Abstract
The addition effect of date liquid sugar (DLS, 1–9 % v/v) to yoghurt milk on the physical (colour, firmness and syneresis), chemical (pH, total titratable acidity (TTA), total phenolic content (TPC) and antioxidant activity), rheological (viscosity and flow behaviour), and sensory attributes was scrutinized. Results showed that the pH value decreased by increasing DLS concentration from 1 to 6 %, while the TTA decreased with an increase in DLS from 6 to 9 % (p < 0.05). The whey syneresis, firmness and viscosity values were considerably influenced by the DLS content and acidity of the yoghurts (p < 0.05). A noticeable increase in antioxidant activity and TPC was found by the increasing DLS content (p < 0.05). Yoghurts containing 6 % v/v DLS also had the lowest syneresis and the highest firmness among the different samples. Moreover, a pseudoplastic rheological behaviour was detected for all the produced yoghurts. An increase in DLS concentration of manufactured yoghurts led to an increase in a, b and total colour difference (TCD) values and a decrease in L value (p < 0.05). The sensory evaluation revealed that there was no significant different in the colour scores. However, the used panelists determined the yoghurt supplemented with 6 % DLS had the highest scores for other investigated attributes.
Keywords: Fruit-flavoured yoghurt, Date liquid sugar, Syneresis, Quality physicochemical properties, Sensory evaluation
Introduction
Yoghurt as a healthy and popular food is produced with lactic fermentation of milk by Streptococcus thermophilus and Lactobacillus delbrueckii ssp. bulgaricus. High functionality of this product is attributed to the presence of living microorganisms such as lactic acid bacteria (LAB), streptococci, bifidobacteria or their combinations, which originate from the starter cultures (Cruz et al. 2013; Parvez et al. 2006). There are different types of yoghurt in various countries and regions. From a technological point of view, they can be classified to full-fat, low-fat, non-fat, flavored, probiotic, frozen and drink yoghurts (Fiszman et al. 1999).
Recently, the use of natural food additives and the incorporation of health promoting substances into the flavored yoghurts have been attracting increased attention. These yoghurts are prepared by adding flavored syrups or fruit concentrates to cultured milk before or after incubation in order to develope novel formulations for babies and children as well as for adults (Atasoy 2009; Gonzalez et al. 2011; Sengul et al. 2012). Fruit-flavoured yoghurt containing a number of fruity flavours (strawberry, banana, blueberry and peach), dessert flavours (key lime pie, chocolate, vanilla and peanut butter), pulps (mango, passion fruit, sour cherry), juices (grape, carrot, carob), jams, herbs, spices and sweeteners (honey, corn syrup) (Gundogdu et al. 2009; Sengul et al. 2012). Cakmakci et al. (2012, 2014) reported that the addition of fruit or vegetable mixtures to yoghurt formulations improves the nutrition functions and sensory characteristics for consumer acceptability.
Date palm or date (Phoenix dactylifera L.) as a unique fruit in the world plays an important role in the economic and political life in its growing areas. It is usually marketed as semi-finished and ready-to-use date products or derived products like date juice, date syrup, date spread and date liquid sugar (DLS) (Ashraf and Hamidi-Esfahani 2011).
DLS with a soluble solid content of 75 % is made from refined date syrup by removing all minerals. It is used mostly in soft drinks, cakes, jellies, preserved fruits, and confectionary products (Ashraf and Hamidi-Esfahani 2011). DLS as a natural and nutritional additive is one of the best choices for milk flavouring and a safe alternative to added sugar to produce dairy products. Moreover, most of the carbohydrates in this product are in the form of fructose and glucose, which are easily absorbed by the human body (Mousazadeh et al. 2011). There are several studies on development of flavored milk drink, frozen yoghurt dessert and ice cream formulated with date juice, date concentrate and date syrup. However, to the best of our knowledge, no scientific studies conducted on DLS-flavoured yoghurt. Thus, the aims of this study were to develop new fruit yoghurts by adding DLS and to determine the effect of different concentrations of DLS on their physicochemical, textural, rheological, and sensory characteristics.
Materials and methods
Chemicals
NaOH, gallic acid and 2,2-diphenyl-1-picryl hydrazyl radical (DPPH•) were provided by Merck Chemical Co. (Darmstadt, Germany). Folin–Ciocalteau and rhodamine-B were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA).
Materials
Cows’ milk was provided from dairy pilot plant of Department of Food Science and Engineering (University of Tehran, Karaj, Iran). The fat, ash, total acids and pH of this fresh milk were 2.3 %, 0.906 %, 13 % and 6.4, respectively. The freeze-dried starter yoghurt culture (YO-FAST-88), containing Streptococcus thermophilus and Lactobacillus delbrueckii spp. bulgaricus was purchased from Chr. Hansen Co. (Denmark). DLS was also provided from Shahdbab Pars Co. (Tabriz, Iran).
Yoghurt preparation
Milk used for the yoghurt production was heated at 80 °C for 15 min and then rapidly cooled to 42 ± 1 °C for inoculating the starter. After the cooling, DLS was added to milk at ratios of 1 to 9 % (v/v). The inoculated milk samples were transferred to plastic cups, incubated at 42 ± 1 °C until their pH reached to 4.6. Both the control and DLS-added yoghurts were stored overnight at 4 °C until the time of tests.
Analysis of pH and acidity
The pH values of the produced yoghurts were measured using a digital pH-meter (MettlerToldo, MP230, Switzerland) according to the method 945.10 of AOAC (1990). The total titratable acidity (TTA) was determined by mixing 9 g of yoghurt with 9 g of distilled water and titrating with 0.1 N NaOH using phenolphthalein colour-indicator to determine the end-point. This parameter was expressed as percentage of lactic acid (AOAC 1990).
Determination of phenolic compounds and antioxidant activity
Preparation of the extracts
For the extraction process, 10.0 mg of each yoghurt samples was extracted with 10 mL of distilled water at room temperature using a laboratory shaker. The mixture was centrifuged at 4000 × g for 20 min (Hermle BHG centrifuge model Z-230, Germany), and the supernatant was thus collected. The suspension was filtered through a Whatman No. l filter paper (Whatman International Ltd, Maidstone, UK). The filtrate was further used as a stock solution for the analysis of antioxidant activity and phenolic compounds.
Determination of total phenolic content
Total phenolic contents (TPCs) in the yoghurt extracts were determined by the Folin–Ciocalteau colorimetric method (Gulcin et al. 2002). Briefly, 1.0 mL of the solution (contains 1.0 mg sample) extract in water was pipetted into a flask. Then, 46 mL of distilled water and 1 mL of Folin and Ciocalteau’s reagent were added and mixed thoroughly. The mixture was left to stand for 3 min, and 3.0 mL of 2 % sodium carbonate was added. After 2 h incubation at ambient temperature, the resulting absorbance was triplically measured at 760 nm using a UV-visible spectrophotometer (Cecil BioQuest CE 2502, UK). The calibration curve was performed using analytical grade gallic acid as standard, and the results were expressed as microgram of gallic acid equivalents (GAEs) per milligram of sample (μg GAE/mg of sample).
DPPH radical scavenging activity
To evaluate the free radical scavenging activity, the extracts were allowed to react with DPPH• free radical. In brief, 0.1 mM solution of DPPH was prepared in ethanol, and 0.5 mL of this solution was added to 1.5 mL of extract solution in ethanol at different concentrations (20–100 μg/mL). The solutions were thoroughly vortexed and incubated in dark for 30 min. The absorbance was thus measured at 517 nm against blank samples lacking the scavenger (Gulcin 2010).
| 1 |
where Ac is the absorbance at 517 nm of the control reaction (containing DPPH solution without the extract), and As is the absorbance of the test sample.
Syneresis measurement
The syneresis amount was determined according to the method described by Rodarte et al. (1993). Briefly, 5 mL yoghurt was centrifuged at 5000 rpm for 20 min and then the whey accumulated after 1.0 min was measured. Syneresis percentage was expressed as volume of drained whey per 100 mL yoghurt.
Textural analysis
Puncture test was carried out to evaluate the yoghurt texture using an Instron Universal Testing Machine (Testometric machine, M350-10CT, England) equipped with a 50 kg compression load cell and integrator. The firmness was determined by means of a stainless steel probe with 3 mm diameter and test velocity was 1 mm/s. Maximum peak force (N) was measured from the penetration curve and expressed as yoghurt firmness (Ghasemlou et al. 2013; Gharibzahedi et al. 2014).
Apparent viscosity determination
The apparent viscosity was measured using a steady-stress viscometer (Brookfield DV-II+ programmable viscometer, Middleboro, MA, USA) equipped to a spindle (no. 4), with a sample size of about 100 mL yoghurt at room temperature. Flow behavior of the yoghurts was also explained by fitting the experimentally measured shear stress-shear rate data to the power-law model.
Measurement of colour attributes
The colour attributes were determined using a colourimeter (Minolta CR 300 Series, Minolta Camera Co., Ltd., Osaka, Japan). The yoghurt samples were placed on a white standard plate (L* = 93.49, a* = −0.25 and b* = 0.1) and the lightness (L) and chromaticity parameters a (red–green) and b (yellow–blue) were measured. Total colour difference (TCD, ∆E), chroma (CH), hue angle (HA), and colour index (CI) were calculated based on the following Eqs. (2–6) (Chouchouli et al. 2013):
| 2 |
| 3 |
| 4 |
| 5 |
rearranging Eqs. (2)–(4) we get,
| 6 |
where L*, a*, and b* are the colour parameter values of the standard and L, a, and b are the colour parameter values of the sample.
Microstructural observations
The selected yoghurt samples were prepared for microscope images after 24 h of product storage at 4 °C. For this analysis, the yoghurt samples were firstly mixed and lyophilized. Several drops of rhodamine-B solution were added to the resultant powders and placed on a microscope slide, covered with a cover slip. Microstructure was visualized by a fluorescence microscope (Leica DMRHC, Germany) equipped with suitable filters.
Sensory analysis
Sensory evaluation of the yoghurts developed with the different concentrations of DLS carried out by 30 trained panelists including graduate students and staff members of Tehran University’s Food Engineering Department who were familiar with the characteristic qualities of produced yogurts. Panelists were seated in sensory booths with standard lighting. Panelists were also asked to indicate on a questionnaire whether they found any difference between samples with regard to these parameters, and if so, which sample they most preferred. Each sample was presented twice, and the samples were presented in random order. This evaluation was based on the texture, colour, sweetness, aroma, flavour and overall acceptability of the samples. A hedonic 9-point structured scale, in which 9 corresponded to most liked and 1 to most disliked.
Statistical analysis
All analytical experiments were carried out in triplicates and the results presented as a mean of the obtained values with the standard deviation. The obtained data were subjected to analysis of variance (ANOVA) using SPSS 13 software (SPSS Inc., Chicago, Illinois, USA). Correlation analysis for studying the relationships between considered traits was also performed employing Pearson’s test.
Results and discussion
pH and acidity
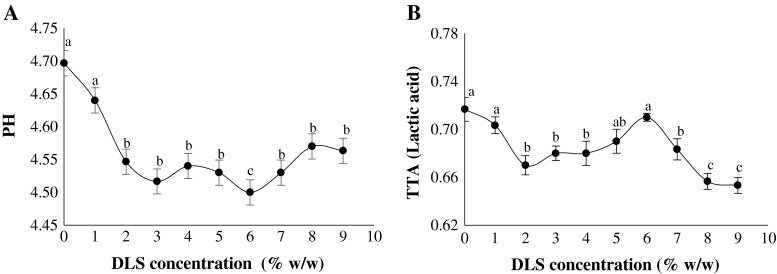
Figure 1a shows the pH values for yoghurts containing the different concentrations of DLS. As considered in this figure, an increase in DLS content from 1 to 6 % v/v led to a significant decrease in pH value (p < 0.05). This could be attributed to the fact that further metabolic activity of starter cultures is available by providing the simple sugars of fructose and glucose in DLS structure (Bonczar et al. 2002). Similar results are reported by Cakmakci et al. (2014) for supplementing set-type yoghurts with carrot juice and sugar. However, it gradually increased by increasing the DLS content from 6 to 9 % v/v (Fig. 2a). This finding also was in agreement with those of Celik and Bakirci (2003), who found that the adding of mulberry concentrated juice to yoghurt by inhibiting the growth of the starter cultures could lead to the higher the pH of the final product. Figure 1b depicts the TTA changes of yoghurts as a function of DLS concentration from 1 to 9 % v/v. In general, a reverse change in pH and TTA amounts of the experimental yoghurts was observed. The lowest TTA value was for the yoghurt containing 9 % v/v DLS which may be due mainly to the high pH of this DLS-supplemented sample. As a reasonable rule, the increase in TTA during the fermentation increases the resistant the gel network was to syneresis. Thus, a low acidification in the product structure can cause loosening of the network and coincide with the whey appearance (Atasoy 2009; Cakmakci et al. 2014).
Fig. 1.
The pH (a) and TTA (b) values of yoghurts produced as a function of DLS content. Means with same letters are not significantly different (p < 0.05)
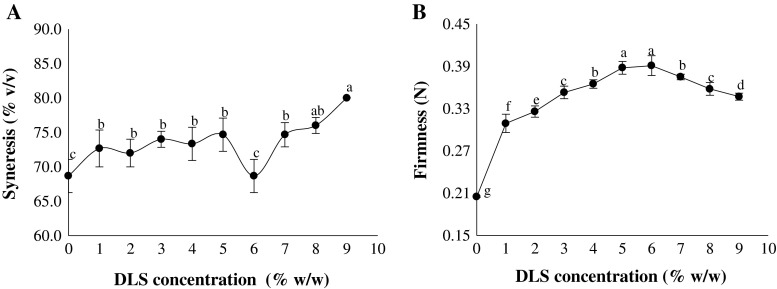
Fig. 2.
The syneresis (a) and firmness (b) values of yoghurts produced as a function of DLS content. Means with same letters are not significantly different (p < 0.05)
Syneresis and firmness characteristics
The syneresis changes of produced yoghurts as a function of DLS content are shown in Fig. 2a. The degree of whey syneresis was considerably influenced by the DLS concentration and acidity of the yoghurts (p < 0.05). The lowest amount of syneresis was found for the control and yoghurts supplemented with 6 % v/v DLS. The higher TTA of this sample led to a contraction in the casein particles resulting in an increase in water-binding capacity (WBC) of proteins and resistance to the syneresis (Ozturk and Oner 1999; Celik and Bakirci 2003). Moreover, low syneresis at optimum DLS content can be attributed to high WBC of fructose monosaccharide in DLS composition (Staley 1987). The similar results reported by Sert et al. (2011) in relation to the sunflower honey addition to set-type yoghurts. However, the highest syneresis level was observed for yoghurts containing 9 % v/v DLS with the lowest TTA. It seems that the increase of whey separation at high amounts of DLS is also due to damage or substantial structural rearrangement of the gel network (Narayana and Gupta 2013). The results of texture analysis also revealed an increase in DLS content from 1 to 6 % could lead to a significant increase in firmness value, while further increase up to 9 % caused to a considerable decrease in amount of this parameter (Fig. 2b). The positive effect of 6 % v/v DLS on yogurt texture can be elucidated by its WBC, which increases the viscosity of the serum phase and, therefore, the serum-holding capacity of yogurt (Fig. 3a). Some texture modifiers such as starches, gums, pectins, fibers and etc. should be used to prevent the consistency decrease of yoghurts at DLS concentrations higher than 6 % v/v (Sodini et al. 2004).
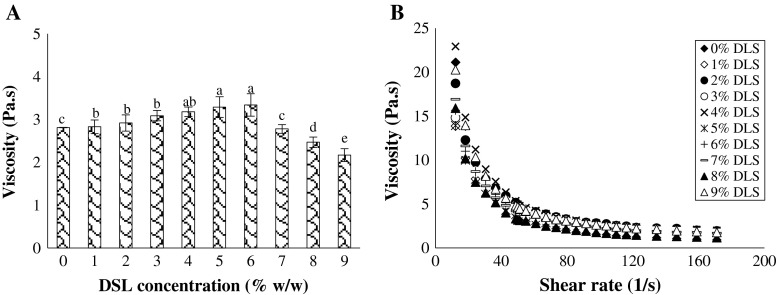
Fig. 3.
(a) Viscosity versus shear rate plot and (b) viscosity value at a constant shear rate (=79.5 1/s) of the produced yoghurts as a function of DLS content. Means with same letters are not significantly different (p < 0.05)
Apparent viscosity and flow behaviour characteristics
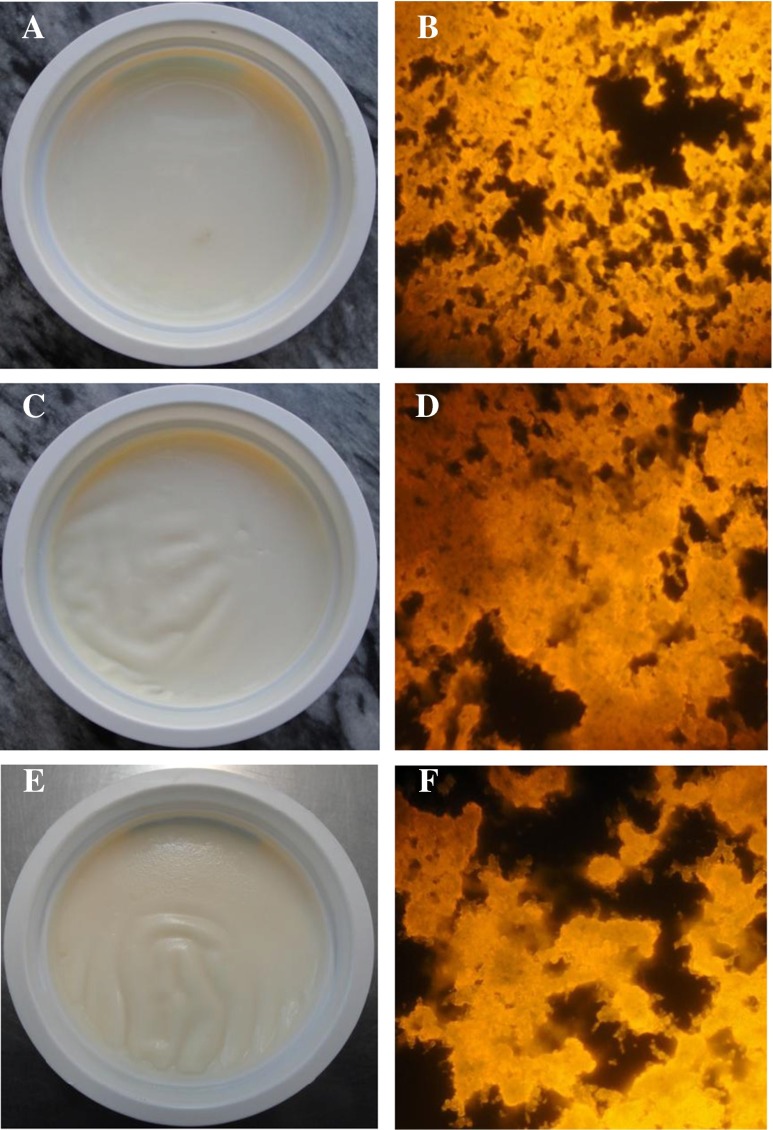
The viscosity increased by increasing DLS content from 1 to 6 % v/v (Fig. 3a). The results showed that all of the prepared DLS yoghurts exhibited a pseudoplastic rheological behavior (Fig. 3b). The increase in Brookfield viscosity observed when fructose is added instead of sucrose or when lactose is hydrolyzed in glucose and galactose is related to the higher WBC of monosaccharides compared to disaccharides (Sodini et al. 2004; Sert et al. 2011). In this condition, there is an aggregation due to the conjunction of the agglutination of fat globules and certain proteins at low temperatures (Fig. 4). Figure 4 clearly revealed that the yoghurts supplemented with an optimum concentration of DLS (6 % v/v) had a more compact microstructure than those formulated with 9 % v/v DLS. Nevertheless, a significant decrease was found for the viscosity values of yoghurts containing more than 6 % v/v DLS (Fig. 3a). The lower viscosity of these yoghurts could be due mainly to the lower WBC of proteins, the higher pH value, and followed by the lower the lactic acid production (Chouchouli et al. 2013). Higher whey repulsion and lower viscosity are common problems for fruit yoghurt. Similarly, a significant decrease in the viscosity value as the fruit sugar increases has been noted by Ozturk and Oner (1999) for grape juice yoghurt, Celik and Bakirci (2003) for mulberry concentrated juice yoghurt, Atasoy (2009) for carob juice concentrate yoghurt and Cakmakci et al. (2014) for carrot juice yoghurt. The correlation analysis also showed there is a highly positive association between viscosity and syneresis values (r = 0.93, p < 0.01). Moreover, the viscosity values had a positive correlation with the firmness values (r = 0.97, p < 0.01) of the yoghurts manufactured here.
Fig. 4.
Appearance (a,c,d) and microstructural (b,d,f) of the set-yoghurts produced with 0 % (a,b), 6 % (c,d), and 9 % (e,f) v/v DLS
Colour attributes
Table 1 reveals the addition of DLS in the yoghurt production significantly influenced the colour values for L, a and b (p < 0.05). The a and b values increased by increasing DLS concentration from 1 to 9 % v/v, while the L value considerably decreased (Table 1). The yellow and red colour of DLS can be contributed to the colour groups, degradation products of reducing sugars, melanoidines and iron polyphenolic complexes (Fathi et al. 2013). The relationship between a, b and L values and DLS concentration can be represented by the following regression equations (Eqs. 7–9):
| 7 |
| 8 |
| 9 |
Table 1.
Colour attributes of the prepared yoghurts as a function of DSL concentration*
| DSL content (% w/w) | Colour parameters | ||||||
|---|---|---|---|---|---|---|---|
| a | b | L | TCD | CH | HA | CI | |
| 0 | −1.9 ± 0.1c | 6.5 ± 0.2d | 89.5 ± 0.4a | 4.6 ± 0.1d | 6.7 ± 0.0c | −73.7 ± 0.2a | 3.0 ± 0.1c |
| 1 | −1.9 ± 0.2c | 6.8 ± 0.3c | 81.2 ± 1.1b | 12.5 ± 1.2c | 7.0 ± 0.0c | −74.4 ± 0.5a | 3.4 ± 0.2b |
| 2 | −2.1 ± 0.0b | 6.9 ± 0.4c | 80.1 ± 0.6b | 13.6 ± 1.1c | 7.2 ± 0.4c | −73.0 ± 0.4a | 3.5 ± 0.0b |
| 3 | −2.2 ± 0.0b | 7.2 ± 0.0c | 79.2 ± 0.8b | 14.5 ± 0.5c | 7.5 ± 0.1b | −73.0 ± 0.3a | 3.5 ± 0.0b |
| 4 | −2.4 ± 0.3b | 7.5 ± 0.9b | 74.5 ± 2.1c | 19.2 ± 0.9b | 7.9 ± 0.0b | −72.2 ± 0.8a | 3.8 ± 0.1a |
| 5 | −2.4 ± 0.2b | 7.6 ± 0.5b | 74.2 ± 1.5c | 19.5 ± 0.7b | 8.0 ± 0.1b | −72.5 ± 0.4a | 3.8 ± 0.0a |
| 6 | −2.5 ± 0.0ab | 7.7 ± 0.0b | 74.0 ± 1.3c | 19.7 ± 0.7b | 8.0 ± 0.2b | −72.0 ± 0.6a | 3.8 ± 0.1a |
| 7 | −2.6 ± 0.1a | 8.0 ± 0.1b | 71.5 ± 1.2d | 22.2 ± 1.0a | 8.4 ± 0.3a | −72.0- ± 0.3a | 4.0 ± 0.2a |
| 8 | −2.8 ± 0.1a | 8.1 ± 0.5ab | 70.7 ± 1.2d | 23.0 ± 0.8a | 8.6 ± 0.1a | −71.0 ± 0.1b | 4.0 ± 0.0a |
| 9 | −2.9 ± 0.3a | 8.6 ± 0.1a | 70.3 ± 1.6d | 23.4 ± 1.1a | 9.0 ± 0.0a | −71.4 ± 0.1b | 4.1 ± 0.0a |
*Values in the same columns followed by different letters (a–d) are significantly different (p < 0.05)
The significant difference of TCD value between the blank sample and yoghurts supplemented with DLS could thus be due to decrease the L value by increasing DLS content. Similar observations were reported by Sert et al. (2011) and Cakmakci et al. (2014) respectively for incorporating sunflower honey and carrot juice into set-type yoghurts. Due to the main colour parameters (a, b and L), a significant difference for CH, HA and CI attributes was also found among the various formulation (Table 1).
Total phenolic content and antioxidant activity
The TPCs in yoghurt samples were shown in Table 2. TPCs of yoghurt samples ranged from 0.03 to 1.15 (mg/dL) of sample at the production time. The addition of DLS at the range of 0 to 9 % to yogurt led to an increase in TPCs of the yogurts. The yoghurts containing DLS showed higher concentrations of TPC compared with the control (P < 0.05). Phenolic compounds are products of secondary metabolism in plants and are good sources of natural antioxidants in human diets. They play important role in delaying the development of chronic diseases such as cardiovascular diseases, cancer, inflammatory bowel syndrome and Alzheimer’s diseases (Chun et al. 2005). DPPH radical is a stable nitrogen-centered on free radical with an unpaired electron and decreases significantly on exposure to proton radical scavengers. The odd electron of DPPH radical is responsible for the strong absorbance at 517 nm and its solution appears deep purple (Gharibzahedi et al. 2015). The principle of DPPH analysis is based on the color change of the solution from purple to yellow as radicals are quenched by antioxidant compounds which can be quantitatively measured from the changes in absorbance (Gharibzahedi et al. 2013). High antioxidant potential of the DLS-fortified yogurts in term of DPPH radical scavenging activity may be due to the existence of numerous hydroxyls in DLS molecule, which could be as electron donator and transfer electron to DPPH free radical (Krings and Berger 2001). As shown in Table 2, the percentage of free radical DPPH• inhibition was enhanced by the increasing DLS concentration. A significant difference in the amount of DPPH• scavenging activity were found among the yoghurt samples containing 6 to 9 % DLS compared with samples containing lower percentages of DLS. DPPH• scavenging activity was varied from 34 % in the sample containing 6 % DLS to about 41 % in samples containing 9 % DLS.
Table 2.
The TPC and antioxidant activities of produced yogurts as a function of DLS concentration
| DSL content (% w/w) | TPC (μg GAE⁄mg of yogurt) | DPPH• scavenging activity (%) |
|---|---|---|
| 0 | 0.03 ± 0.001c | 1.85 ± 0.002c |
| 1 | 0.12 ± 0.001c | 4.43 ± 0.005c |
| 2 | 0.36 ± 0.001ab | 8.23 ± 0.004c |
| 3 | 0.53 ± 0.002b | 10.13 ± 0.003bc |
| 4 | 0.88 ± 0.003b | 20.25 ± 0.008b |
| 5 | 1.04 ± 0.003b | 29.11 ± 0.004b |
| 6 | 1.38 ± 0.002a | 34.81 ± 0.006a |
| 7 | 1.29 ± 0.001a | 37.97 ± 0.003a |
| 8 | 1.24 ± 0.003a | 39.24 ± 0.001a |
| 9 | 1.15 ± 0.005a | 40.67 ± 0.000a |
*Values in the same columns followed by different letters (a–c) are significantly different (p < 0.05)
Sensory attributes
Evaluation of consumer preference of an essential product as yoghurt is one of the most important methods for determining its sensory quality. Based on the scores that given by the used panelists, there was no significant different in the colour of yoghurts (Table 3). The best sample with the highest score of texture, aroma, flavour, sweetness and overall acceptability was for the yoghurt supplemented with 6 % v/v DLS (Table 3). However, the lowest scores for flavour, sweetness, aroma and texture respectively were for samples containing 0, 1, 2 and 9 % v/v DLS. From the panelists view, the yoghurts containing high contents of DLS (7–9 % v/v) were unsuitable for manufacturing in the industrial scale. This fact could probably be due to their high syneresis in results of low firmness and viscosity.
Table 3.
Sensory attributes of the prepared yoghurts as a function of DSL concentration*
| DSL content (% w/w) | Sensory characteristics | |||||
|---|---|---|---|---|---|---|
| Texture | Colour | Sweetness | Aroma | flavour | Overall acceptability | |
| 0 | 7.50 ± 1.08a | 7.60 ± 1.07a | 6.60 ± 1.35b | 6.40 ± 1.07b | 5.60 ± 1.07c | 6.80 ± 0.79b |
| 1 | 7.60 ± 0.79a | 7.30 ± 1.00a | 5.70 ± 0.95c | 6.40 ± 1.07b | 6.20 ± 1.69b | 6.60 ± 0.70b |
| 2 | 7.60 ± 0.97a | 7.30 ± 1.34a | 7.20 ± 0.92a | 5.70 ± 1.16c | 5.90 ± 0.74c | 6.70 ± 0.48b |
| 3 | 7.60 ± 0.74a | 7.60 ± 0.52a | 7.70 ± 0.67a | 6.80 ± 0.92b | 7.10 ± 0.88a | 6.80 ± 0.42b |
| 4 | 7.80 ± 0.63a | 7.70 ± 0.48a | 7.50 ± 1.08a | 6.90 ± 0.99ab | 6.90 ± 1.29a | 7.40 ± 0.97a |
| 5 | 6.90 ± 0.74b | 7.40 ± 0.52a | 6.40 ± 0.84b | 6.20 ± 1.03b | 6.50 ± 0.53b | 6.50 ± 0.71b |
| 6 | 7.90 ± 0.52a | 7.70 ± 0.67a | 7.60 ± 0.71a | 7.50 ± 0.97a | 7.40 ± 1.26a | 7.60 ± 0.84a |
| 7 | 6.70 ± 0.88b | 7.70 ± 0.48a | 7.60 ± 0.52a | 6.40 ± 0.97b | 6.20 ± 0.92b | 6.40 ± 1.07c |
| 8 | 6.10 ± 0.57c | 7.70 ± 0.48a | 7.10 ± 0.74a | 6.40 ± 1.43b | 5.80 ± 1.48c | 6.30 ± 1.06c |
| 9 | 5.90 ± 0.82c | 7.60 ± 0.52a | 7.40 ± 0.70a | 6.40 ± 1.58b | 6.20 ± 2.04b | 6.40 ± 0.97c |
*Values in the same columns followed by different letters (a–c) are significantly different (p < 0.05)
Conclusion
In this study, the production of new set-type yoghurts based on the different concentrations of DLS was developed. Results showed that the yoghurts supplemented with 6 % v/v in comparison to other samples had the optimal pH, TTA and colour characteristics, the high firmness and viscosity and the low syneresis. All the produced yoghurts showed a pseudoplastic rheological behavior. The present study demonstrated that yoghurts containing DLS were rich in phenolic compounds and had a high antioxidant activity in comparison to the control. The sample formulated with 6 % v/v also had the highest scores of sensory evaluation for texture, aroma, flavour and overall acceptability. DLS as a functional ingredient can thus be applied in the yoghurts production in the natural product industry.
Acknowledgments
The authors gratefully acknowledge financial support granted by the University of Tehran.
References
- AOAC (1990) Official methods of analysis of the association of official analytical chemists. No. 945.10, Virginia, USA
- Ashraf Z, Hamidi-Esfahani Z. Date and date processing: a review. Food Rev Int. 2011;27:101–133. doi: 10.1080/87559129.2010.535231. [DOI] [Google Scholar]
- Atasoy A. The effects of carob juice concentrates on the properties of yoghurt. Int J Dairy Technol. 2009;62:228–233. doi: 10.1111/j.1471-0307.2009.00465.x. [DOI] [Google Scholar]
- Bonczar G, Wszoleka M, Siuta A. The effects of certain factors on the properties of yoghurt made from ewe’s milk. Food Chem. 2002;79:85–91. doi: 10.1016/S0308-8146(02)00182-6. [DOI] [Google Scholar]
- Cakmakci S, Cetin B, Turgut T, Gurses M, Erdogan A. Probiotic properties, sensory qualities, and storage stability of probiotic banana yoghurts. Turk J Vet Anim Sci. 2012;36:231–237. [Google Scholar]
- Cakmakci S, Tahmas-Kahyaoglu D, Erkaya T, Cebi K, Hayaloglu AA. β-carotene contents and quality properties of set type yoghurt supplemented with carrot juice and sugar. J Food Process Preserv. 2014;38:1155–1163. doi: 10.1111/jfpp.12075. [DOI] [Google Scholar]
- Celik S, Bakirci I. Some properties of yoghurt produced by adding mulberry pekmez (concentrated juice) Int J Dairy Technol. 2003;56:26–29. doi: 10.1046/j.1471-0307.2003.00070.x. [DOI] [Google Scholar]
- Chouchouli V, Kalogeropoulos N, Konteles SJ, Karvela E, Markis DP, Karathanos VT. Fortification of yoghurts with grape (Vitis vinifera) seed extracts. LWT Food Sci Technol. 2013;53:522–529. doi: 10.1016/j.lwt.2013.03.008. [DOI] [Google Scholar]
- Chun S, Vattem DA, Lin Y, Shetty K. Phenolic antioxidants from clonal oregano (Origanum vulgare) with antimicrobial activity against Helicobacter pylori. Process Biochem. 2005;40:809–816. doi: 10.1016/j.procbio.2004.02.018. [DOI] [Google Scholar]
- Cruz AG, Cadena RS, Alvaro MBVB, Sant’Ana AS, Oliveira CAF, Faria JAF, Bolini HMA, Ferreira MMC. Assessing the use of different chemometric techniques to discriminate low-fat and full-fat yoghurts. LWT Food Sci Technol. 2013;50:210–214. doi: 10.1016/j.lwt.2012.05.023. [DOI] [Google Scholar]
- Fathi G, Labbafi M, Rezaei K, Emam-Djomeh Z, Hamedi M. Decolorization of Iranian date syrup by ultrafiltration. J Agric Sci Technol. 2013;15:1361–1371. [Google Scholar]
- Fiszman SM, Lluch MA, Salvador A. Effect of addition of gelatin on microstructure of acidic milk gels and yoghurt and on their rheological properties. Int Dairy J. 1999;9:895–901. doi: 10.1016/S0958-6946(00)00013-3. [DOI] [Google Scholar]
- Gharibzahedi SMT, Razavi SH, Mousavi SM. Comparison of antioxidant and free radical scavenging activities of biocolourant synthesized by Dietzia natronolimnaea HS-1 cells grown in batch, fed-batch and continuous cultures. Ind Crop Prod. 2013;49:10–16. doi: 10.1016/j.indcrop.2013.03.019. [DOI] [Google Scholar]
- Gharibzahedi SMT, Emam Djomeh Z, Razavi SH, Jafari SM. Mechanical behavior of lentil seeds in relation to their physicochemical and microstructural characteristics. Int J Food Prop. 2014;17:545–558. doi: 10.1080/10942912.2011.642448. [DOI] [Google Scholar]
- Gharibzahedi SMT, Razavi SH, Mousavi SM. Optimal development of a new stable nutraceutical nanoemulsion based on the inclusion complex of 2-hydroxypropyl-β-cyclodextrin with canthaxanthin accumulated by Dietzia natronolimnaea HS-1 using ultrasound-assisted emulsification. J Dispers Sci Technol. 2015;36:614–625. doi: 10.1080/01932691.2014.921188. [DOI] [Google Scholar]
- Ghasemlou M, Gharibzahedi SMT, Emam-Djomeh Z. Relating consumer preferences to textural attributes of cooked beans: development of an industrial protocol and microstructural observations. LWT Food Sci Technol. 2013;50:88–98. doi: 10.1016/j.lwt.2012.06.018. [DOI] [Google Scholar]
- Gonzalez NJ, Adhikari K, Sancho-Madriz MF. Sensory characteristics of peach-flavored yoghurt drinks containing prebiotics and synbiotics. LWT Food Sci Technol. 2011;44:158–163. doi: 10.1016/j.lwt.2010.06.008. [DOI] [Google Scholar]
- Gulcin I. Antioxidant properties of resveratrol: a structure–activity insight. Innov Food Sci Emerg Technol. 2010;11:210–218. doi: 10.1016/j.ifset.2009.07.002. [DOI] [Google Scholar]
- Gulcin I, Oktay M, Kufrevioglu I, Aslan A. Determination of antioxidant activity of lichen Cetraria islandica (L) Ach. J Ethnopharmacol. 2002;7:325–329. doi: 10.1016/S0378-8741(01)00396-8. [DOI] [PubMed] [Google Scholar]
- Gundogdu E, Cakmakci S, Dagdemir E. The effect of garlic (Allium sativum L.) on some quality properties and shelf-life of set and stirred yoghurt. Turk J Vet Anim Sci. 2009;33:27–35. [Google Scholar]
- Krings U, Berger RG. Antioxidant activity of some roasted foods. Food Chem. 2001;72:223–229. doi: 10.1016/S0308-8146(00)00226-0. [DOI] [Google Scholar]
- Mousazadeh AK, Sarshar M, Javadian S, Zarefard MR, Amirifard Haghighi Z. Separation of fructose and glucose from date syrup using resin chromatographic method: experimental data and mathematical modeling. Sep Purif Technol. 2011;79:72–78. doi: 10.1016/j.seppur.2011.03.014. [DOI] [Google Scholar]
- Narayana NMNK, Gupta VK. Effect of total milk solid content adjusted by adding ultrafiltered milk retentate on quality of set mango yoghurt. Int J Dairy Technol. 2013;66:570–575. [Google Scholar]
- Ozturk BA, Oner MD. Production and evaluation of yoghurt with concentrated grape juice. J Food Sci. 1999;64:530–532. doi: 10.1111/j.1365-2621.1999.tb15077.x. [DOI] [Google Scholar]
- Parvez S, Malik KA, Ah Kang S, Kim HY. Probiotics and their fermented food products are beneficial for health. J Appl Microbiol. 2006;100:1171–1185. doi: 10.1111/j.1365-2672.2006.02963.x. [DOI] [PubMed] [Google Scholar]
- Rodarte CW, Galvan MV, Farres A, Gallardo F, Marshal VE, Garibay MG. Yoghurt production from reconstituted skim milk powders using different polymer and non-polymer forming starter cultures. J Dairy Res. 1993;60:247–254. doi: 10.1017/S0022029900027564. [DOI] [Google Scholar]
- Sengul M, Erkaya T, Sengul M, Yildiz H. The effect of adding sour cherry pulp into yoghurt on the physicochemical properties, phenolic content and antioxidant activity during storage. Int J Dairy Technol. 2012;65:429–436. doi: 10.1111/j.1471-0307.2012.00838.x. [DOI] [Google Scholar]
- Sert D, Akin N, Dertli E. Effects of sunflower honey on the physicochemical, microbiological and sensory characteristics in set type yoghurt during refrigerated storage. Int J Dairy Technol. 2011;64:99–107. doi: 10.1111/j.1471-0307.2010.00635.x. [DOI] [Google Scholar]
- Sodini I, Remeuf F, Haddad S, Corrieu G. The relative effect of milk base, starter, and process on yogurt texture: a review. Crit Rev Food Sci Nutr. 2004;44:113–137. doi: 10.1080/10408690490424793. [DOI] [PubMed] [Google Scholar]
- Staley Manufacturing Co Crystalline fructose: a breakthrough in corn sweetener process technology. Food Technol. 1987;41:66–67. [Google Scholar]