This study investigated whether CXCL12 gene therapy promotes remyelination after middle cerebral artery occlusion in adult mice. CXCL12 gene therapy at 1 week after ischemia protected myelin sheath integrity in the perifocal region, increased the number of platelet-derived growth factor receptor-α (PDGFRα)-positive and PDGFRα/bromodeoxyuridine-double positive oligodendrocyte progenitor cells in the subventricular zone, and further enhanced their migration to the ischemic lesion.
Keywords: C-X-C chemokine ligand 12, Ischemia, Oligodendrocyte progenitor cell, Remyelination, White matter
Abstract
Remyelination is an important repair process after ischemic stroke-induced white matter injury. It often fails because of the insufficient recruitment of oligodendrocyte progenitor cells (OPCs) to the demyelinated site or the inefficient differentiation of OPCs to oligodendrocytes. We investigated whether CXCL12 gene therapy promoted remyelination after middle cerebral artery occlusion in adult mice. The results showed that CXCL12 gene therapy at 1 week after ischemia could protect myelin sheath integrity in the perifocal region, increase the number of platelet-derived growth factor receptor-α (PDGFRα)-positive and PDGFRα/bromodeoxyuridine-double positive OPCs in the subventricular zone, and further enhance their migration to the ischemic lesion area. Coadministration of AMD3100, the antagonist for CXCL12 receptor CXCR4, eliminated the beneficial effect of CXCL12 on myelin sheath integrity and negatively influenced OPC proliferation and migration. At 5 weeks after ischemia, CXCR4 was found on the PDGFRα- and/or neuron/glia type 2 (NG2)-positive OPCs but not on the myelin basic protein-positive mature myelin sheaths, and CXCR7 was only expressed on the mature myelin sheath in the ischemic mouse brain. Our data indicated that CXCL12 gene therapy effectively protected white matter and promoted its repair after ischemic injury. The treatment at 1 week after ischemia is effective, suggesting that this strategy has a longer therapeutic time window than the treatments currently available.
Significance
This study has demonstrated for the first time that CXCL12 gene therapy significantly ameliorates brain ischemia-induced white matter injury and promotes oligodendrocyte progenitor cell proliferation in the subventricular zone and migration to the perifocal area in the ischemic mouse brain. Additional data showed that CXCR4 receptor plays an important role during the proliferation and migration of oligodendrocyte progenitor cells, and CXCR7 might play a role during maturation. In contrast to many experimental studies that provide treatment before ischemic insult, CXCL12 gene therapy was performed 1 week after brain ischemia, which significantly prolonged the therapeutic time window of brain ischemia.
Introduction
Ischemic stroke leads to not only gray matter injury but also white matter injury. Ischemia induces the death of mature oligodendrocytes. Pathological changes of oligodendrocytes and myelinated axons appear as early as 30 minutes after brain ischemia, indicating that white matter is highly vulnerable to ischemic attack [1]. This is possibly because blood flow in the white matter is much lower than that in the gray matter and little collateral blood supply exists in the deep white matter [1–3]. White matter injury results from axonal injury induced by demyelination. Because myelin ensures fast nerve impulse conduction and is necessary for the maintenance of the axonal cytoskeleton, the loss of myelin or oligodendrocytes negatively influences axon integrity and signal transduction, even in the presence of preserved neuronal integrity [4–7].
Oligodendrocyte progenitor cells (OPCs) reside in the subventricular zone (SVZ) and can proliferate, migrate, and differentiate into mature oligodendrocytes to repair the damaged myelin sheaths during the process of remyelination [8, 9]. Efficient remyelination protects axons from demyelination-associated axon loss [5]. The endogenous oligodendrogenesis and remyelination lasts up to several months in spinal cord injury-induced demyelination [10]. However, remyelination often fails to ameliorate white matter injury because of limited recruitment of OPCs to the site of demyelination or unsuccessful OPC differentiation into oligodendrocytes [11]. These previous findings suggested that promoting remyelination is an important therapeutic strategy for the treatment of ischemia-induced white matter injury.
C-X-C chemokine ligand 12 (CXCL12), also known as stromal-derived factor-1 (SDF-1), is a chemokine known to regulate the migration, proliferation, and differentiation of neural progenitor cells (NPCs) within the developing central nervous system (CNS). CXCL12 can bind with two receptors, CXCR4 and CXCR7. Increasing evidence has shown that CXCL12 can recruit not only NPCs, but also other types of endogenous stem/progenitor cells, such as endothelial progenitor cells, mesenchymal stem cells, and hematopoietic stem cells, mainly by interacting with CXCR4 [12–14]. Through binding with CXCR4, CXCL12 regulates the survival and outward chemotactic migration of OPCs during embryonic and postnatal CNS myelination [15, 16]. In the cuprizone-induced demyelination model, CXCR4 signaling was found to promote the differentiation of OPCs and remyelination [17]. In vitro studies showed that CXCL12 could also promote the proliferation of OPCs [16, 18].
Our previous study has shown that CXCL12 gene therapy promoted angiogenesis and neurogenesis after brain ischemia [19]. However, to date, no studies have been done to elucidate the effect of CXCL12 in the repair of ischemia-induced demyelination. In the present study, we characterized the expression pattern of endogenous CXCL12 during the acute and subacute phases after brain ischemia. Next, we used adeno-associated virus (AAV) to mediate CXCL12 gene transfer to the ischemic perifocal area at 1 week after middle cerebral artery occlusion (MCAO). We examined whether CXCL12 gene expression protected myelin sheath integrity or promoted the proliferation and migration of OPCs, and we also examined the expression of receptors CXCR4 and CXCR7 in OPCs and mature oligodendrocytes.
Materials and Methods
Experimental Protocol
Animal studies were reported in accordance with the Animal Research: Reporting of In Vivo Experiments guidelines. The Institutional Animal Care and Use Committee of Shanghai Jiao Tong University (Shanghai, China) approved the procedure for the use of laboratory animals. During the animal studies, the guidelines for the regulation of the administration of affairs concerning experimental animals of China enacted in 1988 were followed.
The experimental design is demonstrated in Figure 1. Adult male Institute of Cancer Research (ICR) mice received AAV-CXCL12 or AAV-green fluorescent protein (GFP) gene transfer at 1 week after MCAO. The viral vector constructs were identical to those reported previously [19]. AMD3100 (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com), a CXCR4 antagonist, was injected intraperitoneally 2 weeks after MCAO. AMD3100 was dissolved with normal saline to an injection concentration of 0.3 mg/ml. The dose of AMD3100 was 1 mg/kg/day, sufficient to block CXCR4 without causing stem cell mobilization [20, 21]. The same amount of normal saline was also used in the AAV-GFP and AAV-CXCL12 groups as a control for AMD3100. Bromodeoxyuridine (BrdU) powder (Sigma-Aldrich) was dissolved in normal saline in a concentration of 10 mg/ml. The BrdU solution was injected intraperitoneally at 50 mg/kg once a day for 7 consecutive days at 2 and 4 weeks after MCAO. The mice were sacrificed and the brains sectioned for immunohistochemistry at 3 and 5 weeks after MCAO.
Figure 1.
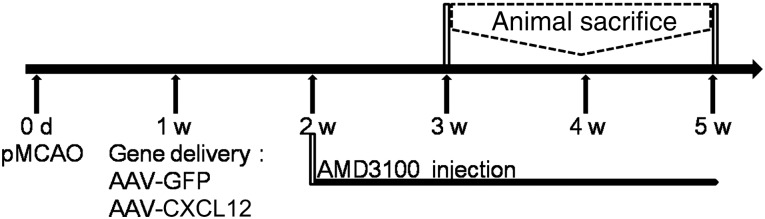
Diagram of the experimental design. The mice underwent MCAO surgery at day 0 and received a stereotactic injection of AAV-CXCL12 or AAV-GFP (as control). AMD3100 was injected intraperitoneally from 2 weeks of ischemia. The mice were sacrificed at 3 and 5 weeks after ischemia. Abbreviations: AAV, adeno-associated virus; CXCL12, C-X-C chemokine ligand 12; d, day; GFP, green fluorescent protein; pMCAO, permanent middle cerebral artery occlusion; w, week.
MCAO in Mice
A total of 47 adult male ICR mice (Sippr-BK, Shanghai, China, http://www.slarc.org.cn/shlarc/website/root/) weighing 30 ± 2 g were used in the present study. The mice were anesthetized using ketamine/xylazine (100:10 mg/kg; Sigma-Aldrich). MCAO was performed as described previously [22]. In brief, after isolation of the common carotid artery and external and internal carotid arteries, the left middle carotid artery was occluded by inserting a blunt 6-0 nylon suture coated with silica gel. The body temperature of the mice was maintained at 37°C throughout the surgery using a thermal blanket. Successful occlusion was verified by the decrease of surface cerebral blood flow to less than 15% of the baseline flow measured by laser Doppler flowmetry (Moor Instruments, Axminster, Devon, U.K., http://www.moor.co.uk).
AAV-CXCL12 Viral Vector Injection
One week after MCAO, the mice were anesthetized as described in the previous section. The virus injection was performed, as described previously [19]. In brief, the mice were immobilized on a stereotaxic apparatus (RWD Life Science, Shenzhen, China, http://www.rwdstco.com), a linear skin incision was made over the bregma and a burr hole was drilled in the skull at 2 mm lateral to the bregma using a handheld driller. A 10-μl syringe (World Precision Instruments, Sarasota, FL, http://www.wpiinc.com) was slowly inserted into the brain until reaching 3.5 mm under the dura and then slowly withdrawn for 1 mm. A total volume of 5 μl of saline solution containing 5 × 108 AAV-CXCL12 or AAV-GFP (as a control) viral particles was injected stereotactically at a rate of 200 nl/min. After finishing the injection, the needle was maintained still for 20 minutes before withdrawal. The bone hole was sealed with bone wax, and the wound was stitched. After awakening from anesthesia, the mice were returned to their cages for long-term recovery.
Immunohistochemistry
The mice were anesthetized with chloral hydrate and transcardially perfused first with normal saline and then with freshly prepared 4% paraformaldehyde in normal saline. The brains were postfixed for 4–5 hours, followed by 24 hours of immersion in 30% sucrose in phosphate-buffered saline (PBS) and frozen before being sectioned using a cryostat (Leica Biosystems, Solms, Germany, http://www.leicabiosystems.com); 20-μm-thick coronal sections were cut. Immunohistochemistry was performed according to the protocol described previously [19]. Care was taken to sample sections at similar anatomical levels. Floating coronal sections were treated with 0.3% Triton-100 in PBS for 30 minutes, blocked by 5% normal donkey serum, incubated with primary antibodies at the following dilutions: CXCR4 (1:100 dilution) and myelin basic protein (MBP; 1:300 dilution; Abcam, Cambridge, MA, http://www.abcam.com), glial fibrillary acidic protein (GFAP), neuronal nuclei protein (NeuN), ionized calcium binding adaptor molecule 1 (Iba1), platelet-derived growth factor-α (PDGFRα), and neuron/glia type 2 (NG2; 1:100 dilution; EMD Millipore, Billerica, MA, http://www.emdmillipore.com), CD31 (1:200 dilution; R&D Systems, Minneapolis, MN, http://www.rndsystems.com) at 4°C overnight. Finally, the sections were incubated with proper biotinylated or fluorescence-conjugated secondary antibodies. The stained sections were mounted after thorough rinsing. For biotinylated immunostaining, the brain sections were developed for the same amount of time.
PDGFRα and BrdU Double Staining
Floating coronal sections were first treated with 2 mol/liter HCl for 30 minutes at 37°C and then neutralized twice with sodium borate for 10 minutes each. The sections were then treated with 0.3% Triton-100 in PBS for 30 minutes, blocked by 5% normal donkey serum, incubated with anti-BrdU (1:200; Santa Cruz Biotechnology, Santa Cruz, CA, http://www.scbt.com) and anti-PDGFRα (1:200), antibody at 4°C overnight. Finally, the sections were incubated with proper secondary antibodies for 60 minutes at room temperature. The stained sections were mounted after rinsing.
PDGFRα+ OPCs and PDGFRα+/BrdU+ Cell Counting
The sections were imaged under the same conditions, and the cells were counted and quantified by an investigator who was unaware of the experimental design. Four serial sections, spaced 400 μm apart (bregma 1.10 mm to −0.10 mm), were selected from each mouse. Positive cells were counted from a single optical fraction in 4–6 sections for each mouse. Each group included three mice.
For PDGFRα+ cell counting, 6 fields were randomly selected from perifocal region under a ×20 objective lens. PDGFRα+ cells in the SVZ were counted for each image (DM2500; Leica Microsystems, Wetzlar, Germany, http://www.leica-microsystems.com). For PDGFRα+/BrdU+ cell counting, 4 fields were randomly selected from the perifocal region under a ×40 objective lens (TCS SP5II; Leica Microsystems). PDGFRα+/BrdU+ cells in the SVZ were counted for each image. The numbers were averaged, and the data were presented as numbers of positive cells per microfield.
MBP+ Myelin Sheath Fluorescence Integrated Optical Density
MBP staining intensity was computed as the mean integrated optical density (IOD), as previously described [23]. In brief, microphotographs were taken using a ×5 objective lens (DM2500; Leica Microsystems). The fluorescence images were first converted to binary images with inverted color. Next, the images were automatically analyzed by the pathology function of the Image Pro Plus, version 6.0 (Media Cybernetics, Bethesda, MD, http://www.mediacy.com) for quantitative IOD calculation. Brain sections incubated without the primary antibody were used to estimate the background staining. Four serial sections, spaced 400 μm apart, were selected from each of the 3 mice in each group. The results are presented as the IOD ratio of the ipsilateral/contralateral hemisphere.
Enzyme-Linked Immunosorbent Assay Analysis
The mice were anesthetized by injection of ketamine/xylazine intraperitoneally. The brain was quickly removed to a cooled brain mold and cut into 4 sections by 3 blades that were 2 mm apart. The second rostral section that included the ischemic core was collected. The protein extracted from ipsilateral striatum was used for enzyme-linked immunosorbent assay (ELISA) analysis. The protein levels of CXCL12 were quantified using an ELISA kit (Mouse SDF-1α ELISA Kit; RayBiotech, Norcross, GA, http://www.raybiotech.com) according to the manufacturer’s protocol. Readings from each sample were normalized for the protein concentration.
Statistical Analysis
The parametric data from different groups were compared using a one-way analysis of variance followed by the Student-Newman-Keuls test using GraphPad Prism, version 3.05 (GraphPad Software, Inc., La Jolla, CA, http://www.graphpad.com). All data were presented as the mean ± SD. A probability value of p < .05 was considered statistically significant.
Results
Endogenous CXCL12 Expression Profile Varies in Acute and Postacute Phases of Ischemic Mouse Brain
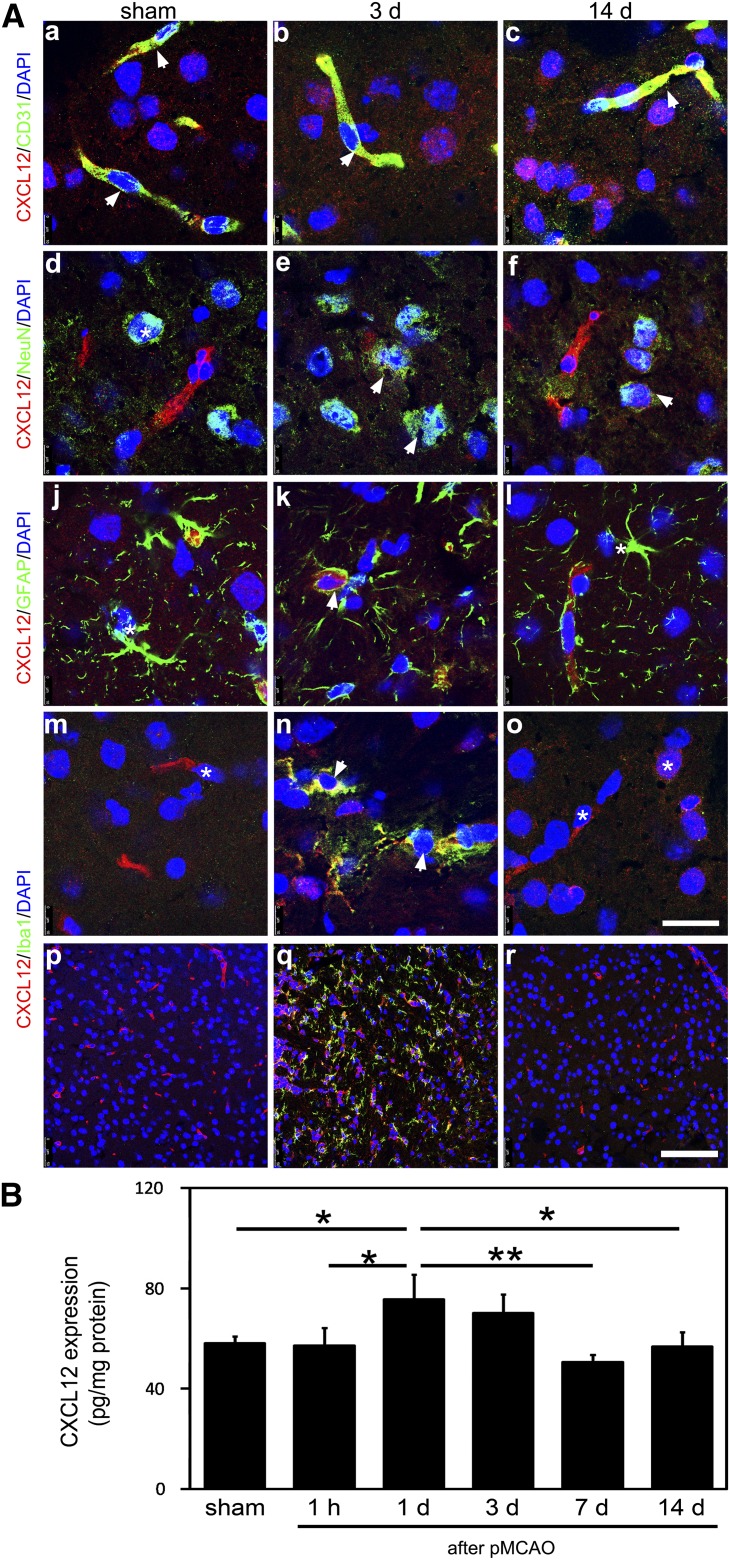
To identify the types of cells that express CXCL12 during the acute and postacute phases of the ischemic mouse brain, double immunostaining was performed. The results showed that CXCL12 was only expressed by CD31+ microvessels in the sham mouse brain (Fig. 2A, left column). In contrast, CXCL12 was expressed, not only by CD31+ microvessels, but also by NeuN+ neurons, GFAP+ astrocytes, and Iba1+ microglia in the 3-day postischemic mouse brain (Fig. 2A, middle column). CXCL12 was no longer detected in GFAP+ astrocytes in the 14-day postischemic mouse brain but was still present in the microvessels and neurons (Fig. 2A, right column). Lower magnification images showed that Iba1+ microglia was not detectable in the sham or 14-day postischemic mouse brain but were abundant in the 3-day postischemic mouse brain (Fig. 2Ap–Ar). CXCL12 was found to colocalize with Iba1+ microglia in the 3-day postischemic mouse brain (Fig. 2An). To quantify endogenous CXCL12 expression after stroke onset, we examined CXCL12 expression at 1 hour and 1, 3, 7, and 14 days (Fig. 2B). CXCL12 protein expression peaked at 1 day after ischemia and started to decrease after 3 days of ischemia.
Figure 2.
Endogenous CXCL12 expression pattern in acute and postacute phases of ischemic mouse brain. (A): Confocal images of immunofluorescent double staining showed CXCL12 (red) expression in CD31+ (green) microvessels (Aa–Ac), NeuN+ (green) neurons (Ad–Af), GFAP+ (green) astrocytes (Aj–Al), and Iba1+ (green) microglials (Am–Ao) of sham mouse brain (column 1), 3-day postischemic mouse brain (column 2), and 14-day postischemic mouse brain (column 3). Lower magnification images of Iba1 immunostaining in the sham mouse brain (Ap) and 3 days (Aq) and 14 days (Ar) of ischemic mouse brain are also shown. (B): Enzyme-linked immunosorbent assay quantification of CXCL12 in the postischemic mouse brain at 1 hour and 1, 3, 7, and 14 days of MCAO (n = 3 per group; numbers indicated on each column). Scale bars = 20 μm (Ao), 100 μm (Ar). Data are presented as mean ± SD; ∗, p < .05; ∗∗, p < .01. Abbreviations: CXCL12, C-X-C chemokine ligand 12; d, day; DAPI, 4′,6-diamidino-2-phenylindole; GFAP, glial fibrillary acidic protein; h, hour; pMCAO, permanent middle cerebral artery occlusion.
CXCL12 Gene Expression Protects Myelin Sheath Integrity
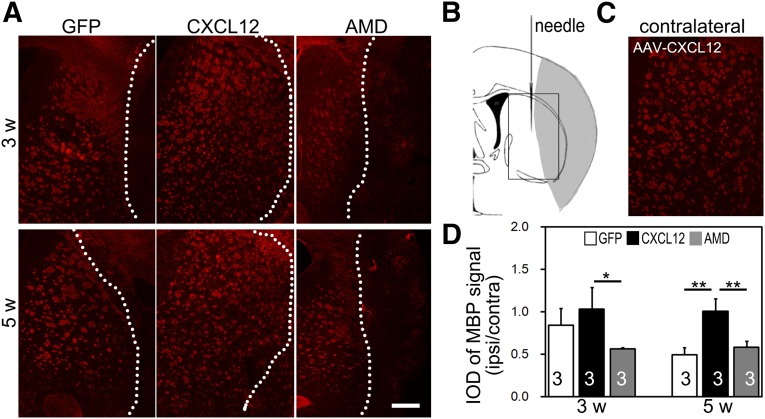
The successful expression of exogenous CXCL12 mediated by gene therapy was confirmed in our previous study [19]. To determine whether CXCL12 gene therapy can protect white matter integrity, MBP immunostaining for myelin basic protein was performed. After ischemia, many lesions were formed around the perifocal area, resulting in serious damage to striatum white matter axons and a breakdown of neurofilaments in the ipsilateral hemisphere (Fig. 3A, 3B). The striatum white matter was maintained morphologically intact in the contralateral hemisphere (Fig. 3C). As an indicator of white matter integrity, the ratio of MBP intensity of the ipsilateral compared with the contralateral hemispheres in the CXCL12 gene therapy group was significantly higher than that of the GFP control group at 5 weeks after ischemia (Fig. 3D). Continuous coadministration of AMD3100 that blocks CXCL12/CXCR4 signaling pathway profoundly diminished the beneficial effect of CXCL12 gene therapy in protecting myelin sheath integrity (Fig. 3D).
Figure 3.
Postacute CXCL12 gene therapy protects myelin sheath integrity. (A): Immunofluorescent staining of MBP+ myelin sheath in the perifocal region of the ipsilateral hemisphere. (B): Hollow box in schematic brain diagram shows the area of interest, the perifocal striatum in ischemic mice. (C): Immunofluorescent staining of MBP+ myelin sheath in the contralateral hemisphere of an AAV-CXCL12 transferred mouse. (D): IOD quantification of MBP+ signal after MCAO in AAV-GFP, AAV-CXCL12, and AAV-CXCL12/AMD3100 treated groups (n = 3 per group; numbers indicated in each column). Scale bar = 500 μm. Data are presented as mean ± SD. ∗, Statistical significance, p < .05; ∗∗, p < .01. Abbreviations: AMD, adeno-associated virus-C-X-C chemokine ligand 12-AMD3100; CXCL12, adeno-associated virus-C-X-C chemokine ligand 12; GFP, adeno-associated virus-green fluorescent protein; IOD, integral optical density; ipsi/contra, ipsilateral/contralateral; MBP, myelin basic protein; w, week.
Postacute CXCL12 Gene Expression Promotes OPC Proliferation and Migration
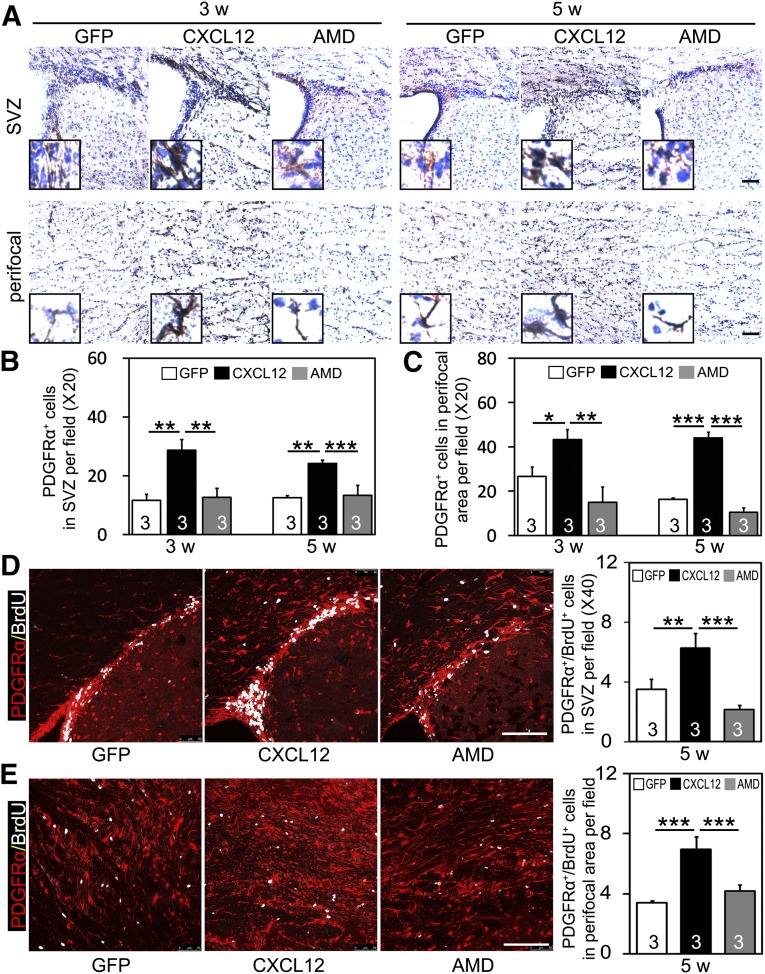
We examined the brain sections of mice that were sacrificed at 3 and 5 weeks after ischemia. The results showed that the numbers of PDGFRα+ cells and PDGFRα+/BrdU+ cells in the SVZ were augmented in the CXCL12 gene therapy group compared with AAV-GFP control group (Fig. 4A, 4B, 4D). Parallel with that, the numbers of PDGFRα+ cells and PDGFRα+/BrdU+ cells in the perifocal area were also significantly increased in the CXCL12 gene therapy group (Fig. 4A, 4C, 4E). Because CXCR4 is expressed on OPCs, the role of CXCR4 in OPC proliferation and migration was determined by treating CXCL12 gene expression in ischemic mice with the CXCR4 antagonist AMD3100 [20, 21]. Continuous coadministration of AMD3100 for 1 or 3 weeks, starting from 2 weeks after ischemia, eliminated the beneficial effect of CXCL12 on increasing the number of PDGFRα+ cells and PDGFRα+/BrdU+ cells in the SVZ (Fig. 4A, 4B, 4D) and perifocal area (Fig. 4A, 4C, 4E).
Figure 4.
Postacute CXCL12 gene therapy promotes OPC proliferation and migration in ischemic mice. (A): Representative photomicrographs of 3,3′-diaminobenzidine-stained coronal sections showing PDGFRα+ cells in SVZ (panel 1) and ipsilateral perifocal region (panel 2). Insets show higher magnifications from SVZ and perifocal region, respectively. Quantifications of PDGFRα+ cells in SVZ (B) and perifocal region (C) after middle cerebral artery occlusion (MCAO) in AAV-GFP, AAV-CXCL12, and AAV-CXCL12/AMD3100 treated groups (n = 3 per group; numbers indicated in each column). Double immunostaining of PDGFRα (red) and BrdU (white) positive cells in SVZ (D) and perifocal area (E) after 5 weeks of MCAO; quantification shown by each bar graph (n = 3 per group). Scale bars = 100 μm. Data are presented as mean ± SD; ∗, p < .05; ∗∗, p < .01; ∗∗∗, p < .001. Abbreviations: AMD, adeno-associated virus-C-X-C chemokine ligand 12-AMD3100; BrdU, bromodeoxyuridine; CXCL12, adeno-associated virus-C-X-C chemokine ligand 12; GFP, adeno-associated virus-green fluorescent protein; OPCs, oligodendrocyte progenitor cells; PDGFRα, platelet-derived growth factor receptor-α; SVZ, subventricular zone; w, week.
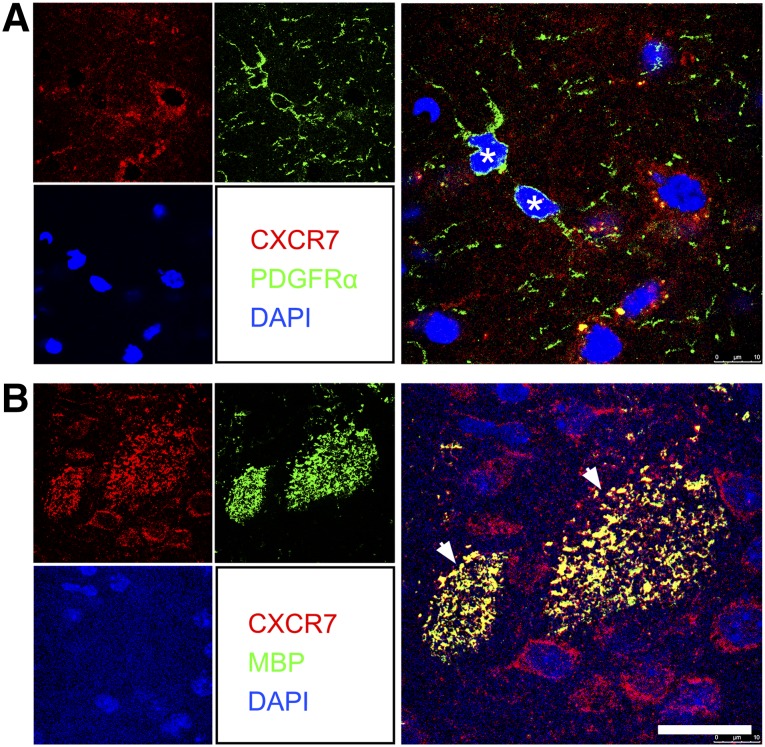
CXCR4 Was Found on OPCs but not on Mature Myelin Sheaths, and CXCR7 Was Found Only on Mature Myelin Sheaths
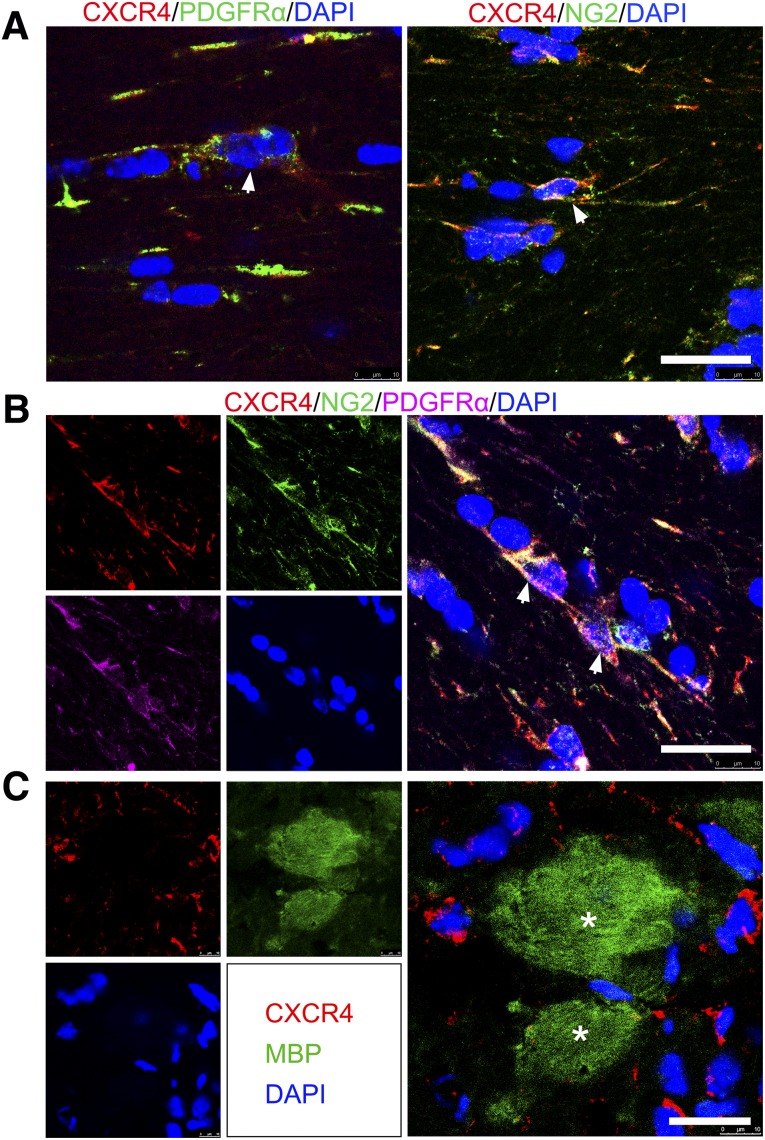
To probe the specific roles of receptors for CXCL12 during the process of OPC proliferation, migration, and maturation, we examined the expression of CXCR4 and CXCR7. Confocal images showed that CXCR4 was expressed by PDGFRα+ and NG2+ OPCs (Fig. 5A). PDGFRα+/NG2+ double-positive OPCs also expressed CXCR4 (Fig. 5B). However, CXCR4 could not be found on the MBP+ mature myelin sheath (Fig. 5C). In contrast to the expression pattern of CXCR4, the other receptor of CXCL12, CXCR7, was barely found on the surface of the OPCs (Fig. 6A) but was observed on mature myelin sheaths (Fig. 6B).
Figure 5.
CXCR4 was expressed on OPCs but not on mature myelin sheaths in the 5-week postischemic mouse brain. (A): Confocal images showing CXCR4 (red) expressed on PDGFRα+ and NG2+ cells (green, arrows). (B): Confocal images of immunofluorescent triple staining of frozen coronal sections showing that CXCR4 (red) is coexpressed by cells that stained double positive for PDGFRα (purple, arrows) and NG2 (green, arrows). (C): CXCR4 (red) was not detected on MBP+ myelin sheath cells (green, asterisks). Scale bars = 20 μm. Abbreviations: CXCR, C-X-C receptor; DAPI, 4′,6-diamidino-2-phenylindole; MBP, myelin basic protein; OPCs, oligodendrocyte progenitor cells; PDGFRα, platelet-derived growth factor receptor-α.
Figure 6.
CXCR7 was not detected on OPCs but on mature myelin sheaths in the 5-week postischemic mouse brain. Double immunostaining confocal images showed CXCR7 (red) was barely detected on PDGFRα+ cells (green, asterisks) (A) but coexpressed with MBP+ mature myelin sheath (green, arrows) (B). Scale bar = 20 μm. Abbreviations: CXCR, C-X-C receptor; DAPI, 4′,6-diamidino-2-phenylindole; MBP, myelin basic protein; OPCs, oligodendrocyte progenitor cells; PDGFRα, platelet-derived growth factor receptor-α.
Discussion
The results that showed the success of AAV-CXCL12 transduction in the mouse brain were reported in our previous study [19]. The expression of CXCL12 can be observed at 1 week and lasts at least 8 weeks after gene transfer based on the GFP fluorescent signal. AAV-CXCL12 mainly transfected neurons and astrocytes but not endothelial cells in the brain. ELISA quantification of CXCL12 has shown that CXCL12 protein significantly increased after 4 weeks of gene transfer. Intraperitoneal injection of AMD3100 does not influence the expression of CXCL12 protein [19]. Based on these data, AAV-CXCL12 gene therapy applied in mice at 1 week after MCAO was used to examine whether it can protect against white matter injury, because the mice that underwent MCAO surgery presented obvious myelin sheath degradation in our study. The data showed that CXCL12 gene therapy significantly ameliorated white matter injury and promoted the proliferation and migration of OPCs. Blocking CXCL12/CXCR4 signaling by AMD3100 essentially eliminated the benefit of CXCL12 gene therapy. Further study showed that CXCR4 receptor was mainly expressed on OPCs, but not on mature oligodendrocytes, and CXCR7 receptor was only found on mature oligodendrocytes.
The expression pattern of CXCL12 during the acute and postacute phases after ischemia was very different, not only in the expression level, but also in the cellular source. Activated astrocytes and microglials are key inflammatory components to ischemic injury during the acute phase [24–26]. CXCL12 is secreted by all major cell types in the brain, namely neurons, astrocytes, microglia, and endothelial cells, and probably plays a role in the acute inflammation response. It has been shown that blocking CXCL12 signaling with AMD3100 in the acute phase significantly reduced acute inflammation [22]. CXCL12 expression in the acute phase is involved in the recruitment of inflammatory cells to the ischemic boundary [22, 27]. However, CXCL12 can also recruit many kinds of stem/progenitor cells, such as neural stem cells and bone marrow stem cells, to the injured area to repair damage [28–30]. In the postacute phase, CXCL12 secreted by neurons and endothelial cells might promote brain repair by enhancing the recruitment of endogenous progenitor cells. The detailed functions of CXCL12 in different cells remain to be fully elucidated with further investigation.
Loss of myelin sheath integrity negatively influences neurological function [6, 7]; thus, ischemia-induced demyelination requires attention during the search of efficient therapeutic treatment for stroke. Remyelination of demyelinated axons requires the appropriate proliferation, migration, and maturation of OPCs, which reside in SVZ, distant from white matter injury areas within the ischemic mouse brain. Chemokines, which regulate these processes during development, are therefore critical elements of the intrinsic ischemic brain injury response. Upregulation of these genes induced by ischemia might represent the brain’s effort in repairing itself. Our previous study has shown that CXCL12 gene therapy in the postacute phase significantly reduced brain atrophy and improved neurobehavioral recovery in ischemic mice [19]. In the present study, we further showed that CXCL12 gene therapy protected myelin sheath integrity and promoted the proliferation of PDGFRα+ OPCs in the SVZ and OPC migration to the perifocal area.
Our data indicate that CXCL12 gene expression in the perifocal area at 1 week after ischemia is beneficial for the preservation of white matter integrity within the striatum and the proliferation and migration of OPCs into demyelinated lesions in vivo. The primary receptor for CXCL12, CXCR4, is expressed by PDGFRα+ and/or NG2+ OPCs in the 5-week ischemic mouse brain and both of these OPCs have been validated for their remyelination ability [31–34]. However, CXCR4 can rarely be found on MBP+ mature myelin sheaths, suggesting that CXCR4 expression might modulate OPC proliferation and migration and is downregulated after fulfilling its duty when OPCs maturate into oligodendrocytes [15, 35]. Blocking CXCL12/CXCR4 signaling pathway with AMD3100 abrogated the beneficial effect of CXCL12 gene therapy in protecting striatum white matter integrity at 5 weeks after ischemia and decreased the number of PDGFRα+ OPCs in the SVZ and perifocal area in the 3-week ischemic brain. More damage from the white matter injury was observed in AMD3100-treated mice than in the AAV-GFP group at 3 weeks of ischemia. This might have been because AMD3100 inhibited the binding of both endogenous and exogenous CXCL12 with CXCR4. Because endogenous CXCL12 plays a beneficial role in this phase, blocking its binding with CXCR4 would further exacerbate the injury.
In the cuprizone-induced demyelination model, CXCL12/CXCR4 signaling was found to regulate OPC proliferation and maturation but did not affect OPC migration from the SVZ into the corpus callosum [36]. In the mouse hepatitis virus-induced intracranial infection demyelinating disease model, CXCL12/CXCR4 contributes to the maturation of OPCs, and administration of AMD3100 increases the number of OPCs [37]. Our data have demonstrated that CXCL12/CXCR4 is important for OPC proliferation and migration. These differences observed in different studies suggest that CXCL12/CXCR4 might function differently in different disease models.
The receptor CXCR7 was abundant on MBP+ mature myelin sheaths but was hardly detected on PDGFRα+ OPCs in the 5-week ischemic mouse brain, which might suggest that CXCR7 plays a part only in the process of OPC maturation. During cuprizone-induced demyelination, CXCR7 was expressed on OPCs, and CXCR7 antagonism augmented OPC proliferation [38]. Cuprizone-induced demyelination is closely associated with inflammation. However, during the postacute ischemic phase, inflammation is much less significant. This difference might account for the different observations regarding CXCR7 expression on OPCs in different models. Further studies using CXCR7 inhibitor in murine ischemic models would help reveal information on its role in promoting OPC maturation.
Conclusion
The present study has identified CXCL12 as a critical regulator for protecting white matter integrity and remyelination in the adult ischemic mice brain. We showed that overexpression of CXCL12 in the perifocal region of the injured striatum protected myelin sheath integrity and enhanced the proliferation and migration of OPCs, suggesting that CXCL12 and/or its receptors could be promising therapeutic candidates to promote recovery from ischemia-induced demyelination.
Acknowledgments
This study was supported by National Natural Science Foundation of China Grant 81100868 (to Y.W.) and the Major State Basic Research Development Program of China (973 program) Grant 2011CB504405 (to G.Y.Y., Y.W.).
Author Contributions
Y. Li: conception and design, provision of study material or patients, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; G.T.: provision of study material or patients, final approval of manuscript; Y. Liu, X.H., J.H., X.L., and Z.Z.: collection and/or assembly of data, final approval of manuscript; G.-Y.Y. and Y.W.: conception and design, financial support, administrative support, provision of study material or patients, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Pantoni L, Garcia JH, Gutierrez JA. Cerebral white matter is highly vulnerable to ischemia. Stroke. 1996;27:1641–1647. doi: 10.1161/01.str.27.9.1641. [DOI] [PubMed] [Google Scholar]
- 2.Arai K, Lo EH. Experimental models for analysis of oligodendrocyte pathophysiology in stroke. Exp Transl Stroke Med. 2009;1:6. doi: 10.1186/2040-7378-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 4.Fork M, Bartels C, Ebert AD, et al. Neuropsychological sequelae of diffuse traumatic brain injury. Brain Inj. 2005;19:101–108. doi: 10.1080/02699050410001726086. [DOI] [PubMed] [Google Scholar]
- 5.Irvine KA, Blakemore WF. Remyelination protects axons from demyelination-associated axon degeneration. Brain. 2008;131:1464–1477. doi: 10.1093/brain/awn080. [DOI] [PubMed] [Google Scholar]
- 6.Kinnunen KM, Greenwood R, Powell JH, et al. White matter damage and cognitive impairment after traumatic brain injury. Brain. 2011;134:449–463. doi: 10.1093/brain/awq347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flygt J, Djupsjö A, Lenne F, et al. Myelin loss and oligodendrocyte pathology in white matter tracts following traumatic brain injury in the rat. Eur J Neurosci. 2013;38:2153–2165. doi: 10.1111/ejn.12179. [DOI] [PubMed] [Google Scholar]
- 8.Horner PJ, Power AE, Kempermann G, et al. Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J Neurosci. 2000;20:2218–2228. doi: 10.1523/JNEUROSCI.20-06-02218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: From biology to therapy. Nat Rev Neurosci. 2008;9:839–855. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- 10.Hesp ZC, Goldstein EZ, Miranda CJ, et al. Chronic oligodendrogenesis and remyelination after spinal cord injury in mice and rats. J Neurosci. 2015;35:1274–1290. doi: 10.1523/JNEUROSCI.2568-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fancy SP, Baranzini SE, Zhao C, et al. Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes Dev. 2009;23:1571–1585. doi: 10.1101/gad.1806309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robin AM, Zhang ZG, Wang L, et al. Stromal cell-derived factor 1alpha mediates neural progenitor cell motility after focal cerebral ischemia. J Cereb Blood Flow Metab. 2006;26:125–134. doi: 10.1038/sj.jcbfm.9600172. [DOI] [PubMed] [Google Scholar]
- 13.Hayakawa J, Migita M, Ueda T, et al. Dextran sulfate and stromal cell derived factor-1 promote CXCR4 expression and improve bone marrow homing efficiency of infused hematopoietic stem cells. J Nippon Med Sch. 2009;76:198–208. doi: 10.1272/jnms.76.198. [DOI] [PubMed] [Google Scholar]
- 14.Sharma M, Afrin F, Satija N, et al. Stromal-derived factor-1/CXCR4 signaling: Indispensable role in homing and engraftment of hematopoietic stem cells in bone marrow. Stem Cells Dev. 2011;20:933–946. doi: 10.1089/scd.2010.0263. [DOI] [PubMed] [Google Scholar]
- 15.Dziembowska M, Tham TN, Lau P, et al. A role for CXCR4 signaling in survival and migration of neural and oligodendrocyte precursors. Glia. 2005;50:258–269. doi: 10.1002/glia.20170. [DOI] [PubMed] [Google Scholar]
- 16.Maysami S, Nguyen D, Zobel F, et al. Modulation of rat oligodendrocyte precursor cells by the chemokine CXCL12. Neuroreport. 2006;17:1187–1190. doi: 10.1097/01.wnr.0000227985.92551.9a. [DOI] [PubMed] [Google Scholar]
- 17.Patel JR, McCandless EE, Dorsey D, et al. CXCR4 promotes differentiation of oligodendrocyte progenitors and remyelination. Proc Natl Acad Sci USA. 2010;107:11062–11067. doi: 10.1073/pnas.1006301107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kadi L, Selvaraju R, de Lys P, et al. Differential effects of chemokines on oligodendrocyte precursor proliferation and myelin formation in vitro. J Neuroimmunol. 2006;174:133–146. doi: 10.1016/j.jneuroim.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Huang J, He X, et al. Postacute stromal cell-derived factor-1α expression promotes neurovascular recovery in ischemic mice. Stroke. 2014;45:1822–1829. doi: 10.1161/STROKEAHA.114.005078. [DOI] [PubMed] [Google Scholar]
- 20.Rubin JB, Kung AL, Klein RS, et al. A small-molecule antagonist of CXCR4 inhibits intracranial growth of primary brain tumors. Proc Natl Acad Sci USA. 2003;100:13513–13518. doi: 10.1073/pnas.2235846100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Theiss HD, Vallaster M, Rischpler C, et al. Dual stem cell therapy after myocardial infarction acts specifically by enhanced homing via the SDF-1/CXCR4 axis. Stem Cell Res (Amst) 2011;7:244–255. doi: 10.1016/j.scr.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Huang J, Li Y, Tang Y, et al. CXCR4 antagonist AMD3100 protects blood-brain barrier integrity and reduces inflammatory response after focal ischemia in mice. Stroke. 2013;44:190–197. doi: 10.1161/STROKEAHA.112.670299. [DOI] [PubMed] [Google Scholar]
- 23.He X, Li Y, Lu H, et al. Netrin-1 overexpression promotes white matter repairing and remodeling after focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2013;33:1921–1927. doi: 10.1038/jcbfm.2013.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller JT, Bartley JH, Wimborne HJ, et al. The neuroblast and angioblast chemotaxic factor SDF-1 (CXCL12) expression is briefly up regulated by reactive astrocytes in brain following neonatal hypoxic-ischemic injury. BMC Neurosci. 2005;6:63. doi: 10.1186/1471-2202-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stoll G, Jander S, Schroeter M. Inflammation and glial responses in ischemic brain lesions. Prog Neurobiol. 1998;56:149–171. doi: 10.1016/s0301-0082(98)00034-3. [DOI] [PubMed] [Google Scholar]
- 26.Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184:53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruscher K, Kuric E, Liu Y, et al. Inhibition of CXCL12 signaling attenuates the postischemic immune response and improves functional recovery after stroke. J Cereb Blood Flow Metab. 2013;33:1225–1234. doi: 10.1038/jcbfm.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Falco E, Porcelli D, Torella AR, et al. SDF-1 involvement in endothelial phenotype and ischemia-induced recruitment of bone marrow progenitor cells. Blood. 2004;104:3472–3482. doi: 10.1182/blood-2003-12-4423. [DOI] [PubMed] [Google Scholar]
- 29.Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 30.Thored P, Arvidsson A, Cacci E, et al. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells. 2006;24:739–747. doi: 10.1634/stemcells.2005-0281. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka K, Nogawa S, Ito D, et al. Activation of NG2-positive oligodendrocyte progenitor cells during post-ischemic reperfusion in the rat brain. Neuroreport. 2001;12:2169–2174. doi: 10.1097/00001756-200107200-00025. [DOI] [PubMed] [Google Scholar]
- 32.Chang A, Nishiyama A, Peterson J, et al. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J Neurosci. 2000;20:6404–6412. doi: 10.1523/JNEUROSCI.20-17-06404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rivers LE, Young KM, Rizzi M, et al. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci. 2008;11:1392–1401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sim FJ, McClain CR, Schanz SJ, et al. CD140a identifies a population of highly myelinogenic, migration-competent and efficiently engrafting human oligodendrocyte progenitor cells. Nat Biotechnol. 2011;29:934–941. doi: 10.1038/nbt.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Banisadr G, Frederick TJ, Freitag C, et al. The role of CXCR4 signaling in the migration of transplanted oligodendrocyte progenitors into the cerebral white matter. Neurobiol Dis. 2011;44:19–27. doi: 10.1016/j.nbd.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel JR, Williams JL, Muccigrosso MM, et al. Astrocyte TNFR2 is required for CXCL12-mediated regulation of oligodendrocyte progenitor proliferation and differentiation within the adult CNS. Acta Neuropathol. 2012;124:847–860. doi: 10.1007/s00401-012-1034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carbajal KS, Miranda JL, Tsukamoto MR, et al. CXCR4 signaling regulates remyelination by endogenous oligodendrocyte progenitor cells in a viral model of demyelination. Glia. 2011;59:1813–1821. doi: 10.1002/glia.21225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams JL, Patel JR, Daniels BP, et al. Targeting CXCR7/ACKR3 as a therapeutic strategy to promote remyelination in the adult central nervous system. J Exp Med. 2014;211:791–799. doi: 10.1084/jem.20131224. [DOI] [PMC free article] [PubMed] [Google Scholar]