Abstract
Rheum emodi is principally known to consist 1,8-dihydroxyanthraquinones (DHAQs) that find immense use in the chemical, pharmaceutical, cosmetic industries and in herbal medication and food sector. The aim of this study was to compare non-conventional and classical methods for extraction of anthraquinones from R. emodi. Optimisation of the extraction parameters for various methods was done and their extraction efficiency was evaluated. In preliminary screening experiments, choice of solvent and solid : solvent ratio was optimised. Comparison of extraction efficiency for classical methods like maceration, heat-reflux, soxhletion and non-conventional methods like ultra-sonication and sublimation was done for five DHAQs - aloe emodin, rhein, emodin, chrysophanol and physcion using HPLC-UV and fluorescence detection in native and acid hydrolysed samples. It was observed that ethanol was the best solvent for extraction of anthraquinones with a solid : solvent ratio of 1:20. A prior acid hydrolysis led to significant increase in anthraquinone extraction. Among the extraction methods heat reflux for 45 min was the most prominent extraction method with highest recovery of the DHAQs. In ultrasonic assisted extraction, an increase in the anthraquinone extraction was seen till 45 min after which the concentration declined. A novel, solvent-free, green and selective method of extraction by sublimation was found to be effective for extraction of anthraquinones.
Keywords: Rheum emodi; Rhubarb, 1,8-dihydroxyanthraquinones; Anthraquinones; Extraction efficiency; Sublimation
Introduction
In recent years a shift in the paradigm has occurred from, categorising foods and medicinal plants as different entities to value added foods with increased nutritional, sensory and therapeutic potential. Amongst such plants, Rheum emodi (Polygonaceae) also known as Rhubarb has been known to be one of the ancient plants mentioned in the Chinese and Indian systems of medicine. Rheum emodi constitutes an important food source in various different forms and used as a culinary plant across the world. The rhizomes are often cooked after drying, the stalks when stewed yields a tart sauce and also used in making wine and baked goods (Clementi and Misiti 2010; Nazir et al. 2013).
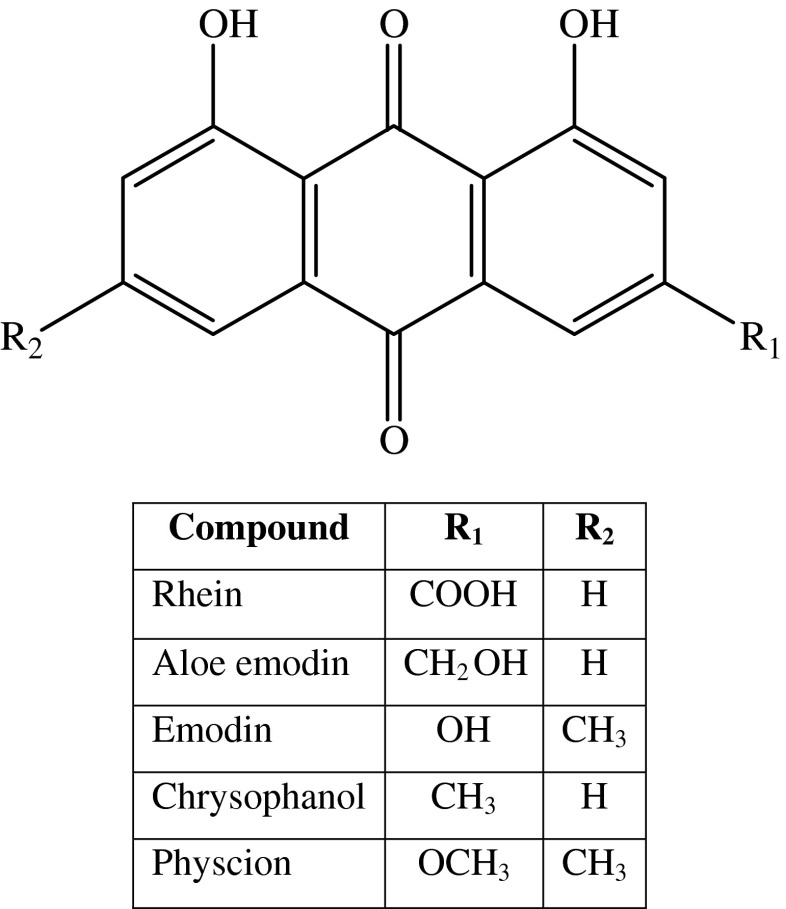
Chemically the rhizomes of R. emodi are known to contain principally free 1,8-dihydroxy anthraquinones (DHAQs) and their glycosides. Rhein, aloe emodin, emodin, chrysophanol and physcion are the aglycones among the anthraquinones that are also present in their glycosidic form (Fig. 1). The DHAQs manifest diverse important pharmacological activities apart from the classical laxative action like antioxidant, anticancer, antimicrobial, antifungal, antidiabetic, hepato-protective, nephro-protective, immune-modulatory and in treating neurodegenerative diseases like Parkinson’s disease (Alam et al. 2005; Hatano et al. 1999; Ibrahim et al. 2008; Kong et al. 2004; Kounsar et al. 2011; Radhika et al. 2010; Rajkumar et al. 2010). Apart from the bio-activites, the DHAQs find significant role in the cosmetic industry as natural pigments and food grade colorants (Caro et al. 2012). They also play a vital role as a bio-catalyst in the paper industry for delignification process and as a catalyst in commercial production of hydrogen peroxide (Goor et al. 2007; Rodriguez et al. 2008). In view of the increasing demand of these compounds in the chemical, pharmaceutical, cosmetic and paper industry and also in herbal medication and food sector it is imperative to develop technologies with high extraction efficiency, yield and selectivity using environmentally friendly approach and low cost of raw materials for the separation of the industrially and pharmacologically important anthraquinones.
Fig. 1.
1,8-di-hydroxyanthraquinones from R. emodi
Although the fact, modern separation and characterization techniques make analysis of bioactive compound easier, the success still depends on the extraction method enabled. The most common factors affecting the extraction efficiency are optimisation of various parameters like nature and type of solvent used, the sample : solvent ratio (feed : solvent), temperature and time. Today modern extraction setups enable the use of non-conventional methods like sonication, supercritical fluid extraction, enzyme digestion, pulsed electric field etc. so as to increase the efficiency, yield, decrease the cost of production and utilizing more environment friendly procedures (Azmir et al. 2013).
The extraction of anthraquinones is a time and solvent consuming phenomena owing to the different intrinsic polarities and solubility. Genovese et al. (2010) compared the extraction methods of anthraquinones from Rhamnus alpinus. Also non-conventional methods for extraction of DHAQs facilitated by pressurized hot water from Morinda citrifolia and using root cell culture from M. aungustifolia have been previously attempted (Aobchey et al. 2002; Pongnaravanea et al. 2006). Quantitative analysis of the anthraquinones from R. palmatum and R. tangiticum using HPLC-diode array detection and mass spectrometry has also been reported (Wei et al. 2013). However optimisation of extraction of the anthraquinones from R. emodi has not been done. Besides in the reported extraction methods, the screening experiment that constitutes the most vital part in deciding the type of solvent, amount of sample : solvent ratio and pre-optimisation of the individual extraction methods has not been studied. The current work envisages comparison of conventional and novel methods for extraction efficiency of the anthraquinones from R. emodi. In the initial part of this work, preliminary screening experiments were carried that determined the choice of extracting solvent and the sample : solvent ratio. Further a prior pre-optimisation of the extraction parameters for the individual extraction methods was done and then comparison of the methods for DHAQ concentration was assessed. In addition, a new promising method for extraction of bio-active compounds by aid of sublimation has been developed.
Materials and methods
General experimental procedures
The dried tubers of R. emodi were obtained from Yucca Enterprises, Mumbai, India, authenticated and voucher specimen was deposited at the Medicinal Natural Products Research Laboratory, Institute of Chemical Technology, Mumbai. All chemicals used were of analytical grade, unless specified, and were obtained from S. D. Fine Chemicals Limited, Mumbai. The reference standards rhein, aloe emodin, emodin, chrysophanol and physcion were isolated in-house. The standards exhibited a purity of >98 %, as confirmed by HPLC and spectral data. HPLC-grade methanol was sourced from Merck (India). Water for HPLC was made in-house after filtration through 0.45 μm filter.
The rhizomes of R. emodi (1 kg) were powdered and stored at 4 °C until subsequent use. Reagent grade solvents were used for extraction; HPLC grade solvents were employed for chromatographic analysis. HPLC analysis was performed by JASCO system, using 250 × 4.6 mm, RP-18 (5-μm particle size) Purospher star column (Merck), with a flow rate of 1.00 ml/min, and monitoring was done using a Jasco UV-1575 detector and a JASCO FP-2020 Plus Intelligent fluorescence detector in series, with an isocratic elution program of methanol : water containing 0.1 % v/v of o-phosphoric acid (85: 15) at 254 nm (for UV detection) and excitation and emission wavelengths of 440 and 540 nm (for fluorescence detection) were employed (He et al. 2009).
Sample preparation
Two sets of samples were prepared during the entire optimisation of extraction parameters. One of these comprised the direct use of R. emodi rhizome powder, while the other set consisted of the acid hydrolysate of the rhizome powder. For preparing the acid hydrolysate (used in the subsequent extraction procedures), the requisite amount of powder sample was refluxed with 10 % HCl for 2 h, cooled and filtered. The filtrate was discarded and residue was dried at 50 °C in oven and used for extraction and analysis.
HPLC analysis
All the samples obtained by different extraction methods were analysed for the individual anthraquinones viz. rhein, aloe emodin, emodin, chrysophanol and physcion. Stock solutions of the standards were prepared for the five anthraquinones by dissolving 10.1 mg aloe emodin, 9.98 mg rhein, 10.2 mg emodin, 11.8 mg chrysophanol and 9.9 mg physcion in 10 ml methanol and diluted using methanol to requisite concentrations. Calibration curves for DHAQ were prepared in the range aloe emodin (0.10–2.02 μg/ml), rhein (0.079–2.99 μg/ml), emodin (0.51–10.2 μg/ml), chrysophanol (1.475–11.8 μg/ml) and physcion (0.124–9.9 μg/ml) in triplicate. The limit of detection of the DHAQs was determined as per ICH guidelines.
Preliminary screening experiments
Selection of solvent
In order to determine the best solvent for extraction for the DHAQs solvents like petroleum ether, chloroform, ethyl acetate and ethanol were employed for extraction. 0.5 g of the powdered drug was extracted with the solvent (sample to solvent ratio - 1:50), shaken vigorously for 15 min and left undisturbed for 24 h. The extract obtained was centrifuged at 3000 rpm for 5 min and 1 ml of the supernatant was diluted with methanol (1:100) and subjected for HPLC analysis. The same procedure was used for the acid hydrolysed samples to select the solvent for extracting the enriched quantity of DHAQs generated by acid hydrolysis.
Optimisation of solid: solvent ratio
To ascertain the requisite amount of solvent for optimum extraction, various sample to solvent ratios like 1:4, 1:8, 1:10, 1:15, 1:20, 1:25 and 1:32 were employed. 0.5 g powdered sample was subjected with different quantities of solvent (ethanol) in a volumetric flask, shaken for 15 min and kept undisturbed for 24 h. The extract obtained was centrifuged and 1 ml of the supernatant was diluted with methanol (1:100) and subjected for HPLC analysis.
Optimisation of different extraction methods
Maceration assisted extraction (ME)
For extraction using maceration, 1 g of native R. emodi sample and the acid hydrolysate obtained from 1 g powder were suspended seperately in 20 ml ethanol, shaken intermittently after every 15 min for first 2 h and then kept undisturbed for 24 h. The extract was then centrifuged and 0.1 ml of supernatant was diluted using methanol (1:100) and injected for HPLC analysis.
Ultra-sonication assisted extraction (UAE)
To study effect of sonication on extraction of DHAQs, 1 g of powdered sample and the acid hydrolysate were transferred in a volumetric flask containing 20 ml ethanol and immersed in an ultra-sonicator bath (Spectralab UCB-30), sonicated at 40 Hz frequency at 100 W for time intervals of 0, 15, 30 and 45 min. The extracts obtained were centrifuged and 0.1 ml of supernatant was diluted with methanol (1:100) and injected for HPLC analysis. For sampling at zero time point, 1 g of the powdered sample and the acid hydrolysate were suspended in 20 ml ethanol, shaken vigorously after which the mixture was centrifuged and supernatant was diluted with methanol (1:10) and injected for HPLC analysis.
Reflux assisted extraction (RE)
For extraction assisted with reflux, 1 g of powdered sample and the acid hydrolysate were transferred to a RBF containing 20 ml ethanol and refluxed for time intervals of 0, 15, 30 and 45 min respectively on water bath. The extract was centrifuged; the supernatant was diluted with methanol (1:100) and subjected to HPLC analysis. The zero time point sample was prepared as that of the zero time point sample for sonication.
Sublimation assisted extraction (SAE)
In order to extract the DHAQs by sublimation process, 1 g of thoroughly dried powder and the acid hydrolysate were transferred in a 50 ml round bottom flask attached to a reflux condenser (length > 1 m) with cold water circulation and placed in a heating mantle. The flask was strongly heated (8–10 min), until yellow fumes ceased from the sample. On cooling to room temperature, divided portions of 100 ml ethanol were used to dissolve the sublimed DHAQ adhered the walls of condenser and volume was made to 100 ml of which 0.1 ml was diluted with methanol (1:100) and subjected to HPLC.
Soxhlet extraction (SE)
For determination of the total amount DHAQs, 1 g powdered sample and acid hydrolysate was soxhleted in 100 ml ethanol for 4, 8 and 12 h respectively. 0.1 ml of this extract was diluted with methanol (1:100) and subjected to HPLC analysis.
Statistical data
All the experiments for optimisation of various extraction process were performed in triplicate and the data presented as mean ± standard deviation (n = 3). Statistical comparison between various methods of extraction was done by applying One way Anova followed by Holm-Sidak’s multiple comparison test. Statistical data was processed using GraphPad Prism 6.
Results and discussion
HPLC analysis
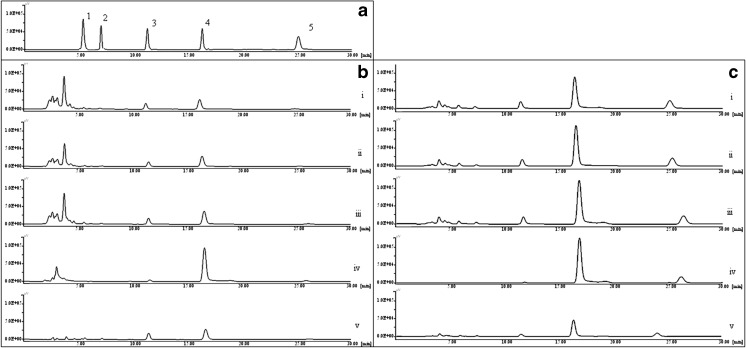
The calibration curves for the five DHAQs showed excellent R2 values and linearity in the entire range as shown in Table 1. Use of fluorescence detector enabled a better detection limit as compared to that of UV detector especially for the analysis of rhein and aloe emodin; since these compounds were present in very meagre quantities evident from Fig. 2. This was further supported by LOD values for rhein and aloe emodin to be 4.25 and 3.85 ng/ml respectively using fluorescence detection as against 375 and 350 ng/ml using UV detection (He et al. 2009). Besides, fluorescence being a characteristic property of the DHAQs, the fluorescence detection could specifically show the peaks of the DHAQs without the interference of other eluting compounds as observed in the UV detection.
Table 1.
Calibration results, linearity and limit of detection for five DHAQ by HPLC-fluorescence detection
| Reference standard | Slope1 | Intercept | Linearity (ng/ml) | R2 | LOD |
|---|---|---|---|---|---|
| Aloe emodin | 257.04 ± 30.87 | 988.71 ± 188.24 | 10.1–2020 | 0.9998 | 4.25 |
| Rhein | 128.56 ± 14.53 | 1601.95 ± 200.21 | 79.84–2994 | 0.9999 | 3.85 |
| Emodin | 226.48 ± 28.32 | 27299.69 ± 2043.67 | 510–10200 | 0.9988 | 10.87 |
| Chrysophanol | 189.74 ± 26.87 | 20374.28 ± 2474.13 | 1475–11800 | 0.9998 | 4.21 |
| Physcion | 451.64 ± 40.68 | 17847.16 ± 956.12 | 124–9900 | 0.9997 | 1.98 |
1Values expressed as Mean ± SD, n = 3
Fig. 2.
HPLC chromatograms for a. DHAQs reference standards 1. Aloe emodin 2. Rhein 3. Emodin 4. Chrysophanol 5. Physcion b. Extraction methods (i) ME (ii) UAE (iii) RE (iv) SAE (v) SE at UV-254 nm detection c. Detection of extraction methods by fluorescence excitation/emission - 440/540 nm
Preliminary screening of extraction conditions
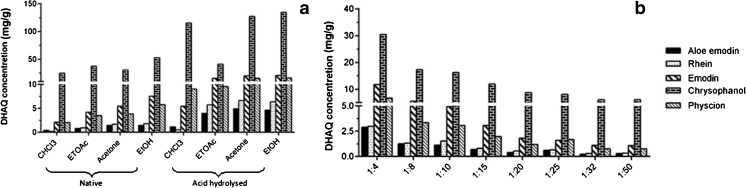
Among the various solvents employed for extraction of the DHAQs, it can be seen from Fig. 3a that ethanol is the best solvent for extraction for the samples, both in the native form and the acid hydrolysed ones. In order to check the best solvent for extraction, along with maximum solubility of the DHAQs; extraction was facilitated using solvents from non polar, mid polar and polar range. As shown in Fig. 1, the DHAQs have a basic 1,8-dihydroxy-anthraquinone scaffold with changes in the functional groups of different polarities at R1 and R2 positions. This is why non polar solvents like petroleum ether cannot solubilise the polar DHAQs rhein, aloe-emodin and emodin. Mid-polar solvents like choloroform and ethyl acetate also possess a sparing solubility for these quinones. Acetone showed a good solubility for DHAQs with 174.06 mg/g of total anthraquinones in acid hydrolysed samples, while ethanol demonstrated a higher concentration of total anthraquinones of 182.75 mg/g and hence was selected as the solvent for extraction for DHAQs. On the other hand the weakly polar DHAQs chrysophanol and physcion showed good solubility in all the solvents employed for the study. The rate of extraction was high with absolute ethanol when compared to ethanol-water mixtures to extract the anthraquinone aglycones. DHAQs are known to be practically insoluble in water and hence mixtures of aqueous and organic solvents seldom might find a possibility to facilitate better extraction; despite earlier reports (Anonymous 1983; Pongnaravanea et al. 2006). Conversely the anthraquinone glycosides exhibit a fair solubility in aqueous-organic solvent mixtures and can used for extraction of the DHAQ glycosides (Pongnaravanea et al. 2006).
Fig. 3.
Preliminary screening experiments: a Effect of various solvents on extraction of DHAQs on native (unhydrolyzed) and acid hydrolyzed samples b Effect of various solid: solvent ratios on extraction of DHAQs
Various sample : solvent ratios were employed to determine the exact amount of solvent needed for the optimum extraction using ethanol (Fig. 3B), amongst which highest extraction efficiency was seen with solid : sample ratios ranging from 1:4 to 1:15. The ratios less than 1:4 did not wet the powder properly and hence could not be used, whilst those from 1:15 to 1:50 did not show substantial improvement in the extraction efficiency. However, in view of the increase in the DHAQ content on hydrolysis, a solid : solvent ratio of 1:20 was selected for optimum extraction.
In all the extraction methods employed, a 1.3 to 3 fold rise in the DHAQ concentration was observed in the acid hydrolysed samples when compared to the native sample of R. emodi which proves that a prior acid hydrolysis is necessary considering the augmented extraction yield by hydrolysis.
Optimisation of different extraction methods
Extraction using ME
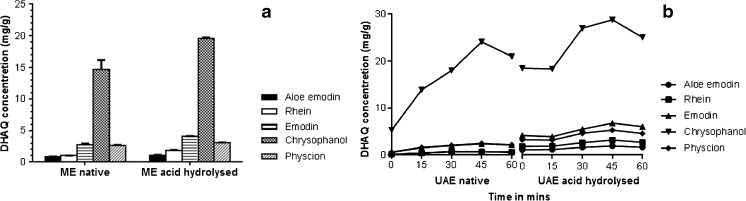
Maceration facilitates the extraction of phytoconstitutents by softening the cell wall with subsequent diffusion across the cell membrane and solubilising the secondary metabolites. No information was available for extraction of DHAQs from R. emodi by ME in the literature. Therefore, a pre-optimised solid: sample ratio (1:20) was used during the maceration process. Figure 4a shows the extraction efficiency by ME after 24 h. The yield of total DHAQ/g of raw material, after ME in native and acid hydrolysed samples was found to be 21.86 mg/g and 37.31 mg/g respectively. Chrysophanol was extracted in highest amount (19.69 mg/g) while rhein and aloe emodin were extracted in low amounts (1.89 and 1.09 mg/g), corresponding to the abundance in concentration in R. emodi.
Fig. 4.
Extraction using maceration (ME) (a) and sonication (UAE) (b) on extraction of DHAQs on native (unhydrolyzed) and acid hydrolyzed samples
Extraction by UAE
UAE increases solubility of DHAQs by cavitation process, thus increasing the diffusion process and enhancing the mass transfer. Figure 4b shows an increase in the rate of extraction with time of all DHAQs at the set frequency of 40 kHz, however after a threshold time (45 min), the DHAQ concentration ceased. This drop in DHAQ concentration was consistently seen in the native as well as acid hydrolysed samples after a time interval of 45 min. This can be attributed to a saturation of the extraction solvent by DHAQs that can occur at a particular time point. Due to continued sonication after this point, other components from the plant matrix also get solubilised; diminishing the solubility of the anthraquinones and thus reducing the extraction rate. A maximum recovery of the total anthraquinones was seen at 45 min and found to be 30.34 mg/g and 46.08 mg/g respectively for native and acid hydrolysed samples was of R. emodi indicating a 1.5 fold increase in the concentration of quinones by acid hydrolysis.
Extraction by RE
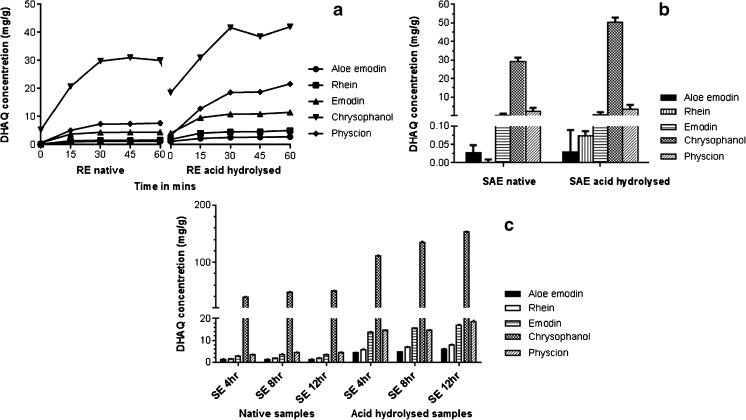
An increase in the temperature assists the solubility and thus the extraction rate of DHAQs, when refluxed with ethanol (Fig. 5a). Among the native samples of R. emodi, there is no significant increase in the extraction of anthraquinones after 30 min (44.2 mg/g total DHAQ content); the highest extraction being observed at 45 min (45.63 mg/g total quinone content). In acid hydrolysed samples saturation of the DHAQs occur at 45 min (83.14 mg/g total quinone content) indicating a 1.8 fold increase in the concentration of anthraquinones by acid hydrolysis.
Fig. 5.
Effect of reflux (RE) (a), sublimation (SAE) (b) and soxhletion (SE) (c) on extraction of DHAQs on native (unhydrolyzed) and acid hydrolyzed samples
Extraction by SAE
Anthraquinones are known to be recrystallized using sublimation (Anonymous 1983; Burnett and Thomson 1967). However so far, no reports of using sublimation as an extraction technique for DHAQs or other bioactives have been made. Selective extraction of DHAQs is possible by SAE because of the characteristic property of DHAQs to sublime, leaving other phytoconstituents trapped in the plant matrix. The HPLC chromatogram of SAE shows a very meagre quantity of other components that get eluted along with the DHAQs (Fig. 2), unlike to those in the chromatograms for UAE and RE. It is interesting to observe that the polar anthraquinones rhein and aloe emodin get extracted in very less quantities (Fig. 5B), which is also evident in the HPLC chromatogram with fluorescence detection. This indicates that this process can be selectively used for isolation of the comparatively non polar DHAQs like chrysophanol and physcion. Figure 5b shows that among the five DHAQs, chrysophanol increased radically in the acid hydrolysed samples (50.20 mg/g) as compared to native samples (20.02 mg/g).
The experimental setup for extraction using SAE consisted of an open system due to which certain loss of the DHAQs due to escaping was evident. To minimise the losses at an increased scale of operation (sample quantity- 50 g), a closed system was enabled with aid of vacuum and chilling. However when sublimation was attempted at 100 g sample quantity in a closed system with aid of vacuum and chilling, the vapours of anthraquinones were produced in large amount which were unable to condense in the cooling assembly and a high pressure is built up in the system. Our ongoing work is optimisation of sublimation process considering the increased scale of operation for lessened degraded product by uncontrolled heat and also developing a method to chill and contain the vapours generated during sublimation. Furthermore, this process can also be used to extract DHAQs from other plant sources like Cassia aungustifolia or Morinda citrifolia (data not shown), that contain a reasonable concentration of these quinones.
Extraction by SE
To determine the total amount of the DHAQs in the native and acid hydrolysed samples at various time points, extraction was assisted using SE. It was observed that no further increase in the extraction of DHAQs was observed in the native samples after 8 h (total DHAQ content 58.58 mg/g), while in the acid hydrolysed samples saturation of DHAQs was seen at 12 h (total DHAQ content 121.29 mg/g) as shown in Fig. 5c, indicating an increase by 2 fold in the total DHAQ content by acid hydrolysis. Aloe emodin, rhein and emodin did not show any variation in concentration at 8 and 12 h time-point, but extraction of chrysophanol and physcion increased by 10 mg and 2 mg respectively.
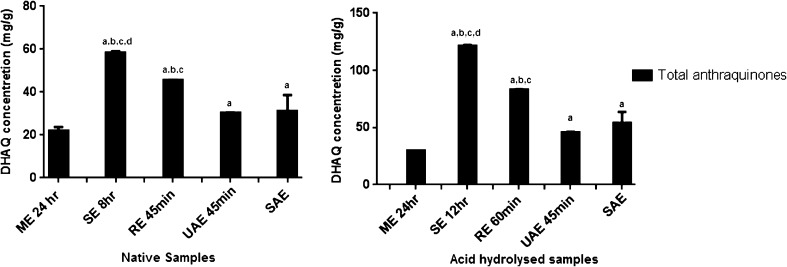
Comparison of extraction methods
Amongst the extraction methods used in the present study, ME, RE and SE are conventionally used for the commercial extraction of most of the phyto-constitutents. A recently preferred method for extraction like UAE finds a great significance in extraction of heat-sensitive natural products. The study enables use of a novel method of extraction by sublimation process, which is highly selective for the DHAQs. The approach was to compare the conventional industrial methods with the novel methods considering factors like extraction time, volume of solvent and suitability at a commercial extent. To compare the aforesaid optimised extraction methods for highest DHAQ recovery of native and acid hydrolysed extracts, the total anthraquinone content was calculated as the sum of individual DHAQs in various extraction processes used. From Fig. 6 it is evident that amongst the native samples of R. emodi, >RE was the best method of extraction (77.9 % recovery of total DHAQs), as evaluated against other methods of extraction like SAE (53.52 %), UAE for 45 min (51.79 %) and ME for 24 h (37.31 %), when the recoveries were compared to soxhletion for 8 h. Similarly in the acid hydrolysed samples, RE for 60 min gave the best extraction of DHAQs (68.54 % recovery of total DHAQs), followed by SAE (44.7 %), UAE for 45 min (37.99 %) and ME for 24 h (24.49 % recovery), when the recoveries were compared to soxhletion for 12 h. It should be noted that all the methods are pre-optimised considering the individual extraction parameters and feasibility during use of the method. Extraction by reflux was the best method for extracting the DHAQs from R. emodi. The comparative less recovery of the DHAQs by RE (77.9 % recovery in native and 68.54 % in the acid hydrolysed samples), when compared to SE can be explained on the fact that the solvent extracting the quinones attains a saturation point thus exhibiting a limiting value for extraction yield of the DHAQs. However, the considerable reduction in extraction time and feasibility of this process are indeed appreciable at a commercial scale extraction or isolation of these bioactive quinones, where parameters like volume and cost of operation needed for this method stand crucial. Besides RE can be easily scaled up to an industrial scale as against soxhletion that can be only used at a laboratory scale and remains unfeasible considering commercial scale-up.
Fig. 6.
Comparison of different optimized extraction methods in native and acid hydrolyzed samples aME, bUAE, cSAE and dRE groups compared by One way Anova followed by Holm-Sidak’s multiple comparison test at p < 0.05
Considering the methods of extraction for DHAQs from various plants, literature reveals several novel methods like supercritical fluid extraction (SCFE), aqueous two phase extraction (ATPS) and pressurised hot water extraction (Genovese et al. 2010; Pongnaravanea et al. 2006; Tan et al. 2013). However SCFE fails to extract the anthraquinones per-se as the DHAQs are polar compounds that cannot be extracted by the comparably less polar carbon dioxide, while the later methods need a very sophisticated setup that may not be viable at a higher scale of operation. It can be seen that extraction using sublimation can be a modest alternative to the conventional methods as it requires minimum infrastructure, cost effective and free from toxic solvents and hence is a ‘green’ method of extraction.
Impurity profiling is an important aspect of standardization of herbal formulations, as also in the extraction process. Generally an extraction process with high selectivity is desirable which consists of increased yields of the actives with minimum quantities of the impurities that get extracted by the extraction solvent. In extraction of DHAQs using ethanol as extracting solvent, it was found that ME and SE carried the maximum impurities while SAE carried the least impurities. Thus sublimation process offers a high selectivity in the extraction of anthraquinones from R. emodi. Also it does not employ the use of solvent making it a solvent-free, cost effective and an environmentally friendly method. This process can also be extended to other plants containing DHAQs for their selective extraction.
Conclusion
Optimisation of extraction parameters for conventional and novel methods of extraction of DHAQs from R. emodi was studied and the extraction efficiency of these processes was evaluated. Ethanol was found to be the best solvent for extraction with solid: solvent ratio 1: 20. In all the extraction methods employed, a 1.3 to 3 fold rise in the DHAQ concentration was observed by acid hydrolysis considering the augmented extraction yield by hydrolysis of corresponding anthraquinone glycosides. Among the pre-optimised extraction methods, refluxing was found to be the most effective method of extraction. A more green, solvent-free and selective process of extraction using sublimation was found efficient for isolating an anthraquinone rich extract which can also be used as a method of extraction for DHAQs from other plant sources. Our findings helps to select the best possible method of extraction at an industrial or commercial level in terms of yields offered, minimum extraction time and offers an economic approach wherein amount of solvents, other utilities like power and time are minimised. Besides in the traditional and modern herbal prescriptions for laxative formulations containing R. emodi; the activity of which is solely due to anthraquinones, designing the most optimum extraction of DHAQs would offer better efficacy. In addition our work assists the quality control of herbal formulations where-in complete extraction of the analytes is crucial for standardization and control.
Acknowledgments
The authors are grateful to the University Grant Commission – Special Assistance Programme (UGC-SAP), New Delhi - India, for providing the financial assistance for this research work.
Footnotes
Research highlights
• Classical and novel methods of extraction for anthraquinones were compared.
• Ethanol was found to be best extracting solvent with solid: sample ratio 1:20.
• Refluxing for 45 min in ethanol gave highest recovery of the anthraquinones.
• A green method was developed for selective extraction of DHAQ by sublimation.
Aditya U Arvindekar, holds a M.S. Institute of Chemical Technology
Galvina R Pereira, holds a PhD, Institute of Chemical Technology
Kirti S Laddha, holds a PhD, Institute of Chemical Technology
References
- Alam MM, Javed K, Jafri MA. Effect of Rheum emodi (Revand Hindi) on renal functions in rats. J Ethnopharmacol. 2005;96:121–125. doi: 10.1016/j.jep.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Anonymous . The merck index. 10. USA: Merck & Co., Inc; 1983. p. 296. [Google Scholar]
- Aobchey P, Sriyam S, Praharnripoorab W, Lhieochaiphant S, Phutrakul S. Production of red pigment from the root of Morinda angustifolia Roxb. var. scabridula Craib. by root cell culture. CMU J. 2002;1:66–78. [Google Scholar]
- Azmir J, Zaidul ISM, Rahman MM, Sharif KM, Mohamed A, et al. Techniques for extraction of bioactive compounds from plant materials: a review. J Food Eng. 2013;117:426–436. doi: 10.1016/j.jfoodeng.2013.01.014. [DOI] [Google Scholar]
- Burnett AR, Thomson RH. Naturally occurring quinones. Part X. The quinonoid constituents of Tabebuia avellanedae (Bignoniaceae) J Chem Soc C. 1967 [Google Scholar]
- Caro Y, Anamale L, Fouillaud M, Laurent P, Petit T, Dufosse L. Natural hydroxyanthraquinoid pigments as potent food grade colorants: an overview. Natl Prod Bioprospect. 2012;2:174–193. doi: 10.1007/s13659-012-0086-0. [DOI] [Google Scholar]
- Clementi EM, Misiti F. Potential health benefits of Rhubarb. In: Watson RR, Preedy VR, editors. Bioactive foods in promoting health: Fruits and vegetables. UK: Academic; 2010. pp. 407–409. [Google Scholar]
- Genovese S, Tammaro F, Menghini L, Carlucci G, Epifano F, Locatelli M. Comparison of three different extraction methods and HPLC determination of the anthraquinones aloe-emodine, emodine, rheine, chrysophanol and physcione in the bark of Rhamnus alpinus L. (Rhamnaceae) Phytochem Anal. 2010;21:261–267. doi: 10.1002/pca.1195. [DOI] [PubMed] [Google Scholar]
- Goor G, Glenneberg J, Jacobi S (2007) Hydrogen peroxide. In: Ullmann’s encyclopedia of industrial chemistry. Wiley-VCH Verlag GmbH & Co. KGaA, Germany. doi:10.1002/14356007.a13_443
- Hatano T, Uebayashi H, Ito H, Shiota S, Tsuchiya T, Yoshida T. Phenolic constituents of cassia seeds and antibacterial effect of some naphthalenes and anthraquinones on methicillin-resistant Staphylococcus aureus. Chem Pharm Bull. 1999;47:1121–1127. doi: 10.1248/cpb.47.1121. [DOI] [PubMed] [Google Scholar]
- He D, Chen B, Tian Q, Yao S. Simultaneous determination of five anthraquinones in medicinal plants and pharmaceutical preparations by HPLC with fluorescence detection. J Pharm Biomed Anal. 2009;49(4):1123–1127. doi: 10.1016/j.jpba.2009.02.014. [DOI] [PubMed] [Google Scholar]
- Ibrahim M, Khaja MN, Aara A, Khan AA, Habeeb MA, Devi YP, Narasu ML. Hepatoprotective activity of Sapindus mukorossi and Rheum emodi extracts: In vitro and in vivo studies. World J Gastroenterol. 2008;14:2566–2571. doi: 10.3748/wjg.14.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong LD, Cheng CH, Tan RX. Inhibition of MAO A and B by some plant derived alkaloids, phenols and anthraquinones. J Ethnopharmacol. 2004;91:351–355. doi: 10.1016/j.jep.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Kounsar F, Rather MA, Ganai BA, Zargar MA. Immuno-enhancing effects of the herbal extract from himalayan rhubarb Rheum emodi Wall. ex Meissn. Food Chem. 2011;126:967–971. doi: 10.1016/j.foodchem.2010.11.103. [DOI] [Google Scholar]
- Nazir S, Sharma M, Saxena M, Abrar M, Ajaz M. Rheum emodi: phytochemistry, bioactive compounds and their biological activity. Int J Phytopharm. 2013;4:272–276. [Google Scholar]
- Pongnaravanea B, Gotob M, Sasakib M, Anekpankula T, Pavasanta P, Shotipruka A. Extraction of anthraquinones from roots of Morinda citrifolia by pressurized hot water: Antioxidant activity of extracts. J Supercrit Fluids. 2006;37:390–396. doi: 10.1016/j.supflu.2005.12.013. [DOI] [Google Scholar]
- Radhika R, Kumari DK, Sudarsanam D. Antidiabetic activity of Rheum emodi in alloxan induced diabetic rats. Int J Pharm Sci Res. 2010;1:296–300. [Google Scholar]
- Rajkumar V, Guha G, Ashok Kumar R. Antioxidant and anti-cancer potentials of Rheum emodi rhizome extracts. Evid Based Complement Alternat Med. 2010 doi: 10.1093/ecam/neq048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A, Moral A, Serrano L, Labidi J, Jimenez L. Rice straw pulp obtained by using various methods. Bioresour Technol. 2008;99:2881–2886. doi: 10.1016/j.biortech.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Tan Z, Li F, Xu X. Extraction and purification of anthraquinones derivatives from Aloe Vera L. using alcohol/salt aqueous two-phase system. Bioprocess Biosyst Eng. 2013;36:1105–1113. doi: 10.1007/s00449-012-0864-4. [DOI] [PubMed] [Google Scholar]
- Wei SY, Yao WX, Ji WY, Wei JQ, Peng SQ. Qualitative and quantitative analysis of anthraquinones in rhubarbs by high performance liquid chromatography with diode array detector and mass spectrometry. Food Chem. 2013;141:1710–1715. doi: 10.1016/j.foodchem.2013.04.074. [DOI] [PubMed] [Google Scholar]