Tumor lysate-loaded dendritic cells (DCs) used in two phase I clinical trials of glioblastoma immunotherapy were prepared using a Good Manufacturing Practice-compliant protocol. Results of quality control tests on batches of the prepared DCs were analyzed retrospectively for yield of mature DCs and their quality in terms of microbiological safety and immunological efficacy. The DC preparation protocol is highly reproducible and produces large numbers of safe and functional DCs.
Keywords: Immunotherapy, Glioblastoma, Dendritic cells, Good Manufacturing Practices, Quality controls
Abstract
Cell therapy based on dendritic cells (DCs) pulsed with tumor lysate is a promising approach in addition to conventional therapy for the treatment of patients with glioblastoma (GB). The success of this approach strongly depends on the ability to generate high-quality, functionally mature DCs (mDCs), with a high level of standardization and in compliance with Good Manufacturing Practices. In the cell factory of the Carlo Besta Foundation, two phase I clinical trials on immunotherapy with tumor lysate-loaded DCs as treatment for GB are ongoing. From 2010 to 2014, 54 patients were enrolled in the studies and 54 batches of DCs were prepared. We retrospectively analyzed the results of the quality control tests carried out on each produced batch, evaluating yield of mDCs and their quality in terms of microbiological safety and immunological efficacy. The number of mDCs obtained allowed the treatment of all the enrolled patients. All 54 batches were sterile, conformed to acceptable endotoxin levels, and were free of Mycoplasma species and adventitious viruses. During culture, cells maintained a high percentage of viability (87%–98%), and all batches showed high viability after thawing (mean ± SD: 94.6% ± 2.9%). Phenotype evaluation of mDCs showed an evident upregulation of markers typical of DC maturation; mixed lymphocyte reaction tests for the functional evaluation of DCs demonstrated that all batches were able to induce lymphocyte responses. These results demonstrated that our protocol for DC preparation is highly reproducible and permits generation of large numbers of safe and functional DCs for in vivo use in immunotherapy approaches.
Significance
Cell therapy based on antigen-pulsed dendritic cells (DCs) is a promising approach for the treatment of glioblastoma patients. The success of this approach strongly depends on the ability to generate high-quality, functional DCs with a high level of standardization, ensuring reproducibility, efficacy, and safety of the final product. This article summarizes the results of the quality controls on 54 batches, to demonstrate the feasibility of producing a therapeutic cell-based vaccine via a well-controlled Good Manufacturing Practices (GMP)-compliant production process. The findings may be of scientific interest to those working in the field of preparation of GMP-compliant products for cell-therapy applications.
Introduction
Glioblastoma (GB) is the most common and aggressive primary brain malignancy, with a yearly incidence of 2.5 per 100,000 people, and is virtually incurable. Conventional treatment, which includes surgical resection followed by radiation and concurrent chemotherapy, allows a median survival of 12–18 months, with 90%–95% of patients surviving for less than 2 years and with a high probability of relapse [1–3]. As current knowledge suggests that the continued tumor growth and limited survival is, in part, due to failure of an effective immune response [4], it seems likely that new strategies based on immunotherapy in combination with conventional therapy could provide a more effective treatment for GB.
Dendritic cell (DC) immunotherapy could represent a well-tolerated, long-term, tumor-specific treatment to kill residual tumor cells that infiltrate adjacent areas of the brain. Preclinical investigations for the development of therapeutic vaccines against high-grade gliomas, based on the use of DCs loaded with a mixture of glioma-derived tumor antigens, have been carried out in rat [5–7] and mouse models [8–10], and have shown the capacity to generate a glioma-specific immune response. Mature DCs (mDCs) loaded with autologous tumor lysate used for the treatment of patients with recurrent malignant brain tumors have shown no major adverse effects [11–15].
The success of immunotherapy approaches based on DCs strongly depends on the ability to generate functional mDCs with a high level of standardization. Moreover, the whole process must be done in compliance with Good Manufacturing Practices (GMP), as required by regulatory authorities, to ensure reproducibility, efficacy, and safety of DCs considered as Advanced-Therapy Medicinal Products (ATMP). GMP rules require that the quality and safety of ATMP must be maintained throughout the entire preparation process to ensure the safety of a product that will be administered to patients. In particular, the cell-therapy product should be extensively analyzed in terms of purity, potency, and suitability for the intended use. mDCs are consistently produced and controlled during every step of preparation to ensure the quality standard at different stages: the collection and manipulation of raw materials; the processing of intermediate products; and the storage, labeling, packaging, and release of the final product.
Since 2010, 2 phase I clinical trials involving the use of DCs have been ongoing at the Neurological Institute C. Besta Foundation for the immunotherapeutic treatment of patients with either newly diagnosed or recurrent GB. DCs are prepared in the clean-room facility of the Cell Therapy Production Unit (UPTC), in compliance with European GMP guidelines for pharmaceutical products.
In this report, we provide data on routinely obtaining large numbers of high-quality, safe, and functional mDCs, from a retrospective analysis of the quality control test results carried out for each batch of mDCs. Quality control tests were performed both “in process,” during the different steps of preparation, and for “batch release” at the end of the production process.
In particular, we evaluated the safety profile of DCs in terms of sterility and absence of contamination with Mycoplasma species, bacterial endotoxin, and adventitious viruses; and their efficacy in terms of viability, maturation status, and potency. Compendial methods, according to European Pharmacopoeia (EP) [16], were used to test sterility, measure endotoxin levels, and detect Mycoplasma species and adventitious viruses. Noncompendial methods, developed in our laboratory for specific processes, were applied to evaluate DC viability, phenotype, maturation status, and potency. The results of this study demonstrate that our protocol for DC production is highly reproducible and permits consistent generation of large numbers of safe and functional mDCs for in vivo use in immunotherapy approaches.
Materials and Methods
Clinical Trials and Patients
The UPTC facility of Neurological Institute C. Besta Foundation was licensed in 2010 by the Italian Medicines Agency (Agenzia Italiana del Farmaco) for the production of autologous DCs pulsed with tumor lysate for the treatment of GB-affected patients.
From April 2010 to April 2014, 54 patients diagnosed with GB were enrolled in 2 ongoing phase I clinical trials on immunotherapy with tumor lysate-loaded mDCs (Newly diagnosed GBM Immuno-Trial-Italy [DENDR1], EudraCT number 2008-005035-15; and Recurrent GBM Immuno-Trial-Italy [DENDR2], EudraCT number 2008-005038-62); the DENDR1 trial is expected to enroll and treat patients at first diagnosis; the DENDR2 is expected to enroll and treat patients at recurrence.
Fifty-four batches of autologous mDCs were prepared (27 for DENDR1 and 27 for DENDR2) and 245 vaccines were administered to the patients (149 in DENDR1 and 96 in DENDR2). These studies were authorized by national authorities and the local ethical committee. Written informed consent was obtained from all patients.
Generation of DCs From Peripheral Blood Mononuclear Cells
All batches were prepared under GMP conditions. Methodological details of DC preparation were previously reported [17]. Briefly, peripheral blood mononuclear cells (PBMCs) were obtained using the closed COBE Spectra Apheresis System (Spectra Cell Separator; Terumo BCT Inc., Tokyo, Japan, http://www.terumobct.com). The isolation of CD14+ monocytes was performed by immunomagnetic labeling of the target cells using the CliniMACS Technology (Miltenyi Biotec, Teterow, Germany, http://www.miltenyibiotec.com). The positive fraction was cultured at 3 × 106 to 5 × 106 cells/ml in VueLife closed culture systems in CellGRO medium (CellGenix GmbH, Freiberg, Germany, http://www.cellgenix.com) implemented with 20 ng/ml interleukin (IL)-4 and 50 ng/ml granulocyte-macrophage colony-stimulating factor (GM-CSF) (CellGenix GmbH). On day 5 of culture, immature DCs (iDCs) were pulsed with autologous tumor lysate, prepared as previously described [17], at the concentration of 50 μg/106 living cells plus 50 μg/ml keyhole limpet hemocyanin (EMD Millipore Corp., Billerica, MA, http://www.emdmillipore.com) with addition of 10 ng/ml IL-4 and 25 ng/ml GM-CSF for 24 hours. On day 6, antigen-loaded DCs (aDCs) were cultured with a proinflammatory cytokine cocktail including 10 ng/ml of tumor necrosis factor (TNF)-α, IL-1β, IL-6 (CellGenix GmbH), and 1 μg/ml prostaglandin E2 (PGE2) (Pfizer Inc., New York, NY, http://www.pfizer.com). After 24 hours, mDCs were collected and frozen at the concentration of 5 × 106 to 6 × 106 viable cells per vial in sodium chloride (NaCl) (B. Braun Inc., Hessen, Germany, http://www.bbraun.de), 10% dimethyl sulfoxide (Li Starfish S.r.l., Milan, Italy, http://www.listarfish.it), and 5% human albumin (Kedrion SpA, Barga, Italy, http://www.kedrion.com), in 2-ml cryogenic vials (Nalgene Nunc International Corp., Rochester, NY, http://nalgene.com). A controlled-rate freezing curve (Planer Kryo 360-3.3; Planer Products PLC, Middlesex, U.K., https://www.planer.com) was used prior to preservation in nitrogen gas. All samples were stored in the GMP-dedicated area of the biobank and managed with Good Automated Manufacturing Practices 4 software (International Society of Pharmaceutical Engineering, Tampa, FL, http://www.ispe.org).
Quality Control
Analytical methods were applied to keep under control contaminants that could be introduced during the manipulations and to analyze the morphological and functional characteristics of the final product. Analytical methods included compendial methods described by the EP and used for microbiological controls (sterility, bacterial endotoxin content, Mycoplasma species, adventitious viruses (e.g., herpes simplex virus type I and adenovirus serotype 5), and noncompendial methods developed just for the process of interest to detect cell viability and number, phenotype (by flow cytometry analysis), and potency via a mixed lymphocyte reaction (MLR) test.
Compendial Methods: Safety Profile of the Batches
Microbiological controls were carried out by a GMP-certified laboratory under aseptic conditions and according to EP. All protocols were validated and specific limits of detection (LOD) were determined.
Sterility
Sterility was assessed by the BacT/Alert 3D Culture System (bioMérieux, Marcy-l’Étoile, France, http://www.biomerieux.com). This is an automated, nondestructive, and noninvasive system that continuously monitors culture of aerobic and anaerobic bacteria and fungi (EP 2.6.27 Microbiologic control of cellular product [16]). The samples were first inoculated in bottles containing the specific culture medium, then the bottles were incubated in a system that works on the colorimetric principle of detection of carbon dioxide produced by the organisms. The LOD of this method was set at 10 cfu.
Bacterial Endotoxin Level
The Limulus amebocyte lysate (LAL) kinetic test was applied to detect and quantify Gram-negative bacterial endotoxins, according to EP 2.6.14 [16]. This test uses a preparation of LAL, in combination with an incubating photometer and appropriate software, to detect, photometrically, the endotoxin level. The cutoff limit was set at a value of 2.86 endotoxin units (EU)/ml.
Mycoplasma Detection
The presence of Mycoplasma species was assessed by nucleic acid amplification techniques (NATs), according to EP 2.6.7 [16], using the Venor GeM Mycoplasma Detection Kit (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com), which includes specific oligonucleotides for the detection of a wide range of Mycoplasma species.
Adventitious Viruses Detection
Adventitious herpes simplex virus type I and adenovirus serotype 5 were detected and quantified by a real-time polymerase chain reaction (PCR) assay, using selected oligonucleotides and probes for the amplification of viral genome (EP 2.6.21 Nucleic acid amplification techniques [16]).
Noncompendial Methods: Functional Testing
Viable Cell Count
Viable cell count was assessed in a Bürker chamber using the vital stain trypan blue, a negatively charged chromophore that enters the cell if the membrane is damaged. Therefore, all the cells that exclude the dye are viable, whereas all nonviable cells are stained. The cut-off limit for cell viability was set at 75%.
Flow Cytometry
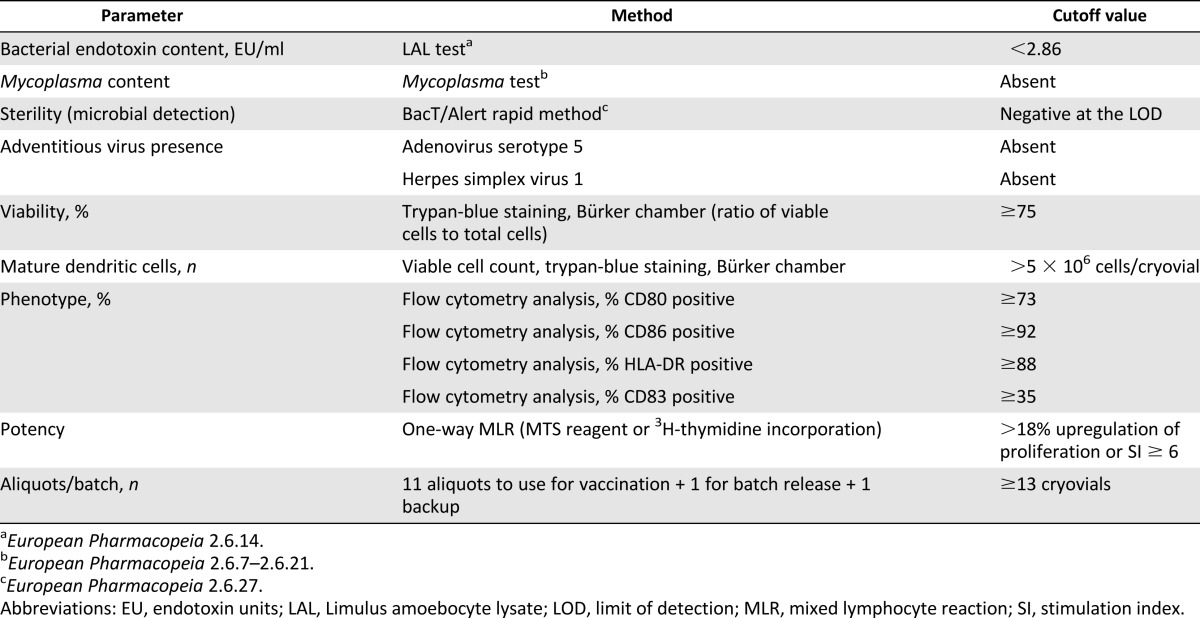
Flow cytometry analysis was performed on CD14+ monocytes, after immunomagnetic selection, to assess the purity of selected cells, and on iDCs, aDCs, and mDCs to analyze their maturation status at different culture steps. Monoclonal antibodies (mAbs) used in this study for phenotype analysis included fluorescein- and phycoerythrin-labeled anti-CD14, anti-CD80, anti-CD83, anti-CD86, and anti-HLA-DR (Becton, Dickinson and Company, Franklin Lakes, NJ, http://www.bd.com). Nonspecific staining was determined with the appropriate isotype control. The appropriate mAbs were used to stain 3 × 105 cells/tube. The tubes were incubated for 30 minutes at 4°C in the dark and washed twice with cold flow cytometry (FACS) buffer. At least 20,000 events were acquired for each sample, immediately or after fixation with 4% paraformaldehyde, on a FACScalibur flow cytometer (Becton, Dickinson and Company). Data were analyzed using CellQuest software (Becton, Dickinson and Company). The cut-off limits for each marker expression on mDCs are summarized in Table 1.
Table 1.
Parameters for batch release
Mixed Lymphocyte Reaction
PBMCs were isolated from donor blood by Ficoll-Paque density gradient centrifugation and resuspended in CellGRO medium (CellGenix GmbH). Unidirectional MLRs were performed by coculturing 2 × 105 PBMCs (responder cells) with stimulating cells (S) in a 96-well plate (Corning, Corning, NY, http://www.corning.com). S cells were represented by 1 × 104 DCs, 2 × 105 autologous PBMCs (for auto-MLR, negative control), or 2 × 105 allogeneic PBMCs (for allo-MLR, positive control). S cells were pretreated with mitomycin-C (50 μg/ml; Sigma-Aldrich Co.) for 20 minutes at 37°C and used after extensive wash.
After 5 days, 1 μCi of 3H-thymidine (GE Healthcare, Amersham, Buckinghamshire, U.K., http://www.gelifesciences.com) was added for further 18 hours. The radioactivity incorporated into DNA was measured in a β-scintillation counter (Trilux 1450; PerkinElmer Inc., Waltham, MA, http://www.perkinelmer.com). Results were expressed as stimulation index (SI) to allow comparison of results between donors. The SI was calculated as follows: mean counts per minute (cpm) from stimulated cells per mean cpm from nonstimulated cells. MLR responses were considered positive when the SI was ≥3 for PBMC-induced stimulation and ≥6 for DC-induced stimulation.
Alternatively, the colorimetric nonradioactive MTS proliferation assay was used. Briefly, after 5 days of culture, the MTS reagent was added as one-tenth of the volume of the cell volume into wells for another 4 hours. Cell proliferation was assessed by evaluating with a spectrophotometer absorbance at wavelength of 540 nm. MLR responses were considered positive when the upregulation of proliferation was ≥18%.
Stability of the Final Product
We tested the stability of the product conserved in nitrogen vapors, considering a cryopreservation period exceeding 12 months; it is not possible to define exactly the period of maximum storage of the finished product in nitrogen vapors, because of clinical variables that can change the time of administration. For the evaluation of the stability, three representative batches were analyzed. One representative vial for each batch analyzed was thawed and centrifuged at 300g for 10 minutes without addition of buffer; the supernatant was removed and the cells resuspended in sodium chloride plus 0.5% human serum albumin. Resuspended cells were then subjected to the following tests: evaluation of cell number and viability, phenotype analysis by flow cytometry, and analysis of the potency by MLR test.
It is also not possible to define, exactly, the period of time from sample preparation (thawing and resuspension) to its inoculation in the patient. For this reason, we tested the stability of the product over a range of time that covered the longest waiting time expected before inoculation (2 hours).
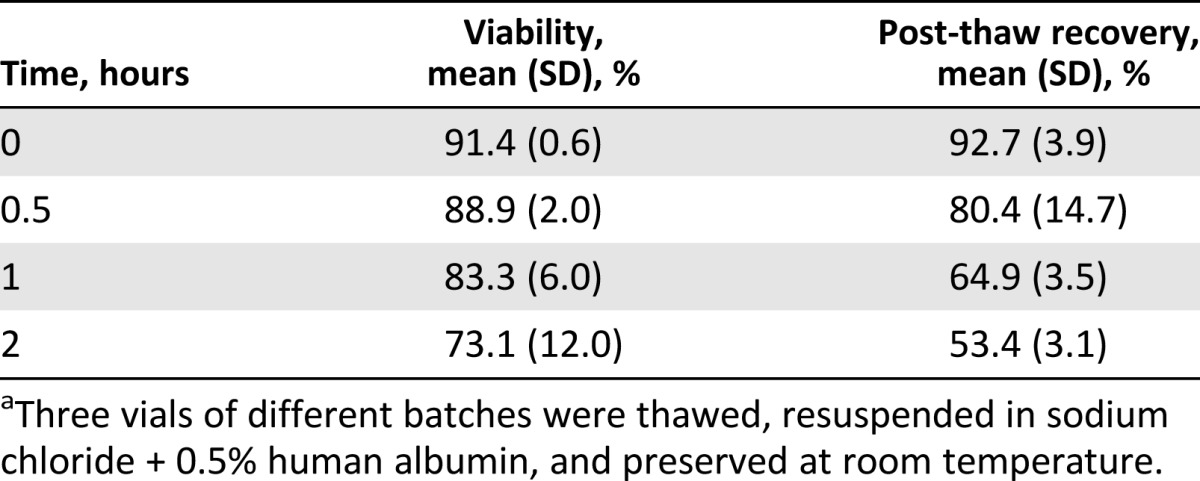
Three representative batches were thawed and the cells resuspended in 500 µl of NaCl plus 0.5% human albumin. The cells were preserved at room temperature. Cell concentration and viability were determined at time (T) = 0, T = 0.5 hour, T = 1 hour, and T = 2 hours after thawing. The post-thaw recovery was calculated for each batch as follows: Percentage of recovery = Final number of cells × 100/Initial number of cells.
Statistical Analysis
Results are expressed as mean ± SD. One-way analysis of variance and a two-tailed test were used for all statistical analyses and performed with GraphPad Prism software version 4.0 (GraphPad Software Inc., La Jolla, CA, http://www.graphpad.com). p values of less than .05 were considered significant.
Results
Batch Release and Patients’ Treatment
Fifty-four batches of DCs were prepared. The batch-release parameters and the defined cut-off values are given in Table 1. Fifty-three of 54 batches (26 in DENDR1, 27 in DENDR2) were approved as complying to the specifications (Table 1) and suitable for the treatment of patients, whereas one of the 54 batches (1 in DENDR1) was not compliant because of an insufficient number of mDCs obtained at the end of the process. This was because of a low number of white blood cells (WBCs) after leukapheresis. A total of 245 vaccines were administered to the patients (149 in DENDR1 and 96 in DENDR2).
Compendial Methods: Safety Profile of the Batches
Sterility
Sterility was assessed by BacT/Alert 3D Culture System. All 54 batches were found to comply within the LOD at the time of collection, on leukapheresis, in tumor lysate, in culture supernatant at day 7, and in mDCs.
LAL Test and Detection of Mycoplasma Species and Adventitious Viruses
The LAL kinetic test was applied to detect and quantify Gram-negative bacterial endotoxins on mDC. The cutoff limit of the LAL kinetic test was 2.86 EU/ml. All 54 batches conformed to the specifications (mean ± SD: 0.52 ± 0.22 EU/ml).
Mycoplasma Detection
The absence of Mycoplasma was assessed by NATs on mDCs, according to EP 2.6.7. In all 54 batches, mycoplasma was absent.
Adventitious Viruses Detection
Adventitious viruses herpes virus type I and adenovirus serotype 5 were detected and quantified by a real-time PCR assay (EP 2.6.21), Adventitious viruses were assessed on tumor lysate and during the batch-release quality controls. In all 54 batches, herpes simplex virus type I and adenovirus serotype 5 were absent.
Noncompendial Methods: Functional Testing
Viable Cell Count and Yield
Fifty-four preparations were analyzed. The recovery rate (mean ± SD) from monocytes to iDCs (differentiation) on day 5 was 32.7% ± 16.4% and to mDC (maturation) on day 7 was 10.0% ± 3.8%. No differences were observed between DCs from DENDR1 patients and those from DENDR2 patients in terms of yield, purity, or recovery rate during the production process (Table 2).
Table 2.
Yield and viability of dendritic cells
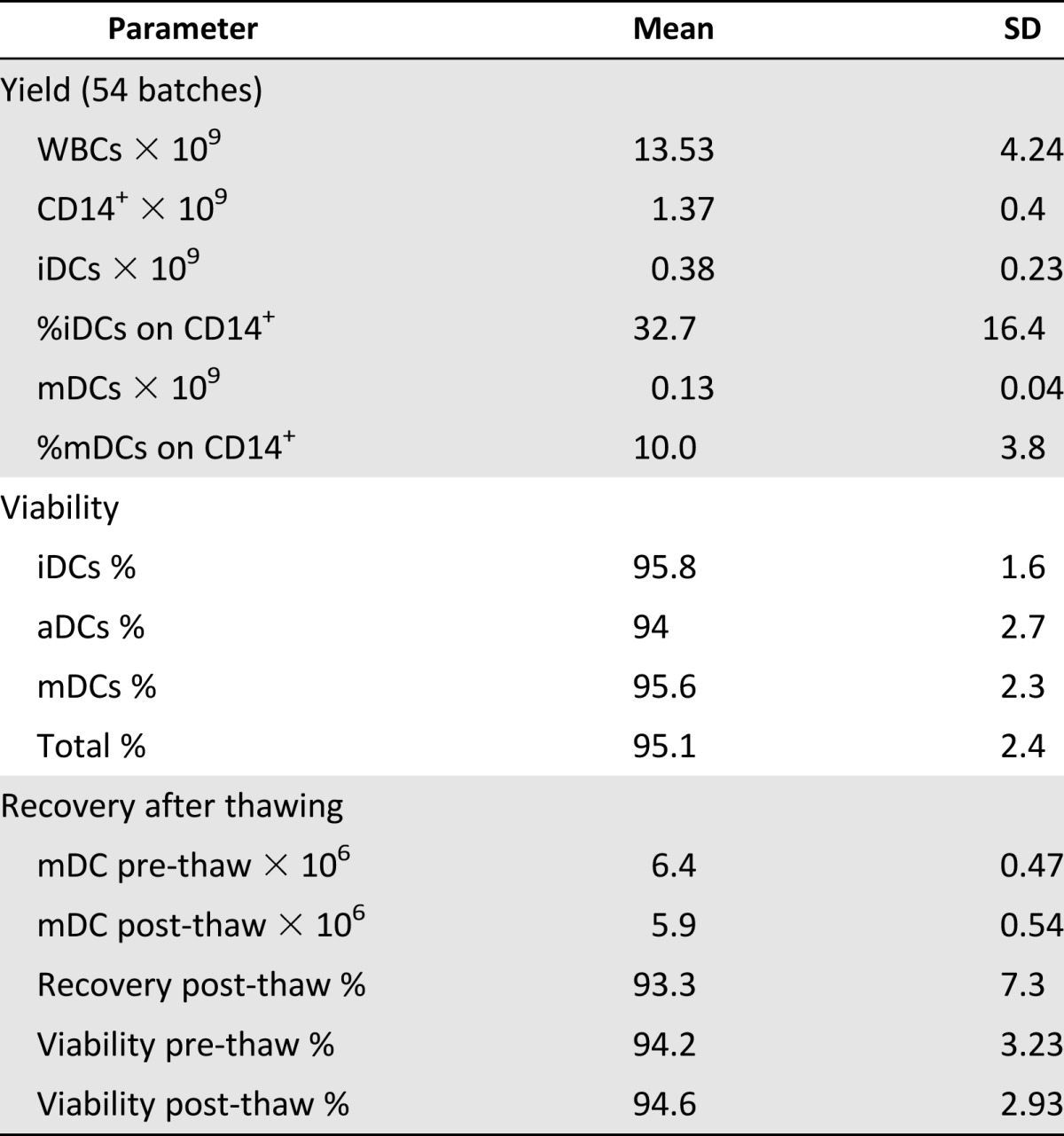
During culture, cells maintained a high percentage of viability, as evaluated with trypan blue staining at different steps of culture, with an absolute range between 87% and 98% and the following percentages (mean ±SD): iDCs, 95.8% ± 1.6%; aDCs, 94.0% ± 2.7%; and mDCs, 95.6% ± 2.3%, as reported in Table 2.
Recovery After Thawing
We evaluated recovery rate and cell viability of final product after cryopreservation in nitrogen vapors. Cells were thawed at room temperature and immediately centrifuged at 300g for 10 minutes without the addition of buffer. Supernatant was removed, and cells were resuspended in 0.9% NaCl plus 0.5% human albumin, at a concentration of 10 × 106 cells per milliliter (0.5 ml per vial). Cells were then counted and viability evaluated by the trypan blue exclusion test.
The mean number (±SD) of cells cryopreserved per vial was 6.4 × 106 ± 0.47 × 106; after thawing, the mean number (±SD) of recovered cells was 5.9 × 106 ± 0.54 × 106 (p = .000032), representing a recovery rate after thawing of 93.3%. Viability was unchanged (prethaw: 94.2% ± 3.2%; post-thaw: 94.6% ± 2.9%; p = not significant) (Table 2). Thawed cells were used for noncompendial analysis for batch release.
Flow Cytometry
To assess the purity of the CD14+ fraction, selected monocytes were analyzed by flow cytometry for CD14 and CD3 expression. Monocytes obtained with the use of the CliniMACS device (Miltenyi Biotec), in 54 preparations, showed a purity (CD14 positivity; mean± SD) of 98.2% ± 2.5% and negativity for CD3 (residual CD3 content of 1.7% ± 1.7%).
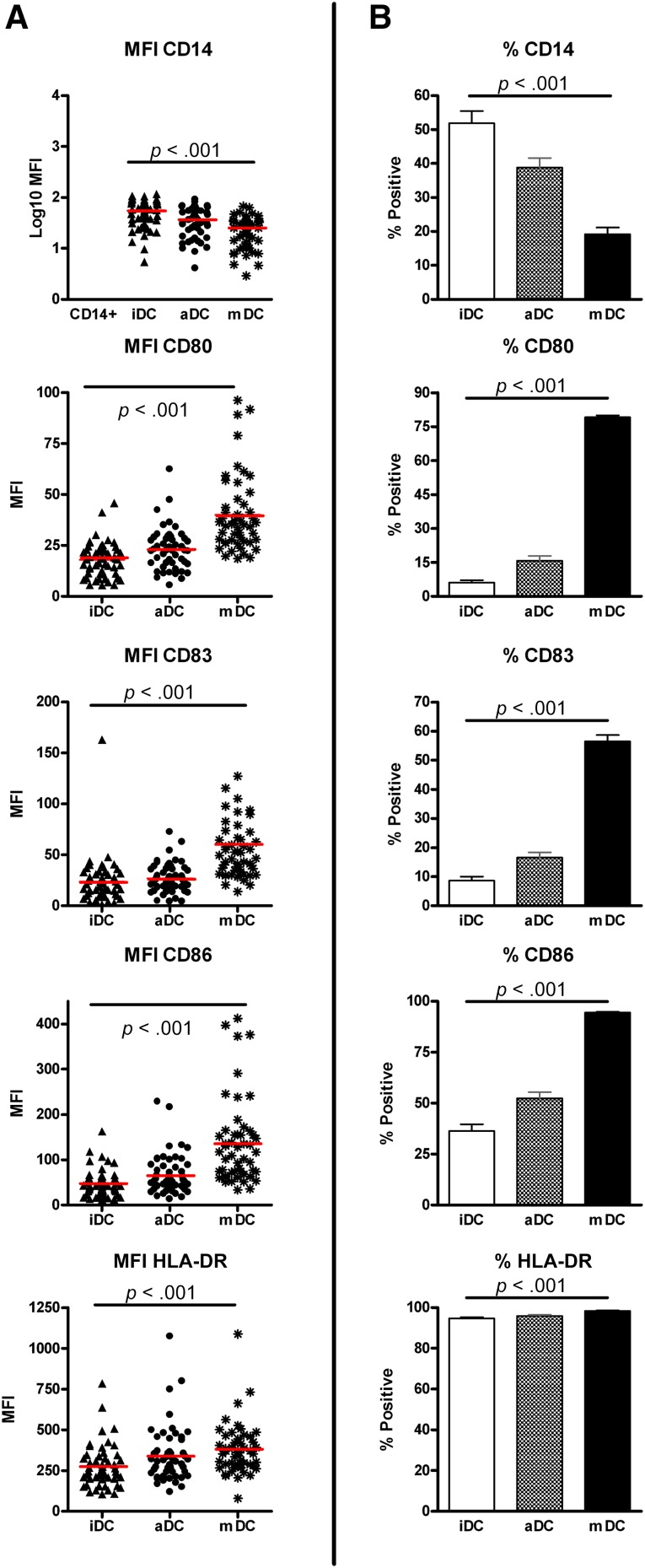
We analyzed differentiation and maturation markers on monocytes and DCs during different steps of the production process on all 54 batches. We evaluated mean fluorescence intensity (MFI) of markers and percentage of positive cells in culture. Moreover, we decided to evaluate the increase in MFI between iDCs and mDCs as an indication for maturation and differentiation during the production process. mDCs, relative to iDCs and in terms of percentage of positive cells, downregulated CD14 (% ± SD: iDCs, 51.9% ± 26.0%; mDCs, 19.2% ± 14.5%; p < .0001) and upregulated costimulatory markers such as CD80 (% ± SD: iDCs, 6.2% ± 8.0%; mDCs, 79.2% ± 5.9%; p < .0001), CD86 (% ± SD: iDCs, 36.4% ± 23.0%; mDCs, 94.56% ± 2.3%; p < .001), and CD83 (% ± SD: iDCs, 8.7% ± 9.0%; mDCs, 56.1% ± 15.5%; p < .001) (Fig. 1B). The MFI results were proportionally modulated during differentiation steps (Fig. 1A), with a strong downregulation of CD14 from iDCs to mDCs (MFI ± SD: iDCs, 55.7 ± 24.3; mDCs, 25.34 ± 15.1; p < .001) and upregulation of constitutively expressed markers such as CD86 (MFI ± SD: iDCs, 47.7 ± 29.2; mDCs, 136.8 ± 93.9; p < .001) and HLA-DR (MFI ± SD: iDCs, 275.0 ± 85.9; mDCs, 380.4 ± 158.1; p = .0004). The evaluation of the increase in MFI of mDCs compared with iDCs revealed that maturation markers (CD80, CD86, and CD83) increased at least 2-fold during maturation and CD14 decreased (mean ± SD: 0.48 ± 0.19); there was a smaller upregulation of HLA-DR (mean ± SD: 1.7 ± 1.1) (Fig. 1A).
Figure 1.
Flow cytometry analysis of the DCs at different steps of maturation/differentiation. The figure shows the analysis of differentiation (CD14 downregulation) and maturation markers (CD80, CD86, CD83, and HLA-DR upregulation) during different steps of culture. (A): MFI was compared with the total percentage of positive cells for the same markers (B). The comparable trends confirmed the success of the differentiation/maturation procedure. Statistical analysis comparing iDCs and mDCs showed a significant difference in all 54 batches for all the markers analyzed. Red lines indicate the mean values. Abbreviations: DC, dendritic cell; iDC, immature dendritic cell; mDC, mature dendritic cell; MFI, mean fluorescence intensity.
Mixed Lymphocyte Reaction
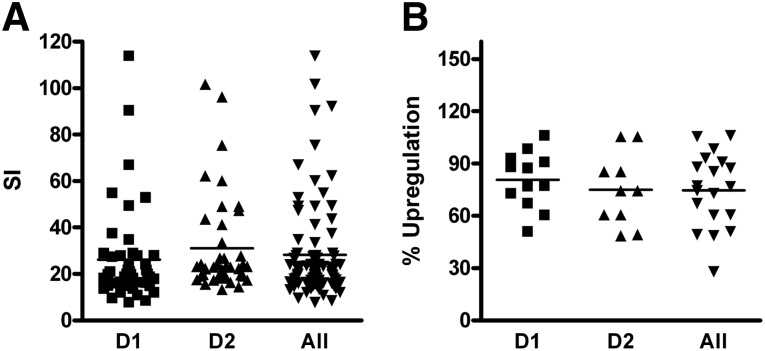
DC functionality was evaluated via one-way MLR using PBMCs as responder cells and 3H-thymidine incorporation to detect proliferation. The acceptance limit for the MLR test was SI ≥ 6, where SI was calculated as the ratio of mean cpm of stimulated cells to mean cpm of nonstimulated cells. As shown in Figure 2A, mDCs are potent stimulatory cells, with a mean SI (± SD) of 25.1 ± 13.7. The comparison between DENDR1 and DENDR2 mDCs did not show differences in their ability to induce allogeneic MLR (SI ± SD for DENDR1: 22.7 ± 12.6, 46 tests; for DENDR2: 27.6 ± 14.5, 42 tests; p = .1002).
Figure 2.
Potency test by mixed lymphocyte reaction (MLR). Dendritic cell functionality was evaluated via one-way MLR. 3H-thymidine incorporation (A) or colorimetric variation of MTS reagent (B) was used to detect cell proliferation. All mature dendritic cells (mDCs) analyzed were potent stimulatory cells by both 3H-thymidine and MTS analysis. The comparison between D1 and D2 mDCs showed that there are no differences in their ability to induce allogeneic MLR. A total of 54 preparations were tested with 3H-thymidine incorporation, and 22 of these also were tested with MTS incorporation. Abbreviations: D1, DENDR1; D2, DENDR2; SI, stimulation index.
We validated an alternative method to evaluate proliferation in the one-way MLR, using the colorimetric assay MTS rather than 3H-thymidine incorporation. We first compared the linearity of 3H-thymidine incorporation and colorimetric MTS assay using PBMCs as responder cells in a concanavalin A (ConA)-induced proliferation test (0–4 µm/ml ConA). Linearity regression revealed that MTS incorporation was comparable to 3H-thymidine incorporation (MTS R2 = 0.8814, 3H-thymidine R2 = 0.9181) (supplemental online Fig. 1). For the validation of the method, we ran 24 parallel MLR tests with 3H-thymidine and MTS between PBMCs as responder/stimulator cells and 14 parallel MLR tests with 3H-thymidine and MTS between PBMCs as responder cells and DCs as stimulator cells. Obtained data defined the acceptance criteria for the MTS test (negative test: percentage upregulation of proliferation <18%; positive test: percentage upregulation of proliferation >18%). To date, we have performed 22 tests with MTS incorporation: The upregulation of proliferation (mean ± SD) was 85.4% ± 26.5%. No statistically significant differences were observed between DENDR1 and DENDR2 DC (12 tests for DENDR1: 91.3% ± 26.3%; 10 tests for DENDR2: 70.7% ± 22.4%; p = .1075) (Fig. 2B).
Stability of the Product
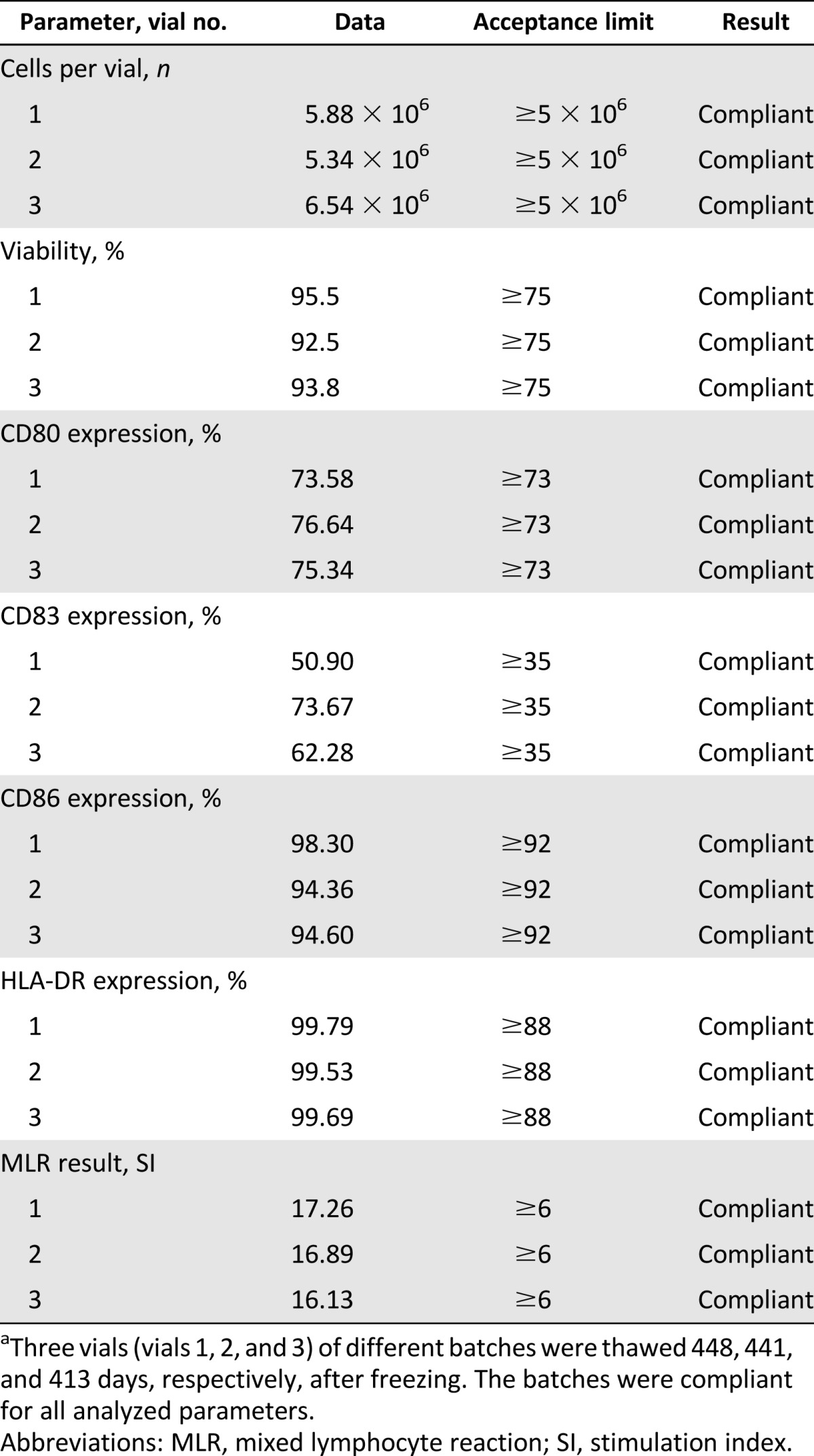
The stability of the final product cryopreserved in nitrogen vapors for a period exceeding 12 months was evaluated. Three runs of validation were conducted for the evaluation of viability, phenotype, and functionality. The mean cryopreservation period was 434 days. Data are summarized in Table 3. The validation of the stability of the vaccine was determined by the absence of out-of-specification values in the three validation runs. Based on the data obtained, we concluded that the final product is stable after cryopreservation in nitrogen vapor for a period exceeding 12 months. We planned to complete the vaccination schedule of each patient within 12 months from the end of production.
Table 3.
Stability of the vaccine preserved in nitrogen vapors for >12 monthsa
The stability of the final product after 0, 0.5, 1, and 2 hours after thawing was evaluated. Three batches were considered for the validation. The viability of cells resulted in >75% up to 1 hour after thawing (mean ± SD at 1 hour: 83.3% ± 6%). The recovery rate was 64.9% ± 3.5% after 1 hour and 80.4% ± 14.7% after 0.5 hour. To guarantee the correct cell dose administered to the patient, we considered that the final product is stable at room temperature, after thawing, for a maximum of 0.5 hour. Results are summarized in Table 4.
Table 4.
Stability of vaccine 0–2 hours after thawinga
Discussion
Advanced-therapy medicinal products are new medical products based on genes (gene therapy), cells (cell therapy), and tissues (tissue engineering) that require a long preparation as well as extensive manipulation before administration to patients. Thus, it is mandatory that ATMP are prepared in cell factories, following the GMP rules.
In the last decade, growing attention has been placed on the generation of DC-based ATMP suitable for clinical applications. A number of papers reported protocols for reproducible monocyte-derived DC generation [18, 19], mainly based on large-scale immunomagnetic selection of CD14+ monocytes [20]. One of the major challenges of DC vaccination is the establishment of harmonized DC production protocols.
Here, we retrospectively analyzed the results of the quality control tests carried out on 54 GMP preparations of autologous DCs pulsed with tumor lysate. To our knowledge, this is the first time that the results of such a large number of DC preparations performed with a highly standardized protocol in a GMP facility are reported in detail. Mainly, we evaluated (a) the quality of DCs both in terms of safety profile (sterility, and Mycoplasma, endotoxin, and adventitious viruses content) and immunological efficacy (viability, maturation status, and potency), (b) the stability of our ATMP product, and (c) the feasibility of obtaining a large number of autologous DCs and the reproducibility of our production protocol.
Compared with other GMP-like protocols, we introduced three innovative changes in methods for DC preparation, as previously described [17]: (a) processing of the tumor was performed with an automated, closed system, the GentleMACS dissociator (Miltenyi Biotec), that minimizes interpreparation variability and the risk of contamination; (b) DC differentiation, loading, and maturation were done in Teflon bags, which allow a more rapid and standardized handling, minimizing the risk of contamination; and (c) cryopreservation of loaded and matured DCs was carried out 7 days after immunoselection, shortening the production time without loss of function of the final product, relative to longer culture protocols.
The analyses carried out during the production process and during the quality control cycle confirmed that the DCs prepared using our GMP protocol are safe for administration to patients; results of compendial tests showed, in fact, that all 54 batches conformed in terms of sterility and absence of Mycoplasma species, endotoxins, and adventitious viruses. In support of the data concerning the safety of cells prepared with our protocol, is, to date, 245 vaccine doses (149 in DENDR1 and 96 in DENDR2) have been administered to the 54 patients enrolled in this study, without any adverse event related to the vaccinations.
Another major safety issue that we must take in consideration is the lack of viable cells in the tumor-lysate preparations. Our in vitro data, as well as safety data from 245 administered vaccines, allow us to assume that the combined thermal (snap-frozen tissue) and mechanical stresses (GentleMACs; Miltenyi Biotec) safely avoid the persistence of viable cells in the tumor lysate and that trypan-blue staining is a valid tool, sufficient to rule out such persistence.
Phenotypical evaluation of the products performed by flow cytometry analysis indicated that MFI was proportionally modulated during differentiation steps, with a strong downregulation of CD14 monocytes markers during the maturation process from iDC to mDC, and an upregulation of constitutively expressed markers such as CD86 and HLA-DR. Maturation markers CD80, CD86, and CD83 increased at least twofold during maturation. These data are fully in agreement with those reported by Eyrich and colleagues, who described that DCs downregulated CD14 and upregulated costimulatory markers such as CD80, CD86, HLA-DR, and CD83 during the culture process [21].
Flow cytometry analysis confirmed the functionality of the “standard” GMP-maturation cocktail (TNF-α, IL-1β, IL-6, and PGE2) that, at the end of the procedure, induced high levels of HLA-DR and costimulatory molecules on mDC surfaces [22], revealing that maturation is complete.
We also evaluated the potency of DCs via one-way MLR using PBMCs as responder cells. As described in other papers, MLR is the gold standard to test the functional ability of DCs as antigen-presenting cells [23]. In accordance with the data reported in such papers, our results confirmed the potential immunological action of all 54 preparations.
With the application of our standardized production protocol, cells maintained a high percentage of viability during culture (87%–99%), with yields of roughly 15% mDCs from initial monocytes or 1% from WBCs, in accordance with results obtained by other groups using large-scale DC-generation technologies [24–26]. Furthermore, our GMP protocol involved the use of a controlled-rate freezing curve to guarantee optimal cryopreservation of the product. The recovery rate of the final product after cryopreservation in nitrogen vapors was 93.34% and cell viability after thawing was unchanged with respect to the fresh final product.
We also evaluated the stability of the final product cryopreserved in nitrogen vapors, showing that this protocol for the cryopreservation of DC guarantees the stability for a period exceeding 12 months. The confirmation that the product is stable for more than 12 months ensures that the patient completes the vaccination schedule without loss of functionality of the ATMP.
Our DC protocol allowed successful generation of mDCs with sufficient numbers of cells to cover the complete vaccination schedule. In fact, the number of DC aliquots was compliant with the specifications except for 1 case out of 54 (1.8% in our series), in which the number of aliquots obtained was lower, still permitting 5 of the 7 DC injections required by the protocol.
Two studies are ongoing at our institution based on the use of DCs loaded with whole tumor lysate in patients affected by glioblastoma at first diagnosis (DENDR1; EudraCT 2008-005035-15) or at recurrence (DENDR2; EudraCT 2008-005038-62). Both studies are based on a Simon’s two-stage design [27]. Despite the difference in clinical characteristics of the patients enrolled in the two trials, we have not found any difference between DENDR1 and DENDR2 in terms of morphological and functional characteristics of the final product. While data for DENDR2 are not yet mature, the survival analysis of the first stage of DENDR1 is positive and allows the passage to stage 2.
Conclusion
Our findings suggest that the protocol used for DC production is highly reproducible and permits, routine generation of large numbers of safe, autologous DCs. The functional characterization showed that these cells exhibit a mature phenotype and fulfill all requirements for in vivo use in immunotherapy approaches. Our data show that a DC production process could be successfully translated to a GMP-compatible technology in a running vaccination program. Our protocol is open for use by other groups and will promote the realization of larger, multicenter immunotherapy trials; further multicenter studies, involving other Italian or European facilities, are needed to confirm our results on a larger scale.
Supplementary Material
Acknowledgments
We thank all colleagues at the Department of Neurosurgery, Istituto Neurologico Carlo Besta, for providing glioblastoma specimens, Dr. Marica Eoli and Dr. Elena Anghileri for patient management, Dr. Bianca Pollo and Dr. Raffaele Nunziata for tumor tissue evaluation, Dr. Carlo Antozzi for the leukapheresis, and Piero Tieni and CryoManagement of SOL Company. This work was supported by “Il Fondo di Gio.”
Author Contributions
S.N. and D.L.: collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; S. Pogliani, M.D.: collection and/or assembly of data, final approval of manuscript; A.B.: final approval of the manuscript; S. Pellegatta: conception and design, final approval of manuscript; E.P.: financial support, final approval of manuscript; G.F.: conception and design, financial support, provision of study material or patients, final approval of manuscript; S.F.: conception and design, coordination of the production activities, final approval of the manuscript.
Disclosure of Potential Conflicts of Interest
A.B. is an uncompensated consultant. The other authors indicated no potential conflicts of interest.
References
- 1.Holland EC. Glioblastoma multiforme: The terminator. Proc Natl Acad Sci USA. 2000;97:6242–6244. doi: 10.1073/pnas.97.12.6242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reardon DA, Wen PY. Therapeutic advances in the treatment of glioblastoma: Rationale and potential role of targeted agents. The Oncologist. 2006;11:152–164. doi: 10.1634/theoncologist.11-2-152. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, van den Bent MJ, Hegi ME. Optimal role of temozolomide in the treatment of malignant gliomas. Curr Neurol Neurosci Rep. 2005;5:198–206. doi: 10.1007/s11910-005-0047-7. [DOI] [PubMed] [Google Scholar]
- 4.Bielamowicz K, Khawja S, Ahmed N. Adoptive cell therapies for glioblastoma. Front Oncol. 2013;3:275. doi: 10.3389/fonc.2013.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liau LM, Black KL, Prins RM, et al. Treatment of intracranial gliomas with bone marrow-derived dendritic cells pulsed with tumor antigens. J Neurosurg. 1999;90:1115–1124. doi: 10.3171/jns.1999.90.6.1115. [DOI] [PubMed] [Google Scholar]
- 6.Siesjö P, Visse E, Sjögren HO. Cure of established, intracerebral rat gliomas induced by therapeutic immunizations with tumor cells and purified APC or adjuvant IFN-gamma treatment. J Immunother Emphasis Tumor Immunol. 1996;19:334–345. [PubMed] [Google Scholar]
- 7.Witham TF, Erff ML, Okada H, et al. 7-Hydroxystaurosporine-induced apoptosis in 9L glioma cells provides an effective antigen source for dendritic cells and yields a potent vaccine strategy in an intracranial glioma model. Neurosurgery. 2002;50:1327–1334; discussion 1334–1335. doi: 10.1097/00006123-200206000-00025. [DOI] [PubMed] [Google Scholar]
- 8.Pellegatta S, Poliani PL, Corno D, et al. Dendritic cells pulsed with glioma lysates induce immunity against syngeneic intracranial gliomas and increase survival of tumor-bearing mice. Neurol Res. 2006;28:527–531. doi: 10.1179/016164106X116809. [DOI] [PubMed] [Google Scholar]
- 9.Heimberger AB, Crotty LE, Archer GE, et al. Bone marrow-derived dendritic cells pulsed with tumor homogenate induce immunity against syngeneic intracerebral glioma. J Neuroimmunol. 2000;103:16–25. doi: 10.1016/s0165-5728(99)00172-1. [DOI] [PubMed] [Google Scholar]
- 10.Insug O, Ku G, Ertl HC, et al. A dendritic cell vaccine induces protective immunity to intracranial growth of glioma. Anticancer Res. 2002;22(2A):613–621. [PubMed] [Google Scholar]
- 11.De Vleeschouwer S, Fieuws S, Rutkowski S, et al. Postoperative adjuvant dendritic cell-based immunotherapy in patients with relapsed glioblastoma multiforme. Clin Cancer Res. 2008;14:3098–3104. doi: 10.1158/1078-0432.CCR-07-4875. [DOI] [PubMed] [Google Scholar]
- 12.Rutkowski S, De Vleeschouwer S, Kaempgen E, et al. Surgery and adjuvant dendritic cell-based tumour vaccination for patients with relapsed malignant glioma, a feasibility study. Br J Cancer. 2004;91:1656–1662. doi: 10.1038/sj.bjc.6602195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wheeler CJ, Black KL. Dendritic cell vaccines and obstacles to beneficial immunity in glioma patients. Curr Opin Mol Ther. 2005;7:35–47. [PubMed] [Google Scholar]
- 14.Yamanaka R, Abe T, Yajima N, et al. Vaccination of recurrent glioma patients with tumour lysate-pulsed dendritic cells elicits immune responses: results of a clinical phase I/II trial. Br J Cancer. 2003;89:1172–1179. doi: 10.1038/sj.bjc.6601268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu JS, Liu G, Ying H, et al. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. 2004;64:4973–4979. doi: 10.1158/0008-5472.CAN-03-3505. [DOI] [PubMed] [Google Scholar]
- 16.Council of Europe. European Pharmacopeia. 8th ed. Strasbourg, France: Council of Europe; 2014.
- 17.Nava S, Dossena M, Pogliani S, et al. An optimized method for manufacturing a clinical scale dendritic cell-based vaccine for the treatment of glioblastoma. PLoS One. 2012;7:e52301. doi: 10.1371/journal.pone.0052301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bohnenkamp HR, Noll T. Development of a standardized protocol for reproducible generation of matured monocyte-derived dendritic cells suitable for clinical application. Cytotechnology. 2003;42:121–131. doi: 10.1023/B:CYTO.0000015833.34696.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Babatz J, Röllig C, Oelschlägel U, et al. Large-scale immunomagnetic selection of CD14+ monocytes to generate dendritic cells for cancer immunotherapy: A phase I study. J Hematother Stem Cell Res. 2003;12:515–523. doi: 10.1089/152581603322448222. [DOI] [PubMed] [Google Scholar]
- 21.Eyrich M, Schreiber SC, Rachor J, et al. Development and validation of a fully GMP-compliant production process of autologous, tumor-lysate-pulsed dendritic cells. Cytotherapy. 2014;16:946–964. doi: 10.1016/j.jcyt.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 22.Gilboa E. DC-based cancer vaccines. J Clin Invest. 2007;117:1195–1203. doi: 10.1172/JCI31205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinman RM, Witmer MD. Lymphoid dendritic cells are potent stimulators of the primary mixed leukocyte reaction in mice. Proc Natl Acad Sci USA. 1978;75:5132–5136. doi: 10.1073/pnas.75.10.5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dohnal AM, Graffi S, Witt V, et al. Comparative evaluation of techniques for the manufacturing of dendritic cell-based cancer vaccines. J Cell Mol Med. 2009;13:125–135. doi: 10.1111/j.1582-4934.2008.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berger TG, Strasser E, Smith R, et al. Efficient elutriation of monocytes within a closed system (Elutra) for clinical-scale generation of dendritic cells. J Immunol Methods. 2005;298:61–72. doi: 10.1016/j.jim.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 26.Sorg RV, Ozcan Z, Brefort T, et al. Clinical-scale generation of dendritic cells in a closed system. J Immunother. 2003;26:374–383. doi: 10.1097/00002371-200307000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.