Abstract
The tree Pistacia atlantica subsp. mutica, namely Bene, is widely distributed in Iranian mountains. Recent studies revealed that the oil of Bene was stable, even more stable than sesame oil, with antioxidant properties. This can give versatile applications for the oil. The volatile composition of this oil has not chemically been investigated so far. In this study, sixty three compounds were identified in the essential oil (EO) of Bene hull. The major components were determined to be α-pinene (20.8 %), camphene (8.4 %), β-myrcene (8.2 %) and limonene (8 %). Antioxidant activities of the essential oil from Bene hull were evaluated by using 2,2′- diphenyl-1-picrylhydrazyl (DPPH) free radical-scavenging, ferric reducing antioxidant power (FRAP), β-carotene bleaching test, thiobarbituric acid reactive species (TBARS) and Rancimat assays. The Bene essential oil exhibited significant antioxidant activities in FRAP and TBARS assays as compared with positive controls. In addition, the oil was evaluated for its antimicrobial activity against both Gram positive (Staphylococcus aureus) and Gram negative (Escherichia coli) bacteria. It showed significant antibacterial activities against S. aureus and E. coli with minimum inhibitory concentration (MIC) values of 6 and 12.5 μg/mL, respectively.
Keywords: Pistachio, FRAP, Antioxidant activity, Antibacterial activity, Rancimat
Introduction
Iran is the leading and largest exporter of pistachio (Pistacia vera) in the world with a total production of 472,097 tons (Iran production) in 2012. The genus Pistacia belongs to the family of Anacardiaceae comprising tree species that are dense bushes with green hull and aromatic properties (Gardeli et al. 2008). Pistacia atlantica subsp. mutica mainly occurs in Zagross mountain of Iran (Farhoosh et al. 2011a). The fruits of Pistacia atlantica subsp. mutica, namely Bene, are round to oval shape with 0.5-0.7 cm diameter. It appears in a wooden hard shell coated with dark green hull containing highly stable and antioxidative oil (Farhoosh et al. 2009). Interestingly, the antioxidant activity of the oil (non-volatile) during frying of sunflower oil has been higher than those of sesame and rice bran oils (Farhoosh et al. 2011b). Local people use the fruits of Bene after grinding and mixing with other ingredients as foods, and unripe Bene are also used as jam. Oleoresin of Bene has been used for making chewing gum in Iran (Hatamnia et al. 2014). Recently, Bene hull oil has been used as a natural alternative for stabilizing the common Kilka fish oil (Pazhouhanmehr et al. 2014). The methanol extract of Bene hull has been shown to have a potential anticancer properties (Rezaei et al. 2012). Extraction yield, phenolics content, and antioxidant activities of the materials extracted conventionally and/or ultrasonically from Bene hull by a number of aqueous and organic solvents (polar protic, polar aprotic and non-polar solvents) have been investigated. The highest oxidative stability index (OSI) value and antioxidant activities were observed in the methanol extract (Rezaie et al. 2015).
Previous researches on Bene hull were focused on the physicochemical and antioxidant activities of its oil. Information about the chemical composition and biological properties of the essential oil of Bene hull has not been reported so far. Therefore, this study was carried out in order to determine the chemical composition and antioxidant and antibacterial activities of the essential oil of Bene hull.
Materials and methods
Plant material
The ripe fruits of Bene were collected in Octobr 2013 from the fields of Marvdasht in the province Fars, Iran. Bene was air-dried in shadow at ambient temperature for 48 h. The dark green hulls of Bene fruits were divided from dark brown hard shells with a mechanichal instrument which was made for this purpose (electric mill with steel brush). The Bene hull was stored at –18 °C until use. A specimen was deposited in the herbarium of the Department of Pharmacognosy, School of Pharmacy, Mashhad University of Medical Sciences, Mashhad, Iran.
Chemicals
Sodium acetate trihydrate 2, 4, 6-tripyridyl-s-triazine (TPTZ), ethanol, 2,2-diphenyl-1-picrylhydrazyl (DPPH˙) and hydrochloric acid were obtained from Sigma company (St. Louis, MO, USA). Ascorbic acid and butylated hydroxyanisole (BHA) was supplied from Fluka Company (Switzerland). Acetic acid, Fe2 (SO4)3 · 7H2O and sodium sulphate were obtained from Merck company (Darmstadt, Germany).
Isolation of essential oil
Two hundred grams of the Bene hull was subjected to hydro-distillation of air-dried material for 3 h, with a clevenger-type apparatus for isolation of essential oil. EO was dried over anhydrous sodium sulphate and stored at 4 °C in dark until analysis. EO was diluted in n-hexane for gas chromatography and mass spectrometry analyses (Beatovic et al. 2015).
Gas chromatography and gas chromatography mass spectrometry (GC & GC–MS)
The GC analysis was performed using a Varian CP-3800 equipped with a FID detector, fused-silica column (CP-Sil 8CB, 50 m × 0.25 mm, film thickness 0.12 m). The operating conditions were: oven temperature 50 °C (5 min), 50 °C-250 °C (3 °C /min), 250 °C (10 min); injector temperature 260 °C, split ratio 1:5, with the carrier gas, N2 (2 mL/min); detector temperature 280 °C.
The GC-MS analyses were performed using an Agilent 5975 apparatus with a HP-5 ms column (30 m x 0.25 mm i.d., 0.25 μm film thickness) interfaced with a quadruple mass detector and a computer equipped with Wiley 7n.l library; oven temperature 50 °C (5 min),50–250 °C (3 °C /min), 250 °C (10 min); injector temperature 250 °C; volume injection, 0.1 μL; split ration, 1:50; carrier gas Helium at 1.1 mL min; ionization potential,70 eV; ionization current, 150 μA; ion source temperature, 250 °C; mass range, 35-465 mui.
The constituents of the essential oil (EO) were identified by calculation of their retention indices under temperature programmed conditions for n-alkanes (C8-C20) and the EO on a CP-Sil 8CB column. Then retention indices of compounds were calculated using the formula, I = 100 × [n + (N-n) (log tunknown – log tn)/(log tN – log tn)], where I is kovats retention index, N and n are the number of carbons in the larger and the smaller n-alkanes, respectively . Identification of individual compounds was made by comparison of their mass spectra and retention indices (RI) with those authentic samples and those given in the literature. Quantification of the relative amount of the individual components was performed according to the area percentage method without consideration of calibration factor (Kucukbay et al. 2014).
Antioxidant activity assays
DPPH free radical-scavenging assay
The radical scavenging ability of the essential oil (EO) was carried out using the stable free radical DPPH (2, 2-diphenyl-1-picrylhydrazyl). DPPH assay involves the reaction of antioxidants with the DPPH radical, changing the complex from a deep violet colour to a yellow complex. The degree of decolorization indicates the scavenging potential of the different concentrations of EO. Radical-scavenging activity of samples was measured by the method of Brand-Williams et al. (1995) slightly modified, as described below. Briefly, a 0.3 mM solution of DPPH in ethanol was prepared. An aliquot (50 μL) of samples (at four different concentrations: 100, 50, 25 and 12.5 μg/mL) were added to 150 μL of the DPPH solution in each well of a 96-well plate. For blank, only 50 μL of solvent was added to the DPPH solution. The decrease in absorbance was measured at 515 nm after 30 min of incubation at 37 °C using the BioTek micro plate reader (Synergy H4, USA). All tests were performed in triplicate (Sulaiman et al. 2011). Percentage scavenging effect = [(ADPPH − AS)/ADPPH] × 100, where AS is the absorbance of the solution when the sample has been added at a particular level and ADPPH is the absorbance of the DPPH solution. The sample concentration providing 50% inhibition (EC50) was calculated from the graph of scavenging effect percentage against sample concentration (Fernandez-Agullo et al. 2013).
Ferric reducing antioxidant potential (FRAP) assay
The FRAP assay was performed according to a previous work (Sulaiman et al. 2011). FRAP reagent was prepared by adding 10 mL of acetate buffer 300 mM, pH 3.6 (3.1 g sodium acetate trihydrate), to 1.0 mL of ferric chloride hexahydrate 20 mM (dissolved in distilled water) and 1.0 mL of 2,4,6-tri-(2-pyridyl)-s-triozine (TPTZ) 10 mM (dissolved in HCl 40 mM). In a well of a 96-well plate, an aliquot (10 μL) of EO (at five different concentrations: 100, 50, 25, 12.5 and 6.25 mg/mL) was added to 190 μL of the FRAP solution. After 30 min of incubation at 37 °C, absorbance of the reaction mixture was measured at 593 nm using BioTek micro plate reader (Synergy H4, USA). All tests were carried out in triplicate. Gallic acid was used as a reference to produce a standard curve. Data were expressed as milligram gallic acid equivalent per gram dry weight on the basis of dried sample (mg GAE/g dw basis).
β-Carotene bleaching test (BCB)
β-Carotene (0.1 mg) was added to a boiling flask together with linoleic acid (20 mg) and Tween 40 (100 mg), all dissolved in chloroform. The solvent was evaporated, under vacuum at 50 °C by a rotary evaporator, and then oxygenated distilled water (50 mL) was added and the mixture was emulsified for 1 min in a sonicator to form emulsion A. Then 200 μL of ethanolic stock solution of each antioxidant (concentrations of stock solutions were 0.1, 0.5, 1.0, 2.0, 3.0 and 4.0 g/l) was mixed with 5 mL of emulsion A in open-capped cuvettes. A control was prepared without antioxidant, consisting of 200 μL of ethanol and 5 mL of emulsion A. In addition, a second emulsion (B) consisting of 20 mg of linoleic acid, 100 mg of Tween 40 and 50 mL of oxygenated water was prepared. Ethanol (200 μL) was added to 5 mL of emulsion B and used to zero the spectrophotometer. The absorbances of the samples were read immediately (t = 0) and every 15 min intervals for 120 min on a CECIL 9000 spectrophotometer at 470 nm. The cuvettes were thermostated at 50 °C between measurements. The average percent of inhibition was calculated from the data with the formula:
where AA(120) is the absorbance of the antioxidant sample after 120 min, AC(120) is the absorbance of the control after 120 min, and AC(0) is the absorbance of the control at the beginning of experiment (t = 0) All determinations were run in triplicate (Guimaraes et al. 2010) .
Thiobarbituric acid reactive species (TBARS) assay
The antioxidant activity of EO was evaluated by quantifying the ability of different concentrations of EO to suppress Fe3+/ascorbate induced lipid peroxidation in soybean phosphatidylcholine liposomes (Duh et al. 1999). The formation of lipid peroxidation products was assayed by measuring malondialdehyde (MDA) levels on the basis of MDA reacted with thiobarbituric acid (TBA) at 532 nm (Serbetci et al. 2012). Soybean phosphatidylcholine liposomes (5 mg/mL in KH2PO4-K2HPO4 buffer) was sonicated under cooling conditions to yield a milky solution. The reaction mixture contained 500 μL of this mixture, 300 μL buffer, containing different concentrations of EO (Tween 80 as co-solvent for dissolving EO in buffer), 100 μL FeCl3 (1 mM) and 100 μL ascorbic acid (1 mM) to start peroxidation. Samples were incubated at 37 °C for an hour. Afterwards, lipid peroxidation was measured by the reaction with TBA. TBA (1 mL, 1% in 50 mM NaOH) and acetic acid (1 mL, 20 %) were added, and the resulting mixture heated at 100 °C for 30 min. 5 mL butanol was added to each tube, vigorously vortexed and centrifuged at 1500 g for 15 min. The absorbance of the organic layer was measured at 532 nm. The results of the samples were compared with those of controls not treated with the oil to calculate the percentage inhibition of lipid peroxidation using the following equation. All the values were based on the percentage antioxidant index (AI %):
Where AC is the absorbance value of the fully oxidized control and AT is the absorbance of the test sample.
Purification and preparation of the sunflower oil (SFO)
For the removal of naturally occurring antioxidant from the SFO, 200 g of the sunflower oil (SFO) was passed through 150 g aluminum oxide (activity degree 1, neutral), which had been activated at 200 °C for 3 h immediately before use. The alumina column (25–2.5 cm i.d.) and collection vessels were wrapped in aluminum foil, and the SFO was drawn through the column by suction without solvent. This procedure was repeated twice to ensure complete elimination of antioxidants (Yoshida et al. 1992). The purified SFO (PSFO) was mixed with antioxidant compounds and then was exposed to the following stability tests.
Rancimat test
A Metrohm Rancimat model 743 (Herisau, Switzerland) was used to measure the oxidative stability index (OSI). The tests were performed with 3 g of the PSFO containing 500 mg/kg concentrations of EO at 100 °C and an airflow rate of 15 L/h (Farhoosh et al. 2008). Protection factors (PF) were calculated as the ratio between the OSI of the PSFO containing an antioxidant compound and the PSFO without adding any antioxidant compound.
Antibacterial assay
Minimum inhibitory concentration (MIC) values were determined using serial two-fold dilutions of EO. For the first concentration (15 %, v/v equivalent to 135 μg/mL), 1.20 mL of EO was added to a mixture of polysorbate 80 (400 μl) and Soya Bean Casein Digest Broth (SCDB) (6.40 mL). The second dilution (10 %, v/v equivalent to 90 μg/mL) was similarly prepared. Then, two-fold serial dilutions were prepared by mixing 4 mL of the previous concentration with the same volume of culture broth, to reach the final concentration of 1.4 μg/mL. All samples were homogenized by vortexing before each dilution. Then, 200 μl from different concentration of EO in a sterile 96-well microtitre plate was inoculated with 20 μl of a 106 CFU/mL bacterial culture overnight. Neubauer hemocytometer under light microscope was used to determine the bacteria density. Staining of bacteria was carried out with trypan blue. All measurements were performed in triplicate. Moreover, negative and positive controls were used for each tested strain. To show the sterility of media, just Mueller-Hinton broth (MHB) was used as negative control. The test bacteria also were inoculated separately in culture media as positive control. After an overnight incubation at 37 °C, 20 μL of 2, 3, 5-triphenyltetrazolium chloride (TTC) (5 mg/mL) was added to each well as a colorimetric indicator of bacterial growth and incubated for 30 min at 37 °C (Shakeri et al. 2014).
Statistical analysis
All experiments and measurements were performed in triplicate, and data were subjected to analysis of variance (ANOVA). ANOVA and regression analyses were conducted with the MStat C and Excel softwares. Duncan’s multiple range test was used for mean separation. P values less than 0.05 were considered significant statistically.
Results and discussion
Essential oil composition
Volatile compounds were identified by comparing GC–MS retention data with the retention indices of the components of EO with n-alkanes. The yield of EO was 0.07 % (v/w dried material). Sixty three components, representing 99.8 % of the total oil, were identified. Retention indices and percentages are shown in Table 1. EO contained a high percentage of monoterpene hydrocarbons (75.7 %), followed by oxygenated monoterpenes (13.4 %), sesquiterpene hydrocarbons (6.0 %) and oxygenated sesquiterpene (1.4 %). The major components of Bene hull were α-pinene (20.8 %), camphene (8.4 %), β-myrcene (8.2 %), limonene (8.0 %), cis-ocimene (5.4 %), and trans-ocimene (5.2 %).
Table 1.
Chemical composition of Bene (Pistacia atlantica subsp. mutica) hull essential oil
| No | Compound name | % | KIa |
|---|---|---|---|
| 1 | Heptanal | 0.8 | 904 |
| 2 | Tricyclene | 0.9 | 924 |
| 3 | α-pinenec | 20.8 | 943 |
| 4 | Camphene | 8.4 | 956 |
| 5 | Verbenene | 4.1 | 963 |
| 6 | Sabinene | 3.4 | 978 |
| 7 | β-pinene | 4.2 | 981 |
| 8 | β-ocimene | 0.1 | 983 |
| 9 | β-myrcene | 8.2 | 993 |
| 10 | δ-2-carene | 0.2 | 1002 |
| 11 | α-phellandrene | 0.7 | 1005 |
| 12 | δ-3-carene | 1.4 | 1011 |
| 13 | Unknown | 0.3 | 1015 |
| 14 | α-terpinene | t b | 1019 |
| 15 | ρ-cymene | 2.4 | 1028 |
| 16 | Limonene | 8.0 | 1032 |
| 17 | cis-ocimene | 5.4 | 1044 |
| 18 | trans-ocimene | 5.2 | 1053 |
| 19 | γ-terpinene | 0.1 | 1063 |
| 20 | Terpinolene | 1.9 | 1090 |
| 21 | α-pinene oxide | 1.0 | 1096 |
| 22 | Linalool | 0.7 | 1103 |
| 23 | n-nonanal | 2.1 | 1108 |
| 24 | α-campholene oxide | 1.3 | 1128 |
| 25 | cis-limonene oxide | 0.1 | 1133 |
| 26 | trans-pinocarveol | 1.1 | 1140 |
| 27 | cis-verbenol | 0.3 | 1142 |
| 28 | Camphor | 0.4 | 1144 |
| 29 | trans-verbenol | 1.1 | 1147 |
| 30 | trans-pinocamphone | 0.2 | 1161 |
| 31 | Pinocarvone | 0.6 | 1163 |
| 32 | Borneol | 0.4 | 1167 |
| 33 | 4-terpineol | 0.3 | 1178 |
| 34 | ρ-methyl acetophenone | 0.4 | 1184 |
| 35 | ρ-cymen-8-ol | 0.8 | 1187 |
| 36 | Myrtenal | 0.6 | 1194 |
| 37 | Myrtenol | 0.2 | 1195 |
| 38 | Dodecane | 0.2 | 1200 |
| 39 | Verbenone | 0.5 | 1206 |
| 40 | trans-carveol | 0.2 | 1218 |
| 41 | Cumin aldehyde | 0.1 | 1241 |
| 42 | Carvone | 0.1 | 1245 |
| 43 | Bornyl acetate | 3.1 | 1287 |
| 44 | ρ-cymen-7-ol | 0.1 | 1292 |
| 45 | β-bourbonene | 0.1 | 1383 |
| 46 | Tetradecane | 0.1 | 1401 |
| 47 | Longifolene | 0.2 | 1404 |
| 48 | α-gurjunene | 0.1 | 1410 |
| 49 | trans-caryophyllene | 3.1 | 1418 |
| 50 | cis-thujopsene | 0.4 | 1429 |
| 51 | γ-elemene | 0.3 | 1435 |
| 52 | α-humulene | 0.4 | 1452 |
| 53 | Allo-aromadendrene | 0.1 | 1460 |
| 54 | γ-muurolene | 0.2 | 1475 |
| 55 | Germacrene D | 0.2 | 1479 |
| 56 | α-chamigrene | 0.1 | 1499 |
| 57 | cuparene | 0.4 | 1502 |
| 58 | β-bisabolene | 0.1 | 1507 |
| 59 | γ-cadinene | 0.1 | 1510 |
| 60 | δ-cadinene | 0.1 | 1519 |
| 61 | Unknown | 0.1 | 1536 |
| 62 | Spathulenol | 0.4 | 1574 |
| 63 | Caryophyllene oxide | 0.9 | 1578 |
| Major groups of compounds | |||
| Monoterpene hydrocarbons | 75.7 | ||
| Oxygenated monoterpenes | 13.4 | ||
| Sesquiterpene hydrocarbons | 6.0 | ||
| Oxygenated sesquiterpenes | 1.4 | ||
| Miscellaneous compounds | 3.3 | ||
| Total identified | 99.8 | ||
KI: The Kovats retention indices relative to C8-C20 n-alkanes were determined on CP-Sil 8CB capillary column
t: trace (≤0.05 %)
Major compounds are shown in bold
Antioxidant activities
As shown in table 2, EO (IC50 = 23.02 ± 0.01 μg/mL) showed moderate DPPH radical-scavenging activity and was lower than that of the standard antioxidant BHT (IC50 = 8.34 ± 0.01 μg/mL) and ascorbic acid (5.01 ± 0.02). Monoterpenes are not typically considered as potent radical scavengers. Therefore, a moderate antioxidant activity of EO can be possibly attributed to the relative high percentage of monoterpenes present in EO (Wojtunik et al. 2014).
Table 2.
Antioxidant activities of the essential oil of Bene hull and some standard antioxidants using DPPH radical-scavenging activity (EC50, μg/mL), ferric reducing antioxidant potential (FRAP, mmol/g), β-carotene bleaching (BCB,% inhibition of β-carotene) and protection factor (PF, concentrations of the sample = 500 ppm) assays
| Sample | DPPH | FRAP | BCB | PF* |
|---|---|---|---|---|
| Essential oil | 23.02 ± 0.01a | 5.29 ± 0.02a | 35.24 ± 0.01c | 0.91 ± 0.00d |
| BHT | 8.34 ± 0.01b | 0.45 ± 0.00d | 91.83 ± 0.03a | 2.30 ± 0.01c |
| Ascorbic acid | 5.01 ± 0.02c | 1.34 ± 0.02c | 6.12 ± 0.00d | 4.13 ± 0.00b |
| α-Tocopherol | 3.11 ± 0.00d | 2.51 ± 0.03b | 75.65 ± 0.02b | 6.2 ± 0.00a |
*The OSI value of control sunflower oil without any antioxidants was 1.96 h
Values are given as mean ± SD (n = 3). Means in each column followed by different letters are significantly different (P < 0.05)
Considering FRAP assay, EO was approximately two times more potent than α-tocopherol. BHT was almost three times less active than ascorbic acid which showed an activity of about half of that of α-tocopherol. On the basis of this assay, EO showed a remarkable antioxidant acitivity compared to the references including α-tocopherol, ascorbic acid, and BHT.
The presence of antioxidants can slow down the rate of β-carotene bleaching. This fact was used in the antioxidant activity assessment of EO of Bene hull in comparison with BHT, ascorbic acid and α-tocopherol. The antioxidant power decreased in the order of BHT > α-tocopherol > EO > ascorbic acid. The percentage inhibition of EO was about one third that of BHT. The reason for the weak activity of EO might be due to the absence of phenolic compounds (Dorman et al. 2000). Due to relatively high polarity, ascorbic acid did not show a considerable antioxidant property in the BCB assay. According to the polar paradox theory, the polar antioxidants residual in the aqueous phase of the emulsion are more diluted in lipid phase and are thus less effective in protecting the linoleic acid (Ahmadi et al. 2007).
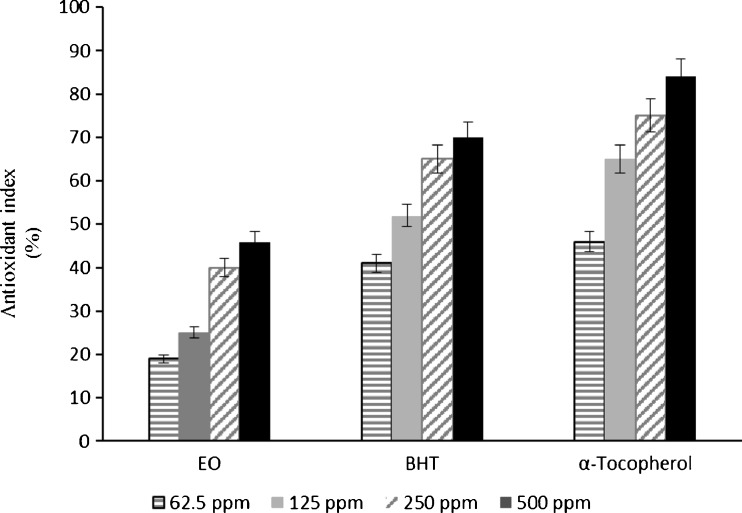
We also tested antioxidant activities of EO using the other antioxidant assay TBARS. Free radicals are known to attack polyunsaturated fatty acids of the membrane system to induce lipid peroxidation. TBARS will be over produced through increasing the free radicals; however it could be the end product of lipid peroxidation process and commonly used as a marker of oxidative stress (Serbetci et al. 2012). The inhibitory effect of EO and reference antioxidants (BHT and α-tocopherol) in different concentrations on lipid peroxidation in phosphatidylcholine liposomes were evaluated by the amount of TBARS accumulated 60 min after the incubation of liposomes with Fe3+/ascorbate (Fig. 1). EO indicated a dose-dependent relationship in inhibiting lipid peroxidation. Although BHT and α-tocopherol showed significantly higher activities, EO possessed a considerable antioxidant effect in TBARS assay. This level of effect can be considered remarkable for an essential oil as a combination of versatile components compared to a pure standard antioxidant compound.
Fig. 1.
Antioxidant activity (%) of Bene hull essential oil, α-tocopherol and BHT at different concentrations (62.5, 125, 250, and 500 ppm) were measured by TBARS assay with ABAP. Values are given as mean ± SD (n = 3)
Rancimat test is frequently used to estimate the antioxidant activity and is alluded to the increase of electrical conductivity due to the formation of volatile carboxylic acids as a result of lipid oxidation. The addition of EO and reference antioxidants to the PSFO prolonged the induction time of lipid oxidation. The OSI value of control PSFO without any antioxidant was only 1.96 h. The PF values of EO and reference compounds with 500 ppm concentration are shown in Table 2. The higher induction period of the PSFO with antioxidant added compared to that of a control (pure oil) means improved antioxidant activity. The α-tocopherol, ascorbic acid, BHT, and EO showed the highest inhibition potency, respectively. Our findings (Table 2) revealed that BHT was destroyed at 100 °C temprature in agreement with previous studies (Kulisic et al. 2005). The method used in this study proved that EO possessed a weak inhibitory effet against the SFO autooxidation process under the conditions (100 °C and an airflow rate of 15 L/h). These results were in agreement with an opinion established by previous studies (Yanishlieva et al. 1999) Low inhibitory effect of EO observed in this method, can be attributed, at least in part, to the loss of monoterpenes at high tempratures.
Antibacterial activities
Recently, there has been a remarkable interest in essential oils from aromatic plants with antimicrobial activities to control pathogens or toxin producing microorganisms in foods. Essential oils which are considered as additional intrinsic determinants to increase the safety and shelf life of foods have been known as antimicrobial agents in food systems (Teixeira et al. 2013). In this study, antibacterial activity of EO extracted from Bene hull was evaluated for the first time. EO showed antibacterial activity against the Gram-positive bacteria Staphylococcus aureus and the Gram-negative bacteria Escherichia coli O157:H7 with MIC values of 6 and 12.5 μg/mL, respectively.
Conclusion
Bene hull EO was rich in monoterpenes. This is the first time that its components have been characterized. The major components of the oil were to be α-pinene, camphene, β-myrcene, limonene, cis-ocimene and trans-ocimene. The results of the DPPH and BCB assay indicated that the essential oil has moderate capacity of scavenging free radicals. The results of FRAP assay were more interesting, indicating good antioxidant properties of EO but the Rancimat test showed no activity. Regarding remarkable antioxidant activity of Bene hull EO, it has a potential to be used as a preservative in foods and cosmetic products. EO can be an inexpensive source of natural antibacterial substances to increase the shelf life of foods and prevent the growth of bacteria; however, further studies are needed.
Acknowledgments
This research was financially supported by grants from the Mashhad University of Medical Sciences Research Council (Grant No. 89185). This study was also a part of the dissertation of Mrs. Mitra Rezaie for the degree of Doctor of Food Science and Technology submitted to, Faculty of Agriculture, Ferdowsi University of Mashhad.
References
- Ahmadi F, Kadivar M, Shahedi M. Antioxidant activity of Kelussia odoratissima Mozaff in model and food systems. Food Chem. 2007;105:57–64. doi: 10.1016/j.foodchem.2007.03.056. [DOI] [Google Scholar]
- Beatovic D, Krstic-Milosevic D, Trifunovic S, Siljegovic J, Glamoclija J, Ristic M, Jelacic S. Chemical Composition, antioxidant and antimicrobial activities of the essential oils of twelve Ocimum basilicum L. cultivars grown in Serbia. Rec Nat Prod. 2015;9:62–75. [Google Scholar]
- Brand-Williams W, Cuvelier M, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Dorman H, Figueiredo AC, Barroso JG, Deans SG. In vitro evaluation of antioxidant activity of essential oils and their components. Flavour Fragr J. 2000;15:12–16. doi: 10.1002/(SICI)1099-1026(200001/02)15:1<12::AID-FFJ858>3.0.CO;2-V. [DOI] [Google Scholar]
- Duh PD, Tu YY, Yen GC. Antioxidant activity of water extract of Harng Jyur (Chrysanthemum morifolium. Ramat) LWT-Food Sci Technol. 1999;32:269–277. doi: 10.1006/fstl.1999.0548. [DOI] [Google Scholar]
- Farhoosh R, Niazmand R, Rezaei M, Sarabi M. Kinetic parameter determination of vegetable oil oxidation under Rancimat test conditions. Eur J Lipid Sci Technol. 2008;110:587–592. doi: 10.1002/ejlt.200800004. [DOI] [Google Scholar]
- Farhoosh R, Khodaparast MHH, Sharif A. Bene hull oil as a highly stable and antioxidative vegetable oil. Eur J Lipid Sci Technol. 2009;111:1259–1265. doi: 10.1002/ejlt.200900081. [DOI] [Google Scholar]
- Farhoosh R, Tavassoli-Kafrani MH, Sharif A. Antioxidant activity of the fractions separated from the unsaponifiable matter of Bene hull oil. Food Chem. 2011;126:583–589. doi: 10.1016/j.foodchem.2010.11.047. [DOI] [Google Scholar]
- Farhoosh R, Tavassoli-Kafrani MH, Sharif A. Antioxidant activity of sesame, rice bran and Bene hull oils and their unsaponifiable matters. Eur J Lipid Sci Technol. 2011;113:506–512. doi: 10.1002/ejlt.201000402. [DOI] [Google Scholar]
- Fernandez-Agullo A, Pereira E, Freire M, Valentao P, Andrade P, Gonzaalez-Alvarez J, Pereira J. Influence of solvent on the antioxidant and antimicrobial properties of walnut (Juglans regia L.) green husk extracts. Ind Crops Prod. 2013;42:126–132. doi: 10.1016/j.indcrop.2012.05.021. [DOI] [Google Scholar]
- Gardeli C, Vassiliki P, Athanasios M, Kibouris T, Komaitis M. Essential oil composition of Pistacia lentiscus L. and Myrtus communis L.: evaluation of antioxidant capacity of methanolic extracts. Food Chem. 2008;107(3):1120–1130. doi: 10.1016/j.foodchem.2007.09.036. [DOI] [Google Scholar]
- Guimaraes R, Sousa MJ, Ferreira IC. Contribution of essential oils and phenolics to the antioxidant properties of aromatic plants. Ind Crops Prod. 2010;32:152–156. doi: 10.1016/j.indcrop.2010.04.011. [DOI] [Google Scholar]
- Hatamnia AA, Abbaspour N, Darvishzadeh R. Antioxidant activity and phenolic profile of different parts of Bene (Pistacia atlantica subsp. kurdica) fruits. Food Chem. 2014;145:306–311. doi: 10.1016/j.foodchem.2013.08.031. [DOI] [PubMed] [Google Scholar]
- Kucukbay FZ, Kuyumcu E, Celen S, Azaz AD, Arabac T. Chemical composition of the essential oils of three Thymus taxa from Turkey with antimicrobial and antioxidant activities. Rec Nat Prod. 2014;8(2):110–120. [Google Scholar]
- Kulisic T, Radonic A, Milos M. Inhibition of lard oxidation by fractions of different essential oils. Grasas y Aceites. 2005;56:284–291. doi: 10.3989/gya.2005.v56.i4.94. [DOI] [Google Scholar]
- Pazhouhanmehr S, Farhoosh R, Esmaeilzadeh Kenari R, Sharif A. Oxidative stability of purified common Kilka (Clupeonella cultiventris caspia) oil as a function of the Bene kernel and hull oils. Int J Food Sci Technol. 2014 [Google Scholar]
- Rezaei PF, Fouladdel S, Ghaffari SM, Amin G, Azizi E. Induction of G1 cell cycle arrest and cyclin D1 down-regulation in response to pericarp extract of Baneh in human breast cancer T47D cells. Daru J Pharm Sci. 2012;20:101. doi: 10.1186/2008-2231-20-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaie M, Farhoosh R, Iranshahi M, Sharif A, Golmohamadzadeh S. Ultrasonic-assisted extraction of antioxidative compounds from Bene (Pistacia atlantica subsp. mutica) hull using various solvents of different physicochemical properties. Food Chem. 2015;173:577–583. doi: 10.1016/j.foodchem.2014.10.081. [DOI] [PubMed] [Google Scholar]
- Serbetci T, Ozsoy N, Demirci B, Can A, Kulur S, Baser K. Chemical composition of the essential oil and antioxidant activity of methanolic extracts from fruits and flowers of Hypericum lydium Boiss. Ind Crops Prod. 2012;36:599–606. doi: 10.1016/j.indcrop.2011.11.002. [DOI] [Google Scholar]
- Shakeri A, Khakdan F, Soheili V, Sahebkar A, Rassam G, Asili J. Chemical composition, antibacterial activity, and cytotoxicity of essential oil from Nepeta ucrainica L. subsp. kopetdaghensis. Ind Crops Prod. 2014;58:315–321. doi: 10.1016/j.indcrop.2014.04.009. [DOI] [Google Scholar]
- Sulaiman SF, Sajak AAB, Ooi KL, Seow EM. Effect of solvents in extracting polyphenols and antioxidants of selected raw vegetables. J Food Compos Anal. 2011;24:506–515. doi: 10.1016/j.jfca.2011.01.020. [DOI] [Google Scholar]
- Teixeira B, Marques A, Ramos C, Neng NR, Nogueira JM, Saraiva JA, Nunes ML. Chemical composition and antibacterial and antioxidant properties of commercial essential oils. Ind Crops Prod. 2013;43:587–595. doi: 10.1016/j.indcrop.2012.07.069. [DOI] [Google Scholar]
- Wojtunik KA, Ciesla LM, Waksmundzka-Hajnos M. Model studies on the antioxidant activity of common terpenoid constituents of essential oils by means of the 2, 2-diphenyl-1-picrylhydrazyl method. J Agric Food Chem. 2014;62:9088–9094. doi: 10.1021/jf502857s. [DOI] [PubMed] [Google Scholar]
- Yanishlieva NV, Marinova EM, Gordon MH, Raneva VG. Antioxidant activity and mechanism of action of thymol and carvacrol in two lipid systems. Food Chem. 1999;64:59–66. doi: 10.1016/S0308-8146(98)00086-7. [DOI] [Google Scholar]
- Yoshida H, Kondo I, Kajimoto G. Participation of free fatty acids in the oxidation of purified soybean oil during microwave heating. JAOCS. 1992;69:1136–1140. [Google Scholar]