Abstract
Electrochemical behavior of artificial antioxidant, butylated hydroxyanisole (BHA), was investigated at a glassy carbon electrode modified with poly L- cysteine [poly (L- Cys/GCE)]. BHA exhibits a pair of well - defined redox peak on L- cysteine modified GCE with Epa = 69 mV and Epc = 4 mV. The modified electrode showed good electrocatalytic activity towards the oxidation of BHA under optimal conditions and exhibited a linear response in the range from 1.0 × 10−5 to 1.0 × 10−6 M with a correlation coefficient of 0.998. The limit of detection was found to be 4.1 × 10−7 M. The kinetics parameters of the proposed sensor such as heterogeneous electron transfer rate, ks, and charge transfer coefficient,α, was calculated and found to be 1.20 s−1 and 0.575 respectively. The average surface concentration of BHA on the surface of poly (L- Cys/GCE) was calculated to be 3.18 × 10−4 mol cm−2. The analytical utility of the proposed sensor was evaluated by the successful determination of BHA in coconut oil and sesame oil samples.
Keywords: BHA, L- cysteine, Sensor, Heterogeneous electron transfer rate
Introduction
Antioxidants are substances that when present in food at low concentrations compared to that of an oxidizable substrate markedly delay or prevent the oxidation of the substrate (Halliwell et al. 1995; Halliwell 1999). Antioxidants have also been of interest to biochemists and health professionals because they may help the body to protect itself against damage caused by reactive oxygen species (ROS) as well as those of nitrogen (RNS) and chlorine (RCS) (Shahidi 1997). Antioxidants are usually classified into two: natural and synthetic antioxidants.
Synthetic antioxidants are widely used as food additives owing to their high performance, low cost and wide availability. Butylated hydroxyanisole (BHA, E-320), a synthetic phenolic antioxidant, has been added to vegetable oils as it prevents rancidification of food which creates objectionable odors (Lam et al. 1979). The conjugated aromatic ring of BHA is able to stabilize free radicals sequestering them. However, studies have proved that the addition of artificial phenolic antioxidants may cause a loss of nourishment and even produce toxic effects. In many countries, the use of these antioxidants are controlled (Page and Charbonneau 1989) and consequently, it is important to determine reliably the amount of these substances in food products.
Various methods such as spectrophotometry (Prasad et al. 1987), liquid chromatography (HPLC) coupled with different detection systems (Ruiz et al. 1999; Tagliabue et al. 2004), gas chromatography (González et al. 1998), micellar electrokinetic capillary chromatography (Guan et al. 2006) etc. have been reported in literature for the determination of BHA in oils and fats. Although analysis of BHA by the above mentioned methods is appropriate, most require time-consuming preliminary steps such as extraction and clean-up. Voltammetric technique using modified electrodes offer alternative methods to those discussed above due to its rapid and sensitive procedures.
Electrochemical sensors are based on the electron transfer (ET) occurring at the interfaces during redox reactions: electrons flow from redox species within the bulk solution towards the electrode surface generating an electron flow that can be collected and measured. Cyclic voltammetric technique can be employed to characterize the kinetics of electron transfer reactions.
Polymer-modified electrodes (PMEs) have received extensive interest in the detection of analytes because of their selectivity, sensitivity and homogeneity in electrochemical deposition, strong adherence to electrode surface and chemical stability of the film (Volkov et al. 1980; Ohnuki et al. 1983; Cosnier 2003). Modification of electrodes by electropolymerization of amino acids has received great attention. L-Cysteine (2-amino-3-mercaptopropanoic acid, L-cys), one of the sulfur amino acids, is non-essential amino acid present in the human body. The objective of the present work is to establish a convenient and sensitive electrochemical sensor for the determination of BHA in food samples, based on electro catalytic activity of electropolymerised film of L - Cysteine on a glassy carbon electrode
Following our research of developing new electrochemical procedures for food analysis, (Thomas et al. 2012; Vikraman et al. 2013; Chandran et al. 2014), herein we report the development of a poly (L - Cysteine) modified glassy carbon electrode sensor for the determination of BHA. The resulting sensor showed excellent reproducibility and stability when the experimental conditions for the fabrication and the analytical performance of modified electrode were optimized. Heterogeneous electron transfer constant, ks, on bare and poly(L-Cys)/GCE electrodes are calculated using Laviron’s (1979) method. Much less information regarding ks for the oxidation of BHA is available in literature. The experimental results indicated that the developed BHA sensor can be used for determination of BHA in oil samples.
Experimental procedures
Electrochemical measurements
Electrochemical measurements were carried out with a CHI600C electrochemical analyzer (CH Instruments Inc., USA) controlled by a personnel computer. A three-electrode configuration (Scheme 1) was employed in which the poly (L-Cys)/ GCE (working electrode) was placed into a cell with clean platinum wire (counter electrode) and an Ag/AgCl electrode (reference electrode). The morphology of poly (L- Cys)/ GCE were investigated with a scanning electron microscope (SEM). SEM images were obtained on a JEOL 6390LV. The pH measurements were carried out in a Metrohm pH meter.
Scheme 1.
Schematic representation of three electrode system
Fabrication of poly (L- cysteine) modified glassy carbon electrode
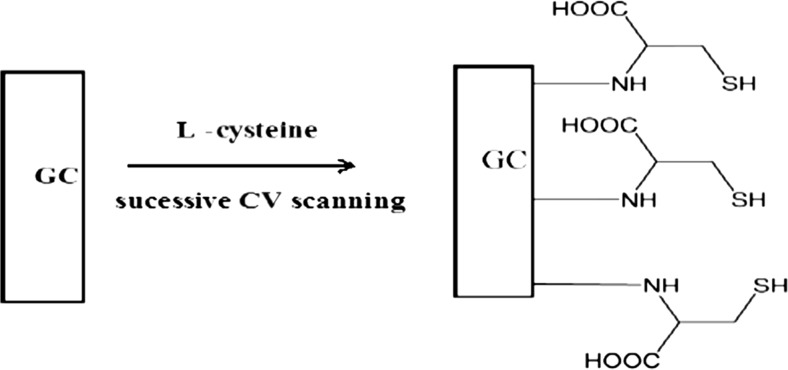
Prior to modification, the unmodified GCE was polished to a mirror finish using alumina slurries with different powder size down to 0.05 μm. After polishing, the electrode was sonicated in ethanol and doubly distilled water for 5 min, successively, in order to remove any adsorbed substances on the electrode surface. The cleaned GCE was immersed in 5 mM L- cysteine solution and 30 cyclic scans were carried out between −0.8 and 2.0 V at a scan rate of 0.1 Vs−1 (Wang et al. 2006). The resulting poly (L- cysteine) modified glassy carbon electrode (poly (L-Cys)/GCE) was thoroughly rinsed with ethanol to remove the physically adsorbed L-cysteine monomers. A schematic representation for fabrication of electrode (poly (L-Cys)/GCE) is represented in Scheme 2.
Scheme 2.
Schematic representation for the fabrication of poly (L-Cysteine) on GCE
Analytical procedure
10.0 mL of 0.1 M citrate buffer solution of pH 6.0 was used as the supporting electrolyte. A desired volume of BHA was pipetted into an electrochemical cell, followed by deareation with pumping O2− free nitrogen. After a quiescent interval of 2 s, differential pulse voltammograms (DPV) were recorded from – 0.50 to 0.30 V at a scan rate of 0.1 Vs−1, with an amplitude of 0.05 V, pulse width of 0.06 s, sample width of 0.02 s and pulse period of 0.5 s. The peak current for oxidation of BHA were measured at 0.024 V. Prior to and after each measurement, the L- cysteine modified GCE was activated by successive cyclic voltammetric sweeps from – 0.50 to 0.30 V at 0.1 Vs−1 in the electrolyte solution until the voltammograms kept unchangeable to achieve a reproducible electrode surface.
Sample preparation
Treatment of vegetable oil samples
5.0 g of a vegetable oil sample was placed into a 100 ml Erlenmeyer flask (with a screw cap) and 5.0 ml of pure methanol was added. After being shaken, with the use of a laboratory shaker for 5 min, this mixture was transferred to a 10 mL centrifuging tube and centrifuged at 3000 rpm for 5 min. After a settling time of 2 min, the extracts were transferred into a 25 mL flask. The above extraction procedure was repeated twice, all the extracts were collected, and transferred into the 25 ml flask; and then the solution was diluted to the mark with methanol (Ni et al. 2000). A 1.0 mL aliquot of this sample solution was analysed by DPV technique.
Results and discussions
Electrochemical behavior of BHA
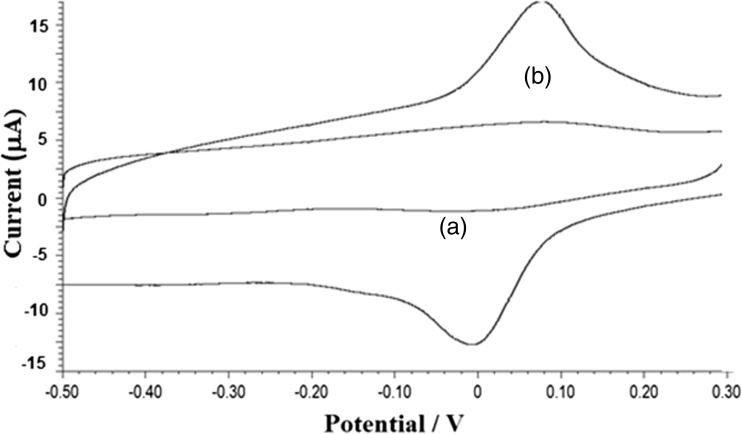
Cyclic voltammetry was used to investigate the electrochemical behavior of 1.0 × 10−5 M BHA in 0.1 M citrate buffer solution (pH 6.0) on a GCE and a poly (L- Cys)/GCE at a scan rate of 0.10 Vs−1. At bare GCE (Fig. 1a), BHA shows an irreversible behavior with an oxidation peak at 0.115 V (2.3 μA). However, on poly (L- cys)/GCE, (Fig. 1b), BHA exhibits a pair of well – defined redox peak with Epa = 0.069 V (15 μA) and Epc = 0.004 V (10 μA). The overpotential of BHA lowered on poly (L- cys)/GCE compared to bare GCE with a shifting of 0.045 V and an enhancement in peak current was observed. The separation between the anodic and cathodic peaks, (ΔEp = 0.065 V), was greater than the value of mV (n = 2), indicating that the electrochemical behavior of BHA under optimized conditions is a quasi-reversible two electron process. The formal potential, of the electrode was 0.036 V.
Fig. 1.
Electrochemical response of 1 × 10−5 M BHA at a bare glassy carbon electrode and poly (L- Cys)/GCE in 0.1 M citrate buffer of pH 6.0
The differential pulse votammetric (DPV) studies for the oxidation of 1 × 10−5 M BHA occurred at a lower potential of 0.020 V (38.8 μA) compared to cyclic voltammetry (CV). Therefore DPV technique was chosen for further studies.
Surface area study
The cyclic voltammetric analysis with redox reactions of 2.0 mM of K3Fe(CN)6 was used to evaluate the electrochemical behavior of the bare GCE and poly(L- cys)/GCE at a scan rate of 0.1 V s−1. The effective surface area of the modified electrode can be determined using the Randles–Sevcik equation (Randles 1948)
| 1 |
For K3Fe(CN)6, n = 1 and D = 7.6 × 10−5 cm2 s−1. From Eq. (1), the effective surface area (A) is proportional to the value of Ip/ѵ1/2. The effective surface areas of the bare and poly (L- cys)/GCE were calculated from the Randle’s slope and are 0.017 cm2 and 0.0351 cm2 respectively. The effective surface area of poly (L- Cys)/GCE was found to be about two times greater than that of bare GCE which is a strong evidence for the successful and effective modification of glassy carbon electrode using L- cysteine.
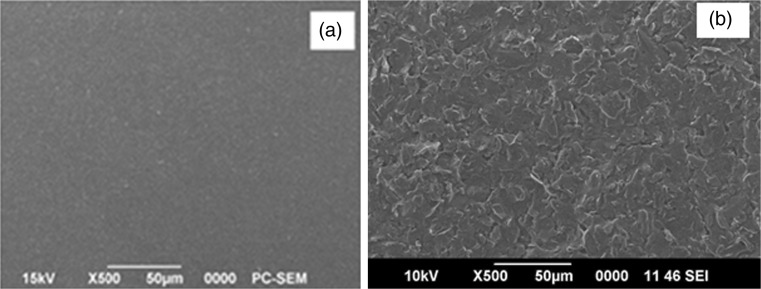
Further evidence for the modification of glassy carbon electrode was obtained from the surface morphology studies using SEM. Figure 2a and b show the changes in morphology obtained by scanning electron microscopy for bare GCE and poly (L-cys)/GCE respectively. After L-cys was electrochemically polymerized on the surface of the GCE, homogenous film of poly (L-cysteine) was observed.
Fig. 2.
SEM images of a bare GCE and b poly (L- Cys)/GCE
Optimization studies
Effect of supporting electrolyte and pH
The electrochemical behavior of 1.0 × 10−5 M BHA at poly (L- Cys) modified GCE in different supporting electrolytes (0.1 M), (such as citrate buffer, phosphate buffer, acetate buffer, HCl, NaOH, KNO3) were examined by DPV. Since oxidation peak current of BHA in citrate buffer solution was more sensitive and the peak shape was more preferable than in the other supporting electrolytes, it was selected for further studies.
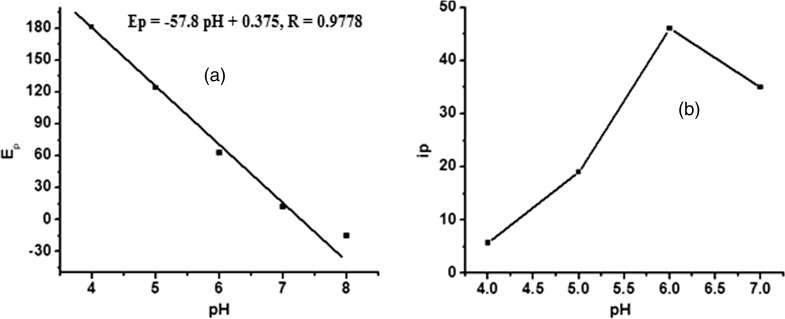
The effect of pH on the oxidation peak potential of BHA at L- cysteine modified GCE was investigated (Fig. 3a). The peak potential corresponding to the BHA oxidation shifted negatively at a slope of −57.8 mV/pH in the range of 1–7, revealing that proton takes part in the oxidation of BHA (Borrego et al. 2001). A linear relationship between peak potential (Ep) and pH was observed for BHA following the equation, Ep = −50.4 pH + 375.4, R = 0.9778. The slope was in agreement with the theoretical value (59 mV/pH), indicating that the oxidation process of BHA occurred with the involvement of equal number of electrons and protons. However, a maximum catalytic peak current was observed at pH 6.0 (Fig. 3b), beyond pH 6.0; the peak current exhibited a gradual decrease. Therefore, citrate buffer of pH 6.0 was used for the determination of BHA to achieve higher sensitivity.
Fig. 3.
Effect of pH on a peak potential and b peak current of 1 × 10−5 M BHA
Effect of scan rate
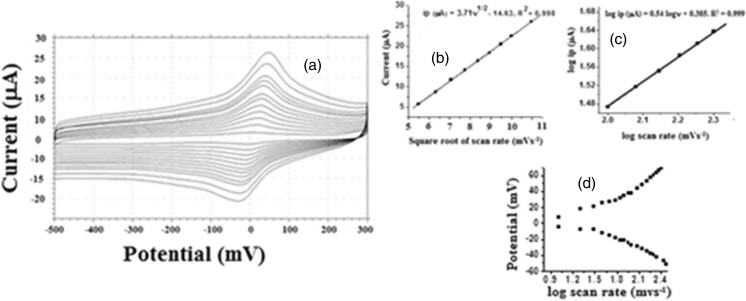
In order to study the nature of electrode process occurring at the electrode surface, the effect of scan rate on the oxidation peak current of 1 × 10−5 M BHA was studied by cyclic voltammetry (Fig. 4a). The plot of peak current vs. square root of scan rate (ν 1/2) is linear over the whole range of scan rate studied (Fig. 4b), which indicates that it is a typical diffusion controlled current system, and the equation can be expressed as Also, a plot of logarithm of peak current, log (ip), versus the logarithm of scan rate, log ѵ, was studied. This relationship was found to be linear, (log ip(μA) = 0.54 log ν + 0.38, R = 0.999) (Fig. 4c) with a slope, 0.54, which is near to the theoretical value of 0.5 for a diffusion controlled process (Wang 2000).
Fig. 4.
Scan rate study: a overlay of cyclic voltammogram for oxidation BHA at different scan rates b Plot of peak current with square root of scan rate c Plot of logarithm of peak current vs. logarithm of scan rate d Plot of peak potential vs. logarithm of scan rate d
The number of electrons involved in the oxidation of BHA was calculated using the Eq. (2);
| 2 |
where ip represents the anodic peak current, Q is the amount of charge integrated from the area of cyclic voltammetric peak, T is the temperature in Kelvin (298 K), R is the universal gas constant (8.314Jmol−1 K−1), F is the Faraday constant (96500 Cmol−1) and n is the number of electrons transferred. From the slope of ip versus ν, n was calculated to be 1.79 (≈2). Hence it can be concluded that the oxidation of BHA involves two electrons and two protons. The mechanism for the oxidation of BHA is given in Scheme 3 (Ceballos et al. 2006).
Scheme 3.
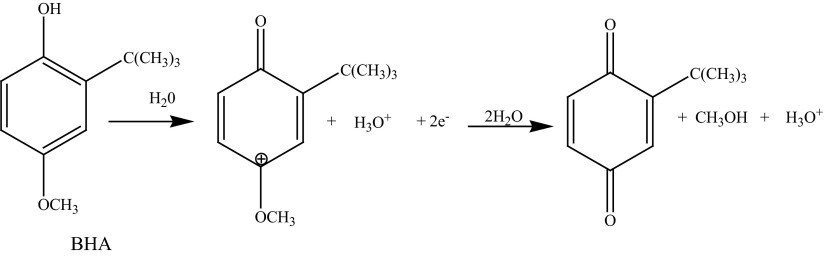
Mechanism for the oxidation of BHA
The study of rates of electron transfer reactions at the electrode electrolyte solution interface is a fundamental issue in electrochemistry. Based on Laviron’s theory (Laviron 1979), the apparent charge transfer rate constant, ks, and the charge transfer coefficient α, of a surface confined redox couple can be measured from CV experiments by using the variation of anodic and cathodic peak potentials as a function of logarithm of scan rate. Figure 4d shows plot of peak potentials (Ep) vs logarithm of scan rate and from the slopes of the plots the transfer coefficients can be calculated using the equations:
| 3 |
| 4 |
Where Epa is anodic peak potential and Epc is cathodic peak potential respectively. The evaluated values for α is 0.58. The heterogeneous electron transfer rate in the redox probe can be determined using the Eq. (5):
| 5 |
The rate constant for the electron transfer process depends on the nature of the electrode material. The value of ks on bare GCE and poly (L- Cys/GCE) was found to be 2.6 × 10−3 s−1 and 1.20s−1 at 0.1 Vs−1. The higher value of ks on modified GCE indicated that L-cys polymerized on the GCE significantly promoted the redox reaction of BHA.
The average surface concentration (Γ) of BHA on the surface of modified glassy carbon electrode could be estimated based on the slope of ip vs. ν using the equation (Yang et al. 2010):
| 6 |
and was found to be 3.18 × 10−4 mol cm−2.
Linearity range, limit of detection, stability and reproducibility
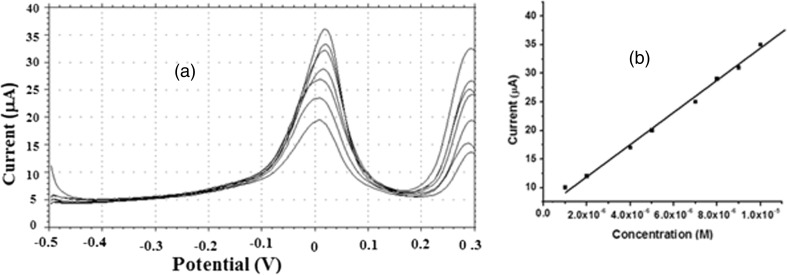
Figure 5a displays the differential pulse voltammograms of different concentration of BHA under optimized working conditions at a poly (L- cys)/ GCE. A linear relationship could be established between ip and the concentration of BHA in the range of 1.0 × 10−5 to 1.0 × 10−6 M (Fig. 5b). The linear regression equation and correlation coefficient are: ip(μA) = 2.8C(M) + 0.41, (R = 0.999) where ip is the oxidation peak current in μA and c is the concentration of BHA in M. The limit of detection was evaluated to be 4.1 × 10−7 M.
Fig. 5.
Concentration study a Overlay of DP voltammograms of BHA at poly (L- Cys)/GCE at different concentrations b Inset is the plot of oxidation peak current versus concentration of BHA
The performance of the modified electrode can be evaluated by its repeatability, stability and reproducibility. Five parallel determinations using same poly (L- Cys)/ GCE were carried out in 1.0 × 10−5 M BHA and the relative standard deviation was 3.9 %. This result infers the good repeatability of the modified electrode. The developed sensor retained 95 % of its response for more than a week. In order to establish the reproducibility of the proposed sensor five determinations using different poly (L- Cys)/ GCE was carried out and the relative standard deviation obtained was 4.2 %. These results proved the good stability and reproducibility of the poly (L-Cys)/GCE sensor.
Table 1 demonstrates the comparison between the results obtained with the proposed sensor and other reported voltammetric sensors.
Table 1.
Comparison of proposed sensor with other reported voltammetric sensors for the determination of BHA
| Electrode | Ep (V) | Linear range (M) | LODa (M) | References |
|---|---|---|---|---|
| BDD | 0.896 | 3.3 × 10−6 – 5.5 × 10−6 | 7.7 × 10−7 | Medeiros et al. 2010 |
| NiHCF / GWCE | 0.312 | 1.2 × 10−6 - 1.07 × 10−3 | 6.0 × 10−7 | Prabakar and Narayanan 2010 |
| Pt | 0.700 | 3.0 × 10−5 to 1.0 × 10−3 | – | Michalkiewicz et al. 2004 |
| Pt/MWCNT | 0.340 | 1.0 × 10−6 to 1.0 × 10−7 | 9.5 × 10−8 | Rasheed et al. 2014 |
| Poly(L-Cys)/GCE | 0.020 | 1.0 × 10−6 to 1.0 × 10−5 | 4.1 × 10−7 | Proposed sensor |
aLimit of detection
BDD boron doped diamond electrode, NiHCF / GWCE nickel hexacyanoferrate (NiHCF) surface modified graphite wax composite electrode, Pt bare platinum electrode, Pt/MWCNT multiwalled carbon nanotube modified platinum electrode
Interference of coexisting substances
In order to evaluate the selectivity of the poly (L- Cys) modified electrode towards the oxidation of BHA, the effect of various substances, which are commonly present with the antioxidant in commercial food samples, such as butylated hydroxy toluene (BHT), tert- butyl hydroquinone (TBHQ), ascorbic acid, sodium sulfite, citric acid, acetic acid and EDTA, were studied using the proposed sensor.
It was found that a 100 fold excess of BHT, TBHQ, sodium sulfite, citric acid, NaCl, acetic acid and EDTA had no influence on the voltammetric determination of 1 × 10−5 M BHA at poly (L-cys/GCE). A 1:1 ratio of ascorbic acid and propyl gallate were found to interfere in the determination of BHA. From the above results, it could be ascertained that majority of the coexisting substances do not interfere with the determination of BHA. These results confirmed the acceptable selectivity of the proposed electrode.
Analytical applications
In order to evaluate the analytical applicability of the proposed sensor, it was applied to the determination of BHA in coconut oil and sesame oil samples by adapting standard addition method. Each test was conducted six times. The good recoveries presented in Table 2 revealed the practical utility of poly (L-cys) modified GCE based BHA sensor.
Table 2.
Results obtained in the determination of BHA in oil by the proposed method
| Sample | Added (M) | Found (M) | R.S.Da (%) | Recoveryb (%) |
|---|---|---|---|---|
| Coconut oil | 1.00 × 10−5 | 0.982 × 10−5 | 4.4 | 98.2 |
| 5.00 × 10−5 | 5.09 × 10−5 | 1.8 | 101.8 | |
| Sesame oil | 1.0 × 10−5 | 1.02 × 10−5 | 2.7 | 102.0 |
| 5.0 × 10−5 | 5.10 × 10−5 | 1.9 | 102.0 |
aRelative standard deviation
bAverage of six replicates
Conclusion
Poly (L-Cys) film has been fabricated on GCE by electropolymerization method and the modified electrode was characterized by SEM. The electropolymerized film showed promising electrocatalytic activity toward the oxidation of BHA. The film showed very good linear range of 1.0 × 10−5 to 1.0 × 10−6 M with a detection limit of 4.1 × 10−7 M. The film shows high stability, very less fouling effect, good repeatability and excellent anti-interference ability. The good recoveries achieved in the real sample studies revealed the promising practical utility of the proposed sensor.
Acknowledgments
The authors acknowledge the financial assistance from Defense Research and Development Organization (DRDO), India, for the support of this work. One of the authors is grateful to Council for Scientific and Industrial Research (CSIR), India, for fellowship.
Footnotes
Highlights
• Development of a voltammetric sensor for butylated hydroxyanisole (BHA) was described
• Experimental parameters were studied and optimized
• Kinetic parameters were studied
• Application studies were carried out
References
- Borrego E, Sicilia D, Rubio S, Pérez- Bendito D. The mixed aggregate method: a useful approach for the determination of amphiphilic substances. TrAC Trend Anal Chem. 2001;20:241–254. doi: 10.1016/S0165-9936(01)00063-2. [DOI] [Google Scholar]
- Ceballos CD, Zón MA, Fernández H. Using square wave voltammetry on ultramicroelectrodes to determine synthetic antioxidants in vegetable oils. J Chem Educ. 2006;83:1349–1352. doi: 10.1021/ed083p1349. [DOI] [Google Scholar]
- Chandran S, Lonappan LA, Thomas D, Jos T, Kumar KG. Development of an electrochemical sensor for the determination of Amaranth: a synthetic dye in soft drinks. Food Anal Methods. 2014;7:741–746. doi: 10.1007/s12161-013-9676-7. [DOI] [Google Scholar]
- Cosnier S. Biosensors based on electropolymerized films: new trends. Anal Bioanal Chem. 2003;377:507–520. doi: 10.1007/s00216-003-2131-7. [DOI] [PubMed] [Google Scholar]
- González M, Ballesteros E, Gallego M, Valcárcel M. Continuous-flow determination of natural and synthetic antioxidants in foods by gas chromatography. Anal Chim Acta. 1998;359:47–55. doi: 10.1016/S0003-2670(97)00659-4. [DOI] [Google Scholar]
- Guan Y, Chu Q, Fu L, Wu T, Ye J. Determination of phenolic antioxidants by micellar electrokinetic capillary chromatography with electrochemical detection. Food Chem. 2006;94:157–162. doi: 10.1016/j.foodchem.2005.01.015. [DOI] [Google Scholar]
- Halliwell B. Food derived antioxidants. Evaluating their importance in food and in vivo. Food Sci Agric Chem. 1999;1:67–109. [Google Scholar]
- Halliwell B, Aeschbach R, Loliger J, Aruoma OI. The characterization of antioxidants. Food Chem Toxicol. 1995;33:601–617. doi: 10.1016/0278-6915(95)00024-V. [DOI] [PubMed] [Google Scholar]
- Lam LK, Pai RP, Wattenberg LW. Synthesis and chemical carcinogen inhibitory activity of 2-tert-butyl-4-hydroxyanisole. J Med Chem. 1979;22:569–71. doi: 10.1021/jm00191a020. [DOI] [PubMed] [Google Scholar]
- Laviron E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J Electroanal Chem Interfacial Electrochem. 1979;101:19–28. doi: 10.1016/S0022-0728(79)80075-3. [DOI] [Google Scholar]
- Medeiros RA, Rocha-Filho RC, Fatibello- Filho O. Simultaneous voltammetric determination of phenolic antioxidants in food using a boron-doped diamond electrode. Food Chem. 2010;123:886–891. doi: 10.1016/j.foodchem.2010.05.010. [DOI] [Google Scholar]
- Michalkiewicz S, Mechanik M, Malyszko J. Voltammetric study of some synthetic antioxidants on platinum microelectrodes in acetic acid medium. Electroanal. 2004;16:588–595. doi: 10.1002/elan.200302826. [DOI] [Google Scholar]
- Ni Y, Wang L, Kokot S. Voltammetric determination of butylated hydroxyanisole, butylated hydroxytoluene, propyl gallate and tert-butylhydroquinone by use of chemometric approaches. Anal Chim Acta. 2000;412:185–193. doi: 10.1016/S0003-2670(00)00720-0. [DOI] [Google Scholar]
- Ohnuki Y, Ohsaka T, Matsuda H, Oyama N. Permselectivity of films prepared by electrochemical oxidation of phenol and amino-aromatic compounds. J Electroanal Chem. 1983;158:55–67. doi: 10.1016/S0022-0728(83)80338-6. [DOI] [Google Scholar]
- Page BD, Charbonneau CF. Liquid chromatographic determination of seven antioxidants in dry food. J Assoc Off Anal Chem. 1989;72:259–265. [PubMed] [Google Scholar]
- Prabakar RSJ, Narayanan SS. Flow injection analysis of BHA by NiHCF modified electrode. Food Chem. 2010;118:449–455. doi: 10.1016/j.foodchem.2009.04.104. [DOI] [Google Scholar]
- Prasad VU, Divakar TE, Hariprasad T, Sastry CSP. Spectrophotometric determination of some antioxidants in oils and fats. Food Chem. 1987;25:159–164. doi: 10.1016/0308-8146(87)90065-3. [DOI] [Google Scholar]
- Randles JEB. Cathode ray polarograph. Trans Faraday Soc. 1948;44:322–327. doi: 10.1039/tf9484400322. [DOI] [Google Scholar]
- Rasheed Z, Vikraman AE, Thomas D, Jagan JS, Kumar KG. Carbon-nanotube based sensor for the determination of butylated hydroxyanisole in food samples. Food Anal Methods. 2014 [Google Scholar]
- Ruiz MA, García-Moreno E, Barbas C, Pingarrón JM. Determination of phenolic antioxidants by HPLC with amperometric detection at a nickel phthalocyanine polymer modified electrode. Electroanal. 1999;11:470–474. doi: 10.1002/(SICI)1521-4109(199906)11:7<470::AID-ELAN470>3.0.CO;2-F. [DOI] [Google Scholar]
- Shahidi F. Measurement of lipid oxidation and evaluation of antioxidant activity. In: Shahidi F, editor. Natural antioxidants: chemistry, health effects and applications. Champaign: AOCS Press; 1997. pp. 1–11. [Google Scholar]
- Tagliabue S, Gasparoli A, Bella DL, Bondioli P. Quali-quantitative determination of synthetic antioxidants in biodiesel. Riv Ital Sostanze Gr. 2004;81:37–40. [Google Scholar]
- Thomas D, Rajith L, Lonappan LA, Issac S, Kumar KG. Sensitive determination of nitrite in food samples using voltammetric techniques. Food Anal Methods. 2012;5:752–758. doi: 10.1007/s12161-011-9292-3. [DOI] [Google Scholar]
- Vikraman AE, Rasheed Z, Rajith L, Lonappan LA, Kumar KG. MWCNT modified gold electrode sensor for the determination of propyl gallate in vegetable oils. Food Anal Methods. 2013;6:775–780. doi: 10.1007/s12161-012-9485-4. [DOI] [Google Scholar]
- Volkov A, Tourillon G, Lacaze PC, Dubois JE. Electrochemical polymerization of aromatic amines: IR, XPS and PMT study of thin film formation on a Pt electrode. J Electroanal Chem. 1980;115:279–291. doi: 10.1016/S0022-0728(80)80332-9. [DOI] [Google Scholar]
- Wang J. Analytical Electrochemistry. 2. New York: Wiley-VCH; 2000. p. 37. [Google Scholar]
- Wang C, Li C, Wang F, Wang C. covalent modification of glassy carbon electrode with L- Cysteine for the determination of acetaminophen. Microchim Acta. 2006;155:365–371. doi: 10.1007/s00604-006-0616-8. [DOI] [Google Scholar]
- Yang D, Zhu L, Jiang X. Electrochemical reaction mechanism and determination of Sudan I at a multi wall carbon nanotubes modified glassy carbon electrode. J Electroanal Chem. 2010;640:17–22. doi: 10.1016/j.jelechem.2009.12.022. [DOI] [Google Scholar]