Abstract
The present work reports studies on biofortification of milk and cheese with microelements. The diet of goats was supplemented with soya-based preparations with Cu(II), Fe(II), Zn(II) and Mn(II), produced by biosorption, instead of mineral salts. In innovative preparations, soya was the biological carrier of microelements. The utilitarian properties of the new preparations were tested in two groups (8 goats in each): experimental and control. The concentration of supplemented microelements was monitored in milk during the experiment. The collected milk was then used to produce cheese by enzymatic and acidic coagulation method. The effect of milk and cheese biofortification in microelements was confirmed. In milk, the level of the following microelements was higher than in the control: Cu(II) – 8.2 %, Mn(II) – 29.2 %, Zn(II) – 14.6 %. In cheese the content of Zn(II) obtained in enzymatic (19.8 %) and in acidic (120 %) coagulation was higher when compared to the control group. By using bio-preparations with microelements it was possible to produce new generation of functional food biofortified with microelements, by agronomic, and thus sustainable and ethically acceptable way. Biofortified milk and cheese can be used as designer milk to prevent from micronutrient deficiencies.
Graphical Abstract.
ᅟ
Keywords: Soya bean meal, Biosorption, Microelements, Feed additive, Goats, Biofortified milk and cheese
Introduction
In our previous reports, it was shown that enriched biomass (macroalgae, microalgae, soya bean meal) with microelement ions via biosorption process can be used in animal nutrition as biological dietary supplement – laying hens (Michalak et al. 2011; Witkowska et al. 2014) and pigs (Saeid et al. 2013a, b). This approach enables biofortification of food products for humans (eggs, meat, milk) by agronomic means.
Feed materials which are used to compose a complete feed for different species of animals should provide the sufficient amount of valuable protein, carbohydrates and fat, should have a low content of anti-nutritional substances and should be inexpensive and readily available (Jamroz 2013; Jamroz et al. 2013). The selection of specific feed materials is made by taking into account the species of animal for which the feed is intended (Pesti and Miller 1993). In many dietary premixes, soya bean meal is the main component (Titi 2003).
Previously, basic research on cations binding by biomass via biosorption process (kinetics, equilibrium) was carried out for selected feed materials (triticale, oat, barley, corn beans, soya beans, wheat and rye). On this basis the biomass for further research was selected—soya beans, which was characterized by good maximum biosorption capacity – 43.1 mg/g (determined by Langmuir equation) (Witkowska et al. 2013). The system for biosorption in a large laboratory scale was configured – it consisted of stirred tank reactor and a fluid bed column reactor (Witkowska and Chojnacka 2013). The next step was to develop the project and build a pilot plant installation. This plant was used for the production of the biomass enriched with microelements (soya bean meal): Zn(II), Cu(II), Fe(II), Cr(III) and Mn(II) ions (Chojnacka et al. 2013). The enriched biomass was used in feeding trials. In the present work, soya bean meal was used as the biological carrier of microelements in the diet of goats. The goal was to biofortify milk and its product (cheese) with microelements.
The carrier of microelements in goats diet was a popular feeding material—soya bean meal. Soya is one of the oldest cultivated plants. It originates from China and until the beginning of the nineteenth century was cultivated only in Asia. Currently, among the legumes, ranks as first in the world trade (Houbowicz-Kliza 2007). Soya bean meal is the primary by-product of the process of removing the oil from soya beans (Gao et al. 2013). The oil may be extracted with organic solvents by heating and pressing the raw material (Jezierny et al. 2010). Thus, produced meal is the main source of protein for various livestock species in Europe, North and South America and in Asia (da Silva et al. 2012). In China alone in 2010, the use of soya bean meal in animal nutrition amounted to 56 million tonnes (Teng et al. 2012). The demand for soya bean meal increased due to the increase in livestock production.
In 2008–2012, world production of soya bean meal increased by 14 % (da Silva et al. 2012). In the European Union the importance of soya bean meal increased strongly since 2005 due to significantly limiting the use of animal proteins in livestock nutrition (Commission Regulation No 1292/2005). Today the demand of the European Union for protein feed materials is covered in 63 % by the soya bean meal. Total annual EU demand for this material is more than 31 million tonnes, of which about 97 % is imported (European Feed and Manufacturers’ Federation 2012).
Currently, the demand for protein in Poland for farmed animals is 1 million tonnes, of which 80 % coincides with soya bean meal (Hanczakowska and Księżak 2012). Over the past century, post-extraction soya bean meal increased as a valuable source of protein for livestock, covering 65 % of global demand poultry, goats, sheep, pigs and cattle protein (da Silva et al. 2012; Van Eys 2002). In the United States the share of soya bean meal pellets derived from all oilseed in the feeding of livestock is 92 % (Cromwell 1999). The utility of soya bean meal in the feeding of livestock is preferred due to its chemical composition, the high protein content and relatively low price and global accessibility (Jezierny et al. 2010; Teng et al. 2012; Van Eys 2002). In soya beans there are no components that lower digestibility: toxic alkaloids or tannins, although it contains a certain amount of trypsin inhibitors, which can, however, be disactivated using fermentation (Gao et al. 2013). Soya also contains saponins and oligosaccharides, which may adversely affect feed intake and nutrient utilization, however, in the production of soya bean meal most of the anti-nutritional components were removed or deactivated (Frikha et al. 2012).
In the present work soya bean meal enriched with Zn(II), Cu(II), Fe(II) and Mn(II) via biosorption process (using inorganic salts: ZnSO4 · 7H2O, CuSO4 · 5H2O, FeCl2 · 4H2O, MnSO4 · H2O) in the a pilot plant installation (Chojnacka et al. 2013) was tested in goats nutrition as bioavailable source of micronutrients to produce functional milk and cheese enriched with microelements. Inorganic salts used as feed additives in the diet were replaced by the enriched biomass. The effect of biofortification of milk and cheese was examined. The general scheme of our research is presented in Fig. 1.
Fig. 1.
General scheme of our research
Materials and methods
Feeding experiments on goats
The work has been carried out in accordance with EU Directive 2010/63/EU for animal experiments. The feeding trials on goats were approved by the Second Local Ethical Committee on Animal Testing at Wrocław University of Environmental and Life Sciences (No 119/2009, 16 November 2009). The trial was conducted at the farm Kozio–lek in Wrocław. In the experiment, the goat breed white Polish ennobled was used, at the age of 3 years. Goats (16) were randomly divided into two groups: experimental (E) and control group (C), eight goats each. The duration of the experiment was 14 days. The animals were given individual numbers.
Prior to the experiment, all the goats were fed with a volumetric (hay) with water available ad libitum and fed with barley screemings in the morning in an amount of 200 g, together with trace elements supplemented in the form of inorganic salts. The mineral composition of hay and barley screemings is presented in Table 1.
Table 1.
Mineral composition of hay and barley screemings (mg/kg d.m., ±SD, N = 3)
| Element | Hay | Barley screemings |
|---|---|---|
| B | 2.71 ± 0.41 | 0.845 ± 0.127 |
| Ca | 3571 ± 714 | 439 ± 66 |
| Cu | 4.87 ± 0.73 | 5.11 ± 0.77 |
| Fe | 49.1 ± 0.74 | 40.7 ± 6.1 |
| K | 9623 ± 1925 | 4714 ± 943 |
| Mg | 1166 ± 233 | 1087 ± 217 |
| Mn | 174 ± 26 | 12.9 ± 1.9 |
| Na | 620 ± 93 | 122 ± 18 |
| P | 1592 ± 318 | 3798 ± 760 |
| S | 1024 ± 205 | 1233 ± 247 |
| Zn | 28.1 ± 4.2 | 28.7 ± 4.3 |
Micronutrient requirement of animals in both groups was covered in 100 %. The doses of micronutrients were selected according to recommended dosages and goat nutrition standards (Directive of the Minister of Agriculture and Rural Development 2004; Dobrowolska 1993). During the experiment, the control group received a mixture of micronutrients in the inorganic form (Cargill Poland Sp., Botulinum), which was added to the barley feed with morning milking. The experimental group received instead of inorganic salts, supplementation with enriched soya bean meal in the morning (Soya–Zn, Soya–Cu, Soya–Fe, Soya–Mn, “Soya” – signifies supplementation with soya bean meal), so the dose of delivered trace elements in both groups was the same. Milk samples were collected from the evening milking of the goats. Table 2 shows the types of supplements and their feed rations in each group.
Table 2.
The types of feed supplements in feed doses in given groups in feeding experiment on goats
| Group | Feed | Feed supplement |
|---|---|---|
| C | Barley | Inorganic salts: CuSO4·5H2O, MnSO4·H2O, ZnSO4·H2O, FeSO4·H2O |
| E | Barley | Soya bean meal enriched separately in: Cu(II), Mn(II), Zn(II), Fe(II) |
where: (C) control, (E) experimental group
Collection of milk
Milk samples (100 ml) were drawn from each individual goat before the experiment and at the end (day 14). In addition, on the last day of the experiment after taking individual samples from each animal, the remaining milk was sampled collectively for each group and separated into the fatty layer (cream), cheese (casein) and whey. The study was conducted in the Department of Food Science of the Wrocław University of Life Sciences in Poland and in the Department of Animal Resources Technology and Quality Management.
For this purpose, the fat was centrifuged in a centrifuge at 5100 rpm at 0 °C for 20 min. After the completion of centrifugation, the milk was left in the centrifuge for another hour. After this time, the cream was separated and harvested by collecting from the surface of the milk. Skimmed milk was filtered through sterile cotton wool to remove residual fat. Prior to the analysis, the milk obtained from the milking of each group (MC – milk from the control group, ME – milk from the experimental group) was divided into two parts (MC1 and MC2, ME1 and ME2), followed by separation of the skim milk cheese (casein curd) and whey. Two methods were used for each group: by acid coagulation and coagulation by enzymatic method.
Acidic coagulation of milk
Milk (100 ml) from the group MC1 and ME1 was titrated with 1 M HCl until a flocculent precipitate appeared. The amount of acid used was converted to the volume of milk MC1 and ME1. When added to the cooled milk from both groups, suitable amount of HCl, was placed in an incubator at 35 °C for 30 min. After precipitation of the casein clot, the curd was separated from the whey.
Enzymatic coagulation of milk
Milk (50 ml) was taken from the group MC2 and ME2 and placed in Erlenmeyer flasks. To each flask, 1 ml of the microbial rennet was added and the time required for precipitation was recorded. Then the converted amount of rennet to be added to milk was calculated in order to clot casein in MC2 and ME2. Milk from both groups was heated to 35 °C in a water bath and then the appropriate amount of rennet was added, mixed and allowed to stand for 30 min. After precipitation of the casein clot, cheese was separated from the whey. Table 3 reports the amount of milk that was used in the analysis and the amount of used reagents.
Table 3.
Experimental setup in acidic and enzymatic coagulation of milk
| Milk-group | Volume of milk (ml) | Type of coagulation | Volume of HCl (ml) | Volume of rennet (ml) | |
|---|---|---|---|---|---|
| MK | MC1 | 1700 | Acidic | 110 | – |
| MC2 | 1700 | Enzymatic | – | 0.5 | |
| ME | ME1 | 1350 | Acidic | 80 | – |
| ME2 | 1350 | Enzymatic | – | 0.4 | |
where: (MC) milk from the control group, (ME) milk from the experimental group
Analytical methods
The collected milk samples (3 g) were purified from organic matter with concentrated nitric acid—69 % m/m (5 ml), spectrally pure (Suprapur, Merck) in Teflon bombs in microwave oven Milestone Start D (USA). The selected parameters of the process assured the complete digestion of samples. After mineralization, samples were diluted with re–demineralized water (Millipore Simplicity) to 50 g. Digested samples underwent multielemental analysis. The content of elements was determined by ICP–OES iCAP 6500 Duo, Thermo Scientific, USA. Quality assurance of the test results was achieved by using Combined Quality Control Standard from ULTRA SCIENTIFIC, USA. The samples were analyzed in three repeats (the reported results of analyses were arithmetic mean, the relative standard deviation was <5 %). The analyses were carried out in quality management system according to PN-EN ISO/IEC 17025:2005.
Statistical analysis
In our paper, two independent groups of goats were compared: first—fed with inorganic salts as feed additive (the control group) and the second – fed with enriched soya bean meal (the experimental group). Wilcoxon test was used. The results were elaborated statistically by Statistica ver. 10.0. Results were considered significantly different when p < 0.05 and p < 0.10.
Results and discussion
The main purpose of feeding studies conducted on goats was to evaluate the impact of soya bean meal enriched with microelements on mineral composition of milk and its products (cheese produced by two distinctive methods and whey). In feeding trials on goats, the formulations produced in a pilot plant quarter-technical scale by biosorption were used.
Based on the content of micronutrients in enriched biomass (Witkowska et al. 2014), the mass of soya bean meal enriched with the microelement that is to be added to the feed was calculated, to meet the needs of goats for these elements, taking into account the Polish and European legislation in this area and dietary recommendations (Directive of the Minister of Agriculture and Rural Development 2004; Dobrowolska 1993). Table 4 shows the dose of microelements, which were given to animals to cover their demand for these minerals in 100 %.
Table 4.
Doses of microelements in feeding trials on goats
| M* | Recommended supplementation of microelements (mg/kg feed) | Content of microelements in enriched soya bean meal (mg/g) (±SD, N = 3) | Mass of supplemented enriched soya bean meal (g/kg feed) |
|---|---|---|---|
| Cu(II) | 10 | 11.7 ± 1.5 | 0.90 |
| Fe(II) | 40 | 11.9 ± 1.5 | 3.40 |
| Zn(II) | 50 | 9.06 ± 1.18 | 5.50 |
| Mn(II) | 60 | 5.92 ± 0.77 | 10 |
M* microelement
Effect of enriched soya bean meal on mineral composition of milk
Table 5 shows the concentration of microelements in the milk at the beginning and at the end of the experiment. Although the experiment was designed as randomized trial, statistically significant differences in the composition of milk between control (C) and experimental (E) group were observed. The concentration of Cu(II) in the milk at the beginning of experiment was almost half that in the experimental group (p 0.00589), as compared to the control group. Despite this, after the end of the experiment, the concentration of this element in the milk in this group was higher by 8.2 % as compared to the control group. The concentration of Fe(II) at the beginning of the experiment was also significantly lower in the experimental group than in the control (p 0.0172). After the completion of the experiment, the level of Fe(II) was still lower in the experimental group than in the control, but the difference between the groups was significantly lower and was 3.2 %. The concentration of Mn(II) at the beginning of the experiment was similar in both groups, while at the end of the experiment, in the experimental group was 29.2 % higher than in the control group. The concentration of Zn(II) in milk at the beginning of the experiment was significantly higher in the experimental group (p 0.0393) and also at the end of the experiment remained significantly increased by 14.6 % (p 0.0223).
Table 5.
Microelement concentrations in milk in groups at the beginning and at the completion of the experiment (±SD, N = 8)
| Group | Content of microelements in milk (μg/100 g) | |||
|---|---|---|---|---|
| Cu | Fe | Mn | Zn | |
| Beginning | ||||
| C | 9.0 ± 2.3a,1 | 50.8 ± 15.2a | 11.4 ± 4.8 | 272 ± 91a,I |
| E | 5.3 ± 2.2a | 35.4 ± 5.2a,1 | 12.0 ± 5.9 | 421 ± 162a |
| Completion | ||||
| C | 4.5 ± 2.41 | 43.1 ± 5.1 | 10.1 ± 5.3 | 350 ± 57a,I |
| E | 4.9 ± 2.1 | 41.7 ± 3.51 | 13.1 ± 4.7 | 401 ± 98a |
a,b,c – statistically significant differences for the same measurement period for p ≤ 0.05
1,2,3 – statistically significant differences between the start and completion of the experiment for p ≤ 0.05
I,II,III – significant differences between the start and completion of the experiment for p ≤ 0.10
Summarizing, the milk was enriched in the experimental group with copper, manganese and zinc, while the level of iron was comparable to the concentration of this element with milk from goats in the control group.
Comparing the concentrations of trace elements at the beginning and at the end of the experiment within a group, there was a statistically significant reduction of Cu(II) level by 49.6 % (p 0.0136) and a significant increase of Zn(II) by 28.5 % (p 0.0591) in the milk of the control group. The concentration of Fe(II) and Mn(II) in this group decreased by 15.1 % and 11.2 %, respectively. On the other hand, in the experimental group a statistically significant increase of Fe(II) by 17.9 % (p 0.0125) was shown. Copper and zinc level was reduced by 8.1 % and 4.8 %, while manganese content increased by 9.0 %. In the group which was fed with the diet supplemented with enriched soya bean meal such a large decrease in elements content was not observed (in comparison with the control group). This may indicate higher bioavailability of microelements in biologically bound form. It should be taken into account that the content of macro- and microelements in milk depends on a variety of environmental, genetic and physiological factors, including for example: the health status, stage of lactation, production season, region of production (Kędzierska-Matysek et al. 2015; Strzałkowska et al. 2008). In the work of Kędzierska-Matysek et al. (2015) the concentration of microelements in goat milk also decreased over the course of lactation. Strzałkowska et al. (2008) showed that the content of basic minerals in goat milk during lactation undergoes some changes. The highest fluctuations in the composition of goat milk were found for zinc (32 %) and copper (39 %) contents. Hejtmankova et al. (2002) observed also that the levels of Ca, Cu, Zn, at the beginning of the lactation period were higher at the beginning than at its end.
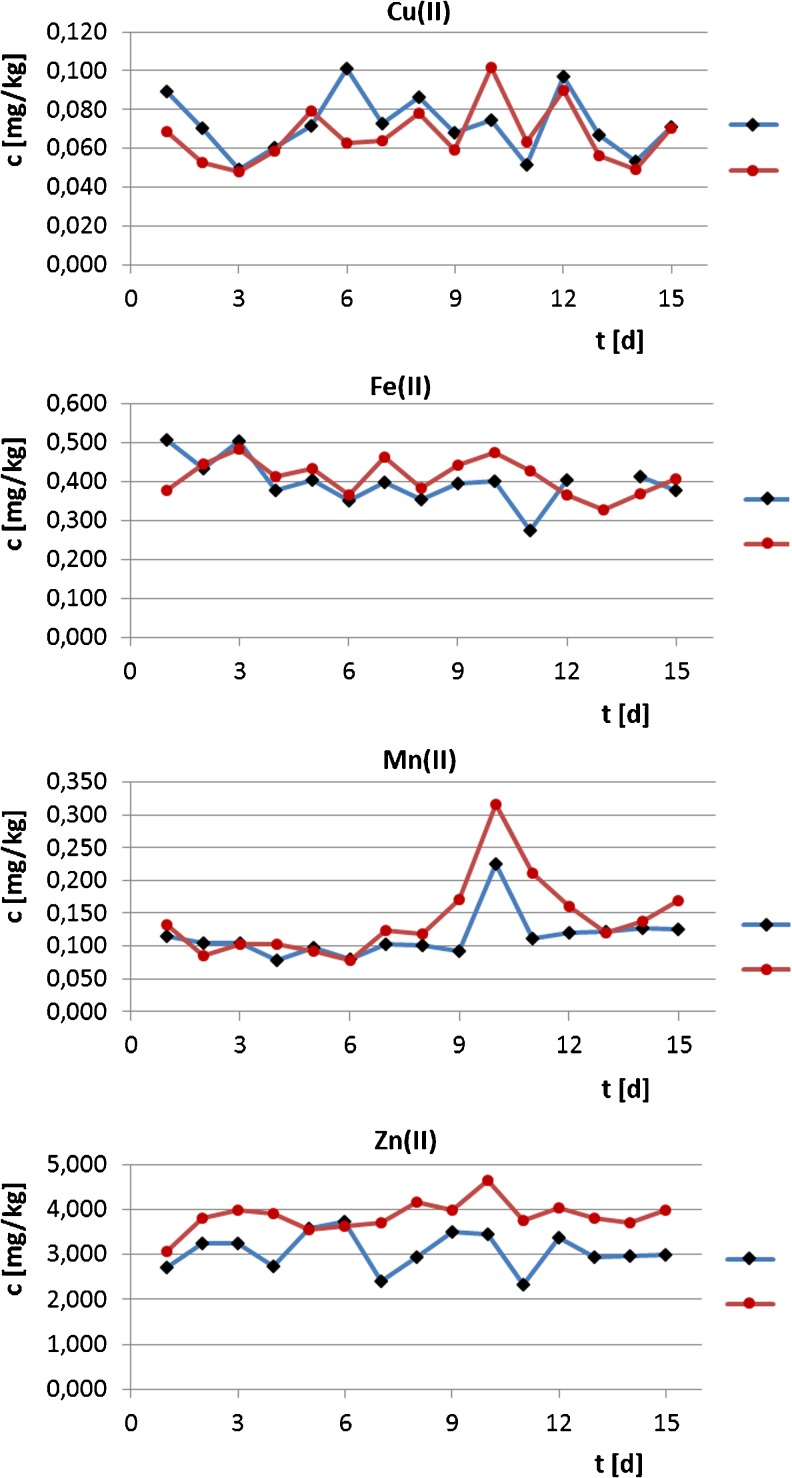
In order to observe changes in the level of microelements in milk during the experiment, samples were collected daily from the milk of goats. Figure 2 shows graphs of the microelement content in the milk on the duration of the experiment. Wilcoxon test has shown that the change of the copper and iron concentration during the experiment was similar in both groups, but changing the content of manganese and zinc was higher in the experimental group. The differences between the groups were statistically significant (p 0.0171 and p 0.00121, respectively).
Fig. 2.
The dependence of the concentration of microelements in milk on the duration of the experiment
Effect of enriched soya bean meal on mineral composition of cheese and whey
Table 6 shows the concentration of microelements in bulk milk collected from all animals in a given group, on the last day of the experiment. This milk was used in cheese production. In this process whey was collected. There was a lower concentration of all the studied trace elements in milk derived from goats in the control group. The content of Cu(II) was higher in the experimental group by 61.0 %, Fe(II) content by 4.0 %, Mn(II) content by 18.6 % and Zn(II) content by 28.7 %.
Table 6.
The concentration of microelements in milk used in the production of cheese (±SD, N = 3)
| Group | Content of microelement in milk for cheese production (μg/100 g) | |||
|---|---|---|---|---|
| Cu (II) | Fe (II) | Mn (II) | Zn (II) | |
| C | 3.7 ± 0.01 | 41.9 ± 1.9 | 11.4 ± 0.1 | 329 ± 2 |
| E | 5.9 ± 0.38 | 43.6 ± 1.2 | 13.6 ± 0.1 | 424 ± 15 |
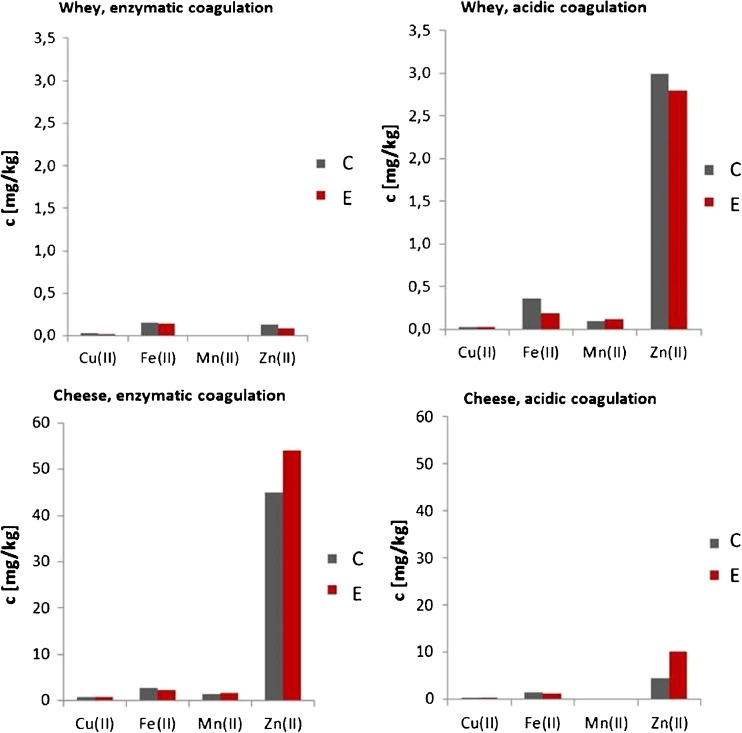
For separation of milk curd and whey, two methods were used: acidic and enzymatic in order to compare their usefulness in obtaining foods rich in microelements. There were observed differences in the content of trace elements in cheese and whey by these two methods (Fig. 3).
Fig. 3.
The concentration of microelements in cheese and whey obtained by enzymatic and acidic method
Using enzymatic coagulation, whey obtained contained lower levels of calcium, magnesium and microelements, as compared to the coagulation with the acid, due to differences in the mechanism and conditions of coagulation (Dłużewski 2008; Pijanowski and Gaweł 1986; Ziajka 2008). At the same time it was observed that whey derived from milk from the control group, contained slightly higher levels of microelements than in the experimental group. In turn, using the enzymatic coagulation, the cheese obtained was richer in micronutrients as compared to acid coagulation. In both cases, the cheese obtained from milk from the experimental group was richer in zinc: 19.8 % by using enzymatic coagulation and 120.2 % by using the coagulation with an acid.
Biosorption as a method of production of bio-based feed supplements
The concept of producing innovative bio-based feed supplements by biosorption method involves the binding of microelement cations to the biomass which is a traditional component of compound feed. It has been shown that the best material for the production of new formulations for animals was soya bean meal (Witkowska et al. 2013). A biosorption pilot plant in which the preparation for the feeding tests was manufactured, was designed and constructed (Chojnacka et al. 2013). The technology bases on a column reactor which allowed for the efficient production of the supplement and elimination of certain difficulties as compared to previously proposed formulations whereby biomass of algae was used (Michalak et al. 2011; Saeid et al. 2013a, b). In order to evaluate new formulations, feeding studies were carried out previously on laying hens (Michalak et al. 2011; Witkowska et al. 2014).
Also in the present study on goats, in the experimental group inorganic salts of trace elements were withdrawn from the premixes and replaced in a new—biological form. It has been proven that innovative microelement preparations basing on the biomass, which is conventional and accessible component of compound feed, may be used in supplementation of livestock diet with microelements and improve production parameters and quality of the products: eggs (Michalak et al. 2011; Witkowska et al. 2014), meat (Saeid et al. 2013a, b), milk – present study. It was observed that the new formulations also increased the density of micronutrients in the food products and therefore can be used in the production of eggs, meat and milk and its products biofortified with micronutrients.
The application of enriched via biosorption biomass in biofortification of food
Micronutrient deficiencies can be corrected by using pills containing inorganic salts of micronutrients or by consuming fortified foods. Foods can be fortified industrially, at home or by agronomic means (biofortification of food of plant or animal origin). It is expected that bioavailability of nutraceuticals would depend on the method of fortification. Probably the most bioavailable, but less efficient in terms of degree of enrichment would be agronomic fortification. In the production of food of animal origin, fortification is achieved by supplementation of livestock feed with more available or slightly increased dose of nutraceuticals that would be then transferred to food products (meat, eggs, milk). Home fortification is carried out by adding microelement salts during preparation of food. The same can be done on the industrial scale.
“Designer food” (“functional food”, “fortified food”) is food with other than nutritional health benefits, which was fortified/enriched with a nutraceutical (micronutrient, PUFAs, vitamins, probiotics) (Rajasekaran and Kalaivani 2013). Among designer foods, the following are available: designer eggs (enriched with PUFAs, microelements (Michalak et al. 2011; Witkowska et al. 2014), oil (omega-3-fatty acids), broccoli (sulphoraphane, selenium), probiotics, drinking yogurt, milk, rice, food enriched with phytosterol, grains (Rajasekaran and Kalaivani 2013).
There were some attempts to fortify milk and produce ‘designer milk’. Different approaches (genetic and nutritional) were employed to design milk for human health benefits (Sabikhi 2007). It is thus possible to produce milk containing nutraceuticals, change the structure of casein or lipid profile (Sabikhi 2007). For instance, genetic manipulation enabled to eliminate beta-lactoglobulin (Rajasekaran and Kalaivani 2013) and reduce level of lactose by ectopic expression of intestinal lactase (Whitelaw 1999). Cows, the diet of which was supplemented with sunflower seeds gave milk with higher levels of PUFAs (Rajasekaran and Kalaivani 2013). There are also examples of industrial fortification of milk beverages: with polyphenols, phenolic compounds, lutein, vitamins (Rajasekaran and Kalaivani 2013) or microelements (iron and zinc) (Sazawal et al. 2010).
In the available literature there is lack of studies on the effectiveness of fortified food. Sazawal et al. (2010) investigated the efficiency of milk fortified with Zn (additional 7.8 mg/day), Fe (9.6 mg), Cu (0.27 mg), Se and vitamins (A, C, E) vs. control in children (1–4 years of age) in prevention of anemia and growth faltering. Fortification was achieved by adding micronutrient bundle to milk powder. The study was conducted for 1 year. In the group that was drinking fortified milk, several statistically significant effects were observed: improvement in weight and height gain, mean hemoglobin and serum ferritin, 88 % lower risk of iron deficiency anemia. The authors concluded that milk is a good vehicle to deliver microelements (Zn and Fe) (Sazawal et al. 2010).
Another study on the efficiency of fortified food was carried by De-Regil et al. (2011) who investigated home fortification with multiple micronutrient powders. The study was carried out on small children (below 2 years of age). Efficacy of this method of fortification was assessed as good. Anemia was reduced by 31 % and iron deficiency by 51 % (De-Regil et al. 2011).
Biofortification can be undertaken to improve nutritional quality of food and prevent from multiple micronutrient deficiencies. Fortified food is becoming more popular. Wagner et al. (2005) discussed the situation in Austria and found 470 fortified products on the market: for babies, drinks, milk products, salt, fats, cereals and sweets that were most popularly fortified with: vitamin C, B6, niacin and calcium. The contribution of daily intake for micronutrients from fortified food products was estimated as 10 %. The authors concluded that there was no risk of nutrients overdose from fortified food products (Wagner et al. 2005).
The role of diet in prevention from non-communicable diseases is accepted and causes that the borderline between food and medicine will be very thin (Abuajah et al. 2014). It is certainly a solution, bearing signs of sustainability. The acceptability of designer products is dependent on ethical issues which could be questionable if transgenic animals transfer to milk therapeutic components (insulin, plasma proteins, vaccines, e.g., human lactoferrin, lysozyme, lipase) (Sabikhi 2007).
In the present work, goat milk and cheese biofortified with Zn(II) were obtained by agronomic means, by supplementation of goats diet with micronutrients on a biological carrier (soya bean meal). This method should be acceptable to the consumers ethically. Supplementation of diet with zinc is the problem discussed publically, because zinc deficiency causes impaired growth, dysfunction of immunological system, increases morbidity and mortality, causes problems with pregnancy and abnormal neurobehavioral development (Gupta et al. 2013). The social problem of zinc deficiency is related with antinutritional factors (e.g., phytic acid) found in staple foods (cereals) (Gupta et al. 2013). Therefore, finding bioavailable sources of this element becomes a significant issue.
Conclusions
The feeding study was conducted with the participation of goats in order to assess the impact of new formulations (enriched soya bean meal) on mineral composition of milk and its products. Summarizing, the feeding trials on goats have shown that the use of biological additives resulted in the enrichment of milk in trace elements in the experimental group. It was demonstrated that the use of biological additives resulted in the enrichment of milk in trace elements with copper (about 8.2 %), manganese (29.2 %) and zinc (14.6 %). Wilcoxon signed-rank test showed that the change in the content of manganese and zinc in the course of the experiment was significantly higher in the experimental group.
Using the enzymatic coagulation of milk, cheese biofortified with zinc was obtained (contained above 45 mg/kg Zn). The cheese from the experimental group was richer in this element by 19.8 % when compared to the control group. When using acidic coagulation, the content of this element in the cheese prepared from the milk of the experimental group was higher by 120.2 % when compared to the control group.
The obtained results showed that enriched with microelements biomass can be used as an alternative to inorganic feed additives. It is characterized by higher bioavailability to the animal what results in higher content of microelements in products for human, yielding a new generation of biofortified functional food.
Acknowledgments
This research was financially supported by Polish Ministry of Science and Higher Education (grant No. R05 0014 10).
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- Abuajah CI, Ogbonna AC, Osuji CM (2014) Functional components and medicinal properties of food: a review. J Food Sci Technol. doi:10.1007/s13197-014-1396-5 [DOI] [PMC free article] [PubMed]
- Chojnacka K, Rusek P, Witkowska Z, Tuhy Ł, Witek-Krowiak A, Saeid A, Samoraj M (2013) Układ do biosorpcji mikroelementów. The patent application, No P 404983 (in Polish)
- Cromwell GL (1999) Soybean Meal – The “Gold Standard”, http://www.uky.edu/Ag/AnimalSciences/pubs/soybeanmeal-thegolfstandard.PDF, access: 12.06.2013
- da Silva FRGB, de Souza M, da Costa AMS, Jorge LMM, Paraiso PR. Experimental and numerical analysis of soybean meal drying in fluidized bed. Powder Technol. 2012;229:61–70. doi: 10.1016/j.powtec.2012.06.008. [DOI] [Google Scholar]
- De-Regil LM, Suchdev PS, Vist GE, Walleser S, Peña-Rosas JP. Home fortification of foods with multiple micronutrient powders for health and nutrition in children under two years of age (Review) Evid Based Child Health. 2011;8:112–201. doi: 10.1002/ebch.1895. [DOI] [PubMed] [Google Scholar]
- Directive of the Minister of Agriculture and Rural Development, 2004, 9, 132
- Dłużewski M. Technologia żywności: podręcznik dla technikum. Warsaw: WSiP; 2008. [Google Scholar]
- Dobrowolska D. Feeding standard for cattle, sheep and goats. nutritive value of fodder for ruminants. Warsaw: Omnitech Press; 1993. [Google Scholar]
- Frikha M, Serrano MP, Valencia DG, Rebollar PG, Fickler J, Mateos GG. Correlation between digestibility of amino acids and chemical composition of soybean meals in broilers at 21 days of age. Anim Feed Sci Technol. 2012;178:103–114. doi: 10.1016/j.anifeedsci.2012.09.002. [DOI] [Google Scholar]
- Gao YL, Wang CS, Zhu QH, Qian GY. Optimization of solid-state fermentation with lactobacillus brevis and Aspergillus oryzae for trypsin inhibitor degradation in soybean meal. J Integrative Agricult. 2013;12:869–876. doi: 10.1016/S2095-3119(13)60305-6. [DOI] [Google Scholar]
- Gupta RK, Gangoliya SS, Singh NK (2013) Reduction of phytic acid and enhancement of bioavailable micronutrients in food grains. J Food Sci Technol. doi:10.1007/s13197-013-0978-y [DOI] [PMC free article] [PubMed]
- Hanczakowska E, Księżak J. Krajowe źródła białkowych pasz roślinnych jako zamienniki śruty sojowej GMO w żywieniu świń. Rocz Nauk Zoot. 2012;39:171–187. [Google Scholar]
- Hejtmankova A, Kucerova J, Miholova D, Kolihova D, Orsak M. Levels of selected macro- and microelements in goat milk from farms in the Czech Republic. Czech J Anim Sci. 2002;47:253–260. [Google Scholar]
- Houbowicz-Kliza G (2007) Uprawa soi. Instrukcja upowszechnieniowa. IUNG-PIB: Puławy, 130 (in Polish)
- Jamroz D. Żywienie zwierząt i paszoznawstwo. Warsaw: PWN; 2013. [Google Scholar]
- Jamroz D, Podkówka W, Chachułowa J. Żywienie zwierząt i paszoznawstwo. Warsaw: PWN; 2013. [Google Scholar]
- Jezierny D, Mosenthin R, Bauer E. The use of grain legumes as a protein source in pig nutrition: a review. Anim Feed Sci Technol. 2010;157:111–128. doi: 10.1016/j.anifeedsci.2010.03.001. [DOI] [Google Scholar]
- Kędzierska-Matysek M, Barłowska J, Litwińczuk Z, Koperska N (2015) Content of macro- and microelements in goat milk, including potentially toxic elements, taking into account lactation stage and region of production. J Elem 20(1). doi:10.5601/jelem.2013.18.4.549
- European Feed and Manufacturers’ Federation (2012) Feed and Food. Statistical Yearbook, 2012. http://www.fefac.eu/files/51501.pdf. Accessed 26 Mar 2014
- Michalak I, Chojnacka K, Dobrzański Z, Górecki H, Zielińska A, Korczyński M, Opaliński S. The effect of enriched with microelements macroalgae on egg quality parameters and mineral content of eggs, eggshell, blood, feathers and droppings. J Animal Physiol Animal Nutr. 2011;95:374–387. doi: 10.1111/j.1439-0396.2010.01065.x. [DOI] [PubMed] [Google Scholar]
- Pesti GM, Miller BR. Animal feed formulation. Van Nostrand Reinhold: AVI Books; 1993. [Google Scholar]
- Pijanowski E, Gaweł J. Zarys chemii i technologii mleka. Warsaw: Państwowe Wydawnictwo Rolnicze i Leśne; 1986. [Google Scholar]
- Rajasekaran A, Kalaivani M. Designer foods and their benefits: a review. J Food Sci Technol. 2013;50:1–16. doi: 10.1007/s13197-012-0726-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabikhi L. Designer milk. Adv Food Nutr Res. 2007;53:161–198. doi: 10.1016/S1043-4526(07)53005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeid A, Chojnacka K, Korczyński M, Korniewicz D, Dobrzański Z. Biomass of Spirulina maxima enriched by biosorption process as a new feed supplement for swine. J Appl Phycol. 2013;25:667–675. doi: 10.1007/s10811-012-9901-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeid A, Chojnacka K, Korczyński M, Korniewicz D, Dobrzański Z. Effect on supplementation of Spirulina maxima enriched with Cu on production performance, metabolical and physiological parameters in fattening pigs. J Appl Phycol. 2013;25:1607–1617. doi: 10.1007/s10811-013-9984-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sazawal S, Dhingra U, Dhingra P, Hiremath G, Sarkar A, Dutta A, Menon VP, Black RE. Micronutrient fortified milk improves iron status, anemia and growth among children 1–4 years: a double masked, randomized, controlled trial. PLoS One. 2010;5(8):e12167. doi: 10.1371/journal.pone.0012167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strzałkowska N, Bagnicka E, Jóźwik A, Krzyżewski J. Macro- and micro-elements’ concentration in goat milk during lactation. Zuchtungskunde. 2008;80:404–411. [Google Scholar]
- Teng D, Gao M, Yang Y, Liu B, Tian Z, Wang J. Bio-modification of soybean meal with Bacillus subtilis or Aspergillus oryzae. Biocat Agricult Biotech. 2012;1:32–38. [Google Scholar]
- Titi HH. Replacing soybean meal with sunflower meal with or without fibrolytic enzymes in fattening diets of goat kids. Small Rumin Res. 2003;48:45–50. doi: 10.1016/S0921-4488(03)00003-8. [DOI] [Google Scholar]
- Van Eys JE. Wykorzystanie soi i śruty sojowej w przemyśle paszowym: znaczenie optymalizacji receptur i zapewniania jakości. Pasze Przem. 2002;9:9–10. [Google Scholar]
- Wagner KH, Blauensteiner D, Schmid I, Elmadfa I. The role of fortified foods–situation in Austria. Forum Nutr. 2005;57:84–90. doi: 10.1159/000083771. [DOI] [PubMed] [Google Scholar]
- Whitelaw B. Toward designer milk. Nat Biotechnol. 1999;17:135–136. doi: 10.1038/6134. [DOI] [PubMed] [Google Scholar]
- Witkowska Z, Chojnacka K. Wytwarzanie nowych biologicznych dodatków paszowych z Cr(III) metodą biosorpcji w trybie półokresowym w skali wielkolaboratoryjnej. Przem Chem. 2013;92:1308–1310. [Google Scholar]
- Witkowska Z, Chojnacka K, Michalak I. Application of biosorption in the production of innovative feed supplements: a novel method. Ads Sci Technol. 2013;31:421–431. doi: 10.1260/0263-6174.31.5.421. [DOI] [Google Scholar]
- Witkowska Z, Chojnacka K, Korczyński M, Świniarska M, Saeid A, Opaliński S, Dobrzański Z. Soybean meal enriched with microelements by biosorption—a new biological feed supplement for laying hens. part I. performance and egg traits. Food Chem. 2014;151:86–92. doi: 10.1016/j.foodchem.2013.11.023. [DOI] [PubMed] [Google Scholar]
- Ziajka S. Mleczarstwo. Podręcznik akademicki. Olsztyn: Wyd. UWM; 2008. [Google Scholar]