Abstract
Health awareness has grown to a greater extent among consumers and they are looking for healthy probiotic counterparts. Keeping in this view, the present review focuses recent developments in dairy and non-dairy probiotic products. All over the world, dairy probiotics are being commercialized in many different forms. However, the allergy and lactose intolerance are the major set-backs to dairy probiotics. Whereas, flavor and refreshing nature are the major advantages of non-dairy drinks, especially fruit juices. Phenotypic and genotypic similarities between dairy and non-dairy probiotics along with the matrix dependency of cell viability and cell functionality are reviewed. The heterogeneous food matrices of non-dairy food carriers are the major constraints for the survival of the probiotics, while the probiotic strains from non-dairy sources are satisfactory. Technological and functional properties, besides the viability of the probiotics used in fermented products of non-dairy origin are extremely important to get a competitive advantage in the world market. The functional attributes of dairy and non-dairy probiotic products are further enhanced by adding prebiotics such as galacto-oligosaccharide, fructo-oligosaccharide and inulin.
Keywords: Dairy probiotics, Non-dairy probiotics, Fruit juices, Fermented foods, Lactic acid bacteria, Microencapsulation, Cell viability
Introduction
Probiotic cultures have been with the mankind ever since people started consuming fermented milks and eating fermented foods. However, their health beneficial effects were uncovered only after Metchnikoff in 1907 suggested that the gut microflora had adverse effects on health and called it “Autointoxication”. He further suggested that ingestion of fermented milks ameliorated this condition. Based on the assumption that colonization of the gut is necessary for maximum beneficial effect, he used intestinal strains of Lactobacillus acidophilus for the treatment of constipation (Rettger and Chaplin 1921; Fuller 1991). The word probiotic is coined by Kollath (1953) and is derived from the Greek language, which means “for life”. According to Lilly and Stillwell (1965), probiotics are substances produced by microorganisms that promote the growth of other microorganisms. However, the widely adopted definition states probiotics as “live microorganisms which when administered in adequate amounts confer a health benefit on the host” (FAO/WHO 2001). There are two more essential terms to know, prebiotics and synbiotics. Prebiotics are defined as the indigestible food ingredients that promote the growth or activity of beneficial bacteria, thereby benefiting the host. Synbiotics are combinations of probiotics and prebiotics that are designed to improve the survival of the ingested microorganisms and their colonization of the intestinal tract (de Vrese and Schrezenmeir 2008). Prebiotics are being added to the food products to stimulate the colonic microflora to get health benefits to the consumers, besides providing textural attributes to the foods (Saad et al. 2013). An acidophilus milk product added with a prebiotic inulin was standardized using artificial neural network (Amiri et al. 2010). Supplementation of a probiotic-fermented soymilk with the fructo-oligosaccharide (FOS), inulin and pectin increased the angiotensin I-converting enzyme inhibitory activity and enhanced the in vitro antihypertensive effect (Yeo and Liong 2010). The functional and health benefits and recent developments in the production of the galacto-oligosaccharides (GOS) and its application in fruit juices and beverages have been thoroughly reviewed (Sangwan et al. 2011). Very recently, Rastall and Gibson (2015) reviewed the impact of prebiotics in promoting the growth beneficial microbes and intestinal health. Classification of probiotic foods is shown in Fig. 1.
Fig. 1.
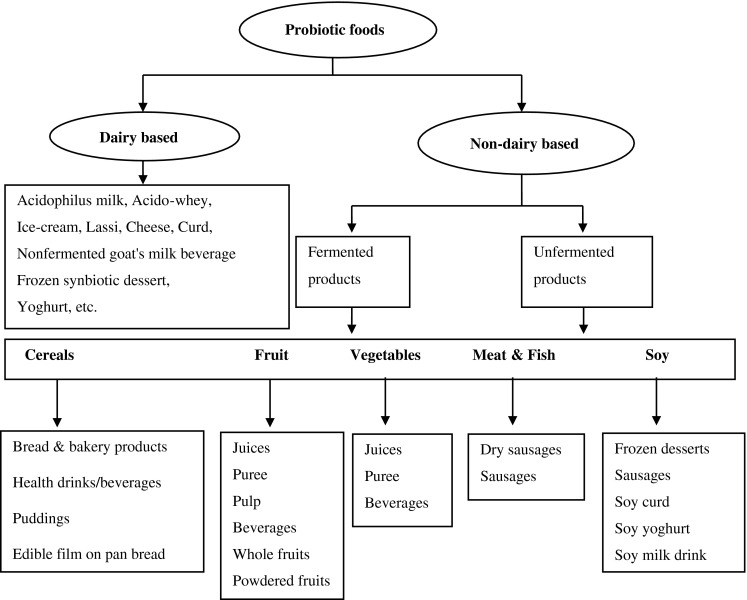
Classification and types of probiotic foods
Several species belonging to the genera of Lactobacillus, Bifidobacterium, Streptococcus, Lactococcus and some species of Enterococcus and Escherichia coli, are widely used as probiotics. Saccharomyces boulardii is the only non-pathogenic yeast being used as a probiotic. Treatment with probiotics involves modulation of the immune system both at the local and systemic levels and the beneficial effects include either shortened duration of infections or lowered susceptibility to pathogens (Antoine 2010). As said earlier by Metchnikoff; not all probiotics colonize gut to confer beneficial health effects (e.g., Bifidobacterium longum), some act in a transient manner by restoring and maintaining the homeostasis in the microbial gut flora (Lb. casei) (Ohland and Macnaughton 2010).
Some of the basic mechanisms by which the probiotics confer health benefits to the host include modulating the mucosal barrier function, decreasing the apoptosis of epithelial cells and by increasing mucin production (Mattar et al. 2002; Gaudier et al. 2005; Yan and Polk 2006; Caballero-Franco et al. 2007; Gogineni et al. 2013; Saad et al. 2013), aiding the increased production of antimicrobial peptides like defensins and cathelcidins by host cells (Schlee et al. 2008; Kelsall 2008; Mondel et al. 2009), production of bacteriocins, microcins and other antimicrobial substances that make the intestinal environment less comfortable for other pathogenic microbes (especially by lowering pH) (Alakomi et al. 2000; Penner et al. 2005; Liévin-Le et al. 2006; Duquesne et al. 2007; Venkateshwari et al. 2010; Vijayendra et al. 2010; Halami et al. 2011; Sharma and Devi 2014), adhering to the epithelial cells in a competitive fashion and by blocking the adherence of pathogens on the epithelial cells either directly or indirectly (Johnson-Henry et al. 2007; Wu et al. 2008), modulating the immune system, by blocking pro-inflammatory molecules and by increasing mucin production (Ogawa et al. 2001; Tien et al. 2006) and interfering with the quorum sensing signaling, process through which the pathogenic microbes communicate with one another (Miller and Bassler 2001; Medellin-Peña et al. 2007).
Many investigations have been carried out to provide the evidence related to the health benefits of probiotics on gastrointestinal infections, antimicrobial activity, improvement in lactose metabolism, reduction in serum cholesterol, immune system stimulation, antimutagenic properties, anti-carcinogenic properties, anti-diarrheal properties, improvement in inflammatory bowel disease and suppression of Helicobacter pylori infection by addition of selected strains to food products (Gotcheva et al. 2002; Nomoto 2005; Imasse et al. 2007; Shah 2007; Vijayendra and Gupta 2012). Some health benefits of probiotics have been reviewed recently (Sanders et al. 2013). Probiotics are conventionally added to dairy products like yogurt, dahi and other fermented dairy foods (Laroia and Martin 1991; Penna et al. 2007; Vijayendra and Gupta 2013a, b) making them as functional foods. Commercially probiotics are being sold throughout the world mainly in the form of fermented foods and fermented dairy products, which play a predominant role as carriers of probiotics (Heller 2001). However, the increasing health concerns of lactose intolerance, milk protein allergy, high cholesterol content and high amounts of saturated fatty acids of dairy based foods are resulting in a shift towards non-dairy foods such as probiotic fermented cereals, fruits and vegetables juices (Gupta and Abu-Ghannam 2012; Peres et al. 2012; Vijaya Kumar et al. 2013, 2015). However, both the dairy and non-dairy consumers are not forsaking their interest in consuming probiotics for their perceived beneficial health effects (Ranadheera et al. 2010). Hence, the non-dairy based probiotic foods are finding their way into our routine life one by one. However, the non-dairy probiotic preparations are not new and many non-dairy preparations of cereals, soy, etc., are traditionally being made for centuries in all parts of the world. Microorganisms used as probiotics are mostly of human or animal origin; however, some studies show that strains recognized as probiotics are also found in non-dairy fermented substrates (Schrezenmeir and de Vrese 2001).
It is necessary for the commercial probiotic preparations to be stable during entire storage time and the matrix in which they are present plays a vital role in their stability and interactions among microbes. Not much is known about the effect of food matrix and product formulation on probiotic functionality and the type of food format plays a key role in affecting survival, physiology and efficacy of probiotic cultures (Sanders and Marco 2010). While developing functional probiotic foods, selection of a suitable food system to deliver probiotics is a vital factor (Ranadheera et al. 2010). Retaining viability and sensory characteristics are the major criteria for the success of these products in the market (Rouhi et al. 2013). Technological conditions while producing the probiotic foods can significantly reduce the viability of probiotic cells due to heat, mechanical damage or due to cell injury caused by osmotic stress (Fu and Chen 2011; Bustos and Bórquez 2013).
For centuries, the preservation and storage of fermented foods involving cereals, soya, meat, etc., are being practiced. However, fermentation process involves mixed cultures such as yeasts, lactic acid bacteria (LAB) and fungi (Blandino et al. 2003) and traditional fermented foods are the potential source of microorganisms and show probiotic characteristics. The information available on these matrices as raw material for probiotic microorganisms is still significantly less when compared to their dairy counterparts. With respect to non-dairy food matrices, the information regarding the survival of microorganisms against the challenges, the criteria for fermentation, their use as starters and their relationship with other microorganisms is minimal (Schrezenmeir and de Vrese 2001). The type of food matrix, rate of moisture content and cell condition play a major role in the survivability of probiotics during long term storage and processing (Endo et al. 2014). Moisture and cell conditions have a great impact on survival of probiotics under severe heat stress while processing and during long-term storage.
Health risks associated with fermented dairy foods
Some health risks are associated with milk based probiotic foods. They mainly include lactose intolerance, allergy to milk proteins, high fat and high cholesterol content. These risks are elaborated below.
Lactose intolerance (LI)
It also known as lactose malabsorption, is the most common type of carbohydrate malabsorption. It is associated with the inability to digest lactose into its constituents, glucose and galactose, due to low levels of lactase enzyme (Hauck et al. 2011). At birth, lactase activity is at the highest and it declines after weaning. The unabsorbed lactose is metabolized by colonic bacteria to produce gases such as hydrogen (H2) and methane (CH4) and short chain fatty acids (Lee 2015). Symptoms related to LI appear 30 min to 2 h after consumption of food products containing lactose. Related symptoms include bloating, cramping, flatulence and loose stool and some reports also suggest that they lead to irritability bowel syndrome (Joachim 1999; Vesa et al. 2000). The rate of LI varies in the population and the rate of incidence of LI in different ethnic races worldwide is provided in Table 1 (Scrimshaw and Murray 1988; de Vrese et al. 2001). As seen from the table, highest rates of LI are found in the Asian populations, Native Americans and African Americans (60–100 %), while lowest rates are found in people of northern European origin (including North Americans). Effect of cholesterol in milk as a consummate effect in addition to allergy to milk proteins and lactose intolerance, high cholesterol content in dairy foods and high amounts of saturated fatty acids add to health concerns of probiotic dairy based foods.
Table 1.
The rate of incidence of lactose intolerance (LI) in different ethnic races
| Ethnicity/ Geographic region | % population with LI |
|---|---|
| East Asian | 90–100 |
| Indigenous (North America) | 80–100 |
| Central Asian | 80 |
| African American (North America) | 75 |
| African (Africa) | 70–90 |
| Indian (Southern India) | 70 |
| French (Southern France) | 65 |
| Ashkenazi Jew (North America) | 60–80 |
| Balkans Region | 55 |
| Latino/Hispanic (North America) | 51 |
| Indian (Northern India) | 30 |
| Anglo (North America) | 21 |
| Italian (Italy) | 20–70 |
| French (Northern France) | 17 |
| Finnish (Finland) | 17 |
| Austrian (Austria) | 15–20 |
| German (Germany) | 15 |
| British (U.K.) | 5–15 |
The consolidated above table is freely adopted from the link as follows http://milk.procon.org/view.resource.php?resourceID=000661. Data compiled from using the references: National Institute of Child Health and Human Development (2006); de Vrese (2001); Scrimshaw and Murray (1988)
Allergy to milk proteins
Atopic dermatitis (AD) is one disease frequently associated with food allergy in children (Ricci et al. 2006; Johnke et al. 2007) and the rate of occurrence of AD during the first year of life is between 2 and 3 % (Host 2002). Some studies have demonstrated that the usage of probiotics reduces the occurrence of AD (Reid and Kirjaivanen 2005). However, not all the preparations of probiotics can be used in children who are sensitive to cow’s milk. With selective strains of probiotics, some studies have reported a reduction in the severity of signs and symptoms in these patients (Isolauri 2001; Kalliomaki et al. 2001, 2003; Sistek et al. 2006; Moro et al. 2006). However, these studies did attract criticisms regarding their favored design towards desired outcome and interpretation of the data (Matricardi 2002). In addition to this, some studies demonstrated that the probiotic supplementation has no significant impact on the symptoms associated with infantile AD (Brouwer et al. 2006) and hence, increased the risk of allergen sensitization in children with a high-risk of atopic diseases (Taylor et al. 2007). It could be potentially unsafe in people sensitive to cow’s milk allergy (Moneret-Vautrin et al. 2006; Lee et al. 2007).
High fat and cholesterol
Milk contains fat and its amount depends on the source of milk. Cow milk has 4–5 % fat, whereas, it’s content in buffalo milk is up to 7–8 %. It has a polyunsaturated to saturated fatty acid ratio of 0.05. Consuming large volumes of milk would increase the total cholesterol and LDL-cholesterol contents in the blood (Levy and Feinleib 1980) and the dietary saturated fat is responsible for the increase of plasma cholesterol levels, which is a major risk factor for coronary heart disease. This risk can be reduced by lowering of low-density lipoproteins (LDL) cholesterol by reducing the saturated fats in the diet (Hill et al. 2009). However, very recently Ebel et al. (2014) have reviewed the impact of probiotics on the risk factors of cardiovascular diseases including its impact on hypercholesterolemia. A significant reduction of 2.63, 4.1 and 4.68 mg/100 ml of serum cholesterol level at the end of 30 days in rats fed with yoghurt, probiotic dahi and probiotic yoghurt, respectively, containing Lb. acidophilus and Bifidobacterium bifidum indicating the hypocholesterolaemic effect of the probiotic cultures was reported recently (Vijayendra and Gupta 2012).
Non-dairy probiotic products
To alleviate the disadvantageous of diary based fermented foods several non-traditional non-dairy based fermented foods have been developed (Table 2). Rivera-Espinoza and Gallardo-Navarro (2010) have reviewed various non-dairy probiotic foods developed worldwide. Among the non-dairy based fermented foods, fruit and vegetable based, cereals based and soy based foods are gaining importance (Prado et al. 2008; Gupta and Abu-Ghannam 2012; Gawkowski and Chikindas 2013; Martins et al. 2013). The major differences between dairy and non-dairy based fermented foods are summarized in Table 3. The following sections highlight some of the recent developments in the area of non-dairy based probiotic fermented foods.
Table 2.
List of some non-dairy probiotic products developed recently
| Category | Product | Reference |
|---|---|---|
| Fruit and vegetable based | Vegetable-based drinks | Lambo et al. (2005) |
| Fermented banana pulp | Tsen et al. (2004) | |
| Fermented banana | Tsen et al. (2009) | |
| Beets-based drink | Yoon et al. (2005) | |
| Tomato-based drink | Yoon et al. (2004) | |
| Many dried fruits | Betoret et al. (2003) | |
| Green coconut water | Prado et al. (2008a) | |
| Peanut milk | Mustafa et al. (2009) | |
| Cranberry, pineapple, and orange juices | Sheehan et al. (2007) | |
| Ginger juice | Chen et al. (2008) | |
| Grape and passion fruit juices | Saarela et al. (2006) | |
| Cabbage juice | Yoon et al. (2006) | |
| Carrot juice | Nazzaro et al. (2008) | |
| Noni juice | Wang et al. (2009b) | |
| Onion | Roberts and Kidd (2005) | |
| Probiotic banana puree | Tsen et al. (2009) | |
| Non fermented fruit juice beverages | Renuka et al. (2009) | |
| Blackcurrant juice | Luckow and Delahunty (2004) | |
| Plum juice | Sheela and Suganya (2012) | |
| Cashew apple juice | Pereira et al. (2011) | |
| Table olives | De Bellis et al. (2010) | |
| Fruit juices (mango, sapota, grape) | Vijaya Kumar et al. (2013) | |
| Soy based | Non fermented soy-based frozen desserts | Heenan et al. (2004) |
| Fermented soymilk drink | Donkor et al. (2007) | |
| Soy-based stirred yogurt-like drinks | Saris et al. (2003) | |
| Soy based products | Bedani et al. (2013) | |
| Soyghurt | Bedani et al. (2014) | |
| Soy curd | Roopashri and Varadaraj (2014) | |
| Soy product fermented with Kefir | Baú et al. (2014) | |
| Cereal based | Cereal-based puddings | Helland et al. (2005) |
| Rice-based yogurt | Boonyaratanakornkit and Wongkhalaung (2000) | |
| Oat-based drink | Angelov et al. (2006) | |
| Oat-based products | Martensson et al. (2002) | |
| Oat milk | Bernat et al. (2014) | |
| Oat, barley, and malt based | Salmerón et al. (2014) | |
| Yosa (oat-bran pudding) | Blandino et al. (2003) | |
| Mahewu (fermented maize beverage) Maize-based beverage | McMaste et al. (2005) | |
| Wacher et al. (2000) | ||
| Wheat, rye, millet, maize, and other cereals fermented probiotic beverages | Blandino et al. (2003) | |
| Malt-based drink | Kedia et al. (2007) | |
| Boza (fermented cereals) | Moncheva et al. (2003) | |
| Maize, sorghum, and millet malt fermented probiotic beverages | Blandino et al. (2003) | |
| Millet or sorghum flour fermented probiotic beverage | Muyanja et al. (2003) | |
| Mixed cereal beverage | Rathore et al. (2012) | |
| Bread and baked products | Côté et al. (2013) | |
| Sorghum based ‘Sorghurt’ | Sanni et al. (2013) | |
| Pseudo cereals (amaranth, buckwheat) | Monika et al. (2013) | |
| As an edible film on pan bread | Soukoulis et al. (2014) | |
| Other non-dairy foods | Starch-saccharified probiotic drink | Oi and KIitabatake (2003) |
| Probiotic cassava-flour product | Molin (2001) | |
| Dosa (rice and Bengal gram) | Soni et al. (1986) | |
| Meat products | Krockel (2006) | |
| Meat based products | Amor and Mayo (2007) | |
| Dry-fermented sausages | Sidira et al. (2014) |
Table 3.
Comparative account of dairy and non-dairy probiotic foods
| Parameter | Dairy probiotic foods | Non-dairy probiotic foods |
|---|---|---|
| Lactose intolerance | Negative effect | No issue |
| Calcium availability | Positive effect | No issue |
| High fat | Negative effect | No issue |
| Cholesterol content | Negative effect | No Issue |
| Dietary fiber | No issue | Positive effect |
| Digestibility | Not easy | Easy to digest |
| Survival rate of probiotics | High | Low |
| Flavour (diacetyl/acetaldehyde) | Positive effect | No issue |
| Phyto-chemicals | No issue | Negative effect |
| Isoflavons | No issue | Positive effect |
Fruit and vegetable based products
Research is being continued in developing alternate solutions to dairy based probiotic products and preference for non-dairy based probiotic products especially using fruit and/or vegetable juice as a major ingredient is a choice. Fruit juices offer several advantages: they are a rich source of nutrients and unlike in dairy products, it obviates the necessity of using starter cultures and hence no competition for nutrients with probiotic cultures. Non-dairy sources are fortified with acidulants which could increase the shelf-life by creating an anaerobic environment that is more optimal for probiotic cultures, which is attained by scavenging the oxygen available. Fruit juices also contain sugars to support the growth of probiotics (Ding and Shah 2008). Besides the advantages said above, they also have a good refreshing taste profile and a choice for people of all age groups. One more advantage is that these juices stay very less time in the stomach and thus the probiotic species spend very less time to the harsh acidic environment of the stomach. Several fruits and vegetables such as apples, oranges, blackcurrant, banana, blueberry, pineapple, cashew apple (Anacardium occidentale L.), cantaloupe melon, raspberry, pomegranate juice, carrot, beetroot, etc. (Savard et al. 2003; Yoon et al. 2005; Pereira et al. 2011; Nualkaekul et al. 2012; Fonteles et al. 2013; Anekella and Orsat 2013) and mixed vegetable juice (Nosrati et al. 2014) are being exploited for this purpose. The viable cell counts of Lb. casei in cashew apple juice even after storage for 6 weeks were found to be more than 8.00 log cfu/mL and, hence, proved to be as efficient as dairy products for its growth (Pereira et al. 2011) and similar trends are seen in pineapple juice (Sheehan et al. 2007) and melon juice (Fonteles et al. 2013). Also, stability and sensory acceptance are considered while developing a probiotic fermented fruit juices (Granato et al. 2010). Novel probiotic Lb. plantarum 299v culture based blackcurrant juices were found to have good aroma and flavour in comparison to non- probiotic blackcurrant juices (Luckow and Delahunty 2004). The encapsulation of probiotic cells is one more advancement to protect them from the acidic environment of the juices by encapsulating with readily available and non-toxic alginates, which further extend the cell viability during shelving (Ding and Shah 2008; Anekella and Orsat 2013; Kailasapathy 2014). These alginate beads are also coated by chitosans to offer extended protection to probiotic cells (Nualkaekul et al. 2012). Microencapsulation of probiotics and their effect on usage in food applications has been reviewed by Heidebach et al. (2012). The cell viability of the probiotic cultures Lb. acidophilus and Lb. casei increased with the incorporation of encapsulating matrices with galacto-oligosaccharides (Krasaekoopt and Watcharapoka 2014). Recently, fermented fruits and vegetables of Asian region have been indicated as a potential source of probiotic cultures (Swain et al. 2014) and its health benefits have been reviewed (Vijayendra and Halami 2015).
As mentioned earlier in this review, the lactose intolerance and cholesterol content of dairy based probiotics is a set-back for its commercialization (Heenan et al. 2004). For our benefit, technological advances have helped in changing the matrix components of the foods in a controlled way to improve the cell viability and cell functionality (Betoret et al. 2003). The modification of the matrix makes them ideal substrates for the culture of probiotics as they readily have beneficial nutrients such as minerals, vitamins, dietary fibers, and antioxidants (Yoon et al. 2004). They lack the dairy allergens, which are avoided by certain segments of the population (Luckow and Delahunty 2004). The appealing tastes and the refreshing profiles offered by the fruit juices have instilled in us a genuine interest for the development of probiotic based fruit juices (Tuorila and Cardello 2002; Yoon et al. 2004; Sheehan et al. 2007). However, unfavorable aromas (perfumery, dairy) and flavors (sour, savory) were observed with the addition of Lb. plantarum to fruit juices (Luckow and Delahunty 2004). The sensory impact study by Luckow and Delahunty (2004) have shown that consumers are interested in conventional orange juices over the probiotic-based ones; however, the awareness of health benefits due to probiotic-based juices might alter their interests. Luckow et al. (2006) proposed that the perceptible off-flavors in juices resulting with the addition of probiotics that contribute to consumer dissatisfaction could be overcome by adding 10 % (v/v) of tropical fruit juices. LAB are the organisms that require essential amino acids and vitamins for growth (Salminen and Von Wrigh 1993). However, some probiotic strains were found to have the capability to grow in fruit matrices. It has been proposed that cell viability is a factor which dependents on the substrate, the oxygen content and the final acidity of the matrix used (Shah 2001). Sheehan et al. (2007) reported extensive differences with respect to the acid resistance property of Lactobacillus and Bifidobacterium in orange, pineapple and cranberry juices. They have observed longer and higher survival rates in orange and pineapple juices which are in contrast to that of cranberry. Lb. casei, Lb. rhamnosus, Lb. paracasei strains have shown higher resistance, surviving over 7.0 log cfu/ml in orange juice and above 6.0 log cfu/ml in pineapple juice for at least 12 weeks. Very recently, a probiotic beverage using coconut water was developed by fermenting it with Lb. plantarum (Prado et al. 2015).
Other non-dairy based products
Cereals have complex nutrient composition and are being consumed on a daily basis all over the world as one of the staple foods. Cereals are considered as healthy non-diary carriers to prepare probiotic foods since they can overcome the disadvantages of fermented dairy products (Prado et al. 2008). Another benefit of consuming fermented cereal based foods is the availability of dietary fiber and presence of non-digestible carbohydrates like oligosaccharides can act as a probiotic which can stimulate the growth of probiotic LAB (Charalampopoulos et al. 2002). Fermentation of cereals by LAB cultures is one of the oldest processing methods in practice, in Asia and African countries for the production of beverages, gruels and porridge. Cereal grains like maize, sorghum, millet, oats, barley, wheat and rye are being used for this purpose. In addition, whole grain consumption reduces type 2 diabetes, cardiovascular disease, obesity and certain type of cancers (Clemens and Pressman 2006). Cereal grains are gaining importance in western countries and have a huge potential for use in the preparation of functional foods (Jideani and Jideani 2011).
Fermentation of cereals increase the bioavailability of minerals such as phosphorous, iron and zinc (Sankara and Deosthale 1983), due to the action of microbial enzymes such as phytases, or due to the organic acids produced during fermentation of cereals (Teucher et al. 2004; Hotz and Gibson 2007). Application of cereals and cereal components in functional foods development was reviewed (Charalampopoulos et al. 2002; Blandino et al. 2003). The health benefits of using cereals (whole grains) and cereal components (brans) in the preparation of probiotic foods have been reviewed (Lamsal and Faubion 2009). Kalui et al. (2010) recently reviewed the probiotic potential of spontaneously fermented cereal based foods. As an alternate to dairy based probiotic foods, single and mixed cereals (barley and malt) based probiotic beverages containing Lb. plantarum and Lb. acidophilus in the range of 7.9 and 8.5 log cfu/mL have been developed (Rathore et al. 2012). Sorghum flour based yoghurt like product ‘sorghurt’ was prepared recently and it had a viable count of >8 log10 cfu/mL of the product, which is higher than the minimum desirable count of 106 cfu/g, with acceptable sensory scores (Sanni et al. 2013). Waters et al. (2015) consolidated the findings of various research workers in determining the role of LAB in the fermentation of cereals used for the preparation of beverages.
Very recently pan bread slices were coated with sodium alginate film impregnated with probiotic cells of Lb. rhamnosus GG and the viability was found to be 6.55–6.91 log cfu/30–40 g portion of bread after in vitro digestion under simulated gastro-intestinal conditions and no impact of the bread crust matrix on cell inactivation was noticed (Soukoulis et al. 2014). Meat mainly in the form of sausages is also being used as an alternate to dairy based probiotic foods. Use of alginate microencapsulated Lb. reuteri and B. longum in the preparation of meat based sausages was reported (Muthukumarasamy and Holley 2006). Rivera-Espinoza and Gallardo-Navarro (2010) have reviewed the cultures used in meat based probiotic products.
Phenotypic and genotypic similarities between dairy and non-dairy probiotics
Both the dairy and non-dairy species show more similarities than discrepancies in their phenotypic and genotypic natures. The Lactococcus lactis is one of the major probiotics used in fermentation of dairy products. However, Lc. lactis is not limited to dairy foods; they are also found on plant surfaces and in other sources (Salama et al. 1995; Ulrich and Müller 1999). Green plant material is a natural source for Lc. lactis subsp. lactis and Lc. lactis subsp. lactis biovar diacetylactis (Salama et al. 1995). Lc. lactis has also been isolated from different sources like soil (Klijn et al. 1995) and termite hindguts (Bauer et al. 2000). Based on the 16 s RNA analyses of nearly 106 isolates of LAB, phenotypic and genotypic characteristics of both dairy isolates and non-dairy isolates were analyzed in order to compare one another in their efficiency of fermenting food sources (Nomura et al. 2006). These isolates were investigated by cluster analysis based on randomly amplified polymorphic DNA profiles. There were no significant differences between isolates from milk and those from plant. The reports were very satisfactory and plant-derived strains showed tolerance for high salt concentration and high pH value, and more kinds of carbohydrates were fermented by non-dairy strains than the milk-derived strains. Reports also suggested no notable differences in the profiles of enzymes, such as lipases, peptidases and phosphatases. One more reliable observation made was that the fermented milks manufactured using the plant-derived strains had the same flavour as that produced by milk-derived strains (Nomura et al. 2006).
Matrix dependence of cell viability and functionality
In the dairy based probiotic foods, the physico-chemical composition of milk, which is rich in protein and lipids (fats), acts as a protective matrix for the probiotics and these factors help the survival of probiotics from adverse conditions of the stomach and small intestine (Saxelin et al. 2003). In addition, milk proteins, as a carrier matrix, can act effectively in protecting the probiotic cells till they reach the site of action in small intestines (Ritter et al. 2009). However, non-dairy food matrices are very different from dairy based; they are more versatile and less understood. Fermented dairy products, such as fermented milks and fresh cheeses, have been the food vehicles with the biggest technological and commercial success for the incorporation of probiotic bacteria (Saxelin 2008; Figueroa-González et al. 2011). Despite the commercial success of dairy probiotics, consumers have a genuine interest in fruit juice based functional beverages prepared with probiotics because they offer varied taste profiles that are appealing to all age groups and also they are perceived as healthy and refreshing in contrast to dairy foods (Rivera-Espinoza and Gallardo-Navarro 2010; do Espirito Santo et al. 2011). However, cell viability is an important attribute to cell functionality (Ouwehand and Salminen 1998) and cell functionality is mainly influenced by the food matrix components (Ranadheera et al. 2010). To assess the viability of Lb. casei cells in commercially available non-dairy drinks, the growth inhibitory capacity of the drinks was studied using well-diffusion agar assay. Out of 13 non-dairy drinks, only citric orange juice affected the growth of both strains with an inhibition zone of 6 to 7 mm for both strains, measured from the edge of the well (Céspedes et al. 2013).
Food matrices and cell conditions influence survival of Lb. rhamnosus GG under heat stresses and during storage (Endo et al. 2014). They have noticed 7.0 to 7.7 log cfu/ml of freeze-dried cells Lb. rhamnosus GG after 20 min heating in oils at 80 °C, whereas, the viable count of the cells suspended in phosphate buffer saline dropped to below one log by 10 min itself indicating the importance of food matrix in the survival of the probiotic bacterial cells. Processed fruits and vegetables have good matrices and are considered as ideal substrates for probiotics due to the presence of minerals, vitamins, antioxidants and fibers (Soccol et al. 2010). It has been observed that the cell wall of the autochthonous LAB is more resistant and thus allows the bacterial adaptation to the environmental conditions like low moisture and antimicrobial compounds, etc., surrounding to the food matrices, like olives (Masuda et al. 2010). Fruits, such as apple, guava, banana and melon, have been found to be potential carriers of probiotic bacteria and strong adherence of these bacteria on fruit tissue was found (Martins et al. 2013).
The efficacy of the bacteria employed in non-dairy drinks or even dairy drinks for that matter must first be tested for their resistance to gastric digestion in stimulatory in vitro studies. But reports suggest that when studying the gastric resistance of probiotic strains, incongruent results were obtained like strains with a well-documented ability to perform beneficially in the human gut didn’t look so much positive in in vitro assays of gastric acid resistance. Hence, these results suggest the necessity of much more refined tests to estimate in vitro and the in vivo resistance to gastric digestion (Morelli 2007). Nonetheless, in vitro tests cannot be ruled out to study the impact of some inherent factors, such as effect of storage or food matrix, on the gastric resistance of probiotic bacteria, as previous reports suggest (Vinderola et al. 2011, 2012).
Cell viability and cell functionality has been found to work according to the phenomena called “cross adaptation”, which states that pre-exposure to sublethal levels of the stress factor will allow cells to adapt to subsequent exposure to higher levels of the same stress factor or to different stresses (Bunning et al. 1990; O’Driscoll et al. 1996). Such results were obtained in a study, where lactobacilli gained higher resistance to simulated gastric digestion over storage that could be due to the exposure to the acidic conditions of juices during refrigeration (Céspedes et al. 2013). Several other reports are also found standing in this line of phenomena, where enhanced resistance to bile salts by nonintestinal lactobacilli due to the prior exposure to gradually increased levels of bile (Burns et al. 2008) and an enhanced resistance to simulated gastric digestion in probiotics in commercial fermented milks during storage (Vinderola et al. 2011) are reported. Similar observation was noticed in bifidobacteria that are grown at low pH values (Vinderola et al. 2012) and in spray-dried lactobacilli due to preliminary heat-treatment and spray-drying (Páez et al. 2012). However, few reports suggested contrasting evidence considering the food matrix, where reduction of gastric resistance in lactobacilli along storage was observed for Lb. casei (Wang et al. 2009a). In another study, bifidobacteria maintained at 4 to 20 °C for 6 weeks has shown reduced resistance to gastric digestion (Saarela et al. 2006). Lactobacillus and Bifidobacterium have shown extensive differences in their resistance to the acid in the orange, pineapple and cranberry juice (Sheehan et al. 2007). Fruit juice being a more heterogeneous food source with different physicochemical properties compared to fermented milks, such erroneous results sometimes are expected and the cell viability and cell functionality are variable and product-dependent.
Future perspectives
The necessity of non-dairy probiotic drinks, the feasibility and development of adaptable technologies for their production are not going together compared to dairy probiotics at least for now. The current research is occurring at a brisk pace and scope looks pretty healthy but not satisfactorily enough when compared to their dairy counterparts. The heterogeneous food matrices of non-dairy food carriers are the major constraints, while the probiotic strains from non-dairy sources are satisfactory. Development of novel, economical and technological matrices is a dire necessity to bring the non-dairy probiotic foods on par with the demand they have to their nature of healthy alternatives to dairy probiotic foods. Although there is a great potential for the use of fruit juices as probiotic products, very few reports on their preparation and production are available. Hence, there is a scope for further research in this area. While developing, functional properties, stability, sensory acceptance, especially related to taste, appeal and price are to be kept in mind, as these factors play a major role in their successful commercialization. Care should be taken while selecting the probiotics to avoid removal of micronutrients from the product or to produce biogenic amines. As all cultures or strains may not have probiotic properties, selection of strain(s) with potential probiotic properties plays a major role in the success of the non-milk probiotic products. Technological issues that can affect the survival of probiotic cultures throughout the production process and during storage should also be addressed while formulating new probiotic products. Functional properties are extremely important to get a competitive advantage in the world market. Hence, care should be taken while confirming the functional attributes of starters before incorporating in the product.
In conclusion, research non-dairy probiotic products can be widened to better understanding and exploiting the benefits of non dairy probiotic products for the mankind. Use of prebiotics in combination with non-dairy probiotic products can also be attempted to produce synbiotic products.
Acknowledgments
We are thankful to the Director, CFTRI, Mysore, for permitting one of the authors to contribute for this review article.
References
- Alakomi HL, Skyttä E, Saarela M, Mattila-Sandholm T, Latva-Kala K, Helander IM. Lactic acid permeabilizes gram-negative bacteria by disrupting the outer membrane. Appl Environ Microbiol. 2000;66:2001–2005. doi: 10.1128/AEM.66.5.2001-2005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiri ZR, Khandelwal P, Aruna BR. Development of acidophilus milk via selected probiotics and prebiotics using artificial neural network. Adv Biosci Biotechnol. 2010;1:224–231. doi: 10.4236/abb.2010.13031. [DOI] [Google Scholar]
- Amor MS, Mayo B. Selection criteria for lactic acid bacteria to be used as functional starter cultures in dry sausage production: an update. Meat Sci. 2007;76:138–146. doi: 10.1016/j.meatsci.2006.10.022. [DOI] [PubMed] [Google Scholar]
- Anekella K, Orsat V. Optimization of microencapsulation of probiotics in raspberry juice by spray drying. LWT Food Sci Technol. 2013;50:17–24. doi: 10.1016/j.lwt.2012.08.003. [DOI] [Google Scholar]
- Angelov A, Gotcheva V, Kuncheva R, Hrstozova T. Development of a new oat-based probiotic drink. Int J Food Microbiol. 2006;112:75–80. doi: 10.1016/j.ijfoodmicro.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Antoine JM. Probiotics: beneficial factors of the defence system. Proc Nutr Soc. 2010;69:429–433. doi: 10.1017/S0029665110001692. [DOI] [PubMed] [Google Scholar]
- Baú TR, Garcia S, Ida EI. Evaluation of a functional soy product with addition of soy fiber and fermented with probiotic kefir culture. Braz Arch Biol Technol. 2014;57:402–409. doi: 10.1590/S1516-89132014005000005. [DOI] [Google Scholar]
- Bauer S, Tholen A, Overmann J, Brune A. Characterization of abundance and diversity of lactic acid bacteria in the hindgut of wood and soil-feeding termite by molecular and culture-dependent techniques. Arch Microbiol. 2000;173:126–137. doi: 10.1007/s002039900120. [DOI] [PubMed] [Google Scholar]
- Bedani R, Rossi EA, Saad SMI. Impact of inulin and okara on Lactobacillus acidophilus La-5 and Bifidobacterium animalis Bb-12 viability in a fermented soy product and probiotic survival under in vitro simulated gastrointestinal conditions. Food Microbiol. 2013;34:382–389. doi: 10.1016/j.fm.2013.01.012. [DOI] [PubMed] [Google Scholar]
- Bedani R, Vieira ADS, Rossi EA, Saad SMI. Tropical fruit pulps decreased probiotic survival to in vitro gastrointestinal stress in synbiotic soy yoghurt with okara during storage. LWT-Food Sci Technol. 2014;55:436–443. doi: 10.1016/j.lwt.2013.10.015. [DOI] [Google Scholar]
- Bernat N, Cháfer M, González-Martínez C, Rodríguez-García J, Chiralt A. Optimisation of oat milk formulation to obtain fermented derivatives by using probiotic Lactobacillus reuteri microorganisms. Food Sci Technol Int. 2014 doi: 10.1177/1082013213518936. [DOI] [PubMed] [Google Scholar]
- Betoret N, Puente L, Diaz MJ, Pagan MJ, Garcia MJ, Gras ML. Development of probiotic enriched dried fruits by vacuum impregnation. J Food Eng. 2003;56:273–277. doi: 10.1016/S0260-8774(02)00268-6. [DOI] [Google Scholar]
- Blandino A, Al-Aseeri ME, Pandiella SS, Cantero D, Webb C. Cereal-based fermented foods and beverages. Food Res Int. 2003;36:527–543. doi: 10.1016/S0963-9969(03)00009-7. [DOI] [Google Scholar]
- Boonyaratanakornkit M, Wongkhalaung C. Development of a yoghurt-type product from saccharified rice. Kasetsart J. 2000;34:107–116. [Google Scholar]
- Brouwer ML, Wolt-Plompen SA, Dubois AE, van der Heide S, Jansen DF, Hoijer MA, Kauffman HA, Duiverman EJ. No effect of probiotics on atopic dermatitis in infancy: a randomized placebo-controlled trial. Clin Exp Allergy. 2006;37:899–906. doi: 10.1111/j.1365-2222.2006.02513.x. [DOI] [PubMed] [Google Scholar]
- Bunning VK, Crawford RG, Tierney JT, Peeler JT. Thermotolerance of Listeria monocytogenes and Salmonella typhimurium after sublethal heat shock. Appl Environ Microbiol. 1990;56:3216–3219. doi: 10.1128/aem.56.10.3216-3219.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns P, Vinderola G, Binetti A, Quiberoni A, de los Reyes-Gavil CG, Reinheimer J. Bile-resistant derivatives obtained from non-intestinal dairy lactobacilli. Int Dairy J. 2008;18:377–385. doi: 10.1016/j.idairyj.2007.10.012. [DOI] [Google Scholar]
- Bustos P, Bórquez R. Influence of osmotic stress and encapsulating materials on the stability of autochthonous Lactobacillus plantarum after spray drying. Dry Technol. 2013;31:57–66. doi: 10.1080/07373937.2012.717325. [DOI] [Google Scholar]
- Caballero-Franco C, Keller K, De Simone C, Chadee K. The VSL#3 probiotic formula induces mucin gene expression and secretion in colonic epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G315–G322. doi: 10.1152/ajpgi.00265.2006. [DOI] [PubMed] [Google Scholar]
- Céspedes M, Cárdenas P, Staffolani M, Ciappini MC, Vinderola G. Performance in nondairy drinks of probiotic Lactobacillus casei strains usually employed in dairy products. J Food Sci. 2013;78:M756–M762. doi: 10.1111/1750-3841.12092. [DOI] [PubMed] [Google Scholar]
- Charalampopoulos D, Wang R, Pandiella SS, Webb C. Application of cereals and cereal components in functional foods: a review. Int J Food Microbiol. 2002;79:131–141. doi: 10.1016/S0168-1605(02)00187-3. [DOI] [PubMed] [Google Scholar]
- Chen INCC, Wang CY, Chang TL. Lactic fermentation and antioxidant activity of Zingiberaceae plants in Taiwan. Int J Food Sci Nutr. 2008;22:1–10. doi: 10.1080/09637480802375531. [DOI] [PubMed] [Google Scholar]
- Clemens R, Pressman P. Heyday in grain land. Food Technol. 2006;60(11):18. [Google Scholar]
- Côté J, Dion J, Burguière P, Casavant L, Eijk VJ. Probiotics in bread and baked products: a new product category. Cereal Foods World. 2013;58:293–296. doi: 10.1094/CFW-58-6-0293. [DOI] [Google Scholar]
- De Bellis P, Valerio F, Sisto A, Lonigro SL, Lavermicocca P. Probiotic table olives: microbial populations adhering on olive surface in fermentation sets inoculated with the probiotic strain Lactobacillus paracasei IMPC2.1 in an industrial plant. Int J Food Microbiol. 2010;140:6–13. doi: 10.1016/j.ijfoodmicro.2010.02.024. [DOI] [PubMed] [Google Scholar]
- de Vrese M, Schrezenmeir J. Probiotics, prebiotics, and synbiotics. Adv Biochem Eng Biotechnol. 2008;111:1–66. doi: 10.1007/10_2008_097. [DOI] [PubMed] [Google Scholar]
- de Vrese M, Stegelmann A, Richter B, Fenselau S, Laue C, Schrezenmeir J. Probiotics-compensation for lactase insufficiency. Am J Clin Nutr. 2001;73(2):421S–429S. doi: 10.1093/ajcn/73.2.421s. [DOI] [PubMed] [Google Scholar]
- Ding WK, Shah NP. Survival of free and microencapsulated probiotic bacteria in orange and apple juices. Int Food Res J. 2008;15:219–232. [Google Scholar]
- do Espirito Santo AP, Perego P, Converti A, Oliveira MN. Influence of food matrices on probiotic viability – a review focusing on the fruity bases. Trends Food Sci Technol. 2011;22:377–385. doi: 10.1016/j.tifs.2011.04.008. [DOI] [Google Scholar]
- Donkor ON, Henriksson A, Vasiljevic T, Shah NP. α-Galactosidase and proteolytic activities of selected probiotic and dairy cultures in fermented soymilk. Food Chem. 2007;104:10–20. doi: 10.1016/j.foodchem.2006.10.065. [DOI] [Google Scholar]
- Duquesne S, Petit V, Peduzzi J, Rebuffat S. Structural and functional diversity of microcins, gene-encoded antibacterial peptides from enterobacteria. J Mol Microbiol Biotechnol. 2007;13:200–209. doi: 10.1159/000104748. [DOI] [PubMed] [Google Scholar]
- Ebel B, Lemetais G, Beney L, Cachon R, Sokol H, Langella P, Gervais P. Impact of probiotics on risk factors for cardiovascular diseases. A review. Crit Rev Food Sci Nutr. 2014;54:175–189. doi: 10.1080/10408398.2011.579361. [DOI] [PubMed] [Google Scholar]
- Endo A, Terasjarvi J, Salminen S. Food matrices and cell conditions influence survival of Lactobacillus rhamnosus GG under heat stresses and during storage. Int J Food Microbiol. 2014;174:110–112. doi: 10.1016/j.ijfoodmicro.2014.01.006. [DOI] [PubMed] [Google Scholar]
- FAO/WHO (2001) Report of a Joint FAO/WHO Expert Consultation on evaluation of health and nutritional properties of probiotics in food including powder milk with live LAB”. Food and Agriculture Organization of the United Nations World Health Organization
- Figueroa-González I, Quijano G, Ramírez G, Cruz-Guerrero A. Probiotics and prebiotics. Perspectives and challenges. J Sci Food Agric. 2011;91:1341–1348. doi: 10.1002/jsfa.4367. [DOI] [PubMed] [Google Scholar]
- Fonteles TV, Costa MGM, de Jesus ALT, Fontes CPML, Fernandes FAN, Rodrigues S. Stability and quality parameters of probiotic cantaloupe melon juice produced with sonicated juice. Food Bioprocess Technol. 2013;6:2860–2869. doi: 10.1007/s11947-012-0962-y. [DOI] [Google Scholar]
- Fu N, Chen XD. Towards a maximal cell survival in convective thermal drying processes. Food Res Int. 2011;44:1127–1149. doi: 10.1016/j.foodres.2011.03.053. [DOI] [Google Scholar]
- Fuller R. Probiotics in human medicine. Gut. 1991;32:439–442. doi: 10.1136/gut.32.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudier E, Michel C, Segain JP, Cherbut C, Hoebler C. The VSL# 3 probiotic mixture modifies microflora but does not heal chronic dextran-sodium sulfate-induced colitis or reinforce the mucus barrier in mice. J Nutr. 2005;135:2753–2761. doi: 10.1093/jn/135.12.2753. [DOI] [PubMed] [Google Scholar]
- Gawkowski D, Chikindas ML. Non-dairy probiotic beverages: the next step into human health. Benefic Microbes. 2013;4:127–142. doi: 10.3920/BM2012.0030. [DOI] [PubMed] [Google Scholar]
- Gogineni VK, Morrow LE, Malesker MA. Probiotics: mechanisms of action and clinical applications. J Prob Health. 2013;1:101. [Google Scholar]
- Gotcheva V, Hristozova E, Hrostozova T, Guo M, Roshkova Z, Angelov A. Assessment of potential probiotic properties of LAB and yeast strains. Food Biotechnol. 2002;16:211–225. doi: 10.1081/FBT-120016668. [DOI] [Google Scholar]
- Granato D, Branco GF, Nazzaro F, Cruz AG, Faria JAF. Functional food and nondairy probiotic food development: trends, concepts, and products. Comp Rev Food Sci Food Saf. 2010;9:292–302. doi: 10.1111/j.1541-4337.2010.00110.x. [DOI] [PubMed] [Google Scholar]
- Gupta S, Abu-Ghannam N. Probiotic fermentation of plant based products: possibilities and opportunities. Crit Rev Food Sci Nutr. 2012;52:183–199. doi: 10.1080/10408398.2010.499779. [DOI] [PubMed] [Google Scholar]
- Halami PM, Badarinath V, Manjulata Devi S, Vijayendra SVN. Partial characterization of heat stable, antilisterial and cell lytic bacteriocin of Pediococcus pentosaceus CFR SIII isolated from a vegetable source. Ann Microbiol. 2011;61:323–330. doi: 10.1007/s13213-010-0145-x. [DOI] [Google Scholar]
- Hauck FR, Thompson JM, Tanabe KO, Moon RY, Vennemann MM. Breastfeeding and reduced risk of sudden infant death syndrome: a meta-analysis. Pediatrics. 2011;128:103–110. doi: 10.1542/peds.2010-3000. [DOI] [PubMed] [Google Scholar]
- Heenan CN, Adams MC, Hosken RW, Fleet GH. Survival and sensory acceptability of probiotic microorganisms in a nonfermented frozen vegetarian dessert. LWT Food Sci Technol. 2004;37:461–466. doi: 10.1016/j.lwt.2003.11.001. [DOI] [Google Scholar]
- Heidebach T, Först P, Kulozik U. Microencapsulation of probiotic cells for food applications. Crit Rev Food Sci Nutr. 2012;52:291–311. doi: 10.1080/10408398.2010.499801. [DOI] [PubMed] [Google Scholar]
- Helland MH, Wciklund T, Narvhus JA. Growth and metabolism of selected strains of probiotic bacteria in milk and water-based cereal puddings. Int Dairy J. 2005;14:957–965. doi: 10.1016/j.idairyj.2004.03.008. [DOI] [Google Scholar]
- Heller KJ. Probiotic bacteria in fermented foods: product characteristics and starter organisms. Am J Clin Nutr. 2001;73:374–379. doi: 10.1093/ajcn/73.2.374s. [DOI] [PubMed] [Google Scholar]
- Hill AM, Fleming JA, Kris-Etherton PM. The role of diet and nutritional supplements in preventing and treating cardiovascular disease. Curr Opin Cardiol. 2009;24:433–441. doi: 10.1097/HCO.0b013e32832f2fb1. [DOI] [PubMed] [Google Scholar]
- Host A. Frequency of cow’s milk allergy in childhood. Ann Allergy Asthma Immunol. 2002;89:33–37. doi: 10.1016/S1081-1206(10)62120-5. [DOI] [PubMed] [Google Scholar]
- Hotz C, Gibson RS. Traditional food-processing and preparation practices to enhance the bioavailability of micronutrients in plant-based diets. J Nutr. 2007;137:1097–1100. doi: 10.1093/jn/137.4.1097. [DOI] [PubMed] [Google Scholar]
- Imasse K, Tanaka A, Tokunaga K, Sugano H, Ishida H, Takahashi S. Lactobacillus reuteri tablets suppress Helicobacter pylori infection in a double blind randomised placebo-controlled cross-over clinical study Kansenshogaku zasshi. J Jpn Assoc Infect Dis. 2007;81:387–393. doi: 10.11150/kansenshogakuzasshi1970.81.387. [DOI] [PubMed] [Google Scholar]
- Isolauri E. Probiotics in the prevention and treatment of allergic disease. Pediatr Allergy Immunol. 2001;12:56–59. doi: 10.1034/j.1399-3038.2001.121413.x. [DOI] [PubMed] [Google Scholar]
- Jideani IA, Jideani VA. Developments on the cereal grains Digitaria exilis (acha) and Digitaria iburua (iburu) J Food Sci Technol. 2011;48:251–259. doi: 10.1007/s13197-010-0208-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joachim G. The relationship between habits of food consumption and reported reactions to food in people with inflammatory bowel disease–testing the limits. Nutr Health. 1999;13:69–83. doi: 10.1177/026010609901300203. [DOI] [PubMed] [Google Scholar]
- Johnke H, Norberg LA, Vach W, Host A, Andersen KE. Patterns of sensitization in infants and its relation to atopic dermatitis. Pediatr Allergy Immunol. 2007;17:591–600. doi: 10.1111/j.1399-3038.2006.00453.x. [DOI] [PubMed] [Google Scholar]
- Johnson-Henry KC, Hagen KE, Gordonpour M, Tompkins TA, Sherman PM. Surface-layer protein extracts from Lactobacillus helveticus inhibit enterohaemorrhagic Escherichia coli O157:H7 adhesion to epithelial cells. Cell Microbiol. 2007;9:356–367. doi: 10.1111/j.1462-5822.2006.00791.x. [DOI] [PubMed] [Google Scholar]
- Kailasapathy K (2014) Microencapsulation for gastrointestinal delivery of probiotic bacteria. In: Kwak HS (ed) Nano-and microencapsulation for foods. Wiley, Hoboken, New Jersey, pp 167–205
- Kalliomaki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. Probiotics in primary prevention of atopic disease: a randomized placebo-controlled trial. Lancet. 2001;357:1076–1079. doi: 10.1016/S0140-6736(00)04259-8. [DOI] [PubMed] [Google Scholar]
- Kalliomaki M, Salminen S, Poussa T, Arvillomi H, Isolauri E. Probiotics and prevention of atopic disease: 4-year follow-up of a randomized placebo-controlled trial. Lancet. 2003;361:1869–1871. doi: 10.1016/S0140-6736(03)13490-3. [DOI] [PubMed] [Google Scholar]
- Kalui CM, Mathara JM, Kutima PM (2010) Probiotic potential of spontaneously fermented cereal based foods—a review. Afr J Biotechnol 9:2490–2498
- Kedia G, Wang R, Patel H, Pandiella SS. Use of mixed cultures for the fermentation of cereal-based substrates with potential probiotic properties. Process Biochem. 2007;42:65–70. doi: 10.1016/j.procbio.2006.07.011. [DOI] [Google Scholar]
- Kelsall BL. Innate and adaptive mechanisms to control of pathological intestinal inflammation. J Pathol. 2008;214:242–259. doi: 10.1002/path.2286. [DOI] [PubMed] [Google Scholar]
- Klijn N, Weerkamp AH, de Vos WM. Detection and characterization of lactose-utilizing Lactococcus spp. in natural ecosystems. Appl Environ Microbiol. 1995;61:788–792. doi: 10.1128/aem.61.2.788-792.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollath W. Nutrition and the tooth system; general review with special reference to vitamins. Dtsch Zahnärztl Z. 1953;8:7–16. [PubMed] [Google Scholar]
- Krasaekoopt W, Watcharapoka S. Effect of addition of inulin and galacto-oligosaccharide on the survival of microencapsulated probiotics in alginate beads coated with chitosan in simulated digestive system, yogurt and fruit juice. LWT Food Sci Technol. 2014;57:761–766. doi: 10.1016/j.lwt.2014.01.037. [DOI] [Google Scholar]
- Krockel L. Use of probiotic bacteria in meat products. Fleischwirtschaft. 2006;86:109–113. [Google Scholar]
- Lambo AM, Oste R, Nyman MGEL. Dietary fibre in fermented oat and barley β-glucan rich concentrates. Food Chem. 2005;85:283–293. doi: 10.1016/j.foodchem.2004.02.035. [DOI] [Google Scholar]
- Lamsal BP, Faubion JM. The beneficial use of cereal and cereal components in probiotic foods. Food Rev Int. 2009;25:103–114. doi: 10.1080/87559120802682573. [DOI] [Google Scholar]
- Laroia S, Martin JH. Effect of pH on survival of Bifidobacterium bifidum and Lactobacillus acidophilus in frozen fermented dairy desserts. Cult Dairy Prod J. 1991;26:13–21. [Google Scholar]
- Lee BH. Fundamentals of food biotechnology. New York: Wiley; 2015. [Google Scholar]
- Lee TT, Morisset M, Astier C, Moneret-Vautrin DA, Cordebar V, Beaudouin E, Codreanu F, Bihain BE, Kanny G. Contamination of probiotic preparations with milk allergens can cause anaphylaxis in children with cow’s milk allergy. J Allergy Clin Immunol. 2007;119:746–747. doi: 10.1016/j.jaci.2006.11.011. [DOI] [PubMed] [Google Scholar]
- Levy RI, Feinleib M. Risk factors for coronary artery disease and their management. In: Braunwald E, editor. Heart disease. Philadelphia: Saunders WB; 1980. pp. 1246–1278. [Google Scholar]
- Liévin-Le Moal V, Servin AL (2006) The front line of enteric host defense against unwelcome intrusion of harmful microorganisms: mucins, antimicrobial peptides, and microbiota. Clin Microbiol Rev 19:315–337 [DOI] [PMC free article] [PubMed]
- Lilly DM, Stillwell RH. Probiotics: growth-promoting factors produced by microorganisms. Science. 1965;147:747–748. doi: 10.1126/science.147.3659.747. [DOI] [PubMed] [Google Scholar]
- Luckow T, Delahunty C. Which juice is healthier? A consumer study of probiotic non-dairy juice drinks. Food Qual Prefer. 2004;15:751–759. doi: 10.1016/j.foodqual.2003.12.007. [DOI] [Google Scholar]
- Luckow T, Sheehan V, Fitzgerald G, Delahunty C (2006) Exposure, health information and flavor-masking strategies for improving the sensory quality of probiotic juice. Appetite 47:315–323 [DOI] [PubMed]
- Martensson O, Oste R, Holst O. The effect of yoghurt culture on the survival of probiotic bacteria in oat-based, non-dairy products. Food Res Int. 2002;35:775–784. doi: 10.1016/S0963-9969(02)00074-1. [DOI] [Google Scholar]
- Martins EMF, Ramos AM, Vanzela ESLB, Stringheta PC, Pinto CLO, Martins JM. Products of vegetable origin: a new alternative for the consumption of probiotic bacteria. Food Res Int. 2013;51:764–770. doi: 10.1016/j.foodres.2013.01.047. [DOI] [Google Scholar]
- Masuda T, Kimura M, Okada S, Yasui H. Pediococcus pentosaceus Sn26 inhibits IgE production and the occurrence of ovalbumin-induced allergic diarrhea in mice. Biosci Biotechnol Biochem. 2010;74:329–335. doi: 10.1271/bbb.90656. [DOI] [PubMed] [Google Scholar]
- Matricardi PM. Probiotics against allergy: data, doubts, and perspectives. Allergy. 2002;57:185–187. doi: 10.1034/j.1398-9995.2002.1a3299.x. [DOI] [PubMed] [Google Scholar]
- Mattar AF, Teitelbaum DH, Drongowski RA, Yongyi F, Harmon CM, Coran AG. Probiotics up-regulate MUC-2 mucin gene expression in a Caco-2 cell-culture model. Pediatr Surg Int. 2002;18:586–590. doi: 10.1007/s00383-002-0855-7. [DOI] [PubMed] [Google Scholar]
- McMaste LD, Kokott SJ, Abratt VR. Use of traditional African fermented beverages as delivery vehicles for Bifidobacterium lactis DSM 10140. Int J Food Microbiol. 2005;102:231–237. doi: 10.1016/j.ijfoodmicro.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Medellin-Peña MJ, Wang H, Johnson R, Anand S, Griffiths MW. Probiotics affect virulence-related gene expression in Escherichia coli O157:H7. Appl Environ Microbiol. 2007;73:4259–4267. doi: 10.1128/AEM.00159-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MB, Bassler BL. Quorum sensing in bacteria. Annu Rev Microbiol. 2001;55:165–199. doi: 10.1146/annurev.micro.55.1.165. [DOI] [PubMed] [Google Scholar]
- Molin G. Probiotics in foods not containing milk or milk constituents, with special reference to Lactobacillus plantarum 299v. Am J Clin Nutr. 2001;73:380–385. doi: 10.1093/ajcn/73.2.380s. [DOI] [PubMed] [Google Scholar]
- Moncheva P, Chipeva V, Kujumzieva A, Ivanova I, Dousset X, Gocheva B (2003) The composition of the microflora of boza, an original Bulgarian beverage. Biotechnol Equip 17:164–168
- Mondel M, Schroeder BO, Zimmermann K, Huber H, Nuding S, Beisner J, Fellermann K, Stange EF, Wehkamp J. Probiotic Escherichia coli treatment mediates antimicrobial human beta-defensin synthesis and fecal excretion in humans. Mucosal Immunol. 2009;2:166–172. doi: 10.1038/mi.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moneret-Vautrin DA, Morisset M, Cordebar V, Codreándu F, Kanny G. Probiotics may be unsafe in infants allergic to cow’s milk. Allergy. 2006;61:507–508. doi: 10.1111/j.1398-9995.2006.01050.x. [DOI] [PubMed] [Google Scholar]
- Monika K, Juraj M, Alzbeta M, Ernest S, Lubomir V. Cereals and pseudocereals as substrates for growth and metabolism of a probiotic strain Lactobacillus rhamnosus GG. J Food Nutr Res. 2013;52:25–36. [Google Scholar]
- Morelli L. In vitro assessment of probiotic bacteria: from survival to functionality. Int Dairy J. 2007;17:1278–1283. doi: 10.1016/j.idairyj.2007.01.015. [DOI] [Google Scholar]
- Moro G, Arslanoglu S, Stahl B, Jelinek J, Wahn U, Boehm G. A mixture of prebiotic oligosaccharides reduces the incidence of atopic dermatitis during the first six months of age. Arch Dis Child. 2006;91:814–819. doi: 10.1136/adc.2006.098251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa S, Shaborin A, Kabeir BM, Yazid AM, Hakim MN, Khahtanan A. Survival of Bifidobacterium pseudocatenulatum G4 during the storage of fermented peanut milk (PM) and skim milk (SM) products. Afr J Food Sci. 2009;3:150–155. [Google Scholar]
- Muthukumarasamy P, Holley RA. Microbiological and sensory quality of dry fermented sausages containing alginate-microencapsulated Lactobacillus reuteri. Int J Food Microbiol. 2006;111:164–169. doi: 10.1016/j.ijfoodmicro.2006.04.036. [DOI] [PubMed] [Google Scholar]
- Muyanja CMBK, Narvhus JA, Treimo J, Langsrud T. Isolation, characterisation and identification of lactic acid bacteria from bushera: a Ugandan traditional fermented beverage. Int J Food Microbiol. 2003;80:201–210. doi: 10.1016/S0168-1605(02)00148-4. [DOI] [PubMed] [Google Scholar]
- National Institute of Child Health and Human Development (2006) Lactose intolerance: information for health care providers. NIH Publication No. 05-5303B
- Nazzaro F, Fratinni F, Sada A, Orlando P. Synbiotic potential of carrot juice supplemented with Lactobacillus spp. and inulin or fructo oligosaccharides. J Sci Food Agric. 2008;88:2271–2276. doi: 10.1002/jsfa.3343. [DOI] [Google Scholar]
- Nomoto K. Review prevention of infections by probiotics. J Biosci Bioeng. 2005;100:583–592. doi: 10.1263/jbb.100.583. [DOI] [PubMed] [Google Scholar]
- Nomura M, Kobayashi M, Narita T, Kimoto-Nira H, Okamoto T. Phenotypic and molecular characterization of Lactococcus lactis from milk and plants. J Appl Microbiol. 2006;101:396–405. doi: 10.1111/j.1365-2672.2006.02949.x. [DOI] [PubMed] [Google Scholar]
- Nosrati R, Hashemiravan M, Talebi M. Fermentation of vegetables juice by probiotic bacteria. Int J Biosci. 2014;4:171–180. [Google Scholar]
- Nualkaekul S, Lenton D, Cook MT, Khutoryanskiy VV, Charalampopoulos D. Chitosan coated alginate beads for the survival of microencapsulated Lactobacillus plantarum in pomegranate juice. Carbohydr Polym. 2012;90:1281–1287. doi: 10.1016/j.carbpol.2012.06.073. [DOI] [PubMed] [Google Scholar]
- O’Driscoll B, Gahan CGM, Hill C. Adaptive acid tolerance response in Listeria monocytogenes: isolation of an acid-tolerant mutant which demonstrates increased virulence. Appl Environ Microbiol. 1996;62:1693–1698. doi: 10.1128/aem.62.5.1693-1698.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa M, Shimizu K, Nomoto K, Takahashi M, Watanuki M, Tanaka R, Tanaka T, Hamabata T, Yamasaki S, Takeda Y. Protective effect of Lactobacillus casei strain Shirota on Shiga toxin-producing Escherichia coli O157:H7 infection in infant rabbits. Infect Immun. 2001;69:1101–1108. doi: 10.1128/IAI.69.2.1101-1108.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohland CL, Macnaughton WK. Probiotic bacteria and intestinal epithelial barrier function. Am J Physiol Gastrointest Liver Physiol. 2010;298:G807–G819. doi: 10.1152/ajpgi.00243.2009. [DOI] [PubMed] [Google Scholar]
- Oi Y, KIitabatake N. Chemical composition of an East African traditional beverage, togwa. J Agric Food Chem. 2003;51:7024–7028. doi: 10.1021/jf0203343. [DOI] [PubMed] [Google Scholar]
- Ouwehand AC, Salminen SJ. The health effects of cultured milk products with viable and non-viable bacteria. Int Dairy J. 1998;8:749–758. doi: 10.1016/S0958-6946(98)00114-9. [DOI] [Google Scholar]
- Páez R, Lavari L, Vinderola G, Audero G, Cuatrin A, Zaritzky N, Reinheimer J. Effect of spray drying on the viability and resistance to simulated gastrointestinal digestion in lactobacilli. Food Res Int. 2012;48:748–754. doi: 10.1016/j.foodres.2012.06.018. [DOI] [Google Scholar]
- Penna ALB, Rao-Gurram S, Barbosa-Ca’novas GV. Effect of milk treatment on acidification, physicochemical characteristics, and probiotic cell counts in low fat yogurt. Milchwissenschaft. 2007;62:48–52. [Google Scholar]
- Penner R, Fedorak RN, Madsen KL. Probiotics and nutraceuticals: non-medicinal treatments of gastrointestinal diseases. Curr Opin Pharmacol. 2005;5:596–603. doi: 10.1016/j.coph.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Pereira ALF, Maciel TC, Rodrigues S. Probiotic beverage from cashew apple juice fermented with Lactobacillus casei. Food Res Int. 2011;44:1276–1283. doi: 10.1016/j.foodres.2010.11.035. [DOI] [Google Scholar]
- Peres CM, Peres C, Hernández-Mendoza A, Malcata FX. Review on fermented plant materials as carriers and sources of potentially probiotic LAB -With an emphasis on table olives. Trends Food Sci Technol. 2012;26:31–42. doi: 10.1016/j.tifs.2012.01.006. [DOI] [Google Scholar]
- Prado FC, Parada JL, Pandey A, Soccol CR. Trends in non-dairy probiotic beverages. Food Res Int. 2008;41:111–123. doi: 10.1016/j.foodres.2007.10.010. [DOI] [Google Scholar]
- Prado FC, Lindner JDD, Inaba J, Thomaz-Soccol V, Brar SK, Soccol CR. Development and evaluation of a fermented coconut water beverage with potential health benefits. J Funct Foods. 2015;12:489–497. doi: 10.1016/j.jff.2014.12.020. [DOI] [Google Scholar]
- Ranadheera RDCS, Baines SK, Adams MC. Importance of food in probiotic efficacy. Food Res Int. 2010;43:1–7. doi: 10.1016/j.foodres.2009.09.009. [DOI] [Google Scholar]
- Rastall RA, Gibson GR. Recent developments in prebiotics to selectively impact beneficial microbes and promote intestinal health. Curr Opin Biotechnol. 2015;32:42–46. doi: 10.1016/j.copbio.2014.11.002. [DOI] [PubMed] [Google Scholar]
- Rathore S, Salmerón I, Pandiella SS. Production of potentially probiotic beverages using single and mixed cereal substrates fermented with LAB cultures. Food Microbiol. 2012;30:239–244. doi: 10.1016/j.fm.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Reid G, Kirjaivanen P. Taking probiotics during pregnancy. Are they useful therapy for mothers and newborns? Can Fam Physician. 2005;51:1477–1479. [PMC free article] [PubMed] [Google Scholar]
- Renuka B, Kulkarni SG, Vijayanand P, Prapulla SG. Fructo oligosaccharide fortification of selected fruit juice beverages: effect on the quality characteristics. LWT-Food Sci Technol. 2009;43:1031–1033. doi: 10.1016/j.lwt.2008.11.004. [DOI] [Google Scholar]
- Rettger LF, Chaplin HA. Treatise on the transformation of the intestinal flora with special reference to the implantation of Bacillus acidophilus. New Haven: Yale University Press; 1921. [Google Scholar]
- Ricci G, Patrizi A, Baldi E, Menna G, Tabanelli M, Masi M. Long-term follow-up of atopic dermatitis: retrospective analysis of related risk factors and association with concomitant allergic diseases. J Am Acad Dermatol. 2006;55:765–771. doi: 10.1016/j.jaad.2006.04.064. [DOI] [PubMed] [Google Scholar]
- Ritter P, Kohler C, Von Ah U. Evaluation of the passage of Lactobacillus gasseri K7 and bifidobacteria from the stomach to intestines using a single reactor model. BMC Microbiol. 2009;9:1–9. doi: 10.1186/1471-2180-9-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Espinoza Y, Gallardo-Navarro Y. Non-dairy probiotic products. Food Microbiol. 2010;27:1–11. doi: 10.1016/j.fm.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Roberts JS, Kidd DR. Lactic acid fermentation of onions. Food Sci Technol. 2005;38:2185–2190. [Google Scholar]
- Roopashri AN, Varadaraj MC. Hydrolysis of flatulence causing oligosaccharides by α-d-galactosidase of a probiotic Lactobacillus plantarum MTCC 5422 in selected legume flours and elaboration of probiotic attributes in soy-based fermented product. Eur Food Res Technol. 2014 [Google Scholar]
- Rouhi M, Sohrabvandi S, Mortazavian AM. Probiotic fermented sausage: viability of probiotic microorganisms and sensory characteristics. Crit Rev Food Sci Nutr. 2013;53:331–348. doi: 10.1080/10408398.2010.531407. [DOI] [PubMed] [Google Scholar]
- Saad N, Delattre C, Urdaci M, Schmitter JM, Bressollier P. An overview of the last advances in probiotic and prebiotic field. LWT Food Sci Technol. 2013;50:1–16. doi: 10.1016/j.lwt.2012.05.014. [DOI] [Google Scholar]
- Saarela M, Virkarjarvi I, Alakomi HL, Matilla PS, Matto J. Stability and functionality of freeze-dried probiotic Bifidobacterium cells during storage in juice and milk. Int Dairy J. 2006;16:1477–1482. doi: 10.1016/j.idairyj.2005.12.007. [DOI] [Google Scholar]
- Salama MS, Musafija-Jeknic T, Sandine WE, Giovannoni SJ. An ecological study of LAB: isolation of new strains of Lactococcus including Lactococcus lactis subsp. cremoris. J Dairy Sci. 1995;78:1004–1017. doi: 10.3168/jds.S0022-0302(95)76716-9. [DOI] [Google Scholar]
- Salmerón I, Thomas K, Pandiella SS. Effect of substrate composition and inoculum on the fermentation kinetics and flavour compound profiles of potentially non-dairy probiotic formulations. LWT-Food Sci Technol. 2014;55:240–247. doi: 10.1016/j.lwt.2013.07.008. [DOI] [Google Scholar]
- Salminen S, von Wright A (1993) Lactic Acid Bacteria. Marcel Dekker Inc., New York
- Sanders ME, Marco ML. Food formats for effective delivery of probiotics. Annu Rev Food Sci Technol. 2010;1:65–85. doi: 10.1146/annurev.food.080708.100743. [DOI] [PubMed] [Google Scholar]
- Sanders ME, Guarner F, Guerrant R, Holt PR, Quigley EM, Sartor RB, Sherman PM, Mayer EA. An update on the use and investigation of probiotics in health and disease. Gut. 2013;62:787–796. doi: 10.1136/gutjnl-2012-302504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangwan V, Tomar SK, Singh RRB, Singh AK, Ali B. Galactooligosaccharides: novel components of designer foods. J Food Sci. 2011;76:R103–R111. doi: 10.1111/j.1750-3841.2011.02131.x. [DOI] [PubMed] [Google Scholar]
- Sankara RDS, Deosthale YG. Mineral composition, ionizable iron and soluble zinc in malted grains of pearl millet and ragi. Food Chem. 1983;11:217–223. doi: 10.1016/0308-8146(83)90104-8. [DOI] [Google Scholar]
- Sanni A, Franz C, Schillinger U, Huch M, Guigas C, Holzapfel W. Characterization and technological properties of LAB in the production of “Sorghurt”, a cereal-based product. Food Biotechnol. 2013;27:178–198. doi: 10.1080/08905436.2013.781949. [DOI] [Google Scholar]
- Saris PEJ, Beasley S, Tourila H. Fermented soymilk with a monoculture of Lactococcus lactis. Int J Food Microbiol. 2003;81:159–162. doi: 10.1016/S0168-1605(02)00196-4. [DOI] [PubMed] [Google Scholar]
- Savard T, Gardner N, Champagne C. Growth of Lactobacillus and Bifidobacterium cultures in a vegetable juice medium, and their stability during storage in a fermented vegetable juice. Sci Aliment. 2003;23:273–283. doi: 10.3166/sda.23.273-283. [DOI] [Google Scholar]
- Saxelin M. Probiotic formulations and applications, the current probiotics market, and changes in the marketplace: a European perspective. Clin Infect Dis. 2008;46:S76–S79. doi: 10.1086/523337. [DOI] [PubMed] [Google Scholar]
- Saxelin M, Korpela R, Mayra-Makinen A. Introduction: classifying functional dairy products. In: Mattila-Sandholm T, Saarela M, editors. Functional dairy products. New York: CRC Press; 2003. pp. 1–15. [Google Scholar]
- Schlee M, Harder J, Köten B, Stange EF, Wehkamp J, Fellermann K. Probiotic lactobacilli and VSL#3 induce enterocyte beta-defensin 2. Clin Exp Immunol. 2008;151:528–535. doi: 10.1111/j.1365-2249.2007.03587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrezenmeir J, de Vrese M. Probiotics, prebiotics and synbiotics approaching a definition. Am J Clin Nutr. 2001;77:361–364. doi: 10.1093/ajcn/73.2.361s. [DOI] [PubMed] [Google Scholar]
- Scrimshaw NS, Murray EB. The acceptability of milk and milk products in populations with a high prevalence of lactose intolerance. Am J Clin Nutr. 1988;48(4):1079–1159. doi: 10.1093/ajcn/48.4.1142. [DOI] [PubMed] [Google Scholar]
- Shah NP. Functional cultures and health benefits. Int Dairy J. 2007;17:1262–1277. doi: 10.1016/j.idairyj.2007.01.014. [DOI] [Google Scholar]
- Shah NP (2001) Functional foods from probiotics and prebiotics. Food Technol 55:46–53
- Sharma M, Devi M. Probiotics: a comprehensive approach toward health foods. Crit Rev Food Sci Nutr. 2014;54:537–552. doi: 10.1080/10408398.2011.594185. [DOI] [PubMed] [Google Scholar]
- Sheehan VM, Ross P, Fitzgerald GF. Assessing the acid tolerance and the technological robustness of probiotic cultures for fortification in fruit juices. Innovative Food Sci Emerg Technol. 2007;8:279–284. doi: 10.1016/j.ifset.2007.01.007. [DOI] [Google Scholar]
- Sheela T, Suganya RS. Studies on anti-diarrhoeal activity of synbiotic plums juice. Int J Sci Res Publ. 2012;2:1–5. [Google Scholar]
- Sidira M, Karapetsas A, Galanis A, Kanellaki M, Kourkoutas Y. Effective survival of immobilized Lactobacillus casei during ripening and heat treatment of probiotic dry-fermented sausages and investigation of the microbial dynamics. Meat Sci. 2014;96:948–955. doi: 10.1016/j.meatsci.2013.09.013. [DOI] [PubMed] [Google Scholar]
- Sistek D, Kelly R, Wickens K, Stanley T, Fitzharris P, Crane J. Is the effect of probiotic on atopic dermatitis confined to food sensitized children? Clin Exp Allergy. 2006;36:629–633. doi: 10.1111/j.1365-2222.2006.02485.x. [DOI] [PubMed] [Google Scholar]
- Soccol CR, Vandenberghe LPS, Spier MR, Medeiros ABP, Yamaguishi CT, Lindner JD, Pandey A, Thomaz-Soccol V. The potential of probiotics. Food Technol Biotechnol. 2010;48:413–434. [Google Scholar]
- Soni SK, Sandhu DK, Vilkhu KS, Kamra N. Microbiological studies on dosa fermentation. Food Microbiol. 1986;3:45–53. doi: 10.1016/S0740-0020(86)80025-9. [DOI] [Google Scholar]
- Soukoulis C, Yonekura L, Gan HH, Behboudi-Jobbehdar S, Parmenter C, Fisk I. Probiotic edible films as a new strategy for developing functional bakery products: the case of pan bread. Food Hydrocoll. 2014;39:231–242. doi: 10.1016/j.foodhyd.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain MR, Anandharaj M, Ray RC, Praveen Rani RP (2014) Fermented fruits and vegetables of Asia: A potential source of probiotics. Biotechnol Res Int. Article ID 250424. doi:10.1155/2014/250424 [DOI] [PMC free article] [PubMed]
- Taylor AL, Dunstan JA, Prescott SL. Probiotic supplementation for the first 6 months of life fails to reduce the risk of atopic dermatitis and increase the risk of allergen sensitization in high-risk children: a randomized control trial. J Allergy Clin Immunol. 2007;119:184–191. doi: 10.1016/j.jaci.2006.08.036. [DOI] [PubMed] [Google Scholar]
- Teucher B, Olivares M, Cori H. Enhancers of iron absorption: ascorbic acid and other organic acids. Int J Vitam Nutr Res. 2004;74:403–419. doi: 10.1024/0300-9831.74.6.403. [DOI] [PubMed] [Google Scholar]
- Tien MT, Girardin SE, Regnault B, Bourhis L, Dillies MA, Coppee JY, Bourdet-Sicard R, Sansonetti PJ, Pédron T. Anti-inflammatory effect of Lactobacillus casei on Shigella-infected human intestinal epithelial cells. J Immunol. 2006;176:1228–1237. doi: 10.4049/jimmunol.176.2.1228. [DOI] [PubMed] [Google Scholar]
- Tsen JH, Lin YP, King VA. Fermentation of banana media by using k-carrageenan immobilized Lactobacillus acidophilus. Int J Food Microbiol. 2004;91:215–220. doi: 10.1016/S0168-1605(03)00376-3. [DOI] [PubMed] [Google Scholar]
- Tsen JH, Lin YP, King AE. Response surface methodology optimisation of immobilized Lactobacillus acidophilus banana puree fermentation. Int J Food Sci Technol. 2009;44:120–127. doi: 10.1111/j.1365-2621.2007.01681.x. [DOI] [Google Scholar]
- Tuorila H, Cardello AV (2002) Consumer responses to an off-flavor in juice in the presence of specific health claims. Food Qual Prefer 13:561–569
- Ulrich A, Müller T. Heterogeneity of plant-associated streptococci as characterized by phenotypic features and restriction analysis of PCR-amplified 16S rRNA. J Appl Microbiol. 1999;84:293–303. doi: 10.1046/j.1365-2672.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Venkateshwari S, Halami PM, Vijayendra SVN. Characterization of the heat stable bacteriocin producing and vancomycin-sensitive Pediococcus pentosaceus CFR B19 isolated from beans. Benefic Microbes. 2010;1:159–164. doi: 10.3920/BM2009.0032. [DOI] [PubMed] [Google Scholar]
- Vesa TH, Marteau P, Korpela R. Lactose intolerance. J Am Coll Nutr. 2000;19:165S–175S. doi: 10.1080/07315724.2000.10718086. [DOI] [PubMed] [Google Scholar]
- Vijaya Kumar B, Sreedharamurthy M, Reddy OVS. Physico-chemical analysis of fresh and probioticated fruit juices with Lactobacillus casei. Int J Appl Sci Biotechnol. 2013;1:127–131. [Google Scholar]
- Vijayendra SVN, Gupta RC. Assessment of probiotic and sensory properties of dahi and yoghurt prepared using bulk freeze-dried cultures in buffalo milk. Ann Microbiol. 2012;62:939–947. doi: 10.1007/s13213-011-0331-5. [DOI] [Google Scholar]
- Vijayendra SVN, Gupta RC. Associative growth behavior of dahi and yoghurt starter cultures with Bifidobacterium bifidum and Lactobacillus acidophilus in buffalo skim milk. Ann Microbiol. 2013;63:461–469. doi: 10.1007/s13213-012-0490-z. [DOI] [Google Scholar]
- Vijayendra SVN, Gupta RC. Performance evaluation of bulk freeze dried starter cultures of dahi and yoghurt along with probiotic strains in standardized milk of cow and buffalo. J Food Sci Technol. 2013;51:4114–4119. doi: 10.1007/s13197-013-0944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayendra SVN, Halami PM (2015) Fermented vegetables-health benefits. In: Tamang JP (ed) Fermented vegetables. CRC Taylor & Francis Publisher, USA, 327–344
- Vijayendra SVN, Rajashree K, Halami PM. Characterization of a heat stable anti-listerial bacteriocin produced by vancomycin sensitive Enterococcus faecium isolated from idli batter. Indian J Microbiol. 2010;50:243–246. doi: 10.1007/s12088-010-0030-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinderola G, Céspedes M, Mateolli D, Cárdenas P, Lescano M, Aimaretti N, Reinheimer J. Changes in gastric resistance of Lactobacillus casei in flavored commercial fermented milks during refrigerated storage. Int J Dairy Technol. 2011;64:269–275. doi: 10.1111/j.1471-0307.2010.00659.x. [DOI] [Google Scholar]
- Vinderola G, Zacarías MF, Bockelmann W, Neve H, Reinheimer J, Heller KJ. Preservation of functionality of Bifidobacterium animalis subsp. lactis INL1 after incorporation of freeze-dried cells into different food matrices. Food Microbiol. 2012;30:274–280. doi: 10.1016/j.fm.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Wacher C, Barzana E, Lappe P, Ulloa M, Owens JD. Microbiology of Indian and Mestizo pozol fermentation. Food Microbiol. 2000;17:251–256. doi: 10.1006/fmic.1999.0310. [DOI] [Google Scholar]
- Wang C, Ng CC, Tzeng W, Shyu YT. Probiotic potential of noni juice fermented with lactic acid bacteria and bifidobacteria. Int J Food Sci Nutr. 2009;1:1–9. doi: 10.1080/09637480902755095. [DOI] [PubMed] [Google Scholar]
- Wang J, Guo Z, Zhang Q, Yan L, Chen W, Liu XM, Zhang HP. Fermentation characteristics and transit tolerance of probiotic Lactobacillus casei Zhang in soymilk and bovine milk during storage. J Dairy Sci. 2009;92:2468–2476. doi: 10.3168/jds.2008-1849. [DOI] [PubMed] [Google Scholar]
- Waters DM, Mauch A, Coffey A, Arendt EK, Zannini E. Lactic acid bacteria as a cell factory for the delivery of functional biomolecules and ingredients in cereal based beverages: a review. Crit Rev Food Sci Nutr. 2015;55:503–520. doi: 10.1080/10408398.2012.660251. [DOI] [PubMed] [Google Scholar]
- Wu X, Vallance BA, Boyer L, Bergstrom KS, Walker J, Madsen K, OKusky JR, Buchan AM, Jacobson K. Saccharomyces boulardii ameliorates Citrobacter rodentium - induced colitis through actions on bacterial virulence factors. Am J Physiol Gastrointest Liver Physiol. 2008;294:G295–G306. doi: 10.1152/ajpgi.00173.2007. [DOI] [PubMed] [Google Scholar]
- Yan F, Polk DB. Probiotics as functional food in the treatment of diarrhea. Curr Opin Clin Nutr Metab Care. 2006;9:717–721. doi: 10.1097/01.mco.0000247477.02650.51. [DOI] [PubMed] [Google Scholar]
- Yeo SK, Liong MT. Angiotensin I-converting enzyme inhibitory activity and bioconversion of isoflavones by probiotics in soymilk supplemented with prebiotics. Int J Food Sci Nutr. 2010;61:161–181. doi: 10.3109/09637480903348122. [DOI] [PubMed] [Google Scholar]
- Yoon KY, Woodamns EE, Hang YD. Probiotication of tomato juice by LAB. J Microbiol. 2004;42:315–318. [PubMed] [Google Scholar]
- Yoon KY, Woodamns EE, Hang YD. Fermentation of beet juice by beneficial LAB. LWT-Food Sci Technol. 2005;38:73–75. doi: 10.1016/j.lwt.2004.04.008. [DOI] [Google Scholar]
- Yoon KY, Woodamns EE, Hang YD. Production of probiotic cabbage juice by LAB. Bioresour Technol. 2006;9:1427–1430. doi: 10.1016/j.biortech.2005.06.018. [DOI] [PubMed] [Google Scholar]


