Abstract
Purpose
Rhodopsin localization and rod photoreceptor (PR) morphology is altered in embryonic transgenic (Tg) Pro23His (P23H) miniswine. At birth, the Tg P23H swine retina lacks rod driven signaling. Curcumin, a neuroprotective food additive, has been shown to rescue Tg P23H rat rod PRs and promote normal trafficking of rhodopsin. We tested the hypothesis that prenatal exposure to curcumin would prevent PR morphological changes in Tg P23H miniswine retinae.
Methods
A domestic sow was inseminated with semen from a Tg P23H miniswine founder. Her daily diet was supplemented with curcumin (100 mg/Kg body weight) from embryonic (E) day 80 to E112. The same diet without curcumin was fed to a second inseminated control sow. At E112, 2 days before parturition, both sows were euthanized. Their embryos were harvested, genotyped, and their eyes enucleated and prepared for morphological evaluation.
Results
In all pigs, we measured mean outer retinal thickness, localization of rhodopsin, and rod PR morphology. Curcumin-treated Tg P23H swine embryonic retinas were similar to WT. Untreated Tg P23H embryonic retinas show significant degenerative effects; their outer retina was thinner, rod PR morphology was abnormal, and rhodopsin was mislocalized to the outer nuclear layer (ONL).
Conclusions
These data support a role for curcumin as a neuroprotective agent that prevents/delays morphological abnormalities associated with rod PR degeneration in this Tg P23H swine model of retinitis pigmentosa (RP).
Translational Relevance
Curcumin, a Food and Drug Administration–approved dietary supplement, may arrest/delay PR degeneration if ingested by individuals at risk for developing RP.
Keywords: retina, retinitis pigmentosa, neuroprotection, electron microscopy, animal models
Introduction
Retinitis Pigmentosa (RP) is the most frequent hereditary retinal degeneration among adults.1 RP causes progressive loss of rod photoreceptors (PRs) and subsequent loss of cones PRs.2 Patients with RP develop symptoms of night blindness as early as adolescence and abnormal electroretinograms (ERGs) have been reported in the first decade of life.3–5 Gene therapy in patients with Leber's Congenital Amaurosis (LCA) who present symptoms at birth or within the first year of life improves visual function at least in the short term (≥3 years).6,7 Despite this success, none of the current medical therapies have proven to be effective for long-term treatment of RP,8–16 which suggests that adjunctive therapy may be necessary. Patients who are known carriers or are at risk of inheriting RP and have not yet experienced symptoms may benefit tremendously from pretreatment strategies that preserve retinal structure and function.
Curcumin, a principal component of the Indian spice turmeric, has been shown to be a morphological neuroprotectant in transgenic rat retinas expressing the Pro-23-His (P23H) rhodopsin mutation.17 This is the same mutation that underlies the most common form of autosomal dominant RP in North America.18 Curcumin, administered from P30 to P70, inhibited severe retinal rod PR degeneration in transgenic P23H rats, which was correlated with dissociated mutant protein aggregation and translocation of mutant rhodopsin to the appropriate cellular compartment. Aggregation and mistrafficking of mutant rhodopsin protein, common across RP models,19–28 is thought to be cytotoxic. Thus, administration prior to these events could prove an effective adjunctive therapy to other strategies that attempt to alter mutant rhodopsin expression. We sought to determine if curcumin administration would have similar neuroprotective effects in a large animal model of RP, which exhibits functional and morphological characteristics similar to P23H retinopathy in man.28,29,30 Because alterations in morphology arise prenatally in this swine RP model, we exposed the embryos prenatally to curcumin. Our results show that curcumin delays structural defects in transgenic P23H miniswine embryo rod PRs.
Materials and Methods
All experimental protocols were approved by the University of Louisville Institutional Animal Care and Use Committee and adhere to the ARVO Statement for Use of Animals in Ophthalmic and Vision Research.
Animals
Two domestic sows were inseminated with semen from a transgenic P23H miniswine founder,29 heterozygous for the P23H transgene to create hybrid (domestic × miniswine) offspring.
Administration of Curcumin
To test the neuroprotective effect of curcumin on rod PRs prior to degeneration, we supplemented the maternal diet of one sow with curcumin (100 mg/Kg body weight/day; Sigma-Aldrich, St. Louis, MO) beginning at gestational age E80, a time prior to the development of PRs outer segments, terminals, and rhodopsin expression.28 The diet was continued until sacrifice at E112, two days prior to parturition. A second domestic sow was inseminated and fed the same diet without curcumin supplementation and her embryos served as untreated controls. Sows were euthanized and their embryos (Wild Type No Treatment [WT No Tx] N = 6; WT Tx, N = 2; P23H No Tx, N = 8; P23H Tx, N = 4) harvested and their eyes enucleated and prepared for morphological analysis. All embryos were genotyped by PCR.29
Retinal Morphology
Retrieval of embryonic retinal tissue has been described previously.28 Eyes were enucleated and immediately immersed in fixative (4% paraformaldehyde for immunohistochemistry and light microscopy; 2% paraformaldehyde/2% glutaraldehyde for transmission electron microscopy) for 24 hours at 4°C. The results are from a total of 40 eyes (20 embryos total).
Morphometric Analysis of the Retina
Plastic sections were prepared as previously described.28 Briefly, a 3-mm wide vertical band of retinal tissue extending from the optic disc to the ora serrata was dissected from the superior retina of the right eye from all embryos and processed for examination at the light microscopic level. Sections were examined at ×40 or ×100 using a NIKON EFD-3 Episcopic-Fluorescence microscope (Nikon Inc., Melville, NY). Photomicrographs were taken on a Moticam 2500 high-resolution camera (Motic, British Columbia, Canada) and digitally processed using Adobe Photoshop (Adobe Systems, San Jose, CA) to adjust brightness and contrast.
Outer nuclear layer (ONL), photoreceptor layer (PRL), and inner nuclear layer (INL) thickness were measured at 2-mm increments along the vertical band of retinal tissue dissected from superior retina. Only the superior retina was measured since the central-to-peripheral pattern of degeneration was shown to be similar across all retinal quadrants.28 To measure ONL and INL thickness, a vertical line was drawn through 10 adjacent vertical columns of photoreceptor nuclei in the ONL and 10 adjacent vertical columns of nuclei in the INL using Moticam Image Plus 2.0 (Motic China Group Co., Ltd., Xiamen, China) in five sections per location/eye and the mean calculated for each location. PRL thickness was measured in the same sections and at the same locations as ONL and INL thickness. To measure PRL thickness, 10 adjacent vertical lines each separated by 5 μm were drawn connecting the external limiting membrane to the apical surface of the retinal pigment epithelium and the mean calculated for each section. Overall ONL, INL, and PRL thickness along the vertical meridian of the superior retina was calculated by averaging the mean thickness across all locations in each eye. A trained masked observer (PAS) measured ONL, INL, and PRL thickness without knowledge of the genotype.
Transmission Electron Microscopy
Sections for transmission electron microscopy were prepared as previously described.28,30 Briefly, a 3-mm wide vertical strip of retinal tissue was removed from the superior retina of the left eye. A 2 × 2 mm piece of retinal tissue was harvested from the vertical band of tissue approximately 5 mm above the superior margin of the optic disc and processed for transmission electron microscopy. Outer retinal morphology was examined with a transmission electron microscope (TEM; Model 300; Phillips, Eindhoven, the Netherlands) and photomicrographs were captured with a digital camera (15 mega pixel digital camera; Scientific Instruments and Applications, Duluth, GA) and Maxim DL Version 5 software (Diffraction Limited, Ottawa, Canada).
Immunohistochemistry
A 3-mm wide vertical band of retinal tissue was dissected from the superior retina immediately temporal to the vertical band that was dissected for light microscopic examination. Sections of retina (20-μm thick) were cut on a cryostat and stored at −80°C. Processing and procedures for immunostaining retinal tissue with monoclonal anti-Rho 1D4 (Cat. # MABN5356, 1:500; Millipore, Chicago, IL,) antibody to immunolabel the expression of rhodopsin in rod PRs has been previously described.28 Sections were examined with a confocal microscope (Olympus FV1000) using a ×40 objective.
Statistics
Morphometric analysis of the retina was analyzed using InStat 3 software for Macintosh (Graphpad Software, Inc., La Jolla, CA). One-way ANOVA and Bonferroni's post-hoc t-test were used to compare mean ONL, PRL, and INL thickness across all groups. A P value less than or equal to 0.05 was interpreted as statistical significance.
Results
Curcumin Prevents the Central-to-Peripheral Retinal Pattern of ONL and PRL Thinning in P23H Miniswine Embryonic Retinas
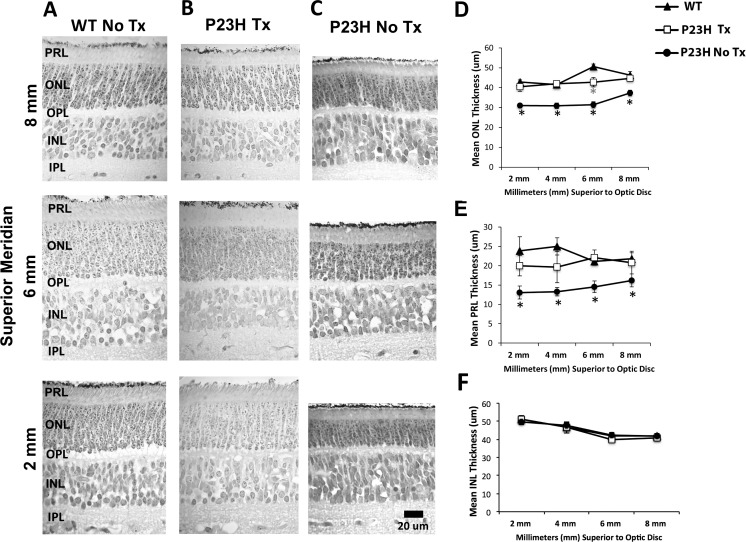
Representative photomicrographs (Fig. 1A) and quantitative measures of WT ONL (Fig. 1D) and PRL (Fig. 1E) thickness across retinal location were similar. WT littermates from curcumin-treated (Tx; n = 2) or untreated (No Tx; n = 6) mothers were the same and these data have been combined. The WT ONL thickness is significantly thicker at 6-mm superior to the disc (P ≤ 0.05). This difference in WT ONL thickness was not observed in either P23H Tx or P23H No Tx embryonic retinas. Further, the difference was not reflected in PRL thickness (Fig. 1E).
Figure 1.
Representative photomicrographs of the retina taken along the superior meridian 2, 6, and 8 mm above the optic disc (A–C). WT (No Tx N = 6; WT Tx N = 2; column A) and Tg P23H Curcumin-Tx (N = 4; column B) sections appeared similar and showed the normal laminar arrangement of the retina. In contrast, Tg P23H No Tx (N = 8; column C) showed obvious thinning of the ONL. Morphometric analysis of the ONL, PRL, and INL (D–F). (D) Tg P23H No Tx miniswine embryos showed a central-to-peripheral thinning of the ONL that was significantly reduced (black asterisks) in several retinal locations compared with WT and Tg P23H Tx, which were similar. The mean thickness in the WT showed a significant increase at 6-mm superior to the disc, whereas the treated Tg P23H Tx remained the same thickness from 2- to 8-mm superior to the disc (gray asterisk). (E) P23H No Tx retinas showed a significant central-to-peripheral thinning of the PRL, while PRL thickness was similar in WT and Tg P23H Tx miniswine embryos. (F) Quantitative measurements of the INL appeared similar across all groups and retinal locations. Scale bar: 20 μm and applies to columns A to C.
The P23H Tx retina was similar to WT over all our outer retinal measures (Figs. 1B, 1D, 1E). In contrast, the P23H untreated (No Tx) retinas showed a central-to-peripheral retinal pattern of ONL and PRL thinning (Figs. 1C, 1D, 1E), similar to our previous study.28 Specifically, across the superior retina of P23H No Tx miniswine embryos (Fig. 1D) the ONL was significantly thinner (black asterisks) up to and including 8 mm compared with either WT or P23H Tx, which were similar. Consistent with ONL thickness, the mean PRL thickness at these same locations differed significantly in the P23H No Tx retina compared with WT or P23H Tx miniswine embryos, which again were similar. Within each group, quantitative measures of the inner nuclear layer (INL; Fig. 1F) were similar at these same locations.
Curcumin Prevents Abnormal Morphological Changes to Rod Photoreceptors
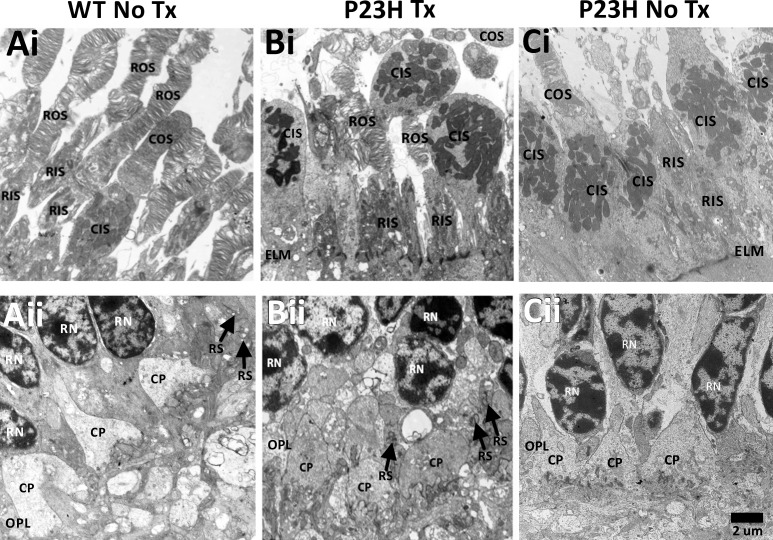
PR morphology was normal in both WT No Tx (Figs. 2Ai, 2Aii) and WT Tx (data not shown) retinas, consistent with our previous observations.28 WT PRs at this embryonic age had inner and outer segments (Fig. 2Ai) and synaptic terminals with triadic profiles (Fig. 2Aii). P23H Tx PRs also had inner and outer segments (Fig. 2Bi) and synaptic terminals with triadic profiles (Fig. 2Bii). In contrast, P23H No Tx rod PRs lacked outer segments (Fig. 2Ci) and spherules with triadic profiles (Fig. 2Cii), consistent with our previous study. Cone PR morphology was normal across all groups.
Figure 2.
Transmission electron micrographs of the PRL (Ai–Ci) and OPL (Aii–Cii). WT (Ai) and P23H Tx (Bi) showed ROS and RIS, and COS and CIS, while P23H No Tx (Ci) lacked identifiable ROS, but did have CIS and COS. The ELM appeared intact in all images. WT (Aii) and P23H Tx (Bi-i) showed RS with triadic profiles (black arrows) and CP in the OPL, while untreated P23H (Cii) lacked RS, but did have CP in the OPL. CIS, cone inner segments; COS, cone outer segments; CP, cone pedicles; ELM, external limiting membrane; OPL, outer plexiform layer; RIS, rod inner segments; RN, rod nuclei; ROS, rod outer segments; RPE, retinal pigment epithelium; RS, rod spherules. Scale bar: 2 μm and applies to all panels.
Curcumin Prevents Mislocalization of Rhodopsin in Rod Photoreceptors
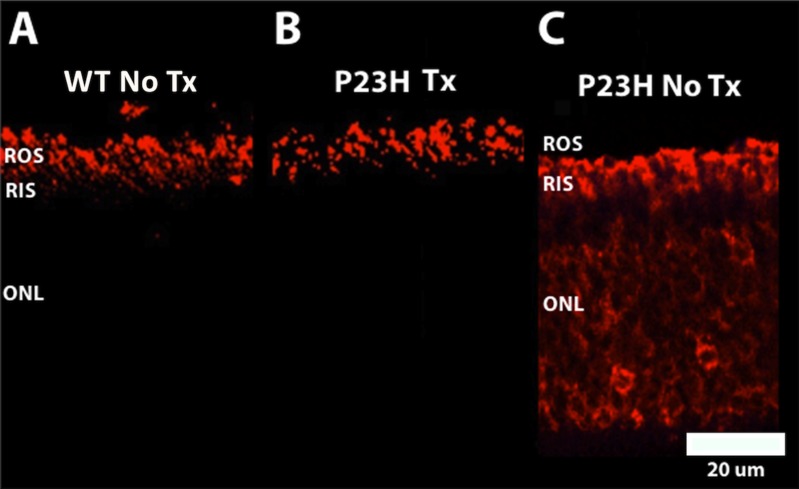
P23H No Tx miniswine embryo rod PR rhodopsin (Fig. 3C) was mislocalized throughout the rod PR cell. Curcumin treatment prevented this mislocalization (Fig. 3B) and rhodopsin expression was confined to rod PR outer segments in all P23H Tx miniswine embryos, similar to their WT littermates (Fig. 3A).
Figure 3.
Representative retinal micrographs of rhodopsin localization detected by immunolabeling with anti-Rho 1D4 (rhodopsin) antibody. WT (A) and P23H Tx (B) showed normal localization of rhodopsin (red labeling) that was confined to the ROS. In contrast, P23H No Tx (C) showed mislocalization of rhodopsin to the RIS and ONL (red labeling). Scale bar: 20 μm and applies to all panels.
Discussion
Our results showed that exposure to curcumin had a neuroprotective effect on rod PRs in P23H miniswine embryos. Because the onset of rod PR degeneration is early in this model we administered curcumin prenatally. Even so, the effects that we observed in curcumin-treated P23H miniswine rod PRs were similar to those reported for curcumin-treated transgenic P23H rats at 70 days of age. We found that curcumin improved rod photoreceptor morphology and prevented abnormal localization of rhodopsin. These similarities suggest that curcumin neuroprotection is likely to be similar across species and age, as long as administration is initiated prior to the onset of rod PR degeneration. Our results support the hypothesis that one of the roles of curcumin neuroprotection is to prevent mistrafficking of mutant rhodopsin protein that results in abnormal expression throughout the rod PR. In our previous studies28,30 we showed that abnormal expression of mutant rhodopsin in P23H miniswine embryos is associated with malformation of rod PR outer segments and spherules, and that P23H piglets never develop rod-driven vision. The amelioration of the morphologic abnormality with curcumin treatment is an important first step because normal rod PRs are a prerequisite to their normal function. Even if normal function is not restored, the presence of intact rod PRs might support cone PR survival and cone driven vision since they may secrete cone survival factors (i.e., rod derived-cone viability factor).31–35
Similar to our results, postnatal delivery of numerous neurotropic compounds alters the progression of photoreceptor degeneration. In the Tg P23H rat, where the onset of photoreceptor degeneration is significantly later compared with the Tg pig.28,30 In addition to curcumin17 other compounds that produce positive results include: safranal,36 cannabinoid agonist HU210,37 tauroursodeoxycholic acid (TUDCA)38 proinsulin,39 Tat-μCL (specific peptide inhibitor of mitochondrial μ-calpain),40 and arimoclomol (heat-shock response coinducer).41 Neither our study nor the work in the rat have explored the long-term effects of these factors. This remains an important issue that needs to be addressed in both models in the future. All of this work indicates that the use of curcumin warrants further investigation as a dietary supplement therapy that may be useful on its own or in conjunction with other therapeutic strategies to preserve vision in RP patients.
Acknowledgements
The authors thank Mr. Doug Emery, Dr. Leslie Sherwood, DVM, and the University of Louisville Large Animal Veterinary Staff for their technical assistance.
Supported by grants from Fight For Sight Grant-In-Aid (PAS); Research to Prevent Blindness, New York City, NY; Kentucky Research Challenge Trust Fund (HJK); KY Science and Engineering Foundation (HJK); EY014701 (MAMc).
Disclosure: P.A. Scott, None; H.J. Kaplan, None; M.A. McCall, None
References
- 1. Berson E. Retinitis pigmentosa: the Friedenwald lecture. Invest Ophthalmol Vis Sci. 1993; 34: 1659–1676. [PubMed] [Google Scholar]
- 2. Milam AH,, Li Z-Y,, Fariss RN. Histopathology of the human retina in retinitis pigmentosa. Prog Retin Eye Res. 1998; 17: 175–205. [DOI] [PubMed] [Google Scholar]
- 3. Berson EL,, Gouras P,, Gunkel RD. Rod response in retinitis pigmentosa dominantly inherited. Arch Ophthalmol. 1968; 80: 58–67. [DOI] [PubMed] [Google Scholar]
- 4. Berson EL,, Simonoff EA. Dominant retinitis pigmentosa with reduced penetrance. Further studies with the electroretinogram. Arch Ophthalmol. 1979; 97: 1286–1291. [DOI] [PubMed] [Google Scholar]
- 5. Berson EL,, Gouras P,, Gunkel RD,, Myrianthopoulos NC. Rod response in sex-linked retinitis pigmentosa. Arch Ophthalmol. 1968; 81: 215–225. [DOI] [PubMed] [Google Scholar]
- 6. Cideciyan AV,, Jacobson SG,, Beltran WA,, et al. Human retinal gene therapy for Leber congenital amaurosis shows advancing retinal degeneration despite enduring visual improvement. Proc Natl Acad Sci. 2013; 110: E517–E525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Simonelli F,, Maguire AM,, Testa F,, et al. Gene therapy for Leber's congenital amaurosis is safe and effective through 1.5 years after vector administration. Mol Ther. 2010; 18: 643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rivas MA,, Vecino E. Animal models and different therapies for treatment of retinitis pigmentosa. Histol Histopathol. 2009; 24: 1295–1322. [DOI] [PubMed] [Google Scholar]
- 9. Chader GJ,, Weiland J,, Humayun MS. Artificial vision: needs functioning, and testing of retinal electronic prosthesis. Prog Brain Res. 2009; 175: 317–332. [DOI] [PubMed] [Google Scholar]
- 10. Rizzo S,, Belting C,, Cinelli L,, et al. The Argus II retinal prosthesis: 12-month outcomes from a single-study center. Am J Ophthalmol. 2014; 157: 1282–1290. [DOI] [PubMed] [Google Scholar]
- 11. Wiley LA,, Burnight ER,, Songstad AE,, et al. Patient-specific induced pluripotent stem cells (iPSCs) for the study and treatment of retinal degenerative diseases. Prog Retin Eye Res. 2015; 44C: 15–35. [DOI] [PubMed] [Google Scholar]
- 12. Burnight ER,, Wiley LA,, Mullins RF,, Stone EM,, Tucker BA. Gene therapy using stem cells [published online ahead of print November 13, 2014]. Cold Spring Harb Perspect Med. doi: http://dx.doi.org/10.1101/cshperspect.a017434. [DOI] [PMC free article] [PubMed]
- 13. He Y,, Zhang Y,, Su G. Recent advances in treatment of retinitis pigmentosa. Curr Stem Cell Res Ther. 2015; 10: 258–265. [DOI] [PubMed] [Google Scholar]
- 14. He Y,, Zhang Y,, Liu X,, Ghazaryan E,, Li Y,, Xie J,, Su G. Recent advances of stem cell therapy for retinitis pigmentosa. Int J Mol Sci. 2014; 15: 14456–14474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Petrs-Silva H,, Linden R. Advances in gene therapy technologies to treat retinitis pigmentosa. Clin Ophthalmol. 2014; 8: 127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garg SJ,, Federman J. Optogenetics, visual prosthesis and electrostimulation for retinal dystrophies. Curr Opin Ophthalmol. 2013; 24; 407–414. [DOI] [PubMed] [Google Scholar]
- 17. Vasireddy V,, Chavali VRM,, Joseph VT,, et al. Rescue of photoreceptor degeneration by curcumin in transgenic rats with P23H rhodopsin mutation. PLoS One. 2011; 6: e21193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dryja TP,, McGee TL,, Reichel E,, et al. A point mutation of the rhodopsin gene in one form of retinitis pigmentosa. Nature. 1990; 343: 364–366. [DOI] [PubMed] [Google Scholar]
- 19. Alfinito PD,, Townes-Anderson E. Activation of mislocalized opsin kills rod cells: a novel mechanism for rod cell death in retinal disease. Proc Natl Acad Sci USA. 2002; 99: 5655–5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sung CH,, Makino C,, Baylor D,, Nathans J. A rhodopsin gene mutation responsible for autosomal dominant retinitis pigmentosa results in protein that is defective in localization to the photoreceptor outer segment. J Neurosci. 1994; 14: 5818–5833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Agarwal N,, Nir I,, Papermaster DS. Expression of opsin and IRBP genes in mutant RCS rats. Exp Eye Res. 1992; 54: 545–554. [DOI] [PubMed] [Google Scholar]
- 22. Roof DJ,, Adamian M,, Hayes A. Rhodopsin accumulation at abnormal sites in retinas of mice with a human P23H rhodopsin transgene. Invest Ophthalmol Vis Sci. 1994; 35: 4049–4062. [PubMed] [Google Scholar]
- 23. Price BA,, Sandoval IM,, Chan F,, Simons DL,, et al. Mislocalization and degradation of human P23H-rhodopsin-GFP in a knockin mouse model of retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2011; 52: 9728–9736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nir I,, Papermaster DS. Immunocytochemial localization of opsin in degenerating photoreceptors of RCS rats and rd and rds mice. Prog Clin Biol Res. 1989; 314: 251–264. [PubMed] [Google Scholar]
- 25. Li YZ,, Wong F. Chang, et al. Rhodopsin transgenic pigs as a model for human retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1998; 39: 808–819. [PubMed] [Google Scholar]
- 26. Tam BM,, Moritz OL. Characterization of rhodopsin P23H-induced retinal degeneration in a Xenopus laevis model of retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2006; 47: 3234–3241. [DOI] [PubMed] [Google Scholar]
- 27. Wang J,, Zhang N,, Beuve A,, Townes-Anderson E. Mislocalized opsin and cAMP signaling: a mechanism for sprouting by rod cells in retinal degeneration. Invest Ophthalmol Vis Sci. 2012; 53: 6355–6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Scott PA,, Fernandez de Castro, JP,, Kaplan HJ,, McCall MAA. Pro23His mutation alters prenatal rod photoreceptor morphology in a transgenic swine model of retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2014; 55: 2452–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ross JW,, Fernandez de Castro JP,, Zhao J,, et al. Generation of an inbred miniature pig model of retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2012; 53: 501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fernandez de Castro JP,, Scott PA,, Fransen JW,, et al. Cone photoreceptors develop normally in the absence of rod photoreceptors in a transgenic swine model of retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2014; 55: 2460–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Léveillard T,, Mohand-Said Lorentz, S,, Hicks D,, et al. Identification and characterization of rod-derived cone viability factor. Nat Genet. 2004; 36: 755–759. [DOI] [PubMed] [Google Scholar]
- 32. Léveillard T,, Sahel JA. Rod-derived cone viability factor for treating blinding diseases: from clinic to redox signaling. Sci Transl Med. 2010; 26:26ps16. [DOI] [PMC free article] [PubMed]
- 33. Yang Y,, Mohand-Said S,, Danan A,, et al. Functional cone rescue by RdCVF protein in a dominant model of retinitis pigmentosa. Mol Ther. 2009; 17: 787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Elachouri G,, Lee-Rivera I,, Clérin E,, et al. Thioredoxin rod-derived cone viability factor protects against photooxidative retinal damage. Free Radic Biol Med. 2015; 81C: 22–29. [DOI] [PubMed] [Google Scholar]
- 35. Byrne LC,, Dalkara D,, Luna G,, et al. Viral-mediated RdCVF and RdCVFL expression protects cone and rod photoreceptors in retinal degeneration. J Clin Invest. 2015; 125: 105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fernández-Sánchez L,, Lax P,, Esquiva G,, Martin-Nieto J,, Pinilla I,, Cuenca N. Safranal a saffron constituent, attenuates retinal degeneration in P23H rats. PLoS One. 2012; 7: e43074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lax P,, Esquiva G,, Altavilla C,, Cuenca N. Neuroprotective effects of the cannabinoid agonist HU210 on retinal degeneration. Exp Eye Res. 2014; 120: 175–185. [DOI] [PubMed] [Google Scholar]
- 38. Fernández-Sánchez L,, Lax P,, Pinilla I,, Martin-Nieto J,, Cuenca N. Tauroursodeoxycholic acid prevents retinal degeneration in transgenic P23H rats. Invest Ophthalmol Vis Sci. 2011; 52: 4998–5008. [DOI] [PubMed] [Google Scholar]
- 39. Fernández-Sánchez L,, Lax P,, Isiegas C,, et al. Proinsulin slows retinal degeneration and vision loss in the P23H rat model of retinitis pigmentosa. Hum Gene Ther. 2012; 23: 1290–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ozaki T,, Ishiguro S,, Hirano S,, et al. Inhibitory peptide of mitochondrial m-calpain protects against photoreceptor degeneration in rhodopsin transgenic S334ter and P23H rats. PLoS One. 2013; 8: e71650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Parfitt DA,, Aguila M,, McCulley CH,, et al. The heat-shock response co-inducer arimoclomol protects against retinal degeneration in rhodopsin retinitis pigmentosa. Cell Death Dis. 2014; 5: e1236. [DOI] [PMC free article] [PubMed] [Google Scholar]