Abstract
Efficacy of mint (Mentha arvensis) leaf and citrus (Citrus aurantium) peel extracts in retarding the quality changes in Indian mackerel during chilled storage was investigated. Mint leaf extract showed higher quantity of phenolics and superior in-vitro antioxidant activities than citrus peel extract. Gutted mackerel were given a dip treatment in mint extract (0.5 %, w/v) and citrus extract (1 % w/v), packed in LDPE pouches and stored at 0–2 °C. The biochemical quality indices viz. total volatile base nitrogen (TVB-N), trimethylamine nitrogen (TMA-N), free fattyacids (FFA) were significantly (p < 0.05) lower in mint extract (ME) treated fishes compared to citrus extract (CE) treated and control fishes (C) without any treatment. Plant extract treatment significantly inhibited lipid oxidation in mackerel as indicated by peroxide value (PV) and thiobarbituric acid reactive substances (TBARS). Aerobic plate count (APC) was markedly higher in C group followed by CE group throughout the storage period. As per sensory evaluation, shelf life of Indian mackerel was determined to be 11–13 days for C group, 13–15 days for CE group and 16–17 days for ME group, during storage at 0–2 °C.
Keywords: Mint leaf, Citrus peel, Indian mackerel, Biochemical quality, Shelf life
Introduction
Presently, in the fisheries sector, both the consumers and manufacturers are increasingly focusing on minimally processed fish products, with less use of synthetic additives and at the same time without compromising food safety. Additionally, the use of synthetic additives as preservative agent is under strict regulation due to their potential carcinogenic effect (Madsen and Bertelsen 1995). Consequently, the stringent laws from legislative bodies coupled with the continuous demand from the consumers for natural preservatives have compelled the manufacturers to restrict the use of synthetic preservatives and to use their natural counterparts. Constituents/extracts from plant sources are generally regarded as safe (GRAS) either because of their traditional use without any documented detrimental impact or the evidences generated from numerous dedicated toxicological studies (Smid and Gorris 1999). In addition to imparting characteristic flavours, certain spices and herbs prolong the storage life of food through their bioactive properties.
The preservative effects of plant constituents are due to certain phytochemicals which have been grouped into several categories including polyphenols, flavonoids, tannins, alkaloids, terpenoids, isothiocyanates, lectins, polypeptides or their oxygen substituted derivatives (Cowan 1999; Edeoga et al. 2005). Natural products, such as plant extract, either as pure compounds or as standardized extracts, provide unlimited opportunities for control of microbial growth owing to their chemical diversity. Numerous studies have been reported on the antibacterial activities of extracts obtained from different plant sources against food born pathogens and food spoilage bacteria (Negi and Jayaprakasha 2003; Shan et al. 2007; Corbo et al. 2008). Polyphenolic compounds present in plant extracts exhibit potential antioxidant properties due to their redox potential; that enable them to act as hydrogen donors, reducing agents, nascent oxygen quenchers, and chelating metal ions in numerous food applications (Gramza et al. 2006). The antioxidant activities of plant extracts are well documented in the literature (Murthy et al. 2002; Guo et al. 2003; Babbar et al. 2011).
The current focus in natural preservatives is on a small number of plant sources, which have been used for many years, and there is a need to expand this list for their food application to ensure safety and quality of the food products. Plants of the Lamiaceae family are very rich in phenolic compounds, and these have been shown to have high antioxidant activity (Ozgen et al. 2006; Tepe et al. 2007). There are several reports on the antioxidant and antibacterial activity of the essential oil and extracts of Mentha species (Marinova and Yanishlieva 1997; Dorman et al. 2003; Moreira et al. 2005; Kanatt et al. 2008). Mint extracts are proven to be potential antioxidants in meat products (Kanatt et al. 2007; Biswas et al. 2012). Citrus peel obtained after the extraction of juice from citrus fruit contributes to a major portion of waste material in the fruit juice industries. The citrus peels are rich in phenolic compounds such as phenolic acids and flavonoids which have been shown to be powerful antioxidant and free radical scavengers (Benavente-Garicia et al. 1997; Wang et al. 2008). Citrus peel extracts are reported to inhibit oxidation in soybean oil (El-aal and Halaweish 2010) and corn oil (Rehman 2006).
Indian mackerel (Rastrelliger kanagurta) is a commercially important pelagic medium-fatty fish species harvested in huge quantities across the Indian coast. This fish is highly popular as whole or gutted style in the domestic as well as export market. Moreover, being a fatty fish, Indian mackerel is attracting a great attention because of the positive role of marine lipids in human nutrition. Despite this, Indian mackerel is highly perishable as it is highly susceptible to oxidation. In dark fleshed fatty/medium fatty fishes, the onset of oxidation and rancidity is fast probably because of large amounts of haemoglobin (a well-known activator of lipid oxidation) and lipids coexist (Richards and Hultin 2002). In fact, this is the major cause of off-flavor development and quality loss of this species during refrigerated storage, the most prevailing preservation method followed in the domestic market. Therefore, effective methods for controlling lipid oxidation and improving quality of fresh Indian mackerel are necessary.
Even though citrus peel and mentha leaf is a potential source of bioactive compounds, both of them are not exploited for its preservative effects in fish. With this background, the present study was undertaken to evaluate the potential of extracts prepared from these two plant sources as preservatives for improving the quality and shelf life of Indian mackerel during chilled storage.
Materials and methods
Materials
The fresh Indian mackerel (weight 200 ± 15 g and length 18 ± 2 cm) was procured from the nearest fish landing centre, Vashi, India and brought to the laboratory in iced condition within 15 min. Mint (Mentha arvensis) leaf was purchased from the local market and citrus (Citrus aurantium) peels were procured from a juice shop nearby Vashi, India. All the chemicals and glasswares used were of reagent grade.
Preparation of extract
The raw materials (mint leaf and citrus peel) were washed in tap water and dried in an oven at 50 °C overnight. Extracts were prepared by modifying the protocol described by Sultana et al. (2009). Dried mint leaf and citrus peel were made into a powder and the powder was then extracted with 60 % ethanol in the ratio 1: 10 in a shaking waterbath at 50 °C for 5 h. The solution after cooling was filtered by Whatmann No. 1 filter paper. The residue was re-extracted with fresh solvent following the procedure aforementioned. The filtrate was pooled and then evaporated in a rotary evaporator (Heidloph, Germany) at 50 °C. To the final thick solution, 10 ml of water was added and evaporated. This step was repeated three times for removing any traces of any solvent if present. The mint leaf extract and citrus peel extract thus obtained was dried at 45 °C in a vacuum drier and stored in refrigerator till further use.
Analysis of extracts
Estimation of total phenolics
Total phenolic content in the extracts was estimated by the method of Singleton and Rossi (1965). 1 ml of the diluted sample (0.5 to 1 mg/ml) was taken in 25 ml volumetric flask containing 9 ml distilled water. 1 ml of Folin-Ciocalteu phenol reagent was added to the flask and mixed well. After 5 min, 10 ml of 7 % Na2CO3 solution was added and mixed well. The solution was allowed to stand for 2 hr at room temperature and absorbance of an aliquot of the solution was measured at 760 nm in a UV–VIS spectrophotometer (Spectronic 21D, Belgium). A blank was prepared with gallic acid standard curve using 0, 5, 10,15,20,25 and 30 μg/ml gallic acid in the same manner.
The concentration of total phenolic compounds in the extract was determined by using the formula:
Where T = Total phenolic content mg/gm of plant extract in GAE,
- C
Concentration of Gallic acid from the calibration curve in mg/ml
- V
volume of the extract in ml
- M
wt of the pure plant extract in gm.
2,2 di phenyl 1 picryl hydrazine (DPPH) assay
DPPH assay of the extracts were done according to the procedure of Nickavar et al. (2008) with little modifications. One ml of 0.15 mM DPPH solution in ethanol was added to 2.5 ml of each extract solutions in ethanol at different concentrations (200, 100, 50, 25, 12.5 and 6.5 μg/ml), Test tubes were vortexed and incubated for 30 min at room temperature in the dark; then, the absorbance values were determined at 518 nm in a UV–VIS spectrophotometer. 1 ml DPPH plus 2.5 ml ethanol were taken as control. The inhibition percentage of DPPH radical was assessed by the following formula:
The IC50 (concentration providing 50 % inhibition) was graphically calculated using a calibration curve in the exponential range by plotting the extract concentration vs the corresponding scavenging effect.
Fe2+ reducing power
Fe2+ reducing power was determined according to the method of Yildirim et al. (2001) with some modifications. Sample solutions (0.1 mg/ml) were mixed with 2.5 ml of 0.2 M phosphate buffer (pH 6.6) and 2.5 ml of 1 % potassium ferricyanide. Mixture was incubated at 50 °C for 20 min. An aliquot of 2.5 ml was then mixed with 2.5 ml distilled water and 2.5 ml of 0.1 % FeCl3 and the absorbance was read at 700 nm. Increased absorbance of the reaction mixture indicated increased reducing power.
Treatment and storage of mackerel
Indian mackerel procured from the landing centre was washed in tap water, beheaded and eviscerated. The gutted mackerel were washed thoroughly in potable water and divided into three lots. One lot was given a dip treatment in 0.5 % (w/v) mint leaf extract solution (ME) for 30 min and another lot was given dip treatment in 1 % (w/v) citrus peel extract solution (CE) for 30 min. The third lot was dipped in distilled water for 30 min, which was served as control (C). Concentration and dipping time of each extract were optimised from a Response Surface Analysis as per a Box-Behnken design (results not shown) where the resulting fish was analysed for microbiological, biochemical and sensory changes during chilled storage; thus, the best combination of concentration and dipping time which improved the keeping quality with less presence of odour and colour of the extracts in fish was chosen. According to this preliminary experiment, the effective concentration of citrus extract and mint extract for retarding the spoilage changes were selected at 1 and 0.5 %, respectively. All the lots were kept in a chiller during the dipping time. After 30 min, all the lots were drained well and three fishes each from each lot were packed in low density polyethylene (LDPE) pouch. The samples were then placed in a polypropylene box with ice and the box was further kept in a chiller maintained at 0–2 °C. The samples were withdrawn periodically for biochemical, microbiological and sensory quality changes. The samples rejected by the sensory panellists during the sampling days were not analysed further. All the analyses were done in triplicate and mean values taken.
Quality assessment
Biochemical analysis
For chemical analysis, the fish muscle was taken and ground using a mixer grinder. Proximate composition of the raw fish was determined by AOAC (1998) method. The fish muscle sample was homogenised in distilled water (1: 5 w/v) and pH was determined by using a glass electrode digital pH meter (Cyberscan 510, Eutech instruments, Singapore). Total volatile base nitrogen (TVB-N) and Tri methyl amine (TMA-N) was estimated by the microdiffusion method (Conway 1950). Oxidative stability of the sample was assessed by measuring Thiobarbituricacid reactive substances (TBARS) value (Tarladgis et al. 1960) as well as Peroxide Value (PV) (Yildiz et al. 2003). Free Fatty Acid (FFA) value was determined as per AOAC (1989) to assess the hydrolytic rancidity.
Microbiological analysis
Aerobic Plate Count (APC) of the samples was analyzed according to the method of Ryser and Schuman (2013). Fifty gram of each sample were drawn aseptically and homogenized with 450 ml of phosphate buffer in a filter stomacher bag using a Stomacher® 400 Circulator (Seward Limited, UK) for 2 min. After decimal dilutions, duplicates of three consecutive dilutions were plated on Plate Count Agar (Difco). Plates were enumerated after incubation at 35 ± 2 °C for 48 ± 2 h for obtaining total aerobic count.
Sensory analysis
Sensory analysis of raw and cooked samples was conducted by a panel of six experienced members. Uniform pieces of raw and cooked (10 min cooking in 1 % brine) samples were assessed by the panellists. Scoring was based on a nine point hedonic scale as described by Amerine et al. (1965). Various sensory characteristics like colour and appearance, texture, odour and flavour were evaluated. The overall acceptability score was determined taking into account the total score obtained for raw and cooked samples. Scores of separate attributes were summed up and divided by the total number of attributes to give an overall acceptability score. An overall score of four was considered as the border line for acceptability.
Statistical analysis
All the analysis was done in triplicate. The data was subjected to ANOVA by statistical software, SPSS version 16. Duncan’s multiple range test was carried out to find out the significant difference between mean values of experimental data of the treatments at 5 % level of significance.
Results and discussion
Total phenolic content
Extraction with 60 % ethanol yielded 15.26 ± 1.24 and 16.14 ± 1.02 % of mint leaf and citrus extract, respectively. The yield of extracts obtained in this study (15–16 % on dry weight basis) was comparatively higher to the yield reported by other workers. Rehman (2006) reported 11.24 ± 0.81 % yield when citrus peel was extracted using ethanol as solvent. Total phenolic content of mint extract was significantly (p < 0.05) higher than that of citrus peel extract (Table 1). The total phenolic content of citrus peel extracts were much lower than those reported by Ghasemia et al. (2009) (132.2–223.2 mg GAE/g DM). Total polyphenol content observed in methanolic extract of M. longifolia L. was 107.20 ± 34.2 mg GAE/g dry wt (Janifer Raj et al. 2010), which was comparable to our results (127 ± 8.2 mg GAE/g). Dorman et al. (2003) have reported a total phenolic content in the range of 128–230 mg gallic acid equivalents/g (dry weight) of extract from different Mentha species. It has been reported that phenolic compounds of plants are present in different bound status depending on species. Thus effective processing steps for liberating phenolic compounds from various plants may be different (Jeong et al. 2004). This reason might have accounted for the significant difference in total phenolic content between citrus peel and mint leaf extracts.
Table 1.
Total Phenolic content and antioxidant activities of the extract
| Parameter /Source | Mint leaf extract | Citrus peel extract | BHT |
|---|---|---|---|
| Total phenolic content (mg gallic acid eqwt/g) | 127b ± 8.2 | 82.8a ± 4.3 | – |
| Fe2+ reducing power (Abs) | 0.302b ± 0.024 | 0.074a ± 0.026 | 0.312c ± 0.03 (25 ppm) |
| DPPH assay (IC50, μg/ml) | 21.86b ± 2.73 | 105.66c ± 3.89 | 11.7a ± 3.84 |
* for each parameter, mean values (n = 3 with standard deviation) followed by different letters (a,b,c) denote significant differences between the sources
DPPH radical scavenging activity
Several free radicals, such as OH·, O2·-, LOO· having different reactivities are formed during lipid oxidation. Antioxidants are able to scavenge these free radicals by donating a H atom. Relatively stable DPPH radical has been widely used to test the ability of compounds to act as free radical scavengers or hydrogen donors and thus to evaluate the antioxidant activity (Jao and Ko 2002). From the results (Table 1), it was evident that, the mint leaf extract had significantly lower IC50 value than citrus peel extracts, indicating that the mint extract have higher free radical scavenging activities than citrus peel extract. The IC50 value obtained for mint extract (21.86 μg/ml) in the present study was comparable to that of the synthetic antioxidant BHT (11.7 μg/ml). IC50 values for the ethanolic extracts of different species of Mentha varied between 13.32 and 87.91 μg/ml (Nickavar et al. 2008). Kanatt et al. (2007) observed an IC50 of 25.8 μg/ml when aqueous extracts of Mentha spicata was tested. Lagha-Benamrouchea and Madani (2013) reported an IC50 value of 0.568 ± 0.182 mg/ml for the methanolic extracts of C. aurantium peel which was found to be the lowest when compared to that of other six Citrus varieties. However, the comparison of antioxidant capacity is difficult as there is wide variations among the solvent used, its concentration, extraction methods etc. between those reported in the literature. The differences in DPPH scavenging activities between mint leaf and citrus peel extract could be due to the higher amount of phenolic compounds in mint extracts. Various authors have reported a positive relationship between total phenolic content free radical scavenging activities and total phenolic content (Wangensteen et al. 2004; Kanatt et al. 2007). The active hydroxyl groups present in the molecular structure of polyphenols are the active components that can interact with the free radicals to inhibit lipid oxidation (Mitsumoto et al. 2005).
Fe2+ reducing power
The presence of reducing agents in the extracts induces reduction of the ferric ions to ferrous ion which absorbs at a wavelength of 700 nm, the increase in absorbance indicates increase in concentration of ferrous ions. ie., higher reducing power of the compounds. The result of this assay further confirmed the antioxidant potential of the extracts. The analysis of reducing power of extracts @ 0.1 mg/ml indicated significantly higher absorbance by mint extract (0.302) than citrus extract (0.074). Reducing power of mint extract was comparable to that of 25 ppm BHT (Table 1). The reducing power of ethanolic extracts of M. arvensis @1 mg/ml was found to be 0.469 which was comparable to that of trolox standard (0.661) (Milosevic et al. 2011). Lagha-Benamrouchea and Madani (2013) demonstrated that the reducing power of methanolic extracts of Citrus varieties ranges from 0.075 to 0.25 at 1 mg/ml. The diversity of the reducing power of the extracts used in the present work is probably due to the diversity of phenolic compounds present in the crude extracts. It has been reported that the reducing power might be due to hydrogen-donating ability (Shimada et al. 1992), and is generally associated with the presence of reductones (Duh 1998). In our results, the trend of reducing abilities for the extracts was similar to the result of the scavenging effect on DPPH radicals. Similar findings for extracts from other plant sources were reported by Chen et al. (2007).
Quality assessment of Indian mackerel
Proximate composition analysis of fish
The fresh mackerel used in this study showed 74.05 ± 1.65 % moisture, 21.75 ± 1.23 % protein, 2.64 ± 0.35 % fat and 1.03 ± 0.07 % ash content. The higher content of protein indicates the nutritive value of Indian mackerel. The fat content indicated a semi fatty nature of mackerel muscle which is susceptible to oxidation during storage. The comparatively higher moisture content contributes to faster spoilage due to microbial growth.
Changes in pH
pH of fresh fish was 6.34, indicating the freshness of the fish. The initial post-mortem pH varies with species to species, catching ground, and the season. No significant change in pH occurred after plant extract treatment on the initial day. Changes in the mean pH values of the samples are presented in the Fig. 1a. pH of all the groups increased marginally and reached a final value of 6.58, 6.59 and 6.61 for control, CE and ME group on their rejection on 13th, 15th and 17th day of storage, respectively. Increase in pH over the storage period is attributed to the production of amines and other volatile bases by the autolytic and microbial action on protein and other compounds (Binsi et al. 2007). pH of the control and treated sample groups didn’t show any significant difference (p > 0.05) till 6 days. The possible reason for this insignificant difference could be due to the insignificant difference between TVB-N values of control and treated samples observed during 9 days storage period. Gao et al. (2014) also observed an insignificant difference between the pH values of control and rosemary extract treated pompano fillets during 15 days chilled storage. After 6th day of storage, pH values were significantly higher (p < 0.05) in control samples followed by CE group than ME treated group. Except for the 15th day, difference in mean pH values between ME and CE groups were insignificant. The result of the present study suggests that pH index is not a good indicator of spoilage in early stages of mackerel storage; instead it can be used as a quality indicator during advanced stages of storage. The pH of fish just after catching is reported between 6.0 and 6.5. The fish is acceptable up to pH 6.8 and the pH of spoiled fish is above 7.0 (Huss 1988). All the samples maintained pH well below this level. The significantly lower pH in treated samples towards the end of storage period might result from the antimicrobial characteristics of mint extract and citrus extract against the bacteria responsible for producing basic compounds.
Fig. 1.

Changes in (a) pH, (b) TVB-N and (c) TMA-N of extract treated mackerel during chilled storage (n = 3, mean ± standard deviation)
Changes in TVB-N
TVB-N is known as a product of bacterial spoilage and endogenous enzymes action and its content is often used as an index to assess the keeping quality and shelf life of products (EEC 1995). TVB-N content in fresh fish was 11.5 mg%. Changes in TVB-N values in fish samples during chilled storage are shown in Fig. 1b. The analysis indicated that the values increased significantly (p < 0.05) in all groups with the advancement of storage period. TVB-N values increased significantly (p < 0.05) from an initial value of 11.5–12.02 mg % to a final value of 25.18, 26.64 and 26.91 mg % respectively, for control, CE and ME groups on their respective rejection days. Starting from 9th day, control group contained significantly (p < 0.05) higher TVB-N value than that of the ME and CE group. TVB-N content of ME and CE groups were not significantly different till 9 days of storage whereas 11th day onwards, the TVB-N values of ME groups remained significantly lower to that of CE group.
The concentration of TVB-N in freshly caught fish is typically between 5 and 20 mg N/100 g, whereas levels of 30–35 mgN/100 g fish are generally regarded as the limit of acceptability for ice-stored cold water fish (Connel 1995). However, various authors have reported different acceptability levels for TVB-N value depending on fish species, specific treatments, and processing conditions: 35–40 mg/100 g (Lakshmanan 2000); 25–30 mg/ 100 g for oyster (Lopez-Caballero et al. 2000); 25–35 mg/100 g for sardine (Ababouch et al. 1996) etc. Even though TVB-N showed an increasing trend, none of the samples crossed the established acceptable limit of TVB-N set by Connel (1995). Huss et al. (1974) reported that not all the bacteria species isolated from fish cause spoilage. Accordingly, although the APC count crossed the acceptable limit in all the groups, TVB-N, a result of bacterial spoilage has not crossed the set limit. Lower levels of TVB-N in treated samples can be attributed to either a decreased bacterial population or reduced capacity of bacteria for oxidative deamination of non-protein nitrogen compounds or both (Manju et al. 2007), which may be attributed by the presence of plant extracts. The result of the present study was consistent with that of several authors who reported the positive effect of various plant extracts in reducing TVB-N values in fish during chilled storage (Li et al. 2012; Pezeshk et al. 2011; Gao et al. 2014). Among the treatments, TVB-N was lowest in ME groups, indicating the more preservative effect of mint extract than citrus extract in controlling the bacterial growth responsible for volatile bases formation during spoilage.
Changes in TMA-N
TMA-N is an important spoilage index, particularly in marine fishes. TMA-N is derived from trimethylamineoxide (TMAO) which is critical for osmoregulation in marine fish. During spoilage, TMAO is reduced by enzymes to TMA. Variations in TMA-N during storage are shown in Fig. 1c. TMA-N was not detected in fresh mackerel used for this study. Compared to TVB-N, the rate of increase of TMA-N was slow in all the samples. On the 1st day, all the samples showed similar level of TMA-N (0.75 mg %), which increased significantly (p < 0.05) in all the groups during the storage. Control and CE groups had significantly higher levels (p < 0.05) of TMA-N than ME group on and after 6th day of storage. TMA-N level in CE group remained lower to that of control group; however significant difference (p < 0.05) was observed only on 11th and 13th day. TMA-N level reached up to 5.96, 5.35 and 5.75 mg% in control, CE and ME groups on their rejection day on 13th, 15th and 17th day, respectively.
From this study, it was appeared that TMA-N index did not provide a good indication of spoilage, as the levels were quite low during storage. According to Teskeredzic and Pfeifer (1987), the offensive fishy odour occurs when the TMA-N concentration of fish exceeds10 mg/100 g. In this study, all the sample groups retained TMA-N value far below this limit during entire storage period. The results of the present study are in agreement with those of Ishida et al. (1976) and Manju et al. (2007) who reported that, in low temperature storage, such as refrigeration above 0 °C; TMA-N formation slows down noticeably. In addition, it has been reported that the level of TMA-N in numerous fatty fish never reached 5 mg % although the limit is 10–15 mg % (Sikorski et al. 1990; Ozogul et al. 2004). Although TMA-N is believed to be generated by the action of spoilage bacteria, the correlation with bacterial numbers is often not very good (Huss 1995). Similarly, in this study, changes in the concentrations of TMA-N in mackerel during chilled storage did not correlate well with APC. Similar observations were made by Ozyurt et al. (2009) during the iced storage of red mullet and goat fish. However, result of TMA-N analysis indicated that the plant extracts, especially mint extract was effective in inhibiting the decarboxylation of trimethyl amine oxide to tri methyl amine by bacteria.
Changes in FFA
The FFA values of fish muscle gives an account of the degree of lipid hydrolysis occurred during spoilage. Although the formation of FFA does not directly result in nutritional losses, examining the extent of lipid hydrolysis need to be studied as FFA is known to oxidise faster than high molecular weight lipid classes like triglycerides and phospholipid by providing a easy accessibility (low stearic hindrance) to oxygen and other pro-oxidant molecules (Lubaza 1971). In brief, FFA values of all the samples showed a significant increase (p < 0.05) as time of storage progressed (Fig. 2a). The FFA value progressively increased from an initial value of 1.59 % oleic acid in fresh fish to a final value of 6.39, 5.98 and 6.42 % oleic acid in C, CE and ME groups, respectively on their final day of storage. The increase in FFA values observed during chilled storage period may be attributed to the hydrolysis of triglycerides and phospholipids mediated by endogenous or microbial lypolytic enzymes.
Fig. 2.
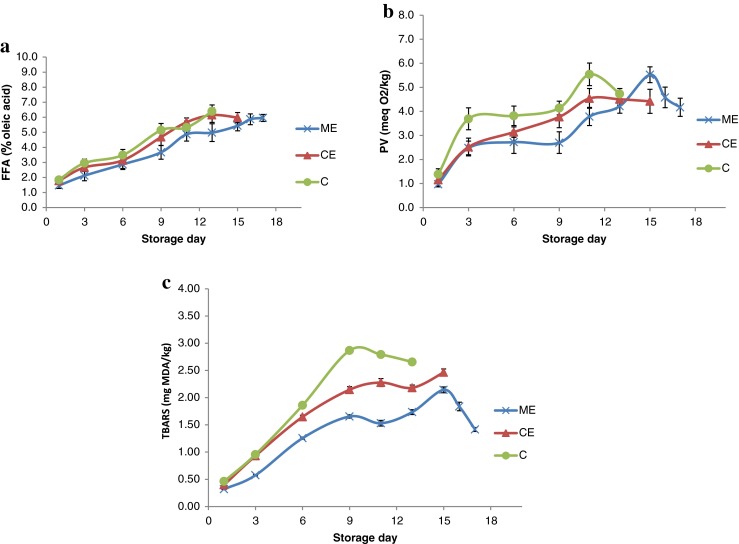
Changes in (a) FFA, (b) PV and (c) TBARS of extract treated mackerel during chilled storage (n = 3, mean ± standard deviation)
There were significant differences (p < 0.05) between the FFA of control and extract treated groups. The rate of lipid hydrolysis was substantially lower in ME treated groups which can be attributed to the inactivation of lipolytic enzymes and inhibition on the growth of lipolytic bacteria, and hence, low degradation of tissue lipids in the same. This observation was in agreement with the result of aerobic plate count where lowest counts were noticed in ME group. The level of FFA in CE group remained slightly lower to that of control group without any significant difference. The results of the study support the use of mint extract for controlling fat degradation in Indian mackerel during chilled storage. Similar to this finding, inhibitory effect on lipid hydrolysis by green tea extract and onion juice was obtained by Sarah et al. (2010) on refrigerated Persian sturgeon.
Changes in PV
Lipid in the fish is susceptible to oxidation and PV measures the amount of hydroperoxides formed i.e., the primary lipid oxidation products in fish muscle. The variations in mean PV values are presented in the Fig. 2b. PV of fresh fish was 0.765 meq O2/kg sample. At day 1, there was no significant difference between the PV of different sample groups. As storage progressed, control, CE and ME groups showed a progressive increase in PV till 11th, 11th and 15th day, respectively, followed by a reduction towards the end of storage period. The maximum value recorded for PV was 5.54, 4.54 and 5.52 meq O2/kg sample for control, CE and ME samples. The values were significantly higher (p < 0.05) in control samples than treated ones. Among the two extracts, mint extract was more effective in controlling the peroxide development compared to citrus extract which was in parallel with the results of in-vitro antioxidant activity assays.
The antioxidant phenolics in the extracts accounted for inhibiting the peroxide development in treated samples. Similar results with different plant extracts viz. potato peel extracts in minced horse mackerel (Farvin et al. 2012), turmeric and shallot extracts in rainbow trout fillet (Pezeshk et al. 2011), grape seed and clove bud extracts on silver carp (Shi et al. 2014) have been reported for retarding the production of PV during chilled storage. The study conducted by Rehman (2006) revealed that citrus peel extract exhibited marked antioxidant activity in terms of inhibiting peroxide formation in refined corn oil which was comparable to that of BHA. The reduction in PV in all the samples towards the end of storage period may be related to the secondary reactions of the hydroperoxides and volatilization (Vidya and Srikar 1996).
Changes in TBARS
TBARS index is widely used as a degree of secondary lipid oxidation in food. TBARS values of different treated groups during chilled storage are shown in Fig. 2c. The results indicated that TBARS values of all the three groups increased gradually from an initial value of 0.298 MDA/kg in fresh fish. The control and ME group recorded a highest value of TBARS as 2.866 and 2.143 MDA/kg, respectively on day 9th and 15th and thereafter showed a reducing trend. Whereas in CE group, the TBARS number indicated a reduction on day 13th; and then increased to 2.46 MDA/kg on day 15. On and after 3rd day of storage, TBARS value in ME groups remained significantly lower (p < 0.05) than that of control and CE samples. Among the control and CE groups, significantly lower (p < 0.05) TBARS values were noticed in CE group after 6 days of storage period.
The result of TBARS index suggests that both the extracts have excellent antioxidant activity in mackerel muscle during storage. Highest inhibition on secondary oxidation was exhibited by mint extract as observed from its lowest TBARS values among the samples. Similar to our observations, the inhibitory effects of plant derived extracts on secondary lipid oxidation of fish during chilled storage has been proven by several authors (Li et al. 2012; Shi et al. 2014; Gao et al. 2014). Kanatt et al. (2007) reported that mint extract (0.1 %) has strongly inhibited secondary lipid oxidation in irradiated meat during chilled storage. Similarly, Biswas et al. (2012) proved that addition of mint extract in pork meat significantly reduced TBARS value than that of control sample during refrigerated storage. Recently, Houicher et al. (2013) reported that addition of M. spicata extract @ 1 % in vacuum packed sardine has effectively reduced TBARS formation and extended the shelf life during refrigerated storage. As many authors reported (Maqsood and Benjakul 2010; Goulas and Kontominas 2007), declining trend in TBARS of ME and control groups towards the end of storage period is attributed to the interaction of these low molecular unstable malonaldehyde compound with other tissue constituents as well as its break down to organic acid, alcoholes etc. which are not determined by TBARS test.
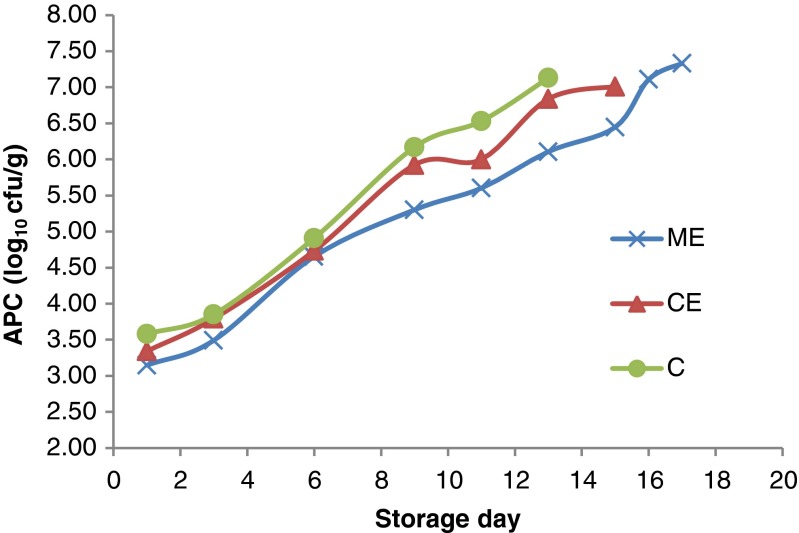
Changes in aerobic plate count
The major changes in fish freshness, for instance unattractive change in food characteristics such as, flavours and odours and colour are largely due to bacterial growth and activity (Huss 1995; Connell 1990). Aerobic plate count (APC) reflects the microbial quality of food and is useful for indicating the potential spoilage of perishable products. Changes in APC of mackerel throughout storage are shown in Fig. 3a. The fresh fish showed a count 4.17 log10 cfu/g, demonstrating its good quality. There were no considerable differences among the APC of different samples up to 6 days of storage. On and after 9th day, the control group showed 1–1.2 log higher counts than ME group. The counts of CE treated samples were 0.2–0.5 log lesser than that of control after 9 days during storage. At the sensory rejection day, the APC reached up to 7.13, 7.01 and 7.33 log10 cfu/g, respectively for control, CE and ME groups. The upper limit (M) for fresh fish proposed by ICMSF (1998) for human consumption is 7 log 10 cfu g −1. In the present study, control attained a count of 7.13 log10 cfu/g on day 11, indicating a microbiological shelf life of about 11 days for the control samples. On the other hand, OE groups and ME groups crossed this limit on day 13 and 16, respectively while it was not reflected in sensory analysis.
Fig. 3.
Changes in aerobic plate count of extract treated mackerel during chilled storage
The significant reduction in APC observed in the treated samples of mackerel can be attributed to the antibacterial effect of plant extracts on aerobic spoilage bacteria, resulting in 2–5 days extension of microbiological shelf life compared to control. The results clearly demonstrated that the mint extract had strong inhibitory effect on microbial growth in mackerel fish which was further in agreement with the result of its in-vitro antibacterial activities (results not shown). Previous work by Sugandhi and Meera Bai (2011) demonstrated that ethanolic extracts of M. arvensis has potential antibacterial activities against P. aeruginosa, S. flexneri, K. pneumonia, S. aureus, and E coli. The result of the present study also confirmed the antibacterial effects of mint and citrus extracts.
Sensory evaluation
Changes in the overall acceptability scores of gutted mackerel samples treated with plant extracts in comparison to control samples are shown in Fig. 4. Sensory deterioration was rapid in control samples and the overall score declined from initial 8.35 to 3.75 on 13th day of storage. The fish treated with plant extracts, particularly by mint extract gave higher sensory scores and showed better characteristics for appearance, flavour, relative to the control during storage while the score reduced up to 3.76 and 3.78 on 15th and 17th day, respectively.
Fig. 4.
Changes in the overall acceptability score of extract treated mackerel during chilled storage (n = 5, mean ± standard deviation)
It was interesting to note that offensive putrid odour/flavour was not detected in the samples during extended storage. This observation was in agreement with that of TVB-N and TMA-N production; which have not crossed the established limits. The fishes were rejected primarily because of loss of its characteristic meaty and juicy flavour and changes in color and appearance. Based on the sensory evaluation, the samples were rejected on the day when the overall score reached beyond 4. Accordingly, the control, CE and ME group samples were considered to be acceptable for consumption up to 11–13, 13–15 and 16–17 days, respectively during storage at 0–2 °C. The study indicated that treatment with mint extract was highly effective in retarding the spoilage indices; extended the shelf life of gutted mackerel by 5 days compared to the control samples. These conclusions agreed well with the results for microbiological and chemical quality analyses.
The preservative action obtained by the mint extract could be due to the presence of rosemarinic acid, caffeic acid, eriocitrin and luteolin which are reported to be the major phenolics in Mentha species (Padmini et al. 2010; Kappa et al. 2013) with potential antibacterial and antioxidant activities (McKay and Blumberg 2006; Singh et al. 2010; Shan et al. 2005). The peel of Citrus fruits is a rich source of flavonoid glycosides, coumarins and glycosides (Shahnan et al. 2007) and the citrus flavonoids have a large spectrum of biological activity including antibacterial, antioxidant and antifungal activities. Manthey and Grohmann (2001) reported that polyphenol compounds such as p-coumaric, ferulic, and sinapic acids and narirutin were present in citrus peel extract. Singh et al. (2010) stated that constituents such as gamma-terpinene, terpinolene, alpha-terpinene, hesperidin, neohesperidin etc. are responsible for the preservative action in citrus species. The result of the present study indicated a positive role of mint extract followed by citrus extract in delaying the spoilage mechanisms of mackerel during chilled storage.
Conclusions
The results of the present study revealed a significant effect of mint and citrus extracts on controlling the biochemical indices like TVB-N, TMA-N, PV, FFA and TBARS during storage. None of the samples crossed the established limits for indices like TVB-N and TMA-N during the storage period. Also, the extract treatment significantly suppressed the growth of bacteria as determined by the counts of total viable bacteria, Enterobacteriaceae and Pseudomonas spp. population. The overall acceptability scores of the extract treated mackerel rated better to that of control. In brief, dipping in citrus and mint extract enhanced the storage stability and extended the shelf life of mackerel by 2 and 5 days, respectively during storage at 0–2 °C. The study also demonstrated a prominent preservative effect of mint extract and hence, mint extract can be considered as a potential natural preservative in fish and fish products. Further research on removal of pigments and characterisation of active compounds in the extracts is envisaged.
Acknowledgments
This research work is supported by Indian Council of Agricultural Research, New Delhi, India. The assistance offered by the technical staff Ms. Priyanka Vichare is deeply acknowledged.
References
- Ababouch LH, Souibri L, Rhaliby K, Ouahdi O, Battal M, Busta FF (1996) Quality changes in sardines (Sardina pilchardus) stored in ice and at ambient temperature. Food Microbiol 13:123–132
- Amerine MA, Pongborn RH, Roescler EB. Principles of sensory evaluation of food. New York: Academic Press; 1965. p. 602. [Google Scholar]
- AOAC . Official methods and recommended practices of American oil chemists society. 5. Champaign: Association of Official Analytical Chemists; 1989. [Google Scholar]
- AOAC . Official methods of analysis. 17. Washington: Association of Official Analytical Chemists; 1998. [Google Scholar]
- Babbar N, Oberoi HS, Uppal S, Patil RT. Total phenolic content and antioxidant capacity of extracts obtained from six important fruit residues. Food Res Int. 2011;44:391–396. doi: 10.1016/j.foodres.2010.10.001. [DOI] [Google Scholar]
- Benavente-Garicia O, Castillo J, Marin FR, Ortuno A, Del Rio JA. Uses and properties of citrus flavonoids. J Agric Food Chem. 1997;45:4505–4515. doi: 10.1021/jf970373s. [DOI] [Google Scholar]
- Binsi PK, Shamasundar BA, Dileep AO. Physico-chemical and functional properties of proteins from green mussel (Perna viridis) during ice storage. J Sci Food Agric. 2007;87:245–254. doi: 10.1002/jsfa.2706. [DOI] [Google Scholar]
- Biswas AK, Chatil MK, Sahoo J. Antioxidant potential of curry (Murraya koenigii L.) and mint (Mentha spicata) leaf extracts and their effect on colour and oxidative stability of raw ground pork meat during refrigeration storage. Food Chem. 2012;133:467–472. doi: 10.1016/j.foodchem.2012.01.073. [DOI] [PubMed] [Google Scholar]
- Chen H, Lin Y, Hsieh C. Evaluation of antioxidant activity of aqueous extract of some selected nutraceutical herbs. Food Chem. 2007;104:1418–1424. doi: 10.1016/j.foodchem.2007.02.004. [DOI] [Google Scholar]
- Connel JJ. Control of fish quality, fishing new books. Cambridge: Blackwell Science Ltd.; 1995. p. 241. [Google Scholar]
- Connell JJ. Control of fish quality. 3. Oxford: Fishing News Books; 1990. pp. 1–227. [Google Scholar]
- Conway EJ. Micro-diffusion analysis and volumetric error. Crosby: Lockwood and Son Ltd; 1950. [Google Scholar]
- Corbo M, Speranza B, Filippone A, Granatiero S, Conte A, Sinigaglia M, Del Nobile M. Study on the synergic effect of natural compounds on the microbial quality decay of packed fish hamburger. Int J Food Microbiol. 2008;127:261–267. doi: 10.1016/j.ijfoodmicro.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12:564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman HJD, Kosar M, Kahlos K, Holm Y, Hiltunen R. Antioxidant properties and composition of aqueous extracts from Mentha species, hybrids, varieties, and cultivars. J Agric Food Chem. 2003;51:4563–4569. doi: 10.1021/jf034108k. [DOI] [PubMed] [Google Scholar]
- Duh PD. Antioxidant activity of Budrock (Arctium lappa Linn): its scavenging effect on free radical and active oxygen. J Am Oil Chem Soc. 1998;75:455–461. doi: 10.1007/s11746-998-0248-8. [DOI] [Google Scholar]
- Edeoga HO, Okwu DE, Mbaebie BO. Phytochemical constituents of some Nigerian medicinal plants. Afric J Biotech. 2005;4:685–688. doi: 10.5897/AJB2005.000-3127. [DOI] [Google Scholar]
- EEC Total volatile basic nitrogen (TVB-N) limit values for certain categories of fishery products and specifying the analysis methods to be used. Commission Decision 95/149/EEC of 8 March 1995. Off J Eur Commun. 1995;L97:84–87. [Google Scholar]
- El-aal A, Halaweish FT. Food preservative activity of phenolic compounds in organic peel extracts (Citrus sinensis L.) Lucrari Stiintifice. 2010;53:457–464. [Google Scholar]
- Farvin KHS, Grejsen HD, Jacobsen C. Potato peel extract as a natural antioxidant in chilled storage of minced horse mackerel (Trachurus trachurus): effect on lipid and protein oxidation. Food Chem. 2012;131:843–851. doi: 10.1016/j.foodchem.2011.09.056. [DOI] [Google Scholar]
- Gao M, Feng L, Jiang T, Zhu J, Fu L, Yuan D, Li J. The use of rosemary extract in combination with nisin to extend the shelf life of pompano (Trachinotus ovatus) fillet during chilled storage. Food Cont. 2014;37:1–8. doi: 10.1016/j.foodcont.2013.09.010. [DOI] [Google Scholar]
- Ghasemia K, Ghasemia Y, Ebrahimzadeh MA. Antioxidant activity, phenol and flavonoid contents of 13 citrus species peels and tissues. Pak J Pharma Sci. 2009;22:277–281. [PubMed] [Google Scholar]
- Goulas AE, Kontominas MG. Combined effect of light salting, modified atmosphere packaging and oregano essential oil on the shelf-life of sea bream (Sparus aurata): biochemical and sensory attributes. Food Chem. 2007;100:287–296. doi: 10.1016/j.foodchem.2005.09.045. [DOI] [Google Scholar]
- Gramza A, Khokhar S, Yoko S, Gliszczynska- Swiglo A, Hes M, Korczak J. Antioxidant activity of tea extracts in lipids and correlation with polyphenol content. Eur J Lipid Sci Tech. 2006;108:351–362. doi: 10.1002/ejlt.200500330. [DOI] [Google Scholar]
- Guo C, Yang J, Wei J, Li Y, Xu J, Jiang Y. Antioxidant activities of peel, pulp and seed fractions of common fruits as determined by FRAP assay. Nutr Res. 2003;23:1719–1726. doi: 10.1016/j.nutres.2003.08.005. [DOI] [Google Scholar]
- Houicher A, Kuley E, Bendeddouche B, Ozogul F. Effect of Mentha spicata L. and Artemisia campestris extracts on the shelf life and quality of vacuum packed refrigerated sardine (Sarda pilchardus) fillets. J Food Prot. 2013;76:1719–1725. doi: 10.4315/0362-028X.JFP-13-118. [DOI] [PubMed] [Google Scholar]
- Huss HH. Fresh fish, quality and quality changes. FAO fisheries series, no. 29. Rome: FAO; 1988. p. 132. [Google Scholar]
- Huss HH (1995) Quality and quality changes in fresh fish. FAO fisheries technical paper, 348
- Huss HH, Dalsgaard D, Hansen L, Ladefoged H, Pedersen ZL. The influence of hygiene in catch handling on the storage life of iced cod and plaice. J Food Sci Technol. 1974;9:213–221. doi: 10.1111/j.1365-2621.1974.tb01765.x. [DOI] [Google Scholar]
- ICMSF (International Commission on Microbiological Specifications for Foods) Microorganisms in foods. 6. Microbial ecology of food commodities. Baltimore: Blackie Academic and Professional; 1998. [Google Scholar]
- Ishida Y, Fuji T, Kodata H. Microbiological studies on salted fish stored at low temperature. In chemical changes of salted fish during storage. Bull Japan Soc Sci Fish. 1976;42:351–358. doi: 10.2331/suisan.42.351. [DOI] [Google Scholar]
- Janifer Raj X, Bajpai PK, Phani Kumar G, Pal Murugan M, Kumar J, Chaurasia OP, Singh SB. Determination of total phenols, free radical scavenging and antibacterial activities of Mentha longifolia Linn. Hudson from the Cold Desert, Ladakh, India. Phcog J. 2010;2:470–475. doi: 10.1016/S0975-3575(10)80033-1. [DOI] [Google Scholar]
- Jao CH, Ko WC. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) radical scavenging by protein hydrolysates from tuna cooking juice. Fish Sci. 2002;68:430–435. doi: 10.1046/j.1444-2906.2002.00442.x. [DOI] [Google Scholar]
- Jeong SM, Kim SY, Kim DR, Jo SC, Nam KC, Ahn DU. Effect of heat treatment on antioxidant activity of citrus peels. J Agric Food Chem. 2004;52:3389–3393. doi: 10.1021/jf049899k. [DOI] [PubMed] [Google Scholar]
- Kanatt SR, Chander R, Sharma A. Antioxidant potential of mint (Mentha spicata L.) in radiation processed lamb meat. Food Chem. 2007;100:451–458. doi: 10.1016/j.foodchem.2005.09.066. [DOI] [Google Scholar]
- Kanatt SR, Chander R, Sharma A. Chitosan and mint mixture: a new preservative for meat and meat products. Food Chem. 2008;107:845–852. doi: 10.1016/j.foodchem.2007.08.088. [DOI] [Google Scholar]
- Kappa K, Hakala E, Orav A, Pohjala A, Vuorela P, Püssa T, Vuorela H, Raal A. Commercial peppermint (Mentha x piperita L.) teas: antichlamydial effects and polyphenolic composition. Food Res Intl. 2013;53:758–766. doi: 10.1016/j.foodres.2013.02.015. [DOI] [Google Scholar]
- Lagha-Benamrouchea S, Madani K. Phenolic contents and antioxidant activity of orange varieties (Citrus sinensis L. and Citrus aurantium L.) cultivated in Algeria: peels and leaves. Ind Crop Prod. 2013;50:723–730. doi: 10.1016/j.indcrop.2013.07.048. [DOI] [Google Scholar]
- Lakshmanan PT. Fish spoilage and quality assessment. In quality assurance in seafood processing. India: Society of Fisheries Technologists; 2000. pp. 28–45. [Google Scholar]
- Li TT, Li JR, Hu WZ, Zhang XG, Li XP, Zhao J. Shelf-life extension of crucian carp (Carassius auratus) using natural preservatives during chilled storage. Food Chem. 2012;135:140–145. doi: 10.1016/j.foodchem.2012.04.115. [DOI] [Google Scholar]
- Lopez-Caballero ME, Perez-Mateos M, Montero P, Borderias AJ. Oyster preservation by high-pressure treatment. J Food Prot. 2000;63:196–201. doi: 10.4315/0362-028x-63.2.196. [DOI] [PubMed] [Google Scholar]
- Lubaza TP. Kinetics of lipid oxidation in foods. Crit Rev Food Technol. 1971;2:355. doi: 10.1080/10408397109527127. [DOI] [Google Scholar]
- Madsen HL, Bertelsen G. Spices as antioxidants. Trend Food Sci Technol. 1995;6:271–277. doi: 10.1016/S0924-2244(00)89112-8. [DOI] [Google Scholar]
- Manju S, Jose L, Gopal TKS, Ravisankar CN, Lalitha KV. Effect of sodium acetate dip treatment and vacuum-packaging on chemical, microbiological, textural and sensory changes of pearl spot (Etroplus suratensis) during chilled storage. Food Chem. 2007;102:27–35. doi: 10.1016/j.foodchem.2006.04.037. [DOI] [Google Scholar]
- Manthey JA, Grohmann K. Phenols in citrus peel by products. Concentrations of hydroxycinnamates and polymethoxylated flavones in citrus peel molasses. J Agric Food Chem. 2001;49:3262–3273. doi: 10.1021/jf010011r. [DOI] [PubMed] [Google Scholar]
- Maqsood S, Benjakul S. Synergistic effect of tannic acid and modified atmospheric packaging on the prevention of lipid oxidation and quality losses of refrigerated striped catfish slices. Food Chem. 2010;121:29–38. doi: 10.1016/j.foodchem.2009.11.086. [DOI] [Google Scholar]
- Marinova EM, Yanishlieva NV. Antioxidative activity of extracts from selected species of the family Limiaceae in sunflower oil. Food Chem. 1997;58:245–248. doi: 10.1016/S0308-8146(96)00223-3. [DOI] [Google Scholar]
- McKay DL, Blumberg JB. A review of the bioactivity and potential health benefits of peppermint tea (Mentha piperita L.) Phyto Res. 2006;20:619–633. doi: 10.1002/ptr.1936. [DOI] [PubMed] [Google Scholar]
- Milosevic S, Zekovic Z, Lepojevic Z, Vidovic S, Radojkovic M, Cvetanovic A. Antioxidant properties of tablets prepared from ginkgo, echinacea and mentha dry extracts. Rom Biotechnol Lett. 2011;16:6481–6487. [Google Scholar]
- Mitsumoto M, Grady MN, Kerry JP, Buckley DJ. Addition of tea catechins and vitamin C on sensory evaluation, colour and lipid stability during chilled storage in cooked or raw beef and chicken patties. Meat Sci. 2005;69:773–779. doi: 10.1016/j.meatsci.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Moreira MR, Ponce AG, Del-Valle CE, Roura SI. Inhibitory parameters of essential oils to reduce a foodborne pathogen. Lebensmittel. 2005;38:565–570. [Google Scholar]
- Murthy CKN, Jayaprakasha GK, Singh RP. Studies on antioxidant activity of pomegranate (Punica granatum) peel extract using in vivo models. J Agric Food Chem. 2002;50:4791–4795. doi: 10.1021/jf0255735. [DOI] [PubMed] [Google Scholar]
- Negi PS, Jayaprakasha GK. Antioxidant and antibacterial activities of Punica granatum peel extracts. J Food Sci. 2003;68:1473–1477. doi: 10.1111/j.1365-2621.2003.tb09669.x. [DOI] [Google Scholar]
- Nickavar B, Alinaghi A, Kamalinejad M. Evaluation of the antioxidant properties of five Mentha species. Iran J Pharma Res. 2008;7:203–209. [Google Scholar]
- Ozgen U, Mavi A, Terzi Z, Yildirim A, Coskun M, Houghton PJ. Antioxidant properties of some medicinal Lamiaceae (Labiatae) species. Pharm Biol. 2006;44:107–112. doi: 10.1080/13880200600592061. [DOI] [Google Scholar]
- Ozogul F, Polat A, Ozogul Y. The effects of modified atmosphere packaging and vacuum packaging on chemical, sensory and microbiological changes of sardines (Sardina plichardus) Food Chem. 2004;85:49–57. doi: 10.1016/j.foodchem.2003.05.006. [DOI] [Google Scholar]
- Ozyurt G, Kuley E, Ozkutuk S, Ozogul F. Sensory, microbiological and chemical assessment of the freshness of red mullet (Mullus barbutus) and goldband goatfish (Upeneus moluccensis) during storage in ice. Food Chem. 2009;114:505–510. doi: 10.1016/j.foodchem.2008.09.078. [DOI] [Google Scholar]
- Padmini EA, Valaemathi M, Ran U. Comparative analysis of chemical composition and antibacterial activities of Mentha spicata I and Camella sinensis. Asia J Exp Biol. 2010;4:772–781. [Google Scholar]
- Pezeshk S, Rezaei M, Hosseini H. Effect of turmeric, shallot extracts and their combinations on quality characteristics of vacuum packaged rainbow trout stored at 4 ± 1 °C. J Food Sci. 2011;76:387–391. doi: 10.1111/j.1750-3841.2011.02242.x. [DOI] [PubMed] [Google Scholar]
- Rehman Z- U (2006) Citrus peel extract-A natural source of antioxidant. Food Chem 99:450–454
- Richards MP, Hultin HO. Contributions of blood and blood components to lipid oxidation in fish muscle. J Agric Food Chem. 2002;50:555–564. doi: 10.1021/jf010562h. [DOI] [PubMed] [Google Scholar]
- Ryser ET, Schuman J. Aerobic plate count, updated september 2013. In: compendium of method for the microbiological examination of foods. Washington: American Public Health Association; 2013. [Google Scholar]
- Sarah H, Hadiseh K, Gholamhossein A, Bahareh S. Effect of green tea (Camellia sinenses) extract and onion (Allium cepa) juice on lipid degradation and sensory acceptance of Persian sturgeon (Acipenser persicus) fillets. Int Food Res J. 2010;17:751–761. [Google Scholar]
- Shahnan SM, Ali S, Ansari H, Bagri P. New sequiterpene derivative from fruit peel of Citrus limon (Linn) Burn F Sci Pharm. 2007;75:65–170. [Google Scholar]
- Shan B, Cai YZ, Sun M, Corke M. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J Agric Food Chem. 2005;53:7749–7759. doi: 10.1021/jf051513y. [DOI] [PubMed] [Google Scholar]
- Shan B, Cai YZ, John D, Corke M. The in vitro antibacterial activity of dietary spice and medicinal herb extracts. Int J Food Microbiol. 2007;117:112–119. doi: 10.1016/j.ijfoodmicro.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Shi C, Cui J, Yin Z, Luo Y, Zhou Z. Grape seed and clove bud extracts as natural antioxidants in silver carp (Hypophthalmichthys molitrix) fillets during chilled storage: effect on lipid and protein oxidation. Food Cont. 2014;40:134–139. doi: 10.1016/j.foodcont.2013.12.001. [DOI] [Google Scholar]
- Shimada K, Fujikawa K, Yahara K, Nakamura T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem. 1992;40:945–948. doi: 10.1021/jf00018a005. [DOI] [Google Scholar]
- Sikorski ZE, Kolakowska K, Burt JR. Postharvest, biochemical and microbial changes. In: Sikorski ZE, editor. Seafood: resources, nutritional composition, and preservation. Boca Raton: CRC Press Inc; 1990. pp. 55–75. [Google Scholar]
- Singh A, Sharma PK, Garg G. Natural products as preservatives. Int J Pharma Bio Sci. 2010;1:601–612. [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am J Enol Viticul. 1965;16:144–158. [Google Scholar]
- Smid EJ, Gorris LGM. Natural antimicrobials for food preservation. In: Rahman MS, editor. Handbook of food preservation. New York: Marcel Dekker; 1999. pp. 285–308. [Google Scholar]
- Sugandhi BRM, Meera Bai G. Antimicrobial activity of Mentha Arvensis L. Lamiaceae. J Adv Lab Res Biol. 2011;2:8–11. [Google Scholar]
- Sultana B, Anwar F, Ashraf M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecul. 2009;14:2167–2180. doi: 10.3390/molecules14062167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarladgis BG, Watts BM, Younthan MT. A distillation method for the quantitative determination of malonaldehyde in rancid foods. J Am Oil Chem Soc. 1960;37:44–52. doi: 10.1007/BF02630824. [DOI] [Google Scholar]
- Tepe B, Daferera D, Tepe A, Polissiou M, Sokmen A. Antioxidant activity of the essential oil and various extracts of Nepta flavida Hud.-Mor. from Turkey. Food Chem. 2007;103:1358–1364. doi: 10.1016/j.foodchem.2006.10.049. [DOI] [Google Scholar]
- Teskeredzic Z, Pfeifer K. Determining the degree of freshness of rainbow trout (Salmo gairdneri) cultured in brackish water. J Food Sci. 1987;52:1101–1102. doi: 10.1111/j.1365-2621.1987.tb14286.x. [DOI] [Google Scholar]
- Vidya SR, Srikar L. Effect of pre-process ice storage on lipid change of Japanese threadfin bream (Nemipterus japonicus) mince during frozen storage. Asia Fish Sci. 1996;9:109–114. [Google Scholar]
- Wang YC, Chuang YC, Hus HW. The flavonoid, carotenoid and pectin content in peels of citrus cultivated in Taiwan. Food Chem. 2008;106:277–284. doi: 10.1016/j.foodchem.2007.05.086. [DOI] [Google Scholar]
- Wangensteen H, Samuelsen AB, Malterud KE. Antioxidant activity in extracts from coriander. Food Chem. 2004;88:293–297. doi: 10.1016/j.foodchem.2004.01.047. [DOI] [Google Scholar]
- Yildirim A, Mavi A, Kara AA. Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J Agric Food Chem. 2001;9:4083–4089. doi: 10.1021/jf0103572. [DOI] [PubMed] [Google Scholar]
- Yildiz G, Wehling R, Cuppett SL (2003) Comparison of four analytical methods for the determination of peroxide value in oxidized soybean oils. J Am Oil Chem Soc 80:103–107