Abstract
Objective
The objective of this narrative review was to summarize the current state of neurostimulation therapies for the treatment of migraine and/or cluster.
Methods
For this narrative review, publications were identified by searching PubMed using the search terms “migraine” or “cluster” combined with “vagal nerve stimulation”, “transcranial magnetic stimulation”, “supraorbital nerve stimulation”, “sphenopalatine ganglion stimulation”, “occipital nerve stimulation”, “deep brain stimulation”, “neurostimulation”, or “neuromodulation”. Publications were chosen based upon the quality of data that were provided and their relevance to the chosen topics of interest for this review. Reference lists of chosen articles and the authors own files were used to identify additional publications. Current clinical trials were identified by searching clinicaltrials.org.
Results and Conclusions
Neurostimulation of the vagal nerve, supraorbital nerve, occipital nerve and sphenopalatine ganglion, transcranial magnetic stimulation, and deep brain stimulation have been investigated for the treatment of migraine and/or cluster. Whereas invasive methods of neurostimulation would be reserved for patients with very severe and treatment refractory migraine or cluster, non-invasive methods of stimulation might serve as useful adjuncts to more conventional therapies. Currently, transcutaneous supraorbital nerve stimulation is FDA approved and commercially available for migraine prevention and transcranial magnetic stimulation is FDA approved for the treatment of migraine with aura. The potential utility of each type of neurostimulation has yet to be completely defined.
Keywords: Migraine, Cluster Headache, Neurostimulation, Vagal Nerve Stimulation, Deep Brain Stimulation, Transcranial Magnetic Stimulation, Occipital Nerve Stimulation, Transcutaneous Supraorbital Nerve Stimulation, Sphenopalatine Ganglion Stimulation
Introduction
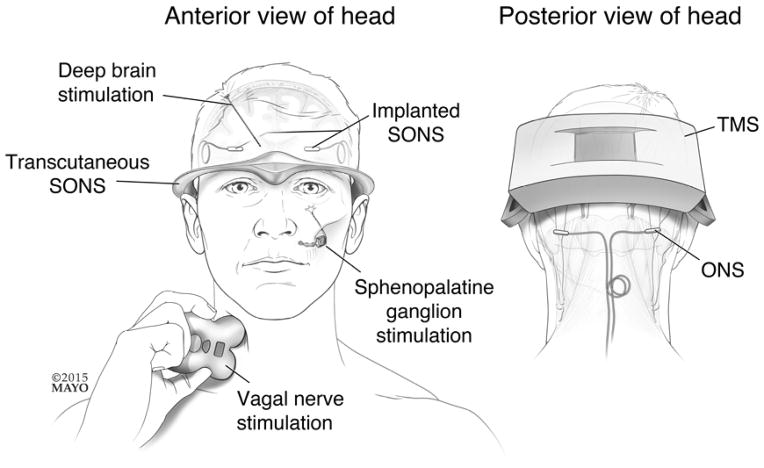
A number of neurostimulation therapies have been investigated for the treatment of migraine and cluster. [Figure 1] Migraine afflicts 38 million Americans, causing substantial pain, hypersensitivity to sensory stimuli, nausea, vomiting and disability.(1) Although cluster affects a smaller population of people compared to migraine, cluster attacks are frequently reported as one of the most painful conditions experienced by mankind. Current pharmacological treatments for migraine and cluster are often suboptimal. First-line abortive migraine treatments provide pain-relief at 2 hours post-treatment in about 60% of patients and pain freedom at 2 hours post-treatment in about 30% of patients, while individual first-line migraine prophylactic medications reduce migraine attack frequency by 50% or more in about 20%–40% of patients.(2, 3) First-line abortive treatments for cluster provide 15 minute pain relief in about 75% of patients and 15 minute pain freedom in about 50% of patients, while first-line prophylactic medications for cluster headache reduce attack frequency by at least 50% in about 70% of patients.(4) Thus, conventional therapies for migraine and cluster are often inadequate and substantial proportions of migraine and cluster patients are considered “refractory” to current therapies. Clearly, non-invasive neurostimulation could serve an important adjunctive role in the abortive and preventive treatment of migraine and cluster headache, while invasive neurostimulation might play a role in the treatment of patients with severe migraine and cluster who are otherwise treatment refractory. In this narrative review, invasive and non-invasive methods of neurostimulation for migraine and cluster are discussed, including vagal nerve stimulation (VNS), transcranial magnetic stimulation (TMS), occipital nerve stimulation (ONS), combined ONS and supraorbital nerve stimulation (SONS), transcutaneous SONS, sphenopalatine ganglion stimulation (SPG), and deep brain stimulation (DBS).
Figure 1. Neurostimulation methods for treatment of migraine and/or cluster.
Invasive and non-invasive methods of neurostimulation for treating migraine and/or cluster are illustrated. ONS = occipital nerve stimulation; SONS = supraorbital nerve stimulation; TMS = transcranial magnetic stimulation.
Vagal Nerve Stimulation
Although the mechanisms by which VNS might effectively abort or prevent migraine attacks are yet to be elucidated, benefits would presumably occur via effects downstream from the nucleus tractus solitarius, a structure with inputs from the vagus nerve. Via the nucleus tractus solitarius, VNS could have activating or inhibiting effects on the thalamus, hypothalamus, reticular activating system, amgydalo-hippocampal complex, cerebral cortex, and trigeminal nucleus caudalis.(5) In animal studies, VNS has been shown to reduce pain-induced activation of neurons in the trigeminal nucleus caudalis, to reduce pain behavior, and to reduce trigeminal allodynia.(6, 7) Case reports of epilepsy patients who had improvements in migraine with VNS and reports of patients with chronic daily headache and depression improving with VNS suggested a possible utility of VNS for migraine treatment.(5, 8, 9)
Non-invasive VNS for the acute treatment of migraine with and without aura has been studied in a small open-label clinical study.(10) Participants were instructed to treat with stimulation once pain became moderate to severe or after 20 minutes of mild pain. Treatment consisted of two, ninety-second transcutaneous stimulations delivered 15 minutes apart, delivered via a hand-held device. Twenty-seven participants treated at least one attack. Pain freedom at two hours was reported for 22% of attacks that were treated when pain was moderate to severe and for 38% of attacks that were treated when mild. Pain relief at two hours was reported for 43% of attacks that were treated when pain was moderate to severe. Vagal nerve stimulation was well tolerated with 13 of 28 treated participants reporting adverse events, all mild or moderate in severity. More common adverse events included stiff neck, frequent urination, shoulder pain or spasm, and lip or facial drooping. Adverse events that were most likely to be treatment-related included neck twitching, raspy voice, and skin redness at the site of stimulation. Despite the vagus nerve having efferent projections to the heart, no cardiac side effects were observed.
Controlled clinical trials of VNS for migraine treatment are required before conclusions can be drawn regarding its potential utility. A pilot study of daily VNS for the prevention of chronic migraine was completed in May 2014 and Data from the Prevention and Acute Treatment of Chronic Cluster Headache (PREVA) study was presented in abstract form at the 2014 European Headache and Migraine Trust International Congress. The PREVA study was a randomized, controlled study investigating the efficacy of VNS delivered twice daily as prophylactic treatment or acutely as abortive treatment versus standard of care for the treatment of chronic cluster. Initial reporting suggested that VNS for the prophylactic and acute treatment of chronic cluster is associated with improvements in several quality of life measures including the EQ-5D-3L, the Headache Impact Test (HIT-6), and the Hospital Anxiety and Depression Scale (HADS).(11) There are several ongoing studies of VNS for the prevention and abortive therapy of episodic and chronic cluster. [clinicaltrials.gov – accessed 10/27/14].
Transcranial Magnetic Stimulation
Single-pulse TMS might be an effective abortive treatment of migraine attacks, while repetitive TMS might be effective for migraine prophylaxis. When TMS is delivered to the scalp, the magnetic field is thought to generate electrical fields that penetrate the brain cortex reaching depths of 1.5 to 3 cm below the skull surface.(12) Single-pulse TMS is presumed to abort attacks of migraine with aura via inhibition of cortical spreading depression. Single-pulse TMS was recently approved by the U.S. FDA for acute treatment of migraine with aura. Repetitive TMS could work as a migraine prophylactic therapy via effects on neurotransmitters and via reducing cortical excitability.(13–15)
Several studies have investigated TMS for the abortive treatment of migraine.(16–19) These studies include a multicenter, randomized, double-blind, sham-controlled study of TMS for acute treatment of migraine with aura.(17) Single-pulse TMS was delivered to the occipital cortex via a portable device. Participants were instructed to treat as soon as possible after migraine aura began and always within one hour of aura onset. Single-pulse TMS was used by 82 subjects while 82 used sham stimulation. TMS was superior to sham stimulation for the primary outcome of pain freedom at 2 hours post-treatment (39% vs. 22%, therapeutic gain 17%; p=.0179]. Treatment-related adverse events were minimal, with 5% of TMS participants reporting adverse events and 2% of sham stimulation participants reporting adverse events. Headache, migraine, and sinusitis were the most commonly reported adverse events.
A few studies, with mixed results, have investigated repetitive TMS for the preventive treatment of migraine.(20–23) All were randomized, sham-stimulation controlled trials of repetitive TMS targeting the dorsolateral prefrontal cortex or motor cortex. The two larger studies investigating TMS for prevention of episodic migraine had mixed results, with one concluding that TMS was superior to placebo for reductions in headache frequency and pain intensity, and the other concluding that TMS was not superior to sham stimulation for several measured outcomes.(22, 23) Differences in stimulation protocols and participant inclusion and exclusion criteria might account for these conflicting results. A study of repetitive TMS for prevention of chronic migraine was negative, with sham-treated patients actually having better outcomes than TMS treated patients, highlighting the substantial placebo-response rates typically seen in migraine studies.(21) Further studies of TMS for prevention of episodic and chronic migraine are needed.
TMS is currently FDA approved for the abortive treatment of migraine with aura.(24) There are several ongoing studies of TMS for the prevention of episodic and chronic migraine. [clinicaltrials.gov – last accessed 10/27/14]
Transcutaneous Supraorbital Nerve Stimulation
The supraorbital and supratrochlear nerves are terminal branches of the frontal nerve, derived from the ophthalmic division of the trigeminal nerve. The supraorbital and supratrochlear nerves provide sensation to the forehead and upper eyelid. Transcutaneous electrical stimulation of these peripheral nerves could provide benefit for the treatment of migraine via inhibition of nociceptive transmission in small pain transmitting fibers and theoretically via modulation of nociceptive activity more centrally in the trigeminal ganglion.
Transcutaneous SONS has been studied for the prevention of migraine in a multicenter, randomized, sham-controlled, double-blind clinical trial and in a large open-label study.(25, 26) In the randomized trial, participants were instructed to treat with transcutaneous supraorbital nerve stimulation for 20 minutes each day for 3 months.(26) For the 67 participants who were randomized, the mean number of migraine days was significantly reduced for those treating with transcutaneous SONS (6.94 to 4.88, p=.02) but not for those in the sham stimulation group (6.54 to 6.22, p=.608). Transcutaneous SONS-treated patients were more likely to have at least a 50% reduction in migraine days per month than those treated with sham (38.1% vs. 12.1%, p=.023). Authors reported that “no adverse events or side effects occurred during the trial, either in the verum or in the sham group”. A large open-label study of 2313 headache sufferers (presumed to be mostly migraineurs) investigated safety and patient satisfaction with transcutaneous supraorbital nerve stimulation in the “general population”.(25) Participants were people who rented the stimulator via the internet and were successfully contacted after the end of the 40-day rental period. Of the 2,312 participants, 53.4% were considered “satisfied” with treatment since they decided to purchase the device at the conclusion of the rental period. At least one adverse event was reported by 4.3% of all participants (5.5% of unsatisfied participants and 3.2% of satisfied participants). The most frequent adverse event was intolerance of the paresthesias induced by stimulation (accounting for 46% of all reported adverse events). Arousal and sleep changes were the second most common adverse events (accounting for 18.6% of all adverse events). No serious adverse events were reported.
There are not currently any transcutaneous SONS studies listed in clincaltrials.gov.
Sphenopalatine Ganglion Stimulation
The SPG is a large extracranial parasympathetic ganglion that innervates meningeal and cerebral blood vessels, nasal mucosa, lacrimal gland, muscles of the upper eyelids and conjunctiva. It has been proposed that SPG stimulation might effectively treat headaches via inhibition of post-ganglionic parasympathetic outflow and subsequent inhibition of pain and cranial autonomic symptoms and via modulating sensory processing in the trigeminal nucleus caudalis.(27)
SPG stimulation for treatment of refractory chronic cluster attacks has been studied in a multicenter, randomized, sham-controlled clinical trial.(28) Twenty-eight participants with chronic cluster completed the randomized component of the trial. Participants were instructed to treat cluster attacks of moderate to severe intensity with 15 minutes of stimulation. Each patient treated 30 attacks for a maximum of 8 weeks. Random insertion of placebo was used, meaning that when a participant initiated stimulation one of three possible stimulation doses was randomly applied in equal proportions: full stimulation, subperception stimulation, or sham stimulation. A total of 566 cluster attacks were treated. SPG stimulation was superior to sham stimulation for the primary efficacy endpoint of pain relief at 15 minutes post-stimulation (67.1% vs. 7.4%, p<.0001). SPG stimulation was also superior to sham stimulation for pain freedom at 15 minutes post-stimulation (34.1% vs. 1.5%, p<.0001). Although measurement of a preventive effect from SPG stimulation was not the primary goal of this study, there was evidence suggesting such an effect. Mean cluster attack frequency dropped from 17.4 attacks per week at baseline to 12.5 attacks per week during the experimental period (p=.005). For the twelve participants who were considered frequency responders there was an average reduction of 88% in the frequency of cluster attacks. Serious adverse events included the need for three stimulator lead revisions and two stimulator explants. Sensory disturbances were experienced by 81% of patients, with sensory loss within distributions of the maxillary nerve being the most common and most but not all resolving over time. Other relatively common adverse events included pain (facial, mouth, temporal, nose, periorbital), swelling, headache, hematoma, and dry eye. Other adverse events included two infections (one at the incision site, one in the maxillary sinus), mild paresis of muscles around the nasolabial fold (2 participants), and operative maxillary sinus puncture (two participants). Long-term follow-up of the study participants is currently underway [clinicaltrials.gov] and preliminary data were presented in abstract form at the 2014 American Academy of Neurology Annual Meeting suggesting a sustained benefit of SPG stimulation for chronic cluster. Among 24 participants completing follow up through 18 months, 66% continued to experience a sustained and clinically significant improvement.(29) Within this subset, 50% were designated as acute responders (91% of attacks effectively treated) and 75% were designated as frequency responders with an overall reduction in baseline attack frequency of 85% compared to baseline.
An open-label pilot study of SPG stimulation for the abortive and preventive treatment of migraine is reportedly complete and sham-controlled SPG stimulation studies for the prevention of migraine and the treatment of chronic cluster are underway. [clinicaltrials.gov]
Occipital and Supraorbital Nerve Stimulation via Implanted Stimulators
ONS has been investigated for the preventive treatment of intractable chronic migraine and medication-refractory cluster. ONS might be effective for the prevention of migraine and cluster via peripheral and central mechanisms. Peripherally, stimulation of large sensory afferents is likely to have a pain-reducing effect via inhibiting nociceptive activity in small c-fiber and a-delta fibers. In support of a central mechanism for ONS, a positron emission tomography (PET) study of 10 drug-resistant chronic cluster patients who were treated with ONS showed that ONS normalized the metabolism of several pain processing brain regions that were hypermetabolic prior to ONS.(30) ONS did not normalize hypothalamic hypermetabolism, a region that is thought to be involved in generation of cluster attacks. Lack of effect on the hypothalamus could explain why cluster attacks tend to recur shortly after cessation of peripheral stimulation.
After several open-label studies suggested potential benefits of ONS for migraine, three randomized sham-controlled trials have been conducted and reported. The ONSTIM feasibility study evaluated ONS therapy in 33 patients with refractory chronic migraine compared to 17 patients receiving sham stimulation and 17 patients who received continued medical management.(31) As a feasibility study, the study was underpowered and numerous efficacy outcomes were evaluated independently. ONS was statistically superior (not necessarily significantly superior) to sham stimulation and medical management for several outcomes including responder rate, reduction in headache days, reduction of days with severe and prolonged headache, and for several measures of disability and quality of life. A second study of patients with chronic migraine randomized 105 patients to active ONS and 52 to sham stimulation.(32) All of these patients had a successful trial of stimulation (defined as at least a 50% reduction in pain or adequate paresthesia coverage in the painful areas) prior to randomization. Patients who did not have a successful trial (n=20) exited the study and were not randomized. ONS was not superior to sham stimulation for the primary outcome (at least a 50% reduction in mean daily visual analog scores) at 12 weeks (active stimulation 17.1% vs. sham stimulation 13.5%, p=.55). ONS was superior to sham stimulation for secondary endpoints, including at least a 30% reduction in mean daily visual analog scores, reduction in number of headache days, and migraine-related disability. Results from the PRISM study of ONS for the treatment of refractory migraine were presented at the 14th Congress of the International Headache Society and published in abstract form.(33) Amongst the 125 subjects who provided 12 week follow-up after implantation, there was no difference in the primary endpoint of change from baseline in the number of migraine days per month in those receiving active ONS (n=63, −5.5 +/− 8.7 days per month) and those receiving sham stimulation (n=62, −3.9 +/− 8.2 days per month, p=.29). An open-label extension of a randomized, sham-controlled trial (lasting 12 weeks) reported long-term safety and efficacy at 52 weeks following ONS implantation.(34) In the intent-to-treat population (n=157), headache days were reduced by 6.7 +/− 8.4 days per month, 47.8% of subjects had at least a 50% reduction in headache days and/or pain intensity, and 65.4% reported having good or excellent headache relief. In this study and others, device and procedure-related adverse events were common, often requiring surgical intervention (e.g. for lead migration).
Nearly 100 patients with cluster headache who have been treated with ONS have been reported in the literature.(35–41) Overall, about 2/3 of such patients have at least a 50% improvement in headache frequency and/or intensity.(40) Thus far, there is a lack of randomized, controlled trials of ONS for treatment of cluster. Plans for a prospective randomized trial of ONS for medically intractable chronic cluster were published in 2013.(42)
Combined supraorbital nerve and occipital nerve stimulation has been investigated for the treatment of chronic migraine.(43, 44) In an open-label study of 7 patients who responded to a percutaneous stimulation trial, all patients had substantial improvements in headache frequency and severity and all felt that combined occipital and supraorbital nerve stimulation was superior to ONS alone.(44) In a second open-label study, 14 patients with chronic migraine were treated with combined supraorbital and occipital nerve stimulation.(43) Ten of 14 patients had at least a 50% reduction in pain severity and the mean reduction in visual analog scale score was 3.92 +/− 2.4. Additional studies would be required to determine the efficacy of combined supraorbital and occipital nerve stimulation and its potential superiority over ONS alone. One recent case report described a patient who obtained excellent relief of occipital pain after ONS, but who continued to have debilitating temporal-distribution pain that responded well to the implantation of bilateral temporal stimulator leads.(45) Unfortunately, explantation was eventually necessary due to complications from infection and component erosion. If peripheral mechanisms are important in determining outcomes from stimulation, combined stimulation might be effective for the management of pain that extends into multiple anatomic distributions; however, there is a need for further studies that investigate the therapeutic outcomes and risks.
Deep Brain Stimulation
DBS of the posterior hypothalamus has been investigated for the treatment of medication-refractory chronic cluster.(46–55) The posterior hypothalamus is thought to play an essential role in the pathophysiology of cluster, and likely accounts for the circadian and diurnal rhythmicity of both cluster periods (i.e. the months during which patients with episodic cluster have cluster attacks) and individual cluster attacks.
Amongst the approximately 60 patients whose outcomes have been published in the literature, about 60% have had at least a 50% reduction in their cluster attack frequency with DBS.(37, 56) One study of 11 patients included a double-blind, prospective, 1 month crossover component during which patients were randomized either to sham stimulation or active stimulation.(48) During the month of blinded treatment, there were no differences in primary or secondary outcome measures for patients receiving active stimulation vs. those randomized to the sham group. However, by the end of the one-year open-label phase, 6 of 11 patients had achieved at least a 50% reduction in cluster attack frequency. The relatively high responder rates found in DBS treatment studies and the persistence of benefits over time suggest that the absence of differential outcomes during the randomized phase of this study are attributable to the outcomes being measured too soon after onset of DBS rather than there being no benefit of DBS beyond sham stimulation.(57) However, additional sham-controlled studies would be needed to determine benefits of hypothalamic DBS. A number of patients have achieved sustained efficacy and tolerability of DBS beyond 2 or more years of treatment.(52)
As would be expected with DBS, side effects and adverse events are a substantial concern when considering a patient for such therapy. Diplopia is a common and temporary side effect of hypothalamic stimulation. Among all cases of DBS for the treatment of cluster, 6 serious adverse events have been reported. All occurred intra-operatively, they included a number of intracranial hemorrhages, and one patient died post-operatively due to intracranial hemorrhage.(53) Given the invasive nature of this procedure and the potential for serious adverse events and outcomes, careful patient selection is critical.
Recommendations include rigorous adherence to the International Classification of Headache Disorders when identifying patients with a diagnosis of cluster, and limiting candidates to those with daily headache who are also refractory to multiple classes of medications, have normal cerebral imaging (including vascular imaging), have been hospitalized in order for the treatment team to witness and classify attacks, have a normal psychological profile, and who have also been refractory for greater than 2 years. Furthermore, it is recommended that candidates for DBS agree to permanently abstain from the use of tobacco and alcohol.(58) Subsequent recommendations suggested further limiting this procedure to those who have failed less-invasive procedures such as ONS.(59)
Conclusions and Future Directions
Current medicinal and non-pharmacological treatments for migraine and cluster are often inadequate for effectively and consistently preventing and aborting migraine and cluster attacks. Safe and effective methods of non-invasive neurostimulation might provide an adjunctive treatment option for migraine and cluster patients who are currently receiving suboptimal treatment outcomes. If found to be effective and relatively safe, invasive methods of neurostimulation might serve an important role in the treatment of patients with severe forms of migraine and cluster that are intractable to less invasive therapies. Further investigations are needed to determine the potential role of neurostimulation for the treatment of migraine and cluster and to identify specific subsets of patients who may better respond to such treatments.
Box 1. Search Strategy.
For this narrative review, publications were identified by searching PubMed using the following search terms: “migraine” or “cluster” and “vagal nerve stimulation” or “migraine” or “cluster” and “transcranial magnetic stimulation” or “migraine” or “cluster” and “supraorbital nerve stimulation” or “migraine” or “cluster” and “sphenopalatine ganglion stimulation” or “migraine” or “cluster” and “occipital nerve stimulation” or “migraine” or “cluster” and “deep brain stimulation” or “migraine” or “cluster” and “neurostimulation” or “migraine” or “cluster” and “neuromodulation”. Publications were chosen based upon the quality of data that were provided and their relevance to the chosen topics of interest for this review. Reference lists of chosen articles and the authors own files were used to identify additional publications. Current clinical trials were identified by searching clinicaltrials.org.
Acknowledgments
Funding: NIH K23NS070891 to TJS
Footnotes
Author Disclosures: within the past 36 months.
Contributor Information
Todd J. Schwedt, Royalties: Up To Date, Cambridge University Press, Consulting/Advisory Boards: Allergan, Zogenix, Supernus, Pfizer, Clinical Trial Investigator: eNeura, Boston Scientific, Alder, Biopharmaceuticals, Autonomic Technologies, Labrys Biologics, Arteaus, Therapeutics, OptiNose US.
Bert Vargas, Consulting/Advisory Boards: Allergan, Zogenix, Avanir, Clinical Trial Investigator: Boston Scientific, Autonomic Technologies, eNeura, Alder Biopharmaceuticals, OptiNose US, Alder, Biopharmaceuticals, Labrys Biologics, Arteaus.
References
- 1.Smitherman TA, Burch R, Sheikh H, Loder E. The prevalence, impact, and treatment of migraine and severe headaches in the United States: a review of statistics from national surveillance studies. Headache. 2013;53:427–36. doi: 10.1111/head.12074. [DOI] [PubMed] [Google Scholar]
- 2.Ferrari MD, Goadsby PJ, Roon KI, Lipton RB. Triptans (serotonin, 5-HT1B/1D agonists) in migraine: detailed results and methods of a meta-analysis of 53 trials. Cephalalgia. 2002;22:633–58. doi: 10.1046/j.1468-2982.2002.00404.x. [DOI] [PubMed] [Google Scholar]
- 3.Shamliyan TA, Kane RL, Taylor FR. Migraine in Adults: Preventive Pharmacologic Treatments. Rockville (MD): 2013. [PubMed] [Google Scholar]
- 4.Ashkenazi A, Schwedt T. Cluster headache--acute and prophylactic therapy. Headache. 2011;51:272–86. doi: 10.1111/j.1526-4610.2010.01830.x. [DOI] [PubMed] [Google Scholar]
- 5.Lenaerts ME, Oommen KJ, Couch JR, Skaggs V. Can vagus nerve stimulation help migraine? Cephalalgia. 2008;28:392–5. doi: 10.1111/j.1468-2982.2008.01538.x. [DOI] [PubMed] [Google Scholar]
- 6.Bohotin C, Scholsem M, Multon S, Martin D, Bohotin V, Schoenen J. Vagus nerve stimulation in awake rats reduces formalin-induced nociceptive behaviour and fos-immunoreactivity in trigeminal nucleus caudalis. Pain. 2003;101:3–12. doi: 10.1016/s0304-3959(02)00301-9. [DOI] [PubMed] [Google Scholar]
- 7.Oshinsky ML, Murphy AL, Hekierski H, Jr, Cooper M, Simon BJ. Noninvasive vagus nerve stimulation as treatment for trigeminal allodynia. Pain. 2014;155:1037–42. doi: 10.1016/j.pain.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basic S, Sporis D, Chudy D, Grahovac G, Nevajda B. The effect of vagus nerve stimulation on migraine in patient with intractable epilepsy: case report. Neurol Sci. 2013;34:797–8. doi: 10.1007/s10072-012-1135-5. [DOI] [PubMed] [Google Scholar]
- 9.Cecchini AP, Mea E, Tullo V, Curone M, Franzini A, Broggi G, et al. Vagus nerve stimulation in drug-resistant daily chronic migraine with depression: preliminary data. Neurol Sci. 2009;30 (Suppl 1):S101–4. doi: 10.1007/s10072-009-0073-3. [DOI] [PubMed] [Google Scholar]
- 10.Goadsby P, Grosberg B, Mauskop A, Cady R, Simmons K. Effect of noninvasive vagus nerve stimulation on acute migraine: An open-label pilot study. Cephalalgia. 2014;34:986–93. doi: 10.1177/0333102414524494. [DOI] [PubMed] [Google Scholar]
- 11.Gaul C, Diener H, Solbach K, et al. Quality of life in subjects treated by non-invasive vagus nerve stimulation using gammacore for the prevention and acute treatment of chronic cluster headache. J Headache Pain. 2014;15:16. [Google Scholar]
- 12.Lipton RB, Pearlman SH. Transcranial magnetic simulation in the treatment of migraine. Neurotherapeutics. 2010;7:204–12. doi: 10.1016/j.nurt.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brighina F, Palermo A, Daniele O, Aloisio A, Fierro B. High-frequency transcranial magnetic stimulation on motor cortex of patients affected by migraine with aura: a way to restore normal cortical excitability? Cephalalgia. 2010;30:46–52. doi: 10.1111/j.1468-2982.2009.01870.x. [DOI] [PubMed] [Google Scholar]
- 14.Keck ME, Welt T, Muller MB, Erhardt A, Ohl F, Toschi N, et al. Repetitive transcranial magnetic stimulation increases the release of dopamine in the mesolimbic and mesostriatal system. Neuropharmacology. 2002;43:101–9. doi: 10.1016/s0028-3908(02)00069-2. [DOI] [PubMed] [Google Scholar]
- 15.Strafella AP, Paus T, Barrett J, Dagher A. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J Neurosci. 2001;21:RC157. doi: 10.1523/JNEUROSCI.21-15-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clarke BM, Upton AR, Kamath MV, Al-Harbi T, Castellanos CM. Transcranial magnetic stimulation for migraine: clinical effects. J Headache Pain. 2006;7:341–6. doi: 10.1007/s10194-006-0329-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lipton RB, Dodick DW, Silberstein SD, Saper JR, Aurora SK, Pearlman SH, et al. Single-pulse transcranial magnetic stimulation for acute treatment of migraine with aura: a randomised, double-blind, parallel-group, sham-controlled trial. The Lancet Neurology. 2010;9:373–80. doi: 10.1016/S1474-4422(10)70054-5. [DOI] [PubMed] [Google Scholar]
- 18.Mohammad YM, Kothari R, Hughes G, Nkrumah M, Fischell S, Robert F, Schweiger J, Ruppel P. Transcranial magnetic stimulation (TMS) relieves migraine headache (Abstract) Headache. 2006;46:839. [Google Scholar]
- 19.Mohammad YM, Hughes G, Nkrumah M, Fischell S, Fischell R, Ruppel P, Schweiger J. Self-administered transcranial magnetic stimulation (TMS), during the aura phase, improves and aborts migraine headache (Abstract) Headache. 2006;46:857. [Google Scholar]
- 20.Brighina F, Piazza A, Vitello G, Aloisio A, Palermo A, Daniele O, et al. rTMS of the prefrontal cortex in the treatment of chronic migraine: a pilot study. J Neurol Sci. 2004;227:67–71. doi: 10.1016/j.jns.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 21.Conforto AB, Amaro E, Jr, Goncalves AL, Mercante JP, Guendler VZ, Ferreira JR, et al. Randomized, proof-of-principle clinical trial of active transcranial magnetic stimulation in chronic migraine. Cephalalgia. 2014;34:464–72. doi: 10.1177/0333102413515340. [DOI] [PubMed] [Google Scholar]
- 22.Misra UK, Kalita J, Bhoi SK. High-rate repetitive transcranial magnetic stimulation in migraine prophylaxis: a randomized, placebo-controlled study. J Neurol. 2013;260:2793–801. doi: 10.1007/s00415-013-7072-2. [DOI] [PubMed] [Google Scholar]
- 23.Teepker M, Hotzel J, Timmesfeld N, Reis J, Mylius V, Haag A, et al. Low-frequency rTMS of the vertex in the prophylactic treatment of migraine. Cephalalgia. 2010;30:137–44. doi: 10.1111/j.1468-2982.2009.01911.x. [DOI] [PubMed] [Google Scholar]
- 24.US Food and Drug Administration. FDA news release: FDA allows marketing of first device to relieve migraine headache pain. 2013 wwwfdagov/NewsEvents/Newsroom/PressAnnouncements/ucm378608htm.
- 25.Magis D, Sava S, d’Elia TS, Baschi R, Schoenen J. Safety and patients’ satisfaction of transcutaneous supraorbital neurostimulation (tSNS) with the Cefaly(R) device in headache treatment: a survey of 2,313 headache sufferers in the general population. J Headache Pain. 2013;14:95. doi: 10.1186/1129-2377-14-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schoenen J, Vandersmissen B, Jeangette S, Herroelen L, Vandenheede M, Gerard P, et al. Migraine prevention with a supraorbital transcutaneous stimulator: a randomized controlled trial. Neurology. 2013;80:697–704. doi: 10.1212/WNL.0b013e3182825055. [DOI] [PubMed] [Google Scholar]
- 27.Khan S, Schoenen J, Ashina M. Sphenopalatine ganglion neuromodulation in migraine: what is the rationale? Cephalalgia. 2014;34:382–91. doi: 10.1177/0333102413512032. [DOI] [PubMed] [Google Scholar]
- 28.Schoenen J, Jensen RH, Lanteri-Minet M, Lainez MJ, Gaul C, Goodman AM, et al. Stimulation of the sphenopalatine ganglion (SPG) for cluster headache treatment. Pathway CH-1: a randomized, sham-controlled study. Cephalalgia. 2013;33:816–30. doi: 10.1177/0333102412473667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lainez M, Jensen R, May A, et al. Long term therapeutic response of sphenopalatine ganglion (SPG) stimulation for cluster headache - Pathway CH-1 study (I9-1.007) Neurology. 2014;82:82. [Google Scholar]
- 30.Magis D, Bruno MA, Fumal A, Gerardy PY, Hustinx R, Laureys S, et al. Central modulation in cluster headache patients treated with occipital nerve stimulation: an FDG-PET study. BMC Neurol. 2011;11:25. doi: 10.1186/1471-2377-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saper JR, Dodick DW, Silberstein SD, McCarville S, Sun M, Goadsby PJ. Occipital nerve stimulation for the treatment of intractable chronic migraine headache: ONSTIM feasibility study. Cephalalgia. 2011;31:271–85. doi: 10.1177/0333102410381142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silberstein SD, Dodick DW, Saper J, Huh B, Slavin KV, Sharan A, et al. Safety and efficacy of peripheral nerve stimulation of the occipital nerves for the management of chronic migraine: results from a randomized, multicenter, double-blinded, controlled study. Cephalalgia. 2012;32:1165–79. doi: 10.1177/0333102412462642. [DOI] [PubMed] [Google Scholar]
- 33.Lipton R, Goadsby P, Cady R, et al. PRISM study: occipital nerve stimulation for treatment-refractory migraine. Cephalalgia. 2009;29(suppl 1):30. [Google Scholar]
- 34.Dodick DW, Silberstein SD, Reed KL, Deer TR, Slavin KV, Huh B, et al. Safety and efficacy of peripheral nerve stimulation of the occipital nerves for the management of chronic migraine: Long-term results from a randomized, multicenter, double-blinded, controlled study. Cephalalgia. 2014 doi: 10.1177/0333102414543331. [DOI] [PubMed] [Google Scholar]
- 35.Burns B, Watkins L, Goadsby PJ. Treatment of medically intractable cluster headache by occipital nerve stimulation: long-term follow-up of eight patients. Lancet. 2007;369:1099–106. doi: 10.1016/S0140-6736(07)60328-6. [DOI] [PubMed] [Google Scholar]
- 36.Burns B, Watkins L, Goadsby PJ. Treatment of intractable chronic cluster headache by occipital nerve stimulation in 14 patients. Neurology. 2009;72:341–5. doi: 10.1212/01.wnl.0000341279.17344.c9. [DOI] [PubMed] [Google Scholar]
- 37.Fontaine D, Vandersteen C, Magis D, Lanteri-Minet M. Neuromodulation in cluster headache. Adv Tech Stand Neurosurg. 2015;42:3–21. doi: 10.1007/978-3-319-09066-5_1. [DOI] [PubMed] [Google Scholar]
- 38.Magis D, Allena M, Bolla M, De Pasqua V, Remacle JM, Schoenen J. Occipital nerve stimulation for drug-resistant chronic cluster headache: a prospective pilot study. The Lancet Neurology. 2007;6:314–21. doi: 10.1016/S1474-4422(07)70058-3. [DOI] [PubMed] [Google Scholar]
- 39.Magis D, Gerardy PY, Remacle JM, Schoenen J. Sustained effectiveness of occipital nerve stimulation in drug-resistant chronic cluster headache. Headache. 2011;51:1191–201. doi: 10.1111/j.1526-4610.2011.01973.x. [DOI] [PubMed] [Google Scholar]
- 40.Magis D, Schoenen J. Advances and challenges in neurostimulation for headaches. The Lancet Neurology. 2012;11:708–19. doi: 10.1016/S1474-4422(12)70139-4. [DOI] [PubMed] [Google Scholar]
- 41.Mueller OM, Gaul C, Katsarava Z, Diener HC, Sure U, Gasser T. Occipital nerve stimulation for the treatment of chronic cluster headache - lessons learned from 18 months experience. Cent Eur Neurosurg. 2011;72:84–9. doi: 10.1055/s-0030-1270476. [DOI] [PubMed] [Google Scholar]
- 42.Wilbrink LA, Teernstra OP, Haan J, van Zwet EW, Evers SM, Spincemaille GH, et al. Occipital nerve stimulation in medically intractable, chronic cluster headache. The ICON study: rationale and protocol of a randomised trial. Cephalalgia. 2013;33:1238–47. doi: 10.1177/0333102413490351. [DOI] [PubMed] [Google Scholar]
- 43.Hann S, Sharan A. Dual occipital and supraorbital nerve stimulation for chronic migraine: a single-center experience, review of literature, and surgical considerations. Neurosurg Focus. 2013;35:E9. doi: 10.3171/2013.6.FOCUS13233. [DOI] [PubMed] [Google Scholar]
- 44.Reed KL, Black SB, Banta CJ, 2nd, Will KR. Combined occipital and supraorbital neurostimulation for the treatment of chronic migraine headaches: initial experience. Cephalalgia. 2010;30:260–71. doi: 10.1111/j.1468-2982.2009.01996.x. [DOI] [PubMed] [Google Scholar]
- 45.Zach KJ, Trentman TL, Zimmerman RS, Dodick DW. Refractory headaches treated with bilateral occipital and temporal region stimulation. Med Devices (Auckl) 2014;7:55–9. doi: 10.2147/MDER.S59719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bartsch T, Pinsker MO, Rasche D, Kinfe T, Hertel F, Diener HC, et al. Hypothalamic deep brain stimulation for cluster headache: experience from a new multicase series. Cephalalgia. 2008;28:285–95. doi: 10.1111/j.1468-2982.2007.01531.x. [DOI] [PubMed] [Google Scholar]
- 47.Broggi G, Franzini A, Leone M, Bussone G. Update on neurosurgical treatment of chronic trigeminal autonomic cephalalgias and atypical facial pain with deep brain stimulation of posterior hypothalamus: results and comments. Neurol Sci. 2007;28 (Suppl 2):S138–45. doi: 10.1007/s10072-007-0767-3. [DOI] [PubMed] [Google Scholar]
- 48.Fontaine D, Lazorthes Y, Mertens P, Blond S, Geraud G, Fabre N, et al. Safety and efficacy of deep brain stimulation in refractory cluster headache: a randomized placebo-controlled double-blind trial followed by a 1-year open extension. J Headache Pain. 2010;11:23–31. doi: 10.1007/s10194-009-0169-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Franzini A, Ferroli P, Leone M, Broggi G. Stimulation of the posterior hypothalamus for treatment of chronic intractable cluster headaches: first reported series. Neurosurgery. 2003;52:1095–9. discussion 99–101. [PubMed] [Google Scholar]
- 50.Franzini A, Messina G, Cordella R, Marras C, Broggi G. Deep brain stimulation of the posteromedial hypothalamus: indications, long-term results, and neurophysiological considerations. Neurosurg Focus. 2010;29:E13. doi: 10.3171/2010.5.FOCUS1094. [DOI] [PubMed] [Google Scholar]
- 51.Leone M, Franzini A, Broggi G, Bussone G. Hypothalamic deep brain stimulation for intractable chronic cluster headache: a 3-year follow-up. Neurol Sci. 2003;24 (Suppl 2):S143–5. doi: 10.1007/s100720300063. [DOI] [PubMed] [Google Scholar]
- 52.Piacentino M, D’Andrea G, Perini F, Volpin L. Drug-resistant cluster headache: long-term evaluation of pain control by posterior hypothalamic deep-brain stimulation. World Neurosurg. 2014;81:442, e11–5. doi: 10.1016/j.wneu.2013.01.130. [DOI] [PubMed] [Google Scholar]
- 53.Schoenen J, Di Clemente L, Vandenheede M, Fumal A, De Pasqua V, Mouchamps M, et al. Hypothalamic stimulation in chronic cluster headache: a pilot study of efficacy and mode of action. Brain. 2005;128:940–7. doi: 10.1093/brain/awh411. [DOI] [PubMed] [Google Scholar]
- 54.Seijo F, Saiz A, Lozano B, Santamarta E, Alvarez-Vega M, Seijo E, et al. Neuromodulation of the posterolateral hypothalamus for the treatment of chronic refractory cluster headache: Experience in five patients with a modified anatomical target. Cephalalgia. 2011;31:1634–41. doi: 10.1177/0333102411430264. [DOI] [PubMed] [Google Scholar]
- 55.Starr PA, Barbaro NM, Raskin NH, Ostrem JL. Chronic stimulation of the posterior hypothalamic region for cluster headache: technique and 1-year results in four patients. J Neurosurg. 2007;106:999–1005. doi: 10.3171/jns.2007.106.6.999. [DOI] [PubMed] [Google Scholar]
- 56.Leone M, Franzini A, Cecchini AP, Broggi G, Bussone G. Hypothalamic deep brain stimulation in the treatment of chronic cluster headache. Ther Adv Neurol Disord. 2010;3:187–95. doi: 10.1177/1756285610370722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leone M, Franzini A, Broggi G, Bussone G. Hypothalamic stimulation for intractable cluster headache: long-term experience. Neurology. 2006;67:150–2. doi: 10.1212/01.wnl.0000223319.56699.8a. [DOI] [PubMed] [Google Scholar]
- 58.Leone M, May A, Franzini A, Broggi G, Dodick D, Rapoport A, et al. Deep brain stimulation for intractable chronic cluster headache: proposals for patient selection. Cephalalgia. 2004;24:934–7. doi: 10.1111/j.1468-2982.2004.00742.x. [DOI] [PubMed] [Google Scholar]
- 59.Sillay KA, Sani S, Starr PA. Deep brain stimulation for medically intractable cluster headache. Neurobiol Dis. 2010;38:361–8. doi: 10.1016/j.nbd.2009.05.020. [DOI] [PubMed] [Google Scholar]