Abstract
Black and green pepper essential oils were used in this study in order to determine the chemical composition, in vitro antimicrobial activity against food spoilage microorganisms and in situ oils effect on food microorganism, after incorporation in chicken soup, by suggested methodology for calculation of Growth inhibition concentrations (GIC50). Chemical analysis revealed a total of 34 components. The major constituent of black pepper oil was trans-caryophyllene (30.33 %), followed by limonene (12.12 %), while β-pinene (24.42 %), δ3-carene (19.72 %), limonene (18.73 %) and α-pinene (10.39 %) were dominant compounds in green pepper oil. Antimicrobial activity was determined by microdilution technique and minimal inhibitory (MIC) and minimal bactericidal/fungicidal concentrations (MBC/MFC) were determined. Green pepper oil showed stronger antibacterial and antifungal activity (MIC 0.50–1.87; MBC 0.63–2.5 mg/ml; MIC 0.07–0.16; MFC 0.13–1.25 mg/ml) against black pepper oil (MIC 0.07–3.75; MBC 0.60–10.00 mg/ml; MIC 0.63–5.00; MFC 1.25–10.00 mg/ml. Oils successfully inhibited the growth of S. aureus in chicken soup in a dose dependent manner. GIC50 values were calculated after 24, 48 and 72 h and were in range of 0.156–0.689 mg/ml. The 50 % inhibitory concentrations (IC50) of EOs were 36.84 and 38.77 mg/ml with in 2, 2-diphenyl-1-picrylhydrazyl (DPPH) assay respectively.
The obtained results revealed that black and green pepper volatiles are efficient in controlling the growth of known food-spoilage microorganisms.
Keywords: Piper nigrum L, Black and green pepper, Essential oil, Antimicrobial activity, Novel methodology for GIC50, Liquid food
Introduction
Despite technological development and food production techniques, food safety remains very important public health issue. According to studies more than 9 million persons each year have a food-borne illness caused by a major pathogen (Scallan et al. 2011). In the United States alone, food recalls and food-borne illnesses bear an annual price tag of approximately 77 billion (including discarded product, revenue, healthcare costs, lost wages and litigation and other expenditures (Scharff 2012). At the same time, Western society appears to be experiencing a trend of ‘green’ consumerism (Chaibi et al. 1997), desiring products with a smaller impact on the environment.
Therefore a need for new methodologies for reducing or eliminating food-borne pathogens, possibly in combination with existing techniques is needed. Many naturally occurring products, such as essential oils from edible and medicinal plants, herbs, spices and isolated compounds have been shown to possess antimicrobial functions and could be used as food additives (Stojković et al. 2011, 2013a, b; Bukvički et al. 2014).
Essential oils (EOs) are plants secondary metabolites. They have been used in medicine, perfumery, cosmetic, and have been added to foods as part of spices or herbs. Particularly, application of essential oils as aroma and flavor ingredients increased recently and became their major employment. Almost 3000 different essential oils are known, and 300 are used commercially in the flavor and fragrances market (Burt and Reinders 2003). The use of essential oils is gaining momentum (Reische et al. 1998) due to the growing consumers demand for the inclusion of natural products and the reduction and elimination of synthetic additives in the food industry. Many authors, in fact, have reported antimicrobial, antifungal, antioxidant and radical-scavenging properties (Škrinjar and Nemet 2009; De Azeredo et al. 2012; Millezi et al. 2012) by spices extracts and essential oils. Only in some cases a direct food-related application has been tested (Fisher et al. 2009; Emiroğlu et al. 2010; Stojković et al. 2011; Bukvički et al. 2014).
Piper nigrum L. is perennial, evergreen woody vines of the family Piperaceae (Srinivasan 2007). Originating from tropical parts of India and Southeast Asia the plant has pleasant smell and more or less bitter taste. There are several types of pepper, black, green, white, orange, red etc. In fact, they all come from the same plant, and differ in the way of preparation for the market, which gives them a different flavor and degree of spiciness. The plant is known to be rich in essential oil (2–3 %) and a source of numerous biologically active constituents such as monoterpenes, sesquiterpenes, and other volatile compounds (Evans 1996; Jagella and Grosch 1999; Chen et al. 2011). P. nigrum is one of the most used spices in the world (Meghwal and Goswami 2013). Pepper has many medicinal properties and it is used in treatment of rheumatism, diabetes, chronic indigestion, colon toxins, obesity, fever and diarrhea. It is also reported that pepper enhance the bioavailability of food, drug, anti-carcinogens and phytochemicals. According to literature P. nigrum sesquiterpenes enhance the trans-dermal delivery of active substances trough skin membrane. Also, increased pancreatic activity and stimulation of gastric acid secretion are recorded (Dorman and Deans 2002; Sashidhar 2002). However, little attention has been given to pepper essential oil research and application. As per our knowledge, there is no report on the preservation of chicken soup with pepper essential oil. In this study, two pepper oils were used. Green pepper oil, which is derived from cooked and dried unripe fruit and black pepper oil obtained from the dried unripe fruit.
Hence, the aims of this study were to determine: i) chemical composition by means of gas chromatography/mass spectrometry (GC/MS); ii) in vitro antimicrobial activity against food poisoning and food spoilage bacteria/fungi; iii) in vitro antiradical properties by using DPPH radical and iiii) to investigate oils inhibitory effects on food spoilage microorganism in situ, after incorporation in soup. The proposed research evaluates the possibility of using pepper essential oils as food preservatives.
Material and methods
Source of the essential oils
Essential oils of Piper nigrum L. were commercial samples. Black pepper and green pepper oils were obtained from SENSIENT ESSENTIAL OILS GMBH, Flughafendamm 11, D-28199 Bremen, Germany.
Essential oils analysis
Procedure used for gas chromatography-flame ionization detector (GC-FID) and GC-MS analyses complies with standards set for Gas Chromatography of essential oils (ISO 7609:1985; ISO 11024–1:1998; ISO 11024–2:1998).
GC-FID analysis was performed on GC Agilent Technologies 7890A apparatus, equipped with the split-splitless injector and automatic liquid sampler (ALS), attached to HP-5 column (30 × 0.32 mm, film thickness 0.25 μm) and fitted to FID. Operating conditions were as follows: carrier gas was H2 (1 ml/min/210 °C); temperatures of injector and detector were set at 250 and 280 °C, respectively, while the column temperature was linearly programmed 40–260 °C at 4 °C/min. Solutions of essential oils’ samples in ethanol (ca. 1 %) were consecutively injected by ALS (1 μl, split-mode). The percentile presence of components in essential oils’ samples were calculated from the peak areas obtained in the area-percent reports (obtained as a result of standard processing of chromatograms) without correction factors, using normalization method.
The GC/MS was performed on HP G 1800C Series II GCD analytical system equipped with HP-5MS column (30 m × 0.25 mm, film thickness 0.25 μm). Carrier gas was He (1 ml/min). Other chromatographic conditions were as those for GC-FID. Transfer line was heated at 260 °C. Mass spectra were recorded in EI mode (70 eV), in a range of m/z 40–450. Solutions of essential oil samples in ethanol (ca. 1 %) were consecutively injected by ALS (0.2 μl, split mode).
The identification of essential oils’ components was based on matching of their mass spectra peaks with those from Wiley 275 and NIST/NBS libraries. The experimental values for Kovats’ retention indices (RI) were determined by using calibrated Automated Mass Spectral Deconvolution and Identification System software (AMDIS (ver. 2.1.)), compared to those from available literature (Adams 2009), and they were used as additional tool to support MS findings.
Microorganisms and culture conditions
The following Gram-positive bacteria were used: Staphylococcus aureus (food isolate), Bacillus cereus (clinical isolate), Listeria monocytogenes (NCTC 7973), and Micrococcus flavus (ATCC 10240), and the following Gram-negative bacteria were used: Pseudomonas aeruginosa (ATCC 27853), Escherichia coli (ATCC 35210), Salmonella typhimurium (ATCC 13311), and Enterobacter cloacae (ATCC 35030). For the antifungal bioassays, eight fungi were used: Penicillium aurantiogriseum (food isolate), Aspergillus niger (ATCC 6275), Aspergillus ochraceus (ATCC 12066), Aspergillus versicolor (ATCC 11730), Aspergillus fumigatus (human isolate), Trichoderma viride (IAM 5061), Penicillium funiculosum (ATCC 36839), Penicillium ochrochloron (ATCC 9112). The organisms were obtained from the Mycological Laboratory, Department of Plant Physiology, Institute for Biological Research “Siniša Stankovic”, Belgrade, Serbia.
Antibacterial assay
The MICs and MBCs were determined using 96-well microtitre plates. The bacterial suspension was adjusted with sterile saline to a concentration of 1.0x105 CFU/ml. Essential oils were dissolved in 5 % DMSO solution containing 0.1 % Tween 80 (v v−1) (100 mg/ml) and added in Tryptic Soy broth (TSB) medium (100 μl) with bacterial inoculum (1.0 × 104 CFU per well) to achieve the wanted concentrations. Microplates were incubated at a rotary shaker (160 rpm) for 24 h at 37 °C. The following day, 30 μl of 0.2 mg/ml solution of INT (p-iodonitrotetrazolium violet) was added to the plates, followed by ½ h incubation so as to ensure adequate color reaction. Inhibition of bacterial growth was indicated by a clear solution or a definite decrease in color reaction (Tsukatani et al. 2012). The lowest concentrations without visible growth (at the binocular microscope) were defined as concentrations that completely inhibited bacterial growth (MICs). The MBCs were determined by serial sub-cultivation of 10 μl into microtitre plates containing 100 μl of broth per well and further incubation for 24 h. The lowest concentration with no visible growth was defined as the MBC, indicating 99.5 % killing of the original inoculum. Streptomycin (Sigma P 7794) and Ampicillin (Panfarma, Belgrade, Serbia) were used as positive controls (1 mg/ml in sterile physiological saline). 5 % DMSO solution containing 0.1 % Tween 80 (v/v) was used as a negative control. All experiments were done in triplicate.
Antifungal activity
In order to investigate the antifungal activity of essential oils, a modified microdilution technique was used. The fungal spores were washed from the surface of agar plates with sterile 0.85 % saline containing 0.1 % Tween 80 (v/v). The spore suspension was adjusted with sterile saline to a concentration of approximately 1.0x105 in a final volume of 100 μl per well. The inocula were stored at 4 °C for further use. Dilutions of the inocula were cultured on solid malt agar to verify the absence of contamination and to check the validity of the inoculum. Minimum inhibitory concentration (MIC) determinations were performed by a serial dilution technique using 96-well microtiter plates. All oils investigated, were dissolved in 5 % DMSO solution containing 0.1 % Tween 80 (v/v) (100 mg/ml) and added in broth Malt medium with inoculum. The lowest concentrations without visible growth (at the binocular microscope) were defined as MICs. The fungicidal concentrations (MFCs) were determined by serial subcultivation of a 10 μl of tested compounds dissolved in medium and inoculated for 72 h, into microtiter plates containing 100 μl of broth per well and further incubation 72 h at 28 °C. The lowest concentration with no visible growth was defined as MFC indicating 99.5 % killing of the original inoculum (Espinel-Ingroff 2001). 5 % dimethyl sulfoxide (DMSO) solution containing 0.1 % Tween 80 (v/v) was used as a negative control, commercial fungicides, bifonazole (Srbolek, Belgrade, Serbia) and ketoconazole (Zorkapharma, Šabac, Serbia), were used as positive controls.
Free radical scavenging activity
The antioxidant activity of black and green pepper oils was determined using the stable radical DPPH method (Blois 1958). Briefly, 500 μl of various concentrations of EO in methanol (20, 40, 60, 80 mg/ml) were mixed with 500 μl of 20 μM methanol solution of DPPH. Absorbance at 517 nm was measured after 30 min in dark. Methanol plus sample solution was used as a blank. DPPH solution and methanol were used as controls. The inhibition percentage was calculated using following formula:
where AC is the absorbance of the negative control and As is absorbance of the samples. The results were compared with butylated hydroxyanisole (BHA).
In situ preservation of chicken soup
Isolation and identification of chicken soup contaminant bacterium
Chicken soup used in this study was purchased from the local market. The packaging stated no artificial preservatives. Chicken soup was left open at 25 ° C for 3 days. After that period, the experiment with dilutions was repeated to check for contaminants. Mueller–Hinton (MH) plates were inoculated with different dilutions of chicken soup in sterilized water and kept at 37 ° C in order to investigate possible bacterial contaminants. Bacterial culture reinoculate in Casein soya bean digest broth, homogenize and incubate at 35 ° C to 37 ° C for 18 to 48 h. Subculture on a plate of Bair–Parker agar and incubate at 35 ° C to 37 ° C for 18 to 72 h. Black colonies of gram positive cocci, surrounded by a clear zone indicate the presence of S. aureus. After confirmation by suitable biochemical tests such as the coagulase test and the deoxyribonuclease test, the culture was identified by M. Sci. Tatjana Stević (Institute for Medicinal Plant Research “Dr Josif Pančić”), as S. aureus and deposited to Bacteria Collection Unit of the Department for Plant Physiology, Institute for Biological Research “Siniša Stanković”, Belgrade, Serbia, with the number Sa-DS-12.
In situ antibacterial assay in chicken soup
Chicken soup was prepared in sterile conditions and according to the manufacturer instructions. Different concentrations of the oils were added to the chicken soup to achieve final concentrations in range of 0.01–105 mg/ml respectively. The controls contained chicken soup but not essential oil. The flasks were homogenized for 30s to ensure mixing of the oils with chicken soup. The soup mixture was inoculated with ~106 cells of foodborne S. aureus that had been prepared by growing overnight bacterium at 37 °C in TSB medium. Cell suspension was adjusted with sterile saline to approximately 1 × 106 cells/ml of chicken soup. The inoculum was mixed thoroughly with the soup, by hands. Experimental microplates were kept at 25 ° C. The inhibition percentage at 25 ° C was calculated by optical density measured by ELISA plate reader (Tecan Austria, GmbH-Austria, Ependorf-AG, Germany) using the following equations:
where OD0 sample and OD sample corresponded to the absorbance at 612 nm of the strain growth in the presence of the extract before and after incubation, respectively; OD0 blank and OD blank corresponded to the broth medium with dissolved compound before and after incubation, respectively; and OD0 growth* and OD growth* to the strain growth in the absence of the oils before and after incubation at 25 ° C, respectively (Stojković et al. 2013a, b; Bukvički et al. 2014).
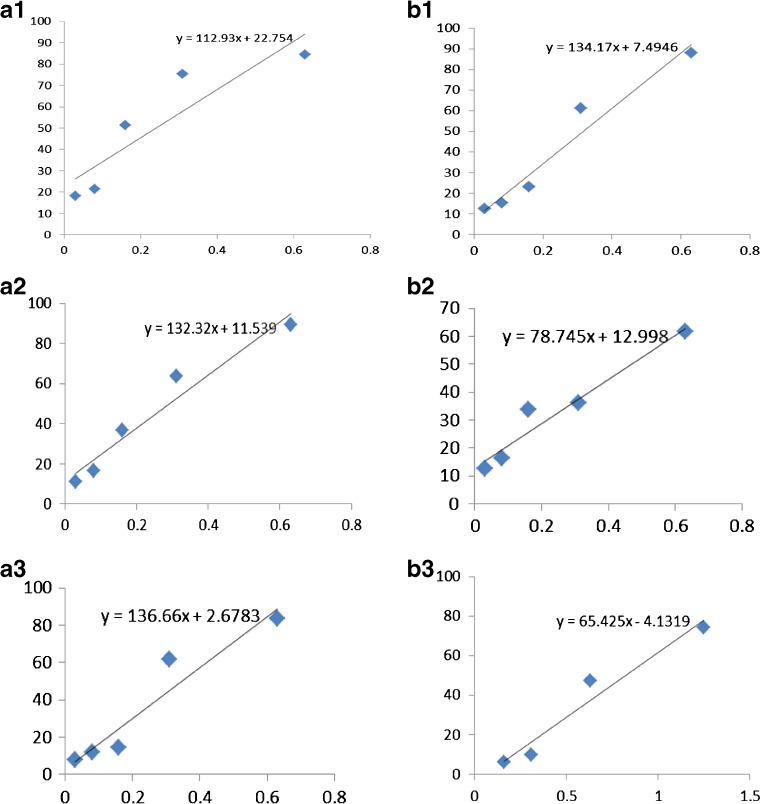
The results were further presented graphically and calculated, using equations presented on Fig. 1, as growth inhibition concentrations (GIC) that cause 50 % retardation of bacterial growth. The concentrations were calculated after 24, 48 and 72 h following the incubation period.
Fig. 1.
Inhibition values (50 %) for green pepper (PG) and black pepper (PN) after 24, 48 and 72 h. x axes is presented in mg/ml, y axis presents percentages of inhibition (%). Figures: A1, A2, A3 present GIC50 values determination for green pepper oil, after 24 h (0.241 mg/ml), 48 h (0.290 mg/ml), 72 h (0.346 mg/ml) of incubation, respectively. Figures: B1, B2, B3 present GIC50 values determination for green pepper oil, after 24 h (0.317 mg/ml), 48 h (0.470 mg/ml), 72 h (0.827 mg/ml) of incubation, respectively
Statistical analysis
The results are expressed as mean values and standard deviation (SD), and analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s HSD Test with α = 0.05. This analysis was carried out using SPSS v. 18.0 program.
Results
The results obtained by GC-FID and GC-MS chemical analysis of black and green pepper essential oils are presented in the Table 1. Results showed that sesquitepene hydrocarbons presented the major portion of black pepper while monoterpene hydrocarbons were major compounds in green pepper oil. Thirty compounds were identified in black pepper oil, which accounts for 100 % of the total oil. The major constituent of the oil was trans-caryophyllene (30.33 %) followed by limonene (12.12 %). Analysis of green pepper oil showed 12 compounds representing 98.44 % of the total oil. The main constituent β-pinene (24.42 %) was followed by δ3-carene (19.72 %), limonene (18.73 %) and α-pinene (10.39 %).
Table 1.
Chemical composition of black and green pepper oils
| No | Constituents | RI | Yield percentage | |
|---|---|---|---|---|
| Black papper oil | Green papper oil | |||
| 1 | α-Thujene | 919 | 0.51 | nd |
| 2 | α-Pinene | 924 | 2.88 | 10.39 |
| 3 | Camphene | 939 | nd | 4.68 |
| 4 | Sabinene | 964 | 7.52 | nd |
| 5 | β-Pinene | 967 | 7.42 | 24.42 |
| 6 | Myrcene | 985 | 0.69 | nd |
| 7 | α-Phellandrene | 997 | 0.33 | nd |
| 8 | δ3-Carene | 1002 | 5.28 | 19.72 |
| 9 | p-Cymene | 1017 | 0.70 | 0.32 |
| 10 | Limonene | 1021 | 12.12 | 18.73 |
| 11 | trans-Pinocarveol | 1131 | nd | 0.31 |
| 12 | cis-Sabinene hydrate | 1062 | 0.31 | nd |
| 13 | p-Mentha-2,4(8)-diene | 1082 | 0.73 | nd |
| 14 | Terpinen-4-ol | 1171 | 0.28 | nd |
| 15 | Myrtenol | 1191 | nd | 2.81 |
| 16 | Geraniol | 1256 | 2.06 | nd |
| 17 | δ-Elemene | 1328 | 0.60 | 4.13 |
| 18 | α-Cubebene | 1340 | 0.99 | nd |
| 19 | α-Copaene | 1365 | 0.56 | 3.15 |
| 20 | β-Elemene | 1382 | 1.74 | nd |
| 21 | cis-Caryophyllene | 1408 | nd | 0.58 |
| 22 | trans-Caryophyllene | 1409 | 30.33 | nd |
| 23 | α-trans-Bergamotene | 1426 | 3.40 | nd |
| 24 | α-Guaiene | 1428 | 0.36 | nd |
| 25 | β-Selinene | 1475 | 3.26 | nd |
| 26 | α-Salinene | 1484 | 5.32 | nd |
| 27 | α-Zingiberene | 1486 | 0.74 | nd |
| 28 | Bicyclogermacrene | 1493 | 0.31 | nd |
| 29 | β-Bisabolene | 1499 | 4.78 | nd |
| 30 | γ-Cadinene | 1513 | 0.65 | nd |
| 31 | γ-trans-Bisabolene | 1522 | 1.39 | nd |
| 32 | Hedycaryol | 1541 | 0.37 | nd |
| 33 | Germacrene B | 1545 | 0.22 | nd |
| 34 | Caryophyllene oxide | 1571 | 4.16 | 9.20 |
| monoterpene hydrocarbons | 38.17 | 78.26 | ||
| oxygenated monoterpenes | 2.65 | 3.12 | ||
| sesquiterpene hydrocarbons | 53.26 | 7.86 | ||
| oxygenated sesquiterpenes | 5.92 | 9.20 | ||
| Total identified | 100.00 | 98.44 | ||
nd, not detected
Antimicrobial activity results, of the essential oils investigated by microdilution method, are summarized in Tables 2 and 3. Oils inhibited the growth of all bacterial and fungal strains tested. Green pepper oil showed slightly stronger antibacterial and antifungal activity (MIC 0.50–1.87; MBC 0.63–2.50 mg/ml; MIC 0.07–0.16; MFC 0.13–1.25 mg/ml) compared to black pepper oil (MIC 0.07–3.75; MBC 0.60–10.0 mg/ml; MIC 0.63–5.00; MFC 1.25–10.00 mg/ml). The most resistant bacterial species was found to be L. monocytogenes while the most susceptible to oils was B. cereus. Fungi appear to be more sensitive compared to bacteria. The most sensitive fungi was A. versicolor while A. niger was the most resistant species. Comparing the results of EOs with that of standard drugs, Ampicillin (MIC 0.05–0.30; MBC 0.10–0.50 mg/ml) and Streptomycin (MIC 0.30–0.80; MBC 0.40–1.25 mg/ml), it was concluded that the oils posses less potent antibacterial activity. However, bifonazole (MIC 0.10–0.20; MFC 0.20–0.30 mg/ml) and ketoconazole (MIC 0.15–1.50; MFC 0.50–2.00 mg/ml) showed lower activity compared to green pepper oil and higher activity in comparison to black pepper oil.
Table 2.
Antibacterial activity of green and black pepper essential oils against food-spoilage bacteria (mg/ml)
| Oils | B.c. | M.f. | S.a. | L.m. | E.c. | En.cl . | P.a. | S.t. | |
|---|---|---|---|---|---|---|---|---|---|
| Green pepper | MIC | 0.50 ± 0.07c | 1.25 ± 0.14b | 0.63 ± 0.22c | 1.87 ± 0.26b | 0.63 ± 0.07b | 1.00 ± 0.00d | 1.00 ± 0.21c | 1.00 ± 0.14c |
| MBC | 0.63 ± 0.22c | 2.5 ± 0.28b | 1.25 ± 0.25c | 2.50 ± 0.28b | 1.25 ± 0.14b | 1.25 ± 0.14d | 1.25 ± 0.14b | 1.25 ± 0.14a | |
| Black pepper | MIC | 0.31 ± 0.09b | 3.75 ± 0.38c | 0.31 ± 0.03b | 2.50 ± 0.28c | 2.50 ± 0.28c | 0.07 ± 0.04a | 1.25 ± 0.14d | 0.63 ± 0.21b |
| MBC | 0.60 ± 0.00bc | 5.0 ± 0.88c | 0.60 ± 0.22b | 10.00 ± 1.44c | 5.00 ± 0.28c | 0.31 ± 0.03a | 5.00 ± 1.44c | 1.25 ± 1.01a | |
| Ampicillin | MIC | 0.1 ± 0.03a | 0.2 ± 0.01a | 0.05 ± 0.03a | 0.2 ± 0.02a | 0.2 ± 0.03a | 0.3 ± 0.02b | 0.2 ± 0.02a | 0.2 ± 0.00a |
| MBC | 0.2 ± 0.01a | 0.3 ± 0.02a | 0.1 ± 0.03a | 0.3 ± 0.09a | 0.3 ± 0.006a | 0.5 ± 0.02b | 0.3 ± 0.02b | 0.3 ± 0.02a | |
| Streptomycin | MIC | 0.3 ± 0.00b | 0.3 ± 0.01a | 0.3 ± 0.003b | 0.4 ± 0.006a | 0.3 ± 0.006a | 0.4 ± 0.02c | 0.8 ± 0.01b | 0.3 ± 0.01a |
| MBC | 0.4 ± 0.01ab | 0.4 ± 0.02a | 0.4 ± 0.006ab | 0.5 ± 0.01a | 0.5 ± 0.006ab | 0.8 ± 0.01c | 1.25 ± 0.02c | 0.5 ± 0.02a |
B.c., Bacillus cereus; M.f., Micrococcus flavus; S.a., Staphylococcus aureus; L.m., Listeria monocytogenes; E.c., Escherichia coli; En.cl., Enterobacter cloacae; P.a., Pseudomonas aeruginosa; S.t., Salmonella typhimurium
In each column, and for each concentration, different letters mean significant differences between samples (p < 0.05)
Table 3.
Antifungal activity of green and black pepper essential oils against food spoilage fungi (mg/ml)
| Oils | A.fum. | A.v. | A.o. | A.n. | T.v. | P.f. | P.o. | P.a. | |
|---|---|---|---|---|---|---|---|---|---|
| Green pepper | MIC | 0.07 ± 0.04a | 0.07 ± 0.05a | 0.01 ± 0.07a | 0.12 ± 0.01a | 0.20 ± 0.16a | 0.16 ± 0.09a | 0.16 ± 0.04a | 0.63 ± 0.21b |
| MFC | 0.16 ± 0.09a | 0.16 ± 0.09a | 0.13 ± 0.02a | 0.16 ± 0.09a | 0.31 ± 0.18a | 0.31 ± 0.13a | 0.31 ± 0.00a | 1.25 ± 0.00ab | |
| Black pepper | MIC | 1.25 ± 0.14a | 0.63 ± 0.21b | 2.50 ± 0.28b | 5.00 ± 0.00c | 2.50 ± 0.28c | 2.50 ± 0.28b | 2.50 ± 0.38c | 2.50 ± 0.32d |
| MFC | 2.50 ± 0.28b | 1.25 ± 0.14c | 5.00 ± 0.28b | 10.0 ± 1.44d | 5.00 ± 0.43c | 5.00 ± 0.00c | 5.00 ± 0.00c | 5.00 ± 1.08c | |
| Ketoconazole | MIC | 0.20 ± 0.01a | 0.20 ± 0.06a | 0.15 ± 0.00a | 0.20 ± 0.03b | 1.00 ± 0.00b | 0.20 ± 0.03a | 1.00 ± 0.30b | 1.50 ± 0.00c |
| MFC | 0.50 ± 0.06a | 0.50 ± 0.06b | 0.20 ± 0.30a | 0.50 ± 0.03a | 1.50 ± 0.30b | 0.50 ± 0.00b | 1.50 ± 0.10b | 2.00 ± 0.00b | |
| Bifonazole | MIC | 0.15 ± 0.03b | 0.10 ± 0.06a | 0.15 ± 0.03a | 0.15 ± 0.03a | 0.15 ± 0.03a | 0.20 ± 0.06a | 0.20 ± 0.06a | 0.20 ± 0.00a |
| MFC | 0.20 ± 0.06a | 0.20 ± 0.06a | 0.20 ± 0.00a | 0.2 ± 0.06a | 0.20 ± 0.00a | 0.25 ± 0.03a | 0.25 ± 0.03a | 0.30 ± 0.00a | |
A.fum., Aspergillus fumigatus; A.v., Aspergillus versicolor; A.o., Aspergillus ochraceus; A.n., Aspergillus niger; T.v., Trichoderma viride; P.f., Penicillium funiculosum; P.o., Penicillium ochrochloron; P.a., Penicillium aurantiogriseum
In each column, and for each concentration, different letters mean significant differences between samples (p < 0.05)
In the DPPH assay, the radical scavenging capacity of the tested EOs increased in a dose dependent manner. The values for 50 % scavenging activity (IC50) are presented in Table 4. The black pepper oil showed slightly better antiradical scavenging activity (36.84 mg/ml) than green pepper oil (38.77 mg/ml). Both oils exhibited weaker antiradical activity compared to control BHA (0.42 mg/ml). However, comparison with the results should be careful, because BHA is an individual compound and not a mixture. The antiradical activity of essential oils might be attributed to the presence of phenolic and related compounds (Sultana et al. 2007).
Table 4.
Free radical scavenging activity of green and black pepper essential oils (mg/ml)
| Oils | Green pepper | Black pepper | BHA |
|---|---|---|---|
| IC50 | 36.84 ± 2.04b | 38.77 ± 11.13b | 0.42 ± 0.01a |
In each column, and for each concentration, different letters mean significant differences between samples (p < 0.05)
The results from the antimicrobial activity tested in situ are summarized in Table 5. It is evident that green pepper essential oil had better results against S. aureus in chicken soup after 24, 48 and 72 h, respectively. Inhibition percentage was dose dependent and inhibition percentage decreased with lower tested doses of oils. The best effect was noticed for the highest concentration used (5 mg/ml) at 25 ° C. Furthermore, inhibition decreased during the days of storage. It could be suggested that S. aureus was able to slightly overcome the effect of active compounds from oils. This could be correlated to the concentration of the oils used.
Table 5.
Percentage of inhibition of S. aureus in chicken soup by essential oils of green and black pepper after 24, 48 and 72 h
| Concentration (mg/ml) | EO | Percentage of inhibition of S. aureus in chicken soup | ||
|---|---|---|---|---|
| 24 h | 48 h | 72 h | ||
| 5.00 | green pepper | 93.15 ± 0.39d | 91.84 ± 1.02e | 89.86 ± 0.95e |
| black pepper | 92.87 ± 2.18e | 93.01 ± 2.21h | 84.85 ± 1.97 | |
| 2.50 | green pepper | 90.66 ± 1.47d | 85.17 ± 2.00e | 83.68 ± 1.46d |
| black pepper | 87.24 ± 2.01c | 84.92 ± 1.73g | 77.44 ± 4.12e | |
| 1.25 | green pepper | 90.49 ± 2.91d | 86.96 ± 2.06e | 89.49 ± 1.40e |
| black pepper | 92.01 ± 2.83de | 81.38 ± 1.07f | 74.08 ± 2.21d | |
| 0.63 | green pepper | 84.33 ± 1.73c | 89.17 ± 1.08e | 83.37 ± 3.69d |
| black pepper | 87.98 ± 1.22cd | 61.79 ± 1.20e | 47.43 ± 5.02d | |
| 0.31 | green pepper | 75.29 ± 4.30b | 63.83 ± 1.40d | 61.62 ± 1.79c |
| black pepper | 61.07 ± 1.18c | 35.98 ± 0.89d | 9.67 ± 1.49c | |
| 0.16 | green pepper | 51.25 ± 2.13b | 36.97 ± 3.18c | 14.52 ± 1.65b |
| black pepper | 22.96 ± 2.83b | 33.64 ± 2.91d | 6.04 ± 0.81b | |
| 0.08 | green pepper | 21.40 ± 3.09a | 16.68 ± 2.34b | 11.69 ± 1.38b |
| black pepper | 15.24 ± 2.82a | 16.19 ± 2.54c | 7.41 ± 4.34ab | |
| 0.03 | green pepper | 18.15 ± 1.72a | 11.15 ± 1.44ab | 7.55 ± 1.70a |
| black pepper | 12.57 ± 2.68a | 12.67 ± 2.74b | 5.17 ± 1.02ab | |
| 0.01 | green pepper | 22.34 ± 6.07a | 5.36 ± 3.86a | 2.68 ± 2.89a |
| black pepper | 15.31 ± 3.34a | 6.35 ± 1.25a | 3.27 ± 1.88a | |
In each column, and for each concentration, different letters mean significant differences between samples (p < 0.05)
GIC50 values for the oils were calculated after 24, 48 and 72 h from the equations presented on Fig. 1. Inhibitory effect on S. aureus was better for green pepper oil after 24 h from the beginning of incubation and GIC50 value was 0.241 mg/ml, while for black pepper oil GIC50 was higher - 0.317 mg/ml. The trend of increasing GIC50 values was continued throughout the experiment and days of storage. GIC50 values showed that green pepper better preserved the chicken soup during days of storage at lower concentrations than black pepper oil. GIC50 had small increase in concentration value for green pepper oil, while the value had larger increase with time for black pepper oil. GIC50 was quiet constant with tendency of negligible small increase in concentration value for green pepper oil, while GIC50 value was almost higher from 0.3 mg/ml after each investigated period for black pepper oil.
The control samples in microliter plates (chicken soup with S. aureus) could grow up to 108 CFU/100 μL of medium, as we have checked by serial dilutions and cultivation of cells on laboratory agar broths (it is widely accepted that bacteria grow up to 109 CFU/mL). From this observation if we assume that bacterium concentration was 108 in control sample (100 % growth) and if we have 95 % inhibition in our experimental well (5 % growth), it means that there are still bacteria in experimental well with concentration of 5 × 108. This value could be read spectrophotometrically, since spectrophotometric measurement is capable of determining cell density of 106 CFU/mL or more. The method was developed to check inhibitory influence of the oil and further to calculate GIC50 values. Though it is not surprising that the results slightly different from microdilution method. Of course 100 % inhibition does not obligatory mean that bacterium was killed. The oil had microbicidal activity at high concentrations, which was checked by subcultivation of bacterium. Thus, the method was developed for validating inhibitory activities of natural preservatives in liquid foods and for the determination of GIC50 value.
Discussion
Previous studies of chemical composition of the P. nigrum essential oil showed that α-pinene, β-pinene, myrcene, α-phellandrene, limonene, linalool, methylpropanal, 2- and 3-methylbutanal, butyric acid, and 3-methylbutyric acid were the main odorants present in the oil (Jagella and Grosch 1999). In the study of Liu et al. (2007) thirty compounds were identified from fruits of P. nigrum. The most dominant were: β-caryophyllene (23.49 %), δ3-carene (22.20 %), D-limonene (18.68 %), β-pinene (8.92 %) and α-pinene (4.03 %) respectively. Our results on chemical profiling of black and green pepper oils are in agreement with studies mentioned above. However, several factors, namely climatic, geographic conditions and growth stage of collected plants may severely affect essential oil yield, their composition and their biological properties, so certain differences in different pepper oil chemical composition are quite common (Zouari et al. 2012).
Overall, both oils, especially green pepper oil showed promising antimicrobial activity. According to literature, few studies have examined the antimicrobial activity of pepper essential oils. In the study of Sasidharan and Nirmala (2010) pepper essential oil showed strong in vitro activity against B. subtilis, P. aeruginosa, A. niger, Penicillium spp, C. albicans, S. cerevisiae and Trichoderma spp.
Rabadia et al. (2011) tested black pepper essential oil and potential synergistic effect against pathogenic fungi C. albicans, C. tropicalis and Trichophyton mentagraphytes by disk diffusion method. All fungal strains were inhibited and Pepper oil exhibited significant inhibition against T. mentagraphytes.
Singh et al. (2005) found that black pepper oil showed complete reduction in number of colonies against B. cereus, and inhibited S. aureus, B. cereus, L. monocytogenes, E. coli, S. typhimurium and P. aeruginosa in concentration dependent manner.
A correlation of the antimicrobial activity of the EOs from our study and their chemical composition suggests that the activity of the oils could be attributed to the presence of the major constituents. Although the major oil constituents are probably not the only responsible for good antimicrobial activity achieved, the involvement of less abundant constituents should also be considered.
In our study none of the oils showed better results than positive control (BHA). In addition, antiradical activities observed in volatile oil could be the synergistic effect of more than one or two compounds that may be present in the system.
Kapoor et al. (2009) concluded that pepper oil showed strong antioxidant activity measured by different techniques and pointed that scavenging power of oil was better than those of BHA and BHT. According to Singh et al. (2004) pepper oil and its extract had almost the same antioxidant activity throughout the experiment as compared with BHA and BHT. However, the results from various studies are difficult for comparison, presumably due to different test methods applied, as well as the different sources of antioxidant and samples used. There are literature reports (Nikolić et al. 2014; Xi et al. 2014) regarding antioxidant activity of various herbs and spice extracts, but, to our knowledge, there is a scare literature about antiradical activity of pepper oils.
To avoid undesirable sensory changes, toxicity and other harmful and undesirable characteristics, application of low amounts of naturally occurring antimicrobial agents as ingredients should be also explored, in order to control spoilage. Research findings from this work provide a basis for developing effective naturally occurring antimicrobial agents to extend shelf life of soup and perhaps other types of traditional foods. The present study provides strong evidence that pepper essential oils could be used in the control of S. aureus in soup.
Conclusion
Medicinal plants are good alternatives to chemically synthesized antibiotics. Presented results from the above showed that green and black pepper oils significantly inhibited the growth of food spoilage microorganisms and they could serve as natural free radical scavengers. The importance of conducting the study on essential oils lies not only in the chemical characterization but also in the possibility of linking the chemical contents with particular functional properties such as preservation of food in vitro and in situ. Inhibition of S. aureus in chicken soup correlates with the functional properties potentially useful in the fight against foodborne pathogens and contaminants. If the active substances are to be added to foods in greater concentrations than is currently practiced for flavorings, further safety studies might be necessary.
Acknowledgments
The authors are grateful to the Ministry of Education, Science and Technological Development of Serbia for financial support (Grant № 173032).
Footnotes
Highlights
• Piper nigrum L. is one of the most used spices in the world.
• Black and green pepper essential oils biological evaluation: in vitro and in situ study.
• Novel tool for determination of GIC50 in liquid foods.
• The oils were efficient in controlling the growth of known food-spoilage microorganisms.
• Food-preservative activity exhibited may be valuable for food industry.
References
- Adams RP. Identification of essential oil compounds by gas chromatography and mass spectrometry. 4. USA Carol Stream, IL: Allured Publishing Corporation; 2009. [Google Scholar]
- AMDIS (ver. 2.1.) Automated Mass Spectral Deconvolution and Identification System software, National Institute of Standards and Technology (NIST), Standard Reference Data Program, Gaithersburg, MD
- Blois MS. Antioxidant determinations by the use of stable free radical. Nature. 1958;181:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- Bukvički D, Stojković D, Soković M, Vannini L, Montanari C, Pejin B, Savic A, Veljic M, Grujic S, Marin PD. Satureja horvatii essential oil: In vitro antimicrobial and antiradical properties and in situ control of Listeria monocytogenes in pork meat. Meat Sci. 2014;96:1355–1360. doi: 10.1016/j.meatsci.2013.11.024. [DOI] [PubMed] [Google Scholar]
- Burt SA, Reinders RD. Antibacterial activity of selected plant essential oils against Escherichia coli O157:H7. Lett Appl Microbiol. 2003;36:162–167. doi: 10.1046/j.1472-765X.2003.01285.x. [DOI] [PubMed] [Google Scholar]
- Chaibi A, Ababouch LH, Belasri K, Boucetta S, Busta FF. Inhibition of germination and vegetative growth of Bacillus cereus T and Clostridium botulinum 62A spores by essential oils. Food Microbiol. 1997;14:161–174. doi: 10.1006/fmic.1996.0075. [DOI] [Google Scholar]
- Chen W, Dou H, Ge C, Congfa L. Comparison of volatile compounds in pepper (Piper nigrum L.) by simultaneous distillation extraction (SDE) and GC-MS. Adv Mater Res. 2011;236:2643–2646. doi: 10.4028/www.scientific.net/AMR.236-238.2643. [DOI] [Google Scholar]
- De Azeredo GA, De Figueiredo RCBQ, De Souza EL, Stamford TLM. Changes in Listeria monocytogenes induced by Origanum vulgare L. and Rosmarinus officinalis L. essential oils alone and combined at subinhibitory amounts. J Food Saf. 2012;32:226–235. doi: 10.1111/j.1745-4565.2012.00372.x. [DOI] [Google Scholar]
- Dorman HJ, Deans SG. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J App Microbiol. 2002;88:308–316. doi: 10.1046/j.1365-2672.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- Emiroğlu ZK, Yemiş GP, Coşkun BK, Candoğan K. Antimicrobial activity of soy edible films incorporated with thyme and oregano essential oils on fresh ground beef patties. Meat Sci. 2010;86:283–288. doi: 10.1016/j.meatsci.2010.04.016. [DOI] [PubMed] [Google Scholar]
- Espinel-Ingroff A. A comparasion of the E-test with the NCCLS M38-P method for antifungal susceptibility testing of common and emerging pathogenic filamentous fungi. J Clin Microbiol. 2001;39:1360–1367. doi: 10.1128/JCM.39.4.1360-1367.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans WC. Trease and Evans’ pharmacognosy. 14. London: WB Saunders; 1996. pp. 63–364. [Google Scholar]
- Fisher K, Phillips C, McWatt L. The use of an antimicrobial citrus vapour to reduce Enterococcus sp. on salad products. Int J Food Sci Technol. 2009;44:1748–1754. doi: 10.1111/j.1365-2621.2009.01992.x. [DOI] [Google Scholar]
- ISO 11024–1 (1998) Essential oils - General guidance on chromatographic profiles - Part 1: Preparation of chromatographic profiles for presentation in standards
- ISO 11024–2 (1998) Essential oils - General guidance on chromatographic profiles - Part 2: Utilization of chromatographic profiles of samples of essential oils
- ISO 7609 (1985) Essential oils - Analysis by gas chromatography on capillary columns - General method
- Jagella T, Grosch W. Flavour and off-flavour compounds of black and white pepper (Piper nigrum L.) II. Odour activity values of desirable and undesirable odorants of black pepper. Eur Food Res Technol. 1999;209:22–26. doi: 10.1007/s002170050450. [DOI] [Google Scholar]
- Kapoor IPS, Singh B, Singh G, De Heluani CS, De Ampasona MP, Catalan CAN. Chemistry and in vitro antioxidant activity of volatile oil and oleoresins of black pepper (Piper nigrum) J Agric Food Chem. 2009;57:5358–5364. doi: 10.1021/jf900642x. [DOI] [PubMed] [Google Scholar]
- Liu L, Song G, Hu Y. GC–MS Analysis of the essential oils of Piper nigrum L. and Piper longum L. Chromatographia. 2007;66:785–790. doi: 10.1365/s10337-007-0408-2. [DOI] [Google Scholar]
- Meghwal M, Goswami TK. Piper nigrum and piperine: an update. Phytother Res. 2013;27:1121–1130. doi: 10.1002/ptr.4972. [DOI] [PubMed] [Google Scholar]
- Millezi AF, Caixeta DS, Rossoni DF. In vitro antimicrobial properties of plant essential oils Thymus vulgaris, Cymbopogon citratus and Laurus nobilis against five important foodborne pathogens. Cienc Tecnol Aliment. 2012;32:167–172. doi: 10.1590/S0101-20612012005000021. [DOI] [Google Scholar]
- Nikolić M, Glamočlija J, Ferreira ICFR, Calhelha RC, Fernandes A, Marković T, Marković D, Giweli A, Soković M. Chemical composition, antimicrobial, antioxidant and antitumor activity of Thymus serpyllum L., Thymus algeriensis Boiss. and Reut and Thymus vulgaris L. essential oils. Ind Crop Prod. 2014;52:183–190. doi: 10.1016/j.indcrop.2013.10.006. [DOI] [Google Scholar]
- Rabadia AG, Kamat SD, Kamat DV. Antifungal activity of essential oils against fluconazole resistant fungi. Int J Phytomed. 2011;3:506–510. [Google Scholar]
- Reische DW, Lillard DA, Eitenmiller RR. Antioxidants in food lipids. In: Ahoh CC, Min DB, editors. In chemistry, nutrition and biotechnology. New York: Marcel Dekker; 1998. pp. 423–448. [Google Scholar]
- Sashidhar NS (2002) Studies on bioactive natural compounds for their antimicrobial and antioxidant properties. Ph.D thesis, Osmania University, Hyedarabad, India
- Sasidharan I, Nirmala MA. Comparative chemical composition and antimicrobial activity of berry and leaf essential oils of Piper nigrum L. Int J Biol Med Res. 2010;1:215–218. [Google Scholar]
- Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis. 2011;17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharff RL. Economic burden from health losses due to foodborne illness in the United States. J Food Prot. 2012;75:123–131. doi: 10.4315/0362-028X.JFP-11-058. [DOI] [PubMed] [Google Scholar]
- Singh G, Marimuthu P, Catalan CMP, De Lampasona MP. Chemical, antioxidant and antifungal activities of volatile oil of black pepper and its acetone extract. J Sci Food Agric. 2004;84:1878–1884. doi: 10.1002/jsfa.1863. [DOI] [Google Scholar]
- Singh G, Marimuthu P, Murali HS, Bawa AS. Antioxidative and antibacterial potentials of essential oils and extracts isolated from various spice materials. J Food Saf. 2005;25:130–145. doi: 10.1111/j.1745-4565.2005.00564.x. [DOI] [Google Scholar]
- Škrinjar MM, Nemet NT. Antimicrobial effects of spices and herbs essential oils. Acta Period Technol. 2009;40:195–209. doi: 10.2298/APT0940195S. [DOI] [Google Scholar]
- Srinivasan K. Black pepper and its pungent principle-piperine: a review of diverse physiological effects. Crit Rev Food Sci Nutr. 2007;47:735–748. doi: 10.1080/10408390601062054. [DOI] [PubMed] [Google Scholar]
- Stojković D, Soković M, Glamočlija J, Džamić A, Ćirić A, Ristić M, Grubišić D. Chemical composition and antimicrobial activity of Vitex agnus-castus L. fruits and leaves essential oils. Food Chem. 2011;128:1017–1022. doi: 10.1016/j.foodchem.2011.04.007. [DOI] [Google Scholar]
- Stojković D, Petrović J, Soković M, Glamočlija J, Kukić-Marković J, Petrović S. In situ antioxidant and antimicrobial activities of naturally occurring caffeic acid, p-coumaric acid and rutin, using food systems. J Sci Food Agric. 2013;93:3205–3208. doi: 10.1002/jsfa.6156. [DOI] [PubMed] [Google Scholar]
- Stojković D, Reis F, Ferreira ICFR, Barros L, Glamočlija J, Ćirić A, Nikolić M, Stević T, Giweli A, Soković M. Tirmania pinoyi: chemical composition, in vitro antioxidant and antibacterial activities and in situ control of staphylococcus aureus in chicken soup. Food Res Int. 2013;53:56–62. doi: 10.1016/j.foodres.2013.03.046. [DOI] [Google Scholar]
- Sultana B, Anwar F, Przybylski R. Antioxidant activity of phenolic components present in barks of Azadirachta indica, Terminalia arjuna, Acacia nilotica and Eugenia jambolana Lam. trees. Food Chem. 2007;104:1106–1114. doi: 10.1016/j.foodchem.2007.01.019. [DOI] [Google Scholar]
- Tsukatani T, Suenaga H, Shig M, Noguchi K, Ishiyama M, Ezoe T, Matsumoto K. Comparison of the WST-8 colorimetric method and the CLSI broth microdilution method for susceptibility testing against drug-resistant bacteria. J Microbiol Meth. 2012;90:160–166. doi: 10.1016/j.mimet.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Xi W, Zhang Y, Sun Y, Shen Y, Ye X, Zhou Z. Phenolic compositions in the pulps of Chinese wild mandarin pulps (Citrus reticulata Blanco.) and their antioxidant properties. Ind Crop Prod. 2014;52:466–474. doi: 10.1016/j.indcrop.2013.11.016. [DOI] [Google Scholar]
- Zouari N, Ayadi I, Fakhfakh N, Rebai A, Zouari S. Variation of chemical composition of essential oils in wild populations of Thymus algeriensis Boiss. et Reut., a North African endemic species. Lipids Health Dis. 2012;11:28. doi: 10.1186/1476-511X-11-28. [DOI] [PMC free article] [PubMed] [Google Scholar]