Abstract
Schizophrenia is associated with subtle abnormalities in day-to-day social behaviors, including a tendency in some patients to “keep their distance” from others in physical space. The neural basis of this abnormality, and related changes in social functioning, is unknown. Here we examined, in schizophrenic patients and healthy control subjects, the functioning of a parietal–frontal network involved in monitoring the space immediately surrounding the body (“personal space”). Using fMRI, we found that one region of this network, the dorsal intraparietal sulcus (DIPS), was hyper-responsive in schizophrenic patients to face stimuli appearing to move towards the subjects, intruding into personal space. This hyper-responsivity was predicted both by the size of personal space (which was abnormally elevated in the schizophrenia group) and the severity of negative symptoms. In contrast, in a second study, the activity of two lower-level visual areas that send information to DIPS (the fusiform face area and middle temporal area) was normal in schizophrenia. Together, these findings suggest that changes in parietal–frontal networks that support the sensory-guided initiation of behavior, including actions occurring in the space surrounding the body, contribute to social dysfunction and negative symptoms in schizophrenia.
Keywords: Schizophrenia, Personal space, Faces, Object processing, Parietal cortex, Premotor cortex
Highlights
-
•
A parietal–frontal network in the primate brain monitors the space near the body.
-
•
“Personal space”, a person's “comfort zone”, is influenced by this network.
-
•
Patients with schizophrenia show an abnormal enlargement of personal space.
-
•
This enlargement correlates with negative symptom levels.
-
•
This enlargement also correlates with parietal responses to personal space intrusions.
1. Introduction
It has been found in a number of studies that abnormalities in social perception are predictive of levels of everyday functioning in schizophrenia (Couture et al., 2006; Green et al., 2012; Hooker and Park, 2002; Mancuso et al., 2011; Rassovsky et al., 2011). Other work indicates that impairments in social functioning may precede and predict the development of schizophrenia in those at risk (Alderman et al., 2014; Cannon et al., 2008; Kwapil, 1998). Thus, abnormalities in processing social information may represent a candidate target for early intervention efforts. However, the neurobiological mechanisms underlying these impairments is poorly understood.
Previously, social perception and cognition have been typically measured in schizophrenia using affect recognition, mentalization or social inferencing paradigms (Green and Leitman, 2008; Pinkham, 2014). These processes are at least partly dependent on semantic or real-world knowledge (e.g., of emotion labels or common social situations), which can be impaired in individuals with schizophrenia as a consequence of their illness. Given this, experimental paradigms that measure low-level, non-verbal processes involved in social behavior are needed (Green et al., 2013).
One such non-verbal process is social spacing, i.e. “personal space”. Personal space is the “comfort zone,” or preferred distance, that one individual maintains from another nearby person (Hayduk, 1983). Like eye gaze and facial expressions, personal space plays an important role in social communication. For example, greater physical proximity during social interactions promotes cooperation and affiliation (Collett, 1971; Kahn and McGaughey, 1977), whereas greater distances between people guard against physical threats and can convey mistrust (Dosey and Meisels, 1969; Graziano and Cooke, 2006; Lourenco et al., 2011). Although personal space is influenced by a number of variables, including familiarity, social status and cultural factors (Hayduk, 1983), there is also evidence for an “optimal distance” for individuals that stabilizes during adolescence (Bar-Haim et al., 2002; Hayduk, 1983).
Findings of enlarged or inflexible personal space have been consistently reported in schizophrenia (Deus and Jokic-Begic, 2006; Duke and Mullens, 1973; Horowitz et al., 1964; Nechamkin et al., 2003; Park et al., 2009; Srivastava and Mandal, 1990). In some studies, personal space abnormalities have been linked specifically to negative symptoms, which can include impairments in social behavior, such as social withdrawal (Nechamkin et al., 2003; Park et al., 2009). However, the cognitive or neural basis of these behavioral abnormalities is not known. One possibility, which we sought to investigate in the current study, is that the functioning of the parietal and frontal regions involved in monitoring and generating actions within the space near the body (“near space”) in primates (Brozzoli et al., 2011; Colby et al., 1993; Fogassi et al., 1996; Graziano et al., 1997; Sereno and Huang, 2006) is altered in patients with schizophrenia.
Although the neural mechanisms responsible for social spacing-related behaviors are incompletely understood, we recently found evidence that the near space-monitoring network in humans is 1) particularly sensitive to social information and 2) appears to influence personal space. In an fMRI study of 21 healthy subjects, we showed that two primary nodes of the near space-monitoring network, the dorsal intraparietal sulcus (DIPS) and the ventral premotor area (PMv), were preferentially responsive to images of human faces (i.e., social stimuli) that appeared to approach or “loom” towards (versus withdraw from) subjects (Holt et al., 2014). This approach-biased activity did not occur in response to non-social stimuli. This network also showed stronger resting-state functional coupling in individuals who preferred physical proximity to others, compared to those who preferred greater social distance, suggesting that it may play a role in determining personal space characteristics and perhaps related social behaviors.
Therefore, based on this prior work, in the current investigation we sought to test whether the function of this parietal–frontal network is (1) altered and (2) predictive of abnormalities in personal space in schizophrenia. Also, since abnormalities in schizophrenia in lower-level visual areas (Javitt, 2009), such as those dedicated to face perception or motion processing, could theoretically influence the function of this near space–monitoring sensory–motor pathway, in another cohort of schizophrenic patients and demographically-matched healthy subjects, we conducted additional control experiments measuring the function of lower-level visual areas in schizophrenia.
2. Materials and methods
2.1. Study 1: participants
For all subjects, the exclusion criteria included severe medical illness, significant head trauma, neurologic illness, substance abuse during the past 6 months and contraindications for MRI scanning (e.g., implanted metal objects, claustrophobia).
Healthy subjects were recruited via advertisement and screened for psychiatric illness using the Structured Clinical Interviewfor DSM-IV (SCID) (First et al., 1995); subjects with past or present psychiatric diagnoses were excluded from this group. Patients who met the DSM-IV criteria for schizophrenia according to the SCID were recruited and characterized by the Massachusetts General Hospital (MGH) Schizophrenia Program. The schizophrenia (n = 15) and control (n = 14, a subgroup of the Holt et al., 2014 cohort) groups were matched with respect to age, mean parental education and socioeconomic status, (see Table 1A). One additional healthy control was included in the functional connectivity analysis; the schizophrenia and control groups remained matched for demographic characteristics with this additional subject. Written informed consent was obtained from all subjects prior to enrollment in accordance with the guidelines of the Partners HealthCare Institutional Review Board. Levels of positive and negative symptoms were evaluated in each schizophrenic patient by one trained rater (DJH) using the Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987) on the day of scanning.
Table 1.
Demographic information about the subjects.
| Control |
Schizophrenia |
P-Value | |||
|---|---|---|---|---|---|
| (n = 14) |
(n = 15) |
||||
| Mean | SD | Mean | SD | ||
| A. Study 1: personal space and parietal–frontal function | |||||
| Age(years) | 26.0 | 6.5 | 30.1 | 9.1 | 0.18 |
| Premorbid IQa | 112.2 | 4.8 | 108.9 | 6.8 | 0.15 |
| Parental education (years) | 14.9 | 2.1 | 14.8 | 3.1 | 0.97 |
| PANSS Total | 52.3 | 12.0 | |||
| PANSS Positive Symptoms Subscale | 13.9 | 5.2 | |||
| PANSS Negative Symptoms Subscale | 12.9 | 5.2 | |||
| PANSS General Symptoms Subscale | 25.5 | 4.7 | |||
| Duration of illness (years) | 9.9 | 8.3 | |||
| Antipsychotic dose in chlorpromazine | 430.7 | 354.7 | |||
| equivalents (n = 7) | |||||
| B. Study 2: lower-level face and motion processing | |||||
| Age(years) | 40.7 | 14.4 | 41.3 | 11.4 | 0.9 |
| Premorbid IQa | 113.7 | 6.1 | 103.4 | 9.7 | 0.001 |
| Parental education (years) | 13.1 | 2.8 | 13.4 | 1.7 | 0.64 |
| PANSS Total | 47.5 | 10.3 | |||
| PANSS Positive Symptoms Subscale | 12.0 | 4.5 | |||
| PANSS Negative Symptoms Subscale | 12.5 | 4.6 | |||
| PANSS General Symptoms Subscale | 23.1 | 4.8 | |||
| Duration of illness (years) | 18.42 | 12.87 | |||
| Antipsychotic dose in chlorpromazine | 542.29 | 479.35 | |||
| equivalents (n = 17) | |||||
Demographic and clinical information about the subjects of Study 1 (A) and Study 2 (B) are listed. In Study 2, the cohort was older and the patients were more likely to be treated with antipsychotic medication, compared to Study 1. Also, p-values of independent Student's t-tests comparing the two groups on key demographic variables (age, premorbid IQ or parental education) are included.
Measured using the North American Adult Reading Test. PANSS, Positive and Negative Syndrome Scale.
2.2. Study 1: MRI data acquisition
All MRI data were collected on a 3 T Siemens Tim Trio scanner(Iselin, NJ). Two anatomical 3D MPRAGE scans were collected for each participant (TR = 2530 ms, TE = 3.39 ms, flip angle = 7°; 256 coronal slices, spatial resolution 3 mm isotropic voxels). 10 functional runs were collected (TR = 2000 ms, TE = 30 ms, flip angle = 90°; 33 axial slices; 3 mm isotropic voxels). In addition, one 6-min-20-s resting BOLD scan (TR = 5000 ms; TE = 30 ms; flip angle = 90°; 55 axial slices, 76 images per slice, 2 mm isotropic voxels) was acquired, during which subjects were instructed to keep their eyes open and blink normally.
2.3. Study 1: stimuli
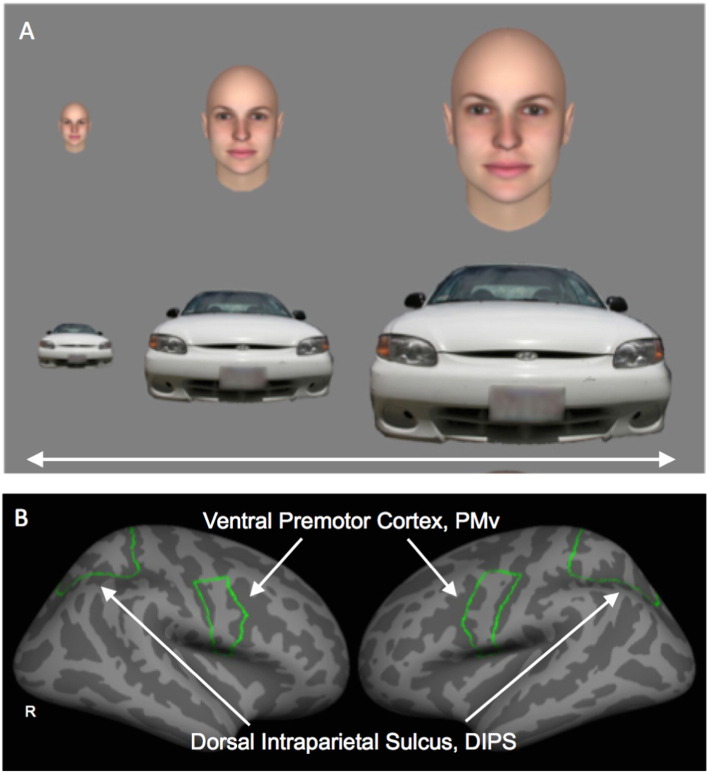
During each functional run, subjects viewed stimuli that appeared to either approach or withdraw from the subject (i.e., expand or contract in size) (Holt et al., 2014), Stimuli were images of human faces (3 males and 3 females, with neutral facial expressions) or cars. Each of the four conditions of interest (i.e., Face Approach, Face Withdrawal, Car Approach, Car Withdrawal) was presented in a block of 16 s duration (Fig. 1A). Two 16-s fixation blocks were presented at the beginning and end of each run. In each run, subjects viewed two blocks of each of the four conditions, randomly presented. The minimum stimulus size was 120 × 120 pixels and the maximum stimulus size was 43,239 × 43,239 pixels. The stimuli changed in size, appearing to approach or withdraw from the subject, at a rate equivalent to a speed of 112 cm/s — a typical speed for walking. The face stimuli were created with FaceGen (http://www.facegen.com), a program used to create realistic human faces. The car stimuli were constructed from photographs of cars.
Fig. 1.
Experimental stimuli and regions-of-interest (Study 1). Examples of each of the stimuli types (faces and cars) used in Study 1 are shown in A. The boundaries of the two anatomically-defined regions-of-interest, the dorsal intraparietal sulcus (DIPS) and the ventral premotor cortex (PMv), are displayed on the FreeSurfer cortical surface template in B. Also see Fig. 3 for posterior views of DIPS. R, Right.
2.4. Study 1: attentional task
To distribute attention evenly across the Approach and Withdrawal conditions, subjects performed a “dummy attention task”, by pressing a button whenever a dot appeared on screen, while maintaining fixation. Five dots appeared per 16-s block, beginning at a random time between 980–1980 ms following trial onset. The duration of the dot stimulus varied from 500–1500 ms, and dot size varied with eccentricity. The percentage of responses for each subject was calculated for each condition.
2.5. Study 1: task-based fMRI analysis
Functional data were sampled onto the cortical surface and projected onto an average spherical representation using the FreeSurfer analysis stream (http://surfer.nmr.mgh.harvard.edu). The two contrasts of interest (Approaching Faces vs. Withdrawing Faces, and Approaching Cars vs. Withdrawing Cars) were examined using a t-statistic at each vertex on the spherical surface using random effects. Only four runs of the data were included for each subject, in order to permit the exclusion of runs confounded by poor performance on the attentional task or head motion, while maintaining approximately the same number of runs per subject (and per group) in the analyses. Thus, the first four runs that met the behavioral and motion inclusion criteria (see below) for each subject were included in the analyses. One subject (a schizophrenic patient) had only three runs that met these criteria. Of note, this procedure did not result in between-group differences in the runs included. Runs 1–4, or runs 1–3 plus one other run, were used for the 14 controls and for 12 of the 15 schizophrenic patients; run 1 plus later runs (runs 5+) were used in 3 patients.
The whole-brain-corrected level of significance of clusters of activated vertices was determined using a Monte Carlo simulation with 10,000 iterations, with a voxel and cluster threshold of p = .05 (the primary analysis). Clusters found within the two anatomically defined regions of interest, the dorsal intraparietal sulcus (DIPS) and the ventral premotor area (PMv), were also reported, for completeness, if they met a more liberal level of significance (p = .01 uncorrected, cluster size of 10 mm2).
As previously described (Holt et al., 2014), the boundaries of the DIPS and PMv regions-of-interest (ROIs) were independently defined in each subject using the cortical parcellation automatically generated by FreeSurfer (Fig. 1B). The boundaries of the superior parietal gyrus were used to delineate DIPS, corresponding to the portion of the dorsal parietal lobe surrounding the intraparietal sulcus (Orban et al., 2003). The ventral boundary of DIPS was composed of the dorsal border of the superior occipital gyrus and the superior and transversalis occipital sulci. PMv was defined as the ventral portion of the precentral gyrus. The dorsal border of PMv was defined as the ventral border of the superior precentral sulcus.
2.6. Study 1: resting-state fMRI analysis
A seed-based functional connectivity analysis was conducted using the resting-state BOLD scans. Standard preprocessing techniques for resting-state functional connectivity analyses (Buckner et al., 2009) were used to selectively capture variance in the BOLD signal corresponding to low-frequency (< .08 Hz) fluctuations in neural activity during the resting-state. Nuisance regressors, including the six parameters computed from the rigid-body motion correction, the averaged signal within a ventricular region-of-interest, a region within the deep white matter, and the signal averaged over the whole brain, were used to remove systematic variance associated with these variables. The first temporal derivative of each regressor was also included to account for temporal shifts in the BOLD signal.
To create whole-brain correlation images, the averaged time series across all voxels within the seed (see below) was used as the variable of interest in a linear regression with the time series corresponding to each voxel across the brain. The statistical analyses of these correlational data were performed on Fisher z transforms (Zar, 1996). Group-level functional connectivity maps (one and two sample t-tests, random effects) were constructed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm) and clusters which met a threshold of p < .05, cluster-level Family Wise Error (FWE)-corrected level of significance across the whole brain, were considered significantly correlated with the seed.
2.7. Study 1: measurements of personal space size and social activity levels
Personal space size was measured using the “Stop-Distance” procedure (Kaitz et al., 2004), a validated method for measuring personal space size with high reliability (kappa ~ .8) (Hayduk, 1983). The procedure is conducted as follows: An experimenter and a participant stand 3 m apart. The experimenter slowly walks towards the participant, maintaining a neutral facial expression and eye contact. Prior to the procedure, the participant had been instructed to say “stop” when he/she feels “slightly uncomfortable”, i.e., when his/her personal space (“the typical distance you stand from someone you've never met before”) has been entered. The experimenter notes this distance as the participant's personal space size (Distance 1, D1). Prior to the procedure the participant was also told that the experimenter would then continue to walk towards him or her, and the participant should say “stop” again when he/she feels “very uncomfortable” (Distance 2, D2). The ratio of D1 and D2 (100 − ((D2 ∗ 100) / D1)) indicates the participant's ability to tolerate personal space intrusion (the “permeability” of personal space). The entire procedure is then repeated with a second experimenter of the opposite gender. The order of the two experimenters (male or female first) is counterbalanced across participants.
In addition, on the day of scanning, a questionnaire was administered which asked each subject to estimate the average percentage of his/her waking hours spent with other people versus alone, and the percentage of his/her waking hours that he/she preferred to spend with other people versus alone (Holt et al., 2014).
2.8. Study 1: quality control
Registration of each subject's functional scans to the T1 scan, as well as slice coverage, was checked manually. As previously described (Holt et al., 2014), a functional run was excluded if the total absolute head motion was greater than 2 mm, or if the response rate during the attentional task was less than 40%. For the resting-state scans, we planned to exclude any run with an SNR less than 125 or absolute head motion greater than 2 mm. All subjects' resting-state data met these criteria (i.e., no runs were excluded).
2.9. Study 1: correlations
The primary goal of this study was to determine whether abnormalities in personal space characteristics in schizophrenia are linked to altered parietal–frontal function. Accordingly, we tested the following hypothesis: Enlargement of personal space in schizophrenia predicts functioning (activation or connectivity) of the DIPS–PMv network. In addition, based on prior work (Nechamkin et al., 2003; Park et al., 2009), we also tested for associations between levels of negative symptoms in the schizophrenic patients and 1) personal space size and 2) functioning of the DIPS–PMv network.
Thus, we calculated correlations between DIPS and PMv responses to looming faces (BOLD responses for the Approaching vs. Withdrawing Faces contrast, extracted from DIPS and PMv (defined in each individual using the FreeSurfer-generated anatomical delineations)) and these behavioral measures, using Spearman's rho.
For correlations between DIPS–PMv resting-state functional connectivity versus personal space size or negative symptoms, we conducted separate voxel-wise, multiple regression analyses using each behavioral measure as an effect of interest, using the DIPS ROI as the seed (Holt et al., 2014). These regression analyses were restricted to the regions showing positive connectivity (p < .05, 20 voxel size threshold) to the DIPS seed. For these analyses, we defined as significant those correlations that met the cluster-level FWE whole-brain correction, p < .05. In addition, we also reported clusters within PMv that met a lower level of significance (p < .01 uncorrected, cluster size ≥100 voxels).
Additional correlations, such as with potential confounds (antipsychotic medication dose, duration of illness), positive symptom severity or sociability levels, were conducted on an exploratory basis.
Lastly, effect sizes (Cohen's d) are reported for any significant correlations and t-tests, in order to provide an estimation of the effect's strength.
2.10. Study 2: participants and MRI data acquisition
19 patients with DSM-IV-diagnosed schizophrenia and 16 healthy control subjects were enrolled in this study, using the same recruitment and assessment approaches described above. Only two subjects (one control and one patient) participated in both Study 1 and 2. As in Study 1, the schizophrenia and control groups were matched with respect to age, gender, and parental education and socioeconomic status (see Table 1B). Also as above, all data were acquired on a Siemens Tim Trio 3 T scanner (with BOLD data resolution of 3 mm isotropic voxels).
2.11. Study 2: stimuli
In this experiment, subjects viewed two sets of stimuli (i.e., two different stimulus contrasts). One was designed to measure activity of the fusiform face area (FFA), a well-characterized face-selective area in the ventral visual stream (Kanwisher et al., 1997). The second stimulus contrast was designed to measure activity of the human middle temporal area (MT+, which likely includes V5), which is a distinctive motion-selective area in the dorsal visual stream (Tootell et al., 1995b; Watson et al., 1993; Zeki et al., 1991). Each experimental paradigm was presented over six 224-s runs. The FFA paradigm included alternating 16-s blocks of photographs of faces or places (rooms or other interior spaces), with 5 blocks of each type (Rajimehr et al., 2009). Each individual photograph (16 per block) was presented for 1 s. For the MT+ paradigm, subjects viewed alternating 16-s blocks of moving and stationary concentric circles (Tootell et al., 1995a). The moving and stationary circles were presented at 5 different contrast levels (1, 3, 10, 30, 100%), at a fixed, ascending order (moving 1%, stationary 1%, moving 3%, stationary 3%, moving 10%, stationary 10%, moving 30%, stationary 30%, moving 100% and stationary 100%) within each run, with 10 blocks per run. All runs of the experiment began and ended with a 32-s-long block during which a fixation cross was presented.
2.12. Study 2: attentional task
Each stimulus of both paradigms included a fixation in the center of the screen that changed color (red to green) for 400 ms, every 5–10 s, 25–27 times during each run. The subjects were asked to press a button when they saw the fixation cross change color.
2.13. Study 2: fMRI data analyses
Cortical maps were constructed for each subject using FreeSurfer. Activation levels were measured for each subject in two functionally-defined regions-of-interest (ROIs), FFA and MT+. Area MT+ has been previously defined as an oval shaped region of ~120 mm2 (Tootell et al., 1995b). In order to compare the responses of these two regions directly, we measured activation levels within these ROIs while keeping the size of the ROIs relatively constant. Thus the ROIs for FFA and MT+ were constructed by manually drawing the boundary of the activation in each individual subject that was centered around the vertex with maximal activation, with an area ~120 mm2 (±5%; areas ranged from 114–126 mm2), in the fusiform gyrus and middle temporal gyrus using the relevant contrast (Faces > Places (FFA) and Moving > Stationary (MT+)). The threshold was varied across subjects in order to maintain a fixed ROI size. For inclusion in the primary analysis, the surface area of each ROI had to meet a minimum threshold of 114 mm2, at a significance level of p < .05.
3. Results
3.1. Study 1: behavior
Consistent with prior work, the size of personal space was significantly larger in the schizophrenic patients compared to the controls (t(27) = 3.9, p = .0006, d = 1.50; Fig. 2A). Moreover, personal space size was significantly correlated with levels of negative (r = .53, p = .04, d = 1.25) (Fig. 2B), but not positive (r = .14, p = .62) symptoms in these patients.
Fig. 2.
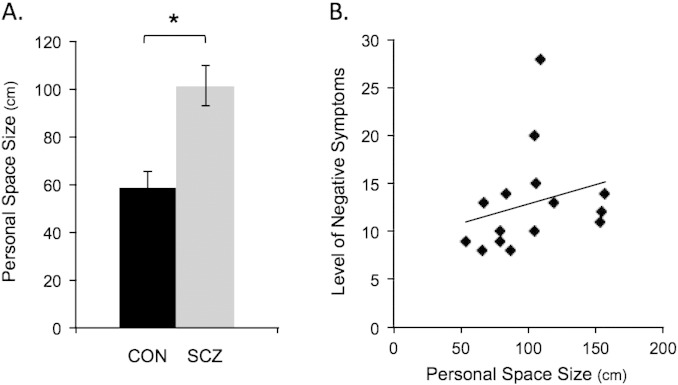
Personal space. The bar plots in A show the mean size of personal space in centimeters for the 14 controls (CON, in black) and 15 schizophrenic patients (SCZ, in gray; * p = .0006). The scatter plot in B shows the significant correlation (r = .53, p = .04, d = 1.25) between personal space size and negative symptom levels (measured using the PANSS, the Positive and Negative Syndrome Scale) in the schizophrenia group. In addition, exploratory analyses revealed that in controls, but not in patients, the size of personal space was negatively correlated with the amount of time preferred with others (r = − .54, p < .05, d = 1.28). PANSS, the Positive and Negative Syndrome Scale; r, Spearman's rho; CON, controls; SCZ, schizophrenic patients; cm, centimeters.
There were no differences between the two groups in personal space permeability (t(27) = .6, p = .56) or in in self-reported percentages of time actually spent or preferred in the company of others (ts < 1.7; ps > .11).
With respect to behavioral responses during fMRI data collection, there were no significant differences in response rates between approaching vs. withdrawing stimuli (overall, as well as within the face or car conditions) or between the face vs. car stimuli (all ps > .26), and there were no significant between-group differences (all ps > .22) (Supplementary Fig. 1).
3.2. Study 1: task-elicited fMRI responses
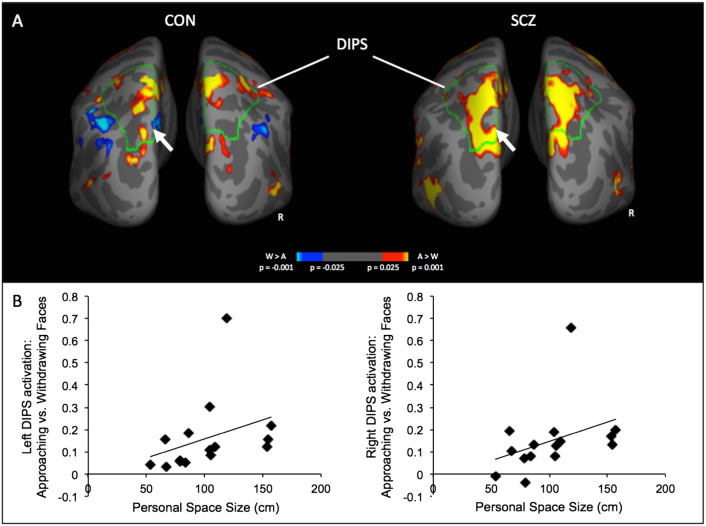
Both the controls and schizophrenic patients showed DIPS and PMv responses to approaching vs. withdrawing faces (see Table 2 for the coordinates of the DIPS and PMv clusters and of other significant loci for this contrast, which were found in the frontal and somatosensory cortices in controls and the frontal and occipitotemporal cortices in the schizophrenic patients). A direct between-group comparison revealed that the schizophrenic patients showed significantly greater activation in the left DIPS (Fig. 3A), as well as in the left lateral frontal cortex and right middle temporal gyrus, than the controls. There were no areas that showed greater activation in the controls compared to the patients.
Table 2.
Clusters showing significant activation to approaching vs. withdrawing faces.
| Region | BA | Tal (x, y, z) | Area (mm2) | Peak p-value | Z |
|---|---|---|---|---|---|
| A. Controls | |||||
| Face Approach > Withdrawal | |||||
| L DIPS | 7 | −14, −49, 59 | 1699 | 2 × 10−5 | 4.24 |
| R DIPS | 7 | −10, −57, 59 | 1520 | 4 × 10−6 | 4.62 |
| L PMv* | 6 | −50, 2, 5 | 26 | 5 × 10−3 | 2.81 |
| 4 | −52, −4, 43 | 14 | 7 × 10−3 | 2.72 | |
| R PMv* | 6 | 55, 5, 29 | 36 | 9 × 10−4 | 3.31 |
| L postcentral | 1/2 | −55, −17, 34 | 591 | 3 × 10−4 | 3.62 |
| R postcentral/superior parietal | 1/7 | 26, −34, 50 | 740 | 7 × 10−4 | 3.37 |
| L caudal middle frontal | 6 | −25, 1, 42 | 585 | 4 × 10−4 | 3.54 |
| R superior frontal/cingulate | 24 | 13, −2, 39 | 639 | 2 × 10−4 | 3.68 |
| Face Withdrawal > Approach | |||||
| L inferior parietal | 7/40 | −40, −58, 43 | 1103 | 2 × 10−4 | 3.72 |
| L caudal middle frontal | 9 | −40, 21, 28 | 617 | 3 × 10−3 | 3.01 |
| B. Schizophrenic patients | |||||
| Face Approach > Withdrawal | |||||
| L DIPS | 7 | −23, −60, 51 | 3309 | 6 × 10−10 | 6.19 |
| R DIPS | 7 | 19, −60, 51 | 3093 | 2 × 10−7 | 5.21 |
| L PMv* | 6 | −45, 1, 28 | 101 | 2 × 10−3 | 3.07 |
| R PMv | 6 | 32, −1, 41 | 1770 | 1 × 10−5 | 4.42 |
| L occipital/middle temporal | 37 | −42, −60, 5 | 823 | 2 × 10−7 | 5.21 |
| L superior/middle frontal | 6 | −20, −2, 48 | 1826 | 3 × 10−6 | 5.14 |
| L superior temporal | 41/42 | −41, −33, 7 | 2044 | 2 × 10−5 | 4.27 |
| C. Schizophrenic patients > controls | |||||
| Face Approach > Withdrawal | |||||
| L DIPS | 7 | −13, −72, 44 | 712 | 2 × 10−4 | 3.75 |
| L caudal middle frontal | 9 | −41, 23, 28 | 982 | 4 × 10−4 | 3.58 |
| R middle temporal | 21 | 48, 4, −30 | 529 | 3.4 × 10−3 | 2.93 |
The location (based on the FreeSurfer parcellation), size, and significance level (z score and p-value of the peak vertex) of clusters of activation (meeting the whole-brain-corrected level of significance) for the approaching vs. withdrawing faces contrast for the controls (A), schizophrenic patients (B) and for the comparison between the two groups (C) are listed. The asterisks indicate clusters in the ventral premotor area (PMv) that met a significance level of p = .01 uncorrected, cluster size = 10 mm2. BA, Brodmann Area; Tal, Talairach coordinates; L, left; R, right; DIPS, dorsal intraparietal sulcus and PMv, ventral premotor area.
Fig. 3.
DIPS responses to approaching vs. withdrawing faces. Posterior views of cortical surface maps of average activation to approaching vs. withdrawing faces for the control subjects (CON, n = 14), schizophrenic patients (SCZ, n = 15) are shown in A. The outlines of the anatomically-defined DIPS region of the cortical surface template is shown in green and labeled. The location of the peak between-group difference in DIPS (SCZ > CON) is indicated with white arrows. Scatter plots of the significant correlations between personal space size and the left and right DIPS responses to approaching vs. withdrawing faces are shown in B (left DIPS: r = .62, p = .013; right DIPS: r = .56, p = .03). After removing one relative outlier evident in these plots who had very high DIPS responses, the correlations remain significant or near significant (left DIPS: r = .54, p = .046; right DIPS: r = .53, p = .051). CON, controls; SCZ, schizophrenic patients; DIPS, dorsal intraparietal sulcus; R, right; A, approaching faces and W, withdrawing faces.
Consistent with our previous findings (Holt et al., 2014), neither group showed approach-biased responses to car stimuli in DIPS or PMv. In other cortical regions, withdrawal-biased responses to the car stimuli were seen in both groups (Table 3), with significant between-group differences in the right lateral orbitofrontal and middle temporal cortices (Control > Schizophrenia for the contrast: Car Withdrawal > Approach).
Table 3.
Clusters showing significant activation to approaching vs. withdrawing cars.
| Region | BA | Tal (x, y, z) | Area (mm2) | Peak p-value | Z |
|---|---|---|---|---|---|
| A. Controls | |||||
| Car Approach > Withdrawal | |||||
| R superior parietal | 19 | 24, −78, 30 | 670 | 1 × 10−4 | 3.89 |
| Car Withdrawal > Approach | |||||
| R pars orbitalis/lateral orbitofrontal | 11 | 36, 50, −10 | 3104 | 7 × 10−6 | 4.50 |
| R isthmus cingulate | 23 | 5, −33, 29 | 836 | 6 × 10−5 | 4.01 |
| L inferior parietal | 7/19 | −30, −61, 41 | 1876 | 1 × 10−4 | 3.89 |
| R inferior parietal | 7/40 | 45, −50, 43 | 1983 | 3 × 10−4 | 3.62 |
| L rostral middle frontal | 46 | −32, 46, 13 | 1694 | 2 × 10−4 | 3.72 |
| R middle temporal | 21 | 64, −34, −9 | 1225 | 2 × 10−4 | 3.72 |
| B. Schizophrenic patients | |||||
| Car Withdrawal > Approach | |||||
| L inferior parietal | 7 | −33, −69, 45 | 705 | 1 × 10−4 | 3.89 |
| R inferior parietal | 40 | 49, −50, 43 | 2313 | 2 × 10−7 | 5.21 |
| L precuneus | 7/31 | −7, −38, 41 | 689 | 1 × 10−4 | 3.89 |
| R precuneus | 31 | 13, −57, 25 | 616 | 3 × 10−6 | 4.53 |
| L lateral orbital frontal | 47/11 | −32 29, −4 | 502 | 1 × 10−4 | 3.89 |
| R rostral middle frontal | 10 | 22, 53, −1 | 551 | 8 × 10−5 | 3.95 |
| 9 | 46, 24, 26 | 1420 | 8 × 10−5 | 3.95 | |
| C. Controls > schizophrenic patients | |||||
| Car Withdrawal > Approach | |||||
| R lateral orbitofrontal | 11 | 17, 35, −18 | 605 | 2 × 10−4 | 3.72 |
| R middle temporal | 21 | 60, −36, −10 | 640 | 3 × 10−4 | 3.62 |
The location (based on the FreeSurfer parcellation), size, and significance level (z score and p-value of the peak vertex) of clusters of activation (meeting a whole-brain-corrected level of significance) for the approaching vs. withdrawing cars contrast for the controls (A), schizophrenic patients (B) and for the comparison between the two groups (C) are listed. BA, Brodmann Area; Tal, Talairach coordinates; L, left; R, right; DIPS, dorsal intraparietal sulcus; PMv, ventral premotor area.
Correlational analyses revealed that in the schizophrenia group, but not in the controls, personal space size correlated with activation of the left (r = .62, p = .013, d = 1.58) and right (r = .56, p = .03, d = 1.35) DIPS to Approaching vs. Withdrawing Faces (Fig. 3B).
3.3. Study 1: resting-state fMRI
We then measured the functional connectivity of the portion of DIPS showing abnormally elevated responses in the schizophrenic patients, in order to determine whether this region showed abnormal functional coupling with PMv or other areas of the brain in the schizophrenia group. Both the controls and patients showed significant functional connectivity between this DIPS seed and a portion of the lateral frontal cortex in or near the anatomically-defined PMv region-of-interest (Supplementary Fig. 2). A between-group comparison revealed that at a whole-brain-corrected level of significance, there was significantly lower connectivity between DIPS and the left fusiform gyrus in the schizophrenic patients compared to the controls (Talairach coordinates of peak [x, y, z] = −38, −49, −6, z = 4.24, p < .0001, 3975 voxels).
Whole-brain regression analyses revealed that levels of negative symptoms in the schizophrenic patients were negatively correlated with the strength of functional connectivity between DIPS and lower-level visual areas (BA 19/39) at a whole-brain-corrected level of significance (Talairach coordinates of peak [x, y, z] = 46, −82, 21, z = 3.87, p < .0001, 3235 voxels).
In addition, at a lower significance threshold, personal space size was negatively correlated with DIPS–PMv connectivity both in the controls, as shown previously (Holt et al., 2014) (34, –5, 26, z = 3.53, p = .0004, 635 voxels; 50, 3, 15, z = 3.19, p = .001, 330 voxels), and in the schizophrenic patients (–38, 13, 20, z = 3.47, p = .0005, 144 voxels; Supplementary Fig. 3). In the schizophrenia group, there was also a negative correlation between levels of negative symptoms and DIPS–PMv connectivity (40, 1, 28, z = 3.83, p = .0001, 221 voxels).
3.4. Study 1: potential confounds
None of the abnormalities observed in the schizophrenic patients were found to be correlated with antipsychotic medication dose or duration of illness.
3.5. Study 2: characteristics of FFA and MT+
One possible interpretation of the findings of Study 1 is that inputs to DIPS from lower-level motion and/or face processing regions could be disrupted in the schizophrenic patients, secondarily resulting in abnormal DIPS responses to moving face stimuli. To examine this possibility, we measured the function of two key brain regions involved in face and motion processing, the fusiform face area (FFA) (Kanwisher et al., 1997) and the middle temporal area (MT+) (Tootell et al., 1995b), respectively, in a second study. To ensure that our findings were not due to heterogeneity among the subjects in FFA or MT+ function (e.g., the presence of a subpopulation of patients showing poor FFA or MT+ function that fails to result in significant between-group differences), we used an individual regions-of-interest approach, in which we defined FFA and MT+ and measured FFA and MT+ responses in each subject.
Using a consistent size criterion (see the Materials and methods section), we found that MT+ was not detectable in the right hemisphere of one control subject, in the left hemisphere of one schizophrenic patient, and in both hemispheres of another schizophrenic patient. Also, FFA was not present in the left hemisphere of one control subject, and in both hemispheres of one schizophrenic patient. There were no significant differences between the two groups in the number of subjects with detectable FFA or MT+ (all ps > .49, Chi Square Test). Also, as expected (given the analysis approach), the mean areas for the FFA and MT+ ROIs, and the mean thresholds at which the ROIs were defined, did not differ between the two groups.
3.6. Study 2: face-selective activation of FFA
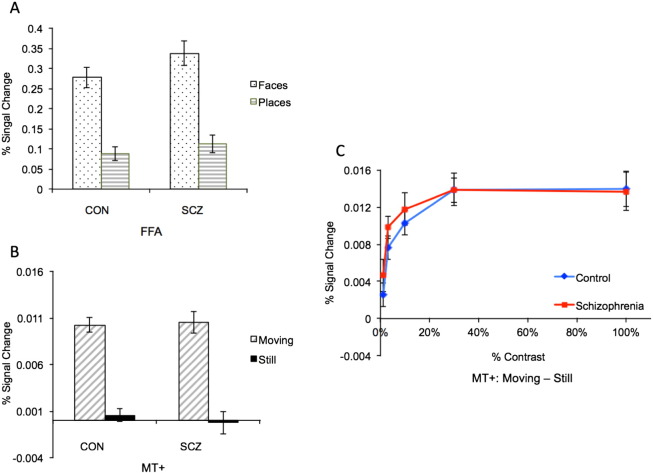
We found no differences between the two groups in FFA activation magnitudes (t(31) = 1.42; p = .17) (Fig. 4A).
Fig. 4.
FFA and MT+ responses. Bar plots of the average responses in the schizophrenic patients (SCZ) and controls (CON) subjects to faces and places (A) and to moving and stationary (i.e., “still”) circles (B) are shown. In C, responses of MT+ to moving vs. stationary circles at different contrast levels reveal that MT+ showed a similar degree of sensitivity to low contrast stimuli (a characteristic feature of MT+) in both groups. FFA, fusiform face area; MT+, middle temporal area; CON, control group and SCZ, schizophrenia group.
3.7. Study 2: contrast-dependent responses of MT+
There was no difference between the two groups in MT+ activation overall, or at any contrast level (i.e., no significant effect of group (F(1,31) = .77; p = .39) or interaction of group by contrast level (F(4) = .47; p = .76) (Fig. 4B, C)).
3.8. Study 2: additional analyses
In addition, we found no significant between-group differences when FFA and MT+ were functionally defined on a group basis using group-averaged maps (i.e., two ROIs (left and right) per region per group) and all subjects were included (16 controls and 19 schizophrenic patients). Lastly, there were no significant differences between the two groups in activation of FFA or MT+ when these group-averaged maps were directly compared, even when the threshold was lowered to p < .05.
4. Discussion
4.1. Summary of the main findings
Consistent with prior findings, we found that the size of personal space was, on average, larger in patients with schizophrenia, as compared to demographically-matched healthy subjects. Moreover, personal space enlargement in the schizophrenic patients predicted both negative symptom severity and elevated responses of a dorsal parietal cortical region known to be involved in monitoring the space surrounding the body. In a second cohort, the function of two key lower-level visual processing areas, FFA and MT+, were found to be intact in schizophrenic patients, consistent with previous reports (Green et al., 2009; Tregellas et al., 2004; Wynn et al., 2008; Yoon et al., 2006), suggesting that basic perceptual inputs subserving social spacing are not grossly impaired in schizophrenia. Taken together, these findings suggest that schizophrenia may be associated with an abnormality in the integration of sensory inputs required for monitoring the environment surrounding the body, with detrimental consequences for social behavior during interactions with others.
4.2. Enlarged personal space in schizophrenia and negative symptoms
Our findings replicate and extend the findings of six prior studies of social spacing in schizophrenia. Remarkably, these prior investigations and ours found roughly similar results, despite the wide range of methods used to measure personal space across these studies, which included indirect paper-and-pencil, projective measures (Duke and Mullens, 1973; Nechamkin et al., 2003), “virtual distance” preferred from a “virtual person” (Park et al., 2009), physical distance preferred from life-sized photographs of people with emotional facial expressions (Srivastava and Mandal, 1990), as well as the physical distance preferred from other people (the approach used here) (Deus and Jokic-Begic, 2006; Horowitz et al., 1964). Also, two of these prior studies found evidence that negative symptoms are linked to abnormalities in social spacing in schizophrenia (Nechamkin et al., 2003; Park et al., 2009), consistent with the correlation we observed between personal space size and negative symptom severity.
Recent evidence for a similar expansion of personal space in patients with autism (Gessaroli et al., 2013), a disorder characterized by deficits in basic social functioning, may provide clues about the mechanisms underlying these behavioral changes. This finding and the association reported here between personal space enlargement and negative symptoms suggest that abnormalities in social spacing may occur in the context of more global abnormalities in day-to-day social behavior. Also, the negative correlation we observed between the size of personal space and the amount of time preferred with others in healthy subjects further supports the possibility that personal space-related behaviors may broadly index an aspect of social skill or motivation that varies dimensionally across both healthy and patient populations.
Similarly, the results of our functional connectivity analyses support a dimensional model of the functioning of this network. Although we found no significant differences between the schizophrenia and control groups in the average connectivity strength of the DIPS–PMv pathway, a larger personal space size in both groups and greater severity of negative symptoms in the schizophrenic patients predicted weaker DIPS–PMv connectivity.
It is possible that discomfort with the physical proximity of others represents a consequence of another type of impairment (e.g., a deficit in a domain of social perception, such as facial affect recognition or mentalizing). If such a deficit were present, increasing personal distance could serve to reduce overall processing loads or arousal levels during social interactions, and provide more time for perceptual processing. Alternatively, a fundamental abnormality in processing information within personal space may be present in some individuals.
4.3. Hyper-responsivity of the parietal cortex in schizophrenia
In this study, the size of personal space also predicted the magnitude of DIPS responses to “looming” face stimuli in the schizophrenic patients. This finding can be interpreted in several ways. First, the change in neural activity may result from the cognitive–behavioral abnormality. In other words, if DIPS neurons increase their firing when salient objects enter personal space, a larger personal space will, in turn, lead to a larger amount of DIPS activity. Alternatively, portions of the parietal cortex may function inefficiently in schizophrenia, leading to parietal cortex hyper-responsivity and a range of behavioral abnormalities, including aberrant social spacing behavior. Distinguishing between these two interpretations will require additional study. However, several pieces of existing evidence support the second, “bottom-up” model.
The parietal cortex plays a role in a variety of sensory integration and sensory-motor coordination processes (Teixeira et al., 2014). Some of these processes may be affected in schizophrenia, including the guidance and initiation of action by sensory input, such as those supporting saccadic eye movements (Lee and Williams, 2000), as well as processes involved in action imitation (Thakkar et al., 2014) and in distinguishing one's body parts (the “bodily self”) from those of others and from the external world (Gallese and Ferri, 2013; Parnas et al., 2005; Thakkar et al., 2011). Several studies have reported findings of abnormal elevations in parietal cortex activity during such processes in schizophrenic patients. For example, one fMRI study found that the intraparietal sulcus showed greater activation bilaterally during a visual saccade task in medication-free schizophrenia patients compared to controls; this abnormality normalized following treatment with antipsychotic medication (Keedy et al., 2009). Another recent study reported abnormally elevated responses in schizophrenic patients, compared to controls, in the left superior parietal cortex (Brodmann area (BA) 7), as well as of other portions of the parietal cortex and the premotor cortex, during a facial expression imitation task (Lee et al., 2014). A third study showed that the right posterior parietal cortex (BA 7/40) was overactive during target anticipation during a working memory task in schizophrenic patients (Quintana et al., 2003a). In addition to these findings in posterior or superior/dorsal parietal cortex, a number of studies have found that the metabolism, task-induced responses or functional connectivity of medial parietal cortex (i.e., the posterior cingulate gyrus and/or precuneus) is abnormally elevated in schizophrenic patients (Andreasen et al., 1997; Ebisch et al., 2014; Haznedar et al., 1997; Holt et al., 2011a; Holt et al., 2011b; Reske et al., 2009). Overall, these reports suggest that the function of both the medial and dorsal parietal cortex may be altered in schizophrenia.
The pathophysiological process(es) underlying these findings are unknown. However, we speculate that since the parietal cortex is one of the last areas to become myelinated in the human brain, it may be particularly vulnerable to the pathological events associated with the onset of schizophrenia (which occurs typically during late adolescence or early adulthood). In light of the large literature documenting changes in brain areas outside of the parietal lobe in schizophrenia, it is unlikely that schizophrenia is associated with a localized “lesion” of the parietal cortex (or of any single region), but instead may result from changes in the coordinated functioning of distributed networks including parietal cortex. Our results specifically suggest that changes in parietal–frontal circuits that support the sensory-guided initiation of behavior, including those occurring in the space surrounding the body, may contribute to the social dysfunction seen in schizophrenia. Recent models proposing that negative symptoms are related to a deficit in the generation of effort in pursuit of rewards (Gold et al., 2012; Strauss and Gold, 2012) are consistent with dysfunction of a network supplying sensory information to the frontal cortex for the purpose of generating actions.
4.4. Reduced connectivity between the intraparietal sulcus and fusiform gyrus in schizophrenia
In the second study described here, responses of two brain areas, the fusiform face area (FFA) and the middle temporal area (MT+), which each provide key input to the dorsal parietal cortex needed for processing dynamic social information (including images of faces moving towards the body), showed responses in schizophrenic patients that were comparable to controls. These negative results are consistent with the findings of many (Green et al., 2009; Wynn et al., 2008; Yoon et al., 2006), although not all (Chen et al., 2008; Martinez et al., 2008; Quintana et al., 2003b) prior studies of these two regions in schizophrenia. However, in contrast to these regional activation findings, we observed a significant reduction in functional connectivity between the fusiform gyrus and the DIPS in schizophrenic patients compared to controls, suggesting that communication between these two regions may be disrupted in schizophrenia.
4.5. A deficit in sensory or sensory-motor integration?
The pattern of DIPS connectivity observed in healthy subjects, with its coupling to both dorsal and ventral visual stream regions (Holt et al., 2014), suggests that it is involved in integrating inputs from spatial and object processing streams for the purpose of initiating motor responses to incoming stimuli. Abnormalities in this circuitry in schizophrenia may lead to impairments in the sensory–motor coupling required for the performance of basic social behaviors. We propose that this impaired coupling leads to changes in behavior during social interactions (including personal space abnormalities), which then may elicit negative feedback from others, which, over time, may result in a tendency to avoid social interactions (i.e., social withdrawal) (Green et al., 2012). Given the evidence for activity-dependent plasticity of parietal-frontal circuits (Albert et al., 2009) and relationships between frontal network strengths and social activity levels (Bickart et al., 2012; Sallet et al., 2011), social avoidance may further worsen any pre-existing structural or functional abnormalities of these pathways. However, the evidence for plasticity in these networks also raises the possibility that deficits within this system could be modified and potentially reversed via interventions designed to engage these regions.
4.6. Limitations
In these studies, parietal–frontal and lower-level visual functioning were not measured in the same group of subjects; thus, additional confirmation of the reported dissociation between the functioning of the near space-monitoring network and its lower-level inputs in schizophrenia is needed. Also, the sample sizes of these two studies were modest. However, fMRI paradigms that engage early–mid stage visual areas (such as the paradigms used here) typically produce robust activation in individual subjects; this aspect of our experimental design and the effect sizes of our findings (ds ≥ 1.25) suggest that our analyses were adequately powered.
4.7. Future directions
Because this system has a developmental trajectory (personal space size tends to stabilize in early adulthood (Hayduk, 1983)), quantifying this trajectory in healthy subjects, as well as understanding whether it is altered in individuals who are at risk for neuropsychiatric illnesses, including schizophrenia, may shed light on the timing of the development of social impairments in these conditions. Also, this network could potentially serve as a quantifiable target of interventions that aim to treat the social impairments seen in a wide range of neuropsychiatric illnesses, including autism and social phobia as well as schizophrenia.
Conflicts of interest
None of the authors report conflicts of interest relevant to this study.
Acknowledgments
This work was supported by the National Institute of Mental Health K23MH076054 (DJH) and a NARSAD Independent Investigator Award (RBHT), as well as by grants (1S10RR023043 and 1S10RR023401) for shared resources provided by the Martinos Center.
Footnotes
Supplementary material for this article can be found online at http://dx.doi.org/10.1016/j.nicl.2015.07.008.
Contributor Information
Daphne J. Holt, Email: dholt@partners.org.
Emily A. Boeke, Email: emilyboeke@nyu.edu.
Garth Coombs, III, Email: gcoombs@nmr.mgh.harvard.edu.
Stephanie N. DeCross, Email: sdecross@mgh.harvard.edu.
Brittany S. Cassidy, Email: britcass@gmail.com.
Steven Stufflebeam, Email: smstufflebeam@mgh.harvard.edu.
Scott L. Rauch, Email: srauch@partners.org.
Roger B.H. Tootell, Email: tootell@nmr.mgh.harvard.edu.
Appendix A. Supplementary data
Supplementary figures.
References
- Albert N.B., Robertson E.M., Miall R.C. The resting human brain and motor learning. Curr. Biol. 2009;19(12):1023–1027. doi: 10.1016/j.cub.2009.04.028. 19427210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderman T., Addington J., Bearden C., Cannon T.D., Cornblatt B.A., McGlashan T.H., Perkins D.O., Seidman L.J., Tsuang M.T., Walker E.F., Woods S.W., Cadenhead K.S. Negative symptoms and impaired social functioning predict later psychosis in Latino youth at clinical high risk in the North American prodromal longitudinal studies consortium. Early Interv. Psychiatry. 2014 doi: 10.1111/eip.12128. 24576057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen N.C., O’Leary D.S., Flaum M., Nopoulos P., Watkins G.L., Boles Ponto L.L., Hichwa R.D. Hypofrontality in schizophrenia: distributed dysfunctional circuits in neuroleptic-naive patients. Lancet. 1997;349(9067):1730–1734. doi: 10.1016/s0140-6736(96)08258-x. 9193383 [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y., Aviezer O., Berson Y., Sagi A. Attachment in infancy and personal space regulation in early adolescence. Attach. Hum. Dev. 2002;4(1):68–83. doi: 10.1080/14616730210123111. 12065031 [DOI] [PubMed] [Google Scholar]
- Bickart K.C., Hollenbeck M.C., Barrett L.F., Dickerson B.C. Intrinsic amygdala–cortical functional connectivity predicts social network size in humans. J. Neurosci. 2012;32(42):14729–14741. doi: 10.1523/JNEUROSCI.1599-12.2012. 23077058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozzoli C., Gentile G., Petkova V.I., Ehrsson H.H. FMRI adaptation reveals a cortical mechanism for the coding of space near the hand. J. Neurosci. 2011;31(24):9023–9031. doi: 10.1523/JNEUROSCI.1172-11.2011. 21677185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R.L., Sepulcre J., Talukdar T., Krienen F.M., Liu H., Hedden T., Andrews-Hanna J.R., Sperling R.A., Johnson K.A. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. J. Neurosci. 2009;29(6):1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. 19211893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon T.D., Cadenhead K., Cornblatt B., Woods S.W., Addington J., Walker E., Seidman L.J., Perkins D., Tsuang M., McGlashan T., Heinssen R. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch. Gen. Psychiatry. 2008;65(1):28–37. doi: 10.1001/archgenpsychiatry.2007.3. 18180426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Grossman E.D., Bidwell L.C., Yurgelun-Todd D., Gruber S.A., Levy D.L., Nakayama K., Holzman P.S. Differential activation patterns of occipital and prefrontal cortices during motion processing: evidence from normal and schizophrenic brains. Cogn. Affect. Behav. Neurosci. 2008;8(3):293–303. doi: 10.3758/cabn.8.3.293. 18814466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby C.L., Duhamel J.R., Goldberg M.E. Ventral intraparietal area of the macaque: anatomic location and visual response properties. J. Neurophysiol. 1993;69(3):902–914. doi: 10.1152/jn.1993.69.3.902. 8385201 [DOI] [PubMed] [Google Scholar]
- Collett P. On training Englishmen in the non-verbal behaviours of Arabs. Int. J. Psychol. 1971;6(3):209–215. [Google Scholar]
- Couture S.M., Penn D.L., Roberts D.L. The functional significance of social cognition in schizophrenia: a review. Schizophr. Bull. 2006;32(Suppl. 1):S44–S63. doi: 10.1093/schbul/sbl029. 16916889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deus V., Jokić-Begić N. Personal space in schizophrenic patients. Psychiatr. Danub. 2006;18(3–4):150–158. 17099605 [PubMed] [Google Scholar]
- Dosey M.A., Meisels M. Personal space and self-protection. J. Pers. Soc. Psychol. 1969;11(2):93–97. doi: 10.1037/h0027040. 5778351 [DOI] [PubMed] [Google Scholar]
- Duke M.P., Mullens M.C. Preferred interpersonal distance as a function of locus of control orientation in chronic schizophrenics, nonschizophreic patients, and normals. J. Consult. Clin. Psychol. 1973;41(2):230–234. doi: 10.1037/h0035141. 4583882 [DOI] [PubMed] [Google Scholar]
- Ebisch S.J., Mantini D., Northoff G., Salone A., De Berardis D., Ferri F., Ferro F.M., Di Giannantonio M., Romani G.L., Gallese V. Altered brain long-range functional interactions underlying the link between aberrant self-experience and self–other relationship in first-episode schizophrenia. Schizophr. Bull. 2014;40(5):1072–1082. doi: 10.1093/schbul/sbt153. 24191160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M.B., Spitzer R.L., Gibbon M., Williams J.B.W. New York State Psychiatric Institute, Biometrics Research; New York: 1995. Structured Clinical Interview for DSM-IV Axis I Disorders. [Google Scholar]
- Fogassi L., Gallese V., Fadiga L., Luppino G., Matelli M., Rizzolatti G. Coding of peripersonal space in inferior premotor cortex (area F4) J. Neurophysiol. 1996;76(1):141–157. doi: 10.1152/jn.1996.76.1.141. 8836215 [DOI] [PubMed] [Google Scholar]
- Gallese V., Ferri F. Jaspers, the body, and schizophrenia: the bodily self. Psychopathology. 2013;46(5):330–336. doi: 10.1159/000353258. 23867974 [DOI] [PubMed] [Google Scholar]
- Gessaroli E., Santelli E., di Pellegrino G., Frassinetti F. Personal space regulation in childhood autism spectrum disorders. PLOS One. 2013;8(9):e74959. doi: 10.1371/journal.pone.0074959. 24086410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold J.M., Waltz J.A., Matveeva T.M., Kasanova Z., Strauss G.P., Herbener E.S., Collins A.G., Frank M.J. Negative symptoms and the failure to represent the expected reward value of actions: behavioral and computational modeling evidence. Arch. Gen. Psychiatry. 2012;69(2):129–138. doi: 10.1001/archgenpsychiatry.2011.1269. 22310503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano M.S., Cooke D.F. Parieto-frontal interactions, personal space, and defensive behavior. Neuropsychologia. 2006;44(6):845–859. doi: 10.1016/j.neuropsychologia.2005.09.009. 16277998 [DOI] [PubMed] [Google Scholar]
- Graziano M.S., Hu X.T., Gross C.G. Visuospatial properties of ventral premotor cortex. J. Neurophysiol. 1997;77(5):2268–2292. doi: 10.1152/jn.1997.77.5.2268. 9163357 [DOI] [PubMed] [Google Scholar]
- Green M.F., Hellemann G., Horan W.P., Lee J., Wynn J.K. From perception to functional outcome in schizophrenia: modeling the role of ability and motivation. Arch. Gen. Psychiatry. 2012;69(12):1216–1224. doi: 10.1001/archgenpsychiatry.2012.652. 23026889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M.F., Lee J., Cohen M.S., Engel S.A., Korb A.S., Nuechterlein K.H., Wynn J.K., Glahn D.C. Functional neuroanatomy of visual masking deficits in schizophrenia. Arch. Gen. Psychiatry. 2009;66(12):1295–1303. doi: 10.1001/archgenpsychiatry.2009.132. 19996034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M.F., Lee J., Ochsner K.N. Adapting social neuroscience measures for schizophrenia clinical trials, Part 1: ferrying paradigms across perilous waters. Schizophr. Bull. 2013;39(6):1192–1200. doi: 10.1093/schbul/sbt131. 24072811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M.F., Leitman D.I. Social cognition in schizophrenia. Schizophr. Bull. 2008;34(4):670–672. doi: 10.1093/schbul/sbn045. 18495642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayduk L.A. Personal space: where we now stand. Psychol. Bull. 1983;94(2):293–335. [Google Scholar]
- Haznedar M.M., Buchsbaum M.S., Luu C., Hazlett E.A., Siegel B.V., Jr., Lohr J., Wu J., Haier R.J., Bunney W.E., Jr. Decreased anterior cingulate gyrus metabolic rate in schizophrenia. Am. J. Psychiatry. 1997;154(5):682–684. doi: 10.1176/ajp.154.5.682. 9137127 [DOI] [PubMed] [Google Scholar]
- Holt D.J., Cassidy B.S., Andrews-Hanna J.R., Lee S.M., Coombs G., Goff D.C., Gabrieli J.D., Moran J.M. An anterior-to-posterior shift in midline cortical activity in schizophrenia during self-reflection. Biol. Psychiatry. 2011;69(5):415–423. doi: 10.1016/j.biopsych.2010.10.003. 21144498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt D.J., Cassidy B.S., Yue X., Rauch S.L., Boeke E.A., Nasr S., Tootell R.B., Coombs G. III. Neural correlates of personal space intrusion. J. Neurosci. 2014;34(12):4123–4134. doi: 10.1523/JNEUROSCI.0686-13.2014. 24647934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt D.J., Lakshmanan B., Freudenreich O., Goff D.C., Rauch S.L., Kuperberg G.R. Dysfunction of a cortical midline network during emotional appraisals in schizophrenia. Schizophr. Bull. 2011;37(1):164–176. doi: 10.1093/schbul/sbp067. 19605517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker C., Park S. Emotion processing and its relationship to social functioning in schizophrenia patients. Psychiatry Res. 2002;112(1):41–50. doi: 10.1016/s0165-1781(02)00177-4. 12379449 [DOI] [PubMed] [Google Scholar]
- Horowitz M.J., Duff D.F., Stratton L.O. Body-buffer zone; exploration of personal space. Arch. Gen. Psychiatry. 1964;11:651–656. doi: 10.1001/archpsyc.1964.01720300081010. 14209746 [DOI] [PubMed] [Google Scholar]
- Javitt D.C. When doors of perception close: bottom-up models of disrupted cognition in schizophrenia. Annu. Rev. Clin. Psychol. 2009;5:249–275. doi: 10.1146/annurev.clinpsy.032408.153502. 19327031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn A., McGaughey T.A. Distance and liking: when moving close produces increased liking. Soc. Psychol. Q. 1977;40(2):138–144. [Google Scholar]
- Kaitz M., Bar-Haim Y., Lehrer M., Grossman E. Adult attachment style and interpersonal distance. Attach. Hum. Dev. 2004;6(3):285–304. doi: 10.1080/14616730412331281520. 15513270 [DOI] [PubMed] [Google Scholar]
- Kanwisher N., McDermott J., Chun M.M. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J. Neurosci. 1997;17(11):4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. 9151747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay S.R., Fiszbein A., Opler L.A. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. 3616518 [DOI] [PubMed] [Google Scholar]
- Keedy S.K., Rosen C., Khine T., Rajarethinam R., Janicak P.G., Sweeney J.A. An fMRI study of visual attention and sensorimotor function before and after antipsychotic treatment in first-episode schizophrenia. Psychiatry Res. 2009;172(1):16–23. doi: 10.1016/j.pscychresns.2008.06.003. 19243925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapil T.R. Social anhedonia as a predictor of the development of schizophrenia-spectrum disorders. J. Abnorm. Psychol. 1998;107(4):558–565. doi: 10.1037//0021-843x.107.4.558. 9830243 [DOI] [PubMed] [Google Scholar]
- Lee J.S., Chun J.W., Yoon S.Y., Park H.J., Kim J.J. Involvement of the mirror neuron system in blunted affect in schizophrenia. Schizophr. Res. 2014;152(1):268–274. doi: 10.1016/j.schres.2013.10.043. 24268934 [DOI] [PubMed] [Google Scholar]
- Lee K.H., Williams L.M. Eye movement dysfunction as a biological marker of risk for schizophrenia. Aust. N. Z. J. Psychiatry. 2000;34(Suppl.):S91–100. doi: 10.1080/000486700228. 11129321 [DOI] [PubMed] [Google Scholar]
- Lourenco S.F., Longo M.R., Pathman T. Near space and its relation to claustrophobic fear. Cognition. 2011;119(3):448–453. doi: 10.1016/j.cognition.2011.02.009. 21396630 [DOI] [PubMed] [Google Scholar]
- Mancuso F., Horan W.P., Kern R.S., Green M.F. Social cognition in psychosis: multidimensional structure, clinical correlates, and relationship with functional outcome. Schizophr. Res. 2011;125(2–3):143–151. doi: 10.1016/j.schres.2010.11.007. 21112743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez A., Hillyard S.A., Dias E.C., Hagler D.J., Jr., Butler P.D., Guilfoyle D.N., Jalbrzikowski M., Silipo G., Javitt D.C. Magnocellular pathway impairment in schizophrenia: evidence from functional magnetic resonance imaging. J. Neurosci. 2008;28(30):7492–7500. doi: 10.1523/JNEUROSCI.1852-08.2008. 18650327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nechamkin Y., Salganik I., Modai I., Ponizovsky A.M. Interpersonal distance in schizophrenic patients: relationship to negative syndrome. Int. J. Soc. Psychiatry. 2003;49(3):166–174. doi: 10.1177/00207640030493002. 14626359 [DOI] [PubMed] [Google Scholar]
- Orban G.A., Fize D., Peuskens H., Denys K., Nelissen K., Sunaert S., Todd J., Vanduffel W. Similarities and differences in motion processing between the human and macaque brain: evidence from fMRI. Neuropsychologia. 2003;41(13):1757–1768. doi: 10.1016/s0028-3932(03)00177-5. 14527539 [DOI] [PubMed] [Google Scholar]
- Park S.H., Ku J., Kim J.J., Jang H.J., Kim S.Y., Kim S.H., Kim C.H., Lee H., Kim I.Y., Kim S.I. Increased personal space of patients with schizophrenia in a virtual social environment. Psychiatry Res. 2009;169(3):197–202. doi: 10.1016/j.psychres.2008.06.039. 19762087 [DOI] [PubMed] [Google Scholar]
- Parnas J., Handest P., Jansson L., Saebye D. Anomalous subjective experience among first-admitted schizophrenia spectrum patients: empirical investigation. Psychopathology. 2005;38(5):259–267. doi: 10.1159/000088442. 16179812 [DOI] [PubMed] [Google Scholar]
- Pinkham A.E. Social cognition in schizophrenia. J. Clin. Psychiatry. 2014;75(Suppl. 2):14–19. doi: 10.4088/JCP.13065su1.04. 24919166 [DOI] [PubMed] [Google Scholar]
- Quintana J., Wong T., Ortiz-Portillo E., Kovalik E., Davidson T., Marder S.R., Mazziotta J.C. Prefrontal–posterior parietal networks in schizophrenia: primary dysfunctions and secondary compensations. Biol. Psychiatry. 2003;53(1):12–24. doi: 10.1016/s0006-3223(02)01435-x. 12513941 [DOI] [PubMed] [Google Scholar]
- Quintana J., Wong T., Ortiz-Portillo E., Marder S.R., Mazziotta J.C. Right lateral fusiform gyrus dysfunction during facial information processing in schizophrenia. Biol. Psychiatry. 2003;53(12):1099–1112. doi: 10.1016/s0006-3223(02)01784-5. 12814861 [DOI] [PubMed] [Google Scholar]
- Rajimehr R., Young J.C., Tootell R.B. An anterior temporal face patch in human cortex, predicted by macaque maps. Proc. Natl. Acad. Sci. U. S. A. 2009;106(6):1995–2000. doi: 10.1073/pnas.0807304106. 19179278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassovsky Y., Horan W.P., Lee J., Sergi M.J., Green M.F. Pathways between early visual processing and functional outcome in schizophrenia. Psychol. Med. 2011;41(3):487–497. doi: 10.1017/S0033291710001054. 20482936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reske M., Habel U., Kellermann T., Backes V., Jon Shah N., von Wilmsdorff M., Gaebel W., Zilles K., Schneider F. Differential brain activation during facial emotion discrimination in first-episode schizophrenia. J. Psychiatr. Res. 2009;43(6):592–599. doi: 10.1016/j.jpsychires.2008.10.012. 19056093 [DOI] [PubMed] [Google Scholar]
- Sallet J., Mars R.B., Noonan M.P., Andersson J.L., O’Reilly J.X., Jbabdi S., Croxson P.L., Jenkinson M., Miller K.L., Rushworth M.F. Social network size affects neural circuits in macaques. Science. 2011;334(6056):697–700. doi: 10.1126/science.1210027. 22053054 [DOI] [PubMed] [Google Scholar]
- Sereno M.I., Huang R.S. A human parietal face area contains aligned head-centered visual and tactile maps. Nat. Neurosci. 2006;9(10):1337–1343. doi: 10.1038/nn1777. 16998482 [DOI] [PubMed] [Google Scholar]
- Srivastava P., Mandal M.K. Proximal spacing to facial affect expressions in schizophrenia. Compr. Psychiatry. 1990;31(2):119–124. doi: 10.1016/0010-440x(90)90015-k. 2311379 [DOI] [PubMed] [Google Scholar]
- Strauss G.P., Gold J.M. A new perspective on anhedonia in schizophrenia. Am. J. Psychiatry. 2012;169(4):364–373. doi: 10.1176/appi.ajp.2011.11030447. 22407079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira S., Machado S., Velasques B., Sanfim A., Minc D., Peressutti C., Bittencourt J., Budde H., Cagy M., Anghinah R., Basile L.F., Piedade R., Ribeiro P., Diniz C., Cartier C., Gongora M., Silva F., Manaia F., Silva J.G. Integrative parietal cortex processes: neurological and psychiatric aspects. J. Neurol. Sci. 2014;338(1–2):12–22. doi: 10.1016/j.jns.2013.12.025. 24398346 [DOI] [PubMed] [Google Scholar]
- Thakkar K.N., Nichols H.S., McIntosh L.G., Park S. Disturbances in body ownership in schizophrenia: evidence from the rubber hand illusion and case study of a spontaneous out-of-body experience. PLOS One. 2011;6(10):e27089. doi: 10.1371/journal.pone.0027089. 22073126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar K.N., Peterman J.S., Park S. Altered brain activation during action imitation and observation in schizophrenia: a translational approach to investigating social dysfunction in schizophrenia. Am. J. Psychiatry. 2014;171(5):539–548. doi: 10.1176/appi.ajp.2013.13040498. 24626638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tootell R.B., Reppas J.B., Dale A.M., Look R.B., Sereno M.I., Malach R., Brady T.J., Rosen B.R. Visual motion aftereffect in human cortical area MT revealed by functional magnetic resonance imaging. Nature. 1995;375(6527):139–141. doi: 10.1038/375139a0. 7753168 [DOI] [PubMed] [Google Scholar]
- Tootell R.B., Reppas J.B., Kwong K.K., Malach R., Born R.T., Brady T.J., Rosen B.R., Belliveau J.W. Functional analysis of human MT and related visual cortical areas using magnetic resonance imaging. J. Neurosci. 1995;15(4):3215–3230. doi: 10.1523/JNEUROSCI.15-04-03215.1995. 7722658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregellas J.R., Tanabe J.L., Miller D.E., Ross R.G., Olincy A., Freedman R. Neurobiology of smooth pursuit eye movement deficits in schizophrenia: an fMRI study. Am. J. Psychiatry. 2004;161(2):315–321. doi: 10.1176/appi.ajp.161.2.315. 14754781 [DOI] [PubMed] [Google Scholar]
- Watson J.D., Myers R., Frackowiak R.S., Hajnal J.V., Woods R.P., Mazziotta J.C., Shipp S., Zeki S. Area V5 of the human brain: evidence from a combined study using positron emission tomography and magnetic resonance imaging. Cereb. Cortex. 1993;3(2):79–94. doi: 10.1093/cercor/3.2.79. 8490322 [DOI] [PubMed] [Google Scholar]
- Wynn J.K., Green M.F., Engel S., Korb A., Lee J., Glahn D., Nuechterlein K.H., Cohen M.S. Increased extent of object-selective cortex in schizophrenia. Psychiatry Res. 2008;164(2):97–105. doi: 10.1016/j.pscychresns.2008.01.005. 18938066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J.H., D’Esposito M., Carter C.S. Preserved function of the fusiform face area in schizophrenia as revealed by fMRI. Psychiatry Res. 2006;148(2–3):205–216. doi: 10.1016/j.pscychresns.2006.06.002. 17095198 [DOI] [PubMed] [Google Scholar]
- Zar J.H. Prentice-Hall; Upper Saddle River: 1996. Biostatistical Analysis. [Google Scholar]
- Zeki S., Watson J.D., Lueck C.J., Friston K.J., Kennard C., Frackowiak R.S. A direct demonstration of functional specialization in human visual cortex. J. Neurosci. 1991;11(3):641–649. doi: 10.1523/JNEUROSCI.11-03-00641.1991. 2002358 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures.