Abstract
The characteristics on physicochemical and morphological properties of starches were investigated in fresh waxy corn kernels. Starches were isolated from eight waxy corn genotypes at the immature kernel stage growing in Thailand. The starch content showed variation with genotypes and ranged from 77.76 to 90.97 %. Granule size distribution showed a two population of starch granules with peak values ranged from 0.8 to 1.1 μm (small) and 9.0 to 12.2 μm (large). Genotypes were also significantly different for average chain length (CL), unit chain length distribution and pasting properties. The small granule (<5 μm) was negatively correlated with CL and degree of polymerization (DP) 25–36 of amylopectin (−0.82 and −0.67, respectively, P < 0.01). And a strong relationship between trough and final viscosity was consistent with the contribution of pasting properties.
Keywords: Microstructure, Granule size, Viscosity, Amylopectin distribution
Introduction
Waxy or glutinous corn (Zea mays L. var. ceratina) is traditional vegetable, consumed similar to sweet corn as cooked green ears. This corn has been developed and cultivated in many Asian countries, including, some parts of China, Japan, Vietnam, Lao, Myanmar, Korea, Taiwan and Thailand (Lertrat and Thongnarin 2008). Waxy corn has similar properties to sticky rice because its endosperm starch consists of mainly amylopectin. Small-scale farmers in Thailand and Southeast Asia have grown waxy corn as a cash crop next to rice for fresh consumption for more than a century. A good eating quality is the main goal for waxy corn breeding program (Lertrat and Thongnarin 2008).
Breeding for fresh kernel has become economically beneficial because the development of niche markets for specialty ear. In addition, it has been increasing numbers of vertically integrated vegetable corn systems can capture the added value in an improved quality food for health benefit. The market share of waxy corn in the seed industry has increased. Seed companies in south-east Asia have shown interests in improving quality of waxy corn and develop new varieties. An objective breeding is to produce starch with novel properties for making new products. New varieties of waxy corn have been developed with improved consistency, taste and shelf life, which affected by the physicochemical properties of starch. Numerous researches exhibited the basic component of starch can affect the textural properties of several food crops such rice (Kang et al. 2006), potato (Sing et al. 2005) and sorghum (Cagampang and Kirleis 1984).
Starch is the main energy storage of plants. The chemical compositions of starch in corn kernels are amylose and amylopectin, and some intermediate materials that retain characteristics of both fractions. Amylose is linear or slightly branched molecules whereas amylopectin is highly branched molecules (Jane et al. 1999). These two polymers have different physical properties. Therefore, lots efforts have be interested to improve novel starch for unique properties by changing the proportions of the two molecules. Functional properties of starch are affected by the amylose content and branched chain length distribution of amylopectin (Hanashiro et al. 1996; Jane et al. 1999). The physicochemical properties of waxy corn starches from mature seeds have been reported (Fiedorowicz and Rebilas 2002; Jane et al. 1999; Jiranuntakul et al. 2011; Singh et al. 2003), but the properties of kernel starches at immature stage are less understood. Including, the variation of varieties are affected to physicochemical properties of waxy corn starch. The immature ears are consumed during kernel development about 16 to 22 days after pollination (DAP), which depend on genetics and environment. Genetic information in the physicochemical properties is important for breeders to select breeding lines that starch characteristics to relate the quality and also for market to select the quality foods with desired properties for consumer. The objectives of this study were to characterization on the physicochemical properties of waxy corn starch at the immaturity stage and study the correlation among physicochemical traits. This information will be useful for developing practical strategies to improve waxy corn for good-eating quality in the future and also this will be useful in selecting the appropriate variety for end use suitability.
Materials and methods
Plant materials
Eight genotypes of waxy corn were used in this study including, KKU-BK, KKU-JP, KKU-UB, KKU-JD, KKU-G2 and KKU-N7 and the two check commercial F1-hybrid varieties (“BigW” and “Violet”). The inbred lines were extracted from a local population with high adaptability, early maturity as well as high and low performance in traits related to eating quality of cooked green ears (Ketthaisong et al. 2014). The breeding lines were selected by Plant Breeding Research Center for Sustainable Agriculture, Khon Kaen University, Thailand. The seeds were grown at Khon Kaen University, Khon Kaen Province, Thailand during November 2011 to January 2012. Standard agronomic practices were used to provide adequate nutrition and kept the plots disease-free. For each variety, fifteen sib-pollinated ears were harvested 16 to19 days after pollination (DAP) and stored at −20 °C in plastic bags until analysis.
Isolation of starches
Waxy corn starches were isolated using a method of Jiranuntakul et al. (2011) with slight modifications. Corn kernels, 400 g for each sample, were steeped in water containing 0.16 % sodium hydrogen sulfite at 45 °C for 12 h to inactivate the enzymes. The grains were ground in a blender and then screened through a 250, 106 and 63 μm sieves, respectively. The cloudy supernatant was removed, and the sediment was re-slurried in 0.2 % sodium hydroxide solution. The supernatant was discarded, and the starch cake was re-suspended in water for five times. The starch slurry was then passed through a 63 μm sieve. The cake was given repeated washings with water until the pH of starch slurry reached seven. The starch cake was then dried in an oven at 40 °C for 24 h.
Amylose and starch contents
The amylose content was determined using the iodine adsorption method of Hoover and Ratnayake (2001), and starch content was obtained using the Total Starch Kit (Megazyme, Co, Wicklow, Ireland).
Starch granule morphology
Starch powders were fixed onto a circular specimen stub with double-side tape, coated with vacuum-coated with a mixture of 60 % gold and 40 % palladium particles then observed using a scanning electron microscope (SEM, JEOL, JSM-5300LV, Japan) with an accelerating voltage of 10 kV.
Particle size analysis
Starch granules were suspended in water, and then the particle size distribution was measured with a laser particle size analyzer Model Master sizer S long bed Ver.2.11.
Unit chain distribution of amylopectin
Unit chain distribution of amylopectin was determined following the procedure described by Jiranuntakul et al. (2012) with slight modifications. Starch 28 mg (dry basis, db) was dispersed in 2.8 mL of distilled water and heated in boiling water until the sample was completely dissolved. Distilled water (3.85 mL) and 0.35 mL of 10 mM acetate buffer (pH 3.5) were added, then mixed with a vortex mixer and incubated at 45 °C for 10 min. A solution of isoamylase (0.03 unit/mg of substrate) from Pseudomonas sp. (1000 U/ml, EC 3.2.1.68, Lot 90701b, Megazyme) was added to sample. Samples were debranched for 12 h at 45 °C in a water bath. After debranching, the enzyme activity was stopped by heating in boiling water for 5 min. The debranched amylopectin solutions were dried using a freeze dryer at −80 °C and kept in a sealed plastic container until analyzed. The unit chain distribution of debranched amylopectin was determined using the High Performance Size Exclusion Chromatograph (HPSEC). The HPSEC system included of a pump (Water 1525; Millipore Waters, USA), an injector, two 6.2 × 250 mm columns connected in series (Zorbax PSM 60S; Agilent Technologies, Santa Clara, CA), packed with 5 mm porous silica microspheres, and a Refractive Index Detector (RI, RID-2414; Waters). A calibration curve for each chromatogram was constructed using maltoheptaose, pullulan 6000 and pullulan 12,000 standards. The data were analyzed by using Breeze two software (Waters, USA), and each sample was analyzed in duplicate. The y-axis of the chromatogram was converted from the RI value to a molar response using the calibration curve.
Pasting properties
The pasting properties of waxy corn starches were measured with a Rapid Visco-Analyzer (RVA-4 Series, Newport Scientific Pvt Ltd, Warriewood, NSW, Australia). The starch slurry at concentration 6 % (w/w, db) were determined, at a fixed a paddle rotation speed of 160 rpm. Each sample was heated from 50 to 95 °C at 12 °C/min, maintained at 95 °C for 2.5 min, and then cooled to 50 °C at the same rate, and finally held at 50 °C for 2 min.
Statistical analysis
The data of starch characteristics and properties were subjected to analysis of variance according to a Randomized Complete Block Design (RCBD) with three replications, and expressed as mean values for standard deviations. The Least Significant Difference (LSD) was used to compare mean difference at P < 0.05. The relationships among traits were calculated by the Pearson’s correlation analysis using accession means (Gomez and Gomez 1984).
Results and discussion
Amylose and starch content
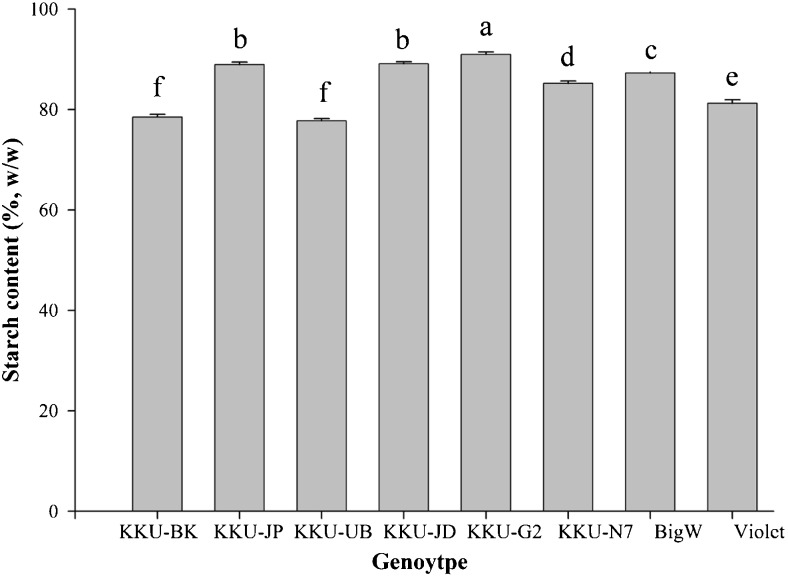
The amylose content of starch separated from different waxy corn genotypes at immature stage were not significantly different (data not shown). Amylose content was very low because every line exhibited inbreeding depression for a waxy gene. Inbreeding is the consequence of the mating between two related individuals. As an effect of selfing, recessive genes, earlier masked in the heterozygous forms, become homozygous (Hallauer et al. 2010). An amylose content of 0.30 % in mature waxy corn starches were reported by Fredriksson et al. (1998), 2.06 % (Jiranuntakul et al. 2011) and 1.00 % (Rocha et al. 2012). The starch content of different genotypes showed significant differences. The variation in starch content ranged from 77.76 to 90.97 %, the KKU-G2 line showed the highest value, whereas the lowest value was observed in KKU-BK line (Fig. 1). In addition, the starch content has been shown to vary among immature botanical sources, with 37 % from kidney bean (Yoshida et al. 2003), 13.70 % from canna (Puncha-arnon et al. 2007) and 74.50 % from yam (Huang et al. 2006). The accumulation of starch depends on developmental stage, organ of plants, types of corn and environment which can affected to physicochemical properties of starch (Li et al. 2007).
Fig. 1.
Starch contents (%, w/w) of eight waxy corn genotypes harvested at immature stage; 16 DAP (KKU-BK) and 19 DAP (KKU-JP, KKU-UB, KKU-JD, KKU-G2, KKU-N7, “BigW” and “Violet”)
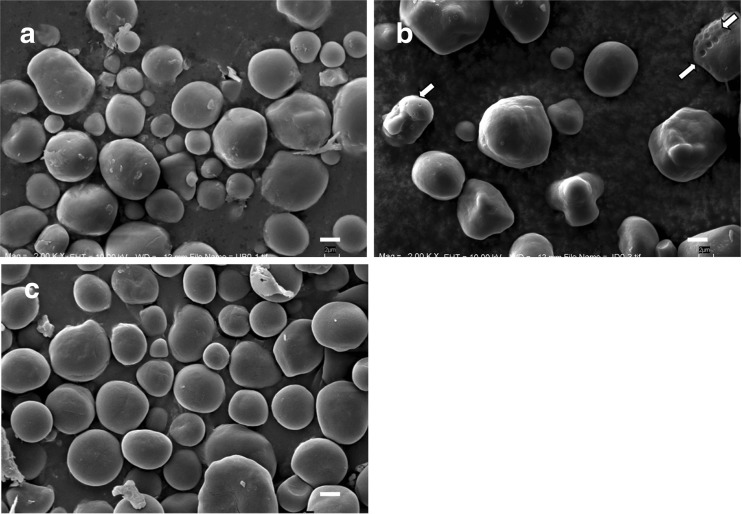
Starch granule morphology
The scanning electron micrographs (SEM) of starch granules isolated from immature kernel are shown in Fig. 2. The granule sizes ranged from 2 to 20 μm in diameter. Granules were found to be oval in shape and have smooth surfaces. The SEM images showed irregularly granule shape similar to the granules of immature sweet corn (Jane and Kasemsuwan 1994), but the surface has been smooth then. Starch from KKU-JD line showed few starch granules with pinholes on the surface at immature kernels (Fig. 2B), which the affects from endogenous enzyme to hydrolyzed starch (Li et al. 2007). The internal structure of corn starch granule is determined during the biosynthesis and development of starch granules by the expression of starch synthase activities during the maturation of kernels (Jane 2007). Whereas, the accumulations of amylose in normal corn kernels were increased during kernel development and correlated with a number of pinholes and activity of granule bound starch synthase I (GBSSI) (Li et al. 2007). The starch granules isolate from normal corn showed few or no pores on immature stage, but those from kernels harvested at fully matured showed a large number of pores (Li et al. 2007). The pinholes could be distributed to endogenous enzyme hydrolysis during kernel development and also are openings to channels for providing an approach to channels that provide an approach to the interior of granules via channel and the surface pores (Miao et al. 2011).
Fig. 2.
SEM of starch granules separated from waxy corn varieties at immature stages; a KKU-UB (2000x), b KKU-JD (2000x), and c “Violet” (2000x). Arrowheads: starch granule with pinholes on the surface and scale bar 2 μm
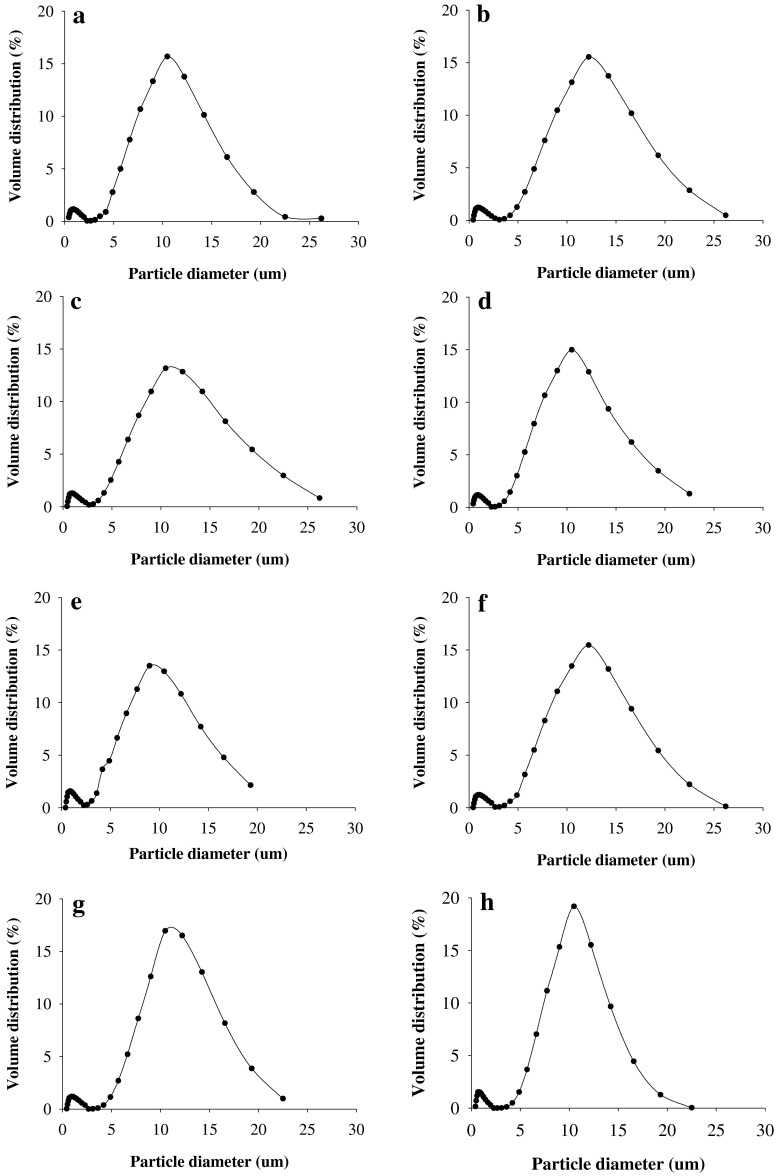
Granule size distribution
Starches from genotypes showed significant differences in distribution and also in the volume of the granules (Fig. 3). The granule sizes exhibited a bimodal distribution with peak values in the ranges of 0.8 to 1.1 μm (small) and 9.0 to 12.2 μm (large) for all starches. The limits between the two populations were described as the minimum of the curves that occurred at ~2.7 μm. The granule sizes were classified into large (>15 μm), medium (5–15 μm), and small (<5 μm) granules (Ketthaisong et al. 2013). Average diameter of large, medium, and small granules ranged between 16.6 to 26.2, 5.7 to 14.2 and 0.4 to 4.9 μm, respectively. Among all starches, medium-granules had a highest contribution to the total volumes (68.5 to 81.5 %), followed by small (11.4 to 21.6 %) and large (5.8 to 19.2 %) granules. “Violet” showed the largest proportion of medium-granules and lowest proportion of large-granules, while KKU-UB starch showed the smallest proportion of medium-granules with 67.3 % (Table 1). The variation in size distribution of starch granules may be due to the difference in genotypes, and it is an important factor that affects the starch quality (Li et al. 2008; Singh et al. 2010).
Fig. 3.
Granules size distribution (%) of starches from immature waxy corn genotypes. a) KKU-BK, b KKU-JP, c) KKU-UB, d KKU-JD, e) KKU-G2, f KKU-N7, g) “BigW” and h “Violet”
Table 1.
Volume distributions of immature waxy corn starch granules (%)
| Genotype | Particle diameter of immature starch granule (μm) | ||
|---|---|---|---|
| Large-granule (>15 μm) | Medium-granule (5–15 μm) | Small-granule (<5 μm) | |
| KKU-BK | 9.5 ± 0.6 b | 76.9 ± 0.7 b | 13.6 ± 0.1 c |
| KKU-JP | 19.2 ± 0.1 f | 68.5 ± 0.4 f | 12.3 ± 0.4 d |
| KKU-UB | 17.4 ± 0.0 g | 67.3 ± 0.0 g | 15.3 ± 0.0 b |
| KKU-JD | 11.1 ± 0.2 c | 74.1 ± 0.0 c | 14.7 ± 0.0 c |
| KKU-G2 | 7.2 ± 0.6 d | 71.2 ± 0.0 d | 21.6 ± 0.6 a |
| KKU-N7 | 17.2 ± 0.1 e | 70.3 ± 0.2 e | 12.4 ± 0.1 e |
| “BigW” | 12.6 ± 0.1 b | 76.7 ± 0.1 b | 11.4 ± 0.7 f |
| “Violet” | 5.8 ± 0.0 a | 81.5 ± 0.0 a | 12.7 ± 0.0 e |
Means with different letters (a, b, …) in the same column are significantly different (P < 0.05). Mean ± standard deviation
Unit chain distribution of debranched immature waxy corn starches
The chain-length distributions as well as the data listed in Table 2. The classification of amylopectin branched chain in the A, B1, B2, and B3 groups conform to chains with a DP of 6–12, 13–24, 25–36 and ≥37, respectively (Hanashiro et al. 1996). In the cluster model of amylopectin, A and B1 chains are located within a single cluster where they form double helices, and form one crystalline lamella, whereas B2 and B3+ chains extend through two or more clusters. Therefore, B2 and longer chains are present in both the crystalline and amorphous lamellae (Hizukuri 1986). Branch chain lengths of amylopectin from the immature starches were significantly different between genotypes for the amount of starch in each group. Average chain length (CL) of starches ranged between 15.2 and 19.4 for KKU-N7 and “BigW”, respectively. The increase of CL can affect the physicochemical properties of starches (Jane et al. 1999). In addition, the numerous reported of mature kernels showed CL with 23.5 (Jane et al. 1999), 20.5 (Wang and Wang 2003), and 16.6 (Jiranuntakul et al. 2012), which differences in stages of plants and also the method of determined.
Table 2.
Average chain length and unit chain distribution of debranched amylopectins from immature waxy corn starchesa
| Genotype | CL | Distribution (%) | |||
|---|---|---|---|---|---|
| DP 6–12 | DP 13–24 | DP 25–36 | DP ≥37 | ||
| KKU-BK | 17.3 ± 0.2 b | 73.3 ± 0.2 ab | 15.1 ± 0.0 cd | 9.6 ± 0.2 d | 2.1 ± 0.1 abc |
| KKU-JP | 18.1 ± 1.6 ab | 73.4 ± 1.2 ab | 15.2 ± 0.7 c | 10.0 ± 0.6 cd | 1.3 ± 0.0 c |
| KKU-UB | 18.3 ± 1.6 ab | 73.9 ± 1.0 ab | 13.8 ± 0.0 d | 10.1 ± 0.4 cd | 2.1 ± 0.6 abc |
| KKU-JD | 18.3 ± 1.5 ab | 73.2 ± 1.7 ab | 15.5 ± 0.5 c | 11.2 ± 0.2 bc | 1.8 ± 1.0 abc |
| KKU-N7 | 15.2 ± 0.1 c | 75.3 ± 0.3 a | 14.6 ± 0.7 cd | 8.3 ± 1.0 e | 1.8 ± 0.1 abc |
| KKU-G2 | 18.4 ± 0.5 ab | 72.3 ± 0.4 b | 15.1 ± 0.1 cd | 10.0 ± 0.1 cd | 2.6 ± 0.3 a |
| “BigW” | 19.4 ± 1.1 a | 67.9 ± 2.3 c | 18.0 ± 1.2 b | 11.7 ± 0.7 b | 2.4 ± 0.4 ab |
| “Violet” | 19.0 ± 1.0 ab | 65.5 ± 0.6 d | 19.9 ± 0.6 a | 13.0 ± 0.5 a | 1.6 ± 0.5 cd |
Means with different letters in the same column are significantly different (P < 0.05). Mean ± standard deviation
CL Average chain length, DP Degree of polymerization
aMolar basis
Eight genotypes exhibited a larger proportion of A chains than of B1, B2 and B3+. KKU-N7 line had the greatest proportions of short branch chain (DP 6–12), 75.3 % and the “Violet” showed smallest proportion, 65.5 %. In mature kernel starches the proportions of amylopectin branch-chains were reported to be DP of 13–24 and DP 25–36 which is lower than those of waxy corn starches (Jiranuntakul et al. 2012). The result suggested that KKU-UB line had 73.9 and 13.8 % of DP 6–12 and 13–24, respectively and KKU-JD line showed a smaller proportion of DP 6–12, but a larger proportion of DP 13–24 with 73.2 and 15.5 %, respectively, whereas, the F1-hybrid varieties showed a smaller proportion with DP 6–12 (65.5 to 67.9 %) than breeding lines (72.3 to 75.3 %). For comparison, waxy corn starch exhibited a small proportions of chains with DP 13–36 but larger proportions of chains with DP 6–12 and >36 (Mendez-Montealvoa et al. 2011). However, some waxy corn starches showed a proportion of debranched amylopectin with DP 13–24 greater than DP 5–12 for 43.3 and 35.2 %, respectively (Wang and Wang 2003). In addition, normal corn starches exhibited a large proportion of branch chains with DP 13–24, 6–12 and 25–36, respectively (Ai et al. 2011; Wang and Wang 2003).
Pasting properties of starches
The pasting properties of immature waxy corn starches showed significant difference between all genotypes. The peak viscosity exhibited a range of 128.9 to 163.9 RVU for KKU-N7 and KKU-JP lines, respectively (Table 3). Sandhu and Singh (2007) reported peak viscosity in the range between 67.0 and 104.5 RVU and break down viscosity range with 27.0 to 115.7 RVU for selected corn lines. Miles et al. (1985) reported that increase in final viscosity might be due to the aggregation of the amylose molecules. Pasting properties are dependent on the rigidity of starch granules. Setback viscosities for starch ranged from 5.0 RVU for KKU-G2 line to 17.0 RVU for KKU-N7 line. In addition, the breeding lines and F1–hybrid varieties exhibited high peak viscosity because it had high proportion of short branch-chains of amylopectin, which can affected to starch properties (Jane et al. 1999). Therefore, the waxy starch samples displayed a similar high peak viscosity, easy breakdown, and low setback viscosity (Jiranuntakul et al. 2011), whereas the rapid increase in viscosity at the early stage of pasting for the gelatinized waxy corn starch powders effected from the rapid hydration and swelling of the amorphous starch powders (Xing and Lim 2012). Pasting properties of starch have been reported to be affected by amylose and branch chain-length distribution of amylopectin. Also, the chain length of amylopectin and molecular size of amylose create synergistic effects on the viscosity of starch pastes (Jane and Chen 1992). This data may be used as criteria for evaluation of physicochemical properties of waxy corn starch between selection lines in breeding program.
Table 3.
Pasting properties of eight waxy corn starches separated from immature kernels
| Genotype | PV (RVU) | TV (RVU) | BV (RVU) | FV (RVU) | SV (RVU) | PT (°C) |
|---|---|---|---|---|---|---|
| KKU–BK | 145.5 ± 0.5 c | 77.0 ± 3.5 e | 68.4 ± 3.4 a | 87.4 ± 3.4 c | 10.8 ± 2.9 b | 77.4 ± 0.4 d |
| KKU–JP | 163.9 ± 1.6 a | 110.8 ± 0.9 a | 53.1 ± 2.4 cd | 123.3 ± 1.4 a | 12.4 ± 2.3 b | 80.1 ± 0.0 b |
| KKU–UB | 161.7 ± 1.6 b | 110.8 ± 1.0 a | 50.9 ± 2.7 d | 124.1 ± 0.5 a | 13.3 ± 1.2 b | 79.3 ± 0.0 c |
| KKU–JD | 136.9 ± 1.6 e | 69.2 ± 1.2 f | 67.8 ± 1.2 a | 76.5 ± 0.4 d | 7.4 ± 0.8 c | 80.7 ± 0.5 ab |
| KKU–G2 | 139.3 ± 0.6 d | 82.7 ± 0.3 d | 56.5 ± 0.3 bc | 87.8 ± 0.8 c | 5.0 ± 0.6 c | 81.0 ± 0.0 a |
| KKU–N7 | 128.9 ± 0.3 f | 106.3 ± 0.5 b | 22.6 ± 0.6 e | 123.3 ± 0.5 a | 17.0 ± 0.9 a | 80.1 ± 0.0 b |
| BigW | 161.0 ± 0.8 b | 103.1 ± 1.8 c | 57.9 ± 2.5 b | 116.5 ± 0.6 b | 13.4 ± 2.4 b | 77.9 ± 0.4 d |
| Violet | 140.2 ± 0.8 d | 71.3 ± 0.5 f | 68.9 ± 1.3 a | 78.4 ± 0.4 d | 7.1 ± 0.1 c | 80.1 ± 0.8 b |
Means with different letters in the same column are significantly different (P ≤ 0.05). Mean ± standard deviation
PV peak viscosity, TV trough viscosity, BV breakdown viscosity, FV final viscosity, SV setback viscosity and PT pasting temperature. RVU rapid visco unit
Correlation of traits
Estimation of simple correlation between various physicochemical characters may provide good information necessary for waxy corn breeders, when, selection is based on two or more traits simultaneously. However, breeding program, precise evaluation of eating quality in early generation is critical. Simple correlation coefficient can help the plant breeders to predict traits. Correlation coefficient is important in plant breeding, because it measures the degree of association, genetic and environment interaction, between two or more characters. If genetic association exists, selection for one trait will cause changes in other traits, basically a correlated response (Hallauer et al. 2010).
Possible correlation coefficients between 14 parameters in eight waxy corn starches at immature stage are summarized in Table 4. The granule size of <5 μm (small) was negatively correlated with CL and DP 25–36 (−0.82 and −0.67, respectively, P < 0.01), and positively correlated with DP 6–12 (0.63, P < 0.01), whereas the granule size 5–15 μm (medium) showed positively correlated with DP 13–24 and DP 25–36 (0.91 and 0.78, respectively, P < 0.01), but exhibited negatively correlated with granule size >15 μm (large) and DP 6–12 (−0.74 and −0.85, respectively, P < 0.01). A strong negative relationship between DP 6–12 to DP 13–24 and DP 25–36 (−0.96 and −0.96, P < 0.01, respectively) was consistent with the contribution of swelling towards pasting properties. Trough viscosity was positively correlated with final viscosity and setback viscosity (0.99 and 0.73, P < 0.01, respectively) and negatively correlated with breakdown viscosity (−0.69, P < 0.01), whereas final viscosity showed highly positive correlation with setback viscosity (0.83, P < 0.01). Similar positive correlation between final viscosity and peak viscosity, and negative correlation between setback viscosity and pasting temperature has been reported by Sandhu and Singh (2007). The structure of amylopectin has been reported to play an important role in the pasting properties of starches. An increase in molecular weight of amylopectin has been effete to decrease the amount of long-branch chain length as well as branching degree of amylopectin, which affected to an increase with peak viscosity and breakdown and decrease in setback and final viscosity (Takeda et al. 1989).
Table 4.
Simple correlation coefficients (r) between physicochemical properties of immature starches separated from different waxy corn genotypes
| Starch content | Smalla | Medium | Large | CL | DP 6–12 | DP 13–24 | DP 25–36 | DP ≥37 | PV | TV | BV | FV | SV | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Small | 0.15 | |||||||||||||
| Medium | 0.01 | −0.37 | ||||||||||||
| Large | −0.12 | −0.33 | −0.74 ** | |||||||||||
| CL | −0.28 | −0.82 ** | 0.40 | 0.18 | ||||||||||
| DP 6–12 | −0.11 | 0.63 ** | −0.85 ** | 0.41 * | −0.75 ** | |||||||||
| DP 13–24 | −0.12 | −0.49 * | 0.91 ** | −0.57 ** | 0.61 ** | −0.95 ** | ||||||||
| DP 25–36 | −0.22 | −0.67 ** | 0.78 ** | −0.32 | 0.75 ** | −0.87 ** | 0.86 ** | |||||||
| DP ≥37 | −0.43 | −0.22 | −0.19 | 0.36 | 0.19 | 0.06 | −0.09 | 0.02 | ||||||
| PV | −0.13 | −0.05 | −0.05 | 0.09 | −0.10 | 0.09 | −0.14 | 0.05 | 0.44 * | |||||
| TV | −0.00 | 0.18 | 0.10 | −0.22 | −0.12 | 0.01 | 0.10 | 0.05 | 0.07 | 0.52 ** | ||||
| BV | −0.11 | −0.25 | −0.15 | 0.33 | 0.05 | 0.07 | −0.23 | −0.01 | 0.29 | 0.26 | −0.69 ** | |||
| FV | −0.06 | 0.17 | 0.12 | −0.24 | −0.10 | −0.02 | 0.13 | 0.06 | 0.07 | 0.48 * | 0.99 ** | −0.71 ** | ||
| SV | −0.28 | 0.10 | 0.17 | −0.24 | 0.02 | −0.13 | 0.22 | 0.09 | 0.03 | 0.22 | 0.73 ** | −0.64 ** | 0.82 ** | |
| PT | 0.40 | −0.11 | 0.15 | −0.07 | 0.14 | −0.13 | 0.17 | 0.08 | −0.30 | −0.52 ** | −0.31 | −0.08 | −0.37 | −0.52 ** |
*, ** Significant at P ≤ 0.05 and 0.01 probability levels, respectively
CL average chain length, DP degree of polymerization, PV peak viscosity, TV trough viscosity, BV viscosity, FV final viscosity and SV setback viscosity
aSmall (<5 μm), medium (5–10 μm) and large (>5 μm) granules
Conclusions
Immature waxy corn starches from different genotypes showed variation on starch contents, granule morphology, granule sizes and chain length distribution of amylopectin. The amount of starch in the small granule population was negative correlation with CL and long branched of amylopectin. Which, the physicochemical properties trait is criteria of choice for evaluation of waxy corn lines in breeding program.
Acknowledgments
We thank The Royal Golden Jubilee Ph.D. Program (RGJ), Choncharean Farm, Chia Tai Ltd., The National Science and Technology Development Agency (NSTDA), Pathumthani, Thailand, The Plant Breeding Research Center for Sustainable Agriculture, Faculty of Agriculture, Khon Kaen University, Thailand and Dr. Paul Scott, USDA-ARD, Corn Insects and Crop Genetics Research Unit.
Footnotes
Highlights
• Immature starches showed variation on starch content, granule sizes and chain length distribution of amylopectin.
• A two population of starch granules with peak values ranged from 0.8 to 1.1 μm (small) and 9.0 to 12.2 μm (large).
• Small granule population was negative correlation with CL and DP 25–36 of amylopectin.
References
- Ai Y, Medic J, Jiang H, Wang D, Jane J. Starch characterization and ethanol production of sorghum. J Agric Food Chem. 2011;59:7385–7329. doi: 10.1021/jf2007584. [DOI] [PubMed] [Google Scholar]
- Cagampang BG, Kirleis AW. Relationship of sorghum grain hardness to selected physical and chemical measurements of grain quality. Cereal Chem. 1984;61:100–105. [Google Scholar]
- Fiedorowicz K, Rebilas K. Physicochemical properties of waxy corn starch and corn amylopectin illuminated with linearly polarised visible light. Carbohydr Polym. 2002;50:315–319. doi: 10.1016/S0144-8617(02)00046-2. [DOI] [Google Scholar]
- Fredriksson H, Silverio J, Andersson R, Eliasson AC, Aman P. The influence of amylose and amylopectin characteristics on gelatinization and retrogradation properties of starches. Carbohydr Polym. 1998;35:119–134. doi: 10.1016/S0144-8617(97)00247-6. [DOI] [Google Scholar]
- Gomez KA, Gomez AA. Statistical procedures for agricultural research. Singapore: John Wiley and Sons; 1984. [Google Scholar]
- Hallauer RA, Carena MJ, Miranda JB. Handbook of plant breeding. Quantitative genetics in maize breeding. New York: Springer; 2010. [Google Scholar]
- Hanashiro I, Abe J, Hizukuri S. A periodic distribution of the chain length of amylopectin as revealed by high–performance anion–exchange chromatography. Carbohydr Res. 1996;283:151–159. doi: 10.1016/0008-6215(95)00408-4. [DOI] [Google Scholar]
- Hizukuri S. Polymodal distribution of the chain lengths of amylopectins and its significance. Carbohydr Res. 1986;147:342–347. doi: 10.1016/S0008-6215(00)90643-8. [DOI] [Google Scholar]
- Hoover R, Ratnayake WS. Determination of total amylose content of starch. In: Wrolstad RE, Acree TE, An H, Decker EA, Penner MA, Reid DS, Schwartz SJ, Shoemaker CF, Sporns P, editors. Current protocols in food analytical chemistry. USA: Wiley; 2001. pp. 2–3. [Google Scholar]
- Huang C, Lin M, Wang CR. Changes in morphological, thermal and pasting properties of yam (Dioscorea alata) starch during growth. Carbohydr Polym. 2006;64:524–531. doi: 10.1016/j.carbpol.2005.11.009. [DOI] [Google Scholar]
- Jane J. Structure of starch granules. J Appl Glyco. 2007;54:31–36. doi: 10.5458/jag.54.31. [DOI] [Google Scholar]
- Jane J, Chen JF. Effects of amylose moleculr size and amylopectin branch chain length on paste properties of starch. Cereal Chem. 1992;69:60–65. [Google Scholar]
- Jane J, Kasemsuwan T. Anthology of starch granule morphology by scanning electron microscopy. Starch-Stärke. 1994;46:121–129. doi: 10.1002/star.19940460402. [DOI] [Google Scholar]
- Jane J, Chen YY, Lee LF, McPherson AE, Wong KS, Radosavljevic M, Kasemsuwan T. Effects of amylopectin branch chain length and amylose content on the gelatinization and pasting properties of starch. Cereal Chem. 1999;76:629–637. doi: 10.1094/CCHEM.1999.76.5.629. [DOI] [Google Scholar]
- Jiranuntakul W, Puttanlek C, Rungsardthong V, Puncha-arnon S, Uttapap D. Microstructural and physicochemical properties of heat-moisture treated waxy and normal starches. J Food Eng. 2011;104:246–258. doi: 10.1016/j.jfoodeng.2010.12.016. [DOI] [Google Scholar]
- Jiranuntakul W, Puttanlek C, Rungsardthong V, Puncha-arnon S, Uttapap D. Amylopectin structure of heat-moisture treated starches. Starch-Stärke. 2012;64:470–480. doi: 10.1002/star.201100160. [DOI] [Google Scholar]
- Kang HJ, Hwang IK, Kim KS, Choi HC. Comparison of the physicochemical properties and ultrastructure of japonica and indica rice grains. J Agric Food Chem. 2006;54:4833–4838. doi: 10.1021/jf060221+. [DOI] [PubMed] [Google Scholar]
- Ketthaisong D, Suriharn B, Tangwongchai R, Lertart K. Changes in physicochemical properties of waxy corn starches at different stages of harvesting. Carbohydr Polym. 2013;98:241–248. doi: 10.1016/j.carbpol.2013.06.016. [DOI] [PubMed] [Google Scholar]
- Ketthaisong D, Suriharn B, Tangwongchai R, Lertart K. Combining ability analysis in complete diallel cross of waxy corn (Zea mays var. ceratina) for starch pasting viscosity characteristics. Sci Hortic. 2014;175:229–235. doi: 10.1016/j.scienta.2014.06.019. [DOI] [Google Scholar]
- Lertrat K, Thongnarin N. Novel approach to eating quality improvement in local waxy corn: Improvement of sweet taste in local waxy corn variety with mixed kernels from super sweet corn. Acta Hortic. 2008;769:145–150. [Google Scholar]
- Li L, Blanco M, Jane J. Physicochemical properties of endosperm and pericarp starches during maize development. Carbohydr Polym. 2007;67:630–639. doi: 10.1016/j.carbpol.2006.08.013. [DOI] [Google Scholar]
- Li W, Yan S, Yin Y, Li Y, Liang T, Gu F, Dai Z, Wang Z. Comparison of starch granule size distribution between hard and soft wheat cultivars in eastern China. Agric Sci China. 2008;7:907–914. doi: 10.1016/S1671-2927(08)60129-7. [DOI] [Google Scholar]
- Mendez-Montealvoa G, Wanga Y, Campbell M. Thermal and rheological properties of granular waxy maize mutant starches after β-amylase modification. Carbohydr Polym. 2011;83:1106–1111. doi: 10.1016/j.carbpol.2010.09.012. [DOI] [Google Scholar]
- Miao M, Zhang T, Mu W, Jiang B. Structural characterizations of waxy maize starch residue following in vitro pancreatin and amyloglucosidase synergistic hydrolysis. Food Hydrocoll. 2011;25:214–220. doi: 10.1016/j.foodhyd.2009.12.004. [DOI] [Google Scholar]
- Miles MJ, Morris VJ, Orford PD, Ring GS. The roles of amylose and amylopectin in the gelation and retrogradation of starch. Carbohydr Res. 1985;135:271–281. doi: 10.1016/S0008-6215(00)90778-X. [DOI] [Google Scholar]
- Puncha-arnon S, Puttanlek C, Rungsardthong V, Pathipanawat W, Uttapap D. Changes in physicochemical properties and morphology of canna starches during rhizomal development. Carbohydr Polym. 2007;70:206–217. doi: 10.1016/j.carbpol.2007.03.020. [DOI] [Google Scholar]
- Rocha ST, Felizardo SG, Jane J, Franco CML. Effect of annealing on the semicrystalline structure of normal and waxy corn starches. Food Hydrocoll. 2012;29:93–99. doi: 10.1016/j.foodhyd.2012.02.003. [DOI] [Google Scholar]
- Sandhu SK, Singh N. Some properties of corn starches II: physicochemical, gelatinization, retrogradation, pasting and gel textural properties. Food Chem. 2007;101:1499–1507. doi: 10.1016/j.foodchem.2006.01.060. [DOI] [Google Scholar]
- Sing KN, Ezekiel L, Guraya R, Sing H. Microstructure, cooking and textural characteristics of potato (Solanum tuberrosum L.) tubers in relation to physicochemical and functional properties of their flours. J Sci Food Agric. 2005;85:1275–1284. doi: 10.1002/jsfa.2108. [DOI] [Google Scholar]
- Singh N, Singh J, Kaur L, Sodhi NS, Gill BS. Morphological, thermal and rheological properties of starches from different botanical sources. Food Chem. 2003;81:219–231. doi: 10.1016/S0308-8146(02)00416-8. [DOI] [Google Scholar]
- Singh S, Singh N, Isono N, Noda T. Relationship of granule size distribution and amylopectin structure with pasting, thermal, and retrogradation properties in wheat starch. J Agric Food Chem. 2010;58:1180–1188. doi: 10.1021/jf902753f. [DOI] [PubMed] [Google Scholar]
- Takeda Y, Takeda C, Suzuki A, Hizukuri S. Structure and properties of sago starches with low and high viscosities on amylograph. J Food Sci. 1989;54:177–182. doi: 10.1111/j.1365-2621.1989.tb08596.x. [DOI] [Google Scholar]
- Wang Y, Wang L. Physicochemical properties of common and waxy corn starches oxidized by different levels of sodium hypochlorite. Carbohydr Polym. 2003;52:207–217. doi: 10.1016/S0144-8617(02)00304-1. [DOI] [Google Scholar]
- Xing Z, Lim S. Pasting viscosity and in vitro digestibility of retrograded waxy and normal corn starch powders. Carbohydr Polym. 2012;87:235–239. doi: 10.1016/j.carbpol.2011.09.038. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Nozaki K, Hanashiro I, Yagi F, Ito H, Honma M. Structure and physicochemical properties of starches from kidney bean seeds at immature, premature and mature stages of development. Carbohydr Res. 2003;338:463–469. doi: 10.1016/S0008-6215(02)00489-5. [DOI] [PubMed] [Google Scholar]