Abstract
Wine production is a complex process both from biochemical and microbiological point of view in which yeast plays a central role. The use of the wine yeast Saccharomyces cerevisiae and non- Saccharomyces yeasts as mixed starter cultures for wine fermentations is of increasing interest to enhance the quality of wine.The most common stress, yeast cells encounter during wine fermentation is the increase in ethanol concentration.To enhance ethanol tolerance, alteration in the cellular lipid composition is one of its defence mechanism. Ethanol tolerance and cellular fatty acid composition of alcohol producing non Saccharomyces forms were compared with enological strains of Sacccharomyces cerevisiae. Saccharomyces cerevisiae used for the study, tolerated 15 % of ethanol and the non Saccharomyces strains such as, Issatchenkia occidentalis and Issatchenkia orientalis tolerated 10 % of ethanol. On exposure of Saccharomyces cerevisiae to ethanol stress, the proportion of monounsaturated fatty acids increased with concomitant decrease in saturated fatty acids. Decrease in monounsaturated fatty acids, exhibited by non-Saccharomyces yeasts when exposed to ethanol stress, could be one of the reasons for their inability to withstand more than 10 % of alcohol. Multivariate techniques of data analysis – principal component analysis and linear discriminant analysis were employed in order to establish differentiation criteria as function of yeast strains, alcohol stress and their fatty acid profile. Based on the data, Chemometrics, such as principal component analysis and discriminant function analysis, can be successfully applied to fatty acid data to categorize the yeast.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-015-1762-y) contains supplementary material, which is available to authorized users.
Keywords: Yeasts, Ethanol stress, Lipid composition, Chemometrics
Introduction
Wine is one of the oldest known medicine which protects against cardiovascular disease, atherosclerosis, hypertension, type 2 diabetes, neurological disorders, certain types of cancer and metabolic syndrome.The alcoholic, polyphenolic and lipid components of wine contribute to these beneficial effects (Guilford and Pezzuto 2011; Fragopoulou et al. 2000).The biological process of wine making involves the interactions of enzymes from different microorganisms, especially yeasts (Moreno-Arribas and Polo 2005). Yeast strains are exposed to a variety of biotic and abiotic stresses during fermentation. These stresses, when prevailing over the cellular defence systems, can affect cell viability, with negative consequences on the progression of fermentative process (Mannazzu et al. 2008). One of the most common stress, that yeast cells encounter during alcohol fermentation is the increased ethanol concentration. High levels of ethanol affects the integrity of cell membrane, damage permeability of the membrane to numerous ionic species and decrease the fluidity of plasma membrane (Ma and Liu 2010). Such levels of alcohol leads to the dissipation of transmembrane electrochemical potential and subsequently acidify intracellular and vacuolar condition (Ma and Liu 2010). At high concentrations, ethanol perturbs protein conformation of key glycolytic enzymes such as pyruvate kinase and hexokinase, causing protein denaturation and dysfunction (Ma and Liu 2010). Increased ethanol concentration affects the uptake of glucose, maltose, ammonium, amino acids and also causes cell leakage of nucleotides, amino acids and potassium ions (Ma and Liu 2010).
Yeast cells have developed appropriate mechanisms to deal with the damages caused by increased ethanol concentration. In response to the damage, yeasts change the membrane compositions to antagonize membrane fluidization and stabilize the plasma membrane. Specifically, the levels of unsaturated fatty acids and ergosterol increased in response to the high concentration of ethanol (Ding et al. 2009). Palmitoleic acid and oleic acid are the key plasma membrane components in S.cerevisiae to compensate the deficits caused by ethanol stress (Ma and Liu 2010). The concentration of these two unsaturated fatty acids in cellular lipids was higher in ethanol tolerant strains (Ma and Liu 2010). Palmitoleic acid and oleic acid were formed by the same catabolic membrane desaturase encoded by OLE1 through oxygen and NADH-dependent desaturation of palmitic acid and stearic acid respectively (Ma and Liu 2010). The role of unstaurated fatty acids in ethanol tolerance was confirmed by synthetic mono-unsaturated fatty acids and expression of insect desaturase TniPVE in yeast (Ma and Liu 2010).
S. cerevisiae, referred to as “the wine yeast” is the most important yeast for wine, responsible for the production of alcohol and secondary metabolites of importance to wine. Non Saccharomyces yeasts are also metabolically active during fermentation and produce a plethora of by-products such as glycerol, acetaldehyde, acetic acid, succinic acid, higher alcohols and fatty acid esters. The use of Saccharomyces and non- Saccharomyces yeasts as mixed starter cultures for wine fermentations is of increasing interest to enhance the quality of wine (Comitini et al. 2011; Jolly et al. 2006).
The Ethanol tolerance of S.cerevisiae is well known (Lee et al. 2011; Mobini-Dehkordi et al. 2006; Moneke et al. 2008). It is widely accepted that, increase in fatty acid composition of yeast is one of the key defence mechanism under ethanol stress (Ding et al.2009). However, ethanol tolerance studies of alcohol producing non Saccharomyces yeasts is very scarce. Multivariate analysis was used to study the effect of ethanol stress, on the cellular fatty acid composition of alcohol producing non Saccharomyces yeasts, in comparison with enological strains of S.cerevisiae.
Materials and methods
Yeast strains
The yeast strains used in the study were isolated using SDA Chloramphenicol medium.These strains were molecularly identified by amplifying the D1/D2 region of large sub unit 28S rDNA gene. In the first experimental setup, we have carried out ethanol tolerance studies and cellular lipid profile of the yeast isolates. The second experimental setup includes, screening of yeast isolates for the production of alcohol and its analysis. All experiments were conducted in triplicates and the average results have been computed.
Culture medium
The culture medium used was yeast extract peptone dextrose (YEPD) medium supplemented with ethanol ranging in concentration from 0 to 20 %.
Effect of ethanol addition on cell growth
To evaluate the effect of ethanol on yeast cell growth, the ethanol supplemented medium was inoculated with 5 % of inoculum and incubated at 25 °C for 72 h in stationary condition. Growth rate was determined every 24 h (Jimenez and Beneitez 1986; Jimoh et al. 2013; Osho 2005).
Alcohol production by the selected isolates
Alcohol production medium (Yeast Extract [1 %], peptone [2 %], dextrose [20 %]) was inoculated with 10 % of the inoculum, incubated at 25 °C for 72 h and checked for the production of alcohol (Jimenez and Beneitez 1986). The amount of alcohol produced was estimated spectrophotometrically (Caputi et al. 1968). Qualitative analysis of the alcohol produced was analysed by Gas Chromatography (GC). Shimadzu GC 2010 equipped with Flame Ionization detector (FID) and Zebron Wax plus capillary column containing (polyethylene glycol) was used with a temperature programming from 40 °C (1 min hold) to 70 °C at a rate of 5 °C min−1 and to 220 °C at a rate of 25 °C min−1 for 3 min. Nitrogen was used as the carrier gas with the flow rate of 1 ml min−1.
Fatty acids analysis
Lipids were extracted with chloroform-methanol (2:1) from the biomass, which was collected after 72 h of incubation. The lyophilized yeast biomass was homogenized at room temperature for 15 min using the chloroform methanol mixture [100 mg in 10 ml of solvent mixture]. The homogenate is centrifuged to recover the liquid phase. For methyl esters preparation of fatty acids, 2 ml of the FAME reagent which consists of methanol: acetyl chloride in 9.5:0.5 ratio was added to the dried lipid portion. The mixture was refluxed for 3 h at 80 °C in a water bath. This was followed by the addition of 5 % sodium chloride solution and the fatty acid methyl esters were extracted using hexane. The hexane layer was washed with 5 ml potassium bicarbonate solution and dried over anhydrous sodium sulphate (Christie 1982). The fatty acid methyl esters were analysed by Shimadzu GC 2010 equipped with FID using RTx - 2330 capillary column with a temperature programming from 120 °C (2 min hold) to 265 °C (10 min hold) at a rate of 5 °C min−1. Nitrogen was used as the carrier gas with the flow rate of 1 ml min−1. The fatty acid methyl esters were further confirmed through GC-MS analysis.
Statistical processing
Multivariate statistical techniques have been employed to extract information from complex data and also to interpret data obtained by instrumental techniques (Arvantoyannis et al. 1999; Cordella et al. 2002; Penza and Cassano 2004). Multivariate techniques of data analysis – principal component analysis (PCA) and Linear discriminant analysis (LDA) – were employed in order to establish differentiation criteria as a function of the yeast strains, as function of alcohol stress and their fatty acid profile. The application of multivariate analysis such as principal component analysis or discriminant analysis provides the possibility to use and understand the data generated based on the properties of the sample for classification among them. Principal component analysis is a popular, most commonly used multivariate technique for dimensionality reduction of the data. It is a mathematical procedure for resolving sets of data into orthogonal components whose linear combinations approximate the original data to any desired degree of accuracy. In this study, PCA was used to extract the first three principal components from the fatty acid data and to examine the possibility of grouping the samples. On the other hand, discriminant function analysis (DFA) is a supervised classification technique, where the number of categories and the sample that belong to each category are pre-defined. This method provides a number of orthogonal linear discriminant functions, equal to the number of categories minus one, that allow the samples to be classified in one or another category (Adams 1995; Martens and Martens 2001; Naes et al. 2002). PCA and DFA was performed using a statistical software - Statistica (v 5.5 StatSoft, Tulsa, OH, USA) on the PCA sample scores on components 1 and 2, which provides the maximum level of separation in models developed.
Result and discussion
Identification of the yeast strains
The rDNA region is variable, to allow reliable separation of all the yeast strains (Kurtzman and Robnett 1997). Based on the amplification of the D1/D2 region of large sub unit 28S rDNA gene, yeast strains were identified as S.cerevisiae designated as ITB with the accession No. FN393977.1; S.cerevisiae AAV2, accession No. KF551990; Issatchenkia occidentalis ApC, accession No. KF551991; Issatchenkia orientalis CL1132, accession No. KF551992; and reference CFTRI strain S.cerevisiae designated as 101.
Ethanol stress and cell growth
The results indicated that, when exposed to ethanol stress, S.cerevisiae strains 101, ITB and AAV2 tolerated 15 % of ethanol, when compared to the non Saccharomyces which resisted upto 10 % (Table 1). Except S.cerevisiae strain ITB, all other yeast isolates used for the study exhibited maximum growth after 72 h of incubation. Growth was not observed, when all these yeast strains were exposed to 20 % of alcohol. Although S. cerevisiae cells adapt to the ethanol stress, increasing ethanol concentration resulting from fermentation eventually lead to growth inhibition and cell death. When the adaptive responses fail, the ultimate consequence of ethanol toxicity is cell death (Carmona-Gutierrez et al. 2012). Earlier Osho (2005) had reported tolerance of S.cerevisiae strains up to 12 % of alcohol. Jolly et al. (2006) observed that, C. stellata tolerated 12 % of ethanol and produced elevated glycerol concentrations of 10–14 g/l. However, Lee et al. (2011) reported, S.cerevisiae and Pichia anamola to resist 15 % of alcohol.
Table 1.
Effect of ethanol stress on growth rate after 72 h of incubation 1) S.cerevisiae ITB 2) S.cerevisiae 101, 3) S.cerevisiae AAV2 4) Issatchenkia orientalis CL1132 5) Issatchenkia occidentalis Ap C
| Sl.No | Yeast strain | Maximum growth attained (Log CFU/ml) | |||
|---|---|---|---|---|---|
| Control | 5 % | 10 % | 15 % | ||
| 1 | ITB | 9.212b | 8.914b | 8.380c | 7.556b |
| 2 | 101 | 9.230b | 9.009b | 8.778c | 7.301b |
| 3 | AAV2 | 9.232b | 8.954b | 8.740c | 7.505b |
| 4 | CL1132 | 7.477a | 7.468a | 6.929b | 0.000a |
| 5 | Ap C | 7.477a | 7.270a | 5.398a | 0.000a |
Values in a column with different letters differ significantly at P < 0.05 by LSD test (Least Significant Difference)
Alcohol production by the selected strains
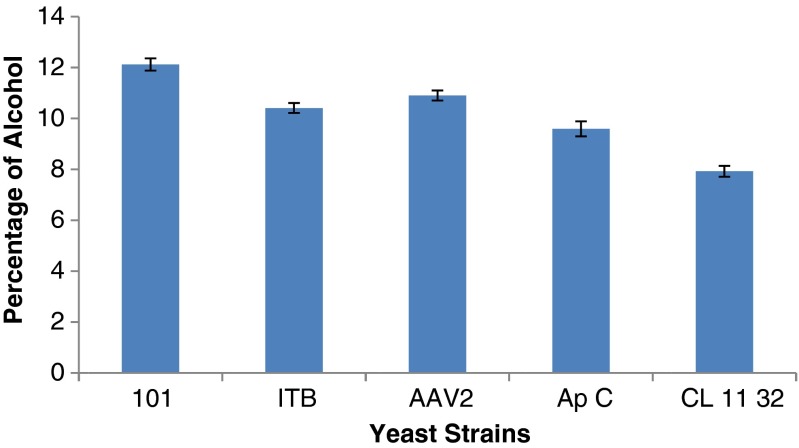
The identified selected yeast isolates were grown in the alcohol production medium, incubated at 25 °C for 72 h. In our study, the yeast strains such as Saccharomyces 101, ITB, AAV2 produced 12, 10.6, 10.7 % and Issatchenkia ApC, CL1132 produced 9.3 and 7.7 % of alcohol respectively (Fig. 1). Qualitative analysis of the alcohol produced by the selected isolates was carried out by Gas Chromatography. The results indicated, ethyl alcohol to be the major alcohol produced by all the isolates with an exception of S.cerevisiae strain ITB, which produced Iso- propyl alcohol in addition to ethyl alcohol. S.cerevisiae strains produced 10.47 % and Hanseniaspora spp. produced 5.02–8.72 % of alcohol (Ndip et al. 2001; Jolly et al. 2006). In our earlier studies with S. cerevisiae (standard culture S-170) we had recorded that the available glucose and the growth rate of the organism (Somshekar and Anu-Appaiah 2013).
Fig. 1.
Production of alcohol by the yeast isolates
Ethanol stress and variation in cellular fatty acid composition
S.cerevisiae strains ITB, 101 and AAV2 produced increased quantities of oleic and palmitoleic acids with a concomitant decrease in lauric and palmitic acids when exposed to extraneously added ethanol. Linolenic acid increased only in strain AAV2. The concentration of the other fatty acids detected, varied in all the three strains when exposed to ethanol stress. The presence and absence of these fatty acids were confirmed by GC-MS analysis.
In this study, we have compared the fatty acid profile of S.cerevisiae strains 101, ITB and AAV2 with alcohol producing non Saccharomyces forms like Issatchenkia orientalis CL1132 and Issatchenkia occidentalis ApC. Both ApC and CL1132 showed increase in caprylic and stearic acids followed by decrease in oleic acid. Myristic and palmitoleic acids increased in ApC with the concomitant decrease of these two fatty acids in CL1132. The concentration of the other fatty acids varied between the non Saccharomyces strains.
The ability of cells to increase the concentration of unsaturated fatty acids in plasma membrane is the principal mechanism used by yeasts to adapt to the presence of ethanol stress. The addition of increasing concentrations of ethanol to the culture medium raises the proportion of monounsaturated fatty acids of phospholipids such as oleic acid (18:1) with concomitant decrease in saturated fatty acids such as palmitic acid (16:0). The proportion of 18:2 acid was higher in the less ethanol tolerant strains (Aguilera et al. 2006). Oleic acid seems to be the most important unsaturated fatty acid in counteracting the toxic nature of ethanol through its effect on plasma membrane fluidity (You et al.2003). The hydrophobic core of the membranes may allocate ethanol molecules in the presence of (C16) monounsaturated fatty acids, which leads to tolerance (Mannazzu et al.2008). Our results pertaining to the variation of cellular fatty acid profile, when exposed to ethanol stress were mostly consistent with those previously studied. In our study, we have observed that, S. cerevisiae strains increased mono unsaturated fatty acids when exposed to ethanol stress, which is depicted in Fig. 2. The non-Saccharomyces strains used for the study produced 8 % of alcohol and also tolerated up to 10 % without manipulating the cultivation conditions. Non Saccharomyces strains exhibited a decrease in mono unsaturated fatty acids when exposed to ethanol stress (Fig. 3). This could be one of the reasons for their inability to withstand more than 10 % of alcohol.
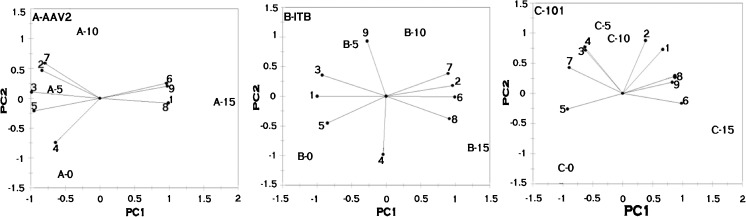
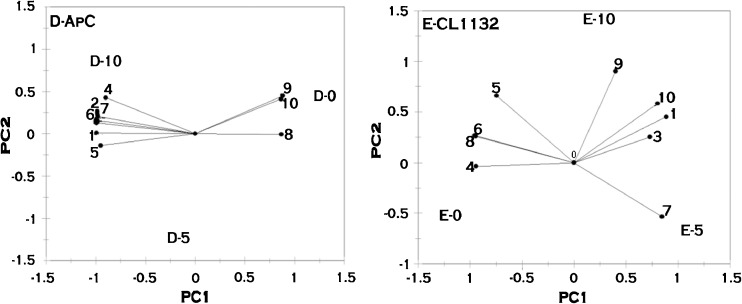
Fig. 2.
Principal component analysis of cellular fatty acid profile of Saccharomyces cerevisiae strains when exposed to ethanol stress. AAV2 - Saccharomyces cerevisiae, ITB - Saccharomyces cerevisiae, 101 - Saccharomyces cerevisiae [0,5,10,15 written adjacent to letters A,B,C,D,E refers to the percentage of ethanol. Fatty acids are designated as follows: 1: Caprylic., 2: Capric., 3: Lauric., 4: Myristic., 5: Palmitic., 6: Palmitoleic., 7: Stearic., 8: Oleic., 9: Linoleic., 10: Linolenic
Fig. 3.
Principal component analysis of cellular fatty acid profile of non Saccharomyces strains when exposed to ethanol stress. Ap C - Issatchenkia occidentalis, CL1132- Issatchenkia orientalis. [0,5,10,15 written adjacent to letters A,B,C,D,E refers to the percentage of ethanol. Fatty acids are designated as follows: 1: Caprylic., 2: Capric., 3: Lauric., 4: Myristic., 5: Palmitic., 6: Palmitoleic., 7: Stearic., 8: Oleic., 9: Linoleic., 10: Linolenic
The role of non Saccharomyces in fermentation is usually limited by their inability to tolerate ethanol. The results of the study by Pina et al. (2004) show that manipulating the culture conditions prior to ethanol challenge, the ethanol tolerance of some non-Saccharomyces strains like H. guilliermondii can be increased. In our study we have the non-Saccharomyces strains which can produce alcohol of 8 % and also withstand 10 % of alcohol without manipulating the cultivation conditions. Alexandre et al. (1994) reported that a significant reduction in the level of phospholipid content was observed when 4 % (v/v) ethanol was added to the growth medium of Kloeckera apiculata, while S.cerevisiae grown in the YPDE maintained the same level of phospholipids as the control cells. High ethanol tolerance is linked to the ability to maintain a high rate of phospholipid biosynthesis.
Statistical study
Principal component analysis
The correlation between the alcohol stress and the fatty acid profile of individual yeast strains are depicted in Fig. 2. In sample A (AAV2), B (ITB) andC (101), it was observed that the fatty acids such as palmitoleic (6) and oleic (8) acids increased with a concomitant decrease in lauric (3) and palmitic (5) acids. The fatty acids which have increased in all the three Saccharomyces strains were present on the positive side of the principal axis and are mostly associated with the samples exposed to 15 % of alcohol.
As depicted in the Fig. 3, sample D (Ap C) indicated the slight increase in fatty acids such as capric (2), caprylic (1) myristic (4) palmitic (5) palmitoleic (6) and stearic (7) acids when exposed to ethanol stress. These fatty acids were present on the negative side of the principal axis 1 and were associated with 10 % alcohol. Linoleic (9), linolenic (10) and oleic (8) acids decreased when exposed to ethanol stress and were present on positive side of the principal axis 1. In sample E (CL1132), it was observed that the fatty acids such as linoleic (9), linolenic (10), caprylic (1), lauric (3), stearic (7) acids were present on the positive side of the principal axis1. These fatty acids increased when exposed to ethanol stress, followed by the concomitant decrease in palmitic (5), palmitoleic (6) and myristic (4) and oleic acids which were present on negative side of the principal axis. From the PCA analysis carried out, it was observed that the fatty acids present on the positive side are oppositely correlated to the fatty acids present on the negative side of the principal axis 1.
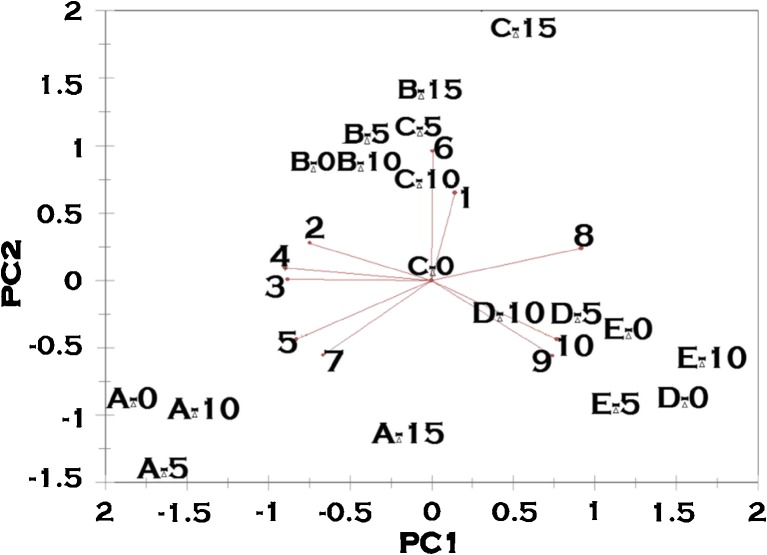
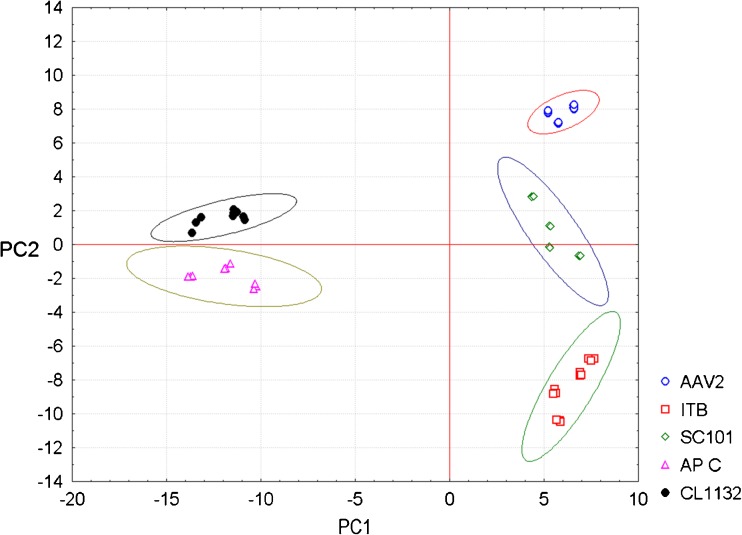
The principal axis 1 accounted 78.91 % of the variance while principal axis 2 for 13.45 % and together accounted for 92.36 % of the total variance of the data matrix. The high variance of the axis also indicates the PCA model developed is capable of explaining the variables and their relationships and also the validity of the model. The PCA resulting plot is shown in Fig. 4. Sample A (AAV2) occupied the third quadrant and associated more with palmitic acid (5) and stearic acid (7). On the other hand, sample B (ITB) that occupied the second quadrant are closely associated with each other and also with palmitoleic acid (6) followed by caprylic(1). Sample C (101) occupied the second quadrant and is associated with Palmitoleic (6) and caprylic (1) acids. Sample D (Ap C), occupied the fourth quadrant and associated more with oleic (8) linoleic (9) and linolenic (10) acids. Sample E (CL1132) showed variations in their positions as the function of alcohol stress, in fourth quadrant and is associated with linoleic (9) and linolenic (10) acids. The above results suggest that PCA is capable of classifying fatty acids with respect to their sample origins and indicated their associations with other variables and isolates.
Fig. 4.
Principal component analysis of cellular fatty acid profile of the yeast strains in the study when exposed to ethanol stress. A: AAV2- Saccharomyces cerevisiae., B: ITB- Saccharomyces cerevisiae., C: 101- Saccharomyces cerevisiae., D: Ap C-Issatchenkia occidentalis., E: CL1132- Issatchenkia orientalis [0,5,10,15 written adjacent to letters A,B,C,D,E refers to the percentage of ethanol]. Fatty acids are designated as follows: 1: Caprylic., 2: Capric., 3: Lauric., 4: Myristic., 5: Palmitic., 6: Palmitoleic., 7: Stearic., 8: Oleic., 9: Linoleic., 10: Linolenic
Multivariate statistical analysis of the data performed using partial least square linear regression modeling indicated a strong correlation between the overall lipid composition and the final ethanol concentration, as an indicator of ethanol tolerance (Henderson et al. 2013). From the Principal Component Analysis (PCA) carried out by Aguilera et al. (2006), it was reported that the best parameters in discriminating the yeast strains found to be the percentages in oleic acid, palmitoleic acid and ATP ase activity. Marullo et al. (2004) reported PCA analysis of the three technological traits such as ethanol tolerance, volatile acidity, H2S production on a yeast population of 48 strains. For the three traits analysed maximal projection conserves 83 % of the information with the two components axis, 1 and 2 that explain 50 % and 33 % of the total inertia.
Discriminat function analysis (DFA)
Discriminat function analysis (DFA) was also performed on the fatty acid data against five different yeast strains as a function of alcohol stress at various concentrations (Fig. 5). The alcohol concentration was increased from 0 to 15 % at four levels (0, 5, 10 and 15). The results of DFA clearly demonstrated its classification power by segregating yeast strains into five distinct clusters. Among the clusters formed, S.cerevisiae strains lie on the positive side of the axis and non Saccharomyces strains ApC and CL1132 lie on the negative side of principal axis.
Fig. 5.
Discriminant function analysis
Conclusions
Our results emphasize the comparison of cellular fatty acid composition in the alcohol producing non-Saccharomyces yeasts and enological strains of S.cerevisiae, when subjected to ethanol stress. The fatty acid profile pattern varied between the alcohol producing non Saccharomyces and Saccharomyces yeasts. Analysis of the fatty acid profiles indicate the different clustering of these forms. This signifies that Chemometrics, such as Principal component analysis (PCA) or Discriminant Function analysis (DFA), can be successfully applied to fatty acid data to categorize the yeast strains. The results of our finding can be more appropriate while studying the ecology of mixed culture fermentation.
Electronic supplementary material
(DOC 243 kb)
Acknowledgments
The authors thank Director, CSIR-Central Food Technological Research Institute (CFTRI) for providing access to the resources necessary for the completion of this study. The first author acknowledges the fellowship by Department of Science and Technology (DST), Government of India under INSPIRE fellowship program.
References
- Adams MJ. Chemometrics in analytical spectroscopy. Analytical spectroscopy monographs. Cambridge: Royal Society of Chemistry; 1995. p. 216. [Google Scholar]
- Aguilera F, Peinado RA, Millán C, et al. Relationship between ethanol tolerance, H+ − ATPase activity and the lipid composition of the plasma membrane in different wine yeast strains. Int J Food Microbiol. 2006;110:34–42. doi: 10.1016/j.ijfoodmicro.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Alexandre H, Rousseaux I, Charpentier C. Relationship between ethanol tolerance, lipid composition and plasma membrane fluidity in Saccharomyces cerevisiae and Kloeckera apiculata. FEMS Microbiol Lett. 1994;124:17–22. doi: 10.1111/j.1574-6968.1994.tb07255.x. [DOI] [PubMed] [Google Scholar]
- Arvantoyannis I, Katsota MN, Psarra P, Soufleros E, Kallithraka S. Application of quality control methods for assessing wine authenticity: use of multivariate analysis (chemometrics) Trends Food Sci Technol. 1999;10:321–336. doi: 10.1016/S0924-2244(99)00053-9. [DOI] [Google Scholar]
- Caputi A, Veda JM, Brown T. Spectrophotometric determination of chromic complex formed during oxidation of alcohol. Am J Enol Vitic. 1968;19:160–165. [Google Scholar]
- Carmona-Gutierrrez D, Sommer C, Andryuskova A, Kroemer G, Madeo F. A higher spirit : avoiding yeast suicide during alcoholic fermentation. Cell Death Differ. 2012;19:913–914. doi: 10.1038/cdd.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie WW. Lipid analysis. New York: Pregamon Press; 1982. pp. 93–96. [Google Scholar]
- Comitini F, Gobbi M, Domizio P, Romani C, Lencioni L, Mannazzu I, Ciani M. Selected non Saccharomyces wine yeats ion controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011;28:873–882. doi: 10.1016/j.fm.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Cordella CH, Moussa I, Martel A-C, Sbirrazzuoli N, Lizzani-Cuvelier L. Recent developments in food characterisation and adulteration detection: technique-oriented perspective. J Agric Food Chem. 2002;50:1751–1764. doi: 10.1021/jf011096z. [DOI] [PubMed] [Google Scholar]
- Ding J, Huang X, Zhang L, et al. Tolerance and stress response to ethanol in the yeast Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 2009;85:253–263. doi: 10.1007/s00253-009-2223-1. [DOI] [PubMed] [Google Scholar]
- Fragopoulou E, Nomikos T, Antonopoulou S, Mitsopoulou CA, Demopoulos CA. Separation of biologically active lipids from red wine. J Agric Food Chem. 2000;48:1234–1238. doi: 10.1021/jf990554p. [DOI] [PubMed] [Google Scholar]
- Guilford JM, Pezzuto JM. Wine and health: a review. Am J Enol Vitic. 2011;4:471–486. doi: 10.5344/ajev.2011.11013. [DOI] [Google Scholar]
- Henderson CM, Lozada-Contreras M, Jiranek V, et al. Ethanol production and maximum cell growth are highly correlated with membrane lipid composition during fermentation as determined by lipidomic analysis of 22 Saccharomyces cerevisiae strains. Appl Environ Microbiol. 2013;79:91–104. doi: 10.1128/AEM.02670-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez J, Beneitez T. Characterisation of wine yeasts for ethanol production. Appl Microbiol Biotechnol. 1986;25(2):150–154. doi: 10.1007/BF00938939. [DOI] [Google Scholar]
- Jimoh SO, Ado SA, Ameh JB, et al. Heat-shock and ethanol-osmotic effect on fermentable yeast cells. Int J Biol Biol Sci. 2013;2(6):91–96. [Google Scholar]
- Jolly NP, Augustyn OPH, Pretorius IS. The role and use of non -Saccharomyces yeasts in wine production. S Afr J Enol Vitic. 2006;27:15–39. [Google Scholar]
- Kurtzman CP, Robnett CJ. Identification of clinically important ascomycetous yeasts based on nucleotide divergence in the 5’ end of the large-subunit (26S) ribosomal DNA gene. J Clin Microbiol. 1997;35:1216. doi: 10.1128/jcm.35.5.1216-1223.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y-J, Choi Y-R, Lee S-Y, et al. Screening wild yeast strains for alcohol fermentation from various fruits. Mycobiology. 2011;39:33–39. doi: 10.4489/MYCO.2011.39.1.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M, Liu ZL. Mechanisms of ethanol tolerance in Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 2010;87:829–845. doi: 10.1007/s00253-010-2594-3. [DOI] [PubMed] [Google Scholar]
- Mannazzu I, Angelozzi D, Belviso S, et al. Behaviour of Saccharomyces cerevisiae wine strains during adaptation to unfavourable conditions of fermentation on synthetic medium: cell lipid composition, membrane integrity, viability and fermentative activity. Int J Food Microbiol. 2008;121:84–91. doi: 10.1016/j.ijfoodmicro.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Martens H, Martens M. Multivariate analysis of quality: an introduction. Chichester: Wiley; 2001. p. 400. [Google Scholar]
- Marullo P, Bely M, Masneuf-Pomarede I, Aigle M, Dubourdieu D. Inheritable nature of enological quantitative traits is demonstrated by meiotic segregation of industrial wine yeast strains. FEMS Yeast Res. 2004;4:711–719. doi: 10.1016/j.femsyr.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Mobini-Dehkordi M, Nahvi I, Ghaedi K, Tavassoli M. Isolation of high ethanol resistant strains of Saccharomyces cerevisiae. Res Pharm Sci. 2006;2:85–91. [Google Scholar]
- Moneke AN, Okolo BN, Nweke AI, Ezeogu LI, Ire FS. Selection and characterisation of high ethanol tolerant Saccharomyces yeasts from orchard soil. Afr J Biotechnol. 2008;7:4567–4575. [Google Scholar]
- Moreno-Arribas MV, Polo M. Winemaking biochemistry and microbiology: current knowledge and future trends. Crit Rev Food Sci Nutr. 2005;45:265–266. doi: 10.1080/10408690490478118. [DOI] [PubMed] [Google Scholar]
- Naes T, Isaksson T, Fearn T, Davies T. A user-friendly guide to multivariate calibration and classification. Chichester: NIR Publications; 2002. p. 420. [Google Scholar]
- Ndip RN, Akoachere JFKT, Dopgima LL, Ndip LM. Characterization of yeast strains for wine production. Appl Biochem Biotechnol. 2001;95:209–220. doi: 10.1385/ABAB:95:3:209. [DOI] [PubMed] [Google Scholar]
- Osho A. Ethanol and sugar tolerance of wine yeasts isolated from fermenting cashew apple juice. Afr J Biotechnol. 2005;4:660–662. doi: 10.5897/AJB2005.000-3119. [DOI] [Google Scholar]
- Penza M, Cassano G. Chemometric characterization of Italian wines by thin-film multisensors array and artificial neural networks. Food Chem. 2004;86:283–296. doi: 10.1016/j.foodchem.2003.09.027. [DOI] [Google Scholar]
- Pina C, Santos C, Couto JA, Hogg T. Ethanol tolerance of five non- Saccharomyces wine yeasts in comparison with a strain of Saccharomyces cerevisiae — influence of different culture conditions. Food Microbiol. 2004;21:439–447. doi: 10.1016/j.fm.2003.10.009. [DOI] [Google Scholar]
- Somshekar KL, Anu-Appaiah KA. Coffee cherry husk- a potential feed stock for alcohol production. Int. J. Environ Waste Manage. 2013;11:410–419. doi: 10.1504/IJEWM.2013.054242. [DOI] [Google Scholar]
- You KM, Rosenfield C, Douglas C, Knipple DC. Ethanol tolerance in the yeast Saccharomyces cerevisiae is dependent on cellular oleic acid content. Appl Environ Microbiol. 2003;69:1499–1503. doi: 10.1128/AEM.69.3.1499-1503.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 243 kb)