Abstract
Background
Long term use of NSAIDs, opioids and corticosteroids was associated with serious adverse effects. Hence, the search for a safer analgesic and anti-inflammatory agent was always going on. It was considered worthwhile to evaluate analgesic and anti-inflammatory activities of Holoptelea integrifolia and Argyreia speciosa.
Aim
To evaluate analgesic and anti-inflammatory activities of aqueous extract of leaves of Holoptelea Integrifolia and methanolic extract of Argyreia Speciosa root powder in mice and rats.
Materials and Methods
After obtaining permission from animal ethics committee, the animals were divided into 7 groups of 6 animals each {control, standard – ibuprofen 100mg/kg, Holoptelea integrifolia (250 and 500 mg/kg), Argyreia speciosa (100 and 300 mg/kg) and combination of Holoptelea integrifolia (250 mg/kg) and Argyreia speciosa (100 mg/kg)}. The analgesic activity of the extracts was evaluated using tail-flick with radiant heat and acetic acid induced writhing method and the anti-inflammatory activity was evaluated using carrageenan induced paw oedema method.
Statistical Analysis
One-way ANOVA followed by post-hoc test. p < 0.05 was considered to be significant.
Results
In tail-flick method, both Holoptelea integrifolia and Argyreia speciosa produced significant (p<0.05) increase in latency as compared to control, their combination showed a significant increase in latency as compared to control as well as to the standard – ibuprofen. In writhing method, Holoptelea integrifolia and Argyreia speciosa, alone and in combination, significantly decreased the number of writhes as compared to control. In paw oedema method, both Holoptelea integrifolia and Argyreia speciosa showed significant inhibition of paw oedema as compared to control and the activity was comparable to ibuprofen.
Conclusion
Extracts of Holoptelea integrifolia and Argyreia speciosa exhibits significant central and peripheral analgesic and anti-inflammatory activity.
Keywords: Acetic acid, Carrageenan, Plant extracts, Tail flick
Introduction
Pain had been defined as an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage [1]. However, for animals, it is harder to know whether an emotional experience has occurred. Therefore, this concept is often excluded. Pain in animals, as provided by Zimmerman, was an aversive sensory experience caused by actual or potential injury that elicits protective motor and vegetative reactions, results in learned avoidance and may modify species specific behaviour, including social behaviour [2].
Inflammation is a reaction of the body against an aggressive agent, characterized by vasodilatation and access of fluid and cells to the target tissue. One of the major signs of inflammation is the pain that can be triggered by direct stimulation of nociceptors or by the action of inflammatory mediators [3]. Inflammation is fundamentally a protective response the ultimate goal of which is to help the organism get rid of both, the initial cause of injury (e.g. microbes, toxins) and the consequences of such injury (e.g. necrotic cells and tissues) [4].
Though inflammation is a protective phenomenon, sometimes it is uncontrolled and becomes the cause of sufferings like disabilities, contractures, disfiguring of body, involvement of surrounding healthy organs and chronic pain. In such situations inflammation needs to be controlled or suppressed by suitable anti-inflammatory agents [4]. Persistent or chronic pain is one of the major causes for people seeking healthcare. It could significantly interfere with the quality of life and general functioning of the patient. Pain therapies currently available were causing uncomfortable to harmful side effects [5]. Drugs commonly used in modern medicine for suppression of pain and inflammation such as non-steroidal anti-inflammatory drugs (NSAIDs), opioids and corticosteroids provide only symptomatic relief. Also, use of these drugs is associated with serious adverse effects [3]. Hence the search for new, safe and effective analgesic and anti-inflammatory drugs is justified.
Holoptelea integrifolia planch (English- Indian elm, Hindi- Chilbil, Marathi- Vavli) belonging to the family Ulmaceae is a medium sized to large deciduous tree. The tree is distributed throughout India in deciduous forests. Its uses in traditional medicine, including analgesic and anti-inflammatory activities, have been reported in literature. The leaves of this plant were used for skin diseases, obesity, in the management of cancer and for wound healing [6].
Argyreia speciosa Linn f (English- Elephant creeper, Hindi- Samundarsokha, Marathi- Samudrasoka), commonly known as Vridhadaraka is a rasayana herb used in many Ayurvedic preparations in the Indian system of medicine. It is a woody climber distributed throughout India up to an altitude of 300 m. It is cultivated in the gardens as an ornamental plant. Roots of A. speciosa are used in Ayurveda as an aphrodisiac, rejuvenator and as a tonic. Roots are also used in rheumatism, gonorrhea, chronic ulcer and diseases of the nervous system. Previous phytochemical studies reveal the presence of lipids, flavonoids, triterpenes, steroids, phenylpropanoids and coumarins in the plant. Several investigations had proposed that this plant possess analgesic and anti-inflammatory activities [7,8].
It is considered worthwhile to evaluate analgesic and anti-inflammatory activities of traditional Indian plants Holoptelea integrifolia and Argyreia speciosa. Hence, the present study was planned to evaluate and compare analgesic and anti-inflammatory activities of Holoptelea integrifolia and Argyreia speciosa in different animal models of pain and inflammation.
Materials and Methods
The study was conducted after obtaining approval (Reg. no. BJMC/IEC/Pharmac/D0910082-35) by the Institutional Animal Ethics Committee and was performed in accordance with the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) guidelines.
Swiss albino mice of either sex weighing 20-25 g and Wistar albino rats of either sex weighing 180-200 g were procured from central animal house of the institute. Aqueous extract of dried leaves of Holoptelea integrifolia (Sumati Bhai Shah Ayurveda College, Pune) and methanolic extract of roots of Argyreia speciosa (raw material from Upavan Society, Nagpur and extract from The Indian Drugs Research Laboratory, Pune) were used in the study.
For evaluation of analgesic and anti-inflammatory activity, mice and rats were divided into seven groups. Each group consisted of six animals. There were three parameters of evaluation. So, total sample size of animals in the study was 126 (7*6*3 = 126). Mice were used in 2 parameters (so, total 84 mice) and rats were used in 1 parameter (so, total 42 rats).The first group served as control. The second group was used as reference standard (Ibuprofen 100 mg/kg p.o.). Two groups received extract of H. integrifolia at two different doses (250, 500 mg/kg p.o.). Two groups received extract of A. speciosa at two different doses (100, 300 mg/kg p.o.). One group received combination of extracts of H. integrifolia (250 mg/kg p.o.) and A. speciosa (100 mg/kg p.o.). Evaluation of analgesic activity was done using tail flick method and acetic acid induced writhing test both in mice. Evaluation of anti-inflammatory activity was done using carrageenan-induced hind paw oedema in rats.
A) Evaluation of analgesic activity
1) Tail flick method in mice [9]
Analgesic activity was assessed by tail-flick response method using analgesiometer; originally described by D’Amour and Smith in 1941 [9].
Mice, placed on the analgesiometer with tail freely protruding out of the holder, were used for noting down the observations. Observations were taken by placing the middle part of the tail on the radiant heat source, that is, heated nichrome wire with platform. The strength of the current passing through the naked nichrome wire was kept constant at 3 amps. A sharp withdrawal of the tail called as “tail-flick response” was taken as the endpoint of the experiment. The time between placing the tail of the mice on the radiant heat source and the sharp withdrawal of the tail was recorded as “reaction time” [9].
During experimentation each animal was tested 4 times keeping a gap of 5 minutes between the two responses while taking the observations. Mean of all the readings was taken as “basal latency”. The screened animals were marked and kept in different cages.
A cut off time of 10 seconds was imposed in all sets of experiments taken as maximum latency so as to rule out thermal injury. In all the groups, tail-flick test was performed prior to drug administration and at the end of 30, 60, 90 and 120 minutes after drug administration and the reaction time at each time interval (test latency) was calculated.
Percentage maximal analgesia was calculated by using the formula:
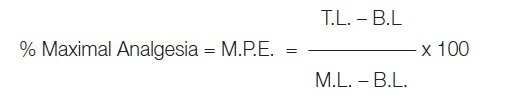 |
Where, M.P.E. = Maximum possible effect.
M.L. = Maximum latency or cut-off time (10 sec)
T.L. = Test latency at the end of particular period of time of testing
B.L. = Basal latency
2) Acetic acid induced writhing test in mice [10]
The writhing model represents a chemical nociceptive test based on the induction of peritonitis like condition in animals by injecting irritant substances intraperitoneally [10].
After 30 minutes of drug administration, 0.1 ml of 1% acetic acid solution was given to mice intraperitoneally (i.p.). The mice were placed individually into glass beakers and five minutes were allowed to elapse. The mice were then observed for a period of ten minutes and the numbers of writhes were recorded for each animal. For scoring purposes, a writhe was indicated by stretching of the abdomen with simultaneous stretching of at least one hind limb. The following formula was used to calculate percentage inhibition [10]:
 |
B) Evaluation of anti-inflammatory activity
1) Carrageenan-induced hind paw oedema in rats [11,12]
The method of Winter, Risley and Nuss (1962) with slight modification was used. Paw oedema was induced by an intradermal injection of 0.1 ml of carrageenan (1% in normal saline) into the plantar surface of the right hind paw of rats.
The acute phase of inflammatory reaction i.e. oedema volume was determined using plethysmometer prior to and 1, 2 and 3 hours after carrageenan injection. All the drugs were administered one hour prior to carrageenan. Percentage inhibition of paw oedema was calculated using the following formula:
 |
Statistical Analysis
Data was analyzed by using Graph Pad Prism software version 5.01. Comparison between different groups was done by ANOVA followed by Bonferroni’s post test for comparison between multiple groups. The p-value less than 0.05 were considered to be statistically significant.
Results
The basal latencies were comparable in all the groups in the tail flick model of analgesia in mice [Table/Fig-1]. Analgesic activity in Holoptelea integrifolia (250 mg/kg) group was observed at 60 and 90 minutes interval, when it was found to be statistically significant as compared to control group (p<0.01). In Holoptelea integrifolia (500 mg/kg) group, analgesic activity was statistically significant when compared with control group (p<0.01). The analgesic activity was maximum at 90 minutes when it was statistically significant as compared to ibuprofen group (p<0.05). Analgesic activity in Argyreia speciosa (100 mg/kg) group was observed at 60 and 90 minutes interval, when it was found to be statistically significant as compared to control group (p<0.01). In Argyreia speciosa (300 mg/kg) group, analgesic activity was statistically significant when compared with control group (p<0.001). The analgesic activity when compared to ibuprofen group was statistically significant at 90 and 120 minutes (p<0.05).Analgesic activity of Holoptelea integrifolia (250 mg/kg) + Argyreia speciosa (100 mg/kg) combination group was found to be statistically significant as compared to control group (p<0.001). When compared to ibuprofen group, the analgesic activity was statistically significant at 60 and 90 minutes (p<0.01). Also, analgesic activity of the combination group was statistically significant as compared to Holoptelea integrifolia group and Argyreia speciosa group (p<0.01).
[Table/Fig-1]:
![[Table/Fig-1]:](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/dfcd/4572971/696c45a758b1/jcdr-9-FF01-g001.jpg)
Effect of different agents on latency to tail flick in radiant heat method of analgesia in mice
Group I – Control
Group II – Ibuprofen
Group III – Holoptelea integrifolia 250 mg/kg
Group IV – Holoptelea integrifolia 500 mg/kg
Group V – Argyreia speciosa 100 mg/kg
Group VI – Argyreia speciosa 300 mg/kg
Group VII – Holoptelea integrifolia 250 mg/kg + Argyreia speciosa 100 mg/kg
At 60 & 90 minutes interval, the Maximum Possible Effect (% maximal analgesia) of the combination group was statistically significant when compared to ibuprofen group, Holoptelea integrifolia (250 and 500 mg/kg) groups and also to Argyreia speciosa (100 and 300 mg/kg) groups (p<0.01) [Table/Fig-2].
[Table/Fig-2]:
![[Table/Fig-2]:](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/dfcd/4572971/d2b8de7b3aaf/jcdr-9-FF01-g002.jpg)
Maximum possible effect (MPE) of agents to tail flick in radiant heat method of analgesia in mice
Group II – Ibuprofen
Group III – Holoptelea integrifolia 250 mg/kg
Group IV – Holoptelea integrifolia 500 mg/kg
Group V – Argyreia speciosa 100 mg/kg
Group VI – Argyreia speciosa 300 mg/kg
Group VII – Holoptelea integrifolia 250 mg/kg + Argyreia speciosa 100 mg/kg
In acetic acid induced writhing model of analgesia, the number of writhes (in 10 minutes) was highest in control group (26.5 ± 0.616) and lowest in ibuprofen group (4.17 ± 0.337). Analgesic activity in Holoptelea integrifolia (250 and 500 mg/kg) groups, Argyreia speciosa (100 and 300 mg/kg) groups and the combination group was statistically significant as compared to control group (p<0.001) [Table/Fig-3].
[Table/Fig-3]:
![[Table/Fig-3]:](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/dfcd/4572971/94699dd7c512/jcdr-9-FF01-g003.jpg)
Effect of different agents in acetic acid induced writhing model of analgesia in mice
Group I – Control
Group II – Ibuprofen
Group III – Holoptelea integrifolia 250 mg/kg
Group IV – Holoptelea integrifolia 500 mg/kg
Group V – Argyreia speciosa 100 mg/kg
Group VI – Argyreia speciosa 300 mg/kg
Group VII – Holoptelea integrifolia 250 mg/kg + Argyreia speciosa 100 mg/kg
Percentage analgesia was highest in ibuprofen group (84.26%), followed by the combination group (72.34%) and was least in Holoptelea integrifolia (250 mg/kg) group (55.36%) and Argyreia speciosa (100 mg/kg) group (60.38%) [Table/Fig-4].
[Table/Fig-4]:
![[Table/Fig-4]:](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/dfcd/4572971/1e7b749f51c3/jcdr-9-FF01-g004.jpg)
Percentage analgesia of agents in acetic acid induced writhing model of analgesia in mice
Group II – Ibuprofen
Group III – Holoptelea integrifolia 250 mg/kg
Group IV – Holoptelea integrifolia 500 mg/kg
Group V – Argyreia speciosa 100 mg/kg
Group VI – Argyreia speciosa 300 mg/kg
Group VII – Holoptelea integrifolia 250 mg/kg + Argyreia speciosa 100 mg/kg
The basal mean paw volume was comparable in all the groups in carrageenan induced paw oedema model in rats [Table/Fig-5]. At 1 and 2 hours, all the groups showed a statistically significant decrease in the paw volume as compared to control group (p<0.01). At 3 hours, all the groups showed a statistically significant decrease in the paw volume as compared to control group (p<0.001). The combination group showed a statistically significant decrease in the paw volume as compared to Holoptelea integrifolia (250 mg/kg) group and Argyreia speciosa (100 mg/kg) group (p<0.01). In addition, Argyreia speciosa (300 mg/kg) group showed a statistically significant decrease in the paw volume as compared to Argyreia speciosa (100 mg/kg) group (p<0.05).
[Table/Fig-5]:
![[Table/Fig-5]:](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/dfcd/4572971/ce088dd9194d/jcdr-9-FF01-g005.jpg)
Effect of different agents on paw volume in carrageenan induced paw oedema in rats
Group I – Control
Group II – Ibuprofen
Group III – Holoptelea integrifolia 250 mg/kg
Group IV – Holoptelea integrifolia 500 mg/kg
Group V – Argyreia speciosa 100 mg/kg
Group VI – Argyreia speciosa 300 mg/kg
Group VII – Holoptelea integrifolia 250 mg/kg + Argyreia speciosa 100 mg/kg
Percentage inhibition of paw oedema at 3 hours interval was calculated. It was observed that the percentage inhibition value of ibuprofen group (51.08%) was greater than the percentage inhibition values of all other groups. Also, the percentage inhibition value of combination group (42.4%) was greater than the percentage inhibition values of Holoptelea integrifolia groups and Argyreia speciosa groups [Table/Fig-6].
[Table/Fig-6]:
![[Table/Fig-6]:](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/dfcd/4572971/e92059596f00/jcdr-9-FF01-g006.jpg)
Percentage inhibition of agents on paw volume in carrageenan
induced paw oedema in rats
Group II – Ibuprofen
Group III – Holoptelea integrifolia 250 mg/kg
Group IV – Holoptelea integrifolia 500 mg/kg
Group V – Argyreia speciosa 100 mg/kg
Group VI – Argyreia speciosa 300 mg/kg
Group VII – Holoptelea integrifolia 250 mg/kg + Argyreia speciosa 100 mg/kg
Discussion
Natural products are a source for bioactive compounds and have potential for developing some novel therapeutic agents. Holoptelea integrifolia and Argyreia speciosa possess many therapeutic activities. More recently there has been an interest in their analgesic and anti-inflammatory activities following reports about their ability to relieve pain and inflammation [6,7,13,14].
The tail flick method of analgesia is very effective in estimating the efficacy and potency of centrally acting analgesic drugs. In this study, both the extracts, when used in higher doses and when combined increased the pain threshold significantly which was comparable to ibuprofen [Table/Fig-1]. Also, the combination group had maximal analgesic activity [Table/Fig-2].
The acetic acid induced writhing model mainly evaluates peripherally acting analgesics. In this study, both the extracts had statistically significant analgesic activity [Table/Fig-3]. But maximal analgesic activity was seen in ibuprofen and the combination group [Table/Fig-4].
Similar results were reported in studies by Bachhav et al., Rizwani et al., and Jeet et al., [7,15,16]. Also, crude extract of Holoptelea integrifolia leaves are reported to contain alkaloids, flavonoids, phenol, steroids, tannins and triterpenoids. In study by Rizwani et al, no acute oral toxicity was observed and extract was considered safe up to dose of 2000 mg/kg [15].
The extracts of Argyreia speciosa are reported to contain fixed oils, fats, phytosterols, glycosides, flavonoids, alkaloids, tannins and phenolic compounds. In study by Jeet et al., the extract was found to be safe up to the dose of 3000 mg/kg [16].
The standard experimental model of acute inflammation is the carrageenan induced hind paw oedema, where it shows a biphasic response [17]. In this study, both the extracts significantly inhibited paw oedema [Table/Fig-5]. Maximum inhibition was observed in the ibuprofen group and the combination group [Table/Fig-6].
Similar results were reported in studies by Sharma et al., Gokhale et al., Sharma Sonu et al., Galani et al and Jeet et al., [13,14,18–20].
Implications
Extracts of Holoptelea integrifolia and Argyreia speciosa demonstrated analgesic and anti-inflammatory activities.
Limitations of the Study
In the present study, we did not investigate the active principle responsible for the analgesic and anti-inflammatory activities of the extracts.
Conclusion
The present study supported the use of Holoptelea integrifolia (aqueous extract of dried leaves) and Argyreia speciosa (methanolic extract of roots) in painful and inflammatory conditions. However, clinical studies are required. Further studies needs to be done to identify the exact active principle which may result in the development of potent analgesic and anti-inflammatory agents.
Acknowledgments
Sumati Bhai Shah Ayurveda College, Pune for providing with the aqueous extract of dried leaves of Holoptelea integrifolia.
Upavan Society, Nagpurfor providing with the roots of Argyreia speciosa.
The Indian Drugs Research Laboratory, Pune for preparing the methanolic extract of roots of Argyreia speciosa.
Financial or Other Competing Interests
None.
References
- [1].Merskey H, Albe-Fessard DG, Bonica JJ, Carmon A, Dubner R, Kerr FWL. Pain terms: a list with definitions and notes on usage. Recommended by the IASP Subcommittee on Taxonomy. Pain. 1979;6:249–52. [PubMed] [Google Scholar]
- [2].Zimmerman M. Physiological mechanisms of pain and its treatment. Klinische Anaesthesiol Intensivether. 1986;32:1–19. [Google Scholar]
- [3].Temponi VDS, da Silva JB, Alves MS, Ribeiro A, de Pinho JRG, Yamamoto CH, et al. Antinociceptive and anti-inflammatory effects of ethanol extract from Vernoniapolyanthes leaves in rodents. Int J Mol Sci. 2012;13(3):3887–99. doi: 10.3390/ijms13033887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kumar V, Abbas AK, Fausto N, Aster JC. Pathologic basis of disease. 8th ed. New Delhi, India: Elsevier Publications; 2010. [Google Scholar]
- [5].Stucky CL, Gold MS, Zhang X. Mechanisms of pain. Proc Natl Acad Sci. 2001;98(21):11845–46. doi: 10.1073/pnas.211373398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kalpana, Upadhyay A. Anti-inflammatory evaluation of ethanolic extract of leaves of Holoptelea integrifolia, Planch. Ann Biol Res. 2010;1(2):185–95. [Google Scholar]
- [7].Bachhav RS, Gulecha VS, Upasani CD. Analgesic and anti-inflammatory activity of Argyreia speciosa root. Indian J Pharmacol. 2009;41(4):158–61. doi: 10.4103/0253-7613.56066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Galani VJ, Patel BG. Psychotropic activity of Argyreia speciosa roots in experimental animals. Ayu. 2011;32(3):380–84. doi: 10.4103/0974-8520.93919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Parmer NS, Prakash S. Screening Methods in Pharmacology. New Delhi, India: Narosa Publishing House; 2006. Evaluation of analgesics, anti-inflammatory and anti-pyretic activity. In: Parmar NS, editor; pp. 232–33. [Google Scholar]
- [10].Parmer NS, Prakash S. Screening Methods in Pharmacology. New Delhi, India: Narosa Publishing House; 2006. Evaluation of analgesics, anti-inflammatory and anti-pyretic activity. In: Parmar NS, editor; pp. 225–26. [Google Scholar]
- [11].Winter CA, Risley EA, Nuss GW. Carrageenin induced oedema in hind paw of the rat as an assay for anti-inflammatory drugs. Proc Soc Exp Biol Med. 1962;111:544–47. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- [12].Singh H, Ghosh MN. Modified plethysmometer for measuring foot volume of unanaesthetized rats. J Pharm Pharmacol. 1968;20(4):316–17. doi: 10.1111/j.2042-7158.1968.tb09747.x. [DOI] [PubMed] [Google Scholar]
- [13].Sharma S, Lakshmi KS, Patidar A, Chaudhary A, Dhaker S. Studies on anti-inflammatory effect of aqueous extract of leaves of Holoptelea integrifolia, Planchin rats. Indian JPharmacol. 2009;41(2):87–88. doi: 10.4103/0253-7613.51348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gokhale AB, Damre AS, Kulkarni KR, Saraf MN. Preliminary evaluation of anti-inflammatory and anti-arthritic activity of S. lappa, A. speciosa and A. aspera. Phytomed. 2002;9(5):433–37. doi: 10.1078/09447110260571689. [DOI] [PubMed] [Google Scholar]
- [15].Rizwani GH, Mahmud S, Shareef H, Perveen R, Ahmed M. Analgesic activity of various extracts of Holoptelea integrifolia (Roxb.) Planch leaves. Pak J Pharm Sci. 2012;25(3):629–32. [PubMed] [Google Scholar]
- [16].Jeet K, Thakur R, Choudhary S, Shukla A, Sharma AK. In vivo analgesic activity of whole aerial part – Argyreia Nervosa. Int J Phytopharmacol. 2012;3(3):221–25. [Google Scholar]
- [17].Vinegar R, Schreiber W, Hugo R. Biphasic development of carrageenin oedema in rats. J PharmacolExpTher. 1969;166(1):96–103. [PubMed] [Google Scholar]
- [18].Sharma S, Rai V, Kapoor B, Rai SB. Phytochemical screening and evaluation of anti-inflammatory activity of leaves extract of Holoptelea integrifolia roxb. IJRPS. 2011;1(1):76–87. [Google Scholar]
- [19].Galani VJ, Patel BG. Analgesic and anti-inflammatory activity of Argyreia speciosa and Sphearanthusindicus in the experimental animals. Global J Pharmacol. 2011;5(1):54–59. [Google Scholar]
- [20].Jeet K, Thakur R. Evaluation of anti-inflammatory activity of whole aerial part – Argyreia nervosa. Int J Pharm Bio Sci. 2012;3(4):150–54. [Google Scholar]


