Abstract
The optimal brewing conditions for Turkish green tea were determined on the basis of extracted catechins and sensory attributes. Green tea infusions were prepared at 75, 85 and 95 °C with brewing times of 1, 2, 3, 5, 10, 20, 30 and 45 min. The amounts of epistructured catechins (EGCG, EGC, ECG, EC), non-epistructured catechins (C, GC, GCG) and caffeine in brewed tea samples were analysed. Sensory analyses were performed by nine trained panelists for infusion colour, taste, aroma and overall acceptability. Brewing at 85 °C for 3 min was found to be the optimal condition, where the EGCG content was at a maximum of 50.69 mg/100 ml with the highest sensory scores. It was observed that the yield of epistructured catechins increased rapidly for the first 3–5 min of brewing at 85 °C, and increased brewing time resulted in a decrease in the yield of epistructured catechins. The amount of nonepistructured catechins continued to increase with longer extraction times. Sensory scores for infusion colour, taste, aroma and overall acceptability were highest at 3 and 5 min brewing times at all temperatures. Sensory scores were very low for 30 and 45 min brewing at 85 and 95 °C due to the bitter taste and dark colour.
Keywords: Green tea, Brewing, EGCG, Catechins, Caffeine
Introduction
Green tea is one of the most popular beverages consumed worldwide. As a result of accumulated scientific evidence on its beneficial effects for human health, its consumption is increasing. It has been reported that daily consumption of green tea decreases blood pressure, decreases the risk of coronary diseases and obesity and improves dental health. Additionally, it has been reported to have antimicrobial activity.
The main beneficial health effects have been attributed to its high content of bioactive compounds, particularly catechins, which make up 30 % of the dry weight of green tea leaves (Graham 1992). Major green tea catechins are: (−)-epicatechin (EC), (−)-epicatechin gallate (ECG), (−)-epigallocatechin (EGC) and (−)-epigallocatechin gallate (EGCG). These epicatechins can change to their epimers that are non-epicatechins, which are known as: (−)- gallocatechin gallate (GCG), (−)-catechin gallate (CG), (−)-gallocatechin (GC) and (+)-catechin (C). EGCG is the most abundant and active catechin, and it is usually used as a quality indicator. Chemically, catechins are water soluble, colourless compounds and impart astringency to tea infusions (Ananingsih et al. 2013; Gramza and Korczak 2005). Tea also contains a significant amount of caffeine (3–5 %), which is unaffected by different processing methods (Chu 1997).
Tea catechins can act as antioxidants by donation of a hydrogen atom, as acceptors of free radicals and interrupters of chain oxidation reactions, or by chelating metals. On the grounds of the results of scavenging free radical activity, Chen and Ho (1995) ranked catechins as follows: EGCG>ECG>EGC>EC>C, where catechin showed half of the activity of EGCG.
Catechin contents of different tea cultivars are reported to vary due to many factors. Essential factors are: tea variety, harvesting season and harvesting conditions, age of the leaves, climate, cultivation practices, and drying and technological processes during tea production (Fernandez et al. 2002; Wu et al. 2012; Wei et al. 2011).
Green tea is typically prepared as an infusion by immersing dried green teas in hot water at around 80-90 °C for a period of 3–4 min. Many parameters may affect the sensory and nutritional quality of tea infusions such as maturity of the leaves, processing parameters and expected sensory quality. It has been reported that both time and temperature of tea infusion have pronounced effects on the extraction of tea catechins, and that the majority of EGCG and caffeine are extracted at 80 °C. However, as infusion time is prolonged, the catechins may undergo chemical changes and the epi forms of the catechins can be converted to non-epi forms. Epimerization is the conversion of tea catechins to their corresponding isomers. The identified epicatechins in green tea (i.e. EGCG, EGC, ECG and EC) are in the cis structure. They can be converted to their epimers that are non-epicatechins (i.e. GCG, GC, CG and C, respectively). This epimerization between pair catechins is reversible. Epimerization can occur at high temperatures. Wang et al. (2008) reported that the concentration of catechins decreased while their isomers increased at increasing temperatures. Degradation of catechins was evident as there was a declining trend in total catechins with increasing temperature. It was also reported that tea catechins could be converted to their corresponding epimers in traditionally brewed tea infusion and in canned tea drinks during brewing, production and storage.
Tea is cultivated in regions with mild climatic conditions. The primary tea producing countries are China, India, Kenya, Japan and Turkey. Among these countries, in China and Japan, tea leaves are mainly processed to green tea due to culinary tradition. Whereas in countries like India and Turkey, the majority of the tea leaves are processed to red or black tea.
Turkey is the largest tea producing country in the vicinity of Europe. Tea production in Turkey is approximately 200,000 t/year, where the Eastern Black Sea region of Turkey is the primary production area. Tea is consumed mainly as black tea in Turkey. The annual consumption of tea in 2009 was approximately 2.8 kg per person (FAOSTAT 2013). In recent years, green tea production has been increasing as a result of increasing consumer demand in Turkey. In this study, the objective was to determine the optimal brewing conditions for Turkish green tea on the basis of extracted catechins and sensory attributes.
Material and methods
Chemicals
Materials purchased from Sigma Aldrich Co included: EC (98 %), ECG (98 %), EGC (95 %), EGCG (95 %), GCG (98 %), CG (98 %), GC (98 %), C (99 %), and caffeine (99 %). Methanol (99.9 %), ethanol (99.8 %) and other solvents were purchased from Merck.
Tea
Fresh tea leaves were collected during the second harvesting period in August 2010, which were then processed into green tea in the CAYKUR Green Tea Factory. Green tea having a particle size of approximately 3 mm and moisture content 4.56 g/100 g, was used in brewing trials. The data analysis for the green tea is shown in Table 1.
Table 1.
Catechin and caffeine contents of green tea leaves (g/100 g dry matter)*
| Catechins | g/100 g dry matter |
|---|---|
| EGCG | 8.69 ± 0.004 |
| EGC | 4.34 ± 0.006 |
| ECG | 2.12 ± 0.004 |
| EC | 0.16 ± 0.004 |
| GCG | 0.21 ± 0.004 |
| GC | 0.85 ± 0.004 |
| C | 0.29 ± 0.004 |
| Total catechins | 16.66 ± 0.004 |
| Caffeine | 2.03 ± 0.004 |
*Values are mean ± standard deviation of three measurements
Preparation of Infusions
Green tea in the amount of 1.6 g was brewed using 110 ml water at 75, 85 and 95 °C for 1, 2, 3, 5, 10, 20, 30 and 45 min. Infusions were prepared in a small porcelain tea pot which was closed with a porcelain lid during brewing. Soft drinking water having a hardness value of 9.24 French degrees and a pH of 7.76 was used. Green tea infusions were filtered using a porcelain filter when each given brewing time was finished. Three samples of green tea infusions were prepared for each brewing condition for catechin analysis, and the mean of the results for the three samples were used.
Sample preparation
Green tea leaf samples were ground in accordance with ISO 1572 (1980), and the particles passing completely through a sieve of aperture size 500 μm were used. Fine homogenized green tea powder in the amount of 0.25 g was weighed into a screw-top extraction tube, and 40 ml of ethanol/water (10:90) was added. Catechins were then extracted for 1 h in an ultrasonic water bath at 50 °C. During the extraction, a sample tube was mixed using a vortex mixer for 1 min every 15 min. The extract was placed again into a shaking water bath at 50 °C for 30 min. After removing the tube from the water bath and cooling it to room temperature, 10 ml of water was added to the tube and placed into a centrifuge at 13,000 rpm for 10 min. Final extract in the amount of 1 ml was filtered through a 0.45 μm pore size syringe filter. Green tea infusions were used directly after the sample filtered through a 0.45 μm pore size syringe filter.
HPLC catechin analysis
Determination of catechins in green tea was performed by using a High Performance Liquid Chromatography (Agilent Series 1100, Waldbronn, Germany) equipped with a diode array detector (DAD) and reversed-phase column, according to ISO 14502-2 (2005). Separation and quantification of catechins were achieved by commercially available reverse-phase 5 μm Kromasil C18 (150 mm × 4.6 mm) with HiChrom 5 μm C18 (300 mm × 4.6 mm) guard columns and gradient mobile phase consisting of 0.1 ml orthophosphoric acid in 100 ml water (v/v) (eluent A) and 0.1 ml orthophosphoric acid in 100 ml methanol (v/v) (eluent B). The gradient was as follows: 0–5 min, 20 % B; 5–7 min, linear gradient from 20 to 24 % B; 7–10 min, 24 % B; 10–20 min, linear gradient from 24 to 40 % B; 20–25 min, linear gradient from 40 to 50 % B; 25–28 min linear gradient from 50 to 20 % B; 28–30 min 20 % B. Elution was performed at a solvent flow rate of 1 ml/min. Detection was accomplished with a diode array detector and chromatograms were recorded at 280 nm. All calculations and individual standards preparation were done using the methods given in ISO 14502–2 (2005). Catechin and caffeine amounts in green tea leaves were calculated as g/100 g dry matter, and in green tea infusions were calculated as mg/100 ml. Three samples of infusions were analysed for catechins, and the mean values were obtained.
Sensory analysis of tea infusions
Sensory analysis was performed by nine trained panelists using EyeQuestion Software in testing booths. Sensory analysis was conducted in three different sections in a day. Tea infusions were prepared according to brewing conditions and were given to panelists. Sensory responses regarding colour, taste, aroma and overall acceptability were evaluated on 10 cm line scales, where the highest intensity was given a value of 10 and the lowest intensity was given a value of 0. Panelists were instructed to eat salt free crackers between samples to minimise any residual effects of previous samples.
Statistical analysis
Analysis of variance was performed using XLSTAT (version 7.5.2) statistical software. Differences between means were evaluated using Fisher (LSD) analysis of differences between groups with a confidence range of 95 %.
Results and discussion
Effect of brewing conditions on the content of individual catechins and caffeine
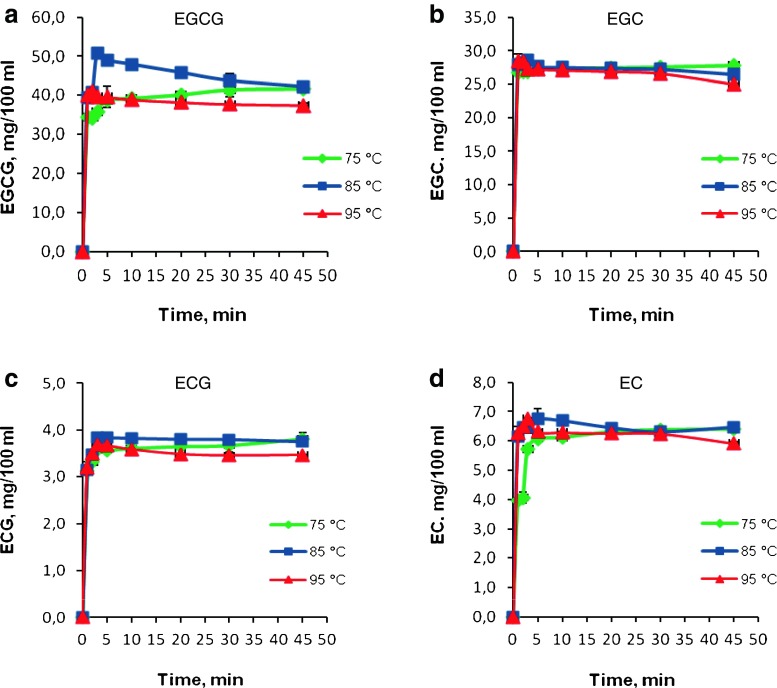
EGCG was the major catechin infused to water among all catechins. Infusion temperature and time was very important to extract the maximum amount of EGCG into a cup of tea. At 75 °C, the extraction of EGCG was very low; the amount of EGCG was 34.41 mg/100 ml after a brewing time of 1 min and increased to maximum as 40.04 mg/100 ml at 20 min (Fig. 1a). Increasing brewing time beyond 20 min was not effective in extracting more EGCG into hot water. A temperature of 75 °C was not sufficient to extract all of the EGCG. At 85 °C, the amount of EGCG increased and reached a maximum as 50.69 mg/100 ml at 3 min (p < 0.05). The EGCG concentration was observed to have decreased at 5 min, and the decrease continued until it reached a relatively stable level of 43.74 mg/100 ml at 30 min. At 95 °C, the maximum observed amount of EGCG was 40.92 mg/100 ml at 2 min. The EGCG level decreased after 2 min of brewing and reached 37.40 mg/100 ml at 45 min. There were no differences observed between the results obtained for 30 and 45 min brewing time for all temperatures (p > 0.05).
Fig. 1.
Effect of brewing time and temperature on the contents of epistructured catechins
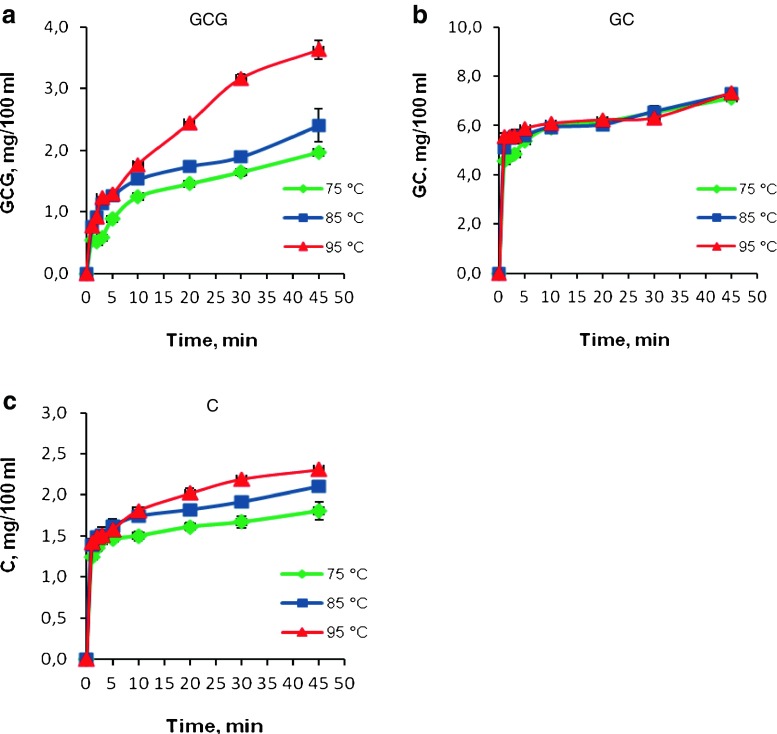
A brewing temperature of 95 °C yielded lower concentrations of EGCG than brewing at 85 °C. This was mainly due to the epimerisation of EGCG to the non-epistructured GCG (Fig. 2a) at temperatures above 85 °C. These observations are in correspondence with other results published in literature that indicate high extraction temperatures improve extraction efficiencies because the heat renders the cell walls more permeable to solvents and components and increases the solubility and diffusion coefficients of the tea components to be extracted (Vuong et al. 2011). However, excessive extraction temperatures above 80 °C can cause degradation of the catechins by promoting the change in epistructured catechins to non-epistructured catechins (Wang et al. 2008). They indicated that the content of non-epistructured catechins (i.e. −GCG, −GC, −C, −CG) increased while the content of the epistructured catechins (i.e. −EGCG, −EGC, −EC, −ECG) decreased. It is advisable to consumers not to use boiling water directly, rather it would be better to wait 3–5 min to decrease the temperature of water to get the maximum amount of EGCG when drinking green tea. Observations from this study demonstrate that above 85 °C and beyond 3 min of brewing, the infusion of EGCG to hot water significantly decreases.
Fig. 2.
Effect of brewing time and temperature on the contents of non-epistructured catechins
EGC was the second highest concentration of catechin found in Turkish green tea. At 75 °C, the infusion rate of EGC was again low (Fig. 1b), where the amount of EGC was 26.91 mg/100 ml at a brewing time of 3 min. The maximum amount of EGC obtained was 28.53 mg/100 ml, again at 85 °C with brewing time of 3 min. At 95 °C, the amount of EGC was 28.30 mg/100 ml at a brewing time of 2 min. At a brewing time of 45 min, the amount of EGC decreased to 25.00 mg/100 ml due to the epimerization to GC (Fig. 2b).
The amounts of EGCG and EGC were observed to have increased at 75 °C by brewing time, however their amounts decreased at 95 °C by brewing time. At 85 °C, their amounts increased to 3 min of brewing time and then decreased.
ECG was the quite stable catechin (Fig. 1c), there was no significant difference in the ECG contents at 3 min (3.59 mg/100 ml) and 30 min (3.68 mg/100 ml) at 75 °C (p > 0.05). At 85 °C, the amount of ECG increased to a maximum as 3.84 mg/100 ml at 3 min (p < 0.05), and no further change was observed due to increasing brewing time. At 95 °C, the amount of ECG increased to 3.68 mg/100 ml at 3 min and then decreased to 3.48 mg/100 ml at 20 min. There was no significant difference (p > 0.05) between the amounts of ECG observed at 20, 30 and 45 min at 95 °C. It was probably due to the very slow epimerization reaction rate of ECG to CG, because CG was not detected in the brewing samples.
The EC content increased continuously at 75 °C (Fig. 1d), where after a 1 min brewing time, it was 3.96 mg/100 ml and reached to 6.31 mg/100 ml at 20 min. At 85 °C, the amount of EC reached its maximum level of 6.74 mg/100 ml (p < 0.05) at 5 min and then decreased to 6.45 mg/100 ml at 20 min. At 95 °C, the amount of EC increased to 6.75 mg/100 ml at 3 min. The EC value was lower at 5 min (6.31 mg/100 ml), and there was no significant change at 30 min (6.25 mg/100 ml) of brewing. Generally, a decrease in the catechin content after 45 min of brewing was very clearly observed, and this is due to epimerization reactions. It is expected that further increases in brewing times would result in even lower concentrations of EC.
Three non-epistructured catechins, GCG, GC and C, were detected in all brewing samples; on the other hand, CG was not detected. Overall, the yield of epistructured catechins reached a plateau around 3–5 min of brewing and decreased slowly after 5 min, especially at 85 °C (Fig. 1a, b, c, d). On the other hand, the non-epistructured catechins (GCG, GC and C) continued to increase with increasing extraction temperature and time (Fig. 2a, b, c). Epistructured catechins are expected to have converted to non-epistructured catechins due to the epimerisation reactions at high temperatures and long brewing times.
Non-epistructured catechin levels were higher after brewing at 95 °C, relative to results at 85 and 75 °C, and the non-epistructured catechin concentrations were lower than the epistructured catechins in the brewed green tea. The maximum amounts of GCG, GC and C were 3.63, 7.33, and 2.31 mg/100 ml at 95 °C, respectively, for 45 min of brewing.
There are other related studies regarding the effects of extraction time and temperature on catechin contents. Vuong et al. (2011) reported trends similar to those found in this study for the extraction of epistructured and non-epistructured catechins. They found that the yield of epistructured catechins reached a plateau around 30 min, whereas the non-epistructured catechins continued to increase with longer extraction times up to 120 min. In our study, the yield of epistructured catechins increased very rapidly for brewing times of 3 to 5 min at 85 °C, and further increases in time resulted in a decrease in the yield of epistructured catechins. Similar to results from Vuong’s study, non-epistructured catechins levels continued to increase with longer extraction times. Zimmerman and Gleichenhagen (2011) studied tea extraction with and without additives. They used 2.5 g of tea leaves and 250 g of water at 70, 80, 90 and 100 °C for 3, 5, 7 min brewing times. They found that the concentrations of EGCG, EC and ECG were highest after 7 min of brewing at 100 °C. Only EGC behaved differently, as it constantly degraded at high temperature. Labbe et al. (2006) used 20 g of green tea with 1000 ml of distilled water at 50, 60, 70, 80 and 90 °C for 5, 10, 20, 40 and 80 min brewing times. They reported that EGC and EC were time dependant compounds, whereas C, EGCG, GCG and ECG were time/temperature dependent compounds. The best combination for EGC and EC was found to be at 50 °C from 20 to 40 min of brewing, while for all the other time/temperature dependent compounds, the best extraction combination was at 90 °C upon 80 min of brewing. Perva-Uzunalic et al. (2006) reported that the maximum extraction efficiency for catechins was achieved when green tea was extracted at 80 °C at 20 min or at 95 °C for 10 min. The differences observed in these studies can be related with sample preparation methodologies since different brewing times and tea/water ratios were used. The comparison of results is sometimes difficult due to the lack of uniformity in the properties of green tea, manufacturing conditions and brewing conditions. The leaf age, leaf size, harvesting season and manufacturing conditions of green tea are all important factors that can affect the results. Unlike the other studies, green tea used in experimental studies was not purchased from the market. Green tea manufactured from newly harvested fresh tea leaves in Rize region of Turkey was used in experimental studies.
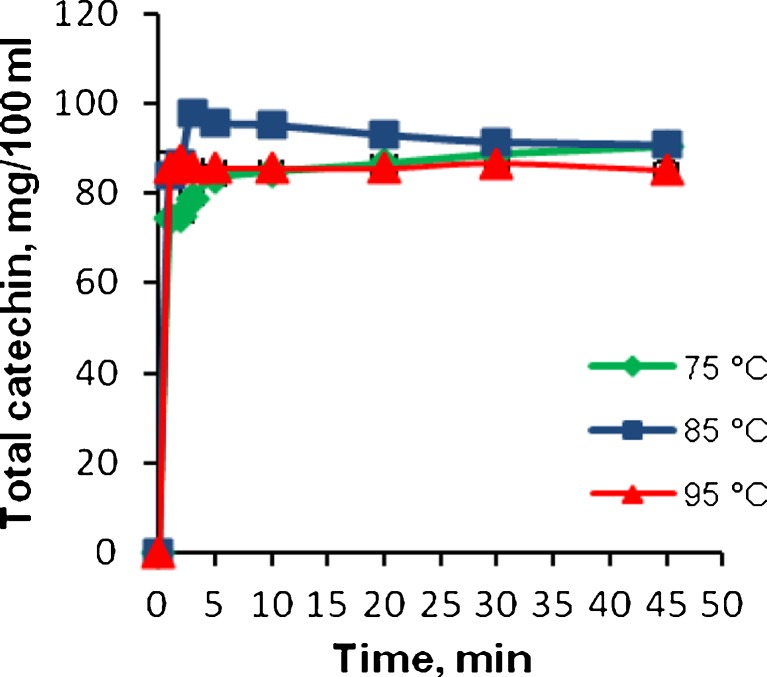
Total catechin contents were also analysed in this study, as can be seen in Fig. 3. At 75 °C, the total catechin content increased with increasing brewing time, where the total catechin was 74.53 mg/100 ml at 1 min and reached 90.47 mg/100 ml upon a 45 min brewing time. At 85 °C, the total catechin content reached its maximum level of 97.71 mg/100 ml at 3 min, then decreased to 91.43 mg/100 ml at 30 min. At 95 °C, there were not any significant changes observed in total catechin content during the brewing time (p > 0.05). The total catechin concentrations were 85.69 and 85.05 mg/100 ml at 1 and 45 min brewing times, respectively. This demonstrates that the total catechin content did not increase with increasing brewing time at high temperature. Brewing at 85 °C for 3 min was found to be the optimal scenario for infusing the maximum catechin into green tea. The consumption of a cup of Turkish green tea may contribute to the intake of 95–97 mg/100 ml of catechins. On a daily basis, two or three cups of green tea intake may provide significant amounts of catechins. Astill et al. (2001) reported that according to the consumer studies in Europe, tea drinkers’ brewing habits vary considerably among countries and among individuals within countries. Brew times from less than 30 s to 5 min are commonly observed, with the majority of consumers brewing for less than 2 min. The tea to water ratio also varies; the tea solids intake per cup can, therefore, vary considerably as a result of brew time and initial concentration alone. They also indicated that green teas are traditionally prepared with water at temperatures lower than the boiling point to avoid the bitterness of green tea infusions. It is usually recommended that a brewing temperature between 70 and 80 °C be used. Lower infusion temperature, initial concentration and brew time are factors that will reduce the amount of polyphenols and caffeine extracted into tea infusion. Our results also showed that a lower brewing temperature (i.e. 75 °C) and brewing time reduced the polyphenol content. It is recommended that tea brewing conditions be written on tea packaging so that consumers can take full advantage of green tea catechins.
Fig. 3.
Effect of brewing time and temperature on the content of total catechin
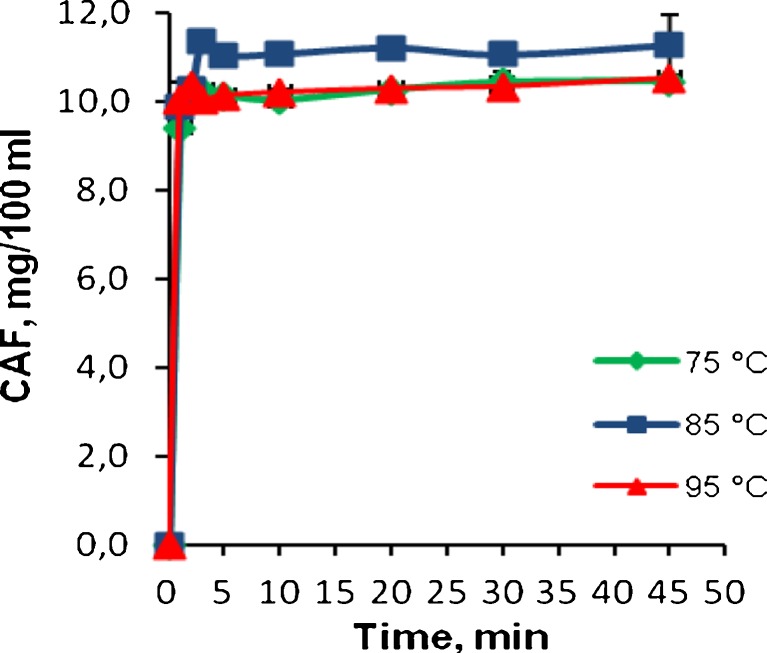
The amount of infused caffeine in green tea was also studied, and the results are given in Fig. 4. At 75 °C, the caffeine level increased to 10.24 mg/100 ml upon 3 min of brewing and did not change significantly thereafter. Caffeine is a very stable molecule, easily soluble in hot water and maintained in the tea infusion. At 85 °C, the caffeine content was at a maximum value of 11.36 mg/100 ml at 3 min, and no significant change was observed (p > 0.05) relative to the concentration obtained at 45 min. When the brewing temperature increased to 95 °C, caffeine contents increased to 10.34 mg/100 ml at 2 min and did not change significantly thereafter.
Fig. 4.
Effect of brewing time and temperature on the content of caffeine
Sensory analysis
Sensory evaluation of all green tea samples is given in Table 2. Infusion colour affects the appearance of green tea, and it is important as the first step of sensory evaluation. The maximum score was 8.7 for tea brewed at 85 °C for 3 min. Sensory colour scores were very similar for 3 and 5 min of brewing at 75, 85 and 95 °C (p > 0.05). As brewing time increased, the infusion colour became more brown, and sensory scores decreased. Samples at 85 °C for 45 min and 95 °C for 20, 30 and 45 min were considered unacceptable given their very dark colour and associated low scores.
Table 2.
Sensory scores for green tea infusions prepared at different brewing conditions*
| Brewing temperature (°C) and time (min) | Infusion colour | Infusion taste | Infusion aroma | Overall acceptability |
|---|---|---|---|---|
| 75 °C, 1 min | 6.8h | 5.9fgh | 5.6gh | 6.1fg |
| 75 °C, 2 min | 7.5cdefg | 7.2cd | 7.3cde | 7.2de |
| 75 °C, 3 min | 8.3abc | 8.1abc | 7.8abcd | 7.5cd |
| 75 °C, 5 min | 8.2abcd | 7.5bcd | 7.7abcd | 7.7bcd |
| 75 °C, 10 min | 7.9abcde | 6.3ef | 7.1de | 7.2de |
| 75 °C, 20 min | 7.5cdefg | 5.8fg | 6.9ef | 6.4ef |
| 75 °C, 30 min | 7.2efgh | 4.7hi | 6.2fg | 5.2gh |
| 75 °C, 45 min | 5.8i | 4.2ij | 4.8hi | 5.0hi |
| 85 °C, 1 min | 6.6h | 6.3ef | 6.0g | 6.1fg |
| 85 °C, 2 min | 7.7cdef | 7.5abcd | 7.4cde | 7.6cd |
| 85 °C, 3 min | 8.7a | 8.4a | 8.3a | 8.6a |
| 85 °C, 5 min | 8.0abcde | 7.8abcd | 8.2ab | 8.3abc |
| 85 °C, 10 min | 7.4defgh | 7.1de | 7.6bcde | 7.8abcd |
| 85 °C, 20 min | 6.7gh | 5.2gh | 6.2fg | 5.7fg |
| 85 °C, 30 min | 5.1i | 4.1ij | 5.5gh | 4.4i |
| 85 °C, 45 min | 3.2k | 1.7k | 2.1j | 2.1jk |
| 95 °C, 1 min | 7.7bcde | 7.3cd | 7.6bcde | 7.3d |
| 95 °C, 2 min | 8.1abcd | 7.9abcd | 8.2ab | 8.2abc |
| 95 °C, 3 min | 8.4ab | 8.3ab | 8.3ab | 8.3abc |
| 95 °C, 5 min | 8.6a | 7.7abcd | 8.0abc | 8.5ab |
| 95 °C, 10 min | 6.8fgh | 4.9hi | 5.5g | 4.8hi |
| 95 °C, 20 min | 4.2j | 3.8j | 4.1i | 2.5j |
| 95 °C, 30 min | 1.6l | 1.2k | 1.2k | 1.2k |
| 95 °C, 45 min | 0.7m | 0.1l | 0.3l | 0.1l |
*Means followed by different letters in each column are significantly different at p ≤ 0.05
Infusion taste scores reached a maximum near 3 to 5 min of brewing time for all temperatures and then decreased after 5 min. Taste scores were very low for 30 and 45 min brewing times. The maximum taste sensory score was 8.4 at 85 °C for 3 min, and sensory taste scores were similar for 2, 3 and 5 min brewing times at all brewing temperatures. Sensory scores for infusion taste at 30 and 45 min brewing times were very low due to the perceived bitterness taste.
Sensory scores for infusion aroma followed the same trend as the sensory colour and taste. The maximum sensory aroma score was 8.3 at 85 °C for 3 min, and sensory aroma scores were similar for 3 and 5 min brewing times at all brewing temperatures. Overall, though, sensory aroma scores were lower at 75 °C, and the panelists perceived less aroma. Aroma scores at 85 °C for 45 min and at 95 °C for 30 and 45 min were very low, because the characteristic green tea aroma was not apparent.
Sensory scores for overall acceptability were very parallel with colour, taste and aroma. Brewing samples for 3 and 5 min at all temperatures were acceptable with highest scores. Overall acceptance scores for 30 and 45 min at 75 °C were around a value of 5, and the samples were considered acceptable. On the other hand, sensory acceptance scores at 85 for 30 and 45 min and 95 °C for 20, 30 and 45 min were very low, and especially scores for tea brewed at 95 °C were unacceptable.
Conclusions
The optimal brewing conditions for Turkish green tea were determined with respect to extracting the highest amount of catechins with acceptable sensory parameters. Brewing at 85 °C for 3 min was found to be the best scenario, where the EGCG content was at its maximum concentration of 50.69 mg/100 ml and sensory scores were at their highest values. It was observed that the yield of epistructured catechins increased very rapidly for the first 3–5 min of brewing at 85 °C, and increased brewing time resulted in a decrease in the yield of epistructured catechins (EGCG, EGC, ECG, EC). The amount of non-epistructured catechins (C, GCG, GC) continued to increase with longer extraction times. Sensory scores for infusion colour, taste, aroma and overall acceptability were highest at 3 and 5 min brewing times for all temperatures. Sensory scores were totally unacceptable at 85 °C for 45 min and at 95 °C for 20, 30 and 45 min of brewing due to the very dark colour, bitter taste and aroma.
Acknowledgments
This research was supported by TUBITAK under the project entitled TARAL 1007 for CAYKUR. We wish to thank the CAYKUR Tea Factory for supplying the green tea used in this study.
References
- Ananingsih VK, Sharma A, Zhou W. Green tea catechins during food processing and storage: a review on stability and detection. Food Res Int. 2013;50(2):469–479. doi: 10.1016/j.foodres.2011.03.004. [DOI] [Google Scholar]
- Astill C, Birch M, Dacombe C, Humphrey PG, Martin PT. Factors affecting the caffeine and polyphenol contents of black and green tea infusions. J Agric Food Chem. 2001;49:5340–5347. doi: 10.1021/jf010759+. [DOI] [PubMed] [Google Scholar]
- Chen CW, Ho CT. Antioxidant properties of polyphenols extracted from green and black teas. J Food Lipids. 1995;2:35–46. doi: 10.1111/j.1745-4522.1995.tb00028.x. [DOI] [Google Scholar]
- Chu DC. Green tea—its cultivation, processing of the tea leaves for drinking materials, and kinds of green tea. In: Juneja LR, Chu DC, Kim M, editors. Chemistry and applications of green tea. Boca Raton: CRC Press; 1997. pp. 1–11. [Google Scholar]
- FAOSTAT (2013) Food supply statistics for tea. http://faostat3.fao.org. Accessed 31 May 2013
- Fernandez PL, Pablos F, Martin MJ, Gonzales AG. Study of catechin and xantine tea profiles as geographical tracers. J Agric Food Chem. 2002;50:1833–1839. doi: 10.1021/jf0114435. [DOI] [PubMed] [Google Scholar]
- Graham HN. Green tea composition, consumption, and polyphenol chemistry. Prev Med. 1992;21(3):334–350. doi: 10.1016/0091-7435(92)90041-F. [DOI] [PubMed] [Google Scholar]
- Gramza A, Korczak J. Tea constituents (Camellia sinensis L.) as antioxidants in lipid systems. Trends Food Sci Technol. 2005;16:351–358. doi: 10.1016/j.tifs.2005.02.004. [DOI] [Google Scholar]
- ISO 1572 (1980) Methods of test for tea–Part 1: Preparation of ground sample of known dry matter content
- ISO 14502-2 (2005) Determination of substances characteristic of green and black tea—Part 2: Content of catechins in green tea—Method using high-performance liquid chromatography
- Labbe D, Tremblay A, Bazinet L. Effect of brewing temperature and duration on green tea catechin solubilisation: basis for production of EGC and EGCG enriched fractions. Sep Purif Technol. 2006;49:1–9. doi: 10.1016/j.seppur.2005.07.038. [DOI] [Google Scholar]
- Perva-Uzunalic A, Skerget M, Knez Z, Weinreich B, Otto F, Grüner S. Extraction of active ingredients from green tea (Camellia sinensis): extraction efficiency of major catechins and caffeine. Food Chem. 2006;96:597–605. doi: 10.1016/j.foodchem.2005.03.015. [DOI] [Google Scholar]
- Vuong QV, Golding JB, Stathopoulo CE, Nguyen MH, Roach PD. Optimizing conditions for the extraction of catechins from green tea using hot water. J Sep Sci. 2011;34:3099–3106. doi: 10.1002/jssc.201000863. [DOI] [PubMed] [Google Scholar]
- Wang R, Zhou W, Jiang X. Reaction kinetics of degradation and epimerization of epigallocatechin gallate (EGCG) in aqueous system over a wide temperature range. J Agric Food Chem. 2008;56:2694–2701. doi: 10.1021/jf0730338. [DOI] [PubMed] [Google Scholar]
- Wei K, Wang L, Zhou J, He W, Zeng J, Jiang Y, Cheng H. Catechin contents in tea (Camellia sinensis L.) as affected by cultivar and environment and their relation to chlorophyll contents. Food Chem. 2011;125:44–48. doi: 10.1016/j.foodchem.2010.08.029. [DOI] [Google Scholar]
- Wu C, Xu H, Heritier J, Andlauer W. Determination of catechins and flavonol glycosides in Chinese tea varieties. Food Chem. 2012;132:144–149. doi: 10.1016/j.foodchem.2011.10.045. [DOI] [PubMed] [Google Scholar]
- Zimmerman BF, Gleichenhagen M. The effect of ascorbic acid, citric acid and low pH on the extraction of green tea: how to get most out of it. Food Chem. 2011;124:1543–1548. doi: 10.1016/j.foodchem.2010.08.009. [DOI] [Google Scholar]