Abstract
BACKGROUND
While a number of pharmacological interventions exist for the treatment of opioid use disorder, evidence evaluating the effect of pain on substance use behavior, attrition rate, and physical or mental health among these therapies has not been well established. We aim to evaluate these effects using evidence gathered from a systematic review of studies evaluating chronic non-cancer pain (CNCP) in patients with opioid use disorder.
METHODS
We searched the Medline, EMBASE, PubMed, PsycINFO, Web of Science, Cochrane Database of Systematic Reviews, ProQuest Dissertations and theses Database, Cochrane Central Register of Controlled Trials, World Health Organization International Clinical Trials Registry Platform Search Portal, and National Institutes for Health Clinical Trials Registry databases to identify articles evaluating the impact of pain on addiction treatment outcomes for patients maintained on opioid agonist therapy.
RESULTS
Upon screening 3,540 articles, 14 studies with a combined sample of 3,128 patients fulfilled the review inclusion criteria. Results from the meta-analysis suggest that pain has no effect on illicit opioid consumption [pooled odds ratio (pOR): 0.70, 95%CI 0.41–1.17; I2 = 0.0] but a protective effect for reducing illicit non-opioid substance use (pOR: 0.57, 95%CI 0.41–0.79; I2 = 0.0). Studies evaluating illicit opioid consumption using other measures demonstrate pain to increase the risk for opioid abuse. Pain is significantly associated with the presence of psychiatric disorders (pOR: 2.18; 95%CI 1.6, 2.9; I2 = 0.0%).
CONCLUSION
CNCP may increase risk for continued opioid abuse and poor psychiatric functioning. Qualitative synthesis of the findings suggests that major methodological differences in the design and measurement of pain and treatment response outcomes are likely impacting the effect estimates.
Keywords: opioid use disorder, chronic pain, opioid substitution therapy, systematic review, guidelines, meta-analysis
Introduction
Chronic non-cancer pain (CNCP) is characterized as a significant pain lasting longer than the standard healing time and that is not directly caused by malignancy.1 There is limited evidence to support the effectiveness of opioids for providing long-term pain relief;2 however, they remain the most commonly employed intervention for managing CNCP.3 This is of concern because of the global rise in opioid-related medication diversion, morbidity, and mortality.4–8 While many trials have evaluated the effectiveness of opioid agonist therapies (OATs) for patients with addiction,9–42 to our knowledge none provides an analysis or discussion as to the mediating effects of pain on substance use behavior, treatment retention, or other patient-important outcomes. Even among the oldest and most commonly employed OAT, namely methadone maintenance treatment (MMT), there exists conflicting evidence that both implicates and refutes the role of chronic pain as a risk for continued opioid abuse.43–47 The management of patients with opioid use disorder poses many challenges, particularly among patients suffering with comorbid psychiatric and physical disorders. For instance, patients with opioid addiction together with comorbid depression are suggested to be at high risk for overdose48 and suicide attempts.49 Efforts to combine the evidence evaluating important risk factors for adverse outcomes in the management of opioid use disorder will prove critical for enhancing our understanding of this complex disorder that is impacted by large variability in treatment effectiveness and prognosis.
A number of OSTs exist, including MMT, levomethadyl acetate (LAAM), and buprenorphine/naloxone. However, the impact of pain on the effectiveness of these therapies among outcomes such as attrition rate, substance use behavior, and physical or mental health has not been well established, leaving many questions unanswered: Are patients with pain responding poorly to opioid maintenance treatment? Is there evidence demonstrating superiority of any OAT in the subpopulation of addiction patients with comorbid pain? We will attempt to answer these questions using evidence gathered from a systematic review of all studies evaluating CNCP in the patient population with opioid use disorder. Findings from this review will serve to provide consensus in establishing whether CNCP is an important risk factor for patients on OST, distinguish the best available OAT treatment for patients with CNCP, and provide an evidence-based knowledge synthesis to enable clinicians managing opioid-dependent and CNCP patients to evaluate risk factors for poor prognosis and tailor treatments accordingly.
Objectives
We aim to 1) evaluate the impact of CNCP on substance use behavior, physical health, psychiatric symptoms, as well as personal and social functioning; 2) determine whether any OAT demonstrates superiority or shows significant benefit for patients with opioid use disorder reporting comorbid pain; 3) provided the data are suitable, combine the evidence from direct and indirect comparisons using network meta-analysis; and 4) identify the most recently published opioid maintenance treatment guidelines from the US, Canada, and the UK to determine how the evidence is being translated into clinical practice for managing chronic pain associated with opioid use disorder.
Research question(s)
Among patients with opioid use disorder being treated with (or randomized to) opioid substitution treatment (OST)
Does CNCP impact OAT outcomes?
-
Which OAT is most effective for improving treatment response in patients with comorbid CNCP?
(Treatment response will be defined by improvements in substance use behavior, physical health, psychiatric symptoms, as well as personal and social functioning.)
Do the most recently published Canadian, American, and UK OAT clinical practice guidelines capture pain as an important factor in opioid use disorder and properly translate the evidence obtained from the studies evaluated in this review?
Materials and Methods
Systematic review
The methods of this systematic review are published50 and registered with PROSPERO (ID: CRD42014014015). Briefly, we performed a systematic review to identify all studies evaluating the impact of chronic pain on different treatment outcomes within the patient population with opioid use disorder. We searched Medline, EMBASE, PubMed, PsycINFO, Web of Science, Cochrane Library, ProQuest Dissertations and theses Database, Cochrane Clinical Trials Registry, World Health Organization (WHO) International Clinical Trials Registry Platform Search Portal, and the National Institutes for Health (NIH) Clinical Trials Registry databases. We searched the Cochrane Library to identify relevant systematic reviews of the topic. The electronic search strategies for each database are presented in the published protocol.50 Independent reviewers later hand-searched reference lists from these reviews for any missed studies. We screened the title, abstract, and full-text articles in duplicate. We report the kappa statistic to demonstrate the level of agreement between reviewers.51
To be included in this review, studies were required to assess the impact of pain on any of the following treatment outcomes: physical, psychological, or social outcomes for patients receiving opioid agonist or antagonist substitution therapy for opioid use disorder. Study participants were required to be on a maintenance therapy for the opioid use disorder. Studies evaluating patients on OAT for the treatment of pain and not opioid use disorder (eg, methadone for pain) were not eligible for this review. While our search did not place any language or time restrictions on retrieved articles, the search was restricted to human studies. We evaluated observational studies using two risk-of-bias tools: cross-sectional studies using the NIH National Heart, Lung, and Blood Institute: Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies,52 and cohort studies using the Newcastle Ottawa Scale.53 We evaluated randomized trials using the Cochrane risk-of-bias tool.54 We assessed the strength of the evidence summarized in this review using Grading of Recommendations Assessment, Development and Evaluation (GRADE).55
Independent reviewers performed full-text extraction in duplicate using pilot-tested data extraction forms. We extracted from each study the following information: author, date of publication, journal of publication, number of study participants, type of population (clinical, incarcerated, pregnant), eligibility criteria, type of OST(s), OAT dose (by chronic pain status), definition of chronic pain, identification of the study primary outcome, definition of treatment response outcome(s), measurement of chronic pain, measurement of response outcome(s), percentage/number of participants with chronic pain, number of patients on prescribed opioids or adjunct pain therapies, statistical analysis performed, study findings, overall statistical findings, factors associated with treatment response (if reported), and authors’ conclusions.
A flow diagram detailing the article selection process as well as detailed tables reporting the key methods and conclusions of studies deemed eligible for this review are reported in accordance with the meta-analysis of observational studies in epidemiology (MOOSE) and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guidelines.56,57
Guideline assessment
We aimed to evaluate how current evidence for managing patients with CNCP is translated to practice and to determine whether current guidelines are using evidence from the studies identified in this review. To identify Canadian and American opioid maintenance treatment guidelines, we searched http://www.guidelines.gov for with the terms “opioid addiction,” “opioid dependence,” “opioid use disorder,” and “opioid substitution treatment.” To identify the most recently published UK guidelines, we searched the National Institute for Health and Care Excellence (NICE) using key words “opioid use disorder,” “methadone,” “opioid use disorder,” “buprenorphine” “naltrexone,” and “opioid dependence.” We extracted specific information including the year of publication, guideline objectives, any information on pain population subgroups, evidence cited by guideline for managing patients with comorbid pain, and any cautions regarding specialized populations.
We intended to evaluate each guideline using the rigor of development and applicability domains from the Appraisal of Guidelines for Research and Evaluation II (AGREE) Instrument. AGREE II is a validated instrument used for the quality assessment of clinical guidelines.58,59 In its entirety, the tool has 23 items organized across six quality domains.58 Our major objective using these guidelines was to distinguish the best quality guidelines by assessing how evidence is being incorporated into guideline development. As such, we only assessed these guidelines on the basis of the rigor of development and applicability domains.
Statistical analysis
Due to the large variations in the definition and measurement of outcomes reported across studies leading to insufficient data to complete a network meta-analysis, we summarize the results of all direct comparisons in this review narratively and statistically where appropriate.
Qualitative summary
Due to the large variations in the definition and measurement of outcomes reported across studies, we chose to provide a qualitative summary for each outcome. We provide a detailed summary of all results according to broader themes that appropriately capture the behavior or attribute of interest. For instance, substance use behavior can capture a wide array of specifically defined and measured outcomes. Whether it is the number of days of crack/cocaine use over the past month or the percentage of participants reporting non-opioid substance abuse, the broader category of illicit substance use adequately captures this behavior. We have chosen a list of categories generated from a larger systematic review of OAT effectiveness,50 which organized outcomes collected from 60 trials into broader domains proposed by commonly used addiction severity indices [ie, the Addiction Severity Index (ASI)60 and Maudsley Addiction Profile (MAP)].61 The identified outcome domains included physical health, psychiatric health and symptoms, abstinence and substance use behavior, personal and social functioning, global quality of life and addiction severity assessments (including global addiction severity measure scores), intervention adherence, acceptance of intervention, and resource utilization (eg, hospital admission).
A summary-of-findings table is presented to demonstrate the impact of pain across each outcome domain. The additional “Findings” column details our conclusions based on the available evidence. To reach a valid conclusion, we decided a priori on the following criterion: ≥50% of the studies for a single intervention (methadone, buprenorphine) must demonstrate a harmful or beneficial effect of pain on the outcome. If less than 50% of the studies demonstrated such an effect, we concluded there was not enough evidence.
Quantitative summary
We conducted meta-analyses using a random-effects model to address the following outcomes: illicit opioid use, illicit substance use, and presence of psychiatric illness. Each of these outcomes was measured as binary variables, whereby the studies provided the number/percentage of participants who reported using opioids, other substances, or a history of psychiatric illness. Since each of our outcomes used for the meta-analysis was dichotomous, we present the summary estimates as pooled odds with 95% confidence intervals. We employed the Mantel–Haenszel method for pooling the results of binary variables, as this method provides the option to estimate between study variations by assessing each study’s final results to a Mantel–Haenszel fixed-effect meta-analysis estimate.62 The results for each meta-analysis are presented in separate forest plots. Due to the small number of studies included in each meta-analysis (maximum of 3), we chose not to assess for publication bias using Egger’s plot. We used the inconsistency index (I2) statistic to determine the level of heterogeneity in the results of the studies, using the I2 values of 0%–40% (might not be important), 30%–60% (moderate heterogeneity), 50%–90% (substantial heterogeneity), and 75%–100% (considerable heterogeneity) as the categorizations set forth by the Cochrane Collaboration.63
As discussed in the published protocol, we anticipated the studies’ quality assessment to important risk of bias assessment items (items assessing adjustment for confounding) as well as differences in measurement selection to be important factors contributing to heterogeneity between studies.50 Our a priori hypotheses for heterogeneity between studies have been previously summarized in detail.50 However, the number of studies eligible for inclusion in the meta-analysis was small enough (n ≤ 3) so that the use of subgroup analyses would be deemed inappropriate.
Results
Study characteristics
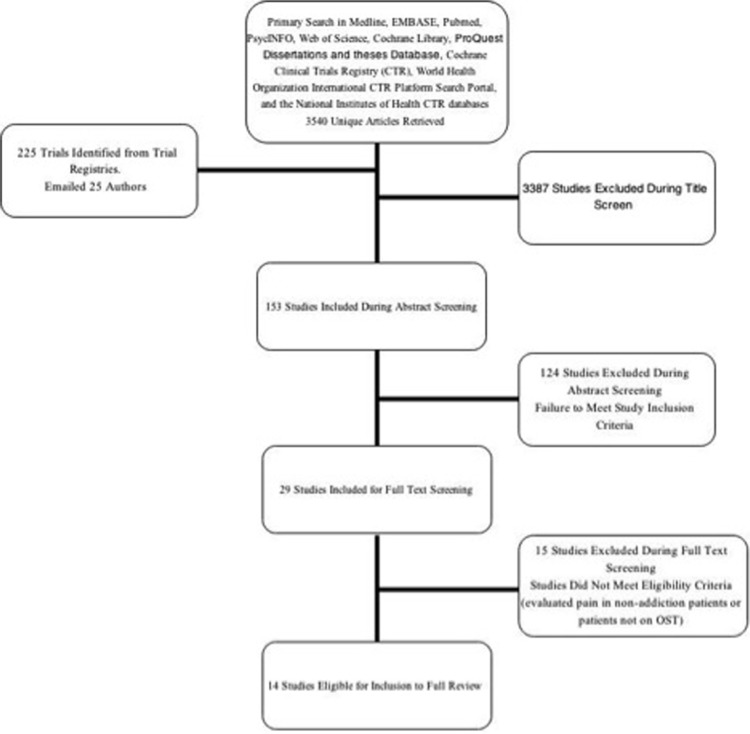
Upon searching seven databases and three clinical trial registries, we reviewed 3,540 unique articles. Independent reviewers screened the title [Kappa (K):0.51, SE 0.04; 95%CI 0.43–0.58), abstract (K:0.41, SE:0.09; 95%CI 0.24–0.58), and full-text articles (K:0.77, SE 0.12; 95%CI 0.53–1.0) with moderate agreement. We identified 14 articles eligible for inclusion in this review.43–45,64–74 Figure 1 provides a flow diagram detailing the screening process at each stage of the literature search.
Figure 1.
Flow diagram of the study selection process.
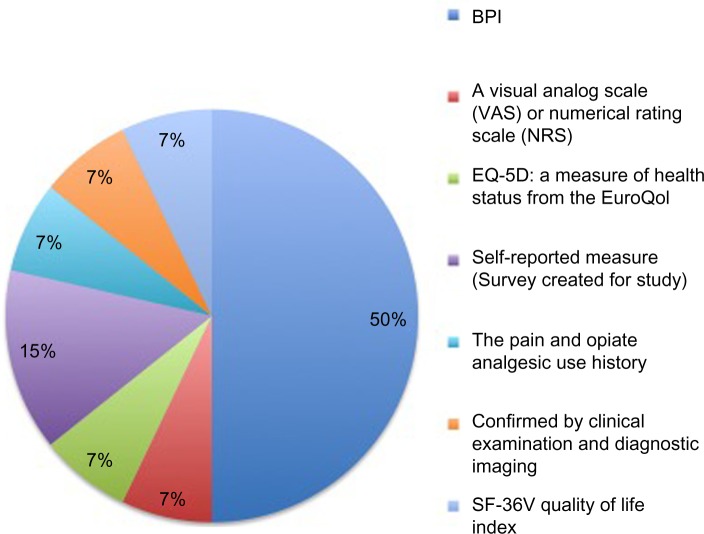
Across a combined population of 3,128 patients, the included studies evaluated the impact of pain on different treatment response outcomes for high-dose methadone (≥60 mg/day), low-dose methadone (<60 mg/day), high-dose LAAM (≥85 mg/day), low-dose LAAM (<85 mg/day), high-dose buprenorphine (≥16 mg/day), low-dose buprenorphine (<16 mg/day), high-dose Suboxone® (buprenorphine ≥16 mg/day + naloxone), and low-dose Suboxone® (buprenorphine <16 mg/day + naloxone). The studies used a range of epidemiologic designs including cross-sectional, randomized controlled trial and prospective cohort. Details of the design characteristics of individual studies including intervention dose, number of participants, mean age of participants, as well as the definitions and measurements used for chronic pain are summarized in Table 1. While the majority of studies used the Brief Pain Inventory (BPI)43,64–66,70,71,74 to measure pain (Fig. 2), the definitions and cut-offs used to determine pain varied greatly, with some studies providing unclear descriptions of both the measurement and definition of pain.69,72 Although some studies report excluding patients using adjunct pain therapies including opioid medication,45,68 the majority of studies neither reported43,44,64,66,69–71,73,74 nor adjusted65,67,72 for the use of adjunct pain therapies including opioids (Table 1).
Table 1.
Description of study design characteristics.
| AUTHOR LAST NAME | YEAR OF PUBLICATION | NUMBER OF PARTICIPANTS | STUDY DESIGN | INTERVENTION(S) EVALUATED | HOW WAS CHRONIC PAIN MEASURED? | HOW WAS CHRONIC PAIN DEFINED | PATIENTS ON PRESCRIBED OPIOIDS OR ADJUNCT PAIN TREATMENTS |
|---|---|---|---|---|---|---|---|
| Peles | 2005 | 170 | Cross-sectional | High-dose methadone (≥60 mg/day) | BPI | Current pain that lasted for at least 6 months | Not reported |
| Dhingra | 2012 | 489 | Cross-sectional | High-dose methadone (≥60 mg/day) | BPI | “Clinically significant pain” was defined by an average pain intensity during the past week of >5 or an average pain interference score during the past week of >5. | 82% of the sample is receiving an adjunct pain therapy (not reported by pain status) |
| Barry | 2009 | 150 | Cross-sectional | High-dose methadone (≥60 mg/day) | BPI | Respondents’ answers to BPI items were used to classify them into one of three pain groups: a) “chronic severe pain” (ie, pain lasting at least 6 months with moderate to severe pain intensity or significant pain interference, respondents who had pain lasting at least 6 months and who scored 5 or higher on the item pertaining to the worst pain intensity in the last 7 days or on any of the items relating to pain interference in the last 7 days were considered to exhibit chronic severe pain; b) “some pain” (ie, pain reported in past week but not CSP); and c) “no pain” (ie, no pain reported in the past week and no CSP). | Not reported |
| Bounes | 2013 | 151 | Cohort study (prospective or retrospective) | Low-dose methadone (<60 mg/day), Low-dose buprenorphine (<16 mg/day) | A Visual Analog Scale (VAS) or Numerical Rating Scale (NRS) was used to assess and quantify the intensity of acute pain at the time of admission, after pain management, and just before hospital discharge. | Acute pain scores rated from 0 to 10 were obtained indiscriminately from one or the other measurement tool. Acute pain exposure was defined as a pain score greater than 0 at the time of admission on any of the rating scales. | Patients without pain: 0% Patients with pain: 5% Total sample: 3% |
| Chakrabarti | 2010 | 69 | Cross-sectional | High-dose Suboxone® (buprenorphine ≥16 mg/day + naloxone) | EQ-5D: a measure of health status from the EuroQol | Degree of pain 1 week before induction, measured as pain or discomfort experienced “today” and coded as 0 = no pain, 1 = some pain, or 2 = extreme pain | Not explicitly reported (although they removed any patients with prescription medications which may interfere with dosing) |
| Dennis | 2014 | 235 | Cross-sectional | High-dose methadone (≥60 mg/day) | Self-report | Participants were categorized as having chronic and/or comorbid pain if they indicated they were currently experiencing or have been diagnosed with chronic pain | Participants on prescribed opioids were removed from analyses |
| Dreifuss | 2013 | 360 | Cross-sectional | High-dose Suboxone® (buprenorphine ≥16 mg/day + naloxone) | The pain and analgesic use history opiate | Not described | Not reported (only brief discussion in study selection criteria that they included patients currently prescribed opioids with approval of attending physician) |
| Dunn | 2014 | 227 | Cross-sectional | High-dose methadone (≥60 mg/day) | BPI | Chronic pain was defined as endorsing question 1 of the BPI, which asked, “Have you had pain other than everyday kinds of pain today?” | Not reported |
| Fox | 2012 | 82 | Cohort study (prospective or retrospective) | High-dose buprenorphine (≥16 mg/day), low-dose buprenorphine (<16 mg/day) | BPI | The Brief Pain Inventory (BPI) asked: “Please rate your pain during the last week by selecting the one number that best describes your pain on the average.” Participants were given a visual analog scale from 0 to 10, with 0 labeled as “no pain” and 10 as “pain as bad as you can imagine.” Similar to prior studies, participants reporting pain scores of ≥5 at the initial interview were considered to have “baseline pain”; those reporting pain scores of ≥5 at all follow-up visits were considered to have “persistent pain” | No participants were on prescribed opioids for pain |
| Jaimison | 2000 | 248 | Cross-sectional | High-dose methadone (≥60 mg/day), low-dose methadone (<60 mg/day) | Self-reported measure (Survey created for study) | Not described | 77% of sample report being prescribed medications for pain, itemized list of different adjunct therapies is provided (summarized for patients with and without pain). Although no adjusted analyses are performed to evaluate the mediating effects of these on study outcomes |
| Neumann | 2013 | 54 | Randomized controlled trial | Low-dose methadone (<60 mg/day), low-dose Suboxone® (buprenorphine <16 mg/day + naloxone) | Confirmed by clinical examination and diagnostic imaging | The diagnosis of a chronic pain condition originating from the spine or large joints was confirmed by clinical examination and the use of diagnostic imaging (eg, radiographs, computed tomography scan, magnetic resonance imaging) | Not reported (patients advised not to continue taking prescribed opioids during course of study) |
| Potter | 2015 | 252 | Cohort study (prospective or retrospective) | Low-dose Suboxone® (buprenorphine <16 mg/day + naloxone) | BPI | Not described | Not reported (only brief discussion in study selection criteria that they included patients currently prescribed opioids with approval of attending physician) |
| Rosenblum | 2003 | 390 | Cross-sectional | High-dose methadone (≥60 mg/day) | BPI | To operationally define a subpopulation of patients with chronic pain that was relatively likely to be clinically significant, an index of “chronic severe pain” was defined as a score of 5 or higher on the BPI item “worst pain in the past week” or of 5 or higher on the BPI pain interference scale, and pain duration for at least 6 months. | Not reported by pain status |
| Trafton | 2004 | 251 | Cross-sectional | High-dose methadone (≥60 mg/day), low-dose methadone (<60 mg/day), high-dose levoacetylmethadol (LAAM) (≥85 mg/day), low-dose levomethadyl acetate hydrochloride (LAAM) (<85 mg/day) | SF-36V Quality-of-Life Index | Reported pain levels were taken from answers to the SF-36V question “How much body pain have you experienced in the last 4 weeks?” Patients answered either “none” (n = 45), “very mild” (n = 28), “mild” (n = 48), “moderate” (n = 60), “severe” (n = 56) or “very severe” (n = 13). For analyses patients were split into those reporting none to mild pain (no-pain group, n = 121) and those reporting moderate to very severe pain (pain group, n = 129). |
Not reported |
Figure 2.
Types of pain measures used across studies.
Using the outcome domain categorizations described earlier, we found the majority of studies evaluated the effects of pain on abstinence from illicit opioids and other substance use related outcomes. Figure 3 provides a summary of all outcome domains with the corresponding number of studies reporting each outcome.
Figure 3.
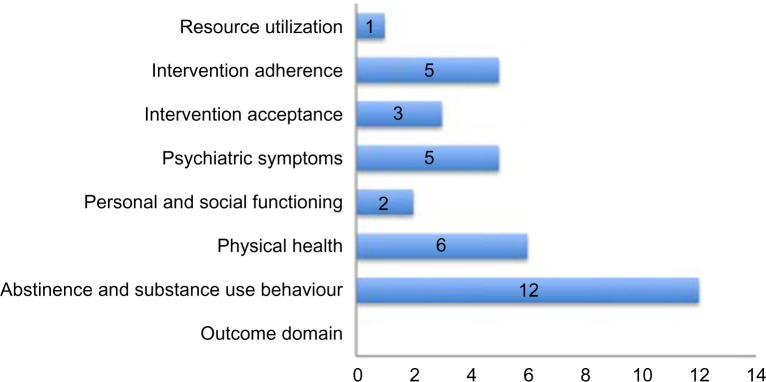
Outcomes evaluated across studies.
Risk of bias assessment
The risk of bias assessment was performed using three instruments52,54 across cross-sectional, cohort, and randomized studies. Results from the quality assessment are summarized in Supplementary Tables 1–3. The majority of studies suffer from a high risk of bias due to the lack of reporting on important issues such as follow-up, missing data, and blinding (Supplementary Tables 1–3). The majority of studies used a cross-sectional design (k = 10) to assess the association between the presence of pain and OAT treatment outcome, while only half of the studies (k = 5) established a “dose–response” relationship between pain severity and treatment outcome, suggesting that an increase in the intensity of the exposure (pain) is associated with an increase in opioid consumption (Supplementary Table 1).
Abstinence and substance use behavior
Illicit opioid use
Among the 14 studies included in this review, 12 evaluated the impact of chronic pain on illicit opioid use behavior. The measurements, definitions, and statistical methodology used to evaluate opioid use are described in Table 2. The majority of studies measured opioid use behavior using urine toxicology screening (Table 2).45,64,65,68–70,73,74 However, some studies relied on a self-report tool generated for the study or the Addiction Severity Index (ASI) to determine the frequency of opioid use.43,44,66,71 We were unable to combine the results of the majority of studies evaluating the same intervention (eg, methadone) because of the large variations in defining illicit opioid use behavior. While some studies reported the number of patients using illicit opioids (separated by pain status),43,45,64–66,68,69,71,73 others chose to report the number of days of illicit opioid use44 as well as the percentage or mean percent of positive opioid screens reported by chronic pain status.45,70 Of the 12 studies that evaluated illicit opioid use behavior, only 2 reported a significant effect of pain on opioid consumption,44,45 whereby both studies were performed in MMT patients and use different measures to assess opioid use behavior. Despite differences in measurements and interventions (eg, methadone, buprenorphine), the majority of studies reported no effect of pain on illicit opioid use.43,64–66,68–71,73
Table 2.
Summary of findings for illicit opioid use outcome.
| AUTHOR LAST NAME | WAS ILLICIT OPIOID ABUSE AN OUTCOME? | WAS ILLICIT OPIOID USE BEHAVIOR THE PRIMARY OUTCOME OF THE STUDY? | HOW WAS ILLICIT OPIOID ABUSE MEASURED? | DEFINITION OF ILLICIT OPIOID USE | OUTCOMES BY CHRONIC PAIN STATUS | STATISTICAL ANALYSIS | PROPORTION OF OPIOID USE OUTCOMES SHOWING PAIN TO IMPACT ILLICIT OPIOID USE BEHAVIOR | STUDY FINDINGS: DID PATIENTS CHARACTERIZED AS HAVING PAIN ALSO HAVE SIGNIFI-CANTLY HIGHER RATES OF ILLICIT OPIOID USE? |
|---|---|---|---|---|---|---|---|---|
| Peles | Yes | No | Urine toxicology screening | Participants were categorized as using opioids if ≥1 urine test in the month preceding the survey was positive. | Chronic pain 15 (16%) positive, non-chronic pain 20 (26.3%) | Chi-square | 0 | No |
| Dhingra | Yes | No | Urine toxicology screening, Self-report | A positive urine toxicology screen or indication by self-report as assessed by the ASI past 30 day drug use history. | In univariate analyses, neither UDS nor self-reported drug use on the ASI was statistically associated with clinically significant pain. (report P-values) | t-Test, Chi-square | 0 | No |
| Barry | Yes | No | Self-report | Participants reported any use in the past week, this was then analyzed as a binary variable. | The pain groups reported comparable levels of psychoactive substance use, illegal drug use and non-medical use of prescription drug in the past week. No specific percentages are reported per group. | ANOVA | 0 | No |
| Chakrabarti | Yes | No | Urine toxicology screening | Participants showing a single positive opioid urine screen were found to be a positive for illicit opioid use behaviour, confirmed by urinalysis. | Opioid-positive urine (%)reported by Degree of pain Week 4 Week 8 Week 12 Extreme pain patients: 22.2% (2/9) 12.5% (1/8) 37.5% (3/8) Some pain patients: 31.3% (10/32) 26.7% (8/30) 37.9% (11/29) No pain: 21.4% (3/14) 20% (2/10) 66.7% (6/9) |
Chi-square | 0 | No |
| Dennis | Yes | Yes | Urine toxicology screening | Continued opioid abuse (COA) was determined by calculating the percentage of positive opioid urine screens provided by participants (number of positive opioid urine screens/total number of opioid urine screens). High COA percentage is indicative of a high number of positive opioid urine screens or, alternatively, a higher rate of illicit opioid consumption. | Mean percentage of positive opioid urine screens among pain patients: 23.99 (SD 27.14) Mean percentage of positive opioid urine screens among non-pain patients: 15.82 (SD 20.11) |
Univariate analysis using only COA outcome as the predictor of comorbid pain in a logistic regression model | 1/1 | Yes |
| Dreifuss | Yes | Yes | Urine toxicology screening, self-report, addiction severity tool score | The Substance Use Report, corroborated by weekly urine drug screens, was administered weekly during treatment and every two weeks during follow-up, and was used as the primary measure to determine “successful outcome.” | Successful with chronic pain: 79 (44.6%) Failure with chronic pain: 70 (38.3%) |
Chi-square | 0 | No |
| Dunn | Yes | Yes | Urine toxicology screening | The mean percent of urine samples provided by each participant that tested positive for opioids, cocaine, or benzodiazepines were evaluated. | Chronic pain: 9, No chronic pain: 11 | Independent group t-tests were used to compare continuous variables | 0 | No |
| Fox | Yes | No | Self-report | Self-reported opioid use was obtained from the substance use survey administered at baseline and follow-up, which inquired as to substance use in the 30 days prior to baseline (heroin, methadone, opioid analgesics, cocaine, alcohol, sedatives, hypnotics, or tranquilizers) and follow-ups. These questions were adapted from the ASI. | Not reported per pain status. However, they report that any opioid use decreased from 89% at baseline to 40% at 1 month, 33% at 3 months, and 26% at 6 months. Similar patterns were observed in those with and without baseline or persistent pain, and showed no significant association between any opioid use and baseline pain (AOR = 1.06, 95% CI: 0.27–4.17, P = 0.93) or persistent pain (AOR = 1.20, 95% CI: 0.31–4.63, P = 0.79), after adjusting for HIV status, depressive symptoms, history of IDU, history of incarceration, baseline opioid use, and time since initiating buprenorphine treatment. | Determined whether pain was associated with use of any opioids during the 6-month follow-up period using nonlinear mixed effects (NLME) models with self-reported use of any opioids as the dependent variable. The NLME approach accounts for non-independence of repeated measures of opioid use within individuals. | 0 | 0 |
| Neumann | Yes | No | Urine toxicology screening | Report the number of patients (%) who have an opioid positive urine screen at 24 week follow-up | Methadone: 2 (15.4%), buprenorphine: 5 (38.5%) P.0.05 Odds ratio: 0.280, 95% CI: 0.042–1.878, P = 0.371 |
Fishers exact test | 0 | No |
| Potter | Yes | No | Urine toxicology screening, Addiction severity tool score | Not described well or reported by pain status. | Not reported by pain status | n/a | n/a | n/a |
| Rosenblum | Yes | No | Self-report | A checklist was used to record drugs, including alcohol, that were used during the patient’s last week of active use. | Drugs used in past 3 months (%) P = 0.05 None (reference for MMTP): CP 156 (42.9%) OR:1.00 1: CP:123 (27.6%) OR: 0.51 95%CI (0.31–0.84) 2: CP: 62 (38.7%) OR: 0.84 (0.46–1.53) $3: CP 49 (36.7%) 0.77 (0.40–1.50) |
Mantel–Haenszel was used for ordinal variables with 3 or more categories | 0 | No |
| Trafton | Yes | Yes | Addiction severity tool score | Number of days of opioid use over last 30, as well as self-reported number of days of opioid use over lifetime. | Opiates GP:1.6 days, 1.9 years; NP: 0.8 days, 0.9 years, P: 2.3 days, 2.9 years 0.03/0.005 | The Kruskal–Wallis test was used to determine if variables significantly differed across pain severity ratings, followed by multiple t-tests to determine which levels of reported pain differed from the group reporting “none”. | 2/3 | Yes |
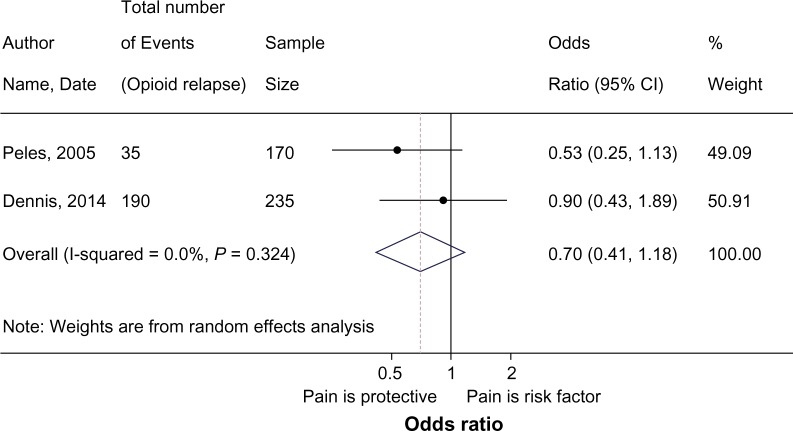
Studies eligible for inclusion in the meta-analysis defined opioid use behavior as a binary outcome, categorizing participants as having engaged in illicit opioid consumption if ≥1 urine test in a designated time period preceding the survey was positive.45,64 While not originally reported in the Dennis et al.45 paper, the authors provided data for the purposes of this review.45 Of the 235 methadone patients assessed in the Dennis et al.45 study, 79.7% of the patients reporting pain and 81.3% of those without pain were found to have ≥1 positive opioid urine screen.45 The meta-analysis presented in the Figure 4 forest plot provides the pooled odds ratio using a random-effects model. Findings from the meta-analysis suggest that there is no effect of pain on illicit opioid consumption pOR:0.70, 95%CI 0.41–1.17; I2 = 0.0). Among the studies that evaluated the impact of pain on opioid consumption among buprenorphine maintained patients, none reported a significant effect.68,69
Figure 4.
Meta-analysis of pain and illicit opioid use.
Illicit substance use (other than opioids)
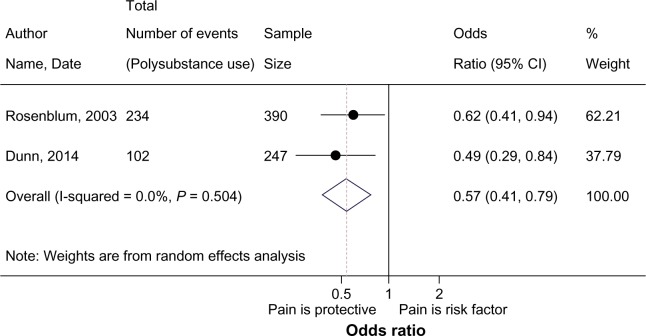
Seven studies assessed the impact of pain on non-opioid illicit substance use,43,44,65–68,70 in which the definition and measurement of what constitutes illicit substance use varied substantially. While some studies assessed the number of participants reporting any illicit substance use (cocaine, benzodiazepine, cannabis) within the last week,66 month,65 or 3 months,43,70 others evaluated the predictors of illicit substance use behavior,68 number of days of substance use in the previous month,44 or the percentage of participants reporting any substance misuse at baseline.67 The stark heterogeneity in defining and measuring illicit substance use precluded the majority of studies from inclusion in the meta-analysis. Studies that measured substance use as the percentage of participants that reported illicit substance use (separated by pain status) were pooled in the meta-analysis. Findings from the meta-analysis are presented in Figure 5, where the presence of pain is shown to be protective against illicit non-opioid substance use (pOR:0.57, 95%CI 0.41–0.79; I2:0.0). These odds of reporting non-opioid illicit substance use are reduced by 43% in participants with comorbid pain.
Figure 5.
Meta-analysis of pain and illicit substance use.
The findings from individual studies revealed that participants with pain reported higher rates of marijuana,44 benzodiaz-apine,70 and sedative44 use. However, the choice of pain measure (eg, BPI) does not appear to impact the relationship between pain and psychoactive substance use.65–67 Trafton et al.44 assessed the impact of pain on the number of days of reported substance use in the previous month (measured using Addiction Severity Index),44 and reported no significant differences in the number of days of reported use between patients with and without pain for alcohol, heroin, and cocaine.44 However, Trafton et al.44 found a significant difference in the number of days as well as lifetime (years) reported use of opiates and marijuana, suggesting participants with pain were more likely to report using these substances.44 The authors44 also found participants with pain to have a longer duration of lifetime history of sedatives use (pain: 2.4 years, no pain: 0.8 years),44 and reported44 no significant differences in health risk behaviors such as injecting or needle-sharing between the pain and no pain groups.44
Bounes et al.67 evaluated the differences in illicit substance use (urine toxicology and self-report) at baseline between pain and no-pain groups; however, they presented the raw data and reported no significant differences between groups for stimulants, hallucinogens, or cannabis use. It appears, however, that cannabis use is reported at a higher rate in patients with pain (28%) in comparison to patients without pain (15%).67 Barry et al.66 (2009) reported similar findings, suggesting, “the pain groups reported comparable levels of psychoactive substance use, illegal drug use and non-medical use of prescription drug in the past week”.66 However, no specific percentages of substance use were reported per group. Dhingra et al.65 did not report any observed differences between pain groups; however, they did suggest that neither urine drug screen (UDS) nor self-reported drug use on the ASI was statistically associated with clinically significant pain in the univariate analysis.65
Dunn et al.70 reported the mean percent of positive urine screens for opiates, benzodiazepine, and cocaine use, finding patients reporting pain to have a significantly higher rate of benzodiazepine use (mean% positive pain 7, mean% positive no pain 3; P = 0.01). However, when evaluating the difference between the number of participants with ≥1 drug urine-screen positive, they found 50 of 90 patients without pain and 52 of 137 patients with pain to be using illicit substances.70 This second measurement was used in the Figure 5 meta-analysis.
Intervention adherence
Among the five studies that evaluated the impact of pain on treatment retention,43,67,71,73,74 one reported a significant effect.67 Among patients treated with low-dose methadone and low-dose buprenorphine, Bounes et al.67 found that retention was lower among patients reporting pain (crude OR: 0.44, 95%CI: 0.22–0.87). Among patients treated with methadone and buprenorphine, Neumann et al.73 found no significant differences between retention rates among patients on buprenorphine (50% retention) and methadone (46.4% retention). While retention was reported as an outcome in the remaining three studies,43,71,74 none reported details of retention by pain status.
Intervention acceptance
Three studies evaluated the impact of pain on intervention acceptance.67,72,73 Jamison et al.72 summarized participants’ views toward methadone treatment, determining whether participants with pain believe they are given enough methadone or are bothered by their dependence on OST. Jamison showed participants with pain 1) did not believe they were given a high-enough dose of methadone, and 2) were extremely bothered by their dependence on methadone.72 Neumann et al.73 chose to report the number of participants who crossed over to a different OAT during the course of the trial, and showed no significant differences in the rate of crossover by pain status. Bounes et al.67 also reported the percentage of participants augmenting prescribed doses of opioid maintenance treatment and found no significant differences between patients with and without pain.67
Resource utilization
Trafton et al.44 provided an analysis of resource utilization to evaluate the impact of pain on physical disability benefit collection, psychiatric disability benefit collection, and the number of hospitalizations reported over the lifetime. They reported a significant difference in the percentage of patients reporting physical disability claims (25% general population, 14% no pain, 35% pain, P < 0.001), and lifetime hospitalizations (3.9% general population, 2.9% no pain, 4.9% pain, P = 0.002).44
Personal and social functioning
Two studies assessed the impact of pain on personal and social functioning.44,72 Though measured and defined differently, both studies showed the presence of chronic pain to be associated with poor personal and social functioning.44,72 Jamison et al.72 found 17.1% of participants with pain reported employment, in comparison to the 32.3% without pain. In addition, the same authors72 found that patients with pain (27%) were more likely to report better family support than patients without pain (21%).72 The differences between groups were tested using X2, both of which were statistically significant.72
Trafton et al.44 evaluated personal and social functioning by examining the participant reported vitality and social functioning using the SF-36V. The authors44 found participants reporting pain to be much less likely to report vitality (35%) and social functioning (45%) in comparison to participants without pain, of which 53% and 76% reported vitality and social functioning, respectively. These results were statistically significant.
Physical health
Of the eight studies assessing the impact of pain on physical health outcomes including adverse events, symptoms related to physical functioning, and the presence of physical comorbidity,43–45,64,65,71–73 seven showed a significant association between the presence pain and worsening physical health.43–45,64,65,71,72 Measures for physical health outcomes varied and included the presence of chronic illness as diagnosed by physician64 or self-report,43,45,65,71–73 inflammatory profile differences by pain status measured using serum levels for inflammatory biomarkers,45 the number of days of reported medical problems,44 percent change in pain/functioning from baseline scores,73 self-reported physical craving for opioids,43 number of participants reporting adverse events by chronic pain status,73 and physical health measured by Health Related Quality of Life (HRLQ) scores65 or SF-36V.44 Of all the studies evaluating physical health outcomes, one did not provide the appropriate data to determine whether pain impacts physical health outcomes.73 However, the same study found no differences in the physical health outcomes of pain patients randomized to low-dose methadone and low-dose suboxone.73 The definitions, measurements, and reported findings for all health outcomes are detailed further in Table 3.
Table 3.
Summary of findings for studies evaluating physical health outcome(s).
| AUTHOR LAST NAME | INTERVENTION(S) EVALUATED | PHYSICAL HEALTH OUTCOME | MEASUREMENT OF PHYSICAL HEALTH OUTCOME(S) | FINDINGS |
|---|---|---|---|---|
| Peles | High-dose Methadone (≥60 mg/day) | The study evaluated the clinical characteristics of patients reporting pain. | All chronic illnesses were diagnosed by internal medicine physician and included 12 categories: Heart [Angina with/without MI]; Endocrinology, metabolic; Cancer; Asthma; Neurology; Digestive system; Muscles/movement; Eyes, ears; Urine system; Coagulation; Gynecological; and Immune system. Patients reporting one or more of the aforementioned illnesses were defined as chronically ill. | Participants with chronic pain were more often diagnosed with physical comorbidities relating to muscle movement, digestive, urinary as well as problems with eyes and ear function. |
| Dhingra | High-dose methadone (≥60 mg/day) | The primary outcome of this study was clinically significant pain, used as the dependent variable in a multi-variable logistic regression model. The study evaluated the physical health symptoms associated with chronic pain. | The study measured physical health using self-report for physical comorbidities and the patients HRQL scores | Clinically significant pain was associated with higher number of comorbid, medical conditions (P < 0.001) and poorer physical HRQL scores (P < 0.001). |
| Dennis | High-dose Methadone (≥60 mg/day) | The study evaluated the clinical and biological characteristics of MMT patients reporting pain. Using a univariate analysis to guide the variable selection, the authors built a multivariable logistic regression model using comorbid pain as the dependent variable. Physical health predictors included in the model were inflammatory markers (IL-6, IL-8, IL-1ra, TNF-alpha, IL-10, IL-1B, and CCL2), and the participants infectious disease status (presence of HIV or hepatitis). | Infectious disease status was measured using self-report, while inflammatory markers were measured using The iMDx™ Prep assays | Infectious disease status (HIV/hepatitis) was not associated with the presence of chronic pain. Of all inflammatory markers tested, IFN-Gamma was shown to be significantly elevated in participants reporting pain (OR]: 2.02; 95% confidence interval [CI]: 1.17, 3.50; P = 0.01) |
| Jaimison | High-dose methadone (≥60 mg/day), low-dose methadone (<60 mg/day) | Evaluated physical health differences in patients reporting pain. | Self-report | Found significant differences in the major health problems reported between patients with main (34.9%) and without pain (9.4%), P < 0.001. Also found major differences between the participants rating their health care as adequate, whereby 75.5% of patients with pain rate their health care as adequate and 94.8% of patients without pain rate their health care as adequate (P, 0.001). Among patients with pain, 36.7% report asthma, 20.4% report angina/chest pain, 11.1% report bleeding problems, 6.6% report a past heart attack, 28.9% report some other unlisted condition, in comparison to the patients without pain who report 16.7%, 7.4%, 3.1%, 1.0%, and 9.4% respectively (all comparisons P < 0.05). |
| Neumann | Low-dose Methadone (<60 mg/day), Low-dose Suboxone® (buprenorphine <16 mg/day + naloxone) | Evaluated physical health using reported side effects and percentchange in pain from baseline | Self-report | The number of patients reporting side effects did not vary significantly between patients on methadone (n = 9) and buprenorphine (n = 8); (OR:1.125 95%CI:0.209–6.04, P = 1.000). The percent change of pain from baseline also did not significantly differ between patients on methadone (mean percent change; SD, 88.6; 24.5), and buprenorphine 87.4; 33.4), P = 0.918. The percent change of functioning from baseline also did not vary significantly between methadone (113.8; 62.5 SD) and buprenorphine groups (121.9; 63.9), P = 0.787. |
| Rosenblum | High-dose Methadone (≥60 mg/day) | The study evaluated the prevalence of comorbid chronic illnesses by pain status, as well as the reported drug cravings. | Self-report | Bivariate analyses were used to compare the prevalence of pain, whereby there was a significant association between reporting chronic illness among patients with chronic severe pain. Among patients with chronic severe pain, 122 (20.5%) report having no concurrent illness (OR: 1.00), whereby 263 (43.7%) reporting having a chronic illness (OR 3.02; 95%CI 1.82,4.98). Additionally, there was a higher number of participants (N = 123, 43.1%) with chronic severe pain reporting high-levels of drug cravings (OR: 1.67; 95%CI 0.99–2.83). |
| Trafton | The study evaluated 1) the number of days of medical problems in the last 30 days, 2) physical functioning as assessed according to SF-36V, and 3) general health. | Self-report according to SF-36V | The study found significant differences across each different physical health outcome evaluated. They report the presence of pain to be associated with an increase in the number of days of reported medical problems (Pain:22.1, No Pain 7.5, P = 0.001), the% of patients with good physical functioning (Pain 55%, No Pain:89%, P < 0.001), and the% with good general physical health (pain: 50%, no pain: 70%, P < 0.001). | |
| Fox | Buprenorphine | The study evaluated baseline differences between patients with and without pain starting an office-based buprenorphine treatment program | Self-report | Patients with pain reported higher rates of HIV |
Psychiatric health and symptoms
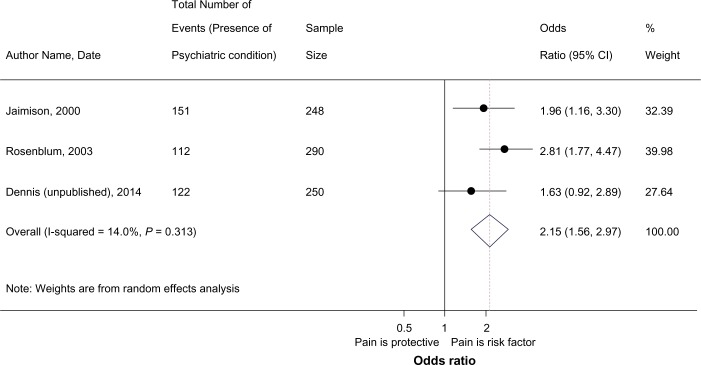
Six studies assessed the association between pain and different psychiatric health outcomes,43,44,65,66,71,72 and all studies reported a significant association between the presence of pain and 1) the presence of psychiatric disorders or 2) an increase in the severity of psychiatry symptoms. The investigation by Fox et al.71 found an increase in depressive symptoms among patients with pain at baseline. Supplementary Table 4 summarizes the findings from all studies that evaluated psychiatric health outcomes including symptom severity and the presence of disorders. The majority of studies chose to present the prevalence of any psychiatric comorbidity stratified by pain status,43,44,72 whereby patients reporting pain showed higher rates of psychiatric comorbidity than their non-pain counterparts.43,44,72 Some studies did, however, evaluate psychiatric symptoms using different psychiatric symptom rating scales.65,66 The studies evaluating the association between pain and specific psychiatric diagnosis (eg, depression, anxiety)44,65,66,72 showed participants reporting pain to have a significant increase in depressive symptoms,44,65,66,72 anxiety,44,66,72 somatization,66 irritability,72 suicidal ideation,44 and violence.44 Only one study reported no significant differences in the suicide attempt histories of pain and non-pain patients.44 Two studies provided suitable data for inclusion into a meta-analysis,43,72 combining the results of studies assessing the percentage of participants reporting psychiatric comorbidity (including all diagnoses) by pain status as the outcome. Dennis et al.45 provided additional data not reported in their original study on the prevalence of psychiatric comorbidity in patients with and without pain. This resulted in the inclusion of three studies into the meta-analysis evaluating the association between pain and psychiatric comorbidity in a combined sample of 788 participants (Fig. 6). Findings from the meta-analysis suggest a significant association between chronic pain and psychiatric comorbidity (pOR: 2.18; 95%CI 1.6–2.9, I2:0.0%, P = 0.324), whereby in comparison to patients without pain, the odds of reporting a psychiatric comorbidity is 2.18 times greater in patients reporting pain, suggesting a significant association between pain and psychiatric disorders.
Figure 6.
Meta-analysis of pain and psychiatric comorbidity.
Summary of included studies
The summary of findings specific to each intervention (eg, methadone, buprenorphine) can be found in Table 4. This table provides an outline of the number of studies evaluating each outcome, as well as those showing risk or benefit based on participants’ exposure status. The table also provides conclusions based on the evidence algorithm discussed previously, whereby ≥50% of the studies must demonstrate an effect. GRADE evidence profiles were constructed to assess our confidence in each meta-analysis estimate. Meta-analyses evaluating the impact of pain on illicit opioid use, illicit substance use, and psychiatric comorbidity were ranked very low, low, and low, respectively. The evidence profiles are summarized in Supplementary Table 5.
Table 4.
Summary of findings across opioid substitution therapies.
| INTERVENTION | OUTCOME | NUMBER OF STUDIES EVALUATING OUTCOME | NUMBER OF STUDIES REPORTING A RISK ASSOCIATION WITH PAIN | NUMBER OF STUDIES REPORTING A PROTECTIVE ASSOCIATION WITH PAIN | FINAL ANALYSIS |
|---|---|---|---|---|---|
| Methadone | Abstinence and illicit substance use: Opioids | 843–45,64–66,70,73 | 244,45 | 0 | Not enough evidence to suggest chronic pain effects treatment |
| Abstinence and illicit substance use: Non-opioids | 643,44,65–67,70 | 244,70 | 267,70 | Not enough evidence to suggest chronic pain effects treatment | |
| Physical health | 743–45,64,65,72,73 | 543–45,64,65,72 | 0 | Pain increases risk for poor physical functioning | |
| Psychiatric health | 543,44,65,66,72 | 543,44,65,66,72 | 0 | Pain increases risk for poor psychiatric functioning | |
| Personal and social functioning | 244,72 | 244,72 | 0 | Pain increases risk for poor personal and social functioning outcomes | |
| Intervention adherence | 343,67,73 | 167 | 0 | No effect | |
| Intervention acceptance | 367,72,73 | 172 | 0 | No effect | |
| Resource utilization | 144 | 144 | 0 | Pain increases resource utilization among patients on MMT | |
| Buprenorphine/Naloxone | Abstinence and illicit substance use: Opioids | 468,69,73,74 | 0 | 0 | No Effect |
| Abstinence and illicit substance use: Non-opioids | 168 | 0 | 0 | No effect | |
| Physical health | 173 | 0 | 0 | No effect | |
| Psychiatric health | 0 | 0 | 0 | Not evaluated | |
| Personal and social functioning | 0 | 0 | 0 | Not evaluated | |
| Intervention adherence | 273,74 | 0 | 0 | No effect | |
| Intervention acceptance | 173 | 0 | 0 | No effect | |
| Resource utilization | 0 | 0 | 0 | Not evaluated | |
| LAAM | Abstinence and illicit substance use: Opioids | 144 | 144 | 0 | Pain increases risk for opioid abuse among patients on LAAM |
| Abstinence and illicit substance use: Non-opioids | 144 | 0 | 0 | No effect | |
| Physical health | 0 | 0 | 0 | Not evaluated | |
| Psychiatric health | 144 | 144 | 0 | Pain increases risk for poor psychiatric functioning | |
| Personal and social functioning | 144 | 144 | 0 | Pain increases risk for poor personal and social functioning outcomes | |
| Intervention adherence | 0 | 0 | 0 | Not evaluated | |
| Intervention acceptance | 144 | 144 | 0 | Patients with pain report less acceptance of their OAT as well as negative views towards OAT in comparison to non-pain patients | |
| Resource utilization | 144 | 144 | 0 | Pain increases resource utilization among patients on LAAM | |
| Buprenorphine | Abstinence and illicit substance use: Opioids | 267,71 | 0 | 0 | Pain has no effect on opioid use behavior |
| Abstinence and illicit substance use: Non-opioids | 167 | n/a | n/a | n/a | |
| Physical health | 171 | 0 | 0 | Not enough evidence to suggest chronic pain impacts treatment (evaluated baseline physical health) | |
| Psychiatric health | 171 | 0 | 0 | Not evaluated (evaluated baseline psychiatric health) | |
| Personal and social functioning | 0 | 0 | 0 | Not evaluated | |
| Intervention adherence | 267,71 | 0 | 0 | No effect | |
| Intervention acceptance | 167 | 0 | 0 | No effect | |
| Resource utilization | 0 | 0 | 0 | Not evaluated |
Guideline evaluation
We identified three of the most recently published national guidelines for opioid use disorder using the national guideline clearinghouse provided by http://www.guideline.gov, and the NICE database.75–78 The guidelines provided minimal information about the effect of pain in the opioid use disorder population.75–78 While some guidelines provide suggestions to manage comorbid CNCP with non-opioid interventions75–77 and refer patients with severe pain to community specialists,75,77 none provides any detail about the risk for psychiatric comorbidity, continued opioid abuse, as well as poor physical, social, and personal functioning among patients with opioid use disorder and comorbid pain.75–78 The summary information including the detailed suggestions for managing patients with pain reported by the guidelines is described in Table 5. Due to the lack of formal recommendations for the management of patients with pain, we were unable to assess each guideline using the rigor of development and applicability domains from AGREE II. The rigor development and applicability domains are used to evaluate how evidence is being incorporated into guideline development. The available guidelines neither provide a formal assessment of the literature nor identify major issues regarding the association between pain and treatment response in opioid use disorder. The lack of formal recommendations for the management of pain during addiction treatment renders the application of tools to assess how evidence is being generated and used to inform recommendations for the management of pain in patients with opioid use disorder unjustified.
Table 5.
Translation of evidence in the opioid maintenance treatment guidelines.
| TITLE OF GUIDELINE | INTERVENTION ASSESSED | DOES THE GUIDELINE PROVIDE SUGGESTIONS FOR MANAGING PATIENTS WITH COMORBID PAIN? | SUGGESTIONS | ARE THESE SUGGESTIONS BASED ON EVIDENCE? | EVIDENCE CITED | ARE ANY RECOMMENDATIONS MADE FOR MANAGING PAIN IN THE OPIOID MAINTENANCE TREATMENT SETTING? | DISCUSSION OF THE RISK FACTORS ASSOCIATED WITH PAIN FOR THIS OST |
|---|---|---|---|---|---|---|---|
| Clinical practice guideline for management of substance use disorders (SUD)76 | Methadone, buprenorphine, naltrexone | Yes | Evaluate opioid dependent patients for severe acute or chronic physical pain that may require appropriate short-acting opioid agonist medication in addition to the medication needed to prevent opioid withdrawal symptoms | No | / | No graded recommendations made | No |
| Buprenorphine/Naloxone Treatment for opioid dependence clinical Practice guidelines77 | Buprenorphine/naloxone | Yes | When managing patients with comorbid chronic non-cancer pain, 1) do not treat them with chronic opioid analgesic therapy for pain, non-opioid alternatives should be aggressively optimized, 2) referral to a reputable multidisciplinary chronic pain clinic regarding pharmacologic and non-pharmacologic non-opioid alternatives is recommended for patients with pain, and 3) if the decision to initiate opioid analgesics has been made, the patient should be monitored by or advice should be sought from a physician experienced in addiction medicine. | No | / | No graded recommendations made | No |
| Methadone maintenance treatment program standards and clinical guidelines75 | Methadone maintenance treatment | Yes | Suggest the management of mild to moderate pain in conditions such as fibromyalgia, low back pain with non-opioid treatments. For patients with severe chronic pain (nociceptive or neuropathic pain condition that usually requires opioid therapy) they suggest 1) non opioid treatments, 2) split methadone dose, 3) codeine or tramadol, and lastly 4) morphine. Suggest strong communication with community physicians managing patients pain, as well as informing non-methadone physicians to also perform routine urine drug screens. | No | / | No graded recommendations made | No |
| Methadone and buprenorphine for the management of opioid dependence78 | Methadone and buprenorphine | No | No suggestions made | / | / | No graded recommendations made | No |
Note: /Indicates this information is not applicable.
Discussion
Findings from a systematic review of 14 studies including a combined sample of 3,128 patients with opioid use disorder suggest that CNCP is an important factor affecting the treatment course for patients on OST. Specifically, patients with CNCP were found to have higher rates of adverse physical, psychiatric, and personal/social functioning than patients without pain. However, these results were only demonstrated in studies evaluating methadone and LAAM.43–45,64–67,70,72 Pain showed no effect on any of the outcomes evaluated for patients on buprenorphine or combination buprenorphine naloxone.67–69,71,73,74 Results from this review also suggest that the current treatment guidelines used for OSTs neither discuss the important impact of pain on treatment prognosis nor provide any formal recommendations for treatment management in this subpopulation. The guidelines only go so far as to suggest 1) managing with non-opioid medications, 2) consulting the specialized pain services for treatment, and 3) maintaining open communication with family physicians managing the patients’ comorbid disorders. These suggestions are made in the supplementary sections of the guideline, with no formal review process or evidence being cited to support their development. Guidelines may be restraining themselves from drawing any conclusions about the appropriate management of patients with comorbid pain because of the inconclusive nature of the evidence. However, the guidelines provide no discussion to suggest that they have evaluated this topic.75–78
While to our knowledge this is the first review to assess the impact of CNCP on the multiple treatment outcomes for patients with opioid use disorder, we are still no closer to reaching firm conclusions as to the optimal therapy for patients with comorbid pain. There is limited evidence evaluating the effects of pain in the addiction setting. Even among the studies available, cadres of measures are employed to assess pain, substance use behavior, and psychiatric comorbidity. This variation in measurements precluded most studies from inclusion in our meta-analysis.
Among the 3,527 unique articles screened for inclusion, only a few studies (n = 14) evaluated the prognostic impact of pain on physical, psychological, and social outcomes. In addition, the studies evaluating this topic suffered from a high risk of bias. The considerable methodological quality issues among the 14 included studies are presented in the individual risk of bias assessments (Supplementary Tables 1–3) and the GRADE evidence profiles (Supplementary Table 5). The strength of the evidence generated by the three meta-analyses determining the impact of pain on illicit opioid use, illicit non-opioid substance use, and the presence of psychiatric comorbidity was downgraded to low, low, and very low. Many of the studies (k = 5) were unable to demonstrate a dose–response relationship between pain severity and treatment response.45,65,69,70,72 The evidence was downgraded as a result of a serious lack of reporting on important methodological study design features such as sample size calculations or power estimation,44,64–66,69,70,72 blinding the outcome assessment,43,44,64–66,68–70,72 and the management of missing data.44,64–66,68–70,72
Among the studies reporting an association between pain and treatment response outcomes such as illicit substance use behavior (opioid and non-opioid),44,45,70 poor physical health,43–45,64,65,72 and psychiatric comorbidity,43,44,65,66,72 a number of studies based their conclusions on relatively imprecise and unadjusted treatment effects. This is of concern, since the majority of evidence stems from small-sample cross-sectional investigations. The experience of pain can be confounded by many variables including age, presence of other physical comorbidities, the use of adjunct pain therapeutics (eg, gabapentin), and the duration on OST. Due to the hyperalgesic effects of some long-acting opioids, patients on OAT may experience higher rates of pain.79 Some of the studies included in this review neither discuss these issues nor adjust for important covariates.43,44,64,66,69,72 In fact, many studies only adjust for variables they find significant in univariate analysis. At times, this may be an inappropriate method since certain variables, while weak in a univariate analysis, may hold an important effect due to biological or other relevance to the outcome such as age or sex. Thus, variables of clinical significance known to impact treatment response such as age, sex, OAT dose (mg/day), use of adjunct therapies, and duration on OAT should always be considered in the analyses.
The definition and measurement of pain across studies requires further consideration. Half of the included studies used BPI as a measure of pain, stating the BPI is a validated tool to assess the presence of pain. This is troubling, since measurement tools are only validated in the population the tools was created and tested within,80 and to our knowledge this tool has never validated in patient population with opioid use disorder. To state the psychometric properties such as internal consistency or test–retest reliability of a tool will be the same in a different population than those for which the tool was developed would be inaccurate. The properties of a reliable measurement tool rest in its ability to capture variance between patients; thus it becomes more difficult to distinguish between individuals of more homogenous populations.80 Tools such as BPI were originally generated and validated within a population of patients with cancer and rheumatoid arthritis.81 Although since then BPI has been widely used in other populations with pain, to our knowledge no proper reliability assessment has been performed in patients with opioid use disorder. Thus, the ability of the BPI to properly capture pain in OAT patients remains questionable and requires formal validation in this population.
Assessment of the overall findings using Table 4 emphasizes the lack of conclusive evidence demonstrating the impact of pain on therapeutic response. For instance, a number of studies suggest that pain has no impact on treatment prognosis for patients on buprenorphine or combination buprenorphine/naloxone; however, a number of outcomes were not evaluated for this intervention. Among patients on methadone – the intervention with the largest body of evidence – pain was found to increase the risk for adverse physical, psychiatric, as well as personal and social functioning. However, there is not enough evidence in this review to establish whether pain increases patients’ propensity to abuse opioids and other illicit substances. The meta-analysis assessing the impact of pain on non-opioid substance use (eg, cocaine, benzodiazepine) suggests that participants with pain have lower odds of abusing non-opioid substances. However, we will refrain from making any firm conclusion based on this analysis since it relies on the findings from two studies,43,70 which represent a fraction of the available evidence assessing this outcome.43,44,65–67,70 The case is similar for illicit opioid use: among the eight43–45,64–66,70,73 studies assessing continued opioid abuse using different definitions and measurements of opioid use (eg, number of positive opioid urine screens, time until opioid relapse), two studies are included in the meta-analysis, both of which suggest protective effects. Two studies that were excluded from the meta-analysis due to measurement variability actually reported a risk association between the presence of pain and opioid use.44,45 Evaluating the differences between the studies reporting a risk effect and those reporting a protective effect of pain on opioid use behavior suggests that the conservative definitions of opioid consumption using a binary categorization of opioid use based on one positive UDS will show a “protective” association between pain and opioid consumption.45,64 For studies evaluating opioid use behavior as a continuous measure such as the mean number of positive opioid urine screens or the number of days of opioid use over the last month, the presence of pain is association with a “risk” association between pain and consumption.44,45 Among the same group of participants, different classifications of opioid use behavior can result in differences in the observed effects of pain.16 Similar findings are noted among studies evaluating illicit non-opioid substance use, where again the evaluation of substance use behavior as a continuous outcome, such as the number of days of illicit substance use or the mean percentage of positive UDS, suggests pain is a risk factor for increase illicit substance consumption.44,70 Again, the evaluation of illicit substance consumption using a binary categorization of illicit substance use based on one positive UDS showed a “protective” association between pain and illicit substance use.67 The fragility of these findings highlights the importance of an a priori selection for defining and measuring substance use outcomes (opioid or non-opioid). These results also emphasize the high susceptibility for selective reporting among studies evaluating pain and opioid use disorder.
In the absence of establishing the most effective therapy for managing opioid addiction patients with comorbid CNCP, it may be worthwhile to consider evidence assessing OAT in the general pain population. Bearing in mind that patients can experience hyperalgesic effects from treatments such as methadone,82,83 other OATs may deliver more therapeutic effects within the pain subpopulation of addiction patients. For instance, recent evidence suggests that patients converting from high-dose full opioid agonists (200–1,370 mg of morphine equivalents) to buprenorphine therapy for more than 60 days exhibit significant improvements in pain severity and quality of life.84,85 It is likely the unique pharmacologic properties of therapies such as buprenorphine (being a partial mu-agonist) enhance the therapeutic effects of the medication, which may also inflate its effect in the pain subpopulation. In light of these findings, future efforts should focus on evaluating the effectiveness of buprenorphine for the chronic pain subpopulation of opioid addiction patients using a randomized study design.
Conclusion
Findings from this review suggest that CNCP may increase the risk for poor physical, psychiatric, as well as personal and social functioning for patients with opioid use disorder and on MMT or LAAM. Important outcomes such as resources utilization (eg, hospitalization), intervention acceptance, and personal/social functioning are understudied. Additionally, we lack evidence on the majority of outcomes for the single formula buprenorphine and combination of buprenorphine/naloxone treatments. We caution the interpretation of evidence from the meta-analyses since these results preclude a substantial portion of the evidence and are based on studies suffering from a high risk of bias. Qualitative synthesis of the findings suggests that major methodological differences in the design and measurement of both pain and treatment response outcomes are likely impacting the observed effect estimates. Does pain really play an important role in mediating the effects of OST? Are patients with pain responding differently? Should patients with pain be managed differently? These questions have yet to be definitively answered. Further research is needed to confirm the association between pain and important outcomes in patients on OAT before making any conclusions as to which treatment is superior for the pain subpopulation. We recommend future studies to establish a larger sample with a demonstrated dose–response relationship between pain and treatment response. Current guidelines neither address nor make any formal recommendations for managing patients with comorbid pain.
Supplementary Material
Table S1. Cross-sectional risk of bias assessment using national institute of health quality assessment tool for observational cohort and cross-sectional studies.
Table S2. Newcastle ottawa scale risk of bias assessment for cohort studies.
Table S3. Risk of bias assessment for randomized controlled trials using the cochrane risk of bias tool.
Table S4. Summary of findings for studies evaluating psychiatric health outcomes.
Table S5. The impact of pain on substitute opioid therapy treatment outcomes.
Acknowledgments
We sincerely thank everyone who contributed to the completion of this study. This project would not have been possible without the great collaboration cemented between GENOA and the CATC network of clinical sites. We extend our deepest appreciation to Jackie Hudson and Sheelagh Rutherford, who both work so hard to ensure the success of this research collaborative. We would also like to thank the funding agencies supporting individual researchers.
Footnotes
ACADEMIC EDITOR: Gregory Stuart, Editor in Chief
PEER REVIEW: Three peer reviewers contributed to the peer review report. Reviewers’ reports totaled 820 words, excluding any confidential comments to the academic editor.
FUNDING: This study is supported by the Peter Boris Centre for Addictions Research and the Canadian Institutes for Health Research (CIHR) Drug Safety and Effectiveness Network (DSEN) grant (grant number: 126639). The funders had no role in study design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. Brittany B. Dennis and Monica Bawor are supported by the Intersections of Mental Health Perspectives in Addictions Research Training (IMPART) research fellowship funded through CIHR and British Columbia Centre of Excellence for Women’s Health. Brittany B. Dennis is also supported by the David L. Sackett Scholarship.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: BBD, LT, ZS. Screened articles eligible for inclusion to the review and abstracted data: BBD, MB, LN, CC, JV. Analyzed the data: BBD, MB, LN, LT, JV, CC, ZS. Wrote the first draft of the manuscript: BBD, MB, LN, CC, JV, JP, MV, JD, GP, AW, DD, LT, ZS, CP, DCM. Contributed to the writing of the manuscript: BBD, MB, LN, CC, JV, JP, MV, JD, GP, AW, DD, LT, ZS, CP, DCM. Agree with manuscript results and conclusions: BBD, MB, LN, CC, JV, JP, MV, JD, GP, AW, DD, LT, ZS, CP, DCM. Jointly developed the structure and arguments for the paper: BBD, MB, LN, CC, JV, JP, MV, JD, GP, AW, DD, LT, ZS, CP, DCM. Made critical revisions and approved final version: BBD, MB, LN, CC, JV, JP, MV, JD, GP, AW, DD, LT, ZS, CP, DCM. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Classification of chronic pain Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl. 1986;3:S1–226. [PubMed] [Google Scholar]
- 2.Chou R, Turner JA, Devine EB, et al. The Effectiveness and Risks of Long-Term Opioid Therapy for Chronic Pain: A Systematic Review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015 Jan 13; doi: 10.7326/M14-2559. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan MD, Edlund MJ, Fan MY, Devries A, Brennan Braden J, Martin BC. Trends in use of opioids for non-cancer pain conditions 2000–2005 in commercial and Medicaid insurance plans: the TROUP study. Pain. 2008 Aug 31;138(2):440–9. doi: 10.1016/j.pain.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Starrels JL, Becker WC, Weiner MG, Li X, Heo M, Turner BJ. Low use of opioid risk reduction strategies in primary care even for high risk patients with chronic pain. J Gen Intern Med. 2011 Sep;26(9):958–64. doi: 10.1007/s11606-011-1648-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Starrels JL, Becker WC, Alford DP, Kapoor A, Williams AR, Turner BJ. Systematic review: treatment agreements and urine drug testing to reduce opioid misuse in patients with chronic pain. Ann Intern Med. 2010 Jun 1;152(11):712–20. doi: 10.7326/0003-4819-152-11-201006010-00004. [DOI] [PubMed] [Google Scholar]
- 6.Chen LH, Hedegaard H, Warner M. Drug-poisoning Deaths Involving Opioid Analgesics: United States, 1999–2011. NCHS data brief. 2014 Sep;(166):1–8. [PubMed] [Google Scholar]
- 7.Volkow ND, McLellan TA. Curtailing diversion and abuse of opioid analgesics without jeopardizing pain treatment. Jama. 2011 Apr 6;305(13):1346–7. doi: 10.1001/jama.2011.369. [DOI] [PubMed] [Google Scholar]
- 8.Dart RC, Surratt HL, Cicero TJ, et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015 Jan 15;372(3):241–8. doi: 10.1056/NEJMsa1406143. [DOI] [PubMed] [Google Scholar]
- 9.Ahmadi J. A controlled trial of buprenorphine treatment for opium dependence: the first experience from Iran. Drug and alcohol dependence. 2002;66(2):111–4. doi: 10.1016/s0376-8716(01)00202-2. http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/791/CN-00384791/frame.html. [DOI] [PubMed] [Google Scholar]
- 10.Ahmadi J, Ahmadi K. Controlled trial of maintenance treatment of intravenous buprenorphine dependence. Irish journal of medical science. 2003 Oct-Dec;172(4):171–3. doi: 10.1007/BF02915283. [DOI] [PubMed] [Google Scholar]
- 11.Ahmadi J, FH, Moosavinasab M, Babaee M, Firoozabadi A, Mohagheghzadeh M, et al. Treatment of heroin dependence. German Journal of Psychiatry. 2004;7(2):1–5. [Google Scholar]
- 12.Anglin MD, Conner BT, Annon J, Longshore D. Levo-alpha-acetylmethadol (LAAM) versus methadone maintenance: 1-year treatment retention, outcomes and status. Addiction. 2007 Sep;102(9):1432–42. doi: 10.1111/j.1360-0443.2007.01935.x. [DOI] [PubMed] [Google Scholar]
- 13.Comer SD, Sullivan MA, Yu E, et al. Injectable, sustained-release naltrexone for the treatment of opioid dependence: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2006 Feb;63(2):210–8. doi: 10.1001/archpsyc.63.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eder H, Jagsch R, Kraigher D, Primorac A, Ebner N, Fischer G. Comparative study of the effectiveness of slow-release morphine and methadone for opioid maintenance therapy. Addiction (Abingdon, England) 2005;100(8):1101–9. doi: 10.1111/j.1360-0443.2005.001128.x. http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/083/CN-00529083/frame.html. [DOI] [PubMed] [Google Scholar]
- 15.Eissenberg T, Bigelow GE, Strain EC, et al. Dose-related efficacy of levomethadyl acetate for treatment of opioid dependence A randomized clinical trial. JAMA: the journal of the American Medical Association. 1997;277(24):1945–51. http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/955/CN-00140955/frame.html. [PubMed] [Google Scholar]
- 16.Fischer G, Gombas W, Eder H, et al. Buprenorphine versus methadone maintenance for the treatment of opioid dependence. Addiction (Abingdon, England) 1999;94(9):1337–47. doi: 10.1046/j.1360-0443.1999.94913376.x. http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/437/CN-00265437/frame.html. [DOI] [PubMed] [Google Scholar]
- 17.Fudala PJ, Bridge TP, Herbert S, et al. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N Engl J Med. 2003 Sep 4;349(10):949–58. doi: 10.1056/NEJMoa022164. [DOI] [PubMed] [Google Scholar]
- 18.Giacomuzzi SM, Ertl M, Kemmler G, Riemer Y, Vigl A. Sublingual buprenorphine and methadone maintenance treatment: a three-year follow-up of quality of life assessment. ScientificWorldJournal. 2005 May 24;5:452–68. doi: 10.1100/tsw.2005.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo S. Efficacy of naltrexone Hydrochloride for preventing relapse among opiate dependent patients after detoxification. Hong Kong Journal of Psychiatry. 2001;11(4):2–8. [Google Scholar]
- 20.Haasen C, Verthein U, Degkwitz P, Berger J, Krausz M, Naber D. Heroin-assisted treatment for opioid dependence: randomised controlled trial. Br J Psychiatry. 2007 Jul;191:55–62. doi: 10.1192/bjp.bp.106.026112. [DOI] [PubMed] [Google Scholar]
- 21.Hartnoll RL, Mitcheson MC, Battersby A, et al. Evaluation of heroin maintenance in controlled trial. Arch Gen Psychiatry. 1980 Aug;37(8):877–84. doi: 10.1001/archpsyc.1980.01780210035003. [DOI] [PubMed] [Google Scholar]
- 22.Jaffe JH, Senay EC, Schuster CR, Renault PR, Smith B, DiMenza S. Methadyl acetate vs methadone A double-blind study in heroin users. JAMA: the journal of the American Medical Association. 1972;222(4):437–42. http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/696/CN-00007696/frame.html. [PubMed] [Google Scholar]
- 23.Johnson RE, Eissenberg T, Stitzer ML, Strain EC, Liebson IA, Bigelow GE. A placebo controlled clinical trial of buprenorphine as a treatment for opioid dependence. Drug Alcohol Depend. 1995 Nov;40(1):17–25. doi: 10.1016/0376-8716(95)01186-2. [DOI] [PubMed] [Google Scholar]
- 24.Johnson RE, Jaffe JH, Fudala PJ. A controlled trial of buprenorphine treatment for opioid dependence. Jama. 1992 May 27;267(20):2750–5. [PubMed] [Google Scholar]
- 25.Kakko J, Svanborg KD, Kreek MJ, Heilig M. 1-year retention and social function after buprenorphine-assisted relapse prevention treatment for heroin dependence in Sweden: a randomised, placebo-controlled trial. Lancet. 2003 Feb 22;361(9358):662–8. doi: 10.1016/S0140-6736(03)12600-1. [DOI] [PubMed] [Google Scholar]
- 26.Kamien JB, Branstetter SA, Amass L. Buprenorphine-naloxone versus methadone maintenance therapy: A randomised double-blind trial with opioid-dependent patients. Heroin Addiction and Related Clinical Problems. 2008;10(4):5–18. http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/368/CN-00754368/frame.html. [Google Scholar]
- 27.King VL, Kidorf MS, Stoller KB, Schwartz R, Kolodner K, Brooner RK. A 12-month controlled trial of methadone medical maintenance integrated into an adaptive treatment model. J Subst Abuse Treat. 2006 Dec;31(4):385–93. doi: 10.1016/j.jsat.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 28.Kosten TR, Schottenfeld R, Ziedonis D, Falcioni J. Buprenorphine versus methadone maintenance for opioid dependence. The Journal of nervous and mental disease. 1993 Jun;181(6):358–64. doi: 10.1097/00005053-199306000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Krook AL, Brors O, Dahlberg J, et al. A placebo-controlled study of high dose buprenorphine in opiate dependents waiting for medication-assisted rehabilitation in Oslo, Norway. Addiction. 2002 May;97(5):533–42. doi: 10.1046/j.1360-0443.2002.00090.x. [DOI] [PubMed] [Google Scholar]
- 30.Krupitsky E, Zvartau E, Blokhina E, et al. Randomized trial of long-acting sustained-release naltrexone implant vs oral naltrexone or placebo for preventing relapse to opioid dependence. Archives of general psychiatry. 2012;69(9):973–81. doi: 10.1001/archgenpsychiatry.2012.1a. http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/942/CN-00841942/frame.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krupitsky EM, Zvartau EE, Masalov DV, et al. Naltrexone for heroin dependence treatment in St. Petersburg, Russia. J Subst Abuse Treat. 2004 Jun;26(4):285–94. doi: 10.1016/j.jsat.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 32.Krupitsky EM, Zvartau EE, Masalov DV, et al. Naltrexone with or without fluoxetine for preventing relapse to heroin addiction in St. Petersburg, Russia. J Subst Abuse Treat. 2006 Dec;31(4):319–28. doi: 10.1016/j.jsat.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 33.Ling W, Charuvastra C, Collins JF, et al. Buprenorphine maintenance treatment of opiate dependence: a multicenter, randomized clinical trial. Addiction. 1998 Apr;93(4):475–86. doi: 10.1046/j.1360-0443.1998.9344753.x. [DOI] [PubMed] [Google Scholar]
- 34.Ling W, Charuvastra C, Kaim SC, Klett CJ. Methadyl acetate and methadone as maintenance treatments for heroin addicts. A veterans administration cooperative study. Arch Gen Psychiatry. 1976 Jun;33(6):709–20. doi: 10.1001/archpsyc.1976.01770060043007. [DOI] [PubMed] [Google Scholar]
- 35.Ling W, Wesson DR, Charuvastra C, Klett CJ. A controlled trial comparing buprenorphine and methadone maintenance in opioid dependence. Archives of general psychiatry. 1996;53(5):401–7. doi: 10.1001/archpsyc.1996.01830050035005. http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/562/CN-00124562/frame.html. [DOI] [PubMed] [Google Scholar]
- 36.Lintzeris N, Ritter A, Panjari M, Clark N, Kutin J, Bammer G. Implementing buprenorphine treatment in community settings in Australia: experiences from the Buprenorphine Implementation Trial. Am J Addict. 2004;13(Suppl 1):S29–41. doi: 10.1080/10550490490440799. [DOI] [PubMed] [Google Scholar]
- 37.March JC, Oviedo-Joekes E, Perea-Milla E, Carrasco F. Controlled trial of prescribed heroin in the treatment of opioid addiction. Journal of substance abuse treatment. 2006;31(2):203–11. doi: 10.1016/j.jsat.2006.04.007. http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/751/CN-00571751/frame.html. [DOI] [PubMed] [Google Scholar]
- 38.Mattick RP, Ali R, White JM, O’Brien S, Wolk S, Danz C. Buprenorphine versus methadone maintenance therapy: a randomized double-blind trial with 405 opioid-dependent patients. Addiction. 2003 Apr;98(4):441–52. doi: 10.1046/j.1360-0443.2003.00335.x. [DOI] [PubMed] [Google Scholar]
- 39.Neri S, Bruno CM, Pulvirenti D, et al. Randomized clinical trial to compare the effects of methadone and buprenorphine on the immune system in drug abusers. Psychopharmacology (Berl) 2005 May;179(3):700–4. doi: 10.1007/s00213-005-2239-x. [DOI] [PubMed] [Google Scholar]
- 40.Oviedo-Joekes E, Brissette S, Marsh DC, et al. Diacetylmorphine versus methadone for the treatment of opioid addiction. N Engl J Med. 2009 Aug 20;361(8):777–86. doi: 10.1056/NEJMoa0810635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oviedo-Joekes E, March JC, Romero M, Perea-Milla E. The Andalusian trial on heroin-assisted treatment: a 2 year follow-up. Drug Alcohol Rev. 2010 Jan;29(1):75–80. doi: 10.1111/j.1465-3362.2009.00100.x. [DOI] [PubMed] [Google Scholar]
- 42.Pani PP, Maremmani I, Pirastu R, Tagliamonte A, Gessa GL. Buprenorphine: a controlled clinical trial in the treatment of opioid dependence. Drug Alcohol Depend. 2000 Jul 1;60(1):39–50. doi: 10.1016/s0376-8716(99)00140-4. [DOI] [PubMed] [Google Scholar]
- 43.Rosenblum A, Joseph H, Fong C, Kipnis S, Cleland C, Portenoy RK. Prevalence and characteristics of chronic pain among chemically dependent patients in methadone maintenance and residential treatment facilities. JAMA. 2003 May 14;289(18):2370–8. doi: 10.1001/jama.289.18.2370. [DOI] [PubMed] [Google Scholar]
- 44.Trafton JA, Oliva EM, Horst DA, Minkel JD, Humphreys K. Treatment needs associated with pain in substance use disorder patients: implications for concurrent treatment. Drug Alcohol Depend. 2004 Jan 7;73(1):23–31. doi: 10.1016/j.drugalcdep.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 45.Dennis BB, Samaan MC, Bawor M, et al. Evaluation of clinical and inflammatory profile in opioid addiction patients with comorbid pain: results from a multicenter investigation. Neuropsychiatric disease and treatment. 2014;10:2239–47. doi: 10.2147/NDT.S72785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barry DT, Beitel M, Joshi D, Schottenfeld RS. Pain and substance-related pain-reduction behaviors among opioid dependent individuals seeking methadone maintenance treatment. Am J Addict. 2009 Mar-Apr;18(2):117–21. doi: 10.1080/10550490902772470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ilgen MA, Trafton JA, Humphreys K. Response to methadone maintenance treatment of opiate dependent patients with and without significant pain. Drug Alcohol Depend. 2006 May 20;82(3):187–93. doi: 10.1016/j.drugalcdep.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 48.Bartoli F, Carra G, Brambilla G, et al. Association between depression and nonfatal overdoses among drug users: a systematic review and meta-analysis. Drug Alcohol Depend. 2014 Jan 1;134:12–21. doi: 10.1016/j.drugalcdep.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 49.Carra G, Bartoli F, Crocamo C, Brady KT, Clerici M. Attempted suicide in people with co-occurring bipolar and substance use disorders: systematic review and meta-analysis. J Affect Disord. 2014;167:125–35. doi: 10.1016/j.jad.2014.05.066. [DOI] [PubMed] [Google Scholar]
- 50.Dennis B, Bawor M, Paul J, et al. The impact of chronic pain on opioid addiction treatment: a systematic review protocol. Systematic reviews. 2015;4(1):49. doi: 10.1186/s13643-015-0042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cohen J. A coefficient of agreement for nominal scales. Educational and Psychological Measurement. 1960;20(1):37–46. [Google Scholar]
- 52.National Institutes of Health . Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. National Heart, Lung, and Blood Institute; 2014. [Google Scholar]
- 53.Wells GA, SB, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. 2009. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm.
- 54.Collaboration TC. Research Projects:Cochrane Risk of Bias tool. 2013. http://bmg.cochrane.org/research-projectscochrane-risk-bias-tool.
- 55.Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011 Apr;64(4):383–94. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 56.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Bmj. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stroup DF, BJ, Morton SC, Olkin I, Williamson GD, Rennie D. Meta-analysis of Observational Studies in Epidemiology. JAMA. 2000;283(15):2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 58.Brouwers MC, Kho ME, Browman GP, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. J Clin Epidemiol. 2010 Dec;63(12):1308–11. doi: 10.1016/j.jclinepi.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 59.Brouwers MC, Kho ME, Browman GP, et al. Development of the AGREE II, part 2: assessment of validity of items and tools to support application. CMAJ: Canadian Medical Association journal = journal de l’Association medicale canadienne. 2010 Jul 13;182(10):E472–8. doi: 10.1503/cmaj.091716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McLellan AT, Kushner H, Metzger D, et al. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9(3):199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 61.Marsden J, Gossop M, Stewart D, et al. The Maudsley Addiction Profile (MAP): a brief instrument for assessing treatment outcome. Addiction. 1998 Dec;93(12):1857–67. doi: 10.1046/j.1360-0443.1998.9312185711.x. [DOI] [PubMed] [Google Scholar]
- 62.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–48. [PubMed] [Google Scholar]
- 63.The Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. [Google Scholar]
- 64.Peles E, Schreiber S, Gordon J, Adelson M. Significantly higher methadone dose for methadone maintenance treatment (MMT) patients with chronic pain. Pain. 2005 Feb;113(3):340–6. doi: 10.1016/j.pain.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 65.Dhingra L, Masson C, Perlman DC, et al. Epidemiology of pain among outpatients in methadone maintenance treatment programs. Drug Alcohol Depend. 2012 Aug 27; doi: 10.1016/j.drugalcdep.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barry DT, Beitel M, Garnet B, Joshi D, Rosenblum A, Schottenfeld RS. Relations among psychopathology, substance use, and physical pain experiences in methadone-maintained patients. J Clin Psychiatry. 2009 Sep;70(9):1213–8. doi: 10.4088/JCP.08m04367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bounes V, Palmaro A, Lapeyre-Mestre M, Roussin A. Long-term Consequences of Acute Pain for Patients under Methadone or Buprenorphine Maintenance Treatment. Pain Physician. 2013 Nov-Dec;16(6):E739–47. [PubMed] [Google Scholar]
- 68.Chakrabarti A, Woody GE, Griffin ML, Subramaniam G, Weiss RD. Predictors of buprenorphine-naloxone dosing in a 12-week treatment trial for opioid-dependent youth: secondary analyses from a NIDA Clinical Trials Network study. Drug and alcohol dependence. 2010;107(2–3):253–6. doi: 10.1016/j.drugalcdep.2009.10.014. http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/172/CN-00743172/frame.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dreifuss JA, Griffin ML, Frost K, et al. Patient characteristics associated with buprenorphine/naloxone treatment outcome for prescription opioid dependence: Results from a multisite study. Drug and Alcohol Dependence. 2013 Jul;131(1–2):112–8. doi: 10.1016/j.drugalcdep.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dunn KE, Brooner RK, Clark MR. Severity and Interference of Chronic Pain in Methadone-Maintained Outpatients. Pain Medicine. 2014 Sep;15(9):1540–8. doi: 10.1111/pme.12430. [DOI] [PubMed] [Google Scholar]
- 71.Fox AD, Sohler NL, Starrels JL, Ning YM, Giovanniello A, Cunningham CO. Pain Is Not Associated with Worse Office-Based Buprenorphine Treatment Outcomes. Substance Abuse. 2012;33(4):361–5. doi: 10.1080/08897077.2011.638734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jamison RN, Kauffman J, Katz NP. Characteristics of methadone maintenance patients with chronic pain. J Pain Symptom Manage. 2000 Jan;19(1):53–62. doi: 10.1016/s0885-3924(99)00144-x. [DOI] [PubMed] [Google Scholar]
- 73.Neumann AM, Blondell RD, Jaanimagi U, et al. A Preliminary Study Comparing Methadone and Buprenorphine in Patients with Chronic Pain and Coexistent Opioid Addiction. Journal of Addictive Diseases. 2013 Jan;32(1):68–78. doi: 10.1080/10550887.2012.759872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Potter JS, Dreifuss JA, Marino EN, et al. The multi-site prescription opioid addiction treatment study: 18-month outcomes. Journal of Substance Abuse Treatment. 2015 Jan;48(1):62–9. doi: 10.1016/j.jsat.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Methadone Maintenance Treatment Program Standards and Clinical Guidelines. Toronto Canada: The College of Physicians and Surgeons of Ontario; 2011. [Google Scholar]
- 76.National Guideline C VA/DoD clinical practice guideline for management of substance use disorders (SUD) [Accessed September 29, 2014]. http://www.guideline.gov/content.aspx?id=15676&search=methadone.
- 77.National Guideline C Buprenorphine/naloxone for opioid dependence: clinical practice guideline. [Accessed September 10, 2014]. http://www.guideline.gov/content.aspx?id=39351&search=methadone.
- 78.NCCMH . Drug Misuse: Opioid Detoxification. Leicester and London: The British Psychological Society and the Royal College of Psychiatrists; 2008. [PubMed] [Google Scholar]
- 79.Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L. A comprehensive review of opioid-induced hyperalgesia. Pain Physician. 2011 Mar-Apr;14(2):145–61. [PubMed] [Google Scholar]
- 80.Streiner DLN, GR . Health Measurement Scales: A Practical Guide to Their Development and Use. 4ed. New York City, United States of America: Oxford University Press; 2008. [Google Scholar]
- 81.Cleeland C. The Brief Pain Inventory: User Guide. The University of Texas MD Anderson Cancer Center; Texas, USA: 1991. [Google Scholar]
- 82.Compton P, Charuvastra VC, Ling W. Pain intolerance in opioid-maintained former opiate addicts: effect of long-acting maintenance agent. Drug Alcohol Depend. 2001 Jul 1;63(2):139–46. doi: 10.1016/s0376-8716(00)00200-3. [DOI] [PubMed] [Google Scholar]
- 83.Pohl M, Smith L. Chronic pain and addiction: challenging co-occurring disorders. J Psychoactive Drugs. 2012 Apr-Jun;44(2):119–24. doi: 10.1080/02791072.2012.684621. [DOI] [PubMed] [Google Scholar]
- 84.Daitch D, Daitch J, Novinson D, Frey M, Mitnick C, Pergolizzi J., Jr Conversion from high-dose full-opioid agonists to sublingual buprenorphine reduces pain scores and improves quality of life for chronic pain patients. Pain Med. 2014 Dec;15(12):2087–94. doi: 10.1111/pme.12520. [DOI] [PubMed] [Google Scholar]
- 85.Baron MJ, McDonald PW. Significant pain reduction in chronic pain patients after detoxification from high-dose opioids. J Opioid Manag. 2006 Sep-Oct;2(5):277–82. doi: 10.5055/jom.2006.0041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Cross-sectional risk of bias assessment using national institute of health quality assessment tool for observational cohort and cross-sectional studies.
Table S2. Newcastle ottawa scale risk of bias assessment for cohort studies.
Table S3. Risk of bias assessment for randomized controlled trials using the cochrane risk of bias tool.
Table S4. Summary of findings for studies evaluating psychiatric health outcomes.
Table S5. The impact of pain on substitute opioid therapy treatment outcomes.