pacini S, Petrini I. Are MSCs angiogenic cells? New insights on human nestin-positive bone marrow-derived multipotent cells. Front Cell Dev Biol. 2014;2:20. doi: 10.3389/fcell.2014.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fehrer C, Lepperdinger G. Mesenchymal stem cell aging. Exp Gerontol. 2005;40:926–930. doi: 10.1016/j.exger.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Macias MI, Grande J, Moreno A, et al. Isolation and characterization of true mesenchymal stem cells derived from human term decidua capable of multilineage differentiation into all 3 embryonic layers. Am J Obstet Gynecol. 2010;203:495.e9–495.e23. doi: 10.1016/j.ajog.2010.06.045. [DOI] [PubMed] [Google Scholar]
- 5.Bieback K, Brinkmann I. Mesenchymal stromal cells from human perinatal tissues: From biology to cell therapy. World J Stem Cells. 2010;2:81–92. doi: 10.4252/wjsc.v2.i4.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parolini O, Alviano F, Bagnara GP, et al. Concise review: Isolation and characterization of cells from human term placenta: Outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells. 2008;26:300–311. doi: 10.1634/stemcells.2007-0594. [DOI] [PubMed] [Google Scholar]
- 7.Hemberger M, Yang W, Natale D, et al. Stem cells from fetal membranes—A workshop report. Placenta. 2008;29(suppl A):S17–S19. doi: 10.1016/j.placenta.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Marcus AJ, Woodbury D. Fetal stem cells from extra-embryonic tissues: Do not discard. J Cell Mol Med. 2008;12:730–742. doi: 10.1111/j.1582-4934.2008.00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broxmeyer HE, Srour E, Orschell C, et al. Cord blood stem and progenitor cells. Methods Enzymol. 2006;419:439–473. doi: 10.1016/S0076-6879(06)19018-7. [DOI] [PubMed] [Google Scholar]
- 10.McNiece IK, Almeida-Porada G, Shpall EJ, et al. Ex vivo expanded cord blood cells provide rapid engraftment in fetal sheep but lack long-term engrafting potential. Exp Hematol. 2002;30:612–616. doi: 10.1016/s0301-472x(02)00805-6. [DOI] [PubMed] [Google Scholar]
- 11.Bossolasco P, Montemurro T, Cova L, et al. Molecular and phenotypic characterization of human amniotic fluid cells and their differentiation potential. Cell Res. 2006;16:329–336. doi: 10.1038/sj.cr.7310043. [DOI] [PubMed] [Google Scholar]
- 12.Roubelakis MG, Pappa KI, Bitsika V, et al. Molecular and proteomic characterization of human mesenchymal stem cells derived from amniotic fluid: Comparison to bone marrow mesenchymal stem cells. Stem Cells Dev. 2007;16:931–952. doi: 10.1089/scd.2007.0036. [DOI] [PubMed] [Google Scholar]
- 13.Tamagawa T, Oi S, Ishiwata I, et al. Differentiation of mesenchymal cells derived from human amniotic membranes into hepatocyte-like cells in vitro. Hum Cell. 2007;20:77–84. doi: 10.1111/j.1749-0774.2007.00032.x. [DOI] [PubMed] [Google Scholar]
- 14.Wang H-S, Hung S-C, Peng S-T, et al. Mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem Cells. 2004;22:1330–1337. doi: 10.1634/stemcells.2004-0013. [DOI] [PubMed] [Google Scholar]
- 15.Portmann-Lanz CB, Schoeberlein A, Huber A, et al. Placental mesenchymal stem cells as potential autologous graft for pre- and perinatal neuroregeneration. Am J Obstet Gynecol. 2006;194:664–673. doi: 10.1016/j.ajog.2006.01.101. [DOI] [PubMed] [Google Scholar]
- 16.Soncini M, Vertua E, Gibelli L, et al. Isolation and characterization of mesenchymal cells from human fetal membranes. J Tissue Eng Regen Med. 2007;1:296–305. doi: 10.1002/term.40. [DOI] [PubMed] [Google Scholar]
- 17.Fukuchi Y, Nakajima H, Sugiyama D, et al. Human placenta-derived cells have mesenchymal stem/progenitor cell potential. Stem Cells. 2004;22:649–658. doi: 10.1634/stemcells.22-5-649. [DOI] [PubMed] [Google Scholar]
- 18.Igura K, Zhang X, Takahashi K, et al. Isolation and characterization of mesenchymal progenitor cells from chorionic villi of human placenta. Cytotherapy. 2004;6:543–553. doi: 10.1080/14653240410005366-1. [DOI] [PubMed] [Google Scholar]
- 19.Huang Y-C, Yang Z-M, Chen X-H, et al. Isolation of mesenchymal stem cells from human placental decidua basalis and resistance to hypoxia and serum deprivation. Stem Cell Rev. 2009;5:247–255. doi: 10.1007/s12015-009-9069-x. [DOI] [PubMed] [Google Scholar]
- 20.In ’t Anker PS, Scherjon SA, Kleijburg-van der Keur C, et al. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22:1338–1345. doi: 10.1634/stemcells.2004-0058. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Z, Lin H, Shi M, et al. Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. J Gastroenterol Hepatol. 2012;27(suppl 2):112–120. doi: 10.1111/j.1440-1746.2011.07024.x. [DOI] [PubMed] [Google Scholar]
- 22.Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109:235–242. doi: 10.1046/j.1365-2141.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- 23.Nazarov I, Lee JW, Soupene E, et al. Multipotent stromal stem cells from human placenta demonstrate high therapeutic potential. Stem Cells Translational Medicine. 2012;1:359–372. doi: 10.5966/sctm.2011-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park S, Kim E, Koh S-E, et al. Dopaminergic differentiation of neural progenitors derived from placental mesenchymal stem cells in the brains of Parkinson’s disease model rats and alleviation of asymmetric rotational behavior. Brain Res. 2012;1466:158–166. doi: 10.1016/j.brainres.2012.05.032. [DOI] [PubMed] [Google Scholar]
- 25.Lu GH, Yong WS, Xu ZM, et al. Human placental decidua basalis-derived mesenchymal stem cells differentiate into dopamine neuron-like cells with no response to long-term culture in vitro. Neuroreport. 2012;23:513–518. doi: 10.1097/WNR.0b013e328353fbb4. [DOI] [PubMed] [Google Scholar]
- 26.Suşman S, Soriţău O, Rus-Ciucă D, et al. Placental stem cell differentiation into islets of Langerhans-like glucagon-secreting cells. Rom J Morphol Embryol. 2010;51:733–738. [PubMed] [Google Scholar]
- 27.Prather WR, Toren A, Meiron M, et al. The role of placental-derived adherent stromal cell (PLX-PAD) in the treatment of critical limb ischemia. Cytotherapy. 2009;11:427–434. doi: 10.1080/14653240902849762. [DOI] [PubMed] [Google Scholar]
- 28.Kinzer M, Hingerl K, König J, et al. Mesenchymal stromal cells from the human placenta promote neovascularization in a mouse model in vivo. Placenta. 2014;35:517–519. doi: 10.1016/j.placenta.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Ventura C, Cantoni S, Bianchi F, et al. Hyaluronan mixed esters of butyric and retinoic acid drive cardiac and endothelial fate in term placenta human mesenchymal stem cells and enhance cardiac repair in infarcted rat hearts. J Biol Chem. 2007;282:14243–14252. doi: 10.1074/jbc.M609350200. [DOI] [PubMed] [Google Scholar]
- 30.Mayer L, Pandak WM, Melmed GY, et al. Safety and tolerability of human placenta-derived cells (PDA001) in treatment-resistant Crohn’s disease: A phase 1 study. Inflamm Bowel Dis. 2013;19:754–760. doi: 10.1097/MIB.0b013e31827f27df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chambers DC, Enever D, Ilic N, et al. A phase 1b study of placenta-derived mesenchymal stromal cells in patients with idiopathic pulmonary fibrosis. Respirology. 2014;19:1013–1018. doi: 10.1111/resp.12343. [DOI] [PubMed] [Google Scholar]
- 32.Ramot Y, Meiron M, Toren A, et al. Safety and biodistribution profile of placental-derived mesenchymal stromal cells (PLX-PAD) following intramuscular delivery. Toxicol Pathol. 2009;37:606–616. doi: 10.1177/0192623309338383. [DOI] [PubMed] [Google Scholar]
- 33.Kim EY, Lee KB, Kim MK. The potential of mesenchymal stem cells derived from amniotic membrane and amniotic fluid for neuronal regenerative therapy. BMB Rep. 2014;47:135–140. doi: 10.5483/BMBRep.2014.47.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hass R, Kasper C, Böhm S, et al. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9:12–25. doi: 10.1186/1478-811X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nombela-Arrieta C, Ritz J, Silberstein LE. The elusive nature and function of mesenchymal stem cells. Nat Rev Mol Cell Biol. 2011;12:126–131. doi: 10.1038/nrm3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wegmeyer H, Bröske A-M, Leddin M, et al. Mesenchymal stromal cell characteristics vary depending on their origin. Stem Cells Dev. 2013;22:2606–2618. doi: 10.1089/scd.2013.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, Charif N, Mainard D, et al. Donor’s age dependent proliferation decrease of human bone marrow mesenchymal stem cells is linked to diminished clonogenicity. Biomed Mater Eng. 2014;24(suppl):47–52. doi: 10.3233/BME-140973. [DOI] [PubMed] [Google Scholar]
- 38.Siddappa R, Licht R, van Blitterswijk C, et al. Donor variation and loss of multipotency during in vitro expansion of human mesenchymal stem cells for bone tissue engineering. J Orthop Res. 2007;25:1029–1041. doi: 10.1002/jor.20402. [DOI] [PubMed] [Google Scholar]
- 39.Alcayaga-Miranda F, Cuenca J, Luz-Crawford P, et al. Characterization of menstrual stem cells: Angiogenic effect, migration and hematopoietic stem cell support in comparison with bone marrow mesenchymal stem cells. Stem Cell Res Ther. 2015;6:32. doi: 10.1186/s13287-015-0013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heazlewood CF, Sherrell H, Ryan J, et al. High incidence of contaminating maternal cell overgrowth in human placental mesenchymal stem/stromal cell cultures: A systematic review. Stem Cells Translational Medicine. 2014;3:1305–1311. doi: 10.5966/sctm.2014-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dmitrieva RI, Minullina IR, Bilibina AA, et al. Bone marrow- and subcutaneous adipose tissue-derived mesenchymal stem cells: Differences and similarities. Cell Cycle. 2012;11:377–383. doi: 10.4161/cc.11.2.18858. [DOI] [PubMed] [Google Scholar]
- 42.Foy KC, Liu Z, Phillips G, et al. Combination treatment with HER-2 and VEGF peptide mimics induces potent anti-tumor and anti-angiogenic responses in vitro and in vivo. J Biol Chem. 2011;286:13626–13637. doi: 10.1074/jbc.M110.216820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang L, Yang Y, Zhu Y, et al. Characterization of placenta-derived mesenchymal stem cells cultured in autologous human cord blood serum. Mol Med Rep. 2012;6:760–766. doi: 10.3892/mmr.2012.1000. [DOI] [PubMed] [Google Scholar]
- 44.Kern S, Eichler H, Stoeve J, et al. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 45.Hwang JH, Shim SS, Seok OS, et al. Comparison of cytokine expression in mesenchymal stem cells from human placenta, cord blood, and bone marrow. J Korean Med Sci. 2009;24:547–554. doi: 10.3346/jkms.2009.24.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Castro-Manrreza ME, Mayani H, Monroy-García A, et al. Human mesenchymal stromal cells from adult and neonatal sources: A comparative in vitro analysis of their immunosuppressive properties against T cells. Stem Cells Dev. 2014;23:1217–1232. doi: 10.1089/scd.2013.0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim MJ, Shin KS, Jeon JH, et al. Human chorionic-plate-derived mesenchymal stem cells and Wharton’s jelly-derived mesenchymal stem cells: A comparative analysis of their potential as placenta-derived stem cells. Cell Tissue Res. 2011;346:53–64. doi: 10.1007/s00441-011-1249-8. [DOI] [PubMed] [Google Scholar]
- 48.Wulf GG, Viereck V, Hemmerlein B, et al. Mesengenic progenitor cells derived from human placenta. Tissue Eng. 2004;10:1136–1147. doi: 10.1089/ten.2004.10.1136. [DOI] [PubMed] [Google Scholar]
- 49.Lo YMD, Lau TK, Chan LYS, et al. Quantitative analysis of the bidirectional fetomaternal transfer of nucleated cells and plasma DNA. Clin Chem. 2000;46:1301–1309. [PubMed] [Google Scholar]
- 50.Srivatsa B, Srivatsa S, Johnson KL, et al. Maternal cell microchimerism in newborn tissues. J Pediatr. 2003;142:31–35. doi: 10.1067/mpd.2003.mpd0327. [DOI] [PubMed] [Google Scholar]
- 51.Chen CP, Lee MY, Huang JP, et al. Trafficking of multipotent mesenchymal stromal cells from maternal circulation through the placenta involves vascular endothelial growth factor receptor-1 and integrins. Stem Cells. 2008;26:550–561. doi: 10.1634/stemcells.2007-0406. [DOI] [PubMed] [Google Scholar]
- 52.Battula VL, Treml S, Bareiss PM, et al. Isolation of functionally distinct mesenchymal stem cell subsets using antibodies against CD56, CD271, and mesenchymal stem cell antigen-1. Haematologica. 2009;94:173–184. doi: 10.3324/haematol.13740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mariotti E, Mirabelli P, Abate G, et al. Comparative characteristics of mesenchymal stem cells from human bone marrow and placenta: CD10, CD49d, and CD56 make a difference. Stem Cells Dev. 2008;17:1039–1041. doi: 10.1089/scd.2008.0212. [DOI] [PubMed] [Google Scholar]
- 54.Kaltz N, Ringe J, Holzwarth C, et al. Novel markers of mesenchymal stem cells defined by genome-wide gene expression analysis of stromal cells from different sources. Exp Cell Res. 2010;316:2609–2617. doi: 10.1016/j.yexcr.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 55.Chang Y-J, Shih DT, Tseng C-P, et al. Disparate mesenchyme-lineage tendencies in mesenchymal stem cells from human bone marrow and umbilical cord blood. Stem Cells. 2006;24:679–685. doi: 10.1634/stemcells.2004-0308. [DOI] [PubMed] [Google Scholar]
- 56.Barlow S, Brooke G, Chatterjee K, et al. Comparison of human placenta- and bone marrow-derived multipotent mesenchymal stem cells. Stem Cells Dev. 2008;17:1095–1107. doi: 10.1089/scd.2007.0154. [DOI] [PubMed] [Google Scholar]
- 57.Gordeladze JO, Drevon CA, Syversen U, et al. Leptin stimulates human osteoblastic cell proliferation, de novo collagen synthesis, and mineralization: Impact on differentiation markers, apoptosis, and osteoclastic signaling. J Cell Biochem. 2002;85:825–836. doi: 10.1002/jcb.10156. [DOI] [PubMed] [Google Scholar]
- 58.Meisel R, Zibert A, Laryea M, et al. Human bone marrow stromal cells inhibit allogeneic T-cellresponses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619–4621. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 59.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 60.Krampera M, Cosmi L, Angeli R, et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24:386–398. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 61.Selmani Z, Naji A, Zidi I, et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26:212–222. doi: 10.1634/stemcells.2007-0554. [DOI] [PubMed] [Google Scholar]
- 62.Cuerquis J, Romieu-Mourez R, François M, et al. Human mesenchymal stromal cells transiently increase cytokine production by activated T cells before suppressing T-cell proliferation: Effect of interferon-γ and tumor necrosis factor-α stimulation. Cytotherapy. 2014;16:191–202. doi: 10.1016/j.jcyt.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 63.Lee JM, Jung J, Lee HJ, et al. Comparison of immunomodulatory effects of placenta mesenchymal stem cells with bone marrow and adipose mesenchymal stem cells. Int Immunopharmacol. 2012;13:219–224. doi: 10.1016/j.intimp.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 64.Fazekasova H, Lechler R, Langford K, et al. Placenta-derived MSCs are partially immunogenic and less immunomodulatory than bone marrow-derived MSCs. J Tissue Eng Regen Med. 2011;5:684–694. doi: 10.1002/term.362. [DOI] [PubMed] [Google Scholar]
- 65.Lu G, Zhu S, Ke Y, et al. Transplantation-potential-related biological properties of decidua basalis mesenchymal stem cells from maternal human term placenta. Cell Tissue Res. 2013;352:301–312. doi: 10.1007/s00441-013-1560-7. [DOI] [PubMed] [Google Scholar]
- 66.Erkers T, Nava S, Yosef J, et al. Decidual stromal cells promote regulatory T cells and suppress alloreactivity in a cell contact-dependent manner. Stem Cells Dev. 2013;22:2596–2605. doi: 10.1089/scd.2013.0079. [DOI] [PubMed] [Google Scholar]
- 67.Han ZB, Wang YW, Wang T, et al. [Isolation and biological characteristics of mesenchymal stem cells derived from human placenta decidua basalis] Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2013;21:754–759. doi: 10.7534/j.issn.1009-2137.2013.03.042. [DOI] [PubMed] [Google Scholar]
- 68.Zhu Y, Yang Y, Zhang Y, et al. Placental mesenchymal stem cells of fetal and maternal origins demonstrate different therapeutic potentials. Stem Cell Res Ther. 2014;5:48. doi: 10.1186/scrt436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang Y, Zhao S. Vascular Biology of the Placenta. San Rafael, CA: Morgan & Claypool Life Sciences; 2010. [PubMed] [Google Scholar]
- 70.Tran TC, Kimura K, Nagano M, et al. Identification of human placenta-derived mesenchymal stem cells involved in re-endothelialization. J Cell Physiol. 2011;226:224–235. doi: 10.1002/jcp.22329. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.