Abstract
Rheological properties of salep-milk mixture as hot drink were evaluated using a rotational viscometer at different temperature (45, 50, 55, 60, and 65 °C) and salep concentration (0.75, 1.00, and 1.25 w/v, %). All salep-milk mixtures exhibited non-Newtonian behavior. The shear rate /shear stress data obtained from forward and backward directions were examined by common rheological models such as power law, Herschel-Bulkey, Casson and Bingham plastic models. Among the common models, the power-law model fitted the shear rate and shear stress data for 1.00 and 1.25 % salep concentration at all temperature. The Bingham plastic model described well the flow behavior of the salep-milk mixtures in 0.75 % salep concentration at all temperature. Flow behavior index (n), according to the power law and Herschel-Bulkey models decreased with an increase in salep concentration and a decrease of temperature. The consistency coefficient decreased with temperature and increased with salep concentration.
Keywords: Rheology, Salep, Flow models, Shear-thinning
Introduction
Salep tubers are generally gathered from orchids growing in forested mountainous regions. Tubers grow best in soil with a high lime content, and those with the finest aroma and richest in starch are found at altitudes of around 1000 m. In Anatolia most orchid species belong to the genera Orchis and Ophrys. Wild orchids are most abundant in the provinces of Kahramanmaraş, Adıyaman, Bitlis, and the Black Sea provinces, particularly Kastamonu. They flower in April and May, and then seed. Some of the flowers are scentless, while others produce a sweet scent that is strongest in the evening, and their colours vary from white to various tones of purple (Cankan 2001).
Salep is made from the powdered root of wild orchids used as food and drugs. The orchid tubers are gathered while the plant is in flower. Each orchid has two tubers, one the main tuber from which the flower springs, and the other its younger offshoot. Only the young tuber is harvested, leaving the main tuber untouched. The beige tubers are either egg-shaped or forked. The root and tuber are washed and then tossed into boiling milk or water for a short while to remove the bitter flavour, inhibit the enzymatic activity and reduce the loss of water soluble ingredient. They are dried either in the open air or in ovens to speed up the process. After drying they may be stored whole or grounded. The principal substances contained in salep vary according to the species, but basically consist of glucomannan, starch, protein and ash (Tekinşen and Guner 2010). The colour is generally beige. Salep is the traditional thickening ingredient in Turkish ice cream. Salep plays an important role as a stabilizers used in the production of Kahramanmaraş-type ice cream in Türkiye (Tekinşen and Karacabey 1984). It is also commonly used mixed with milk to make the hot drink known as salep, which is served sprinkled with cinnamon. In times of Ottoman salep was an ingredient of invigorate pastes prepared for the sultans, along with ginger, coriander, senna, black cumin seeds, coconut, aniseed and numerous herbs and spices (Tekfidan 2001).
In Turkey, with the coming of cold winter days, salep is the most popular hot drink and is sold by coffee and pastry shops and street vendors outside who kept it warm in large copper jugs on a brazier. Nowadays, it is produced by some companies as ready-to-drink mixtures including milk, sugar, starch, and hydrocolloids.
The viscosity of food is important in the design of food processes and processing equipment, quality evaluation and control of food products, and understanding the structure of food materials (Krokida et al. 2001). The rheological data of food materials are a requirement for process engineering analyses (extrusion, pumping, mixing, agitation, heating, coating, and process control), quality control and shelf-life estimation, texture evaluation, product development, and the development of constitutive equations for rheological characterization (Ofoli 1990). The rheological properties of salep-milk mixture as hot drink to the best of our knowledge were only examined by Arduzlar and Boyacioğlu (2003). Researchers investigated effects of starch type on rheological properties of salep drink. Kaya and Tekin (2001) and Güven et al. (2003) studied rheological properties of ice cream mix with prepared ground salep. Farhoosh and Rıazı (2007) studied the rheological properties of salep as a function of concentration and temperature. Yılmaz et al.(2010) reported that the flow behaviour of salep beverage prepared with aspartame and lemon fiber was adequately described by power law model. The other studies regarding the glucomannan of salep were investigated. The polysaccharide of salep orchid tuber was shown to be a glucomannan with a glucose/mannose ratio of 2.6 and an acetyl content of 2.1 % (Juers et al. 1967). Glucomannans found in the hemicellulose as well as in the roots, bulbs and tubers of many plants are generally considered linear random block copolymers even though species with side chains of α- (1–6) linked D-galactose units are known (Benincasa et al. 2002). Salep glucomannans are widely distributed as reserve materials of certain plants. The structure of glucomannan of salep is constituted of acetylated D-mannopyranosyl units linked by a (1 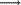 4)-β linkage whereas the latter contains acetylated (1
4)-β linkage whereas the latter contains acetylated (1 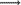 4)- β-D glucopyranosyl randomly distributed in the backbone with a ratio of mannose to glucose of about 3:1 (Marchessault et al. 1981). Glucomannan extracted from the tubers of Orchis morio contain D-glucopyranosyl and D-mannopyranosyl residues in the molar ratio ca 1:3.3 (Buchala et al. 1974). Some researchers (Kayacier and Dogan 2006; Yasar et al. 2009) investigated dynamic rheological characterization of salep glucomannan/galactomannan-based milk beverages.
4)- β-D glucopyranosyl randomly distributed in the backbone with a ratio of mannose to glucose of about 3:1 (Marchessault et al. 1981). Glucomannan extracted from the tubers of Orchis morio contain D-glucopyranosyl and D-mannopyranosyl residues in the molar ratio ca 1:3.3 (Buchala et al. 1974). Some researchers (Kayacier and Dogan 2006; Yasar et al. 2009) investigated dynamic rheological characterization of salep glucomannan/galactomannan-based milk beverages.
In food industry, an extensively employed glucomannan is konjac glucomannan. It has high water holding capacity and it forms highly viscous solutions and a thermally stable gel (Yoshimura et al. 1998; Yoshimura and Nishinari 1999; Nishinari et al. 2000). The glucomannans such as konjac glucomannan and salep are mainly used as thickening, stabilizing agents, and food emulsifiers (Tye 1991; Tian et al. 1998; Üçüncü 1992).
The objective of this study is to determine the flow properties of salep-milk mixture as hot drink by varying the salep concentrations at different temperatures.
Materials and methods
Materials
Ground salep was purchased from a local spice seller in Mersin, Türkiye. Sterilized and homogenized cow milk (Güney, Türkiye) was used in all salep-milk mixtures.
Salep-milk mixtures were prepared by adding 0.75 %, 1.00 %, and 1.25 % (w/v) of ground salep in sterilized milk at room temperature. The samples were heated until 85 °C and kept for 2 min at this temperature. Prior to flow property measurement, the temperature of each mixture was decreased to 45, 50, 55, 60, or 65 °C. All samples were prepared daily.
Methods
Flow properties measurement
Viscosity and torque measurements were made on ground salep-milk mixtures using a Brookfield viscometer (RVDV-E model, Brookfield Engineering Laboratories Inc., MA, USA) at temperatures of 45, 50, 55, 60, and 65o C with nine spindle speeds (1, 3, 5, 10, 20, 30, 50, 60, and 100 rpm). The temperature was maintained using a thermostatically controlled water bath. All data were taken after 30 s in each sample, with a rest in time between the measurements at different spindle speeds. Appropriate spindles (spindle no: 1 or 2) were used to get all readings within the scale. A 500 ml beaker was used for all measurements with the guard leg on and enough samples were added to just cover the immersion grooves on the spindle shafts. The flow curves of salep-milk mixtures were measured in the temperature range of 45–65 °C by increasing (forward measurements) and decreasing (backward measurements) the rotational of spindle speed. All experiments were replicated three times. Average shear stress and shear rates were calculated by the method of Mitschka (1982).
The flow curves of salep-milk mixtures were modeled using common rheological models such as Powerlaw, Casson and Herschel-Bulkley models
-
Power law model:
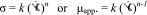
1 Here, σ is shear-stress (Pa),
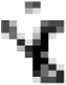 is shear-rate (s−1), k is consistency index (Pa sn), n is flow behavior index (dimensionless), μapp. is apparent viscosity (Pa s) at different shear rate calculated from power law parameter.
is shear-rate (s−1), k is consistency index (Pa sn), n is flow behavior index (dimensionless), μapp. is apparent viscosity (Pa s) at different shear rate calculated from power law parameter. -
Casson model:
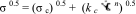
2 Here, σc and kc are Casson yield stress (Pa) and Casson viscosity (Pa0.5 s0.5), respectively.
-
Herschel-Bulkley model:
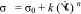
3 Here, σ0 is the yield stress (Pa).
The flow behavior index (n) and consistency index (k) were estimated by employing non-linear regression analysis of shear-rate/shear-stress data using the Statistica software (STATSOFT 1995).
The suitability of the rheological models was compared by determining the root mean square (RMS) error (%) according to the following Eq. (4).
| 4 |
where; N is the number of data point and τi and τp indicated the experimental and predicted shear stress data, respectively.
Statistics
Analysis of variance was applied to determine the effect of salep concentration and temperature on the flow behavior index and apparent viscosity at shear rate of 1 s−1 values. For each main effect and interaction, a multiple comparison of means was performed using the least significant difference test (LSD) (p < 0.05). These analyses were performed with the Statistica software (STATSOFT 1995).
Results and discussion
Characterization of flow properties
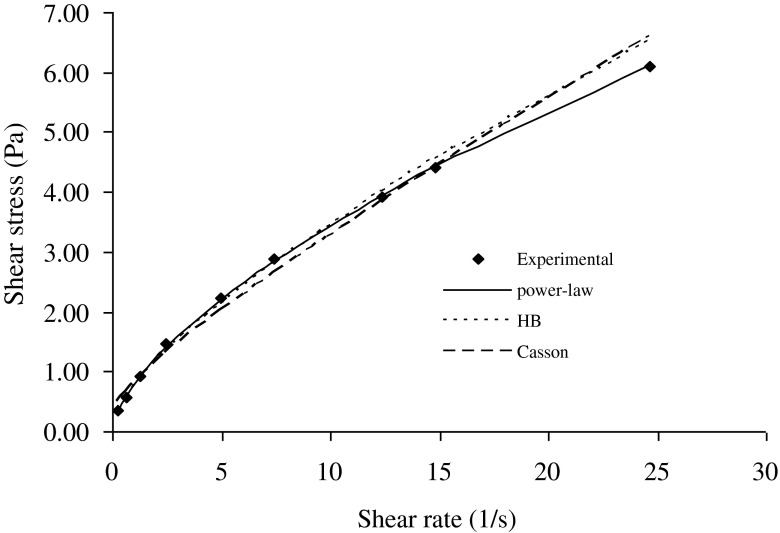
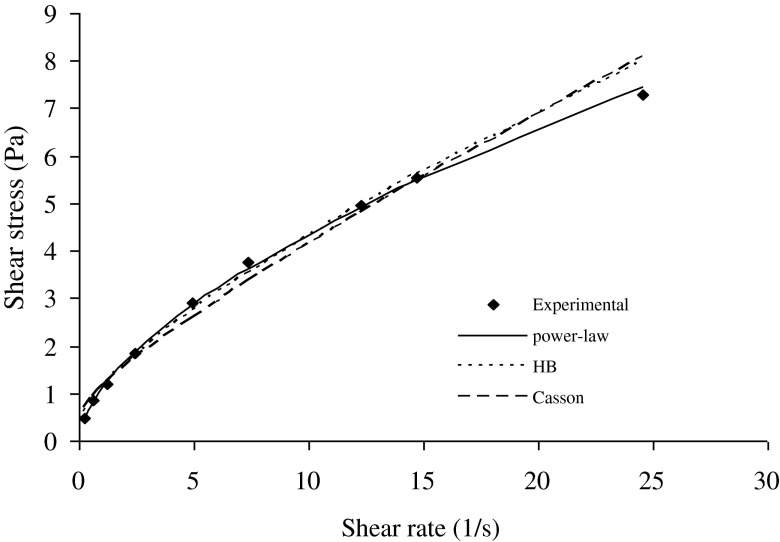
Mixtures of salep and milk were prepared at different concentrations of salep, with salep contents of 0.75 %, 1.0 % and 1.25 %. The shear rate /shear stress data obtained from forward and backward directions were analyzed to examine of fitting by common rheological models. The summary data for all three models was shown in Tables 1, 2, 3 and 4 respectively. The variation of shear stress values with shear rate were determined at different concentration of salep. When the salep concentration was 1.0 and 1.25 %, at low shear rates (<1.23 s−1), the predicted values by using Herschel-Bulkey, and Casson models were higher than the experimental values (Figs. 1 and 2). The predicted values were lower than the experimental values 12.24 s−1 shear rate, while the predicted values were again higher than the experimental values at high shear rates (>12.24 s−1). This difference between power law and Herschel-Bulkey or Casson models can be explained in terms of yield stress. Latha et al. (2002) reported that the calculated yield stress in rice flour dispersions as determined by Herschel-Bulkey or Casson models, were higher than those determined experimentally. Similar result by using Casson model had been reported for rice-blackgram dispersions (Bhattacharya and Bhat 1997). In our study, the yield stress of salep-milk mixture was not determined experimentally. However, the calculated yield stress values may be much higher than the experimental ones and due to the absence of yield stress term in power-law model, the experimental data on shear-rate/shear-stress fits well to power-law model. For power-law model, these results were supported by low RMS error values ranging between 0.02 and 1.27 %. In 1.00 and 1.25 % salep concentrations, similar trends were observed for increasing and decreasing shear rate curves at all temperatures.
Table 1.
Rheological parameter of the power law (Eq. 1) used to describe the flow curves of salep milk mixtures at different salep content and different temperature
| Salep concentration (%) |
Temperature (°C) |
Forward measurement | Backward measurement | ||||
|---|---|---|---|---|---|---|---|
|
k
(Pa.sn) |
n | R2(a) |
k
(Pa.sn) |
n | R2(a) | ||
| 0.75 | 65 | 0.124 | 0.811 | 0.987 | 0.135 | 0.802 | 0.991 |
| 60 | 0.158 | 0.754 | 0.993 | 0.144 | 0.785 | 0.997 | |
| 55 | 0.181 | 0.749 | 0.992 | 0.182 | 0.746 | 0.992 | |
| 50 | 0.179 | 0.750 | 0.999 | 0.185 | 0.752 | 0.999 | |
| 45 | 0.211 | 0.735 | 0.994 | 0.218 | 0.729 | 0.994 | |
| 1.00 | 65 | 0.429 | 0.697 | 0.999 | 0.439 | 0.701 | 0.999 |
| 60 | 0.543 | 0.668 | 0.999 | 0.513 | 0.691 | 0.999 | |
| 55 | 0.733 | 0.661 | 0.998 | 0.715 | 0.648 | 0.999 | |
| 50 | 0.804 | 0.634 | 0.999 | 0.719 | 0.663 | 0.999 | |
| 45 | 0.886 | 0.615 | 0.999 | 0.818 | 0.629 | 0.999 | |
| 1.25 | 65 | 0.727 | 0.685 | 0.996 | 0.809 | 0.663 | 0.998 |
| 60 | 0.902 | 0.645 | 0.999 | 0.905 | 0.647 | 0.997 | |
| 55 | 1.111 | 0.595 | 0.999 | 1.106 | 0.605 | 0.997 | |
| 50 | 1.212 | 0.589 | 0.999 | 1.284 | 0.582 | 0.998 | |
| 45 | 1.400 | 0.584 | 0.998 | 1.419 | 0.582 | 0.997 | |
aR2 is determination coefficient
Table 2.
Rheological parameter of the Casson model (Eq. 2) used to describe the flow curves of salep milk mixtures at different salep content and different temperature
| Salep concentration (%) |
Temperature (°C) |
Forward measurement | Backward measurement | ||||
|---|---|---|---|---|---|---|---|
| kc
(Pa0.5 s0.5) |
σc
(Pa) |
R2(a) | kc
(Pa0.5 s0.5) |
σc
(Pa) |
R2(a) | ||
| 0.75 | 65 | 0.249 | 0.008 | 0.999 | 0.245 | 0.016 | 0.999 |
| 60 | 0.247 | 0.026 | 0.998 | 0.248 | 0.022 | 0.998 | |
| 55 | 0.250 | 0.017 | 0.998 | 0.263 | 0.022 | 0.999 | |
| 50 | 0.279 | 0.018 | 0.999 | 0.280 | 0.019 | 0.999 | |
| 45 | 0.280 | 0.028 | 0.999 | 0.277 | 0.033 | 0.999 | |
| 1.00 | 65 | 0.365 | 0.082 | 0.994 | 0.368 | 0.088 | 0.991 |
| 60 | 0.348 | 0.092 | 0.986 | 0.410 | 0.085 | 0.987 | |
| 55 | 0.416 | 0.173 | 0.980 | 0.417 | 0.180 | 0.992 | |
| 50 | 0.419 | 0.236 | 0.989 | 0.424 | 0.179 | 0.985 | |
| 45 | 0.415 | 0.295 | 0.986 | 0.419 | 0.239 | 0.988 | |
| 1.25 | 65 | 0.443 | 0.181 | 0.982 | 0.437 | 0.227 | 0.983 |
| 60 | 0.463 | 0.217 | 0.983 | 0.464 | 0.214 | 0.972 | |
| 55 | 0.452 | 0.363 | 0.984 | 0.446 | 0.393 | 0.973 | |
| 50 | 0.455 | 0.432 | 0.977 | 0.470 | 0.423 | 0.966 | |
| 45 | 0.495 | 0.460 | 0.969 | 0.497 | 0.463 | 0.965 | |
aR2 is determination coefficient
Table 3.
Rheological parameter of the Herschel-Bulkley (Eq. 3) used to describe the flow curves of salep milk mixtures at different salep content and different temperature
| Salep concentration % |
Temperature (°C) |
Forward measurement | Backward measurement | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
k
(Pa.sn) |
n | σ0
(Pa) |
R2(a) |
k
(Pa.sn) |
n | σ0
(Pa) |
R2(a) | ||
| 0.75 | 65 | 0.072 | 1.006 | 0.047 | 0.998 | 0.080 | 0.976 | 0.063 | 0.998 |
| 60 | 0.083 | 0.996 | 0.080 | 0.999 | 0.078 | 1.068 | 0.081 | 0.999 | |
| 55 | 0.083 | 0.971 | 0.061 | 0.998 | 0.085 | 1.027 | 0.069 | 0.993 | |
| 50 | 0.094 | 0.995 | 0.090 | 0.998 | 0.097 | 0.989 | 0.087 | 0.995 | |
| 45 | 0.106 | 0.972 | 0.099 | 0.998 | 0.106 | 0.982 | 0.099 | 0.995 | |
| 1.00 | 65 | 0.300 | 0.839 | 0.095 | 0.995 | 0.299 | 0.855 | 0.101 | 0.990 |
| 60 | 0.407 | 0.7355 | 0.086 | 0.999 | 0.442 | 0.772 | 0.092 | 0.998 | |
| 55 | 0.596 | 0.724 | 0.163 | 0.995 | 0.476 | 0.792 | 0.209 | 0.995 | |
| 50 | 0.624 | 0.725 | 0.149 | 0.997 | 0.590 | 0.728 | 0.128 | 0.996 | |
| 45 | 0.721 | 0.682 | 0.163 | 0.996 | 0.647 | 0.708 | 0.157 | 0.997 | |
| 1.25 | 65 | 0.587 | 0.751 | 0.167 | 0.995 | 0.677 | 0.709 | 0.167 | 0.997 |
| 60 | 0.632 | 0.799 | 0.186 | 0.990 | 0.658 | 0.770 | 0.176 | 0.976 | |
| 55 | 0.751 | 0.724 | 0.349 | 0.996 | 0.880 | 0.669 | 0.279 | 0.995 | |
| 50 | 0.821 | 0.717 | 0.365 | 0.991 | 0.907 | 0.694 | 0.370 | 0.991 | |
| 45 | 1.125 | 0.654 | 0.269 | 0.994 | 1.037 | 0.684 | 0.232 | 0.990 | |
aR2 is determination coefficient
Table 4.
Rheological parameter of the Bingham model (Eq. 5) used to describe the flow curves of salep milk mixture at 0.75 % salep content and different temperature
| Temperature (°C) | Forward measurement | Backward measurement | ||||
|---|---|---|---|---|---|---|
| μ (Pa s) |
σo
(Pa) |
R2(a) | μ (Pa s) |
σo
(Pa) |
R2(a) | |
| 65 | 0.072 | 0.040 | 0.999 | 0.073 | 0.064 | 0.999 |
| 60 | 0.080 | 0.089 | 0.999 | 0.077 | 0.081 | 0.999 |
| 55 | 0.085 | 0.087 | 0.999 | 0.085 | 0.091 | 0.999 |
| 50 | 0.091 | 0.091 | 0.999 | 0.091 | 0.099 | 0.999 |
| 45 | 0.094 | 0.121 | 0.999 | 0.095 | 0.132 | 0.998 |
aR2 is determination coefficient
Fig. 1.
Sample rheogram of a salep-milk mixture (1.00 % salep content at 55 °C) showing the fitting by power law, Herschel-Bulkley and Casson models
Fig. 2.
Sample rheogram of a salep-milk mixture (1.25 % salep content at 55 °C) showing the fitting by power law, Herschel-Bulkley and Casson models
The flow of chocolate milk beverages has been characterized by Yanes et al. (2002a) as Newton, Ostwald-de Waale, and Bingham models, depending on temperature, on the types of milk used in formulation and on both concentration and type of hydrocolloid. This qualitative differences in the rheological parameters among chocolate milk beverages has been attributed to the possible interactions between type of hydrocolloid and casein micelles. The shape of rheogram (shear-stress versus shear rate plot) of salep-milk mixture at 0.75 % salep content was different from the other rheograms obtained with 1.00 and 1.25 % salep content. When shear rate was increased, the relation between shear rate and shear stress was apparently linear. However, at this salep concentration, apparent viscosity was not constant and apparent viscosity was dependent on shear rate. When shear rate was increased apparent viscosity showed decreased. Therefore the shear rate /shear stress data were examined by power-law, Herschel-Bulkey, Casson and Bingham plastic models. Equation 5 was used for Bingham plastic model.
| 5 |
where; μ is plastic viscosity (Pa s) and σo is yield stress (Pa).
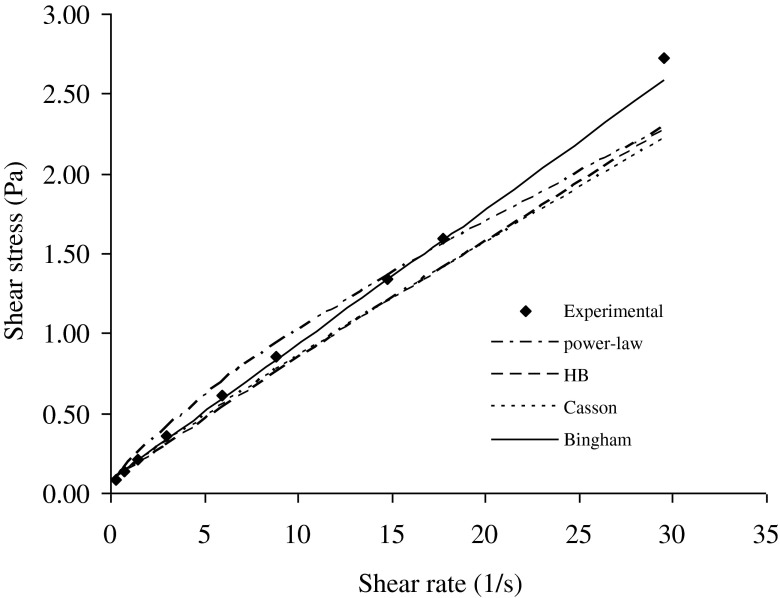
When the salep concentration was 0.75 %, the predicted values by using Herschel-Bulkey, and Casson models were generally lower than the experimental values (Fig. 3). However, at low shear rate, the predicted values by using power-law model were higher than the experimental values, while the predicted values were lower than the experimental values at above the 14.75 s−1. The nearly straight line in Fig. 3 indicated that it seems Newtonian behavior. However, Bingham plastic model describes well the flow data with the highest R2 and the lowest RMS error values for data especially obtained at 55 °C, 60 °C, and 65 °C. The experimental results showed that the salep-milk mixtures exhibited more linear trend when temperature was higher. The maximum RMS error values of power law, Herschel-Bulkey, Casson and Bingham plastic models at all temperature were 1.96 %, 1.60 %, 1.86 %, 0.12 %, respectively.
Fig. 3.
Sample rheogram of a salep-milk mixture (0.75 % salep content at 55 °C) showing the fitting by power law, Herschel-Bulkley, Casson and Bingham models
At all salep concentrations, the values of flow behavior index (n) were less than 1, and so, the mixtures of salep and milk had a shear thinning behavior. At 0.75 % salep concentration, flow behavior index (n) values were close to 1, and mixtures exhibited yield stress with a small magnitudes. However above this stress, mixture showed simple Newtonian flow. The mixture had more observed Newtonian behavior when temperature was getting high. Similar results were obtained by Kaya and Tekin (2001). They reported that n values of the water-salep-sugar mixes showed a gradual change from Newtonian to non-Newtonian behavior with increasing salep concentration.
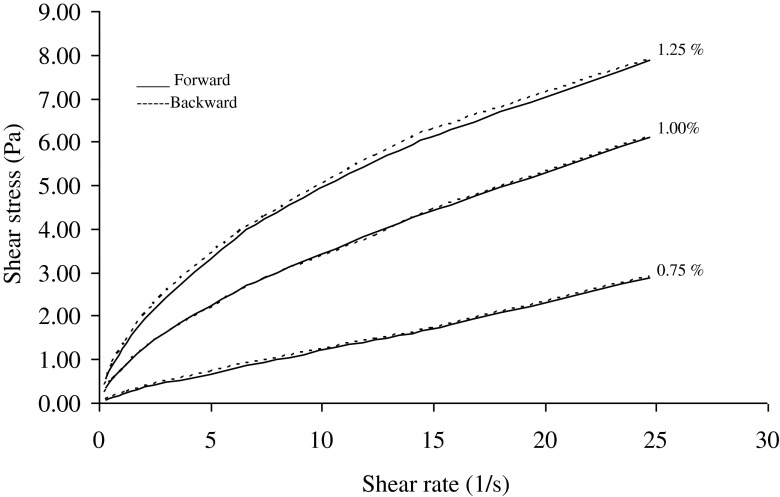
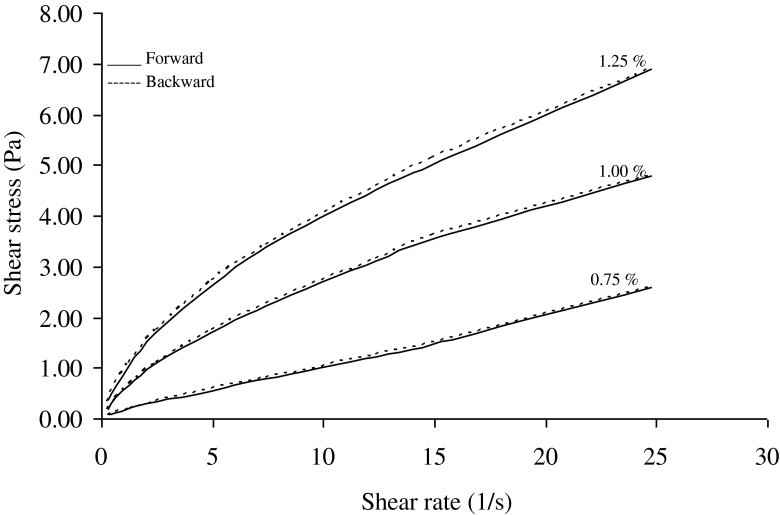
The flow behavior index (n) values, calculated according to the power law and Herschel-Bulkey models for 1.00 % salep concentration were higher than n values calculated for 1.25 % salep concentration (Tables 1 and 3). Flow behavior index (n), according to the power law and Herschel-Bulkey models for 1.00 and 1.25 % salep concentration decreased when temperature were decreased. Similar pattern was also observed for backward curve. These experimental results showed that salep-milk mixtures at 1.25 % salep concentration were more shear thinning when the temperature was lower. Flow behavior index (n) values of Herschel-Bulkey model were higher than those of power-law model because of the existence of yield stress term in the Herschel-Bulkey model. When the n values of increasing shear-rate curves were compared to the decreasing curves, the n values were very close. Figures 4 and 5 showed the flow curves of mixtures of salep and milk at 50 and 60 °C of both in the forward (increasing shear rate) and backward (decreasing shear rate) directions. Forward and backward curves of mixtures of salep and milk fit into one another. In the other temperature (45, 55 and 65 °C) forward and backward curves of mixtures showed similar trend to curves obtained at 50 and 60 °C. This behavior and very close n values indicated that the shearing (over) time did not affect the structure of mixture.
Fig. 4.
The shear rate /shear stress data obtained from forward and backward directions of salep milk mixtures at 50 °C
Fig. 5.
The shear rate /shear stress data obtained from forward and backward directions of salep and milk mixtures at 60 °C
The consistency index (k) and flow behavior index values depended on the model employed for computation, and the use of power law equation lead to prediction of high k values (Tables 1, 2 and 3). As expected, the consistency index (k) or plastic viscosity value (μ) decreased with temperature for all using models. An increase in salep content markedly increased the consistency index (k). This result agreed with some previous studies by Latha et al. (2002), Gόmez-Diaz and Navaza (2003), Nickerson et al. (2003), Toğrul and Arslan (2003), and Bhattacharya et al. (2004), regarding steamed rice flour dispersions, food additives such as carboxymethyl cellulose and alginate, gellan solutions, sugar beet pulp cellulose, and blackgram four dispersions, respectively.
Effect of temperature and salep concentration on rheological parameter
Mixtures of salep and milk prepared with 1.25 % salep concentration exhibited the highest viscosity because of increasing the water holding capacity (Figs. 4 and 5). Arrhenius relationship has been used to describe the rheological parameter by many researchers (İbanoğlu, and İbanoğlu 1999; Maskan and Göğüş 2000; Gόmez-Diaz and Navaza 2003; Herceg and Lelas 2005). Yanes et al. (2002b) reported that using of apparent viscosity at a shear rate of 1 s−1, instead of consistency index values, is to be acceptable when comparison of experimental data with same unit. Therefore in our study, apparent viscosity values at shear rate of 1 s−1 were used for comparison of experimental data obtained from 1.00 to 1.25 % salep concentration. The temperature dependency of the apparent viscosity values at shear rate of 1 s−1 (μ1) for 1.00 and 1.25 % salep concentration, according to the power law, was analyzed using an Arrhenius type equation.
| 6 |
where; μ10= The Arrhenius constant (Pa s) at a reference temperature (T = ∞), Ea= activation energy (kJ/kmol), R= universal gas constant (8.314 kJ/kmol K), and T= absolute temperature (K). The same equation was applied to the plastic viscosity value (μ) for 0.75 % salep concentration. Activation energy values for apparent viscosity at shear rate of 1 s−1 for 1.00 and 1.25 % salep concentration were 32,945 kJ/kmol (R2 = 0.981) and 28,697 kJ/kmol (R2 = 0.991), respectively. In 0.75 % salep concentration, activation energy (Ea) value for plastic viscosity (μ) was 11,972 kJ/kmol (R2 = 0.968). The lowest energetic barrier to viscous flow was found in the mixtures prepared with 0.75 % salep content.
The flow behavior index values of the mixtures of 1 % salep concentration were higher than that of mixtures of 1.25 % salep concentration in all temperature (Table 1). Temperature increasing from 45 to 65 °C leads to lowered the pseudoplastic properties of mixtures in both salep concentration. The increasing of flow behavior index with increasing of temperature can be explained by the milk protein structure changes. When temperature was increased, milk proteins such as whey proteins unfold and hidden hydrophobic groups inside globules become accessible and therefore the water binding capacity significantly decreases. As a result, flow behavior index increased and apparent viscosity at 1 s −1 decreased.
Conclusions
Salep-milk mixture at different salep concentrations (0.75, 1.00, 1.25 % w/v) and at different temperatures (45, 50, 55, 60, and 65 °C) indicates that they behave as shear-thinning fluids. Salep-milk mixture rheology at 0.75 % salep concentration can be described by Bingham model during successive forward and backward shearing sequence. However, power law model fitted the shear rate/shear stress quite well at 1.00 and 1.25 % salep concentrations.
Both in the forward and backward measurements, the flow behavior index, n, is less than unity, and consistency coefficient, decreased with temperature but increased with salep concentration.
References
- Arduzlar D, Boyacioğlu H (2003) The effect of starch type on rheological properties of traditional salep drink. Poster 372, AACC Annual Meeting, September 28 - October 2, Oregon Convention Center, Portland, Oregon, USA
- Benincasa MA, Cartoni G, Delle FC. Flow field-flow fractionation and characterization of ionic and neutral polysaccharides of vegetable and microbial origin. J Chromatogr A. 2002;967(2):219–234. doi: 10.1016/S0021-9673(02)00756-2. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S, Bhat KK. Steady shear rheology of rice-blackgram suspensions and suitability of rheological models. J Food Eng. 1997;32:241–250. doi: 10.1016/S0260-8774(97)00027-7. [DOI] [Google Scholar]
- Bhattacharya S, Latha RB, Bhat KK. Controlled stress rheological measurement of blackgram flour dispersions. J Food Eng. 2004;63:135–139. doi: 10.1016/S0260-8774(03)00291-7. [DOI] [Google Scholar]
- Buchala AJ, Franz G, Meier H. A glucomannan from the tubers of Orchis morio. Phytochemistry. 1974;13:163–166. doi: 10.1016/S0031-9422(00)91286-X. [DOI] [Google Scholar]
- Cankan K (2001) Beverages: beyond Turkish coffee and ayran. http://www.Cankan.com/gturkishcuisine/47tc-beverages.htm
- Farhoosh R, Rıazı A. A compositional study on two current types of salep in Iran and their rheological properties as a function of concentration and temperature. Food Hydrocoll. 2007;21(4):660–666. doi: 10.1016/j.foodhyd.2006.07.021. [DOI] [Google Scholar]
- Gόmez-Diaz D, Navaza JM. Rheology of aqueous solutions of food additives: effect of concentration, temperature and blending. J Food Eng. 2003;56:387–392. doi: 10.1016/S0260-8774(02)00211-X. [DOI] [Google Scholar]
- Güven M, Karaca OB, Kacar A. The effects of the combined use of stabilizers containing locust bean gum and of the storage time on Kahramanmaraş-type ice creams. Int J Dairy Technol. 2003;56:223–228. doi: 10.1046/j.1471-0307.2003.00108.x. [DOI] [Google Scholar]
- Herceg Z, Lelas V. The influence of temperature and solid matter content on the viscosity of whey protein concentrates and skim milk powder before and after tribomechanical treatment. J Food Eng. 2005;66:433–438. doi: 10.1016/j.jfoodeng.2004.04.012. [DOI] [Google Scholar]
- İbanoğlu Ş, İbanoğlu E. Rheological properties of cooked tarhana, a cereal-based soup. Food Res Int. 1999;32:29–33. doi: 10.1016/S0963-9969(99)00069-1. [DOI] [Google Scholar]
- Juers DH, Swenson HA, Kurath SF. Configuration and hydrodynamic properties of fully acetylated salep glucomannan. J Polym Sci. 1967;5:361–375. doi: 10.1002/pol.1967.110050502. [DOI] [Google Scholar]
- Kaya S, Tekin AR. The effect of salep content on the rheological characteristics of a typical ice-cream mix. J Food Eng. 2001;47:59–62. doi: 10.1016/S0260-8774(00)00093-5. [DOI] [Google Scholar]
- Kayacier A, Dogan M. Rheological properties of some gums-salep mixed solutions. J Food Eng. 2006;72:261–265. doi: 10.1016/j.jfoodeng.2004.12.005. [DOI] [Google Scholar]
- Krokida MK, Maroulis ZB, Saravacos GD. Rheological properties of fluid fruit and vegetable puree products: compilation of literature data. Int J Food Prop. 2001;4:179–200. doi: 10.1081/JFP-100105186. [DOI] [Google Scholar]
- Latha RB, Bhat KK, Bhattacharya S. Rheological behaviour of steamed rice flour dispersions. J Food Eng. 2002;51:125–129. doi: 10.1016/S0260-8774(01)00048-6. [DOI] [Google Scholar]
- Marchessault RH, Buleon A, Deslandes Y. Structural studies on triacetates of mannan and glucomannan. Carbohydr Polym. 1981;1:129–138. doi: 10.1016/0144-8617(81)90005-9. [DOI] [Google Scholar]
- Maskan M, Göğüş F. Effect of sugar on the rheological properties of sunflower oil–water emulsions. J Food Eng. 2000;43:173–177. doi: 10.1016/S0260-8774(99)00147-8. [DOI] [Google Scholar]
- Mitschka P. Simple conversion of Brookfield R.V.T. readings into viscosity functions. Rheol Acta. 1982;21:207–209. doi: 10.1007/BF01736420. [DOI] [Google Scholar]
- Nickerson MT, Paulson AT, Speers RA. Rheological properties of gellan solutions: effect of calcium ions and temperature on pre-gel formation. Food Hydrocoll. 2003;17:577–583. doi: 10.1016/S0268-005X(02)00075-9. [DOI] [Google Scholar]
- Nishinari K, Zhang H, Ikeda S. Hydrocolloid gels of polysaccharides and proteins. Colloid Interface Sci. 2000;5:195–201. doi: 10.1016/S1359-0294(00)00053-4. [DOI] [Google Scholar]
- Ofoli RY. Interrelationships of rheology, kinetics, and transport phenomena in food processing. In: Faridi H, Faubion JM, editors. Dough rheology and baked product texture. New York: AVI; 1990. pp. 497–512. [Google Scholar]
- STATSOFT (1995) Statistica for Windows. Release 5.0. Statsoft Inc Tulsa, OK, USA
- Tekfidan N. Warm winter’s drink: Salep. Skylife. 2001;1:40–48. [Google Scholar]
- Tekinşen C, Karacabey A (1984) Bazı stabilizer karışımlarının Kahramanmaraş tipi dondurmanın fiziksel ve organoleptik nitelikleri üzerine etkisi. Tübitak, Proje No: VHAG 594
- Tekinşen KK, Guner A. Chemical composition and physicochemical properties of tubera salep produced from some Orchidaceae species. Food Chem. 2010;121:468–471. doi: 10.1016/j.foodchem.2009.12.066. [DOI] [Google Scholar]
- Tian B, Dong C, Chen L. Preparation of konjac glucomannan ester of palmitic acid and its emulsification. J Appl Polym Sci. 1998;67:1035–1038. doi: 10.1002/(SICI)1097-4628(19980207)67:6<1035::AID-APP10>3.0.CO;2-#. [DOI] [Google Scholar]
- Toğrul H, Arslan N. Flow properties of sugar beet pulp cellulose and intrinsic viscosity-molecular weight relationship. Carbohydr Polym. 2003;54:63–71. doi: 10.1016/S0144-8617(03)00146-2. [DOI] [Google Scholar]
- Tye RJ. Konjac flour: properties and application. Food Technol. 1991;45:82–92. [Google Scholar]
- Üçüncü M (1992) Süt Teknolojisi, Ege.Üniversitesi. Mühendislik Fakültesi Yayınları. No:88: 152–157 İzmir, Türkiye
- Yanes M, Durán L, Costell E. Rheological and optical properties of commercial chocolate milk beverages. J Food Eng. 2002;51:229–234. doi: 10.1016/S0260-8774(01)00061-9. [DOI] [Google Scholar]
- Yanes M, Durán L, Costell E. Effect of hydrocolloid type and concentration on flow behaviour and sensory properties of milk beverages model systems. Food Hydrocoll. 2002;16:605–611. doi: 10.1016/S0268-005X(02)00023-1. [DOI] [Google Scholar]
- Yasar K, Kahyaoğlu T, Şahan N. Dynamic rheological characterization of salep glucomannan/galactomannan-based milk beverages. Food Hydrocoll. 2009;23(5):1305–1311. doi: 10.1016/j.foodhyd.2008.11.005. [DOI] [Google Scholar]
- Yılmaz MT, Sert D, Karakaya M, Tıske SS. Optimization of the effect of sweetener and dietary fiber on rhelogical and sensory properties of salep beverage. J Texture Stud. 2010;41:804–824. doi: 10.1111/j.1745-4603.2010.00256.x. [DOI] [Google Scholar]
- Yoshimura M, Nishinari K. Dynamic viscoelastic study on the gelation of konjac glucomannan with different molecular weights. Food Hydrocoll. 1999;13:227–233. doi: 10.1016/S0268-005X(99)00003-X. [DOI] [Google Scholar]
- Yoshimura M, Takaya T, Nishinari K. Rheological studies on mixtures of corn starch and konjac-glucomannan. Carbohydr Polym. 1998;35:71–79. doi: 10.1016/S0144-8617(97)00232-4. [DOI] [Google Scholar]