Abstract
The antioxidative capacity of six different tissue hydrolysates (porcine colon, heart and neck and bovine lung, kidney and pancreas) were tested by three different assays monitoring iron chelation, ABTS radical scavenging and inhibition of lipid oxidation in emulsions, respectively. The hydrolysates were also investigated with respect to amino acid composition and peptide size distribution. The hydrolysates contained peptides ranging from 20 kDa to below 100 Da with a predominance of peptides with low molecular weight (53.8 to 89.0 % below 3 kDa). All hydrolysates exhibited antioxidant activity as assessed with all three methods; inhibition of lipid oxidation ranging from 72 to 88 % (at a final protein concentration of 7 mg/mL), iron chelation capacity from 23 to 63 % (at 1.1 mg/mL), and ABTS radical scavenging from 38 to 50 % (at 10 μg /mL). The antioxidant activity did not correlate with the proportion of low molecular weight peptides in the hydrolysed tissues, but with the content of specific amino acid residues. The ABTS radical scavenging capacity of the tissues was found to correlate with the content of Trp, Tyr, Met and Arg, whereas the ability to inhibit the oxidation of lineoleic acid correlated with the content of Glu and His. The chosen animal by-products thus represent a natural source of antioxidants with potential for food application.
Keywords: Enzymatic hydrolysis, Antioxidant capacity, Animal by-products, ABTS, Size-exclusion chromatography, Amino acid composition
Introduction
Enzymatic hydrolysates of food proteins have been exploited for decades, traditionally for their functional properties, e.g. flavor enhancement and emulsification, but with an increasing focus worldwide on their potential bioactive properties. A variety of bioactive properties has been reported for food protein hydrolysates, e.g. antimicrobial, angiotensin I-converting enzyme-inhibitory and antioxidant (Udenigwe and Aluko 2011). Antioxidative hydrolysates have gained increasing interest for use in foods to provide natural protection against oxidation (Samaranayaka and Li-Chan 2011). Lipid peroxidation in food is associated with off-flavors and unfavorable changes in texture and color, which ultimately lead to product deterioration and shortened shelf life, and therefore is of great economic concern for the food industry (Chaiyasit et al. 2007). Protein hydrolysates added to food may protect the food from lipid oxidation (and oxidation of other macromolecules) by physical or chemical means. Physically, due to the amphipathic nature of peptides, they are believed to be capable of adsorbing at the interface of lipid droplets, thereby providing a physical protection of the lipid against reactive oxygen species (Donnelly et al. 1998). Chemically, protection provided by protein hydrolysates is related to the amino acid composition and primary structure of the peptides present. However, other factors than the amino acids also contribute to the activity, e.g. the peptide context of the amino acid and the peptide size. For example, histidine (His) has been shown to have a stronger hydroxyl radical quenching activity when incorporated into a dipeptide with Gly or Ala, than when mixed with either of the free amino acids, implying a potential role of the peptide bond and of the amino acid composition (Chan et al. 1994).
Several studies employing molecular fractionation techniques to separate protein hydrolysates, have reported that low molecular weight fractions (<3 kDa) possess higher antioxidant activity than fractions of higher molecular weights (Ajibola et al. 2011; Irshad et al. 2013).
Studies on antioxidative properties of food protein-derived hydrolysates have included substrates derived from various sources, such as plant, milk, fish and meat proteins (Sarmadi and Ismail 2010; Pihlanto 2006; Joseph et al. 2011). Edible by-products and low value cuts (including organ meats) from the meat industry represent an interesting protein source for release of antioxidant peptides (Damgaard et al. 2014). Such products are highly diverse regarding composition and functionality, and many represent a significant source of protein and essential amino acids (Anderson 1988). Furthermore, many organ meats, (e.g. liver, kidney and pancreas) are high in a variety of vitamins and minerals (http://ndb.nal.usda.gov/ NDB no.10110, 10106, 10115, respectively) compared to lean tissue. The use of enzymatic hydrolysis of slaughterhouse by-products therefore offers a strategy to improve the utilization of such products. Very few studies have been conducted on the antioxidative properties of hydrolysates from organ meats. In our previous work on the antioxidant capacity of six hydrolysed porcine tissues, we found large differences among tissues (Damgaard et al. 2014), with colon providing the highest and heart the overall lowest capacity. Furthermore, bioactivity screening of a variety of different porcine and bovine tissue hydrolysates revealed that bovine kidney, lung, and pancreas as well as porcine neck were good sources of antioxidant hydrolysates (unpublished results; Lene Meinert, DMRI). Based on these results, six tissues were chosen as substrates for antioxidant peptides/hydrolysates in this study.
The purpose of the present study is to investigate the antioxidative capacity of hydrolysates of the six selected animal tissues, and to relate the activity to the amino acid composition and the molecular size distribution of peptides in the hydrolysates. Since there is no single antioxidant standard method to test for antioxidant capacity, it is recommended to use different methods for investigating the different mechanisms of antioxidant capacity. Hence, the antioxidative capacity of the hydrolysates was tested by monitoring the oxidation of linoleic acid in an emulsion system as well as by in vitro tests for two different antioxidant mechanisms involved, namely radical scavenging and iron chelation.
Methods and materials
Chemicals
Met-myoglobin, Tween-20, linoleic acid, cytochrome C, aprotinin and leucine enkephaline were purchased from Sigma-Aldrich (St. Louis, USA) and glycyl-L-tyrosine from Sigma-Aldrich (Buchs, Switzerland). 6-Hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), ascorbic acid, acetonitrile and trifluoric acid were purchased from Sigma-Aldrich (Steinheim, Germany). Ethanol (99.9 %) was purchased from Chemethyl A/S (Køge, Denmark). Hydrochloric acid was from JT. Baker (Deventer, Holland). Tyr-bradykinin was purchased from Sigma-Aldrich Denmark A/S (Brøndby, Denmark) and β-lactoglobulin was purified at the department (Kristiansen et al. 1998).
Preparation of protein hydrolysates
Colon, heart and neck from pigs and kidney, pancreas and lung tissue from cows were collected from a slaughterhouse and hydrolysed with equal amounts of Alcalase and Protamex (enzyme:substrate ratio of 1:1000 (w/w)) for 2 h as previously described (Damgaard et al. 2014). Hydrolysates were frozen and kept at −20 °C until use. Before analysis, hydrolysates were thawed at 4 °C overnight and centrifuged at 20.000×g for 25 min, and the supernatant was filtered through a 10 mL syringe stuffed with approx. 2 mL of fiberglass to remove any aggregates and lipids.
Dry matter determination
The dry matter (DM) content was determined with a moisture analyzer (Sartorius MA30, Goettingen, Germany) as described previously (Damgaard et al. 2014) but using a canned meat standard (FAPAS, York, UK) as a control for the calibration. Samples were analysed in triplicate.
Amino acid analysis
A full amino acid analysis was performed at Eurofins (Holsterbro, Denmark) following the reference method from the European Union commission regulation, EU 152/2009 (G) (www.agriculture.gov.ie) for tryptophan and EU 152/2009 (F) for other amino acids. Asn and Gln were transformed into acids and were determined together with Asp and Glu, respectively. Total protein content of each hydrolysate was calculated as the sum of all amino acids.
Size exclusion chromatography
Size exclusion chromatography (SEC) was performed on a Superdex Peptide® HR 10/300 column (fractionation range 7000–100 Da) coupled to a FPLC AKTA-purifier system (GE healthcare). From each hydrolysate (20 mg/mL) a volume of 25 μL were injected and peptides were eluted isocratically with aqueous acetonitrile (30 %) and TFA (0.1 %) at a flow rate of 0.5 mL/min. Elution was monitored at 214 nm and the proximate molecular masses of eluted peptides were determined using the following molecular weight standards: β-lactoglobulin (18.3 kDa), cytochrome C (12.5 kDa), aprotinin (6512 Da), Tyr-bradykinin (1223 Da), Leu-enkephalin (555.5 Da) and glycyl-tyrosine (238 Da). All separations were performed in duplicate.
Inhibition of lipid oxidation in emulsions
Tissue hydrolysates were diluted before analysis to give a final concentration in the assay of 10 mg DM/mL. Emulsions were made by mixing 6 mL of hydrolysate with 13.3 mL of 50 mM phosphate buffer, pH 6.8, and 500 μL linoleic emulsion (0.125 g Tween-20, 0.30 g linoleic acid in 25 mL of 50 mM phosphate buffer (pH 6.8)) to a final volume of 19.8 mL, and the oxidation was initiated by addition of 200 μL of 0.4 mM met-myoglobin. Lipid oxidation was monitored as the oxygen consumption in a closed system under water (25 °C) with a LDO oxygen sensor coupled to a portable Hach HQ30d meter (Hach Lange, Broenshoej, Denmark) and recorded at 10 s intervals. Negative control samples contained water instead of hydrolysate. Trolox and ascorbic acid were used as positive standards. All samples were analysed in duplicate. The oxygen concentration (%) was plotted as a function of time, and the slope of the curve in the linear region was used to calculate the rate (v) of oxygen consumption (μmol O2/s) (Microsoft excel 2010). Inhibition of lipid oxidation was calculated according to the equation
where v (O2)sample is the initial oxygen consumption rate in the presence of the hydrolysate and v (O2) control is the initial oxygen consumption rate when water had replaced the sample.
Determination of iron chelation capacity
The iron chelating capacity of the hydrolysates was investigated as the ability to inhibit the formation of a Fe2+- ferrozine complex according to the procedure described previously (Damgaard et al. 2014). All hydrolysate samples were diluted with distilled water and tested in triplicate at 10 mg DM/mL (1.1 mg/mL in the assay).
ABTS radical scavenging capacity
The radical scavenging capacity of the hydrolysates was assayed at 50 μg DM/mL (10 μg in the assay) with an ABTS assay according to the protocol described by Damgaard et al. (2014). Samples were tested five times (each in triplicate) on different days. Trolox and ascorbic acid were used as positive standards.
Statistical analysis
Data were expressed as means ± standard deviations. Differences in antioxidant capacities among the hydrolysates from the various tissues were analysed by one-way ANOVA and Tukey’s test (Microsoft Excel 2010), and considered statistically significant at p < 0.05. Principal Component Analysis (PCA) was performed in order to retrieve information about systematic variations in the amino acid composition in relation to the tissues. For this analysis, values were normalized and imported into MatLab software (version MATLAB 2014a) (Mathworks, Massachusetts, USA), were preprocessed using auto-scaling, and processed with the MatLab and Partial Least Squares (PLS) Toolbox (Version 7.3.1 Eigenvector Research, Inc.; Wenatchee, WA, USA). Correlations between mean values for antioxidant capacities obtained by the various methods and hydrolysate properties such as proportion of particular amino acids and peptide size fractions were tested with the CORR procedure using SAS 9.4 for windows (SAS institute Inc., Cary, NC, USA).
Results and discussion
Characterization of crude tissue hydrolysates
Dry matter and protein content of hydrolysates
During hydrolysis of the six animal tissues different amounts of peptides were released as revealed by the protein and dry matter (DM) contents of the hydrolysates (Table 1). The DM varied between tissues (from 3.5 to 10.6 %) in a similar manner as the protein content (varying from 2.5 to 6.9 %). The protein contents in DM therefore remained relatively constant ranging from 65.1 to 72.2 % (Table 1). These values are similar to those of goby fish protein hydrolysates digested for 6 h with different proteases (69–79 % protein of DM basis; Nasri et al. 2013). The pancreas hydrolysate had the highest DM and protein content, suggesting that the proteins in pancreas were more accessible to the proteolytic enzymes and/or that the peptides released were less prone to aggregation and precipitation. The DM contents of the heart and colon hydrolysates were lower than those found previously for similar hydrolysates (Damgaard et al. 2014), which could indicate that the process of selecting organs and hydrolyzing them may not be fully reproducible. In order to test the reproducibility of the hydrolysis, the peptide size distributions of the heart and colon tissues used in the two studies were compared, and found to be almost identical (data not shown) indicating that the hydrolysis was reproducible in regard to proteolytic cleavage, and that the lower soluble yield was not caused by different cleavage patterns. Instead, this indicates that the lack of reproducibility is caused by differences in the starting material, i.e. the tissues, which could be related to differences in e.g. gender, age or post mortem storage time.
Table 1.
Dry matter and protein content of the hydrolysed bovine and porcine tissues
| Hydrolysate | DM (g/100 g) | Protein (g/100 g) | Protein (%) |
|---|---|---|---|
| Pancreas (bovine) | 10.6 ± 0.5 a | 6.9 | 65.1 |
| Heart (porcine) | 6.9 ± 0.1 b | 5.0 | 72.5 |
| Neck (porcine) | 6.6 ± 0.1 b | 4.4 | 66.7 |
| Kidney (bovine) | 6.2 ± 0.2 b | 4.2 | 67.5 |
| Lung (bovine) | 6.1 ± 0.2 b | 4.1 | 67.2 |
| Colon (porcine) | 3.5 ± 0.2 c | 2.5 | 70.2 |
Amino acid composition of hydrolysates
The amino acid composition of the hydrolysates is shown in Table 2. The variations in the relative amino acid content between the different tissues are illustrated in the PCA plot (Fig. 1). The principle component 1, which explained 58.8 % of the amino acid variation in the different tissues, is mainly due to the contents of Pro, Gly and Ala, which are most abundant in the lung and neck hydrolysates, as opposed to the higher content of sulphur-containing and more bulky amino acids in hydrolysates from kidney and pancreas. The high amount of Pro, Ala and Gly in the lung and neck hydrolysates, 28.5 and 29.1 %, respectively (Table 2), in comparison to in the remaining four hydrolysates (20.2 to 21.8 %) probably indicates a high proportion of connective tissue, since Pro, Ala and Gly (together with hydroxyproline) are the major constituent amino acids of connective tissue (Eastoe 1955). This is in agreement with the work of Gault and Lawrie (1980), who reported a connective tissue content of 26 % in bovine lung and a lower content in other offal tissues. The large intestines, which colon is a part of, was also found by these authors to contain a quite high amount of connective tissue (23 %; Gault and Lawrie 1980). However, this was not reflected in the amino acid composition of the colon hydrolysate in the present study (Table 2, Fig. 1), and could be due to preferential enzymatic degradation of the non-connective tissue proteins in this tissue. The fact that the colon tissue gave rise to the lowest DM and protein yields (Table 1) confirms that this tissue is more resistant to proteolytic degradation, which might be related to the nature of the connective tissue.
Table 2.
Amino acid content of the six hydrolysed bovine and porcine tissues
| Heart | Lung | Kidney | Pancreas | Neck | Colon | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| g/100 g | % | g/100 g | % | g/100 g | % | g/100 g | % | g/100 g | % | g/100 g | % | |
| Serine | 0.221 | 4.461 | 0.172 | 4.181 | 0.210 | 5.005 | 0.370 | 5.394 | 0.194 | 4.399 | 0.125 | 4.989 |
| Glutamic acid | 0.827 | 16.692 | 0.602 | 14.633 | 0.611 | 14.561 | 0.914 | 13.323 | 0.689 | 15.622 | 0.370 | 14.768 |
| Proline | 0.270 | 5.450 | 0.325 | 7.900 | 0.253 | 6.029 | 0.402 | 5.860 | 0.442 | 10.022 | 0.160 | 6.386 |
| Glycine | 0.366 | 7.387 | 0.533 | 12.955 | 0.375 | 8.937 | 0.609 | 8.877 | 0.500 | 11.337 | 0.222 | 8.861 |
| Alanine | 0.364 | 7.327 | 0.316 | 7.681 | 0.284 | 6.768 | 0.466 | 6.793 | 0.342 | 7.754 | 0.163 | 6.506 |
| Valine | 0.234 | 4.723 | 0.202 | 4.910 | 0.240 | 5.720 | 0.416 | 6.064 | 0.189 | 4.285 | 0.128 | 5.109 |
| Isoleucine | 0.182 | 3.673 | 0.131 | 3.184 | 0.173 | 4.123 | 0.291 | 4.242 | 0.129 | 2.925 | 0.102 | 4.071 |
| Leucine | 0.404 | 8.154 | 0.303 | 7.365 | 0.350 | 8.341 | 0.560 | 8.163 | 0.297 | 6.734 | 0.190 | 7.583 |
| Tyrosine | 0.137 | 2.765 | 0.088 | 2.137 | 0.074 | 1.764 | 0.102 | 1.487 | 0.089 | 2.013 | 0.071 | 2.834 |
| Phenylalanine | 0.184 | 3.714 | 0.149 | 3.622 | 0.174 | 4.147 | 0.283 | 4.125 | 0.133 | 3.016 | 0.096 | 3.832 |
| Lysine | 0.429 | 8.659 | 0.301 | 7.316 | 0.316 | 7.531 | 0.530 | 7.726 | 0.373 | 8.457 | 0.187 | 7.464 |
| Histidine | 0.127 | 2.563 | 0.097 | 2.350 | 0.110 | 2.621 | 0.164 | 2.391 | 0.116 | 2.630 | 0.059 | 2.355 |
| Arginine | 0.335 | 6.762 | 0.264 | 6.417 | 0.250 | 5.958 | 0.430 | 6.268 | 0.272 | 6.167 | 0.173 | 6.905 |
| Aspartic acid | 0.468 | 9.446 | 0.353 | 8.580 | 0.394 | 9.390 | 0.666 | 9.708 | 0.361 | 8.185 | 0.231 | 9.220 |
| Threonine | 0.217 | 4.380 | 0.139 | 3.379 | 0.197 | 4.695 | 0.327 | 4.767 | 0.158 | 3.582 | 0.115 | 4.590 |
| Tryptophane | 0.043 | 0.858 | 0.032 | 0.766 | 0.046 | 1.099 | 0.098 | 1.430 | 0.028 | 0.628 | 0.025 | 0.978 |
| Cysteine | 0.047 | 0.949 | 0.041 | 0.997 | 0.062 | 1.478 | 0.114 | 1.662 | 0.031 | 0.703 | 0.034 | 1.357 |
| Methionine | 0.101 | 2.039 | 0.067 | 1.629 | 0.077 | 1.835 | 0.118 | 1.720 | 0.068 | 1.542 | 0.055 | 2.195 |
Fig 1.
Principle component analysis for characterization of various bovine (B. lung, B. kidney, B. pancreas) and porcine (P. heart, P. neck and P. colon) tissue hydrolysates by their relative amino acid compositions
The principle component 2 explained another 21.7 % of the amino acid variation, which is primarily caused by a high content of Gly in hydrolysates from pancreas, lung and kidney opposed to a high content of charged amino acids (Glu and Arg) and Tyr and Met in the heart hydrolysate (Fig. 1). This confirms previously published values showing that porcine heart contains higher amounts of Met, Glu, and Arg compared to bovine kidney, lung and pancreas (Schweigert et al. 1954; Anderson 1988). However, in contrast to our results, the content of Tyr was reported to be lower in porcine heart compared to bovine kidney and pancreas (Anderson 1988).
Peptide size distribution of hydrolysates
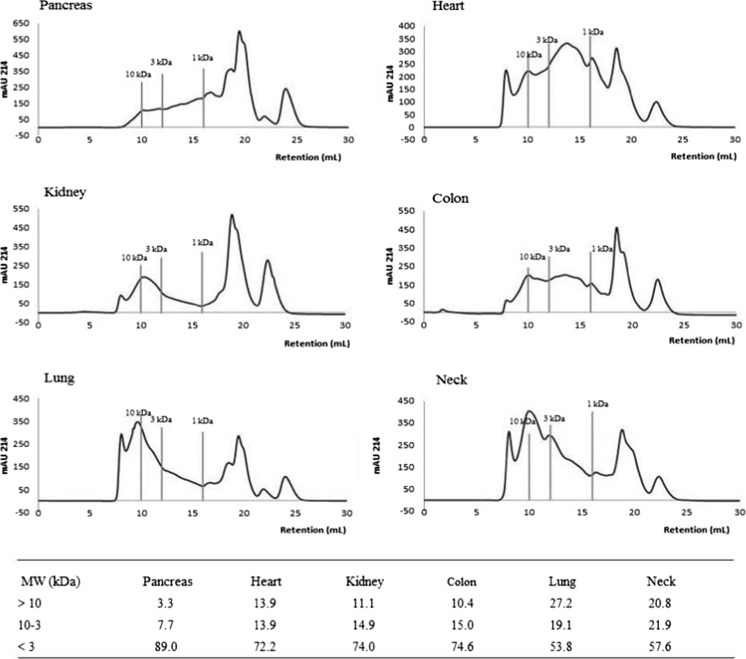
The size exclusion chromatography profiles of the hydrolysed tissues are shown in Fig. 2. Although the size profiles varied with tissue, all hydrolysates, except the pancreas hydrolysate, contained peptides eluting in the whole size range from 20 kDa to less than 0.1 kDa and with two large peaks containing peptides with masses below 1 kDa. In order to quantify the size distribution data, profiles were split into three molecular weight classes (≥10 kDa, 10–3 kDa and ≤ 3 kDa) and the percentage of material in each was quantified based on the area under the curve (Fig. 2). According to these, the hydrolysates can be divided into three groups. The pancreas hydrolysate represents the first group which has the largest proportion of small peptides, with 89 % of the peptides present in the < 3 kDa fraction and only 3.3 % in the > 10 kDa fraction. The high proportion of small peptides indicates that pancreas tissue is very accessible to proteolytic degradation as also revealed by the high DM and protein contents in the hydrolysate (Table 1). The second group comprises the hydrolysates of heart, colon and kidney, which showed quite similar profiles with comparable distribution across all three molecular weight intervals (~12 % > 10 kDa, ~14 %: 10–3 kDa and ~73 % < 3 kDa). The third group comprises the neck and lung hydrolysates, which are the least hydrolysed tissues with 20.8 and 27.2 % of peptides present in the > 10 kDa fraction and only 57.6 and 53.8 % in the < 3 kDa fraction, respectively. This is consistent with the lung and neck hydrolysates having the highest content of connective tissue as suggested from the high proportion of Pro, Ala and Gly (see Amino acid composition of hydrolysates section). High amounts of Pro could sterically hinder enzyme accessibility, which might explain the greater fraction of high molecular weight (>10 kDa) peptides in these hydrolysates. Interestingly, the colon hydrolysate that had a low DM content (Table 1), contained a relatively large proportion of small peptides compared with the neck and lung hydrolysates, once again indicating that some proteins in the colon tissue, probably protein of the connective tissue, are resistant to proteolytic degradation, whereas the accessible proteins in this tissue were more fully degraded. To our knowledge, enzymatic hydrolysates of bovine and porcine tissues have not previously been characterized with respect to peptide size profiles. However, Goby fish protein hydrolysates digested with different proteases, also contained a large proportion of small peptides (67–88 % below 3 kDa; Nasri et al. 2013). This is in agreement with our results showing that, despite the variation, all hydrolysates consisted mainly of low molecular weight peptides (54 to 89 % of peptides were < 3 kDa). This is expected to be beneficial for the antioxidant activity, since small peptides have been shown to exert higher activity than larger peptides (Ajibola et al. 2011; Irshad et al. 2013).
Fig 2.
Size-exclusion profiles (214 nm) of the hydrolysates from porcine tissues (neck, colon and heart) and bovine tissues (kidney, lung and pancreas) with size markers (10, 3 and 1 kDa) included. The distribution (%) of peptides into MW classes is given below the profiles
Antioxidant capacity of crude tissue hydrolysates
Inhibition of lipid oxidation
All tissue hydrolysates were able to inhibit the lipid oxidation in a linoleic acid emulsion system with inhibition percentages between 65 and 88 (Table 3). It seems that not many meat and meat organ/tissue-derived hydrolysates have been tested for their ability to inhibit lipid oxidation. However, a porcine plasma hydrolysate was shown to inhibit TBARS formation in a liposome model system by 25–40 % at 40 mg/mL (Liu et al. 2010). Otherwise, animal sources used for antioxidant protein hydrolysates have primarily been milk and egg as well as various fish proteins. A crude cod hydrolysate inhibited TBARS formation by 20 % at 4.25 mg/mL (Farvin et al. 2014). Since our samples were tested at 10 mg DM/mL in the final assay (peptide concentration around 7 mg/mL), the inhibition potential of the hydrolysed organs seems comparable to that of the cod protein hydrolysates and much better than that of the porcine plasma hydrolysate.
Table 3.
Antioxidant capacities (mean ± std) of the crude bovine and porcine tissue hydrolysates
| Inhibition of lipid oxidation (%) | Iron chelation (%) | ABTS radical scavenging (%) | |
|---|---|---|---|
| Lung (bovine) | 86.2 ± 0.8a | 55.0 ± 2.0 ab | 41.6 ± 3.6 bc |
| Kidney (bovine) | 73.9 ± 2.3 b | 63.4 ± 3.6 a | 41.0 ± 5.3 bc |
| Pancreas (bovine) | 87.9 ± 8.4a | 19.6 ± 19.5 c | 38.7 ± 9.7 c |
| Heart (porcine) | 64.7 ± 0.5 c | 23.0 ± 7.6 c | 44.6 ± 4.7 b |
| Colon (porcine) | 82.9 ± 9.3 a | 35.2 ± 11.0 bc | 49.6 ± 4.0 a |
| Neck (porcine) | 72.4 ± 0.5 b | 32.5 ± 3.4 bc | 37.9 ± 4.4 c |
| TroloxA | 39.6 ± 1.4 | – | 60.6 ± 6.4 |
| Ascorbic acidB | 53.2 ± 0.6 | – | 46.4 ± 4.7 |
DM concentrations in the assays were: inhibition of lipid oxidation (10 mg/mL), iron chelation (1.1 mg/mL) and ABTS radical scavenging (10 μg/mL)
ATrolox was tested at 6.4 μM for ABTS and 30 μM for inhibition of lipid oxidation
BAscorbic acid was tested at 7 μM for ABTS and 50 μM for inhibition of lipid oxidation
* Values with different letters in the same column are significantly different at p < 0.05
The activity of the hydrolysates was not correlated to the proportion of any particular size fraction of peptides in the hydrolysates (results not shown).
The hydrolysates from bovine pancreas and lung, and porcine colon were the most efficient inhibitors of lipid oxidation, with significantly higher activity than the other hydrolysates. Interestingly, these hydrolysates have quite different amino acid composition according to their position in the PCA plot (Fig. 1). Nevertheless, the correlation analysis revealed a positive correlation between the inhibition of lipid oxidation and the percentage of His (r2 = 0.83) and Glu (r2 = 0.87), and especially the sum of these (r2 = 0.91). Glutamic acid residues are able to bind metal ions (Storcksdieck et al. 2007; Saiga et al. 2003), and iron chelation may be one of the mechanisms by which these hydrolysates inhibit lipid oxidation. Also, His residues are known as good metal chelators (Torres-Fuentes et al. 2012) as well as good radical scavengers, which is attributed to the hydrogen donation properties of the imidazol ring (Chan et al. 1994). The presence of these two amino acids may thus contribute significantly to the antioxidant activity of the hydrolysates by the mechanisms mentioned. However, since there was no correlation between the ability to inhibit lipid oxidation in emulsions and either the ABTS radical scavenging capacity or the iron chelating capacities of the various hydrolysates (data not shown), the antioxidant capacity in liposomes could in part be exerted by formation of an interfacial layer around the oil droplets. Such an interfacial layer could protect against oxidation, e.g. by repelling positively charged metals, which could otherwise initiate oxidation (Xiong 2010).
Iron chelating capacity
The kidney and lung hydrolysates had the highest iron chelating capacity (63 and 55 %, respectively) followed by the colon and neck hydrolysates (Table 3). The iron chelating capacity of the pancreas and heart hydrolysates were significantly lower (~3 fold) than that of the kidney hydrolysate. Surprisingly, the colon and heart hydrolysates had much lower capacities compared to our earlier findings (~80 and 20 % at 5 mg/mL) (Damgaard et al. 2014), despite of the same conditions of hydrolysis and assay used, and that the concentrations were twice as high in the present study. This is likely to be caused by variation in organs as pointed out in the first result section. To our knowledge, the iron chelating capacity of hydrolysed organs has not been determined by others, but all had higher iron chelation capabilities compared to that of Alcalase-hydrolysed porcine plasma protein (<20 % at 40 mg/mL; Liu et al. 2010) and the highest capacities were comparable to that of thermolysin-hydrolysed bovine liver sarcoplasmic protein (55 % at 5 mg protein/mL; Di Bernardini et al. 2011). This shows that there is some variation, but that hydrolysed porcine and bovine organ tisues can have a quite good metal chelating capacity.
As already mentioned, the metal chelating capacity of peptides and proteins has often been ascribed to specific amino acids, such as iron binding peptides rich in the acidic amino acids, glutamic and aspartic acid (Storcksdieck et al. 2007; Saiga et al. 2003), or His (Torres-Fuentes et al. 2012). We did not find any correlation between the content of His, Glu or Asp, or any other amino acids, and the iron chelating capacity across the different hydrolysates (data not shown). Since the hydrolysates are crude it is possible that other factors contribute to the antioxidant capacities. Several organ meats and meat by-products contain compounds which can act as antioxidants, e.g. ascorbic acid and the β-alanyl peptides carnosine and anserine (Xiong 2010). For example, bovine lung tissue contains the largest amount of ascorbic acid (~39 mg/100 g; http://ndb.nal.usda.gov, NDB no. 13328), which could contribute to its high iron chelation capacity. However, hydrolysates of bovine kidneys had the highest iron chelating capacity, even though this tissue contains almost four times less ascorbic acid than bovine lung tissue and also less than bovine pancreas tissue (http://ndb.nal.usda.gov, NDB no. 13323), indicating that ascorbic acid is not the primary antioxidant in these hydrolysates. Furthermore, the iron chelating capacities of the hydrolysates did not correlate with the proportion of low molecular weight substances (or any other size categories, data not shown), suggesting that peptides of various molecular size contribute to the iron chelating capacities seen here.
ABTS radical scavenging capacity
The hydrolysates exhibited radical scavenging capacities around 40 % (Table 3, third column), except that from the colon tissue which showed a significantly higher capacity (~50 %). Since this sample contained 50 μg DM/mL, the peptide concentration in colon tissue hydrolysate scavenging 50 % of the radicals (IC50) is around 35 μg/mL (~7 μg /mL in the assay). In general, all the values reported here are lower than the ABTS radical scavenging capacities recently obtained for hydrolysed porcine tissues (Damgaard et al. 2014). Nonetheless, the activity of the hydrolysates in the present study are in the range of those observed for hydrolysates made from tannery fleshings (Balakrishnan et al. 2011) and fermented shrimp biowaste (Sachindra and Bhaskar 2008), and higher than those of hydrolysates from patin (fish) sarcoplasmic protein made with Alcalase or papain (IC50 ~ 0.8–0.9 mg/mL; Najafian and Babji 2014). All the hydrolysates had lower ABTS radical scavenging capacities compared to trolox but similar to that of ascorbic acid, showing their potential for antioxidant ingredients.
Surprisingly, the ABTS radical scavenging activity of the various tissue hydrolysates did not correlate with the proportion of low molecular weight peptides in these (r2 = 0.5), like it has been seen for milk protein hydrolysates (De Gobba et al. 2014).
In order for an amino acid residue to act as a strong antioxidant it is crucial that it oxidizes readily before others, and that it hinders or delay the ongoing oxidation of other molecules. Previous measurements of the pseudo-first-order rate constants for the hydroxyl radical mediated oxidation of amino acid side chains have shown that the relative proneness of amino acids to oxidation follows the order Cys > Trp, Tyr > Met > Phe > His > Ile > Leu > Pro (Sharp et al. 2004). As shown in Fig. 1, the colon and heart hydrolysates, which exhibited the highest ABTS radical scavenging capacity, contained a high proportion of charged amino acids (Glu, and Arg) and also Tyr and Met. Indeed, the ABTS radical scavenging capacity values correlated with the content of Arg (r2 = 0.84), Met (r2 = 0.92), Tyr (r2 = 0.85), and the sum of Tyr and Trp (r2 = 0.94), which is in agreement with the results of several other studies. The aromatic amino acids, Trp and Tyr, are both reported as having good radical scavenging properties. For example, Tyr containing peptides derived from royal jelly protein (Guo et al. 2009) and tilapia (Fan et al. 2012), and synthetic peptides containing Tyr or Trp (Saito et al. 2003), have been shown to have strong radical scavenging activity. The antioxidant capacity of these two amino acids is assumed to be derived from their ability to act as hydrogen donors due to their indolylic and phenolic groups (Hernandez-Ledesma et al. 2005). Furthermore, Hernandez-Ledesma et al. (2005) also reported that peptides containing Trp, Tyr, and Met had the highest antioxidant activity when tested in the ORAC assay, followed by those containing Cys and His. Methionine residues exert antioxidant capacity through sulfur oxidation resulting in methionine sulfoxide (Hernandez-Ledesma et al. 2005). Finally, free arginine has been shown to scavenge ABTS and DPPH radicals as well as oxygen radicals in a dose dependent manner (Lass et al. 2002; Kim et al. 2004) probably attributed to the guanidinium group (Lass et al. 2002). All of these results are consistent with our findings.
The above results show that bovine and porcine tissues as industrial by-products by means of enzymatic hydrolysis can be transformed into hydrolysates with antioxidant capacity, representing natural ingredients that may be applied in certain processed meat products to prolong shelf-life.
Conclusions
Hydrolysis of bovine and porcine organs for 2 h with Protamex and Alcalase resulted in hydrolysates containing peptides of various sizes (most below 3 kDa) and varying in amino acid composition. All the hydrolysates showed antioxidant capacities in all the assays used, although with no correlation between activities obtained by the various assays. The highest inhibition of lipid oxidation was exhibited by the pancreas, lung and colon hydrolysates. The capacity of the lung hydrolysate might primarily be due to its high iron chelating capacity exerted by Glu and His residues. The high capacity of the colon hydrolysate might primarily be due to its high radical scavenging capacity, related to its high content of Tyr, Trp, Met and Arg. The pancreas hydrolysate did exhibit both capacities but not to a high extent. The antioxidant capacity of this hydrolysate might be related to its high content of Phe and Val providing a hydrophobic environment for association to the interface of lipid droplets/molecules. The applicability of these hydrolysates should be tested in meat food systems.
Acknowledgments
Thanks to Erik T. Hansen and Kim Dam Nielsen from Dat-Schaub for producing the hydrolysates and for providing support and help. Thanks to Lene Meinert from the Danish Meat Research Institute for data sharing, support and help, and thanks to Daniel Tsegay Berhe from Food Science, University of Copenhagen, for help with the PCA analysis. This work was financed by inSPIRe and the Pig Levy Fund.
Contributor Information
Trine Damgaard, Email: tdd@food.ku.dk.
René Lametsch, Phone: +45 35333483, Email: rla@food.ku.dk.
Jeanette Otte, Email: jo@food.ku.dk.
References
- Ajibola CF, Fashakin JB, Fagbemi TN, Aluko RE. Effect of peptide size on antioxidant properties of african yam bean seed (sphenostylis stenocarpa) protein hydrolysate fractions. Int J Mol Sci. 2011;12:6685–6702. doi: 10.3390/ijms12106685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA. Composition and nutritional value of edible meat by-products. In: Pearson AM, Dutson TR, editors. Edible meat by-products advances in meat research. Amsterdam: Elsevier Science Publishers Ltd; 1988. pp. 15–45. [Google Scholar]
- Balakrishnan B, Prasad B, Rai AK, Velappan SP, Subbanna MN, Narayan B. In vitro antioxidant and antibacterial properties of hydrolysed proteins of delimed tannery fleshings: comparison of acid hydrolysis and fermentation methods. Biodegradation. 2011;22:287–295. doi: 10.1007/s10532-010-9398-0. [DOI] [PubMed] [Google Scholar]
- Chaiyasit W, Fau ER, Fau MD, Decker EA. Role of physical structures in bulk oils on lipid oxidation. Crit Rev Food Sci Nutr. 2007;47:299–317. doi: 10.1080/10408390600754248. [DOI] [PubMed] [Google Scholar]
- Chan WKM, Decker EA, Lee JB, Butterfield DA. EPR spin-trapping studies of the hydroxyl radical scavenging activity of carnosine and related dipeptides. J Agric Food Chem. 1994;42:1407–1410. doi: 10.1021/jf00043a003. [DOI] [Google Scholar]
- Damgaard TD, Otte J, Meinert L, Jensen K, Lametsch R. Antioxidant capacity of hydrolyzed porcine tissues. Food Sci Nutr. 2014;2:282–288. doi: 10.1002/fsn3.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gobba C, Tompa G, Otte J. Bioactive peptides from caseins released by cold active proteolytic enzymes from Arsukibacterium ikkense. Food Chem. 2014;165:205–215. doi: 10.1016/j.foodchem.2014.05.082. [DOI] [PubMed] [Google Scholar]
- Di Bernardini R, Rai DK, Bolton D, Kerry J, O’Neill E, Mullen AM, Hayes M. Isolation, purification and characterization of antioxidant peptidic fractions from a bovine liver sarcoplasmic protein thermolysin hydrolysate. Peptides. 2011;32:388–400. doi: 10.1016/j.peptides.2010.11.024. [DOI] [PubMed] [Google Scholar]
- Donnelly JL, Decker EA, McClements DJ. Iron-catalyzed oxidation of menhaden oil as affected by emulsifiers. J Food Sci. 1998;63:997–1000. doi: 10.1111/j.1365-2621.1998.tb15841.x. [DOI] [Google Scholar]
- Eastoe JE. The amino acid composition of mammalian collagen and gelatin. Biochem J. 1955;61:589–600. doi: 10.1042/bj0610589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, He J, Zhuang Y, Sun L. Purification and identification of antioxidant peptides from enzymatic hydrolysates of tilapia (oreochromis niloticus) frame protein. Molecules. 2012;17:12836–12850. doi: 10.3390/molecules171112836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farvin KHS, Andersen LL, Nielsen HH, Jacobsen C, Jakobsen G, Johansson I, Jessen F. Antioxidant activity of cod (gadus morhua) protein hydrolysates: in vitro assays and evaluation in 5 % fish oil-in-water emulsion. Food Chem. 2014;149:326–334. doi: 10.1016/j.foodchem.2013.03.075. [DOI] [PubMed] [Google Scholar]
- Gault NF, Lawrie RA. Efficiency of protein extraction and recovery from meat industry by-products. Meat Sci. 1980;4:167–190. doi: 10.1016/0309-1740(80)90047-9. [DOI] [PubMed] [Google Scholar]
- Guo H, Kouzuma Y, Yonekura M. Structures and properties of antioxidative peptides derived from royal jelly protein. Food Chem. 2009;113:238–245. doi: 10.1016/j.foodchem.2008.06.081. [DOI] [Google Scholar]
- Hernandez-Ledesma B, Davalos A, Bartolome B, Amigo L. Preparation of antioxidant enzymatic hydrolysates from α-lactalbumin and β-lactoglobulin identification of active peptides by HPLC-MS/MS. J Agric Food Chem. 2005;53:588–593. doi: 10.1021/jf048626m. [DOI] [PubMed] [Google Scholar]
- Irshad I, Kanekanian A, Peters A, Masud T (2013) Antioxidant activity of bioactive peptides derived from bovine casein hydrolysate fractions. J Food Sci Technol:1–9
- Joseph TR, Reynolds PR, Bolton D, Fitzgerald GF, Stanton C. Bioactive peptides from muscle sources: meat and fish. Nutrients. 2011;3:765–791. doi: 10.3390/nu3090765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Lee KW, Lee HJ. Total antioxidant capacity of arginine-conjugated linoleic acid (CLA) complex. J Agric Food Chem. 2004;52:439–444. doi: 10.1021/jf030186w. [DOI] [PubMed] [Google Scholar]
- Kristiansen KR, Otte J, Ipsen R, Qvist KB. Large-scale preparation of β-lactoglobulin A and B by ultrafiltration and ion-exchange chromatography. Int Dairy J. 1998;8:113–118. doi: 10.1016/S0958-6946(98)00028-4. [DOI] [Google Scholar]
- Lass A, Suessenbacher A, Wölkart G, Mayer B, Brunner F. Functional and analytical evidence for scavenging of oxygen radicals by l-arginine. Mol Pharmacol. 2002;61:1081–1088. doi: 10.1124/mol.61.5.1081. [DOI] [PubMed] [Google Scholar]
- Liu Q, Kong B, Xiong YL, Xia X. Antioxidant activity and functional properties of porcine plasma protein hydrolysate as influenced by the degree of hydrolysis. Food Chem. 2010;118:403–410. doi: 10.1016/j.foodchem.2009.05.013. [DOI] [Google Scholar]
- Najafian L, Babji AS. Production of bioactive peptides using enzymatic hydrolysis and identification antioxidative peptides from patin (pangasius sutchi) sarcoplasmic protein hydolysate. J Funct Foods. 2014;9:280–289. doi: 10.1016/j.jff.2014.05.003. [DOI] [Google Scholar]
- Nasri R, Younes I, Jridi M, Trigui M, Bougatef A, Nedjar-Arroume N, Dhulster P, Nasri M, Karra-Châabouni M. ACE inhibitory and antioxidative activities of Goby (Zosterissessor ophiocephalus) fish protein hydrolysates: effect on meat lipid oxidation. Food Res Int. 2013;54:552–561. doi: 10.1016/j.foodres.2013.07.001. [DOI] [Google Scholar]
- Pihlanto A. Antioxidative peptides derived from milk proteins. Int Dairy J. 2006;16:1306–1314. doi: 10.1016/j.idairyj.2006.06.005. [DOI] [Google Scholar]
- Sachindra NM, Bhaskar N. In vitro antioxidant activity of liquor from fermented shrimp biowaste. Bioresour Technol. 2008;99:9013–9016. doi: 10.1016/j.biortech.2008.04.036. [DOI] [PubMed] [Google Scholar]
- Saiga E, Tanabe S, Nishimura T (2003) Antioxidant activity of peptides obtained from porcine myofibrillar proteins by protease treatment. J Agric Food Chem 51:3661–3667 [DOI] [PubMed]
- Saito K, Jin D, Ogawa T, Muramoto K, Hatakeyama E, Yasuhara T, Nokihara K. Antioxidative properties of tripeptide libraries prepared by the combinatorial chemistry. J Agric Food Chem. 2003;51:3668–3674. doi: 10.1021/jf021191n. [DOI] [PubMed] [Google Scholar]
- Samaranayaka AGP, Li-Chan ECY. Food-derived peptidic antioxidants: a review of their production, assessment, and potential applications. J Funct Foods. 2011;3:229–254. doi: 10.1016/j.jff.2011.05.006. [DOI] [Google Scholar]
- Sarmadi BH, Ismail A. Antioxidative peptides from food proteins: a review. Peptides. 2010;31:1949–1956. doi: 10.1016/j.peptides.2010.06.020. [DOI] [PubMed] [Google Scholar]
- Schweigert BS, Bennet BA, Guthneck BT. Amino acid composition of organ meats. J Food Sci. 1954;19:219–223. doi: 10.1111/j.1365-2621.1954.tb17442.x. [DOI] [Google Scholar]
- Sharp JS, Becker JM, Hettich RL. Analysis of protein solvent accessible surfaces by photochemical oxidation and mass spectrometry. Anal Chem. 2004;76:672–683. doi: 10.1021/ac0302004. [DOI] [PubMed] [Google Scholar]
- Storcksdieck S, Bonsmann G, Hurrell RF. Iron-binding properties, amino acid composition, and structure of muscle tissue peptides from in vitro digestion of different meat sources. J Food Sci. 2007;72:S019–S029. doi: 10.1111/j.1750-3841.2006.00229.x. [DOI] [PubMed] [Google Scholar]
- Torres-Fuentes C, Alaiz M, Vioque J. Iron-chelating activity of chickpea protein hydrolysate peptides. Food Chem. 2012;134:1585–1588. doi: 10.1016/j.foodchem.2012.03.112. [DOI] [PubMed] [Google Scholar]
- Udenigwe CC, Aluko RE. Food protein-derived bioactive peptides: production, processing, and potential health benefits. J Food Sci. 2011;77:R11–R24. doi: 10.1111/j.1750-3841.2011.02455.x. [DOI] [PubMed] [Google Scholar]
- Xiong YL. Antioxidant peptides. In: Mine Y, Li-Chan E, Jiang B, editors. Bioactive proteins and peptides as functional foods and nutraceuticals. Amsterdam: Blackwell Publishing Ltd; 2010. pp. 29–42. [Google Scholar]