Abstract
Wine belongs to a family of products where the quality matters. Its quality can be in principle verified using diverse physicochemical approaches, including the determination of various chemical compounds generally accepted as chemical markers of product quality. Example of such applicable compounds is a family derived from phenols. Next to a more classical approach, infrared spectroscopy can play an important role in this game. Here we sought to develop an easy to use, ultra-fast and robust method based on FT-IR with some important advantages including lower sample and solvent consumptions. The tested and evaluated method was consequently applied in a monitoring of changes in a content of total phenolic compounds (TPC) and total antioxidant activity (TAA) during a process of wine-making. It was found out that total amount of phenolic compounds differs both for individual kind of wines, namely red, white and rose, at each processing stage of the production. The content of phenolic compounds of red and white wine increased while an opposite trend was observed in rose wine. TAA values of analysed wines showed difference between individual kind of wine and indicate the same trend like phenolic profile. Antioxidant activity values relate to changes of phenolic content during production process.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-014-1644-8) contains supplementary material, which is available to authorized users.
Keywords: Phenolic compounds, Antioxidant activity, Chemometrics, FT-IR spectroscopy
Introduction
Control of wine quality presents an important task for both: winemakers and costumers. Knowledge of the chemical composition of wine at every stage of production enables a better control of the production process (Gishen et al. 2010). Such monitoring can be efficiently used as a source of data for a further tuning of the production process potentially leading to the increase of the product quality. Family of compounds of phenolic nature can be labelled as one of the groups with superior impact especially due to their influence on the organoleptic properties of wine (Jackson 2008). Importantly for this study, their content is expected to be considerably changed during a wine-making process.
Wine is a source of large number of natural phenolic compounds such as anthocyanins, flavonoids, procyanidins, hydroxycinnamic acids, and their derivatives (Tarantilis et al. 2008). Content of phenolic compounds in wine does not depend only on the characteristics of raw grapes, influenced mainly by variety, ripening stage, climatic and soil conditions, but also on the winemaking process, which is characteristic for each kind of wine and viniculture (Rodríguez-Delgado et al. 2002; Bai et al. 2013).
The content of phenolic compounds is usually analysed by high performance liquid chromatography (HPLC) (Ginjom et al. 2011), capillary zone electrophoresis (CZE) (Woraratphoka et al. 2007), mass spectrometry (MS) (Bravo et al. 2006) and electroanalytical techniques (Šeruga et al. 2011). Recognized spectrophotometric methods, namely: ferric reducing antioxidant potential (FRAP) (Katalinić et al. 2004), 1,1-diphenyl-2-picrylhydrzyl (DPPH) method (Fernández-Pachón et al. 2004) and oxygen radical absorbance capacity (ORAC) (Villaño et al. 2006) are available too. Albeit the indisputable low limits of detection and increased specificity of these methods, there is still a well described gap which can be filled by procedures able to excel in reliability and speed with a given ability to be potentially used directly on the field. Infrared spectroscopy (FT-IR, NIR) in combination with a proper statistics can be potentially recognized as a technique of choice due to its rapid, non-destructive and versatile nature. FT-IR also requires minimal sample preparation. Moreover, recent developments on the field of miniaturization brought portable and well applicable infrared spectroscopy as described e.g. by Perez-Marin or Sanchez (Peréz-Marín et al. 2009; Sánchez et al. 2011). The portable infrared spectroscopy can be obtained even as an accessory to a smartphone, as described by Friedrich (Friedrich et al. 2014). Handheld NIR spectrometers were applied in a characterization of grapes on-vine ripening (Gonzáles-Caballero et al. 2012) or in an analysis of fruits and vegetables (Plans et al. 2013; Pérez-Marín et al. 2010; Tiwari et al. 2013). FT-IR has already been proved to be suitable for routine qualitative analysis and process control in wineries (Bevin et al. 2006), for example, analysis of raw material (Versari et al. 2008), monitoring of fermentation (Di Egidio et al. 2010), determination of main parameters in wine (ethanol, organic acids, sugar, glycerol, etc.) (Cuadrado et al. 2005; Regmi et al. 2012; Shen et al. 2011; Patz et al. 2004) and like the method for authenticity of wine (Versari et al. 2014). With regard to analysis of phenolic compound, there are also described some applications of infrared spectroscopy to the determination of phenolic compounds in wine (Soriano et al. 2007; Fernández and Agosin 2007), discrimination of red wine cultivars based on phenolic wine extract (Edelmann et al. 2001) and for screening of total phenolic content, total flavonoid content and antioxidant capacity in Moscatel dessert wines (Silva et al. 2014). Based on the previously mentioned statements, here we sought to develop a procedure based on the utilization of infra-red spectroscopy applicable not only for a control of quality of produced wine, but more importantly for an evaluation of winemaking process based on the monitoring of total phenolic compounds as well as monitoring of total antioxidant activity.
Materials and methods
Wine samples
Three kinds of wine (red wine Lemberger, rose wine Lemberger and white wine Cuvée (variety Pinot Blank, Traminer Rot and Sauvignon)) in different stages of production were selected for this study. Individual samples of wines with their processing stage and date of collection are described in Table 1. All kinds of wines were obtained from one producer from Czech viniculture (winery) which is located in wine region Moravia (Velké Pavlovice sub-region) South Moravia (Czech Republic).
Table 1.
Samples of wines in different period of production process
| Red wine | Rose wine | White wine | ||||
|---|---|---|---|---|---|---|
| Collection date | Sample name | Processing stage | Sample name | Processing stage | Sample name | Processing stage |
| 16 Oct 2011 | C1 | Mash | R1 | Mash | B1 | Mash |
| 17 Oct 2011 | C2 | Maceration of skin | R2 | Must after maceration | B2 | Must after maceration |
| 22 Oct 23 Oct 2011 |
C3 | Fermentation | R3 | Fermentation | B3 | Fermentation |
| 1 Nov 2011 | C4 | Fermentation | R4 | Ending fermentation | B4 | Fermentation,SO2 |
| 15 Nov 2011 | C5 | Fermentation | R5_1 | After fermentation | B5_1 | After fermentation |
| R5_2 | After fermentationdf | B5_2 | After fermentationdf | |||
| R5_3 | After fermentationcf | B5_3 | After fermantationcf | |||
| 11 Mar 2012 | C6 | Maturation | R6 | Maturation | B6 | Maturation |
| 25 June 2012 | C7 | Final wine | R7 | Final wine | B7 | Final wine |
SO2 addition of SO2
DF desk filtration
CF cross-flow filtration
All wine samples were collected into 100 ml plastic vessels (made of polypropylene) and were stored at the temperature −20 °C prior to analysis according to recommended protocols (Christian et al. 2014; Harvey 2000).
FT-IR analysis
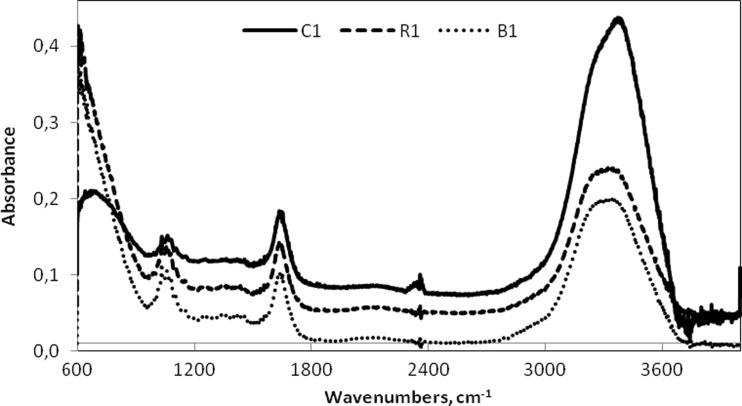
The measurement of each sample was carried out on FT-IR spectrometer (Nicolet 6700, Thermo Scientific, USA) using liquid cell positioned on a single reflection attenuated total reflectance (ATR) accessory equipped by a zinc selenide crystal. The software OMNIC version 8 was used for spectral acquisition. Infrared spectra were obtained in the range of 4000–600 cm−1 with a spectral resolution of 2 cm−1. Each evaluated spectrum is a mean of 32 scans. Spectral data obtained by analysis of red, rose and white wine are shown in Fig 1. Measurements were performed with 60 μL of samples in order to fully utilize total volume of the liquid cell. The reference scan of the ZnSe crystal was taken before each measurement and used for consecutive background corrections. All samples were measured in triplicate if not stated otherwise.
Fig. 1.
FT-IR spectra of red (C1,  ), rose (R1,
), rose (R1,  ) and white (B1,
) and white (B1,  ) wine in first stage of production measured in range 4000–600 cm−1 with a resolution 2 cm−1 and 32 scans
) wine in first stage of production measured in range 4000–600 cm−1 with a resolution 2 cm−1 and 32 scans
A complete spectral range (4000–600 cm−1) of each sample was stored, however, spectral analysis based on PLS models was conducted only using selected parts of IR regions. The full spectra of liquid samples show the presence of interfering compounds, mostly water that absorbs at 1750–1590 cm−1 and 3700–3000 cm−1. However, these regions may contain important spectral data. Selection of spectral region of phenolic compounds can be thus challenging as mentioned before e.g. (Moreira and Santos 2004). The selection was thus based not only on previous works including (Versari et al. 2010; Romera-Fernández et al. 2012; Fragoso et al. 2011), but also on resulting parameters of PLS models including PRESS analysis, statistical spectra and loading spectra. For more information see Online Resource 1, Figures S1–S5. Following spectral regions were used for the prediction of total phenolic content (TPC): 3000–2960 cm−1 and 1457–966 cm−1 for red wine, 1457–966 cm−1 for rose wine and 3000–2960 cm−1 and 1543–966 cm−1 for white wine. For the prediction of total antioxidant activity (TAA) these regions were chosen: 2973–2434 cm−1, 2280–1717 cm−1 and 1445–966 cm−1 for red wine, 3730–1034 cm−1, 1032–627 cm−1 and 626–614 cm−1 for rose wine and 2971–2435 cm−1, 2280–1717 cm−1 and 1543–966 cm−1 for white wine.
Spectral analysis and statistical evaluation
Statistical analysis was performed using TQ Analyst (version 8, Thermo Scientific, USA). Quantitative analysis was performed with PLS regression by relating the sample spectra and the reference values (Wold et al. 2001). The leave-out-one cross validation diagnostic tool was used to determine the maximum number of significant PLS factors to ensure the predictive ability of calibration model and to avoid over fitting. Root mean square error of calibration, root mean square error of cross-validation, root mean square error of prediction (RMSEC, RMSECV, RMSEP, respectively) and correlation coefficient R between the predicted and reference values were used as indicators of the prediction capability of the developed model. A good model should have low and very similar RMSEC and RMSEP values and R values close to unity. In this study, different pre-treatments were applied namely the first (1D) and the second (2D) derivation.
Three calibration models were built between the FT-IR spectra (raw, 1D and 2D) and the reference values of TPC and TAA for each kind of wine. Table 2 shows statistical parameters of PLS models for the determination of total phenolic compounds. All nine PLS models provide good calibration (Rcal > 0.9), although the best results for the determination have only models, where the raw spectra were used. However, only white wine has better results for the first derivation spectra than for raw one. These PLS models had the highest value of calibration correlation coefficient (Rcal) and cross validation correlation coefficient (Rcv) and values of RMSEC, RMSECV were smaller in comparing with other models. Statistical parameters of PLS models for the determination of total antioxidant activity are presented in Table 3. Seven of the nine models obtained provide good calibration (Rcal > 0.9) and for determination of TAA, good results were reached for PLS models where raw FT-IR spectra were used. In both cases, results of cross validation indicated higher RMSECV values than RMSEC values obtained during calibration. On the basis of our results, we concluded, that derivation of FT-IR spectra leads rather to loss of spectral information and downgrade of calibration models. The best calibration models were chosen on the basis of our results for the determination of TPC and TAA in all kinds of wines. In almost all cases, these models were built using raw spectra; only in one case there were better parameters after data pre-treatment (Table 2).
Table 2.
Statistical parameters of PLS models for total phenolic compounds in all kinds of wine
| Data pre-treatment | PLS factors | RMSEC | Rcal | RMSEP | RMSECV | Rcv | |
|---|---|---|---|---|---|---|---|
| Red wine | Raw | 4 | 27.8 | 0.99 | 7.2 | 59.1 | 0.94 |
| 1D | 1 | 1090.0 | 0.90 | 139.0 | 2950.0 | 0.14 | |
| 2D | 4 | 32.3 | 0.98 | 64.5 | 193.0 | 0.27 | |
| Rose wine | Raw | 3 | 9.7 | 0.92 | 8.7 | 16.7 | 0.72 |
| 1D | 7 | 0.6 | 0.99 | 0.2 | 23.6 | 0.25 | |
| 2D | 4 | 4.8 | 0.98 | 5.6 | 29.5 | 0.18 | |
| White wine | Raw | 5 | 8.3 | 0.95 | 10.7 | 34.0 | 0.09 |
| 1D | 5 | 4.0 | 0.99 | 20.9 | 24.5 | 0.40 | |
| 2D | 3 | 8.9 | 0.93 | 26.5 | 24.6 | 0.37 |
RMSEC Root mean square error of calibration, R cal Correlation coefficient of calibration, RMSEP Root mean square error of prediction, RMSECV Root mean square error of cross-validation, R cv Correlation coefficient of cross validation
Table 3.
Statistical parameters of PLS models for total antioxidant activity of all kind of wine
| Data pre-treatment | PLS factors | RMSEC | Rcal | RMSEP | RMSECV | Rcv | |
|---|---|---|---|---|---|---|---|
| Red wine | Raw | 3 | 0.3 | 0.98 | 0.0 | 0.6 | 0.88 |
| 1D | 4 | 0.1 | 0.99 | 0.4 | 0.2 | 0.38 | |
| 2D | 1 | 0.7 | 0.85 | 0.4 | 1.9 | 0.52 | |
| Rose wine | Raw | 4 | 0.1 | 0.99 | 0.0 | 0.4 | 0.93 |
| 1D | 1 | 0.4 | 0.86 | 0.9 | 0.8 | 0.25 | |
| 2D | 5 | 0.0 | 0.99 | 0.7 | 0.8 | 0.26 | |
| White wine | Raw | 2 | 0.2 | 0.92 | 0.0 | 0.4 | 0.80 |
| 1D | 1 | 0.3 | 0.91 | 0.2 | 0.5 | 0.65 | |
| 2D | 3 | 0.1 | 0.98 | 0.2 | 0.5 | 0.64 |
RMSEC Root mean square error of calibration, R cal Correlation coefficient of calibration, RMSEP Root mean square error of prediction, RMSECV Root mean square error of cross-validation, R cv Correlation coefficient of cross validation
Determination of total antioxidant activity
Ferric reducing-antioxidant assay (FRAP) was applied for the determination of total antioxidant activity (TAA) of wine samples by measuring the change of absorbance at 593 nm after appropriate dilution in 1 cm quartz cuvette (Benzie and Strain 1996). UV-visible spectra were obtained on a UV/VIS spectrometer Lambda 25 (Perkin Elmer, USA). The quantification was carried out using calibration line built with ascorbic acid (analytical grade, Lach-Ner, Czech Republic) as the standard at five different concentrations in the range of 1 · 10−3–5 · 10−5 mol L−1. Total antioxidant activity was reported as mmol of ascorbic acid equivalents (mmol L−1 AAE).
Determination of total phenolic compounds
The content of the total phenolic compounds (TPC) in wine samples was determined by measuring the absorbance at 280 nm after proper dilution in 1 cm quartz cuvette (Waterhouse 2002). UV-visible spectra were obtained on the above described UV/VIS spectrometer. The quantification was performed using calibration line built with gallic acid (analytical grade, Fluka, Switzerland) as the standard at five different concentrations in the range of 2–14 mg L−1. Content of total phenolic compounds was expressed as mg gallic acid equivalents (mg L−1 GAE).
Results and discussion
Total phenolic content
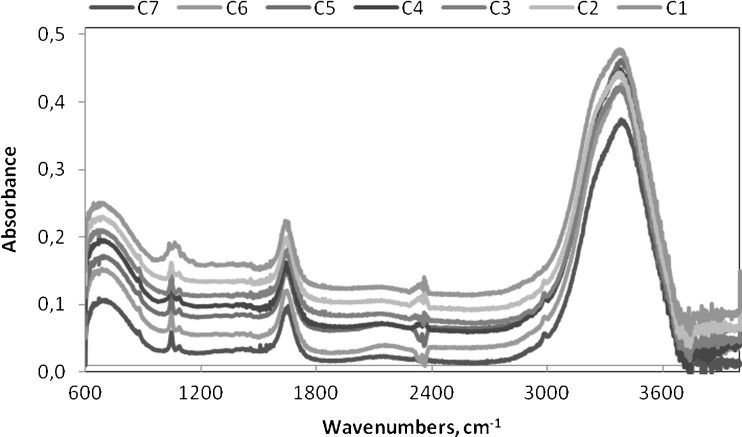
The TPC values of wines were obtained from PLS models on the basis of infrared spectra of these wines. Example of infrared spectra of red wine samples in all stages of production is presented in Fig. 2. Resulting values of TPC of red, rose and white wine with relevant relative standard deviations (RSD, %) are shown in Fig. 3. Values of RSD were in range 0.0–4.7 %. The content of phenolic compounds differs considerably in different kinds of wines, depending on the grape variety, environmental and manufacturing factors. Phenolic compounds are important to characteristics of red wines and are responsible for all the differences among red and white wines (Ribéreaou-Gayon et al. 2006). Our results confirm differences in phenolic content among wine samples studied. The TPC values in red wines were significantly higher than in rose and white wines. These results are well in accordance with data obtained by Paixão et al. (2007), Frankel et al. (1995) and Sato et al. (1996).
Fig. 2.
FT-IR spectra of red wine (C7-C1) in all stages of production measured in range 4000–600 cm−1 with a resolution 2 cm−1 and 32 scans
Fig. 3.
Total phenolic content of all kinds of wines during winemaking process by FT-IR spectroscopy (C1-C7 Red wine; R1-R7 Rose wine; B1-B7 White wine) Value after ± is relative standard deviation RSD (%) calculated for each wine samples
In our work we focused on the changes in the content of phenolic compounds during production process, which includes some basic operations such as harvesting, crushing, fermentation, pressing, aging, finishing operations and bottling. Wine quality is mainly determined by raw materials, but on the other hand, winemaking techniques also play an important role in the extraction of phenolic compounds and their further stability in wine; the quality of wine (phenolic composition) can be influenced by various factors, for expamle fermentation temperature, skin to juice ratio, maceration time and enzyme addition (Cozzolino et al. 2004). From our results it is evident, that content of phenolic compounds increase during manufacturing process of red wine (from 668.5 to 1208.2 mg L−1 GAE) and white wine (from 190.8 to 193.7 mg L−1 GAE), but opposite trend was observed in rose (from 286.7 to 238.0 mg L−1 GAE) wine. These changes of content of TPC were probably caused by different manufacturing processes of each kind of wine.
Values of TPC of red wine increase through the winemaking, from mash to bottling. At the beginning of winemaking process, the fresh mash sample had content of TPC 668.5 mg L−1 and wine intended for bottling showed almost double amount of TPC. Rapid increase of TPC can be seen during maceration step (C1–C2). Maceration is a manufacturing process, when extraction of aromatic compounds (anthocyanins, tannins) from skin and seeds occur. Extraction of these compounds depends on maceration conditions such as duration and temperature and also ethanol formed as result primary fermentation. Ethanol formed contributes both to enhanced solubilization and facilitates anthocyanin escape by increasing membrane porosity. Next slight increase of TPC can be seen at the beginning of alcoholic fermentation after adding of yeast (C3). The alcoholic fermentation produces ethanol, which supported extraction of phenolic compounds from mash (Ginjom et al. 2010). Further, TPC values (C4–C5) do not change significantly during fermentation stage (primary fermentation and malolactic acid fermentation). Slight decrease occurred in sample C6, which could be caused by oxidation taking place in wooden barrels during maturation. Phenolic compounds especially anthocyanins undergo different oxidation, condensation and polymerization reactions (Goméz-Cordovés and González-SanJosé 1995) These reactions lead to decrease of amount of phenolic compounds, as result of transformation of monomeric anthocyanins on polymeric anthocyanins or anthocyanin-derived pigments (Blanco-Vega et al. 2014). Next obvious rise of TPC was reached in sample C7 and it is assigned to wine maturation in bottle. Phenolic content of red wines declines during the maturation and aging stage (Lomolino et al. 2010), but we observed inverse trend in our wine sample. Because this study was not repeated throughout production process, this increase of TPC could not be explained, but similar increase of total phenolic in red wine was published in work of Ginjom et al. (2010).
Values of TPC of rose wine decrease through the production process. At the beginning of this process, fresh mash had content of TPC lower than red wine and higher than white wine, in comparing the same production stage. From results it can be seen, that moderate decline of TPC occurs during maceration stage (R2), that did not lead to rise of TPC, in comparison to red wine. This can be caused by different production practise for example different duration, because rose and red wine was produced from same variety of wine. Next significant decline (R3) comes probably due to addition of bentonite. Generally, bentonite is added to wines primarily for purpose of reduction of content of termolabile proteins (Sauvage et al. 2010). However, it could lead to capture of polyphenols, which secondarily proved decrease of value of TPC. Following fermentation, that was supported addition of yeast, caused rapid increase of content of polyphenolic compounds. One of the technological operations of production process of this wine was a desk filtration and cross flow filtration, which is used for clarification and stabilization of wine (Boissier et al. 2008). After these operations, the small decrease of TPC was achieved. Many researches have demonstrated that polyphenols and polysaccharides are responsible for membrane fouling, that these molecules absorb to membrane materials (Vernhet and Moutounet 2002). Follow-up minimal increase of amount of polyphenols (samples R6 and R7) was attributed to maturation of wine.
Content of polyphenols of white wine reached the lowest values in comparison to red and rose wine, and changes of TPC don’t correspond to individual processing stage, to a certain extent. Slight increase of TPC was observed through wine-making process of this wine. From results it is evident, that significant decrease of TPC was achieved after maceration of mash, nevertheless maceration process itself should lead to its increase and this phenomenon was described in literature (Hernanz et al. 2007; Darias-Martín et al. 2000). Sample B3 reached small drop due to addition of bentonite and effect of bentonite was explained in previous paragraph. However, content of phenolic compounds (R4) did not rise after fermentation, i.e. after addition of yeast. Wine is subject to continuous changes in composition during storage as a consequence of different oxidation reactions. Polyphenols belong among the most readily oxidized wine constituents (Oliveira et al. 2011). From sensory point of view, controlled oxidation could be beneficial for red wine by enhancing and stabilizing colour and reducing astringency, nevertheless, the quality of white wine is generally damaged by exposure to air (Singleton 1987). Therefore, the colour changes in white wines due to browning, which is caused by the oxidation of phenols to quinines, which in turn polymerise to form macromolecules with typical yellow-brown colour (Recamales et al. 2006). Browning of white wines usually starts at the early stages of winemaking by enzymatic oxidation reactions (Cheynier et al. 1990); and after fermentation, polyphenoloxidaze activity decreases and oxidative browning is related to polyphenol chemical oxidation (Singleton 1987). We suppose that the above changes could affect the content of phenolic compounds in our wine samples, on the other hand wine variety and different winemaking practises play an important role in composition of wines. Slight increase of TPC was reached in samples B5, B5_2 and B5_3, which can be caused by addition of SO2. Sulphur dioxide is used for presentation of oxidation reactions (Makhotkina and Kilmartin 2010). After desk filtration, decrease of TPC was observed (B5_2), but opposite trend was observed after cross-flow filtration (B5_3). Next small drop was due to another addition of bentonite (B6). Rapid increase of amount of phenolic compounds was gained in sample B7, which can be results of wine maturation, but we do not explain this change, because the study did not repeat. One of the reasons of increase could be, that our white wine is a mixture of three different grape varieties (Cuvée) and therefore the changes of composition of this wine can be different in comparing with individual grape variety. This comparison could be subject of another study.
Based on our results, we can conclude, that the amount of phenolic compounds varies depending on kind of wine and production process. Here obtained results are in a good agreement with work of Burns et al. (2001).
Total antioxidant activity
It is known, that wines are rich source of polyphenols (flavonoids and non-flavonoids) and many of these compounds (e.g. resveratrol, quercetin, rutin, catechin and their oligomers and proanthocyanidins) have multiple biological activities that are mainly attributed to their antioxidant and antiradical activity (Baroni et al. 2012). The polyphenolic profile of red wines differs basically from that of white wines by effect of differences in composition among red and white grapes, and also in the vinification technology used (Alén-Ruiz et al. 2009). Figure 4 demonstrates resulting values of TAA for red, rose and white wine during production process with relevant relative standard deviations (RSD, %), which were obtained from PLS models on the basis of their infrared spectra. Values of RSD were in range 0.0–10.3 %. The results show, that red wine had marked higher values of TAA than rose and white wine. Similar results were reported in work of Paixão et al. (2007).
Fig. 4.
Total antioxidant activity of all kinds of wines during winemaking process by FT- IR spectroscopy (C1-C7 Red wine; R1-R7 Rose wine; B1-B7 White wine) Value after ± is relative standard deviation RSD (%) calculated for each wine samples
Similarly to the total phenolic content, changes of total antioxidant activity were monitored during production process. Value of TAA increased during production process of red wine (from 7.8 to 10.9 mmol L−1 AAE), but opposite trend was observed in rose (from 3.6 to 2.3 mmol L−1 AAE) and white wine (from 3.2 to 1.9 mmol L−1 AAE). For red wine, marked increase of TAA was monitored during maceration and primary fermentation stage, slight decrease followed during fermentation and aging in oak wooden barrels, next rise of value of TAA was gained for the last wine sample (C7). The gradual decrease of TAA was observed in rose and white wine through whole wine-making process. The comparison of values of TPC and TAA shows that they have a very similar trend in terms of changes throughout the wine-making process. Rose wine sample (R6) was the only exception, when value of TAA was double, but content of phenolic compounds did not show this increase. The influence of sulphur dioxide, which is being added during wine-making process, could be responsible for rapid increase of TAA. Lachman et al. (Lachman et al. 2007) also described the influence of sulphur dioxide on total antioxidant status. In this case timing of sampling will be important, whether the sample should be taken just after addition of SO2 or in another time interval.
The antioxidant activity of a wine is due to polyphenolic compounds (Zafrilla et al. 2003). However, in the literature contrastant and confused data occur about the correlation between the antioxidant activity and the polyphenolic contents of wine (Di Majo et al. 2008). In agreement with few authors (Ghiselli et al. 1998) a linear correlation exists between antioxidant activity and total phenolic compounds; others claim that the statistic correlation is relevant between total polyphenols and just the flavonoids fraction (Katalinić et al. 2004); finaly Arnous et al. (2002) maintain that the anti-radicalic activity is due to the flavan-3-ols fraction and not due to anthocyanins. However, few researches have carried out experiments regarding the relationships among the polyphenolic compounds concentration, total antioxidant capacity of wine and the viticulture practices or effect of wine storage in a bottle (Coletta et al. 2014; Kallithraka et al. 2009). In this study, correlation analysis was performed among values of TPC and TAA. There was statistically significant correlation in red wines (R = 0.79), and weak correlations in rose (R = 0.26) and white (R = 0.40) wines were found. It is evident, that in our case antioxidant activity varies depending on the content of phenolic compounds, as Fernandéz-Pachón et al. (2006), in their study, confirmed.
Conclusions
Study of three kinds of wines in different stages of wine-making process using infrared spectroscopy was performed. PLS models were build on the basis of their infrared spectra, which processed using different pre-treatments (raw, first and second derivate). Total phenolic compounds and total antioxidant activity were evaluated based on the best calibration models.
In summary, this study verified that red wines have higher value of TPC than rose and white ones and the same results were obtained for antioxidant activity. Further, it was found out that the content of phenolic compounds and antioxidant activity differs at each processing stage of the wine-making process. The content of phenolic compounds of red wine and white wine increased while an opposite trend was observed in rose wine. Total antioxidant activity values showed the difference between individual kinds of wine and indicated the same trend like phenolic profile. Positive correlation among total phenolic content and total antioxidant activity was observed.
Overall, infra-red spectroscopy together with statistical analysis is a suitable alternative analytical method for the determination of wine parameters, such as TPC and TAA. Consequently monitoring of changes in phenolic compounds could be important not only for monitoring of wine composition, but also for revelation of undesirable changes during wine-making process, such as polymerization reactions in red wines or browning in white wine.
Electronic supplementary material
(PDF 237 kb)
Acknowledgments
This work was supported by the student project PRF_2014_031 of Palacky University and Operation Program Research and Development for Innovations – European Social Fund (project CZ.1.05./2.1.00/03.0058) of Ministry of Education, Youth and Sports of the Czech Republic.
References
- Alén-Ruiz F, García-Falcón MS, Pérez-Lamela MC, Martínez-Carballo E, Simal-Gándara J. Influence of major polyphenols on antioxidant aktivity in Mencía and Brancellao red wines. Food Chem. 2009;113:53–60. doi: 10.1016/j.foodchem.2008.07.038. [DOI] [Google Scholar]
- Arnous A, Makris DP, Kefalas P. Correlation of pigment and flavanol content wine antioxidant properties in selected aged regional wines from Greece. J Food Compos Anal. 2002;15:655–665. doi: 10.1006/jfca.2002.1070. [DOI] [Google Scholar]
- Bai B, He F, Yang L, Chen F, Reeves MJ, Li J. Comparative study of phenolic compounds in Cabernet Sauvignon wines made in traditional and Ganimede fermenters. Food Chem. 2013;141:3984–3992. doi: 10.1016/j.foodchem.2013.06.074. [DOI] [PubMed] [Google Scholar]
- Baroni MV, Di Paola Naranjo RD, García-Ferreyra C, Otaiza S, Wunderlin DA. How good antioxidant is the red wine? Comparison of some in vitro and in vivo methods to assess the antioxidant capacity of Argentinean red wines. LWT Food Sci Technol. 2012;47:1–7. doi: 10.1016/j.lwt.2012.01.015. [DOI] [Google Scholar]
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Bevin CHJ, Fergusson AJ, Perry WB, Janik LJ, Cozzolino D. Development of a rapid “fingerprinting” system for wine authenticity by Mid-infrared spectroscopy. J Agric Food Chem. 2006;54:9713–9718. doi: 10.1021/jf062265o. [DOI] [PubMed] [Google Scholar]
- Blanco-Vega D, Goméz-Alonso S, Hermosín-Gutiérrez I. Identification, content and distribution of anthocyanins and low molecular weight anthocyanin-derived pigments in Spanish commercial red wines. Food Chem. 2014;158:449–458. doi: 10.1016/j.foodchem.2014.02.154. [DOI] [PubMed] [Google Scholar]
- Boissier B, Lutin F, Moutounet M, Vernhet A. Particles deposition during the cross-flow microfiltration of red wines—incidence of the hydrodynamic conditions and of the yeast to fines ratio. Chem Eng Process. 2008;47:276–286. doi: 10.1016/j.cep.2007.01.027. [DOI] [Google Scholar]
- Bravo MN, Silva S, Coelho AV, Vilas Boas L, Bronze MR. Analysis of phenolic compounds in Muscatel wines produced in Portugal. Anal Chim Acta. 2006;563:84–92. doi: 10.1016/j.aca.2005.11.054. [DOI] [Google Scholar]
- Burns J, Gardner PT, Matthews D, Duthie GG, Lean MEJ, Crozier A. Extraction of phenolic and changes in antioxidant activity of red wines during vinification. J Agric Food Chem. 2001;49:5797–5808. doi: 10.1021/jf010682p. [DOI] [PubMed] [Google Scholar]
- Cheynier V, Rigaud J, Souquet JM, Barillére JM, Moutounet M. Must browning in relation to the behaviour of phenolic compounds during oxidation. Am J Enol Vitic. 1990;41:346–349. [Google Scholar]
- Christian GD, Dasgupta PK, Schug KA. Analytical chemistry. Danvers: Wiley; 2014. [Google Scholar]
- Coletta A, Berto S, Crupi P, Cravero MC, Tamborra P, Antonacci D, Daniele PG, Prenesti E. Effect of viticulture practices on concentration of polyphenolic compounds and total antioxidant capacity of Southern Italy red wines. Food Chem. 2014;152:467–474. doi: 10.1016/j.foodchem.2013.11.142. [DOI] [PubMed] [Google Scholar]
- Cozzolino D, Kwiatkowski MJ, Parker M, Cynkar WU, Dambergs RG, Gishen M, Herderich MJ. Prediction of phenolic compounds in red wine fermentations by visible and near infrared spectroscopy. Anal Chim Acta. 2004;513:73–80. doi: 10.1016/j.aca.2003.08.066. [DOI] [Google Scholar]
- Cuadrado MU, Leque de Castro MD, Pérez Juan PM, Gómez-Nieto MA. Comparison and joint use of near infrared spectroscopy and Fourier transform mid infrared spectroscopy for the determination of wine parameters. Talanta. 2005;66:218–224. doi: 10.1016/j.talanta.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Darias-Martín JJ, Rodríguez O, Díaz E, Lamuela-Raventós RM. Effect of skin contact on the antioxidant phenolics in white wine. Food Chem. 2000;71:483–487. doi: 10.1016/S0308-8146(00)00177-1. [DOI] [Google Scholar]
- Di Egidio V, Sinelli N, Giovanelli G, Mooles A, Casiraghi E. NIR and MIR spectroscopy as rapid methods to monitor red wine fermentation. Eur Food Res Technol. 2010;230:947–955. doi: 10.1007/s00217-010-1227-5. [DOI] [Google Scholar]
- Di Majo D, La Guardia M, Giammanco S, La Neve L, Giammanco M. The antioxidant capacity of red wine in relationship with its polyphenolic constituents. Food Chem. 2008;111:45–49. doi: 10.1016/j.foodchem.2008.03.037. [DOI] [Google Scholar]
- Edelmann A, Diewok J, Schuster KC, Lendl B. Rapid method for the discrimination of red cultivars based on mid-infrared spectroscopy of phenolic wine extracts. J Agric Food Chem. 2001;49:1139–1145. doi: 10.1021/jf001196p. [DOI] [PubMed] [Google Scholar]
- Fernández K, Agosin E. Quantitative analysis of red wine tannins using Fourier-transform mid-infrared spectrometry. J Agric Food Chem. 2007;55:7294–7300. doi: 10.1021/jf071193d. [DOI] [PubMed] [Google Scholar]
- Fernández-Pachón MS, Villaño D, García-Parrilla MC, Troncoso AM. Antioxidant activity of wines and relation with their polyphenolic composition. Anal Chim Acta. 2004;513:113–118. doi: 10.1016/j.aca.2004.02.028. [DOI] [Google Scholar]
- Fernandéz-Pachón MS, Villaño D, Troncoso AM, García-Parrilla MC. Determination of the phenolic composition of sherry and table white wines by liquid chromatography and their relation with antioxidant activity. Anal Chim Acta. 2006;563:101–108. doi: 10.1016/j.aca.2005.09.057. [DOI] [Google Scholar]
- Fragoso S, Aceña L, Guasch J, Busto O, Mestres M (2011) Application of FT-MIR spectroscopy for fast control of red grape phenolic ripening. J Agric Food Chem 59:2175–2183 [DOI] [PubMed]
- Frankel EN, Waterhouse AL, Teissedre PL. Principal phenolic phytochemicals in selected California wines and their antioxidant activity in inhibiting oxidation of human low-density lipoproteins. J Agric Food Chem. 1995;43:890–894. doi: 10.1021/jf00052a008. [DOI] [Google Scholar]
- Friedrich DM, Hulse CA, von Gunten M, Williamson EP, Pederson CG, O’Brien NA (2014) Miniature near-infrared spectrometer for point-of-use chemical analysis. In Soskind YG, Olson C (eds) Photonic instrumentation engineering, SPIE-INT SOC OPTICAL ENGINEERING, Bellingham. doi:10.1117/12.2040669
- Ghiselli A, Nardini M, Baldi A, Scaccini C. Antioxidant activity of different phenolic fractions separated from an Italian red wine. J Agric Food Chem. 1998;46(2):361–367. doi: 10.1021/jf970486b. [DOI] [PubMed] [Google Scholar]
- Ginjom IR, D’Arcy BR, Caffin NA, Gidley M. Phenolic contents and antioxidant activities of major Australian red wines throughout the winemaking process. J Agric Food Chem. 2010;58:10133–10142. doi: 10.1021/jf100822n. [DOI] [PubMed] [Google Scholar]
- Ginjom I, D’Arcy B, Caffin N, Gidley M. Phenolic compound profiles in selected Queensland red wines at all stages of the wine-making process. Food Chem. 2011;125:823–834. doi: 10.1016/j.foodchem.2010.08.062. [DOI] [Google Scholar]
- Gishen M, Cozzolino D, Dambergs RG. The analysis of grapes, wine and other alcoholic beverages by Infrared spekctroscopy. In: Li-Chan E, Griffiths PR, Chalmers JM, editors. Applications of vibrational spectroscopy in food science. Chichester: John Wiley and Sons, Ltd.; 2010. pp. 539–556. [Google Scholar]
- Goméz-Cordovés C, González-SanJosé ML. Interpretation of colour variables during the aging of red wines: relationship with families of phenolic compounds. J Agric Food Chem. 1995;43(3):557–561. doi: 10.1021/jf00051a001. [DOI] [Google Scholar]
- Gonzáles-Caballero V, Sánchez MT, Fernández-Novales J, López MI, Pérez-Marín D. On-vine monitoring of grape ripening using near-infrared spectroscopy. Food Anal Methods. 2012;5:1377–1385. doi: 10.1007/s12161-012-9389-3. [DOI] [Google Scholar]
- Harvey D. Modern analytical chemistry. Boston: Mc Graff Hill; 2000. [Google Scholar]
- Hernanz D, Recamales AF, González-Miret ML, Gómez-Míguez MJ, Vicario IM, Heredia FJ. Phenolic composition of white wines with a prefermentative maceration at experimental and industrial scale. J Food Eng. 2007;80:327–335. doi: 10.1016/j.jfoodeng.2006.06.006. [DOI] [Google Scholar]
- Jackson RS. Wine science principles and applications. London: Academic; 2008. [Google Scholar]
- Kallithraka S, Salacha MI, Tzourou I. Changes in phenolic composition and antioxidant activity of white wine during bottle storage: Accelerated browning test versus bottle storage. Food Chem. 2009;113:500–505. doi: 10.1016/j.foodchem.2008.07.083. [DOI] [Google Scholar]
- Katalinić V, Milos M, Modun D, Musíć I, Boban M. Antioxidant effectiveness of selected wines in comparison with (+)-catechin. Food Chem. 2004;86:593–600. doi: 10.1016/j.foodchem.2003.10.007. [DOI] [Google Scholar]
- Lachman J, Šulc M, Schilla M. Comparison of the total antioxidant status of Bohemian wines during the wine-making process. Food Chem. 2007;103:802–807. doi: 10.1016/j.foodchem.2006.09.024. [DOI] [Google Scholar]
- Lomolino G, Zocca F, Spettoli P, Zanin G, Lante A. A preliminary study in changes on phenolic content during Bianchetta Trevigiana winemaking. J Food Compos Anal. 2010;23:575–579. doi: 10.1016/j.jfca.2010.04.001. [DOI] [Google Scholar]
- Makhotkina O, Kilmartin PA. The use of cyclic voltammetry for wine analysis: determination of polyphenols and free sulfur dioxide. Anal Chim Acta. 2010;668:155–165. doi: 10.1016/j.aca.2010.03.064. [DOI] [PubMed] [Google Scholar]
- Moreira JL, Santos L. Spectroscopic interferences in Fourier transform infrared wine analysis. Anal Chim Acta. 2004;513:263–268. doi: 10.1016/j.aca.2003.09.029. [DOI] [Google Scholar]
- Oliveira CM, Ferreira ACS, De Freitas V, Silva AMS. Oxidation mechanisms occurring in wines. Food Res Int. 2011;44:1115–1126. doi: 10.1016/j.foodres.2011.03.050. [DOI] [Google Scholar]
- Paixão N, Perestrelo R, Marques JC, Câmara JS. Relationship between antioxidant capacity and total phenolic content of red, rosé and white wines. Food Chem. 2007;105:204–214. doi: 10.1016/j.foodchem.2007.04.017. [DOI] [Google Scholar]
- Patz CD, Blieke A, Ristow R, Dietrich H. Application of FT-MIR spektrometry in wine analysis. Anal Chim Acta. 2004;513:81–89. doi: 10.1016/j.aca.2004.02.051. [DOI] [Google Scholar]
- Peréz-Marín D, Sánchez MT, Paz P, Soriano A, Guerrero JE, Garrido-Varo A. Non-destructive determination of quality parameters in nectarines during on-tree ripening and post-harvest storage. Postharvest Biol Technol. 2009;52:180–188. doi: 10.1016/j.postharvbio.2008.10.005. [DOI] [Google Scholar]
- Pérez-Marín D, Paz P, Guerrero JE, Garrido-Varo A, Sánchez MT. Miniature handheld NIR sensor for the on-site non-destructive assessment of post-harvest quality and refrigerated storage behavior in plums. J Food Eng. 2010;99:294–302. doi: 10.1016/j.jfoodeng.2010.03.002. [DOI] [Google Scholar]
- Plans M, Simó J, Casañas F, Sabaté J, Rodriguez-Saona L. Characterization of common beans (Phaseolus vulgaris L.) by infrared spectroscopy: comparison of MIR, FT-NIR and dispersive NIR using portable and benchtop instruments. Food Res Int. 2013;54:1643–1651. doi: 10.1016/j.foodres.2013.09.003. [DOI] [Google Scholar]
- Recamales AF, Sayago A, González-Miret ML, Hernanz D. The effect of time and storage conditions on the phenolic composition and colour of white wine. Food Res Int. 2006;39:220–229. doi: 10.1016/j.foodres.2005.07.009. [DOI] [Google Scholar]
- Regmi U, Palma M, Barroso CG. Direct determination of organic acids in wine and wine-derived products by Fourier transform infrared (FT-IR) spectroscopy and chemometric techniques. Anal Chim Acta. 2012;732:137–144. doi: 10.1016/j.aca.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Ribéreaou-Gayon P, Glories Y, Maujean A, Dubourdieu D. Handbook of enology. The chemistry of wine stabilization and treatments. Chichester: John Wiley & Sons, Ltd.; 2006. [Google Scholar]
- Rodríguez-Delgado MA, González-Hernández G, Conde-González JE, Pérez-Trujill JP. Principal component analysis of the polyphenol content in young red wines. Food Chem. 2002;78:523–532. doi: 10.1016/S0308-8146(02)00206-6. [DOI] [Google Scholar]
- Romera-Fernández M, Berrueta LA, Garmón-Lobato S, Gallo B, Vicente F, Moreda JM. Feasibility study of FT-MIR spectroscopy and PLS-R for the fast determination of anthocyanins in wine. Talanta. 2012;88:303–310. doi: 10.1016/j.talanta.2011.10.045. [DOI] [PubMed] [Google Scholar]
- Sánchez MT, De La Haba MJ, Guerrero JE, Garrido-Varo A, Pérez-Marín D. Testing of a local approach for the prediction of quality parameters in intact nectarines using a portable NIRS instrument. Postharvest Biol Technol. 2011;60:130–135. doi: 10.1016/j.postharvbio.2010.12.006. [DOI] [Google Scholar]
- Sato M, Ramarathnam N, Suzuki Y, Ohkubo T, Takeuchi M, Ochi H. Varietal differences in the phenolic content and superoxide radical scavenging potential of wines from different sources. J Agric Food Chem. 1996;44(1):37–41. doi: 10.1021/jf950190a. [DOI] [Google Scholar]
- Sauvage S-X, Bach B, Moutounet M, Vernhet A. Proteins in white wines: thermo-sensitivity and differential adsorbtion by bentonite. Food Chem. 2010;118:26–34. doi: 10.1016/j.foodchem.2009.02.080. [DOI] [Google Scholar]
- Šeruga M, Novak I, Jakobek L. Determination of polyphenols content and antioxidant activity of some red winesby differential pulse voltammetry, HPLC and spectrophotometric methods. Food Chem. 2011;124:1208–1216. doi: 10.1016/j.foodchem.2010.07.047. [DOI] [Google Scholar]
- Shen F, Ying Y, Li B, Zheng Y, Hu J. Prediction of sugars and acids in Chinese rice wine by mid-infrared spectroscopy. Food Res Int. 2011;44:1521–1527. doi: 10.1016/j.foodres.2011.03.058. [DOI] [Google Scholar]
- Silva SD, Feliciano RP, Boas LV, Bronze MR. Application of FTIR-ATR to Moscatel dessert wines for prediction of total phenolic and flavonoid contents and antioxidant capacity. Food Chem. 2014;150:489–493. doi: 10.1016/j.foodchem.2013.11.028. [DOI] [PubMed] [Google Scholar]
- Singleton VL. Oxygen with phenols and related reactions in musts, wines, and model systems: observations and practical implications. Am J Enol Vitic. 1987;38:69–77. [Google Scholar]
- Soriano A, Pérez-Juan PM, Vicario A, González JM, Pérez-Coello MS. Determination of anthocyanins in red wine using a newly developed method based on Fourier transform infrared spectroscopy. Food Chem. 2007;104:1295–1303. doi: 10.1016/j.foodchem.2006.10.011. [DOI] [Google Scholar]
- Tarantilis PA, Troianou VE, Pappas CS, Kotseridis YS, Polissiou MG. Differentiation of Greek red wines on the basis of grape variety using attenuated total reflectance Fourier transforms infrared spectroscopy. Food Chem. 2008;111:192–196. doi: 10.1016/j.foodchem.2008.03.020. [DOI] [Google Scholar]
- Tiwari G, Slaughter DC, Cantwel M. Nondestructive maturity determination in green tomatoes using a handheld visible and near infrared instrument. Postharvest Biol Technol. 2013;86:221–229. doi: 10.1016/j.postharvbio.2013.07.009. [DOI] [Google Scholar]
- Vernhet A, Moutounet M. Fouling of organic microfiltration membranes by wine constituents: importance, relative impact of wine polysaccharides and polyphenols and incidence of membrane properties. J Membr Sci. 2002;201(1–2):103–122. doi: 10.1016/S0376-7388(01)00723-2. [DOI] [Google Scholar]
- Versari A, Parpinello GP, Mattioli AU, Galassi S. Determination of grape quality at harvest using Fourier-transform mid-infrared spectroscopy and multivariate analysis. Am J Enol Vitic. 2008;59:317–322. [Google Scholar]
- Versari A, Parpinello GP, Scazzina F, Del Rio D. Prediction of total antioxidant capacity of red wine by Fourier transform infrared spectroscopy. Food Control. 2010;21:786–789. doi: 10.1016/j.foodcont.2009.11.001. [DOI] [Google Scholar]
- Versari A, Laurie VF, Ricci A, Laghi L, Parpinello GP. Progress in authentication, typification and traceability of grapes and wines by chemometric approaches. Food Res Int. 2014;60:2–18. doi: 10.1016/j.foodres.2014.02.007. [DOI] [Google Scholar]
- Villaño D, Fernández-Pachón MS, Troncoso AM, García-Parrilla MC. Influence of enological practices on the antioxidant activity of wines. Food Chem. 2006;95:394–404. doi: 10.1016/j.foodchem.2005.01.005. [DOI] [Google Scholar]
- Waterhouse AI. Current protocols in food analytical chemistry. Chichester: Wiley; 2002. Determination of total phenolics; pp. I1.1.1–I1.1.8. [Google Scholar]
- Wold S, Sjöström M, Eriksson L. PLS-regression: a basic tool of chemometrics. Chemom Intell Lab Syst. 2001;58:109–130. doi: 10.1016/S0169-7439(01)00155-1. [DOI] [Google Scholar]
- Woraratphoka J, Intarapichet KO, Indrapichate K. Phenolic compounds and antioxidative properties of selected wines from the northeast of Thailand. Food Chem. 2007;104:1485–1490. doi: 10.1016/j.foodchem.2007.02.020. [DOI] [Google Scholar]
- Zafrilla P, Morillas J, Mulero J, Cayuela JM, Martínez-Cachá A, Pardo F, López Nicolás JM. Changes during storage in conventional and ecological wine: phenolic content and antioxidant activity. J Agric Food Chem. 2003;51:4694–4700. doi: 10.1021/jf021251p. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 237 kb)