It was hypothesized that transplanting human embryonic stem cells (hESCs) lacking human leukocyte antigen class I (HLA-I) expression would not result in a robust immune rejection by allogeneic recipients. β2-Microglobulin-null hESCs transplanted into natural killer cell-depleted immunocompetent mice developed tumors resembling those from control hESCs in severe combined immunodeficiency mice. β2-Microglobulin-null hESCs significantly reduced immunogenicity to CD8+ T cells and might provide a renewable source of cells for tissue regeneration without HLA matching.
Keywords: Immunogenicity of human embryonic stem cells, β2-Microglobulin gene targeting strategy, Human leukocyte antigen class I, Differentiation and characterization
Abstract
Human embryonic stem cells (hESCs) are a promising source of cells for tissue regeneration, yet histoincompatibility remains a major challenge to their clinical application. Because the human leukocyte antigen class I (HLA-I) molecules are the primary mediators of immune rejection, we hypothesized that cells derived from a hESC line lacking HLA-I expression could be transplanted without evoking a robust immune response from allogeneic recipients. In the present study, we used the replacement targeting strategy to delete exons 2 and 3 of β2-microglobulin on both gene alleles in hESCs. Because β2-microglobulin serves as the HLA-I light chain, disruption of the β2-microglobulin gene led to complete HLA-I deficiency on the cell surface of hESCs and their derivatives. Therefore, these cells were resistant to CD8+ T-cell-mediated destruction. Although interferon-γ (IFN-γ) treatment significantly induced β2-microglobulin expression, promoting CD8+ T cell-mediated killing of control hESCs and their derivatives, CD8+ T-cell-mediated cytotoxicity was barely observed with β2-microglobulin-null hESCs and their derivatives treated with IFN-γ. This genetic manipulation to disrupt HLA-I expression did not affect the self-renewal capacity, genomic stability, or pluripotency of hESCs. Despite being relatively sensitive to natural killer (NK) cell-mediated killing due to the lack of HLA-I expression, when transplanted into NK cell-depleted immunocompetent mice, β2-microglobulin-null hESCs developed into tumors resembling those derived from control hESCs in severe combined immunodeficiency mice. These results demonstrate that β2-microglobulin-null hESCs significantly reduce immunogenicity to CD8+ T cells and might provide a renewable source of cells for tissue regeneration without the need for HLA matching in the future.
Significance
This study reports the generation of a novel β2-microglobulin (B2M)−/− human embryonic stem cell (hESC) line. Differentiated mature cells from this line do not express cell surface human leukocyte antigen molecules even after interferon-γ stimulation and are resistant to alloreactive CD8+ T cells. Moreover, this B2M−/− hESC line contains no off-target integration or cleavage events, is devoid of stable B2M mRNA, exhibits a normal karyotype, and retains its self-renewal capacity, genomic stability, and pluripotency. Although B2M−/− hESC-derived cells are more susceptible to natural killer (NK) cells, murine transplantation studies have indicated that they are, overall, much less immunogenic than normal hESCs. Thus, these data show for the first time that, in vivo, the advantages provided by B2M−/− hESC-derived cells in avoiding CD8+ T-cell killing appear significantly greater than any disadvantage caused by increased susceptibility to NK cells.
Introduction
Human embryonic stem cells (hESCs) derived from the inner cell mass of blastocyst-stage embryos possess unlimited self-renewal capacity and can be induced to differentiate into all cell types of three germ layers in culture [1, 2]. These characteristics make hESCs the most reliable source of replacement cells for tissue regeneration. Since the successful establishment of hESCs in culture [1], significant progress has been made in developing techniques to effectively generate hESC-derived cell lineages, such as cardiomyocytes [3], neurons [4], pneumocytes [5], and pancreatic β cells [6]. Therapeutic use of hESC-derivatives for the treatment of currently incurable diseases should have an enormous clinical impact. Recently, hESC-based therapies have been introduced into human clinical trials for spinal cord injury and macular degeneration [7, 8]. Despite these tremendous advancements, hESC-based therapies are still in the early stages of development, with significant challenges remaining that must be addressed before they can be safely and routinely used clinically. One of the most significant challenges for therapeutic transplantation of hESC-derived cells is avoidance of immune-mediated rejection. Transplantation of allogeneic hESCs can induce a robust immune response [9], even in immune privileged sites [10]. Thus, the therapeutic use of allogeneic hESC-derived cells would require extensive immunosuppressive treatment to prevent graft rejection, which substantially increases the risk of developing opportunistic infections and tumorigenesis [11, 12].
Extensive effort has been devoted to generating personalized hESCs to overcome immune rejection. Somatic cell nuclear transfer (SCNT) is a potential method to generate personalized hESCs (SCNT-hESCs). With the SCNT technique, somatic cell nucleus from a patient is transferred to an enucleated oocyte to generate a viable embryo via a reprogramming process from which SCNT-hESCs can be derived. The derived SCNT-hESCs are genetically identical to the patient, except for the mitochondrial DNA contributed by the oocyte and thus are expected to not cause an immune response. However, derivation of SCNT-hESCs has proved difficult because of early SCNT embryo arrest at the 2- to 8-cell stage or failure in the derivation of stable hESCs from SCNT embryos [13–16]. Recently, Tachibana et al. established an efficient methodology for reprogramming somatic cells to SCNT-hESCs [17]. However, in a subsequent study, they showed that SCNT somatic cell reprogramming could result in epigenetic/transcriptional variations in the derived SCNT-hESCs [18]. Whether these abnormally expressed genes will affect their immunogenicity remains uncertain.
Use of patient-derived induced pluripotent stem cells (iPSCs) generated by recently developed methodologies is another possible approach to prevent immune rejection [19]. The original techniques used viral vectors to introduce four reprogramming factors into somatic cells to convert adult cells to autologous iPSCs [19–21]. For safety considerations, extensive effort has been devoted to generating “clinical grade” iPSCs that are virus free and devoid of insertional mutagenesis [22–29]. Despite these efforts, recent studies have demonstrated that reprogramming in itself could result in a number of abnormally expressed genes in iPSC-derived cells, causing immune rejection in syngeneic recipients [18, 30]. Consistent with this finding, Araki et al. subsequently reported that in vitro differentiated cardiomyocytes from iPSCs were highly immunogenic in syngeneic mice [31]. In that same study, however, skin and bone marrow cells derived from iPSCs showed limited immunogenicity in syngeneic mice. Collectively, these findings suggest that the degree of immune response elicited from iPSCs engrafted in syngeneic recipients could be cell type dependent or dependent on the in vitro culture conditions used for iPSC differentiation [31]. Thus, additional research is required before SCNT-hESCs or human iPSCs can be used clinically without concerns of possible immune rejection of transplanted SCNT-hESCs or IPSC-derived cells. Finally, the financial costs of generating individualized IPSCs will be significant, and the ability to incur such costs will not be feasible for most patients.
Another possible strategy to reduce graft rejection is to minimize alloantigenic differences between the donors and patients. The cell surface-expressed human leukocyte antigens (HLAs), molecules encoded by genes located in the human major histocompatibility complex on chromosome 6, are the major mediators of immune rejection. Mismatch of a single HLA gene between the donor and patient can cause a robust immune response [32]. HLA genes are divided into HLA class I (HLA-I) and HLA class II (HLA-II). HLA-I genes (HLA-A, HLA-B, and HLA-C) are expressed in almost all tissue cell types, presenting “non-self” antigen-processed peptides to CD8+ T cells, thereby promoting their activation to cytolytic CD8+ T cells. Transplanted cells expressing “non-self” HLA-I molecules will cause a robust cellular immune response directed at these cells and ultimately resulting in their destruction by activated cytolytic CD8+ T cells. HLA-I proteins are covalently associated with β2-microglobulin (B2M) in the endoplasmic reticulum, which is essential for forming functional HLA-I molecules on the cell surface. Most individuals have at least three major HLA-II genes (HLA-DP, HLA-DQ, and HLA-DR). In contrast to the wide cellular distribution of HLA-I molecules, expression of HLA-II genes is restricted to antigen-presenting cells such as dendritic cells, macrophages, and B cells. A bank of hESCs has been proposed, from which one could find patient HLA-matched hESCs for tissue regeneration [33]. However, HLA antigens are the most polymorphic observed in the human genome, and it has been reported that the number of diploid combinations of HLA-I genes alone is greater than 1 billion [34]. Thus, it would be essentially impossible to build such a large hESC bank.
The generation of a “universal donor” hESC line compatible with any HLA genotype might provide an alternative strategy that could resolve the immune rejection and financial issues associated with that of iPSCs and banked hESCs. To generate such a hESC line, one possible approach would be to functionally disrupt both genetic alleles encoding the HLA-I light chain, B2M. The resulting B2M-null hESC line and its derivatives would be expected to exhibit greatly reduced surface HLA-I expression and, as a consequence, reduced immunogenicity to allogeneic CD8+ T cells. Because HLA molecules serve as major ligand inhibitors to natural killer (NK) cells, one possible caveat to this approach could be that HLA-I-deficient donor cells would be rendered sensitive to NK cell recognition and destruction. Despite this potential problem, the generation and characterization of a HLA-I-deficient hESC line would be the first essential step in the generation of a “universal donor” hESC line for possible clinical use in the future. Recently, the transcription activator-like effector nuclease (TALEN) targeting approach was used in an attempt to generated B2M-deficient hESC lines by deletion of a few nucleotides in exon 2 of the B2M gene [35]. Although the B2M-targeted hESC lines appeared to be surface HLA-I deficient, they were found to still contain mRNAs specific for B2M and HLA-I. The B2M and HLA-I mRNAs were expressed at levels similar to those of untargeted hESCs (both constitutive and interferon-γ [IFN-γ] induced). Thus, concern exists that these TALEN B2M-targeted hESC lines might express residual cell surface HLA-I that would be sufficient to cause immune rejection, such as has been observed with B2M−/− mouse cells that also express B2M mRNA [36]. Although the TALEN B2M-targeted hESC lines were not examined for off-target cleavage events, the occurrence of nonspecific cleavage when using TALENs remains a significant issue that would impose a major safety concern on their clinical use [37, 38]. We report the generation of a novel B2M-null hESC line. This hESC line was created using a replacement targeting strategy, in which exons 2 and 3 were deleted from both genetic alleles of B2M, resulting in complete B2M deficiency, without causing chromosomal abnormalities, alterations in pluripotency, or impairment of self-renewal capacity. The B2M-null hESCs and their differentiated cells were surface HLA-I deficient and resistant to alloreactive CD8+ T cell-mediated killing in vitro and to immune rejection in NK cell-depleted immune competent mice in vivo.
Materials and Methods
Construction of B2M-Targeting Vectors
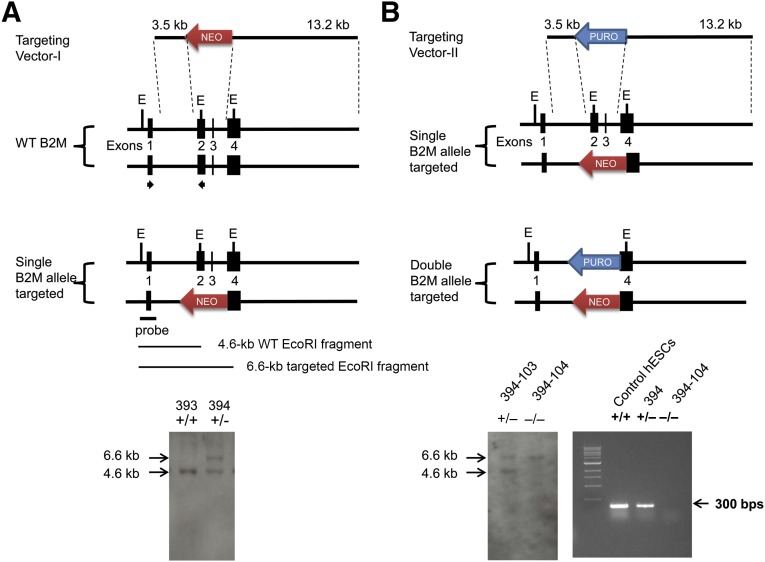
To generate the B2M-targeting vector I, one 3.5-kb DNA fragment homologous to intron 1 of the B2M gene was cloned into KpnI and XhoI sites downstream of neomycin-resistance (NEOR) gene in the pKO Scrambler V901 vector (Lexicon Pharmaceuticals, Inc., Woodland, TX, http://www.lexgen.com). Subsequently, one 13.2-kb DNA fragment that includes part of intron 3 and the entire exon 4 of the B2M gene was digested with BamHI and SalI and added into the site upstream of the NEOR gene (Fig. 1A, top). To produce the B2M-targeting vector II, the NEOR gene of B2M-targeting vector I was replaced with the puromycin-resistance (PUROR) gene (Fig. 1B, top). The targeting vectors were then linearized with KpnI before transfection of hESCs for B2M gene targeting.
Figure 1.
Targeting strategy and identification of B2M-targeted human embryonic stem cells (hESCs). The B2M-targeting vectors harbor a NEOR (targeting vector I) or PUROR gene (targeting vector II), each flanked by a 3.5-kb left arm homologous to intron 1 of the B2M gene and a 13.2-kb right arm identical to the region downstream of exon 3, including exon 4 of the B2M gene. The probe containing exon 1 sequences is upstream of the targeted region and identifies a 4.6-kb WT EcoRI B2M fragment and a 6.6-kb targeted EcoRI B2M fragment. The arrows indicate the locations of B2M forward primer I (5′-GCC TTA GCT GTG CTC GCG CTA C-3′) and reverse primer I (5′-GTC ACA TGG TTC ACA CGG CAG GCA TAC TC-3′) used for screening of B2M-targeted hESC clones. Southern hybridization identified only a 4.6-kb WT EcoRI B2M fragment in hESC-393 ([A], bottom); a 4.6-kb WT EcoRI B2M fragment and a 6.6-kb targeted EcoRI B2M fragment were detected in hESC-394, indicating that 1 of the B2M alleles had been targeted. Southern blot analysis of hESC clones from the targeting vector II transfection showed a 4.6-kb WT EcoRI B2M fragment and a 6.6-kb targeted EcoRI B2M fragment in hESC-394-103 (B2M+/− hESCs) but only a 6.6-kb targeted EcoRI B2M fragment in hESC-394-104 (B2M−/− hESCs), demonstrating that both B2M gene alleles had been disrupted in hESC-394-104 but not in hESC-394-103 ([B], bottom left). Reverse transcription-polymerase chain reaction analysis of B2M expression in the control hESCs, hESC-394 and hESC-394-104, demonstrated no B2M mRNA detected in the hESC-394-104 ([B], bottom right). Abbreviations: B2M, β2-microglobulin; bps, base pairs; E, EcoRI; WT, wild type.
Generation of B2M-Null hESCs
The hESCs (H9.2) were routinely maintained in mitomycin-treated mouse embryonic fibroblast (CF-1 MEF) feeder cells on 6-well plates using hESC medium containing 80% Dulbecco’s modified Eagle’s medium (DMEM)/F12, 20% knockout serum replacement, 1% nonessential amino acid, 1 mM l-glutamine, 0.1 mM 2-mercaptoethanol, and 4 ng/ml basic fibroblast growth factor (bFGF) [5]. To target the B2M gene, approximately 1 × 106 hESCs at passage 38 were resuspended in 100 μl of supplemented mouse embryonic stem cell Nucleofector solution (VAPH-1001, Lonza Inc., Basel, Switzerland, http://www.lonza.com), mixed with 5 μg of linearized B2M-targeting vector I, and then transfected, as previously described [5, 39]. The transfected cells were placed on Matrigel-coated 10-cm plates in MEF-conditioned hESC medium (CM) and selected in the presence of G418 (50 μg/ml; Gibco Invitrogen, Life Technologies, Carlsbad, CA, http://www.lifetechnologies.com) for 14 days [5]. The stably transfected hESC colonies that had survived G418 selection were selected and screened by Southern hybridization analysis to identify single B2M allele-targeted hESC (B2M+/− hESC) clones. To generate double B2M allele-targeted hESCs (B2M−/− hESC) clones, the B2M+/− hESCs were then prepared and transfected with B2M-targeting vector II, as above. B2M-targeting vector II transfected cells were selected by G418 and puromycin (0.5 μg/ml; Sigma-Aldrich, St. Louis, MO, http://www.sigmaladrich.com) for 14 days. Similarly, those hESC colonies that had survived the G418 and puromycin double selection were picked and screened for identification of B2M−/− hESC clones using Southern blot and reverse transcription-polymerase chain reaction (RT-PCR) analysis.
Southern Blot and RT-PCR Analysis of B2M-Targeted hESC Clones
Genomic DNA was isolated from hESC clones and digested with EcoRI for Southern blot analysis. A 400-base pair PCR fragment containing exon 1 sequence of B2M was used as a probe to identify a 4.6-kb wild-type (WT) EcoRI B2M fragment and a 6.6-kb targeted EcoRI B2M fragment (Fig. 1). In addition, RT-PCR was performed to identify B2M-targeted hESC clones with B2M forward primer I located in exon 1 and B2M reverse primer I located in exon 2 using the OneStep RT-PCR Kit (Qiagen, Hilden, Germany, http://www.qiagen.com).
Karyotype Analysis
High-resolution chromosomal G-banding analysis of B2M−/− hESCs at different passages was performed at the Clinical and Research Cytogenetic Laboratory, Texas Children’s Hospital (Houston, TX).
Differentiation of B2M-Null hESCs
To differentiate B2M−/− hESCs, the cells were cultured on Matrigel-coated plates in differentiation medium (DM) for 14 days [5]. The DM contains 80% knockout-DMEM, 20% fetal bovine serum (FBS), 1% nonessential amino acid, and 1 mM l-glutamine. To assess the effect of inflammatory stimuli on expression of B2M, 25 ng/ml IFN-γ (PeproTech, Rocky Hill, NJ, http://www.peprotech.com) was added into the medium for 48 hours before the assay. To produce lung alveolar epithelial type II cell (ATIIC)-enriched cultures, hESCs were cultured on Matrigel-coated plates in serum-free medium supplemented with activin A and wnt3a (20 ng/ml and 10 ng/ml; R&D Systems, Minneapolis, MN, http://www.rndsystems.com) for the first 6 days to promote definitive endoderm differentiation. Next, bFGF (50 ng/ml; catalog no. F-0291; Sigma-Aldrich) was used to induce alveolar lineage-specific differentiation as previously reported [40].
Real-Time Quantitative RT-PCR Analysis
Total RNA was isolated from undifferentiated and differentiated B2M−/− hESCs using RNA Bee (Tel-Test Inc., Friendswood, TX, http://www.tel-test.com) [29]. To examine the B2M mRNA expression levels, quantitative RT-PCR (qRT-PCR) was performed using TaqMan One-Step RT-PCR Master Mix Kit (Applied Biosystems, Foster City, CA, http://www.appliedbiosystems.com) with the following primer pairs and probes: (a) B2M forward primer II, B2M reverse primer II, and B2M probe; and (b) 18S forward primer, 18S reverse primer, and 18S probe.
Flow Cytometry and Immunofluorescent Staining
The B2M−/− hESCs and differentiated B2M−/− hESCs were dissociated into single cell suspensions, as previously described [5]. The cells were blocked with 10% goat serum and then incubated on ice for 1 hour with 1:50 diluted R-phycoerythrin-conjugated mouse anti-B2M (Santa Cruz Biotechnology Inc., Santa Cruz, CA, http://www.scbt.com) or mouse anti-human major histocompatibility complex I (MHC-I; W6/32; Abcam, Cambridge, U.K., http://www.abcam.com), which recognizes HLA-A, HLA-B, and HLA-C, for flow cytometry analysis. For immunofluorescent staining, the B2M−/− hESCs and differentiated B2M−/− hESCs were washed with phosphate-buffered saline (PBS) and fixed in 4% paraformaldehyde at room temperature for 15 minutes. After being blocked in 10% goat serum, the cells were stained with 1:50 diluted mouse anti-human MHC-I or mouse anti-B2M for 2 hours at room temperature. For ATIIC-enriched cultures, 1:150 diluted anti-human MHC-I was used for staining. The surface-expressed HLA-I or B2M was visualized with Alexa Fluor 488 (green) or 546 (red) conjugated goat anti-mouse IgG (1:1,000, Molecular Probes, Eugene, OR, http://probes.invitrogen.com) with Draq5 (1:500, BioStatus, Leicestershire, U.K., http://www.biostatus.com) counterstaining [29]. To stain protein markers expressed by differentiated B2M−/− hESCs, the paraformaldehyde-fixed cells were washed with PBS and permeabilized in 0.2% Triton X (Sigma-Aldrich) for 30 minutes. The cells were then stained with either rabbit anti-human pro-surfactant protein B (SPB), rabbit anti-human pro-SPC (1:500; Chemicon, Temecula, CA, http://www.chemicon.com), mouse anti-glucagon, mouse anti-insulin, mouse anti-myosin 2, mouse anti-α1-fetoprotein, mouse anti-nestin, rabbit anti-tyrosine hydroxylase, or rabbit anti-βIII-tubulin (1:200 dilution; Abcam) for 2 hours at room temperature. Alexa Fluor 546- or 488-conjugated goat anti-mouse or anti-rabbit IgG (1:1,000 dilution) were used to visualize these expressed proteins in the differentiated B2M−/− hESCs with Draq5 counterstaining.
Cytolytic Assays
Human peripheral blood mononuclear cells were isolated by density gradient centrifugation using Ficoll-Paque (MACS; Miltenyi Biotec, Bergisch Gladbach, Germany, http://www.miltenyibiotec.com). The CD8+ T cells and NK cells were then isolated using the CD8+ T-Cell Isolation Kit and NK Cell Isolation Kit (MACS; Miltenyi Biotec), respectively. The purity of isolated CD8+ T cells (88% ± 3.3%) and NK cells (86% ± 2.7%) was evaluated by flow cytometry and then cultured in Roswell Park Memorial Institute 1640-positive 10% FBS with 50 U/ml interleukin-2 for 48 hours before assay. The cytotoxic activity of CD8+ T cells and NK cells against the B2M−/− hESCs and their derivatives was assessed using the Cytotoxicity Detection Kit (Roche Applied Science, Indianapolis, IN, https://www.roche-applied-science.com). In brief, the B2M−/− hESCs and differentiated B2M−/− hESCs were dissociated into single cell suspensions, and 2 × 105 cells in 100 μl of assay medium were added into each well of 96-well plate. The CD8+ T cells or NK cells in 100 μl of assay medium were then added and mixed with B2M−/− hESCs or differentiated B2M−/− hESCs at various effector/target (E/T) ratios. Controls were set up following the manufacturer’s instructions. After being cultured for 3 hours, the 96-well plates were centrifuged at 250g for 10 minutes, and 100 μl of supernatant was carefully collected from each reaction and transferred into the corresponding well of optically clear 96-well plates. Next, 100 μl of reaction mixture was then added into each well and incubated for 30 minutes at 20°C. The absorbance of the samples was measured at a 490-nm wavelength to determine the lactate dehydrogenase activity. To examine in vivo CD8+ T cell- and NK cell-mediated response to transplanted hESCs, 4 × 106 hESCs or B2M−/− hESCs were mixed with 200 ml of growth factor-reduced Matrigel and injected subcutaneously into 10-week-old immunocompetent mice [41]. The implants were harvested after 48 hours for immunofluorescence staining of CD8+ T cells or KLRA1+ NK cells.
Teratoma Formation
To examine in vivo pluripotency and immunogenicity, B2M−/− hESCs were resuspended at 5 × 106 in 100 μl of hESC medium. The isoflurane-anesthetized severe combined immunodeficiency (SCID) mice and immunocompetent mice with a C57BL/6 genetic background (Jackson Laboratory, Bar Harbor, ME, http://www.jax.org) were intramuscularly injected with 5 × 106 cells in the left hind leg [29]. To assess the role of NK cells in the prevention of teratoma formation from B2M−/− hESCs, the mice were depleted of NK cells by i.p. injection of anti-asialo GM1 (Wako Chemicals, Osaka, Japan, http://www.wako-chem.co.jp/english) [42]. Anti-asialo GM1 serum was reconstituted with 1 ml of normal saline, and the mice received anti-asialo GM1 3 times weekly (50 μl per mouse each time) beginning 2 days before cell injection. The tumors were surgically dissected from the mice 10 weeks after injection for histological evaluation by H&E staining.
Statistical Analysis
The data were analyzed using one-way analysis of variance. The resulting pairwise p values < .05 were considered statistically significant.
Results
Generation of the B2M-Null hESC Line
Histoincompatibility is a major obstacle in the therapeutic application of hESC-derived cells for tissue regeneration. Abolition of surface-expressed HLA-I on hESC-derivatives could minimize their immunogenicity to alloreactive T cells. To test this possibility, we sought to generate a hESC line in which surface-expressed HLA-I was ablated. To do so, we aimed to disrupt both alleles of the B2M gene encoding the HLA-I light chain by replacing exons 2 and 3 with NEOR and PUROR cassettes via homologous recombination with 2 B2M-targeting vectors (Fig. 1). To first generate a single B2M allele-targeted hESC line, hESCs were transfected with the linearized targeting vector I and then seeded onto Matrigel-coated 10-cm dishes with CM for G418 selection. Of ∼1 × 106 transfected hESCs, we obtained 528 G418-resistant hESC colonies. From Southern blot analysis, 2 of the G418-resistant clones were found to contain a 4.6-kb WT EcoRI fragment and a 6.6-kb targeted EcoRI fragment, indicating that correctly targeted homologous recombination had occurred in 1 B2M gene allele in each of these 2 clones (B2M+/− hESC). The Southern hybridization data from one of the targeted clones (hESC-394) are shown in Figure 1A, bottom. This correctly targeted clone hESC-394 contained a single copy of the targeting vector as demonstrated by Southern hybridization (data not shown) and was subsequently transfected with the linearized targeting vector II to produce B2M-null hESC lines. Of 3 million transfected hESC-394 cells, 613 stably transfected clones surviving G418 and puromycin double selection were isolated and screened for successful homologous recombination of both B2M gene alleles. Southern hybridization revealed that 2 hESC clones contained a 6.6-kb targeted EcoRI fragment but lacked the 4.6-kb WT EcoRI fragment, indicating that both B2M gene alleles had been genetically disrupted (B2M−/− hESCs). The Southern hybridization results of one of the B2M−/− hESC clones (hESC-394-104) are shown in Figure 1B, bottom left. No random insertion of the targeting vector sequence in the chromosomal DNA was detected in hESC-394-104 (data not shown). In addition, RT-PCR was performed with B2M forward primer I located in exon 1 and reverse primer I located in exon 2 (targeted area) to confirm that both B2M gene alleles had been functionally disrupted in hESC-394-104 (i.e., no B2M mRNA was detected in the hESC-394-104 cells; Fig. 1B, bottom right).
Surface HLA-I Expression Lacking on B2M-Null hESCs Before and After Differentiation
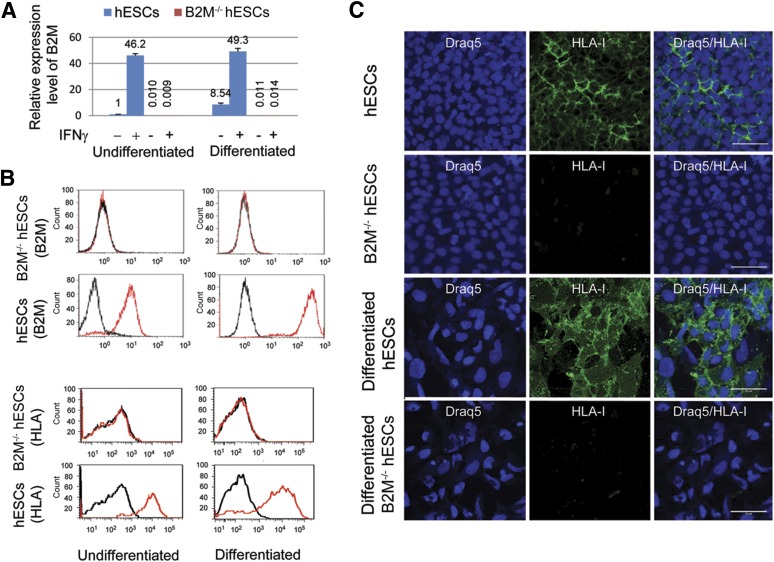
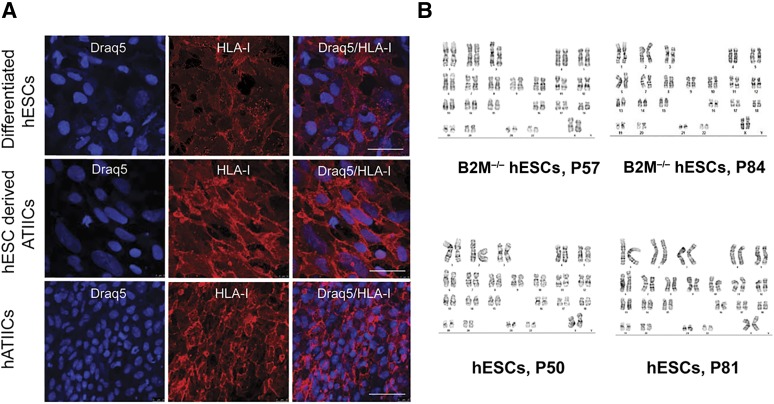
qRT-PCR was performed to evaluate B2M mRNA expression in control hESCs and the B2M−/− hESCs (hESC-394-104) before and after differentiation using B2M-specific primers located in exon 4 (nondisrupted exon; as described in Materials and Methods). B2M mRNA expression was clearly detectable in undifferentiated hESCs and significantly increased in the differentiated cultures by approximately 8.5-fold (Fig. 2A). In contrast, no B2M mRNA transcripts were detected in the B2M−/− hESCs before and after differentiation. Because IFN-γ released in the course of the immune response can increase B2M expression, promoting immune rejection [43], we next examined whether B2M mRNA expressed by hESCs and B2M−/− hESCs can be induced by IFN-γ. As anticipated, B2M mRNA was significantly upregulated in undifferentiated and differentiated hESCs by IFN-γ. In contrast, even after IFN-γ stimulation, no B2M mRNA was detected in the B2M−/− hESCs before or after differentiation (Fig. 2A). Because B2M plays an essential role for surface HLA-I presentation via covalent association with the α-chains of HLA-I molecules, it is anticipated that disruption of the B2M gene will lead to a complete loss of surface HLA-I expression. To test this possibility, surface B2M or HLA-I protein expression on hESCs and B2M−/− hESCs was analyzed by flow cytometry using a mouse anti-B2M or anti-human MHC-I antibody. Flow cytometry analyses revealed that both surface B2M and HLA-I were expressed on hESCs but not detected on B2M−/− hESCs (Fig. 2B). To examine their expression after differentiation, hESCs and B2M−/− hESCs were subjected to spontaneous differentiation by culturing on Matrigel-coated dishes in DM for 14 days. Flow cytometry analyses showed that the both B2M and HLA-I expression levels on hESCs were significantly increased approximately 12-fold and 6-fold, separately, after differentiation (Fig. 2B). Just as was the case with the undifferentiated cells, no surface B2M or HLA-I expression was detected after differentiation of the B2M−/− hESCs. In agreement with these results, HLA-I expression was shown by immunofluorescence staining on control hESCs, with relatively higher staining density observed on the surface of differentiated hESCs (Fig. 2C). Because spontaneously differentiated cultures generate a mixed population of hESC derivatives, including undifferentiated stem and progenitor cell types, they could express lower levels of HLA-I proteins compared with hESC-derived mature cell lineages or adult tissue cells. To test this possibility, we compared by immunohistochemistry the HLA-I expression of the spontaneously differentiated hESCs with that of human hESC-derived ATIIC cultures (hES-ATIICs) and human primary ATIICs (hATIICs). The results showed that HLA-I expression in the hES-ATIICs, in which ∼81% of the cells were ATIIC-specific surfactant protein C positive (data not shown), was significantly higher than that in the spontaneously differentiated hESC cultures and reached levels comparable to that expressed by hATIICs (Fig. 3A). These data suggest that mature hESC derivatives should be of similar immunogenicity as the corresponding adult tissue cells. Moreover, the surface HLA-I proteins could not be detected on B2M−/− hESCs or their derivatives by either flow cytometry analysis or immunofluorescence staining (Fig. 2B, 2C), demonstrating that the functional knockout of the B2M gene had led to complete surface HLA-I protein deficiency.
Figure 2.
Expression of B2M and HLA-I in B2M-null hESCs. (A): Quantitative reverse transcription-polymerase chain reaction analysis of B2M expression levels in undifferentiated and differentiated B2M−/− hESCs (hESC-394-104), with and without IFN-γ treatment, using the following primer pairs and probes: B2M forward primer-II (5′-GAG TGC TGT CTC CAT GTT TGA TG-3′), B2M reverse primer-II (5′-AAG TTG CCA GCC CTC CTA GA-3′), and B2M probe (5′-6-FAM-CTA CCT GTG GAG CAA CCT GCT CAG A-TAMRA-3′); and 18S forward primer (5′-TAA CGA ACG AGA CTCTGG CAT-3′), 18S reverse primer (5′-CGG ACA TCT AAG GGC ATC ACA G-3′), and 18S probe (5′-FAM-TGG CTG AAC GCC ACT TGT CCC TCT AA-TAMRA-3′). The expression levels were normalized to endogenous 18S rRNA control. Bar graphs depict B2M mRNA expression levels relative to hESC control (n = 5). (B): Flow cytometry was performed to analyze surface B2M or HLA-I protein expression in the hESCs and B2M−/− hESCs (hESC-394-104) before and after differentiation using the mouse anti-B2M antibody (top panel, red) or mouse anti-human MHC-I antibody (bottom panel, red) with mouse IgG as the isotype control (black). (C): Immunofluorescent staining demonstrated negative HLA-I expression on the surface of undifferentiated and differentiated B2M−/− hESCs (hESC-394-104), with hESCs serving as the control. Scale bars = 50 μm (human major histocompatibility complex I antibody: 1:50 diluted). Abbreviations: B2M, β2-microglobulin; hESCs, human embryonic stem cells; HLA, human leukocyte antigen; IFN-γ, interferon-γ.
Figure 3.
HLA-I expression in hESC-derived ATIICs and cytogenetic analysis B2M-null hESCs. (A): Immunofluorescent staining for surface HLA-I expression on differentiated hESCs, hESC-derived ATIICs, and hATIICs using 1:150 diluted human major histocompatibility complex I antibody. Scale bars = 50 μm. (B): Cytogenetic evaluation of B2M−/− hESCs (hESC-394-104) with hESC control. Twenty metaphase cells harvested from each cell type were analyzed at different passages. Abbreviations: ATIICs, alveolar epithelial type II cells; B2M, β2-microglobulin; hATIICs, human primary ATIICs; hESCs, human embryonic stem cells; HLA, human leukocyte antigen.
Self-Renewal Capacity, Genomic Stability, and In Vitro Pluripotency of B2M-Null hESCs
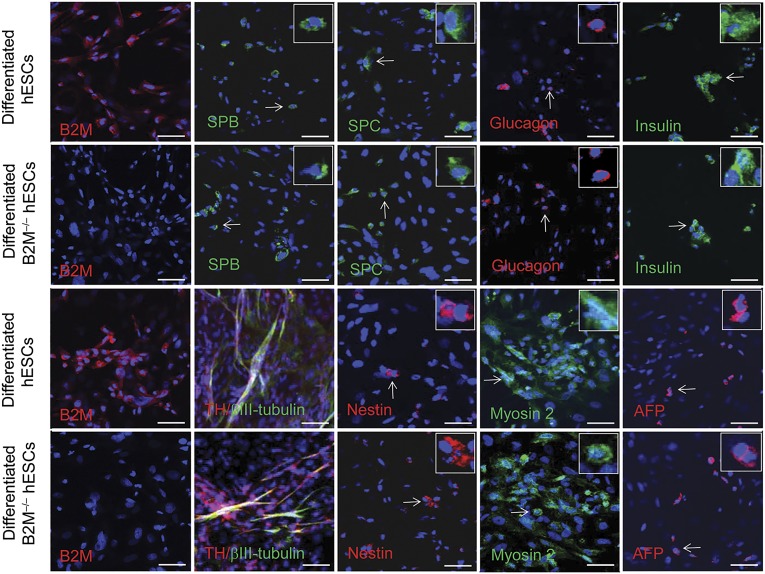
To examine their long-term self-renewal capacity, the B2M−/− hESCs were cultured in hESC-supporting conditions as described in Materials and Methods. The cell line propagated as robustly as did the control hESCs and maintained its defined morphology and self-renewal capacity at least up to passage 87. To examine whether the genetic manipulations had caused chromosomal aberrations, chromosomal karyotype analysis of the B2M−/− hESCs was performed using standard protocols for high-resolution G-banding. Just as did the control hESCs, the B2M−/− hESCs showed the normal karyotype at passages 57 and 84 (Fig. 3B). In addition, the same as the control hESCs, the B2M−/− hESCs possessed the capacity to differentiate into many different cell lineages in vitro, including distal lung epithelial cells, pancreatic cells, neuronal cells, muscle cells, and hepatocyte progenitor cells, as demonstrated by immunocytochemistry (Fig. 4). Taken together, these results indicate that the genetic manipulations to knockout both B2M gene alleles did not affect the self-renewal capacity, karyotype stability, or pluripotency of the B2M−/− hESCs.
Figure 4.
In vitro differentiation of B2M-null hESCs. Immunofluorescent staining of differentiated cultures of B2M-null hESCs was performed to examine their potential to differentiate into various cell lineages from three germ layers with hESC control. The differentiated B2M-null hESCs were B2M negative compared with the differentiated control hESCs (first column images). The SPB- or SPC-expressing distal lung epithelial cell types (green), glucagon-positive pancreatic α cells (red), insulin-producing pancreatic β cells (green), and AFP-positive hepatocyte progenitor cells (red) derived from endoderm were identified in the cultures (the right four images in the top two rows and the fifth images in the bottom two rows). Mesoderm-derived muscle cells showing positive myosin-2 staining were demonstrated in the differentiated cultures (fourth images in the bottom two rows). The ability of B2M-null hESCs to differentiate into ectoderm-derived cells was demonstrated by derived nestin, TH, and/or βIII-tubulin-positive nerve cells in the cultures (second and third images in the bottom two rows). A magnified view of a representative B2M-null hESC-derived cell indicated by a white arrow is inset in each corresponding image. Scale bars = 50 μm. Abbreviations: AFP, α1-fetoprotein; B2M, β2-microglobulin; hESCs, human embryonic stem cells; SPB, surfactant protein B; SPC, surfactant protein C; TH, tyrosine hydroxylase.
Resistance of B2M-Null hESCs to Alloreactive CD8+ T Cell-Mediated Killing In Vitro
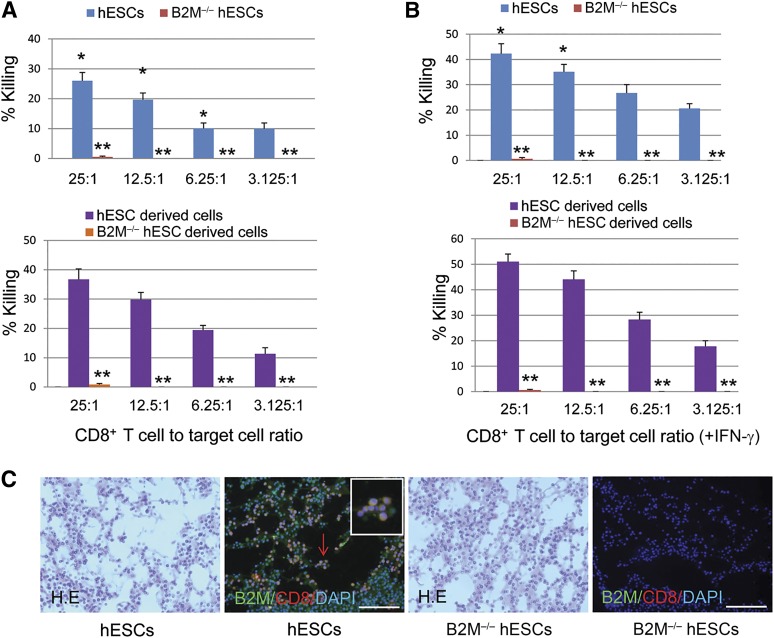
HLA-I proteins expressed by tissue cells represent the primary targets for immune rejection mediated by alloreactive CD8+ T cells. It is expected, therefore, that the HLA-I-deprived B2M−/− hESCs and their derivatives will have significantly reduced the immunogenicity to CD8+ T cells. To investigate this, we analyzed the cytolytic activity of allogeneic CD8+ T cells against hESCs, B2M−/− hESCs and their derivatives at various E/T ratios (Fig. 5). The results showed that CD8+ T cells at all E/T ratios caused significant killing of the hESCs, with the most robust killing occurring at the higher concentrations of CD8+ T cells (26.0% at 25:1; 19.7% at 12.5:1; 10% at 6.25:1; and 10% at 3.125:1; Fig. 5A, top). CD8+ T cell-mediated killing was significantly increased (p < .01) when differentiated hESCs were tested at E/T ratios of 25:1, 12.5:1, and 6.25:1 (Fig. 5A, bottom), which likely resulted from significantly increased HLA-I expression after hESC differentiation, just as had been demonstrated previously (Fig. 2). As expected, only minimal CD8+ T cell-mediated killing of the B2M−/− hESCs occurred (before or after differentiation) with less than 1% killing observed even at the highest E/T ratio of 25:1 (Fig. 5A), demonstrating greatly reduced immunogenicity of B2M−/− cells. Because IFN-γ released in the course of an immune response could upregulate B2M expression, promoting immune-rejection [43], we next assessed whether IFN-γ-treated B2M−/− hESCs become sensitive to CD8+ T cell-mediated lysis. The results shown in Figure 5B demonstrated that although IFN-γ treatment significantly induced B2M expression and promoted CD8+ T cell-mediated killing of control hESCs and their derivatives (p < .01), IFN-γ treatment was completely ineffective in increasing the sensitivity of B2M−/− hESCs or their derivatives to CD8+ T cell-mediated killing. We also used Matrigel-hESC and Matrigel-B2M−/− hESC implant models to test in vivo the CD8+ T-cell response. As expected, an acute CD8+ T-cell response was observed, demonstrated by numerous CD8+ T cells infiltrating into the Matrigel-hESC implants after 2 days (Fig. 5C). In contrast, very limited CD8+ T-cell infiltration was demonstrated in Matrigel-B2M−/− hESC implants at this time point, indicating that transplanted HLA-deficient cells are impaired in their ability to elicit a robust CD8+ T-cell-mediated immune response. These results indicate that the B2M−/− hESCs and their derivatives have acquired significant resistance to alloreactive CD8+ T cells by their loss of surface HLA-I expression.
Figure 5.
Resistance of B2M-null hESCs and their derivatives to alloreactive CD8+ T cell-mediated killing in vitro. Histogram representation of the cytotoxic effects of CD8+ T cells on undifferentiated and differentiated B2M−/− hESCs (A) and on IFN-γ-treated B2M−/− hESCs (B) before and after differentiation with hESC control. The various ratios of CD8+ T cell to target cell are indicated (n = 5; ∗, p < .01 vs. differentiated hESCs with or without IFN-γ treatment; ∗∗, p < .0001 vs. undifferentiated or differentiated hESCs). (C): 4 × 106 hESCs or B2M−/− hESCs were mixed with 200 ml of growth factor-reduced Matrigel and then injected subcutaneously into 10-week-old immunocompetent mice. Implants were harvested after 48 hours and then fixed in 4% formalin, sectioned, and stained with mouse anti-B2M (1:100 dilution; Santa Cruz Biotechnology) and rabbit anti-CD8 antibody (1:100 dilution; Bioss Inc., Woburn, MA, http://www.biossusa.com) or with H&E. Representative immunofluorescence detection of numerous CD8+ T cells (red) infiltrating into B2M-positive (green) Matrigel-hESC implants shown (second image), but rare CD8+ T cells were present in the B2M-negative Matrigel-B2M−/− hESC implants (fourth image). A magnified view of representative CD8+ T cells indicated by red arrow was inserted in each corresponding image. Scale bars = 100 μm. Abbreviations: B2M, β2-microglobulin; DAPI, 4′,6-diamidino-2-phenylindole; hESC, human embryonic stem cell; IFN-γ, interferon-γ.
Susceptibility of the B2M-Null hESC Line to NK Cell-Mediated Killing
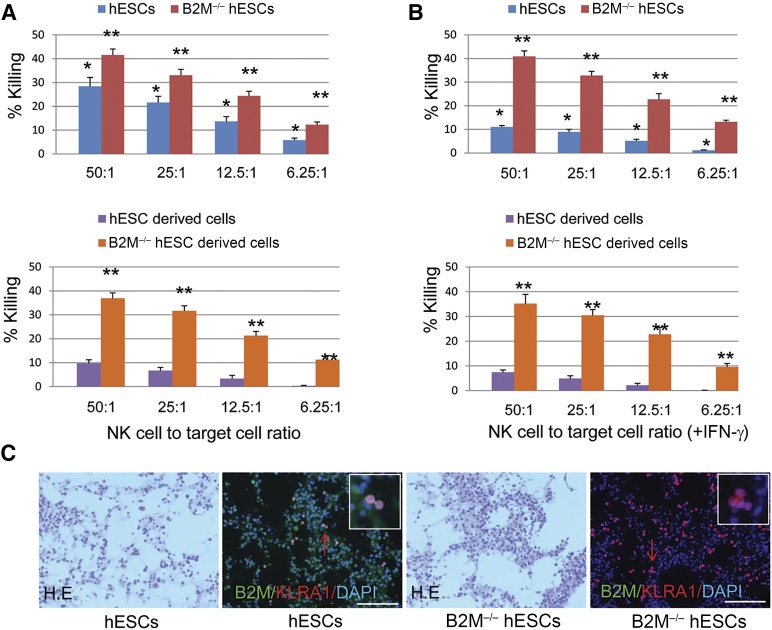
Because surface HLA-I molecules serve as inhibitor ligands to NK cells, it is possible that B2M−/− hESC-derived cells might become sensitive to NK cell-mediated graft rejection. To examine the susceptibility of B2M−/− hESCs and their derivatives to NK cells, the cytolytic activity of human NK cells against hESCs, B2M−/− hESCs, and their derivatives was analyzed at various E/T ratios. Approximately 28.4%, 21.6%, 13.7%, and 5.8% of hESCs were killed by NK cells at E/T ratios of 50:1, 25:1, 12.5:1, and 6.25:1, respectively (Fig. 6A, top). In agreement with higher HLA-I expression levels in differentiated hESCs, the NK cell-mediated killing of the differentiated cells was significantly decreased to levels of 9.7% (E/T 50:1), 6.7% (E/T 25:1), 3.3% (E/T 12.5:1), and 0.3% (E/T 6.25:1; Fig. 6A, bottom). Compared with control hESCs, the NK cell-mediated killing of B2M−/− hESCs was significantly increased at all E/T ratios before and after differentiation owing to the lack of surface HLA-I expression (Fig. 6A). IFN-γ treatment significantly suppressed NK cell-mediated killing of hESCs at all E/T ratios by the effect of IFN-γ in inducing B2M expression (Fig. 6B, top). Because differentiated hESCs already express high levels of HLA-I proteins, IFN-γ treatment only moderately suppressed NK cell-mediated killing of differentiated hESCs (Fig. 6B, bottom). B2M−/− hESCs are essentially devoid of surface HLA-I expression; therefore, IFN-γ treatment did not influence NK cell-mediated lysis of B2M−/− hESCs before or after differentiation (Fig. 6B). We also examined in vivo the NK cell response to B2M−/− hESCs. In agreement with the in vitro data, Matrigel-B2M−/− hESC implants contained significantly more infiltrating KLRA1+ NK cells than did Matrigel-hESC implants (Fig. 6C). Taken together, the results have demonstrated that B2M−/− hESCs and their derivatives are more sensitive to NK cell-mediated killing than normal hESCs owing to the lack of surface HLA-I expression.
Figure 6.
Susceptibility of B2M-null hESCs to NK cell-mediated killing. Bar graph representation of the cytotoxic effects of NK cells on undifferentiated and differentiated B2M−/− hESCs (A) and IFN-γ-treated B2M−/− hESCs before and after differentiation (B) with hESC control. The various ratios of NK cell to target cell are indicated (n = 5; ∗, p < .01 vs. differentiated hESCs treated with or without IFN-γ; ∗∗, p < .01 vs. undifferentiated or differentiated hESCs). (C): 4 × 106 hESCs or B2M−/− hESCs were mixed with 200 ml of growth factor-reduced Matrigel and then injected subcutaneously into 10-week-old immunocompetent mice. Implants were harvested after 48 hours and then fixed in 4% formalin, sectioned, and stained with mouse anti-B2M and rabbit anti-mouse KLRA1 antibody (1:100 dilution; Abcam) or with H&E. Representative immunofluorescence detection of KLRA1-positive NK cells (red) infiltrating into the B2M-positive (green) Matrigel-hESC implants or B2M-negative Matrigel-B2M−/− hESC implants shown. A magnified view of representative KLRA1+ cells indicated by red arrow was inserted in each corresponding image. Scale bars = 100 μm. Abbreviations: B2M, β2-microglobulin; DAPI, 4′,6-diamidino-2-phenylindole; hESC, human embryonic stem cell; IFN-γ, interferon-γ; NK, natural killer.
In Vivo Immunogenicity and Pluripotency of B2M-Null hESCs
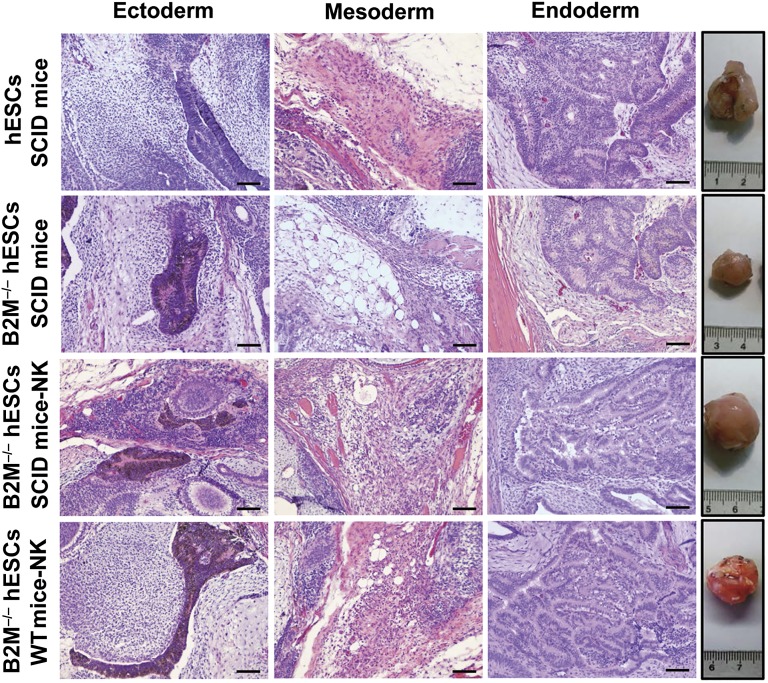
To test the pluripotency and immunogenicity of the B2M−/− hESC line in vivo, the cells were transplanted intramuscularly into the left hind leg of SCID mice and subsequently examined for teratoma formation for 10 weeks. Tumors derived from hESCs and B2M−/− hESCs were noted at 3 weeks and harvested 10 weeks after injection. The size (1.2 × 1.4 × 1.0 cm) of tumors derived from B2M−/− hESCs was significantly smaller than that (2.0 × 2.1 × 1.6 cm) derived from hESCs (Fig. 7, far right). Because this SCID mouse strain possesses functional NK cells [44], the reduced size of the tumors derived from B2M−/− hESCs might have resulted from elevated NK cell-mediated killing of these HLA-I-deficient hESCs. To determine whether this was in fact the case, the ability of B2M−/− hESCs to form teratomas was examined in NK cell-depleted SCID mice. As anticipated, we found that the size (2.1 × 2.1 × 1.8 cm) of B2M−/− hESC-derived tumors in the NK cell-depleted SCID mice was comparable to that derived from control hESCs in the NK+ SCID mice. Because HLA-I molecules are the major target in immune rejection mediated by alloreactive CD8+ T cells and because B2M−/− hESCs and their derived cells showed greatly reduced immunogenicity to CD8+ T cells in vitro (Fig. 5), we hypothesized that the HLA-I-deficient B2M−/− hESCs but not control hESCs would avoid immune rejection and form teratomas when injected into immunocompetent mice that had been NK cell depleted. The results showed that the transplanted B2M−/− hESCs, but not the control hESCs, developed into tumors in the NK cell-depleted immunocompetent mice and that the size (1.8 × 2.0 × 1.8 cm) of the tumors was comparable to the size observed in the NK cell-depleted SCID mice (Fig. 7, bottom). The histological analysis revealed that the teratomas consisted of a large variety of cell types derived from all three germ layers (Fig. 7). Taken together, the results have demonstrated that the B2M−/− hESCs are pluripotent and do not elicit a notable immune response when grafted into xenogeneic hosts, provided that the recipients’ NK cells were depleted before transplantation.
Figure 7.
Immunogenicity and pluripotency of B2M-null hESCs in immunocompetent mice. The ability of B2M−/− hESCs (hESC-394-104) to evade the immune response was examined by teratoma formation assay in SCID and immunocompetent mice with and without NK cell depletion using hESCs as control. As hESCs, B2M−/− hESCs developed into tumors in SCID mice, but the teratoma sizes were significantly smaller than those derived from hESCs due to NK cell-mediated killing (second row, fourth image from left). However, the teratomas derived from B2M−/− hESCs in SCID and immunocompetent mice resembled those derived from control hESCs in SCID mice provided that NK cells were depleted before transplantation (fourth images in bottom two rows). H&E staining of representative teratoma sections showed various tissues derived from hESCs and B2M−/− hESCs. Scale bars = 200 μm. Abbreviations: B2M, β2-microglobulin; hESC, human embryonic stem cell; IFN-γ, interferon-γ; NK, natural killer; SCID, severe combined immunodeficiency; WT, wild type.
Discussion
Allogeneic immune rejection of transplanted hESC-derived cells remains a major obstacle in the clinical application of hESC-based therapies. As a major mediator of immune rejection, HLA-I expression was analyzed early on as a possible predictor of immunogenicity of hESCs [38]. It was shown in these initial studies that the HLA-I expression levels on hESCs, hESC-derived embryoid bodies, and teratomas were lower than that on somatic cell lines, suggesting that hESCs and their cellular derivatives might be less susceptible to immune rejection. However, because very low levels of HLA-I on target cells still elicit a potent cytolytic T-cell response [45], it is improbable that the diminished expression of HLA-I alone will be sufficient to prevent immune rejection of engrafted hESC-derived cells. Moreover, therapeutic applications of hESCs will require, in most cases, the generation and transplantation of mature hESC-derived cells, which likely express HLA-I proteins at similarly high levels as those of their corresponding primary tissue cells [46, 47]. In support of normal HLA-I expression by hESC-derived mature cells, we have shown that HLA-I expression on hESC-derived ATIICs are at levels comparable to those on primary hATIICs. Thus, hESC-based strategies to repair the injured lung alveoli will require the development of strategies to circumvent allogenic immune rejection of transplanted hESC-derived lung cells.
To overcome the histoincompatibility issues of hESC-derived adult tissue cells, we aimed to generate an HLA-I-deficient hESC line that can be used in allogeneic recipients with limited complications in the absence of adjunctive immunosuppression. With our designed targeting strategy, exons 2 and 3 of both B2M alleles were deleted in hESCs, leading to complete B2M expression deficiency. Moreover, our targeting strategy was designed such that no stable B2M mRNA would exist in the resulting B2M−/− hESCs, thus eliminating any possibility that HLA-I peptides could be biosynthesized and presented on the cell surface of B2M-null hESCs or their derivatives. In contrast to our B2M−/− hESCs, which exhibited no stable B2M mRNA, B2M mRNA was detected in B2M-deficient mice [48] in which the B2M gene was targeted by insertion of the NEOR gene into exon 2. Because the inserted NEOR gene in the mouse-targeted ESCs had the same transcriptional orientation with the B2M gene and lacked a polyadenylation signal, B2M mRNA transcripts were still expressed and were significantly induced by IFN-γ. Although it was unclear whether the B2M mRNA in the B2M−/− murine cells were translated into functional B2M proteins, the mice did express low levels MHC-I on cell surfaces [48, 49] that was sufficient to induce robust immune rejection [36]. Therefore, a significant advantage of our B2M-null hESC line is that it contains no stable B2M mRNA and thus no B2M protein, even in the presence of IFN-γ. One might expect that B2M−/− hESC-derived cell types are completely deficient for both surface-expressed HLA-I and HLA-II with diminished immunogenicity as long as they do not differentiate hematopoietically. Our results showed that the B2M−/− hESCs and their derivatives were resistant to alloreactive CD8+ T cells and developed into tumors in immunocompetent mice at the levels resembling those found in SCID mice, provided that NK cells were depleted before transplantation. Although CD8+ T cells are significantly more important in graft rejection than NK cells, we did observe in our investigations that B2M-null hESCs and their derivatives were susceptible to NK cells owing to the lack of HLA-I ligand inhibitors on their cell surface. These findings indicate that the NK cell-mediated immune response could present a problem that must be addressed before the potential therapeutic use of B2M−/− hESCs can be considered in the future. Because NK cells are important in the immune defense against viral infection and tumor formation, such systemic long-term suppression of NK cells to prevent graft rejection could substantially increase the risk of opportunistic infections and tumorigenesis. However, these increased risks in NK cell suppression might not be substantially different from the risks that transplant patients currently experience from the systemic administration of immunosuppressive drugs after transplant surgery. Protection against NK cell-mediated killing could be achieved by expression of soluble MHC-I homolog MIC ligands as decoys for NK cells [50] or interference with activating NK receptor signals [51, 52]. In addition, some ESC-derived cell lineages, such as cardiomyocytes, might not express activating ligands and the adhesion molecule intercellular adhesion molecule 1 to NK cells [53]. Thus, B2M-null hESC-derived cell lineages of certain cell types might be “nonimmunogenic” in allogenic recipients without NK inhibition. Clearly, additional investigations to evaluate and compare different approaches in NK cells and immune response impairment will be necessary for the further development and potential clinical use of B2M−/− hESCs.
ABO blood group antigens are expressed primarily on the surface of red blood cells, but they are also found on certain epithelial and vascular endothelial cells [54, 55]. Therefore, ABO compatibility should also be taken into account in the development of hESC-based strategies [56–58]. The B2M-null hESCs are derived from H9.2 hESCs that carry 2 different ABO genetic alleles, 1 being A1 type (A101) and 1 being O type (O101) [59]. Therefore, the B2M−/− hESC-derived cells we have reported should be compatible donor cells for recipients with either A or AB blood type, the distribution of which is approximately 44% in the U.S. population (Bloodcenter.Stanford.edu; accessed December 28, 2014). Generation of additional B2M-null hESC lines that carry two universally compatible O genetic alleles (e.g., the commonly used H1 hESC line [59]) would provide B2M−/− hESC-derived cells that would avoid the graft rejection issues caused by ABO incompatibility.
Conclusion
We have reported the generation of a novel B2M−/− hESC line. Differentiated mature cells from this line do not express cell surface HLA molecules even after IFN-γ stimulation and are resistant to alloreactive CD8+ T cells. Moreover, this B2M−/− hESC line contains no off-target integration or cleavage events, is devoid of stable B2M mRNA, exhibits a normal karyotype, and retains its self-renewal capacity, genomic stability, and pluripotency. Although B2M−/− hESC-derived cells are more susceptible to NK cells, murine transplantation studies have indicated that they are, overall, much less immunogenic than normal hESCs. Thus, these data have shown for the first time in vivo the advantages provided by B2M−/− hESC-derived cells in avoiding allogeneic CD8+ T cell killing. Further development and genetic modification of B2M−/− hESCs to avoid NK recognition should assist in their therapeutic potential as a possible “universal donor” stem cell line.
Acknowledgments
This work was supported by the Nancy and Clive Runnells Embryonic Stem Cell Research Fund (R.A.W.), U.S. Public Health Service National Institutes Health Grant R21 HL102833-01 (D.W.), University of Texas Medical School Bridging Grant Program (R.A.W. and D.W.), the Welch Foundation Endowment Fund-7712 (D.W.), and the Hans J. Muller-Eberhard and Irma Gigli Distinguished Chair in Immunology (R.A.W.).
Author Contributions
D.W.: conception and design, collection and/or assembly data, data analysis and interpretation, manuscript writing; Y.Q., Q.Y., J.E.M.: collection and/or assembly data, data analysis and interpretation; R.A.W.: conception and design, data analysis and interpretation, manuscript writing.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Reubinoff BE, Pera MF, Fong CY, et al. Embryonic stem cell lines from human blastocysts: Somatic differentiation in vitro. Nat Biotechnol. 2000;18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- 3.Mummery C, Ward-van Oostwaard D, Doevendans P, et al. Differentiation of human embryonic stem cells to cardiomyocytes: Role of coculture with visceral endoderm-like cells. Circulation. 2003;107:2733–2740. doi: 10.1161/01.CIR.0000068356.38592.68. [DOI] [PubMed] [Google Scholar]
- 4.Johnson MA, Weick JP, Pearce RA, et al. Functional neural development from human embryonic stem cells: Accelerated synaptic activity via astrocyte coculture. J Neurosci. 2007;27:3069–3077. doi: 10.1523/JNEUROSCI.4562-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D, Haviland DL, Burns AR, et al. A pure population of lung alveolar epithelial type II cells derived from human embryonic stem cells. Proc Natl Acad Sci USA. 2007;104:4449–4454. doi: 10.1073/pnas.0700052104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang D, Jiang W, Liu M, et al. Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells. Cell Res. 2009;19:429–438. doi: 10.1038/cr.2009.28. [DOI] [PubMed] [Google Scholar]
- 7.Alper J. Geron gets green light for human trial of ES cell-derived product. Nat Biotechnol. 2009;27:213–214. doi: 10.1038/nbt0309-213a. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz SD, Hubschman JP, Heilwell G, et al. Embryonic stem cell trials for macular degeneration: A preliminary report. Lancet. 2012;379:713–720. doi: 10.1016/S0140-6736(12)60028-2. [DOI] [PubMed] [Google Scholar]
- 9.Grinnemo KH, Kumagai-Braesch M, Månsson-Broberg A, et al. Human embryonic stem cells are immunogenic in allogeneic and xenogeneic settings. Reprod Biomed Online. 2006;13:712–724. doi: 10.1016/s1472-6483(10)60663-3. [DOI] [PubMed] [Google Scholar]
- 10.Grinnemo KH, Genead R, Kumagai-Braesch M, et al. Costimulation blockade induces tolerance to HESC transplanted to the testis and induces regulatory T-cells to HESC transplanted into the heart. Stem Cells. 2008;26:1850–1857. doi: 10.1634/stemcells.2008.0111. [DOI] [PubMed] [Google Scholar]
- 11.Simon DM, Levin S. Infectious complications of solid organ transplantations. Infect Dis Clin North Am. 2001;15:521–549. doi: 10.1016/s0891-5520(05)70158-6. [DOI] [PubMed] [Google Scholar]
- 12.Penn I. Post-transplant malignancy: The role of immunosuppression. Drug Saf. 2000;23:101–113. doi: 10.2165/00002018-200023020-00002. [DOI] [PubMed] [Google Scholar]
- 13.Egli D, Chen AE, Saphier G, et al. Reprogramming within hours following nuclear transfer into mouse but not human zygotes. Nat Commun. 2011;2:488. doi: 10.1038/ncomms1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noggle S, Fung HL, Gore A, et al. Human oocytes reprogram somatic cells to a pluripotent state. Nature. 2011;478:70–75. doi: 10.1038/nature10397. [DOI] [PubMed] [Google Scholar]
- 15.Fan Y, Jiang Y, Chen X, et al. Derivation of cloned human blastocysts by histone deacetylase inhibitor treatment after somatic cell nuclear transfer with β-thalassemia fibroblasts. Stem Cells Dev. 2011;20:1951–1959. doi: 10.1089/scd.2010.0451. [DOI] [PubMed] [Google Scholar]
- 16.French AJ, Adams CA, Anderson LS, et al. Development of human cloned blastocysts following somatic cell nuclear transfer with adult fibroblasts. Stem Cells. 2008;26:485–493. doi: 10.1634/stemcells.2007-0252. [DOI] [PubMed] [Google Scholar]
- 17.Tachibana M, Amato P, Sparman M, et al. Human embryonic stem cells derived by somatic cell nuclear transfer. Cell. 2013;153:1228–1238. doi: 10.1016/j.cell.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma H, Morey R, O’Neil RC, et al. Abnormalities in human pluripotent cells due to reprogramming mechanisms. Nature. 2014;511:177–183. doi: 10.1038/nature13551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 20.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 21.Yamanaka S, Takahashi K. [Induction of pluripotent stem cells from mouse fibroblast cultures] Tanpakushitsu Kakusan Koso. 2006;51:2346–2351. [PubMed] [Google Scholar]
- 22.Stadtfeld M, Nagaya M, Utikal J, et al. Induced pluripotent stem cells generated without viral integration. Science. 2008;322:945–949. doi: 10.1126/science.1162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fusaki N, Ban H, Nishiyama A, et al. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85:348–362. doi: 10.2183/pjab.85.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jia F, Wilson KD, Sun N, et al. A nonviral minicircle vector for deriving human iPS cells. Nat Methods. 2010;7:197–199. doi: 10.1038/nmeth.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woltjen K, Michael IP, Mohseni P, et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009;458:766–770. doi: 10.1038/nature07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu J, Hu K, Smuga-Otto K, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim D, Kim CH, Moon JI, et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4:472–476. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warren L, Manos PD, Ahfeldt T, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan Q, Quan Y, Sun H, et al. A site-specific genetic modification for induction of pluripotency and subsequent isolation of derived lung alveolar epithelial type II cells. Stem Cells. 2014;32:402–413. doi: 10.1002/stem.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao T, Zhang ZN, Rong Z, et al. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212–215. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
- 31.Araki R, Uda M, Hoki Y, et al. Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. Nature. 2013;494:100–104. doi: 10.1038/nature11807. [DOI] [PubMed] [Google Scholar]
- 32.Fleischhauer K, Kernan NA, O’Reilly RJ, et al. Bone marrow-allograft rejection by T lymphocytes recognizing a single amino acid difference in HLA-B44. N Engl J Med. 1990;323:1818–1822. doi: 10.1056/NEJM199012273232607. [DOI] [PubMed] [Google Scholar]
- 33.Nieto A, Cobo F, Barroso-Deljesús A, et al. Embryonic stem cell bank: A work proposal. Stem Cell Rev. 2006;2:117–126. doi: 10.1007/s12015-006-0018-7. [DOI] [PubMed] [Google Scholar]
- 34.Rubinstein P. HLA matching for bone marrow transplantation—How much is enough? N Engl J Med. 2001;345:1842–1844. doi: 10.1056/NEJM200112203452511. [DOI] [PubMed] [Google Scholar]
- 35.Lu P, Chen J, He L, et al. Generating hypoimmunogenic human embryonic stem cells by the disruption of beta 2-microglobulin. Stem Cell Rev. 2013;9:806–813. doi: 10.1007/s12015-013-9457-0. [DOI] [PubMed] [Google Scholar]
- 36.Grusby MJ, Auchincloss H, Jr, Lee R, et al. Mice lacking major histocompatibility complex class I and class II molecules. Proc Natl Acad Sci USA. 1993;90:3913–3917. doi: 10.1073/pnas.90.9.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grau J, Boch J, Posch S. TALENoffer: Genome-wide TALEN off-target prediction. Bioinformatics. 2013;29:2931–2932. doi: 10.1093/bioinformatics/btt501. [DOI] [PubMed] [Google Scholar]
- 38.Guilinger JP, Pattanayak V, Reyon D, et al. Broad specificity profiling of TALENs results in engineered nucleases with improved DNA-cleavage specificity. Nat Methods. 2014;11:429–435. doi: 10.1038/nmeth.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang D, Morales JE, Calame DG, et al. Transplantation of human embryonic stem cell-derived alveolar epithelial type II cells abrogates acute lung injury in mice. Mol Ther. 2010;18:625–634. doi: 10.1038/mt.2009.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roszell B, Mondrinos MJ, Seaton A, et al. Efficient derivation of alveolar type II cells from embryonic stem cells for in vivo application. Tissue Eng Part A. 2009;15:3351–3365. doi: 10.1089/ten.tea.2008.0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Domev H, Milkov I, Itskovitz-Eldor J, et al. Immunoevasive pericytes from human pluripotent stem cells preferentially modulate induction of allogeneic regulatory T cells. Stem Cells Translational Medicine. 2014;3:1169–1181. doi: 10.5966/sctm.2014-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sellon DC, Knowles DP, Greiner EC, et al. Depletion of natural killer cells does not result in neurologic disease due to Sarcocystis neurona in mice with severe combined immunodeficiency. J Parasitol. 2004;90:782–788. doi: 10.1645/GE-205R. [DOI] [PubMed] [Google Scholar]
- 43.Drukker M, Katz G, Urbach A, et al. Characterization of the expression of MHC proteins in human embryonic stem cells. Proc Natl Acad Sci USA. 2002;99:9864–9869. doi: 10.1073/pnas.142298299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Welsh RM, O’Donnell CL, Shultz LD. Antiviral activity of NK 1.1+ natural killer cells in C57BL/6 scid mice infected with murine cytomegalovirus. Nat Immun. 1994;13:239–245. [PubMed] [Google Scholar]
- 45.Sykulev Y, Joo M, Vturina I, et al. Evidence that a single peptide-MHC complex on a target cell can elicit a cytolytic T cell response. Immunity. 1996;4:565–571. doi: 10.1016/s1074-7613(00)80483-5. [DOI] [PubMed] [Google Scholar]
- 46.Tang C, Weissman IL, Drukker M. Immunogenicity of in vitro maintained and matured populations: Potential barriers to engraftment of human pluripotent stem cell derivatives. Methods Mol Biol. 2013;1029:17–31. doi: 10.1007/978-1-62703-478-4_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boyd AS, Wood KJ. Variation in MHC expression between undifferentiated mouse ES cells and ES cell-derived insulin-producing cell clusters. Transplantation. 2009;87:1300–1304. doi: 10.1097/TP.0b013e3181a19421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zijlstra M, Bix M, Simister NE, et al. Beta 2-microglobulin deficient mice lack CD4-8+ cytolytic T cells. Nature. 1990;344:742–746. doi: 10.1038/344742a0. [DOI] [PubMed] [Google Scholar]
- 49.Bix M, Raulet D. Functionally conformed free class I heavy chains exist on the surface of beta 2 microglobulin negative cells. J Exp Med. 1992;176:829–834. doi: 10.1084/jem.176.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Groh V, Wu J, Yee C, et al. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734–738. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 51.Terme M, Ullrich E, Delahaye NF, et al. Natural killer cell-directed therapies: Moving from unexpected results to successful strategies. Nat Immunol. 2008;9:486–494. doi: 10.1038/ni1580. [DOI] [PubMed] [Google Scholar]
- 52.Orange JS, Fassett MS, Koopman LA, et al. Viral evasion of natural killer cells. Nat Immunol. 2002;3:1006–1012. doi: 10.1038/ni1102-1006. [DOI] [PubMed] [Google Scholar]
- 53.Frenzel LP, Abdullah Z, Kriegeskorte AK, et al. Role of natural-killer group 2 member D ligands and intercellular adhesion molecule 1 in natural killer cell-mediated lysis of murine embryonic stem cells and embryonic stem cell-derived cardiomyocytes. Stem Cells. 2009;27:307–316. doi: 10.1634/stemcells.2008-0528. [DOI] [PubMed] [Google Scholar]
- 54.Clausen H, Hakomori S. ABH and related histo-blood group antigens: Immunochemical differences in carrier isotypes and their distribution. Vox Sang. 1989;56:1–20. doi: 10.1111/j.1423-0410.1989.tb03040.x. [DOI] [PubMed] [Google Scholar]
- 55.Ito N, Hirota T. Histochemical and cytochemical localization of blood group antigens. Prog Histochem Cytochem. 1992;25:1–85. doi: 10.1016/s0079-6336(11)80056-2. [DOI] [PubMed] [Google Scholar]
- 56.Starzl TE, Tzakis A, Makowka L, et al. The definition of ABO factors in transplantation: Relation to other humoral antibody states. Transplant Proc. 1987;19:4492–4497. [PMC free article] [PubMed] [Google Scholar]
- 57.Paul LC, Baldwin WM., III Humoral rejection mechanisms and ABO incompatibility in renal transplantation. Transplant Proc. 1987;19:4463–4467. [PubMed] [Google Scholar]
- 58.Cooper DK. Clinical survey of heart transplantation between ABO blood group-incompatible recipients and donors. J Heart Transplant. 1990;9:376–381. [PubMed] [Google Scholar]
- 59.Chen YT, Dejosez M, Zwaka TP, et al. H1 and H9 human embryonic stem cell lines are heterozygous for the ABO locus. Stem Cells Dev. 2008;17:853–855. doi: 10.1089/scd.2007.0226. [DOI] [PMC free article] [PubMed] [Google Scholar]