Abstract
The study was carried out to produce and characterize a low-calorie orange nectar using response surface methodology (RSM). To optimize the formulation, three different levels of independent variables; sugar, stevioside and pectin and two responses of Brix and viscosity were selected. In the optimum formulation, sugar content reduced to 70 % (compared to control sample) using stevioside and pectin with maximum levels of 0.06 and 0.03 %, respectively. Physicochemical properties of optimal and control samples were determined at refrigerated (4 °C) and ambient (25 °C) temperatures for 2 months. At refrigerator, the reduction rate for stevioside was 5 % while a decrease of 18 % was observed at ambient temperature. The vitamin C content in low-calorie orange nactar was 13.4 % higher than control sample at both temperatures. Thus the production of low-calorie orange nectar using stevioside could be industrially feasible and recommended to people who looking for dietetic foods.
Keywords: Nutrition, Pectin, Optimization, Storage, Vitamin C
Introduction
Orange juice is popular beverage in all part of the world, as it is rich in ascorbic acid, carotenoids, flavonoids, phenylpropanoids, potassium and folic acid. Its consumption reduces the risk of free-radical attack to body tissues and also decreases outbreak of cancer and cardiovascular diseases (Ashurst 1995; Vieira et al. 2010).
Changing human life style to reduced levels of physical activities and increased consumption of high-calorie food products, threats human healthiness. Therefore, much attention is attracted on production and consumption of low-calorie products (Garcia-Noguera et al. 2010). The easiest method to reduce food calorie is sugar (sucrose) replacement with low-calorie sweeteners (Cardoso and André Bolini 2008). Noncaloric sweeteners such as saccharin, cyclamate, aspartame, acesulfame K and sucralose are widely used for production of low-calorie foods (Ferrer and Thurman 2010). Due to their harmful effects to the health, much attention is attracted to the application of plant-derived sweeteners such as stevioside (Kim and Kinghorn 2002). Stevia (Stevia rebaudiana, Bertoni) discovered by Moises Bertoni in 1899, is native of Paraguay (Ibrahim et al. 2008; Madan et al. 2010). Stevia is a small shrub belongs to Asteraceae family (Kim and Kinghorn 2002). The eight diterpene glycosides are responsible for sweetness taste of stevia leaves that contains stevioside, dulcoside A, steviolbioside, rebaudioside A, B, C, D and E (Bovanová et al. 1998). Stevioside is the most important of these diterpene glycosides with three glucose molecules in its structure. It is a white and crystalline powder and approximately 300 times sweeter than sucrose. The body cannot metabolize stevioside, so its caloric intake is zero (Geuns 2003).
The ideal sweetness in beverages prepared with instant coffee was determined using different sweeteners; sucrose, sucralose, stevia, aspartame, acesulfame K and cyclamate/saccharin (2:1) blend. Moreas and André Bolini (2010)). This study was also conducted on peach nectar and reported that 0.1 % of stevia can be equi-sweet to 10 % sucrose (Cardoso and André Bolini 2007). Parpinello et al. (2001) observed a calorie reduction of 25 % in peach juice by replacement of 34 g/L sucrose with 160 mg/L stevioside without affecting the sensory characteristics of the product.
The objectives of this study were to optimize the formulation of low-calorie orange nectar using stevioside by response surface methodology (RSM) and to evaluate its physicochemical properties during storage period at refrigerated (4 °C) and ambient (25 °C) temperatures.
Material and methods
Raw materials
Orange concentrate was obtained from Urmia Shahdab Co. (Urmia city). Stevioside powder (purity, 85–95 %) was purchased from Isfahan Chocolate Co. (Isfahan city). High methoxyl pectin was obtained from Majid Co. (Ahvaz city). Folin–Ciocalteu reagent, methanol, gallic acid were purchased from Merck Co. (Germany).
Formulation optimization
To prepare orange juice, orange concentrate (70 Brix) was first diluted with water to 13 Brix. The orange nectar was formulated with 60 % orange juice and 40 % sugar syrup (water containing 13 % sucrose). To produce low-calorie nectar, three parameters of sugar, stevioside and pectin content of nectar at three levels were evaluated using RSM (the parameters levels were selected according to similar previous studies). Eighteen formulations were prepared according to D-optimal design and their viscosity and Brix were measured as responses (Table 1). Viscosity was measured by ubbelohde viscometer (Fischer, USA) and Brix was determined by measurement of the refractive index with digital refractometer (DR201-95, Kruss, Germany) at 20 °C (Sánchez-Moreno et al. 2003).
Table 1.
Levels of variables for production of low-calorie orange nectar
| Variables | Levels | ||
|---|---|---|---|
| Code | −1 | 0 | +1 |
| Sugar (percentage of total sugar in blank sample) | 30 | 55 | 80 |
| Stevioside (%) | 0.02 | 0.04 | 0.06 |
| Pectin (%) | 0.02 | 0.03 | 0.04 |
Physicochemical properties of orange nectar
The optimized formula along with control orange nectar (containing sugar and without stevioside and pectin) were prepared, pasteurized at 80 °C for 2 min (Cinquanta et al. 2010), bottled in PET bottles and stored at refrigerated (4 °C) and ambient (25 °C) temperatures for 60 days. The changes in their physicochemical properties were evaluated during storage.
Sugar content
A high-performance liquid chromatography (HPLC) system (Shimadzu, Japan) equipped with a refractive index detector was used for sugar analysis. The HPLC column was SCR-101N (30 cm × 9.7 mm i.d) fitted with a guard column SCR (N) (5 cm × 4 mm i.d). The mobile phase was deionized water at a flow rate of 0.7 ml/min and 60 °C (Kelebek et al. 2009).
Stevioside content
For stevioside measurement, a HPLC system with C18-ODS column (25 cm × 4.6 mm i.d) and UV detector (SPD-6AV) was used. Methanol 68 % (at flow rate of 1 ml/min) was used as mobile phase at 25 °C. Stevioside was detected at 210 nm (Bovanová et al. 1998).
Total phenolic content
Total phenolic content was determined by spectrophotometer using Folin-Ciocalteu reagent. The sample (0.5 ml) was mixed with the reagent, 1:10 diluted with distilled water, (5 ml) and aqueous Na2CO3 (4 ml, 1 M). The mixture was allowed to stand for 15 min and the total phenolics were determined at 765 nm and calculated as mg gallic acid in liter (Pourmorad et al. 2006).
Turbidity
The samples were first centrifuged at 1500 rpm for 10 min and then the turbidity was measured by absorbance reading at 660 nm (Rivas et al. 2006).
Viscosity
An Ubbelohde viscometer (Fisher, USA) was used for viscosity measurement. Viscosity was determined at 25 °C and was expressed in centipoise (cp) (Karangwa et al. 2010).
Vitamin C
Vitamin C (ascorbic acid) content was measured based on the ability of ascorbic acid to reduce 2–6 dichloroindophenol indicator according to the AOAC method 967.21 (AOAC 1995).
Titratable acidity
Ten grams sample was diluted with 100 ml distilled water. The acidity was determined by titration with 0.1N NaOH to pH 8.1 (Sánchez-Moreno et al. 2003).
Statistical analysis
A statistical method of D-optimal was applied to optimize orange nectar formulation using response surface methodology (RSM). The data were analyzed by Design Expert version 7.1.6 (Stat-Ease Inc., Minneapolis, MN) software. During storage, the results were analyzed using split-plot in time according to completely randomized design. The experiments were carried out in triplicate and the data analyzed using SAS software (version 9.1.3). The least significant difference (LSD) was performed to evaluate means at significance level of 99 %.
Results and discussion
Model evaluation
The effect of three independent variables; sugar (X1), stevioside (X2) and pectin (X3) content on dependent variables of viscosity and Brix were evaluated employing RSM. The linear model was selected as the best-fitting model having insignificant lack of fit and reasonable agreement between adjusted and predicted R-squared. The high value of R2 and low standard deviation (SD) showed a close agreement between the experimental results and the theoretical data predicted by the model (Table 2). This evaluation confirmed the accuracy and fitness of the model.
Table 2.
The treatments conducted in RSM and their actual and observed responses of viscosity and Brix
| Treatments | Sugar(% from total sugar) | Stevioside (%) | Pectin (%) | Real viscosity (cp) | Predicted viscosity (cp) | Real Brix (%) | Predicted Brix (%) |
|---|---|---|---|---|---|---|---|
| 1 | 80 | 0.02 | 0.02 | 1.79 | 1.80 | 12 | 11.9 |
| 2 | 30 | 0.06 | 0.04 | 1.82 | 1.84 | 9.4 | 9.3 |
| 3 | 30 | 0.04 | 0.03 | 1.70 | 1.73 | 9.3 | 9.3 |
| 4 | 80 | 0.02 | 0.04 | 2.00 | 2.02 | 11.8 | 11.9 |
| 5 | 80 | 0.04 | 0.03 | 1.95 | 1.91 | 12.1 | 11.9 |
| 6 | 80 | 0.06 | 0.04 | 2.02 | 2.02 | 12 | 11.9 |
| 7 | 30 | 0.06 | 0.02 | 1.60 | 1.63 | 9.4 | 9.3 |
| 8 | 30 | 0.06 | 0.02 | 1.61 | 1.63 | 9.4 | 9.3 |
| 9 | 55 | 0.03 | 0.03 | 1.74 | 1.82 | 10.6 | 10.6 |
| 10 | 30 | 0.02 | 0.04 | 1.83 | 1.84 | 9.3 | 9.3 |
| 11 | 30 | 0.02 | 0.04 | 1.82 | 1.84 | 9.4 | 9.3 |
| 12 | 55 | 0.04 | 0.04 | 1.92 | 1.93 | 10.7 | 10.6 |
| 13 | 55 | 0.04 | 0.04 | 1.90 | 1.93 | 10.7 | 10.6 |
| 14 | 80 | 0.02 | 0.04 | 2.01 | 2.02 | 11.8 | 11.9 |
| 15 | 80 | 0.06 | 0.02 | 1.75 | 1.81 | 11.9 | 11.9 |
| 16 | 30 | 0.02 | 0.02 | 1.63 | 1.63 | 9.5 | 9.3 |
| 17 | 55 | 0.06 | 0.03 | 1.76 | 1.82 | 10.8 | 10.6 |
| 18 | 55 | 0.02 | 0.02 | 1.73 | 1.72 | 10.5 | 10.6 |
The significance of each coefficient was determined by P value. The P value less than 0.05 indicated that among the test factors in the study, model terms of X1 (sugar) and X3 (pectin) in viscosity and X1 (sugar) in Brix were significant variables (Table 3). The coefficients of the test factors for viscosity and Brix responses were given as equations below:
| 1 |
| 2 |
Table 3.
Results of analysis of variance of predicted models for viscosity and Brix in orange nectar
| Coefficients | (SS) | (df) | (MS) | Fvalue | prob>F | |
|---|---|---|---|---|---|---|
| Viscosity | ||||||
| model | 0.29 | 3 | 0.097 | 113.02 | <0.0001 | |
| X0 | +1.304158 | – | – | – | – | – |
| X1 = sugar | +0.00367 | 0.11 | 1 | 0.11 | 125.91 | <0.0001 |
| X 2 = stevioside | −0.43059 | 9.5E-04 | 1 | 9.5E-04 | 1.11 | 0.3096 |
| X3 = pectin | +10.54998 | 0.15 | 1 | 0.15 | 175.14 | <0.0001 |
| Brix | ||||||
| model | 21.03 | 3 | 7.01 | 763.55 | <0.0001 | |
| X0 | +7.79478 | – | – | – | – | – |
| X1 = sugar | +0.051124 | 20.89 | 1 | 20.89 | 2275.21 | <0.0001 |
| X 2 = stevioside | +2.102319 | 0.023 | 1 | 0.023 | 2.47 | 0.1386 |
| X3 = pectin | −0.87673 | 1.034E-03 | 1 | 1.034E-03 | 0.11 | 0.7422 |
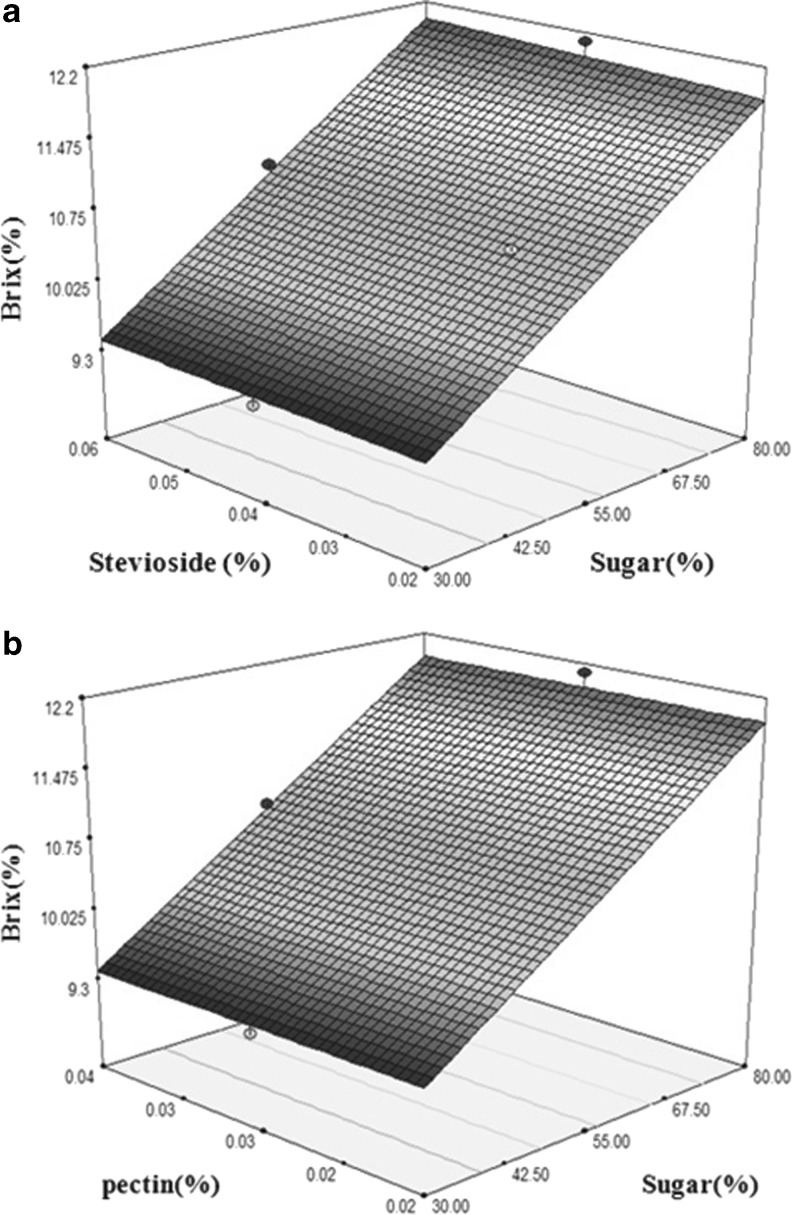
Figure 1 denotes the response surfaces of nectar viscosity as a function of pectin/stevioside, pectin/sugar and stevioside/sugar content. The changes in stevioside content from 0.02 to 0.06 % did not have significant effect on viscosity while the highest viscosity obtained in high content of sugar (80 %) and pectin (0.04 %). The response surfaces regarding the effect of sugar/pectin and sugar/ stevioside content on Brix are shown in Fig. 2. There was no significant interaction between stevioside and pectin content. Based on the response surface plots, as the amount of sugar increased, nectar Brix also increased and the highest Brix was found at 80 % sugar.
Fig. 1.
3D surface showing interaction effects of stevioside and sugar (a), pectin and sugar (b) and pectin and stevioside (c) on viscosity of low-calorie orange nectar
Fig. 2.
3D surface showing interaction effects of stevioside and sugar (a) and pectin and sugar (b) on Brix of low-calorie orange nectar
Formulation optimization
In this study, a formulation of low-calorie orange nectar with the highest viscosity and Brix considered as an optimum product. The optimum concentration of independent variables was predicted by numerical and graphical optimization procedures. For the graphical interpretation and visualizing the relationship between variables and responses, three-dimensional (3D) response surface plotting was used. From the multiple response optimizations, the overall optimum region was achieved by a combined content of 30 % sugar (of total sugar in blank sample), 0.06 % stevioside and 0.03 % pectin.
Physicochemical properties of low-calorie orange nectar during storage
Change in titratable acidity
The acidity is one of the physico-chemical properties, which affects both organoleptic and quality of a product (Hussain et al. 2011). In this study it was observed that titratable acidity increased 23.1 and 33.9 % for blank sample (BS) and 22.1 and 32.6 % for low-sugar nectar (LSN) during 2-month storage at both refrigerated and room temperatures, respectively (Table 4). This increment during storage might be due to the pectin degradation to pectinic acid or sugar fermentation (Ayub et al. 2010; Supraditareporn and Pinthong 2007). Hussain et al. (2011) also reported increase in the acidity of apple and apricot blend juice during 90 days storage.
Table 4.
The content of titratable acidity (g citric acid /100 ml) in blank and optimal samples during storage
| Treatment | Temperature | Time(days) | |||
|---|---|---|---|---|---|
| 0 | 20 | 40 | 60 | ||
| blank | refrigerator | 0.695 ± 0.000 a(B) | 0.720 ± 0.000 b(B) | 0.740 ± 0.005 c(B) | 0.856 ± 0.005 d(C) |
| blank | ambient | 0.695 ± 0.000 a(B) | 0.730 ± 0.000 b(A) | 0.752 ± 0.003 c(A) | 0.931 ± 0.001 d(A) |
| optimal | refrigerator | 0.708 ± 0.000 a(A) | 0.730 ± 0.000b(A) | 0.737 ± 0.003 c(B) | 0.865 ± 0.004 d(B) |
| optimal | ambient | 0. 707 ± 0.003 a(A) | 0.733 ± 0.004 b(A) | 0.744 ± 0.006 c(B) | 0.938 ± 0.002 d(A) |
Values with different letters (capital letters in each column and minuscule letters in each row) represent significant difference at the confidence level of 99 %
Changes in sugar content
The changes in content of sucrose, glucose and fructose are shown in Table 5. Immidiately after preparation, the predominant sugar was sucrose (5.8) followed by glucose (2.4) and fructose (2.4 %) in low LSN while in BS, the content of sucrose, glucose and fructose was 9.3, 2.7 and 2.7 %, respectively. Storage time and temperature had significant effect on the amounts of sugars. At refrigerated temperature, sucrose content was decreased from 9.3 to 9.1 and 5.8 to 5.4 g/100 g in BS and LSN, respectively. This reduction was great at 25 °C in which the content was reduced to 7.7 and 3.9 % in BS and LSN, respectively. On the contrary, the content of fructose and glucose was increased during storage as this increment was higher at ambient temperature. The sucrose in juices might be hydrolyzed and converted to its monosaccharides (glucose and fructose) during storage. This conversion can be accelerated at high temperature (Yousaf et al. 2009). Wang et al. (2006) reported that after 150-day storage of carrot juice concentrate at 25 and 37 °C, amount of sucrose was decreased while the glucose and fructose content was increased. The decrease in sucrose was attributed to the sucrose inversion into glocuse and fructose at higher temperature. Similar data was given by Yousaf et al. (2009) for clarified banana juice fortified with inulin and oligofructose stored for 8 weeks at 4, 25 and 35 °C. The sucrose content of samples stored at 4 °C decreased due to the hydrolysis of sucrose, whereas samples stored at 25 and 35 °C showed a slight increase in sucrose contents because of hydrolysis of inulin and oligofructose. Sandi et al. (2004) studied on yellow passion fruit juice that stored at room temperature (25 ± 5 °C) and refrigeration (5 ± 1 °C) for 120-day. They found the concentration of reducing sugars (fructose and glucose) increased over time while that of sucrose decreased. The increase in the reducing sugars concentration was reported higher in the juices kept at room temperature. The findings showed that sucrose reduction in BS was lower than that of LSN (at both temperatures) which might be due to the partly high acidity of low-calorie sample (Table 4).
Table 5.
The content of stevioside, glucose, fructose and sucrose (g/100 g) in blank and optimal samples during storage
| Sugar | Sample | Temperature | Time(days) | |||
|---|---|---|---|---|---|---|
| 0 | 20 | 40 | 60 | |||
| Sucrose | blank | refrigerator | 9.333 ± 0.004a(A) | 9.292 ± 0.003b(A) | 9.227 ± 0.005cd(A) | 9.195 ± 0.007d(A) |
| blank | ambient | 9.333 ± 0.004a(A) | 8.853 ± 0.004b(B) | 8.298 ± 0.005c(B) | 7.754 ± 0.001d(B) | |
| optimal | refrigerator | 5.832 ± 0.002a(B) | 5.688 ± 0.009b(C) | 5.586 ± 0.008c(C) | 5.457 ± 0.001d(C) | |
| optimal | ambient | 5.834 ± 0.007a(B) | 5.208 ± 0.004b(D) | 4.591 ± 0.015c(D) | 3.980 ± 0.043d(D) | |
| Glucose | blank | refrigerator | 2.702 ± 0.002c(A) | 2.718 ± 0.004c(B) | 2.766 ± 0.004b(A) | 2.804 ± 0.005a(D) |
| blank | ambient | 2.702 ± 0.002d(A) | 2.855 ± 0.006c(A) | 3.024 ± 0.020b(C) | 3.205 ± 0.011a(B) | |
| optimal | refrigerator | 2.460 ± 0.000d(B) | 2.649 ± 0.011c(C) | 2.771 ± 0.012b(C) | 2.952 ± 0.002a(C) | |
| optimal | ambient | 2.460 ± 0.000 d(B) | 2.856 ± 0.007 c(A) | 3.297 ± 0.007 b(A) | 3.717 ± 0.009 a(A) | |
| Fructose | blank | refrigerator | 2.703 ± 0.004c(A) | 2.732 ± 0.003bc(C) | 2.782 ± 0.004ab(C) | 2.822 ± 0.003a(D) |
| blank | ambient | 2.703 ± 0.004d(A) | 3.388 ± 0.004c(A) | 4.265 ± 0.078 b(A) | 4.740 ± 0.000 a(B) | |
| optimal | refrigerator | 2.440 ± 0.000d(B) | 2.589 ± 0.005c(D) | 2.756 ± 0.009b(C) | 2.948 ± 0.002a(C) | |
| optimal | ambient | 2.441 ± 0.000d(B) | 3.308 ± 0.000c(B) | 4.103 ± 0.017b(B) | 4.874 ± 0.000 a(A) | |
| Stevioside | optimal | refrigerator | 0.061 ± 0.000a(A) | 0.061 ± 0.000a(A) | 0.059 ± 0.000b(A) | 0.058 ± 0.000b(A) |
| optimal | ambient | 0.061 ± 0.000a(A) | 0.056 ± 0.000b(B) | 0.053 ± 0.000c(B) | 0.050 ± 0.000d(B) | |
Values with different letters (capital letters in each column and minuscule letters in each row) represent significant difference at the confidence level of 99 %
Changes in stevioside content
The stevioside content was 0.06 % in LSN immediately after production. Similar to sugars, storage time and temperature were effective on stevioside content and its concentration reduced over 60-day storage. This reduction was 5 % at refrigerator while a decrease of 18 % was observed at ambient temperature (Table 5). Acidic pH of orange nectar (≈3.3) might be the reason why stevioside was degraded at ambient temperature over storage. Wolwer-Rieck et al. (2010) studied the stability of stevioside and rebaudioside A in non-alcoholic drinks. They discovered after 72 h storage of soft drinks at 80 °C, the highest degradation of stevioside (71 %) was observed in the caffeinated lemonade (pH = 2.4) whereas in energy drink (pH = 3.5) stevioside was decomposed to only 27 %.
Changes in total phenolic content (TPC)
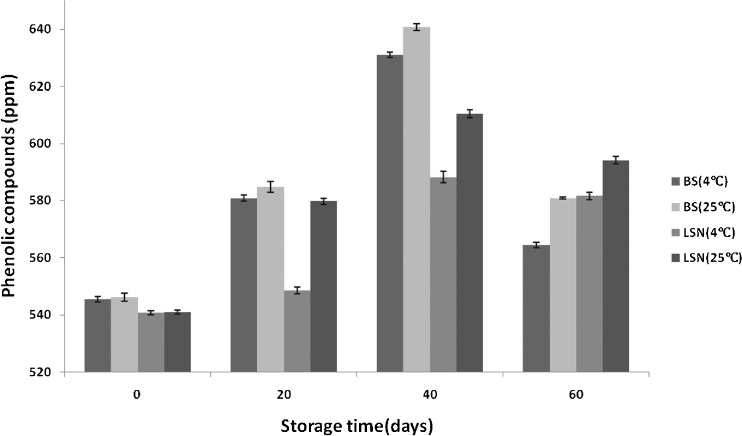
Fruits and vegetables are known as rich sources of polyphenols which have positive health effects on human body (Piljac-Žegarac et al. 2009). Fresh BS and LSN contained about 546 and 540 ppm TPC, respectively (Fig. 3). The storage time showed a significant effect on phenolic content of products as TPC increased in BS (from 545 to 631 ppm) and LSN (from 540 to 588 ppm) until 40th day and after that reduced to 564 and 581 ppm, respectively at 4 °C (Fig. 3). It is possible that during storage, some compounds were formed that reacted with the Folin–ciocalteu reagent and significantly enhanced the phenolic compounds (Piljac-Žegarac et al. 2009). The decrease of polyphenols at the end of storage might be due to the oxidative degradation of phenolic compounds and their polymerization reaction with proteins (Cao et al. 2012). Due to the enhancing effect of temperature on chemical reactions, the similar but great trend in TPC was observed at ambient storage temperature. Piljac-Žegarac et al. (2009) determined TPC in six industrial dark fruit juices during 29-day refrigerated storage. All juices exhibited fluctuations in TP values with a marked increase after 48 h followed by a decrease in TPC for next 13 days. Prolonged storage up to 29 days resulted in another increase in TPC for all juices. The TPC changes in LSN was lower than that of BS which might be due to the presence of pectin in LSN that can have supporting effects on phenolic compounds.
Fig. 3.
The content of phenolic compounds (ppm) in low-sugar nectar (LSN) and blank sample (BS) during storage
Changes in turbidity
One of the most important physical characteristics of orange juice is its tubidity and the main purpose of any preservation treatment in citrus juice is reduction of PME activity, which is responsible for loss of turbidity (Rivas et al. 2006). Turbid fruit juices are complex systems containing fine pulp particles dispersed in serum composed of macromolecules (pectins, proteins, etc.) colloidally dissolved in a true solution of low-molecular weight components (sugars, organic acids, etc.) (Hsieh and ko 2008). At first day, turbidity of BS (0.38) was lower than value of LSN (0.59) which was due to the presence of pectin in formulation of low-calorie juice (Fig. 4). Storage time was significantly effective on turbidity as it decreased about 24 and 14 % for BS and LSN, respectively at 4 °C. The higher reduction (about 34 and 24 %) was observed for BS and LSN, respectively at ambient temperature. Pectin methylesterases (PMEs) are natural cell wall enzymes in citrus juices. PMEs have high heat resistance and become inactive at higher temperature than microorganisms inactivation. After the pasteurization, about 10 % of these enzymes are active and the remaining PME activity could cause the removal of the methoxy groups of pectins producing free carboxylic radicals. Divalent cations could cross-link different pectin chains through free carboxylic groups giving macropolymers and leads to decreases of turbidity during the storage (Gómez et al. 2011). This observation was in accordance with Gómez et al. (2011) who stored orange concentrates for 6 months at 3 °C and found that its turbidity decreased during storage.
Fig. 4.
The turbidity measurement in low-sugar nectar (LSN) and blank sample (BS) during storage
Changes in viscosity
Viscosity is another important quality attribute of juices that influences the consumer’s choice. Activity of some enzymes such as PME and polygalacturonase (PG) are effective in viscosity changes of juices (Aguiló-Aguayo et al. 2008). The changes in viscosity of orange nectars are shown in Table 6. The viscosity of fresh LSN and BS were 1.77 and 1.76, respectively showing that the viscosity of LSN was slightly higher than that of BS. The addition of 0.03 % pectin into LSN might be the reason for viscosity increment in low-calorie sample. Over storage period, this parameter was decreased (about 10 %) as at higher temperature this reduction was greater. The existence of PMEs and dissociation of pectin during the storage is the reason for this viscosity reduction in orange nectar. Aguiló-Aguayo et al. (2008, 2010) also reported the viscosity of tomato and watermelon juices reduced after 77 and 56-day storage, respectively. It was attributed to the degradation of pectic substances by endogenous enzymes such as pectin methylesterase and polygalacturonase. Cao et al. (2012) discovered the viscosity of cloudy strawberry juice was decreased to 66.93 and 70.75 % after 6 months storage at 4 and 25 °C,respectively.
Table 6.
The viscosity (cp) of blank and optimal samples during storage
| Treatment | Temperature | Time(days) | |||
|---|---|---|---|---|---|
| 0 | 20 | 40 | 60 | ||
| blank | refrigerator | 1.764 ± 0.006 a(B) | 1.729 ± 0.005 b(B) | 1.605 ± 0.002 c(C) | 1.590 ± 0.007 d(A) |
| blank | ambient | 1.765 ± 0.005 a(B) | 1.720 ± 0.006 b(C) | 1.594 ± 0.004 c(D) | 1.574 ± 0.008 d(B) |
| optimal | refrigerator | 1.774 ± 0.001 a(A) | 1.749 ± 0.001 b(A) | 1.690 ± 0.000 c(A) | 1.589 ± 0.005 d(A) |
| optimal | ambient | 1.772 ± 0.007 a(A) | 1.692 ± 0.006 b(D) | 1.622 ± 0.005 c(B) | 1.502 ± 0.028 d(C) |
Values with different letters (capital letters in each column and minuscule letters in each row) represent significant difference at the confidence level of 99 %
Change in vitamin C
Ascorbic acid (vitamin C) is a natural antioxidant mainly present in fruits and vegetables (de Quirós et al. 2009). It is evident that the quality and nutritional value of any fruit juice as a source of vitamin C depends on its content and its rate of loss upon storage (Berlinet et al. 2006). Juices with 50 % retention of their initial vitamin C could be considered as the end of their shelf life (Chia et al. 2012). The storage time showed a significant effect on amount of ascorbic acid (Fig. 5) as storage prolonged, the vitamin C content of both orange nectars decreased. At refrigerated temperature, a reduction of 25 and 30 % was observed for LSN and BS, respectively. A greater vitamin loss (51 % for BS and 48 % for LSN) was recorded at ambient temperature. The degradation of Vitamin C during long term storage is due to dissolved oxygen. In addition, ascorbic acid loss might be because of the oxidative mechanism resulting from exposure to light, heat peroxides and enzymes such as ascorbate oxidase and peroxidase (Chia et al. 2012). Moreover, Storage temperature, type of processing and packaging materials could affect on ascorbic acid degradation during storage (Chia et al. 2012). The reduction rate of vitamin C in LSN was lower than that of BS which might be due to the protective effect of stevioside on ascorbic acid. Kroyer (1999) observed a protective effect of stevioside on the degradation of ascorbic acid resulted in a delayed degradation rate of vitamin C in the presence of stevioside.
Fig. 5.
The content of vitamin C (mg/100 ml) in low-sugar nectar (LSN) and blank sample (BS) during storage
Conclusions
Regarding viscosity and Brix as responses in response surface methodology (RSM), optimal levels of sugar, stevioside and pectin was achieved to produce low-calorie orange nectar. Without significant changes in viscosity and Brix, the optimum formulation contained 0.06 % stevioside and 0.03 % pectin and its sugar content reduced to 70 % compared to blank sample. Two-month storage at 4 and 25 °C showed a significant effect on phenolic, ascorbic acid, sugar and stevioside content in low-calorie and blank samples. At the end of storage, turbidity, viscosity and the amount of sucrose, stevioside, and vitamin C decreased while glocuse, fructose and titratable acidity increased. At refrigerator, the reduction rate for stevioside was 5 % while a decrease of 18 % was observed at ambient temperature. Acidic pH of orange nectar might be the reason why stevioside was degraded at ambient temperature over storage. The vitamin C content in low-calorie orange nactar was higher than that of blank sample which might be due to the protective effect of stevioside on ascorbic acid. However, findings revealed that the production of low-calorie orange nectar using stevioside could be industrially feasible and recommended to people who looking for dietetic foods.
References
- Aguiló-Aguayo I, Soliva-Fortuny R, Martín-Belloso O. Comparative study on color, viscosity and related enzymes of tomato juice treated by high-intensity pulsed electric fields or heat. Eur Food Res Technol. 2008;227:599–606. doi: 10.1007/s00217-007-0761-2. [DOI] [Google Scholar]
- Aguiló-Aguayo I, Soliva-Fortuny R, Martín-Belloso O. Color and viscosity of watermelon juice treated by high-intensity pulsed electric fields or heat. Innov Food Sci Emerg. 2010;11:299–305. doi: 10.1016/j.ifset.2009.12.004. [DOI] [Google Scholar]
- AOAC Official method 967.21, ascorbic acid in vitamin preparations and juices. Off Methods Anal Assoc Off Anal Chem. 1995;2:16–17. [Google Scholar]
- Ashurst PR. Production and packaging of non-carbonated fruit juice and fruit beverages. London: Blackie Academic and Professional; 1995. [Google Scholar]
- Ayub M, Ullah J, Muhammad A, Zeb A. Evaluation of strawberry juice preserved with chemical preservatives at refrigeration temperature. Int J Nutr Metab. 2010;2:027–032. [Google Scholar]
- Berlinet CC, Brat P, Brillouet JM, Ducruet V. Ascorbic acid, aroma compounds and browning of orange juices related to PET packaging materials and pH. J Sci Food Agric. 2006;86:2206–2212. doi: 10.1002/jsfa.2597. [DOI] [Google Scholar]
- Bovanová L, Brandšteterová E, Baxa S. HPLC determination of stevioside in plant material and food samples. Z Lebensm Unters Forsch A. 1998;207:352–355. doi: 10.1007/s002170050344. [DOI] [Google Scholar]
- Cao X, Bi X, Huang W, Wu J, Hu X, Liao X. Changes of quality of high hydrostatic pressure processed cloudy and clear strawberry juices during storage. Innov Food Sci Emerg Technol. 2012;16:181–190. doi: 10.1016/j.ifset.2012.05.008. [DOI] [Google Scholar]
- Cardoso JMP, André Bolini HM. Different sweeteners in peach nectar: ideal and equivalent sweetness. Food Res Int. 2007;40:1249–1253. doi: 10.1016/j.foodres.2007.08.004. [DOI] [Google Scholar]
- Cardoso JMP, André Bolini HM. Descriptive profile of peach nectar sweetened with sucrose and different sweeteners. J Sens Stud. 2008;23:804–816. doi: 10.1111/j.1745-459X.2008.00187.x. [DOI] [Google Scholar]
- Chia SL, Rosnah S, Noranizan MA, Wan Ramli WD. The effect of storage on the quality attributes of ultraviolet-irradiated and thermally pasteurised pineapple juices. Int Food Res J. 2012;19:1001–1010. [Google Scholar]
- Cinquanta L, Albanese D, Cuccurullo G, Di Matteo M. Effect on orange juice of batch pasteurization in an improved pilot-scale microwave oven. J Food Sci. 2010;75:46–50. doi: 10.1111/j.1750-3841.2009.01412.x. [DOI] [PubMed] [Google Scholar]
- De Quirós AR-B, Fernández-Arias M, López-Hernández J. A screening method for the determination of ascorbic acid in fruit juices and soft drinks. Food Chem. 2009;116:509–512. doi: 10.1016/j.foodchem.2009.03.013. [DOI] [Google Scholar]
- Ferrer I, Thurman EM. Analysis of sucralose and other sweeteners in water and beverage samples by liquid chromatography/time-of-flight mass spectrometry. J Chromatogr. 2010;1217:4127–4134. doi: 10.1016/j.chroma.2010.02.020. [DOI] [PubMed] [Google Scholar]
- Garcia-Noguera J, Weller CL, Oliveira FIP, Rodrigues S, Fernandes FAN. Dual-stage sugar substitution in strawberries with a Stevia—based sweetener. Innov Food Sci Emerg. 2010;11:225–230. doi: 10.1016/j.ifset.2009.07.001. [DOI] [Google Scholar]
- Geuns JMC. Molecules of interest stevioside. Phytochemistry. 2003;64:913–921. doi: 10.1016/S0031-9422(03)00426-6. [DOI] [PubMed] [Google Scholar]
- Gómez JA, Tárrega A, Bayarri S, Carbonell JV. Clarification and gelation of animally heated orange juice concentrate during its refrigerated storage. J Food Process Eng. 2011;34:1187–1198. doi: 10.1111/j.1745-4530.2009.00411.x. [DOI] [Google Scholar]
- Hsieh C, Ko W. Effect of high-voltage electrostatic field on quality of carrot juice during refrigeration. Food Sci Technol. 2008;41:1752–1757. [Google Scholar]
- Hussain I, Zeb A, Ayub M. Evaluation of apple and apricot blend juice preserved with sodium benzoate at refrigeration temperature. World J Dairy Food Sci. 2011;6:79–85. [Google Scholar]
- Ibrahim AI, Nasr MI, Mohammed BR, El-Zefzafi MM. Plant growth regulators affecting in vitro cultivation of Stevia rebaudiana. Sugar Tech. 2008;10:254–259. doi: 10.1007/s12355-008-0045-6. [DOI] [Google Scholar]
- Karangwa E, Khizar H, Rao L, Nshimiyimana DS, Foh MBK, Li L, Xia SQ, Zhang XM. Optimization of processing parameters for clarification of blended carrot-orange juice and improvement of its carotene content. Adv J Food Sci Technol. 2010;2:268–278. [Google Scholar]
- Kelebek H, Selli S, Canbas A, Cabaroglu T. HPLC determination of organic acids, sugars, phenolic compositions and antioxidant capacity of orange juice and orange wine made from a Turkish cv. Kozan. Microchem J. 2009;91:187–192. doi: 10.1016/j.microc.2008.10.008. [DOI] [Google Scholar]
- Kim N, Kinghorn AD. Highly sweet compounds of plant origin. Arch Pharm Res. 2002;25:725–746. doi: 10.1007/BF02976987. [DOI] [PubMed] [Google Scholar]
- Kroyer G. The low calorie sweetener stevioside: stability and interaction with food ingredients. Lebensm Wiss Technol. 1999;32:509–512. doi: 10.1006/fstl.1999.0585. [DOI] [Google Scholar]
- Madan S, Ahmad S, Singh GN, Kohli K, Kumar Y, Singh R, Garg M. Stevia Rebaudiana(Bert.) Bertoni—a review. Ind J Nat Prod Resour. 2010;1:267–286. [Google Scholar]
- Moreas PCBT, André Bolini HM. Different sweeteners in beverage prepared whit instsnt and roasted coffee: ideal and equivalent sweetness. J Sens Stud. 2010;25:215–225. [Google Scholar]
- Parpinello GP, Versari A, Castellari M, Galassi S. Stevioside as a replacement of sucrose in peach juice: sensory evalution. J Sens Stud. 2001;16:471–484. doi: 10.1111/j.1745-459X.2001.tb00314.x. [DOI] [Google Scholar]
- Piljac-Žegarac J, Valek L, Martinez S, Belščak A. Fluctuations in the phenolic content and antioxidant capacity of dark fruit juices in refrigerated storage. Food Chem. 2009;113:394–400. doi: 10.1016/j.foodchem.2008.07.048. [DOI] [Google Scholar]
- Pourmorad F, Hosseinimehr SJ, Shahabimajd N. Antioxidant activity, phenol and flavonoid contents of some selected Iranian medicinal plants. Afr J Biotechnol. 2006;5:1142–1145. [Google Scholar]
- Rivas A, Rodrigo D, Martíneza A, Barbosa-Cánovasb GV, Rodrigo M. Effect of PEF and heat pasteurization on the physical–chemical characteristics of blended orange and carrot juice. LWT Food Sci Technol. 2006;39:1163–1170. doi: 10.1016/j.lwt.2005.07.002. [DOI] [Google Scholar]
- Sánchez-Moreno C, Plaza L, de Ancos B, Cano MP. Quantitative bioactive compoundsassessment and their relative contributionto the antioxidant capacity of commercialorange juices. J Sci Food Agric. 2003;83:430–439. doi: 10.1002/jsfa.1392. [DOI] [Google Scholar]
- Sandi D, Chaves JBP, de Sousa ACG, Ferreira J, Parreiras M, da Silva MTC, Constant PBL. Hunter color dimensions, sugar content and volatile compounds in pasteurized yellow passion fruit juice (Passiflora edulis var. flavicarpa) during storage. Braz Arch Biol Technol. 2004;47:233–245. doi: 10.1590/S1516-89132004000200011. [DOI] [Google Scholar]
- Supraditareporn W, Pinthong R. Physical, chemical and microbiological changes during storage of orange juices cv. Sai Nam Pung and cv. Khieo Waan in Northern Thailand. Int J Agric Biol. 2007;9:726–730. [Google Scholar]
- Vieira SM, Silva TM, Glória MBA. Influence of processing on the levels of amines and proline and on the physico-chemical characteristics of concentrated orange juice. Food Chem. 2010;119:7–11. doi: 10.1016/j.foodchem.2008.12.069. [DOI] [Google Scholar]
- Wang H, Hu X, Chen F, Wu J, Zhang Z, Liao X, Wang Z. Kinetic analysis of non-enzymatic browning in carrot juice concentrate during storage. Eur Food Res Technol. 2006;223:282–289. doi: 10.1007/s00217-005-0202-z. [DOI] [Google Scholar]
- Wolwer-Rieck U, Tomberg W, Wawrzun A. Investigations on the stability of stevioside and Rebaudioside A in soft drinks. J Agric Food Chem. 2010;58:12216–12220. doi: 10.1021/jf102894v. [DOI] [PubMed] [Google Scholar]
- Yousaf MS, Yusof S, Manap MYBA, Abd-Aziz S. Storage stability of clarified banana juice fortified with Inulin and Oligofroctose. J Food Process Preserv. 2009;34:599–610. doi: 10.1111/j.1745-4549.2009.00419.x. [DOI] [Google Scholar]