Abstract
Purslane (Portulaca oleracea L.) has several health benefits, such as it reduces risk of CVD, obesity and diabetes. The objective of the study was to investigate the effect of different drying on retention of bioactive molecules, such as omega-3 fatty acids, total phenolic content and antiradical activity of purslane. Five different dehydration methods including microwave (100 MW, 5 min), tray, vacuum, low temperature low humidity, infrared were used at 55–60 °C for 5–7 h for dehydration of purslane. Three solvents, viz. water, ethanol and methanol were used for extraction of bioactive molecules from purslane. Total polyphenol content, antiradical activity and rehydration ratio of the bioactive molecules were determined. Results revealed that total PUFA, α-linolenic acid (ALA), total polyphenol content and antiradical activity were found to retain in the dried purslane in the range of (47.9–59.9 %), (42.5–50 %), (188–408GAE/100 g) and (33.0–88.8 mg/100 g) respectively. The highest values of ALA, total polyphenol content and antiradical activity were found to obtain in the vacuum dried sample. Rehydration ratio was found in the range of 3.2–4.3 and vacuum dried purslane showed maximum rehydration. It could be concluded that vacuum dehydration of purslane is an effective method for retention of bioactive molecules and good rehydration behaviour of dried purslane.
Keywords: Dehydration, Purslane, Retention, Bioactive molecules, Rehydration
Introduction
Green leafy vegetables (GLV) are multi-cultural components used in Indian cuisine. They are rich sources of iron, calcium, β-carotene, dietary fiber, vitamin C and many trace minerals. A huge number of leaves from different sources, such as perennial trees, aquatic plants are consumed especially in rural areas. These vegetables are an economic source to ensure the micronutrient intake. GLV are seasonal and also highly perishable due to their high water content. There are heavy losses due to non-availability of sufficient storage, transport and proper processing facilities at the production point (Pande et al. 2000). There is a need to preserve the nature’s storehouse of nutrients through convenient processing techniques. Dehydration seems to be the simplest technology for preserving GLV, especially when they are abundantly available (Sheetal et al. 2013). Interest on these bioactive compounds have arisen because of their ability to exhibit a wide range of biological effects, including antibacterial, antiviral, anti-inflammation, antiallergic, antithrombotic and vasodilatory (Cook and Sammans 1996). As bioactive compounds are very susceptible to temperature, choosing the right drying method can be the input for a successful action. Vegetables growers go on to dry under sun, which yields unhealthy and poor quality product. Leafy vegetables are important in the diet as they are good source of ascorbic acid, β-carotene and fibre. Among the several methods of food preservation described in the literature to assure their availability throughout the year, solar-drying technique has received much emphasis in the tropics (Ajayi and Osifo 1977; Isong 1977) for vegetables, in particular.
Portulaceae is a family of annual or perennial herbs. It contains about 19 genera and 500 species. Portulaca is an important genus of this family includes 150 species. Portulaca oleracea (Portulacaceae family) is listed in the World Health Organization as one of the most used medicinal plants and termed as ‘Global Panacea’ (Dweck 2001; Samy et al. 2004). The mixture of phytochemicals present in many of these plants contributes to their protective and health effects. Purslane has been studied in detail as a prolific weed, but very little is known about its production as a food crop and the effects of cultural conditions on its nutritional value. There have been some studies carried out to determine the best cultural conditions to obtain higher levels of fatty acids and lower levels of oxalic acid in the leaves. The leaves of this plant used for culinary purposes give low yields of oil, but are rich sources of essential fatty acids, such as α-linolenic acid (C18:3 n3) and linoleic acid. P. oleracea is in use since antiquity in Unani and Ayurvedic medicines for various ailments, such as skin diseases, fever, dysentery, diarrhea, bleeding piles, kidney, liver, spleen diseases etc. The versatile biological activities of this plant have been proven on scientific parameters which are attributed to its phytochemical constituents like saponin, flavonoids, omega-3 fatty acids, phenolic acid, etc. It mainly possesses anti-tussive, diuretic, hypolipidemic, antiulcerogenic, anti-inflammatory, anticonvulsant, antimicrobial properties etc. (Arshiya and Khaleequr 2013).
According to Schieber et al. (2001), the loss of macromolecules like flavonoid during heat treatment might be due to the harsh drying conditions, in particular, the temperature and duration used (Davey et al. 2000). However, polyphenolics and fatty acids especially PUFA are sensitive to heat and oxygen. Several studies have evaluated the effects of different drying methods on the biochemical changes of fruit and vegetables (Khanal et al. 2010). The minimum loss of bioactive compounds was found at drying temperature not higher than 50 °C (Raghavan and Orsat 2007). For fully benefit from purslane, it is critical to develop drying conditions that can maximize the retention of bioactive compounds while remaining economically feasible. The conventional hot-air drying of vegetables normally involves thermal and/or chemical pre- treatment and drying at higher temperature for long drying time. This causes darkening in colour, loss in flavour and decrease in rehydration ability. Freeze drying produces a high quality product, but being an expensive process, its application for vegetable drying is limited. Dehydration processes affect to a varying degree of quality attributes, such as colour, texture and nutrient retention (Karel et al. 1993). They can also develop undesirable biochemical reactions such as deterioration of aroma compounds or degradation of nutritional substances (Achanta and Okos 1995). Such variation in nutrient retention and other quality attributes may be due to the type of vegetables, differences in the stage of maturity, harvesting method, cultural practices and type of pre-treatment and drying method (Luh and Woodroof 1975). All the above mentioned physical and biochemical changes certainly cause a reduction in product quality and process efficiency (Chuy and Labuza 1994) particularly when dealing with high-value foods. Therefore, the choice of the right method of preservation could be the key for a successful operation. The information related to the effect of dehydration of purslane on retention of bioactive molecules and its rehydration behaviour is scanty. The objective of the study was to investigate the impact of different dehydration techniques of purslane on retention of bioactive molecules such as omega-3 fatty acids, total phenolic content and antiradical activity and rehydration behaviour of the dehydrated leaves.
Materials and methods
Procurement of chemicals and reagents
All the chemical and solvents procured were of analytical grade. The β-carotene, Folin–Ciocalteu’s phenol reagent and 1,1-Diphenyl-2-picrylhydrazyl (DPPH) were obtained from Sigma-Aldrich, Germany. All the organic solvents used for extraction of fatty acids were of HPLC grade (Rankem, Delhi, India) {Standard mixtures of fatty acid methyl esters (C8-C24) (Cat No.18918-1AMP)} were procured from Supelco-Sigma Aldrich, Bangalore. All the determinations were done in triplicate.
Procurement of plant materials
Purslane was collected directly from the super market of Mysore. The leafy vegetables (LV) were de-rooted, washed thoroughly in tap water to remove adhering mud particles, rinsed in distilled water and excess water was drained off.
Dehydration of purslane leaves
Five different dehydration methods such as microwave (100 MW, 5 min), tray, vacuum, low temperature low humidity and infrared were used at 55–60 °C for 5–7 h for dehydration of purslane. Moisture content of microwave, vacuum, LTLH, tray and infrared dried purslane leaves was analyzed using the AOAC oven method (AOAC 1998).
Preparation of purslane extract
The parts of the plant suitable for consumption were used, which consisted of the soft upper stems of the plant and leaves. The discoloured and insect-damaged portions were discarded. At the initial stage, three different solvents (methanol, ethanol and water) were used for extraction of the plant material to determine the solvent that has the highest extraction efficiency.
Two grams of the plant were weighed; liquid nitrogen was added and ground into fine powder with mortar and pestle. One hundred millilitres of solvent was added to the finely ground plant and the mixture was swirled in an orbital shaker for 1 h. To test for extraction efficiency of each solvent, second and third extractions were made by transferring the residue from the first extraction into another flask. 100 ml of solvent was added to the extracts; the mixture was filtered and stored in a −20 °C freezer. All the analyses were completed subsequently.
Determination of flavonoids from purslane extract
One gram of sample was weighed into a 60 mL test tube with screw cap. An aliquot of 40 mL 80 % methanol, 10 mL 6 M HCl and 80 mg ascorbic acid were added. The tube was flushed with nitrogen for 30 s, sealed tightly, and incubated in a water bath at 90 °C for 2 h. After acid hydrolysis, the tube was allowed to cool, brought up to 50 mL with methanol, sonicated for 10 min, and centrifuged at 4000 g for 10 min. Five milliliters of supernatant was dried with a rotary evaporator. The residual was re-dissolved in 5 mL methanol. Approximately 3 mL was filtered through a Millex_-GN 0.2 lm syringe filter (Millipore, Bedford, MA). The filtrate was mixed with a constant level of internal standard (isoferulic acid, 50 mg/mL) prior to HPLC analysis.
The HPLC–MS system was composed of Shimadzu LC- 20 AD HPLC system, Shimadzu SPD-20AV UV–vis detector, and Shimadzu 2010EV mass spectrometer fitted with an electro-spray interface (Shimadzu, Kyoto, Japan). The column used was Alltech Prevail C-18 (Alltech, Deerfield, IL), 150 mm × 4.6 mm. The mobile phase used was 0.05 % (v/v) aqueous acetic acid (phase A) and 0.05 % acetic acid in 80 % acetonitrile + 20 % methanol (phase B) at a flow rate of 0.8 mL/min. The linear gradient of phase B was 5 % for the first 2 min, increased from 5 % to 40 % from 2 to 48 min, maintained at 40 % from 48 to 57 min, and decreased from 40 to 5 % from 57 to 57.1 min. Finally, isocratic elution with 5 % phase B was maintained until 65 min. UV–vis detector wavelength was set at 280 nm. Mass spectra were acquired in negative ion mode. Ion was scanned from m/z 150–600 with scan speed 1000 amu/s. Nebulizing gas flow used was 1.5 L/min. Drying gas pressure was 0.1 MPa.
Determination of total phenol content (TPC)
Total phenol content was determined using method developed by Singleton and Rossi (1965). One and a half milliliter of Folin–Ciocalteu’s reagent (diluted 10 times) and 1.2 ml of Na2CO3 (7.5 % w/v) solution were added to 300 ml of plant extract. Mixtures were shaken and left to stand at room temperature (25 ± 2 °C) for 30 min before measuring absorbance at 765 nm using spectrophotometer (Anthelie Advanced 5 Secoman, France). The total phenol content (TPC) was expressed as gallic acid equivalent in mg/100 g fresh plant material.
Determination of antioxidant activity
The ability of a compound to donate a hydrogen atom was assessed on the basis of the scavenging activity of the stable 1, 1-diphenyl-2-picrylhydrazyl (DPPH) radical according to a procedure based on (Miliauskas et al. 2004) with slight modifications. Two milliliters of 0.15 mM DPPH was added to 1 ml of extracts in different dilutions. A control was prepared by adding 2 ml of DPPH to 1 ml of methanol. The contents of the tubes were mixed, allowed to stand for 30 min and absorbance was measured at 517 nm using spectrophotometer (Anthelie Advanced 5 Secoman, France). Triplicate tubes were prepared for each extract. The results were expressed as:
| 1 |
Extraction of lipid and preparation of fatty acids methyl ester (FAME)
Total lipids were extracted by the method of Bligh and dyer (1959) with minor modifications. In brief, 0.5 g of dehydrated leaf sample was homogenized with the help of 2:1 chloroform –methanol solution. After homogenization, 1 % KCl (w/v) was added and content was filtered through filter paper. The filtrate was allowed to separate into two layers and lower (chloroform) phase was collected into pre weighted tube, dried in N2 vapour and total lipid content determined gravimetrically. Fatty acid methyl esters (FAME) were prepared by conventional BF3 Method (O’Fallon et al. 2007). About 500 μl of BF3 was added and dried under nitrogen gas and vortex well. The sample was kept on water bath at 65 ° C for 35 min, allowed to cool in refrigerator for 10 min and exposed in ambient temperature for10 min. About 500 μl hexane and 500 μl of water were added to the sample and allowed to vortex well. Then the upper layer was collected in presence of anhydrous sodium sulphate and injected to the GC. Peak identification of fatty acids was performed by comparison of the retention time with respective reference standards.
GC-MS analysis of fatty acids methyl ester (FAME)
FAMEs were analysed using GC-MS (Perkin Elmer, Turbomass Gold, Mass spectrometer) equipped with FID using fused silica Rtx-2330 column (Restek made, 30 m × 0.32 mm ID and 0.20 μm film thickness). Injector port and detector was set up at 230 and 250 ° C respectively. N2 was used as a carrier gas. Initially, column temperature was maintained at 120 ° C, followed by increasing to 220 ° C in 20 min, and hold at 220 ° C for 10 min. The fatty acids were identified by comparing their fragmentation pattern and retention time with authentic standards and also the NIST library.
Rehydration (reconstitution) of dried purslane sample
Rehydration means refreshing the dehydrated or dried products in water. Six beakers of each 500 ml capacity were taken and 150 ml of water and 5 g of dried sample were poured into each beaker and kept for 60 min for pre-soaking. The samples were transferred to another six beakers with 150 ml boiling water. Counting of time rehydration started during boiling. After optimum the leftover liquid was drained off and solid content was transferred to a Buchner funnel (4 in. diameter) separately fitted with filter paper (Whatman No. 1). The excess water was removed by applying a gentle suction for 10 s. The rehydrated materials were removed from the funnel, weighed individually. The rehydration ratio was calculated using the following relation (Pervin et al. 2008).
| 2 |
Measurement of colour
The colour of dehydrated samples was measured by a Konica Minolta, CM5, Japan). The instrument was standardized each time with white and black ceramic plate. The results were expressed as L* (whiteness/ darkness), a* (red/ green), and b* (yellow/ blue) values. The L*, a* and b* values (CIE system) were measured for the different samples at 10° view angle (Ranganna 1997).
Stastistical analysis
Values from triplicate determination of each sample were averaged and represented as mean with standard deviation. Data were analysed statistically by the MS Excel 2010 by one way ANOVA.
Results and discussion
Effect of dehydration on moisture content of purslane leaves
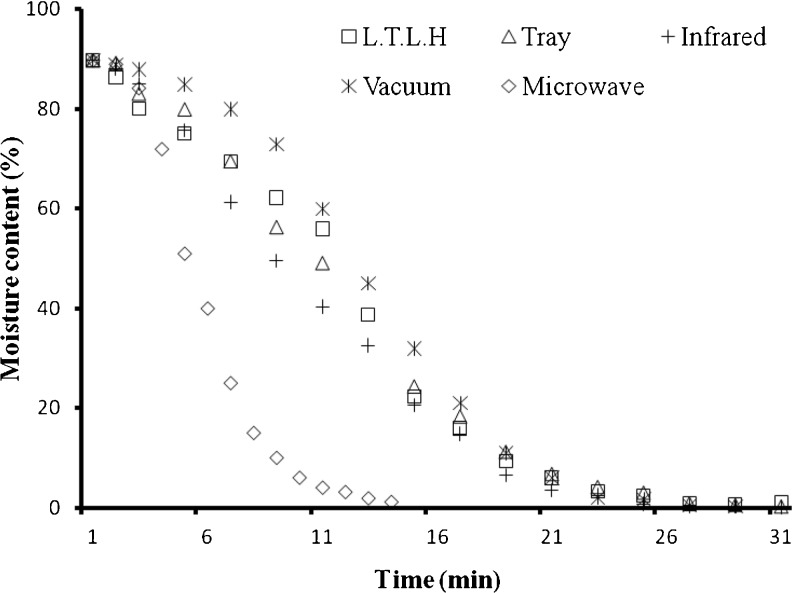
Moisture content (MC) of the dried purslane samples obtained using five drying methods mentioned above was found in between 0.12 and 0.66 % (Table 1), in which tray dried sample resulted retention of highest MC. The vacuum dried purslane leaves showed slow dehydration rates (Fig. 1) as compare to other dehydration techniques that may help to retain the bioactive components in the dried leaves.
Table 1.
Moisture content and rehydration ratio of dried purslane leaves
| Dehydration methods | |||||
|---|---|---|---|---|---|
| Moisture content (%) | Vacuum | L.T.L.H | Microwave | Tray | Infrared |
| 0.31 ± 0.5 | 0.66 ± 0.3 | 0.12 ± 0.8 | 0.54 ± 0.2 | 0.24 ± 0.4 | |
| Rehydration Ratio | 4.26 ± 0.4 | 3.31 ± 0.7 | 3.71 ± 0.3 | 3.2 ± 0.9 | 3.24 ± 0.5 |
Fig. 1.
Comparison of drying curves of purslane leaves at 65 ± 2 ° C using different dryers
Rehydration ratio of dried purslane leaves
In rehydration ratio was found maximum in case of vacuum dried purslane. It can be seen that rehydration ratios varied from different dehydration methods, and the rehydration ratios can be arranged by vacuum>microwave>l.t.l.h>infrared>tray drying (Table 1). The results indicated the best rehydration capacity of purslane from vacuum drying. The cell structure of purslane is expected to be less damaged due to slow drying rate and low temperature process of vacuum drying. The lowest rehydration capacity of purslane leaves was obtained from tray drying, which was conducted at a long time drying to reach desired moisture content and caused a serious contraction of the cell organization, resulting in a greatly damaged structure. It was noticed that rehydration ratios were increased by applying vacuum during drying. The vacuum condition leads to less damage and higher water holding capacity. The rehydration ratio of purslane from vacuum drying was greater than that of hot air drying possibly due to the puffing role of internal heating nature to make leaves less and produce more porous structure, leading to a reduced damage of leaf structure and better rehydration capacity with better product quality.
Effect of dehydration on colour of dried purslane leaves
In Table 2 purslane leaves that were processed by the five drying methods were distinguishable based on both visual and instrumental assessment of colour. Instrumentally, leaves underwent drying using tray, microwave and infrared drying were darker, with less green hues than the l.t.l.h and vacuum dried samples. Vacuum samples had the highest degree of lightness, than the others. The darker appearance of the others dried leaves and compared to the vacuum samples may be due to the exposure to heat during drying. (Abbatemarco and Ramaswamy 1995). In the vacuum drying system, drying at 65 ° C could preserve the color of the sample better than at other conditions. This is due to the fact that the main cause of color change in vacuum drying was chlorophyll degradation and nonenzymatic browning reaction, which is Maillard reaction and ascorbic acid oxidation since oxygen and light, which are the causes of these reactions existed at the lowest level in the system. Because Maillard reaction depends on temperature and duration of heat treatment (Chua et al. 2002), drying at lower temperatures gave better color retention than drying at higher temperatures.
Table 2.
Colour values of dried purslane leaves
| Drying methods | L* | a* | b* | Δ E |
|---|---|---|---|---|
| Vacuum | 43.07 ± 0.7 | −1.84 ± 0.2 | 20.12 ± 0.5 | 47.6 ± 0.6 |
| Tray | 36.49 ± 0.5 | 0.05 ± 0.3 | 11.08 ± 0.3 | 35.1 ± 0.7 |
| L.T.L.H | 39.21 ± 0.6 | −1.38 ± 0.4 | 18.58 ± 0.1 | 35.5 ± 0.1 |
| Microwave | 34.03 ± 0.2 | 0.31 ± 0.7 | 10.99 ± 0.4 | 35.4 ± 0.5 |
| Infrared | 30.54 ± 0.1 | 1.01 ± 0.5 | 6.88 ± 0.5 | 29.7 ± 0.3 |
Polyphenols, widely distributed in plants, contribute to fruit organoleptic and nutritive quality in terms of colour, taste, aroma, and flavour (Serrano et al. 2010), also being involved in astringent and bitter tastes. It is known that, amongst other factors, such as maturity stage or light exposure, phenolic composition varies with the cultivar. In addition, the phenolic profile has already been revealed to be a useful parameter for the discrimination of the different fruit parts (Ferreres et al. 2009). The intake of these compounds is an important health-protecting factor. Evidence for their role in the prevention of degenerative diseases is emerging. Experimental studies on animal and human cell lines have demonstrated that polyphenols can play a role in preventing cancer and cardiovascular diseases, when taken daily in adequate amounts (Wijngaard et al. 2009). Extraction is one of the most important steps in sample pretreatment. Generally, it is a separation process where the distribution of the analyte (in this case, a phenolic compound) between two immiscible phases is made in order to arrive at the appropriate distribution coefficient (Dobiáš et al. 2010). The extraction procedure is sequential and systematically carried out using an aqueous organic solvent to extract phenolic compounds in fruit and vegetable samples. This traditional method is called liquid liquid extraction (LLE) and different extraction solvents have been mentioned in the literature such as ethanol, acetone or methanol, or a mixture with water (Ross et al. 2009). Normally, extraction efficiency increases at higher extraction temperatures, but the working temperature affects the stability of the phenolic compounds, which also depends on their chemical structure. Thus, factors that influence the extraction processes (temperature, polyphenolic structure, pressure, sample characteristics, and other factors) are discussed using examples.
Effect of dehydration on total polyphenol content of dried purslane leaves
The Table 3 showed that among the three solvents, viz. water, methanol and ethanol, the methanol extract yielded the highest TPC (408 mg GAE/100 g dry weight) content. The TPC values of the ethanol extracts and water extracts were found lower, being 349.82 and 293 mg GAE/100 g, respectively in vacuum dried leaves. The water extract exhibited a slightly viscous texture which made it unsuitable for study. It is observed that methanol is the most suitable solvent in the extraction in polyphenolic compounds from plant tissue. This may be due to its ability to inhibit the action of polyphenol oxidase that causes the oxidation of polyphenols and its ease of evaporation as compared to water molecule (Yao et al. 2004). As far as the drying method is concerned, the total polyphenol content was found in the range of (188–408 GAE/100 g) and vacuum dried purslane showed maximum retention due to application of vacuum.
Table 3.
Antiradical activity and total polyphenol content of dried purslane leaves
| Dehydration methods | Antiradical activity (%) |
Total polyphenol content (mg GAE/100DW) |
||||
|---|---|---|---|---|---|---|
| Methanol | Ethanol | Water | Methanol | Ethanol | Water | |
| Vacuum | 107.23 ± 0.57a | 51.18 ± .86a | 54.43 ± 2.4a | 408.12 ± 1.5a | 349.82 ± 0.1a | 293.17 ± 0.6a |
| L.T.L.H | 97.86 ± 0.80b | 55.87 ± 2.03b | 54.80 ± 0.13b | 372.2 ± 1.5b | 349.48 ± 3.1b | 188.9 ± 2.0b |
| Microwave | 89.05 ± 1.38c | 57.28 ± 1.61c | 54.85 ± 2.01c | 322.92 ± 0.7c | 375.05 ± 2.1c | 273.35 ± 2.3c |
| Tray | 74.22 ± 2.27d | 31.26 ± 0.89d | 44.09 ± 0.77d | 273.9 ± 0.7d | 359.6 ± 0.3d | 231.4 ± 2.4d |
| Infrared | 50.58 ± 1.07e | 49.45 ± 1.09e | 96.79 ± 0.14e | 248.75 ± 1.9e | 265.67 ± 1.6e | 225.12 ± 1.5e |
Data followed by different letters within each column are significantly different (p < 0.05)
Effect of dehydration on anti-oxidant activity of dried purslane leaves
The anti-oxidant activity was found in the range of (31.8–97.0 mg/100 g) in which vacuum dried purslane samples showed the highest antiradical activity (Table 3). These bioactive compounds act as radical scavengers and retards or inhibit lipid autoxidation. They are essential antioxidants that protect against the propagation of the oxidative chain (Navarro et al. 2006).
Effect of dehydration on flavonoid content of dried purslane leaves
Five flavonols (0.34 to 4.85 mg/100 g) viz., rosmarinic, ferulic acid, quercetin, kaempferol, coumaric were identified in the dried purslane leaves (Table 4). The HPLC data of flavonol content showed more than 24 peaks. Among all the 5 samples, vacuum dried sample retain maximum content of flavonol content.
Table 4.
Flavonoids content vacuum dried purslane leaves
| Dehydration methods | Flavonoids content (mg/100 g dry wt.) | ||||
|---|---|---|---|---|---|
| Rosmarinic acid | Ferulic acid | Kaempferol | Quercetin | Coumaric acid | |
| Vacuum | 0.55 ± 0.02a | 4.85 ± 0.06 a | 0.41 ± 0.08 a | 0.92 ± 0.03 a | 0.34 ± 0.02 a |
| L.T.L.H | 0.41 ± 0.01 b | 4.01 ± 0.02 b | 0.34 ± 0.04 b | 0.81 ± 0.06 a | 0.21 ± 0.03 b |
| Microwave | 0.36 ± 0.05 c | 3.53 ± 0.02 c | 0.25 ± 0.06 c | 0.70 ± 0.03b | 0.18 ± 0.04 b |
| Tray | 0.31 ± 0.04 c | 3.30 ± 0.07 d | 0.18 ± 0.02 d | 0.63 ± 0.06 b | 0.12 ± 0.02 c |
| Infrared | 0.34 ± 0.02 c | 3.43 ± 0.03 d | 0.21 ± 0.08 d | 0.69 ± 0.04 b | 0.16 ± 0.06 b |
Data followed by different letters within each column are significantly different (p < 0.05)
Fatty acid profile of purslane
The Table 5 showed that the total PUFA and α-linolenic acid (ALA) were observed to retain in the dried purslane to an extent of (47.9–59.9 %) and (42.5–50 %) respectively. The highest value for ALA was observed in the vacuum dried sample. Polyunsaturated fatty acids (PUFAs) (ω-6 and ω-3) are essential lipids for human health. Actually, people from developed countries include large amounts of saturated and ω-6 PUFAs in their diets which are detrimental to their health leading to increasing the risk of CVD and other chronic diseases. Linolenic acid (ALA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are essential PUFAs because mammals cannot synthesize them de novo (Whelan and Rust 2006). They are indispensable components of the cell membrane, important in brain and retina, cell growth and division, platelet aggregation, inflammatory responses, haemorrhage, vasoconstriction and vasodilatation and immune functions. Recent studies have shown their role in prevention and treatment of CVD, diabetes, arthritis, cancer and other disorders. In general terms, the oxidation of one component induces the oxidation of the other. Lipids containing polyunsaturated fatty acids and their esters are easily oxidised by molecular oxygen by a free radical chain mechanism called autoxidation. In general terms, and depending on the storage conditions, storage of fatty products leads to a decrease in the content of the PUFAs fraction.
Table 5.
Fatty acid and distribution of SFA, MUFA and PUFA content of dried purslane leaves in during different drying methods
| Fatty acids composition (% area) | Vacuum | Tray | L.T.L.H | Microwave | Infrared |
|---|---|---|---|---|---|
| Capric acid (C10:0) | 1.97 ± 0.1 | 1.23 ± 0.4 | 0.85 ± 0.1 | 1.43 ± o.6 | 0.29 ± 0.9 |
| Lauric acid (C12:0) | 0.34 ± 0.4 | 0.66 ± 0.3 | 0.95 ± 0.3 | 1.58 ± 0.1 | 0.6 ± 0.5 |
| Myristic acid (C14:0) | 1.91 ± 0.3 | 0.68 ± 0.2 | 2.12 ± 0.4 | 1.73 ± 0.5 | 3.84 ± 0.4 |
| Palmitic acid (C16:0) | 20.02 ± 0.7 | 17.4 ± 0.4 | 19.54 ± 0.3 | 18.4 ± 0.7 | 20.34 ± 0.3 |
| Stearic acid (C18:0) | 2.22 ± 0.8 | 6.25 ± 0.1 | 1.14 ± 0.5 | 3.64 ± 0.8 | 4.87 ± 0.2 |
| Oleic acid (C18:1) | 3.79 ± 0.6 | 8.39 ± 0.7 | 2.93 ± 0.8 | 4.74 ± 0.5 | 4.38 ± 0.1 |
| Linoleic acid (C18:2) | 8.32 ± 0.9 | 10.68 ± 0.7 | 4.34 ± 0.6 | 9.8 ± 0.4 | 5.87 ± 0.6 |
| α-Linolenic acid (C18:3) | 52.65 ± 0.2 | 42.92 ± 0.7 | 50.63 ± 0.5 | 49.62 ± 0.7 | 31.63 ± 0.8 |
| Distribution of SFA, MUFA and PUFA content | |||||
| SFA | 26.46 ± 0.7a | 26.22 ± 0.3a | 24.6 ± 0.1b | 26.78 ± 0.1a | 29.94 ± 0.6c |
| MUFA | 3.79 ± 0.7a | 8.39 ± 0.1c | 2.93 ± 0.1b | 4.74 ± 0.1a | 4.38 ± 0.7a |
| PUFA | 60.97 ± 0.1a | 53.6 ± 0.3b | 54.97 ± 0.2b | 59.42 ± 0.6a | 37.5 ± 0.3c |
| ALA | 52.65 ± 0.1a | 42.92 ± 0.5b | 50.63 ± 0.1c | 49.62 ± 0.5c | 31.63 ± 0.3d |
Data followed by different letters within each row are significantly different (p < 0.05). S saturated, MU monounsaturated, PU polyunsaturated, FA fatty acids, ALA α-Linolenic acid
GC-MS profile of purslane
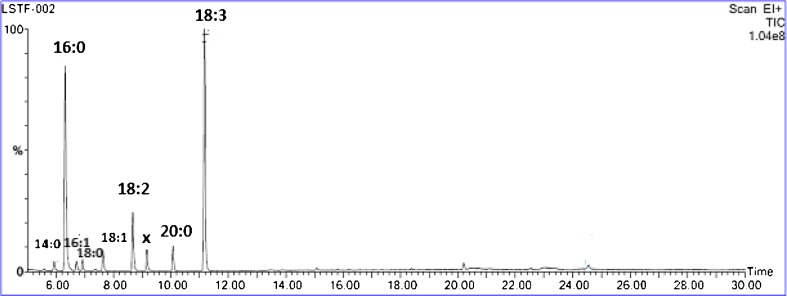
The Fig. 2 showed the chromatogram of dried leaves. The temperatures and methods of drying affect the lipid composition of the oil. In present study, 11 fatty acids were identified by GC-FID/MS in leaves of purslane. In leaves α-linolenic acid was found in highest quantity (42–54 %) followed by palmitic acid (17–23 %), linoleic acid (4–10 %) arachidic acid (1–8 %) (Fig. 2).
Fig. 2.
GC-MS of fatty acid from vacuum dried purslane leaves
Conclusion
As bioactive compounds are very susceptible to temperature, choosing the right drying method can be the input for a successful action. Based on the retention of the bioactive compounds the drying may be followed in the following order: vacuum>ltlh>tray> infrared>microwave. Therefore, vacuum drying is the alternative and suitable method for green leafy vegetables that retains the bioactive compounds significantly. This dehydration technique is especially suitable for products that are prone to heat damage such as fruits and vegetables. In the present work colour, during vacuum drying, rehydration behaviour, nutritional value, colour, and properties of purslane are found to improve as compared to others drying techniques. Moreover, in the dry state, purslane possess a unique colour, texture which could be a desirable property for preservation of this leaves and developing snack type products.
Acknowledgement
The authors thank Prof. Ram Rajasekharan, Director, CSIR-CFTRI, Mysore for his constant encouragement and support. The first author gratefully acknowledges U.G.C for providing the financial assistance in the form of JRF to carry out the present investigation.
References
- Abbatemarco C, Ramaswamy HS. End-over-end thermal processing of canned vegetables: Effect on texture and color. Food Res Int. 1995;27:327–334. doi: 10.1016/0963-9969(94)90188-0. [DOI] [Google Scholar]
- Achanta S, Okos M. R (1995) Impact of drying on biological product quality. In: G. V. Barbosa-Canovas, Welti-Chanes (eds). Food preservation by moisture control: fundamentals and applications pp. 637–657
- Ajayi OA, Osifo OA (1977) Home preservation of fruits and vegetables in Nigeria
- AOAC . Official Methods of Analysis. 16. Washington, DC: Association of Official Analytical Chemists; 1998. [Google Scholar]
- Arshiya S, Khaleequr R. Portulaca oleracea linn: a global panacea with ethnomedicinal and pharmacological potential. Int J Pharm Sci. 2013;5:33–39. [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Chua KJ, Hawlader, MNA, Chou SK, Ho, JC (2002) On the study of time varying temperature drying-effect on drying kinetics and product quality. Drying Technol 20:1559–1577
- Chuy, Labuza Caking and stickiness of dairy-based food powders as related to glass transition. J Food Sci. 1994;59:43–46. doi: 10.1111/j.1365-2621.1994.tb06893.x. [DOI] [Google Scholar]
- Cook NC, Samman S. Flavonoids-chemistry, metabolism, cardioprotective effect and dietary sources. J Nutr Biochem. 1996;7:66–76. doi: 10.1016/0955-2863(95)00168-9. [DOI] [Google Scholar]
- Davey MW, Van-Montagu M, Inze D, Sanmartin M, Kanellis A, Smirnoff N, Benzie IJJ, Strain JJ, Favell D, Fletcher J. Plant l-ascorbic acid: Chemistry, function, metabolism, bioavailability and effects of processing. J Sci Food Agric. 2000;80:825–860. doi: 10.1002/(SICI)1097-0010(20000515)80:7<825::AID-JSFA598>3.0.CO;2-6. [DOI] [Google Scholar]
- Dobiáš P, Pavlíková P, Adam M, Eisner A, Beòová B, Ventura K. Comparison of pressurised fluid and ultrasonic extraction methods for analysis of plant antioxidants and their antioxidant capacity. Cent Eur J Chem. 2010;8:87–95. doi: 10.2478/s11532-009-0125-9. [DOI] [Google Scholar]
- Dweck AC (2001). Purslane (Portulaca oleracea) - the global panacea. http://www.dweckdata.com/Published_papers/Portulaca_oleracea.pdf accessed.
- Ferreres F, Gomes D, Valentão P, Gonçalves R, Pio R, Alves E, Seabra RM, Andrade PB. Improved loquat (Eriobotrya japonica Lindl.) cultivars: variation of phenolics and antioxidative potential. Food Chem. 2009;114:1019–1027. doi: 10.1016/j.foodchem.2008.10.065. [DOI] [Google Scholar]
- Isong NC. Nutrient losses in dehydrated foods. Crit Rev Food Technol. 1977;1:49–55. [Google Scholar]
- Karel M, Buera MP, Roos Y (1993) Effects of glass transitions on processing and storage. In J. M. V. Blanshard, & P. J. Lillford (eds), The glassy state in food. pp 12–34
- Khanal RC, Howard LR, Prior RL. Effect of heating on the stability of grape and blueberry pomace procyanidins and total anthocyanins. Food Res Int. 2010;43:1464–1469. doi: 10.1016/j.foodres.2010.04.018. [DOI] [Google Scholar]
- Luh BS, Woodroof JG. Commercial Vegetable processing. Westport, Corn: Avi. Pub. Co.; 1975. [Google Scholar]
- Miliauskas G, Venskutonis PR, Van Beek TA. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004;2:231–237. doi: 10.1016/j.foodchem.2003.05.007. [DOI] [Google Scholar]
- Navarro JM, Flores P, Garrido C, Martinez V. Changes in the contents of antioxidant compounds in pepper fruits at different ripening stages, as affected by salinity. Food Chem. 2006;96:66–73. doi: 10.1016/j.foodchem.2005.01.057. [DOI] [Google Scholar]
- O’Fallon JV, Busboom JR, Nelson ML, Gaskins CT. A direct method for fatty acid methyl ester synthesis: Application to wet meat tissues, oils, and feedstuffs. J Anim Sci. 2007;85:1511–1521. doi: 10.2527/jas.2006-491. [DOI] [PubMed] [Google Scholar]
- Pande VK, Sonune AV, Philip SK. Solar drying of coriander and methi leaves. J Food Sci Technol. 2000;23:639–641. [Google Scholar]
- Pervin S, Islam MS, Islam MN. Study on Rehydration Characteristics of Dried Lablab Bean (Lablab Purpureus) Seeds. J Agric Rural Dev. 2008;6(1&2):157–163. [Google Scholar]
- Raghavan GSV, Orsat V. Recent advances in drying of biomaterials for superior quality bioproducts. Asia-Pacific J Chem Eng. 2007;2:20–29. doi: 10.1002/apj.51. [DOI] [Google Scholar]
- Ranganna S. Handbook of Analysis and Quality Control of Fruit and Vegetable Products. 2. New Delhi: Tata McGraw Hill Publications. Co; 1997. [Google Scholar]
- Ross KA, Beta T, Arntfield SD. A comparative study on the phenolic acids identified and quantified in dry beans using HPLC as affected by different extraction and hydrolysis methods. Food Chem. 2009;113:336–344. doi: 10.1016/j.foodchem.2008.07.064. [DOI] [Google Scholar]
- Samy J, Sugumaran M, Lee KLW. Herbs of Malaysia: An Introduction to the medicinal, culinary, aromatic and cosmetic use of herbs. Kuala Lumpur: Times Edition; 2004. [Google Scholar]
- Schieber A, Keller P, Carle R. Determination of phenolic acids and flavonoids of apple and pear by high-performance liquid chromatography. J Chromatogr A. 2001;910:265–273. doi: 10.1016/S0021-9673(00)01217-6. [DOI] [PubMed] [Google Scholar]
- Serrano M, Zapata PJ, Castillo S, Guillén F, Martínez-Romero D, Valero D. Antioxidant and nutritive constituents during sweet pepper development and ripening are enhanced by nitrophenolate treatments. Food Chem. 2010;118:497–503. doi: 10.1016/j.foodchem.2009.05.006. [DOI] [Google Scholar]
- Sheetal G, Gowri BS, Lakshmi AJ, Prakash J. Retention of nutrients in green leafy vegetables on dehydration. J Food Sci Technol. 2013 doi: 10.1007/s13197-011-0407-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton VL, Rossi JA., Jr Colorunetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Viticult. 1965;16:144–158. [Google Scholar]
- Whelan J, Rust C. Innovative Dietary Sources of N-3 Fatty Acids. Annu Rev Nutr. 2006;26:75–103. doi: 10.1146/annurev.nutr.25.050304.092605. [DOI] [PubMed] [Google Scholar]
- Wijngaard HH, Rößle C, Brunton NA. Survey of Irish fruit and vegetable waste and byproducts as a source of polyphenolic antioxidants. Food Chem. 2009;116:202–207. doi: 10.1016/j.foodchem.2009.02.033. [DOI] [Google Scholar]
- Yao L, Jiang Y, Datta N, Singanusong R, Liu X, Duan J. HPLC analyses of flavonols and phenolic acids in the fresh young shoots of tea (Camellia sinensis) grown in Australia. Food Chem. 2004;84:253–263. doi: 10.1016/S0308-8146(03)00209-7. [DOI] [Google Scholar]