Abstract
The aim of this work was to develop chitosan edible films added with essential oils obtained from two Thymus species, Thymus moroderi (TMEO) and Thymus piperella (TPEO) to determine their application for enhancing safety (antioxidant and antibacterial properties) and shelf-life of cooked cured ham (CCH) stored at 4 °C during 21 days. Addition of TMEO and TPEO into chitosan films decreased the aerobic mesophilic bacteria (AMB) and lactic acid bacteria (LAB) counts in coated cooked cured ham samples as compared with uncoated samples. Both AMB and LAB showed the lowest counts in CCH samples coated with chitosan films added with TPEO at 2 %. In regard to lipid oxidation, the CCH samples coated with chitosan films added with TMEO or TPEO had lower degrees of lipid oxidation than uncoated control samples. Chitosan films added with TPEO at 2 % showed the lowest values. The addition of TPEO or TMEO in chitosan films used as coated in CCH improved their shelf life.
Keywords: Essential oils, Chitosan, Films, Shelf-life, Cooked cured ham
Introduction
At present there is great variety of ready-to-eat (RTE) foods including pre-packed and precooked meats, in individual or family-size packages, with very different presentations. However, the transformation into RTE food of meat product such as cooked ham, involves additional manipulation such as cutting, slicing, dicing and packaging aimed at facilitating its consumption at home (Gil-Díaz et al. 2009). This may contribute considerably to the contamination of RTE meat products with pathogenic bacteria. For this reason there is a need for continuous supervision of RTE food products to ensure safety to consumers in the industrialize countries. The increased demands by consumers for better quality and improved freshness of RTE food products have given rise to the development and implementation of edible films (Beverlya et al. 2008).
Edible films and coatings are thin layers of edible material formed or placed on or between foods or food components (Bravin et al. 2006) which can play an important role on their preservation, distribution and marketing of food products (Falguera et al. 2011). They are prepared from biopolymers; the major constituents of these are polysaccharides, proteins and lipids.
In particular, chitosan has a great potential for a wide range of food applications due to its biodegradability, biocompatibility, antimicrobial and antioxidant activities, nontoxicity and film-forming capacity (Tharanathan and Kittur 2003). The use of edible films helps to maintain product quality, enhance sensory properties, improve product safety, and increase the shelf life of various RTE food products (Beverlya et al. 2008). Moreover, they can act as carriers of active substances, such as antioxidant, antimicrobial or flavoring compounds, resulting in shelf-life extension and safety improvement of the food product. One of these active substances is the essential oils (EOs). The ability of EOs to protect foods against pathogenic and spoilage microorganisms as well as the oxidation have been reported by several researchers (Ruiz-Navajas et al. 2013; Alves-Silva et al. 2013). In order to achieve effective antimicrobial activity in direct food applications, high concentrations of essential oils are generally needed, which might impact inappropriate flavors and odors in the product (Seydim and Sarikus 2006). To avoid this problem, the EOs could be incorporated into bioactive film coatings which would allow us to fix and retain the compound on the product surface, thus increasing its effectiveness (Sánchez-González et al. 2010). In these coatings, the major compounds are biodegradable polymers and a relatively reduced amount of EOs can be used. Consequently, the application costs of essential oils and/or other problems, such as the intense aroma and potential toxicity, could be minimized (Sánchez-González et al. 2010).
Several studies have shown that incorporation of EOs into chitosan films or coatings may not only enhance the film’s antimicrobial and antioxidant properties but also reduce water vapor permeability and decrease lipid oxidation of the product on which the film is applied (Kanatt et al. 2008) mainly meat and meat products. There are several scientific works where the edible films are elaborated with isolate whey protein, isolate soy protein, alginate and so on and incorporated with essential oils have been used to prevent the lipid oxidation and microbial growth in meat and meat product (Zinoviadou et al. 2009; Emiroğlu et al. 2010; Juck et al. 2010). However, little information is available regarding the applications of chitosan edible films with addition of EOs.
The aim of this work was to develop chitosan edible film added with EOs obtained from two Thymus species, Thymus moroderi and Thymus piperella and to determine their application for enhancing safety (antioxidant and antibacterial properties) and shelf-life of cooked cured ham storage at 4 °C during 21 days.
Materials and methods
Extraction of the essential oils
Thymus moroderi (TM) and Thymus piperella (TP) were collected during their flowering period and the identification of the plant material was made by Prof Dra. Concepcion Obon de Castro, Biology Department of Miguel Hernandez University (Spain). The EOs of TM and TP were extracted from whole plant (stems, leaves and flowers) by hydro-distillation using a Clevenger-type apparatus for 3 h. The oily layer obtained on top of the aqueous distillate was separated and dried with 0.5 g of anhydrous sodium sulphate (Panreac Química, Barcelona, Spain). The extracted EOs were kept in sealed air-tight glass vials and covered with aluminum foil at 4 °C until further analysis.
Preparation of edible films
Chitosan-based film was prepared by dissolving chitosan (high molecular weight 75–85 % deacetylated, Sigma-Aldrich Chemical Co., Steinheim, Germany) in a lactic acid aqueous solution (1 % v/v) (Sigma-Aldrich Chemical Co., Steinheim, Germany) at a concentration of 2 % (w/v) while stirring on a magnetic stirrer/hot plate, following the indications of Ojagh et al. (2010) with some modifications.
The chitosan solution was stirred at room temperature until it was completely dissolved (24 h). The resultant chitosan solution was filtered through a Whatman No 3 filter paper to remove any undissolved particles. After filtration the solution was returned to the magnetic stirrer/hot plate and glycerol (Panreac Quimica) was added to a level of 0.75 mL/g chitosan as a plasticizer. The plasticizer was mixed into the solution for 15 min. Then, Tween 80 (Panreac, Quimica) at level of 0.2 % (v/v) of EO, was added as an emulsifier to assist EO dispersion in film forming solutions. After 15 min of stirring, the following five solutions were prepared by adding T. moroderi (TMEO) or T. piperella (TPEO) EOs into chitosan solution: (i) chitosan without TMEO and TPEO; (ii) chitosan with 1 % TMEO; (iii) chitosan with 2 % TMEO; (iv) chitosan with 1 % TPEO; (v) chitosan with 2 % TPEO. Both chitosan TMEO (CH+TMEO) and chitosan TPEO (CH+TPEO) mixtures were emulsified at room temperature using a rotor-stator homogenizer (Ultraturrax DI 25, Janke & Kunkel, Staufen, Germany) at 20,000 rpm for 3 min. These emulsions were degasified at room temperature using an ultrasonic water bath (Selecta S.A. Barcelona, Spain), without temperature control, during 2 h. The film forming solutions (7 g) were casted into 60 mm inner diameter sterile Petri dishes (0.25 g/cm2) covers and then dried for 48 h at 37 °C. Dried films were peeled and stored in a desiccator at 25 °C and 51 % relative humidity until evaluation. Saturated magnesium nitrate (Panreac Quimica) solution was used to meet required relative humidity.
Film application on cooked cured ham
Cooked cured hams (CCH) were purchased directly from a local producer and transported immediately to the laboratory under refrigerated conditions and then they were sliced (3 mm thick and 12.5 g weight). Each film with different formulations was placed between two slices and was placed in a sterile bags made of polyethylene and polyamide laminate of 1.1 g/m2/24 h water vapor permeability at 23 °C 10 cm3/m2/24 h, nitrogen permeability at 23 °C, 140 cm3/m2/24 h carbon dioxide permeability at 23 °C, and 30 cm3/m2/24 h oxygen permeability at 23 °C (Fibran, Girona, Spain). Slices without any chitosan film were also prepared as control CMH samples. The bags were heat-sealed and stored at 4 ± 1 °C. The packs were stored for 21 days. Samples from each treatment were taken at 0, 7, 14, and 21 days (storage time) and analyzed on the same day.
Physico-chemical analysis
The CIE L*a*b* color space was studied following the procedure of Cassens et al. (1995). The following color coordinates were determined: lightness (L*), redness (a*, ± red-green), and yellowness (b*, ± yellow-blue). Color determinations were made, at 12 ± 2 °C by means of a Minolta CM-2600D (Minolta Camera Co., Osaka, Japan) spectrophotometer with illuminant D65, 10° observer angle, 11 mm aperture for illumination and 8 mm for measurement. American Meat Science Association guidelines for color measurements were followed and spectrally pure glass (CRA51, Minolta Co., Osaka, Japan) was put between the samples and the equipment (AMSA 2012).
The pH was measured by blending a 5 g sample with 50 mL deionized water for 2 min. The pH of the resultant suspension was measured with a Crison pH meter (Model 507, Crison, Barcelona, Spain) equipped with a Crison combination electrode (Cat. n°. 52, Crison, Barcelona, Spain).
Lipid oxidation
Lipid oxidation was assessed in triplicate by the 2-thiobarbituric acid (TBA) test following the recommendations of Buege and Aust (1978). TBARS values were calculated from a standard curve of malonaldehyde (MAD) and expressed as mg MAD/kg sample.
Microbiological analysis
At each time (0, 7, 14, and 21 day), films were removed from the middle of two slices with a forceps and then the slices (two slices from the same treatment, weighing 25 g) were homogenized with sterile 1.5 % peptone water (225 mL) in a Stomacher 400 (Colworth, London, UK) for 2 min. Aerobic mesophilic counts were determined on Plate Count Agar, Enterobacteriaceae using Violet Red Bile Glucose Agar (VRBGA) and lactic acid bacteria (LAB) were counted on double layer MRS Agar at pH 5.6. In all cases, plates were incubated at 37 °C for 48 h. Moulds and yeasts were determined on Rose Bengal plates with chloramphenicol incubated at 28 °C for 5 days. All results are reported as log10 colony forming per gram (CFU/g).
Release of phenolics contents from film into cooked cured hams
On each sampling day (0, 7, 14, and 21 day), films were removed from the middle of two slices and total phenolic content (TPC) of films was determined with Folin-Ciocalteu reagent (Singleton and Rossi 1965). Every sample of each film was extracted with 5 mL of methanol using an ultrasonic water bath (Selecta S.A. Barcelona, Spain) without temperature control during 2 h. Then, the mixtures were centrifuged at 3600 g for 15 min at 4 °C. Then a volume of 0.3 mL of supernatant was introduced into the test tubes followed by 2.5 mL of Folin Ciocalteu’s reagent (SigmaeAldrich Chemical Co., Steinheim, Germany) (diluted 10 times with water) and 2 mL of sodium carbonate (Panreac Quimica) (7.5 % w/v).
The tubes were vortex-mixed, covered with parafilm and incubated at 50 °C for 5 min. Absorbance at 760 nm was measured with an HP 8451 spectrophotometer (Hewlett Packard, Cambridge, UK) and compared to a gallic acid calibration curve. The results were expressed in mg gallic acid equivalents (GAE)/g of films.
Statistical assay
For each experiment and sample day, three independent samples were examined with three replications per sample. Statistical analysis and comparisons among means were carried out using the statistical package SPSS 19.0 (SPSS Inc., Chicago, IL.). All the data collected for pH, color, lipid oxidation, microbiological analysis and release of phenolic compounds were analyzed by two-way analysis of variance (ANOVA) to test the effects of two fixed factors: film samples (levels: control, CH, CH+TPEO 1 %, CH+TPEO 2 %, CH+TMEO 1 % and CH+TMEO 2 %) and time (levels: 0, 7, 14 and 21 days). Tukey’s post hoc test was applied for comparisons of means; differences were considered significant at p < 0.05. Generalized Additive Models were used in order to estimate the relationship between variables with different links and transformations of the variables following the recommendations of Wood (2006).
Results and discussion
The chemical composition of the essential oils used in this work was previously determined by Ruiz-Navajas et al. (2012). In the Thymus moroderi (TMEO) the main components were camphor (26.74 %), 1.8-cineol (24.99 %), myrcene (5.63 %) and α-pinene (4.35 %) while in Thymus piperella (TPEO) the predominant compounds were carvacrol (31.92 %), para-cymene (16.18 %), γ-terpinene (10.11 %) and α-terpineol (7.29 %).
Physico-chemical analysis
Figure 1 showed the effect of chitosan films formulated with TMEO and TPEO essential oils on pH values in cooked cured ham stored at 4 °C for 21 days. In all uncoated and coated samples, the pH values decreased with the storage time. In uncoated CCH samples there was a slight fell (p < 0.05) of pH values (6.24 at day 0 to 6.12 at day 21). Nevertheless, in coated CCH samples the decrease in pH values was most accentuated. At the end of storage time all coated CCH samples showed pH values comprised between 5.63 and 5.68 with no statistical differences (p > 0.05) between them, except for the sample coated with CH+TPEO2% that showed higher (p < 0.05) values (5.81). This decreased in pH values of coated CCH samples could be attributed to the release of lactic acid present in chitosan films into meat product. Additionally, the gradual growth of lactic bacteria in the CCH samples, which could have generate lactic acid, which would lead to the gradual decrease of pH.
Fig. 1.
Effect of chitosan films formulated with Thymus moroderi (TMEO) and Thymus piperella (TPEO) essential oils on pH values in cooked cured ham stored at 4 °C for 21 days
As regards to color parameters, Table 1 shows the effect of chitosan films formulated with TMEO and TPEO essential oils on lightness (L*), redness (a*) and yellowness (b*) coordinates in cooked cured ham storage at 4 °C during 21 days. For lightness (L*), at the end of storage time, the type of film used had no significant (p > 0.05) effect on this coordinate except for the sample coated with CH+TPEO 1 % that showed higher (p < 0.05) values. In the same way, storage time had no significant (p > 0.05) effect in the samples coated with CH, CH+TMEO and CH+TPEO, except for the sample coated with CH+TPEO 1 %. The absence of any modification in this parameter may have been due to the protective effect of the packing film against oxygen. However, the storage time had a significant (p < 0.05) effect in the uncoated CCH sample. In these samples, L* values decreased from 60.43 at the outset to 58.95 at the end of the experiment. Some authors reported that L* values in meat and meat products are related to (i) surface water, (ii) water vapor exchanges between the products and the environment and (iii) modifications of the different states of hemopigments (Fernández-López et al. 2000).
Table 1.
Effect of chitosan films formulated with Thymus moroderi (TMEO) and Thymus piperella (TPEO) essential oils on lightness (L*), redness (a*) and yellowness (b*) coordinates in cooked cured ham storage at 4 °C during 21 days
| Sample | Time (Days) | ||||
|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | ||
| L* | Control | 60.43 ± 0.53aA | 57.92 ± 0.66bA | 55.76 ± 0.18cA | 58.95 ± 0.56bA |
| CH | 60.43 ± 0.53aA | 60.62 ± 1.93aA | 59.65 ± 1.79aB | 59.53 ± 0.64aA | |
| CH+TMEO 1 % | 60.43 ± 0.53aA | 60.62 ± 1.17aA | 58.28 ± 0.76bB | 59.47 ± 0.58aA | |
| CH+TMEO 2 % | 60.43 ± 0.53aA | 57.18 ± 2.38aA | 59.80 ± 1.03aB | 59.53 ± 1.09aA | |
| CH+TPEO 1 % | 60.43 ± 0.53aA | 60.72 ± 2.95abcA | 58.86 ± 0.67bB | 62.99 ± 0.39cB | |
| CH+TPEO 2 % | 60.43 ± 0.53aA | 58.18 ± 1.03bA | 59.45 ± 0.59abB | 59.66 ± 1.09abA | |
| a* | Control | 10.08 ± 0.43aA | 9.58 ± 1.66abA | 10.15 ± 0.56aA | 8.89 ± 0.68bA |
| CH | 10.08 ± 0.43aA | 9.19 ± 2.40abA | 9.52 ± 0.14aA | 8.54 ± 0.37bA | |
| CH+TMEO 1 % | 10.08 ± 0.43aA | 8.99 ± 2.89abA | 10.25 ± 0.54aA | 8.91 ± 0.31bA | |
| CH+TMEO 2 % | 10.08 ± 0.43aA | 10.18 ± 1.14aA | 9.68 ± 0.23aA | 8.77 ± 0.19bA | |
| CH+TPEO 1 % | 10.08 ± 0.43aA | 9.24 ± 1.93aA | 9.66 ± 0.23aA | 5.06 ± 0.30bB | |
| CH+ TPEO 2 % | 10.08 ± 0.43aA | 8.99 ± 3.25abA | 8.71 ± 0.96abA | 8.43 ± 0.99bA | |
| b* | Control | 8.37 ± 0.11aA | 8.74 ± 1.39aA | 8.81 ± 0.46aA | 8.17 ± 0.66aA |
| CH | 8.37 ± 0.11aA | 7.78 ± 2.93aA | 8.69 ± 0.35aA | 7.90 ± 0.75aA | |
| CH+TMEO 1 % | 8.37 ± 0.11aA | 8.54 ± 2.29aA | 8.09 ± 0.47aA | 7.98 ± 0.40aA | |
| CH+TMEO 2 % | 8.37 ± 0.11aA | 9.74 ± 2.44abA | 8.15 ± 0.48aA | 10.05 ± 0.82bB | |
| CH+TPEO 1 % | 8.37 ± 0.11aA | 8.19 ± 1.31abA | 7.72 ± 2.36abA | 11.37 ± 0.93cB | |
| CH+TPEO 2 % | 8.37 ± 0.11aA | 12.26 ± 2.08bB | 8.36 ± 0.22aA | 7.37 ± 0.63cA | |
For the same coordinate, values followed by the same lower case letter, in the same column, are not significantly different (p > 0.05) according to Tukey’s Multiple Range Test
For the same coordinate, values followed by the same upper case letter, in the same row, are not significantly different (p > 0.05) according to Tukey’s Multiple Range Test
As regards redness (a*) coordinate, as occur with lightness, at the end of storage time, there no were differences (p > 0.05) between uncoated and coated CCH samples, except for CH+TPEO 1 % that showed lowest (p < 0.05) values. The storage time, however, had an effect (p < 0.05), and the redness values fell in both uncoated and coated CCH samples. This coordinate is affected by the structural integrity of the food, the pigment content and disposition (water or lipid-soluble) and surface water availability (Fernández-López et al. 2005). Moreover, decreases in redness have been related to oxidation of lipids and hemopigments (Fernández-López et al. 2006).
For yellowness (b*) coordinate, at day 21, there no were differences (p > 0.05) between uncoated CCH samples and samples coated with CH or CH+1%TMEO. Similarly, storage time had no effect on either of these samples. On the other hand, the CCH samples coated with CH+2%TMEO, CH+1%TPEO and CH+2%TPEO were affected (p < 0.05) by type of films used and storage time. The behavior of b* depends to a great extent on the food matrix, and it is recognized that changes (pH, oxidation extent, water activity, etc.) in the matrix have the greatest influence on this coordinate in many foods (Cofrades et al. 2004).
Lipid oxidation
There are several scientific works that has shown the beneficial effects of chitosan on lipid oxidation when it is added to meat and meat products as ingredient (Georgantelis et al. 2007; Sayas-Barberá et al. 2011). However, to our knowledge, few studies have been conducted to analysis the antioxidant effect of chitosan films or chitosan films added with EO on meat and meat products.
Figure 2 shows the effect of chitosan films formulated with Thymus TMEO and TPEO essential oils on TBA values in cooked cured ham storage at 4 °C during 21 days. At day 0, there was no significant difference (p > 0.05) in TBA values between uncoated and coated CCH samples. On day 7, the CCH samples coated with CH+TPEO 2 % and CH+TMEO 2 % showed the lowest (p < 0.05) oxidation degree with TBA values of 2.79 and 2.67 mg MA/kg sample, respectively. The CCH sample coated with chitosan film without EOs added showed a slight reduction in oxidation degree (p < 0.05) with regards to uncoated sample. The antioxidant ability of CH films is thought to be due to chelation of free ion which is released from hemoproteins of meat product during storage (Shahidi et al. 1999). At day 14, again the CCH samples in which CH+TPEO 2 % and CH+TMEO 2 % was used showed the lowest TBA values (p < 0.05) with reductions in the oxidation degree when compared with control of 26.86 and 37.17 % respectively with statistically differences (p < 0.05) between them. At the end of experiment (day 21) the CCH sample coated with CH+TPEO 2 % showed the lowest (p < 0.05) TBA values with a reduction in the oxidation degree regarding to uncoated CCH sample of 28.37 % followed by the CCH sample coated with CH+TMEO 2 % (p < 0.05) with a reduction in the oxidation degree regarding to uncoated CCH sample of 25.25 %. These results were in agreement with Moradi et al. (2011) who reported that a cooked meat product coated with chitosan films containing Zataria multiflora Boiss EO had lower degrees of lipid oxidation than uncoated control sample. In the same way, Suman et al. (2010) showed that coating ground beef patties with chitosan reduced TBARS values and improved the surface red color of patties as compared to uncoated samples. Chitosan film incorporating green tea extract was analyzed as active packaging for shelf life extension of pork sausages by Siripatrawan and Noipha (2012). These authors reported that during the storage time (20 days), significantly higher TBA values were evident in control samples than those coated with chitosan film or chitosan film incorporating green tea extract.
Fig. 2.
Effect of chitosan films formulated with Thymus moroderi (TMEO) and Thymus piperella (TPEO) essential oils on TBA values in cooked cured ham stored at 4 °C for 21 days
The decrease in the lipid oxidation of samples coated with CH, CH+TPEO or CH+TMEO may be, probably, due to a sharp release of active compounds from those films during first days of storage as mentioned Moradi et al. (2011). These results showed that the incorporation of EOs in the films improved the protection of the meat samples against lipid oxidation. The antioxidant activity of EOs obtained from plants belonged to genus Thymus is widely demonstrated (Viuda-Martos et al. 2010a; Zouari et al. 2011). The antioxidant activity of EOs obtained from plants belonging to genus Thymus can be credited to the presence of its major phenolic compounds, particularly thymol and carvacrol, and their recognized impact on lipid oxidation. The antioxidant activity of phenolic compounds is related to the hydroxyl groups linked to the aromatic ring, which are capable of donating hydrogen atoms with electrons and stabilizing free radicals (Dorman et al. 2003; Yanishlieva et al. 2006).
Moreover, the storage conditions (4 °C and protected from light) as well as the low oxygen permeability characteristics of chitosan films may contributed to the inhibition of lipid oxidation.
Microbial counts
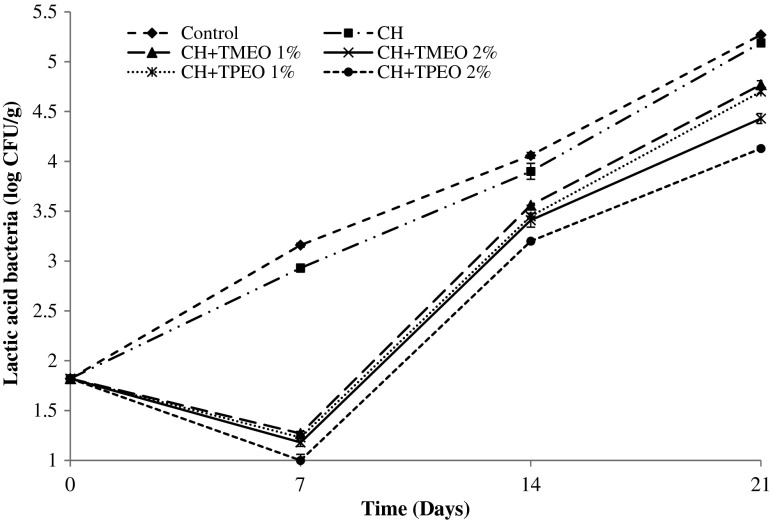
Effect of chitosan films formulated with TMEO and TPEO essential oils on Aerobic Mesophilic Bacteria (AMB) and Lactic Acid Bacteria (LAB) in cooked cured ham storage at 4 °C during 21 days are given in Figs. 3 and 4. Microbial counts (aerobic mesophilic bacteria and lactic acid bacteria) were (p < 0.05) affected by storage time and the application of chitosan edible films added with TMEO or TPEO. In our experiment, no moulds and yeast were found in any of the treatments regardless of time of storage, probably due to the aseptic slicing process, together with the presence of the sodium chloride in the products (Viuda-Martos et al. 2010b). In the same way, no enterobacteria were found. Ouattara et al. (2000) reported that indigenous Enterobacteriaceae in meat products (bologna, beef pastrami and cooked ham) were inhibited by the use of antimicrobial films, containing chitosan and acetic acid.
Fig. 3.
Effect of chitosan films formulated with Thymus moroderi (TMEO) and Thymus piperella (TPEO) essential oils on Aerobic Mesophilic Bacteria (AMB) in cooked cured ham stored at 4 °C for 21 days
Fig. 4.
Effect of chitosan films formulated with Thymus moroderi (TMEO) and Thymus piperella (TPEO) essential oils on Lactic Acid Bacteria (LAB) in cooked cured ham stored at 4 °C for 21 days
As regards to aerobic mesophilic bacteria (Fig. 3), the log CFU/g of the uncoated and those samples coated with chitosan added with TMEO and TPEO showed no significant difference (p > 0.05) at day 0. However, on day 7, the CCH samples coated with CH+TMEO or CH+TPEO at 2 % showed the lowest counts (p < 0.05) with 2.57 and 2.61 log cycle reduction, respectively, with regard to uncoated sample. CCH sample coated with CH showed a 0.62 log reduction relating to uncoated sample demonstrating a certain antibacterial activity of chitosan. The antimicrobial effect of chitosan is thought to be related to electrostatic interaction between a positive charge on the NH+3 group of glucosamine monomer in chitosan molecules and negative charge of microbial cell membrane that leads to the leakage of intracellular constituents (Dutta et al. 2009). At day 14, CCH samples coated with CH+TMEO 2 %, CH+TPEO 1 % and CH+TPEO 2 % showed the lowest counts (p < 0.05) with no statistically differences between them (p > 0.05). At the end of experiment (day 21) the CCH sample coated with CH+TPEO 2 % showed the lowest counts (p < 0.05) in aerobic mesophilic bacteria with a reduction of 0.87 log cycles regarding to uncoated CCH sample. CCH samples coated with CH+TMEO 1 %, CH+TMEO 2 % and CH+TPEO 1 % showed a 0.34, 0.53 and 0.37 log cycle reduction, respectively, in aerobic mesophilic bacteria counts when compared to the control, with no statistically differences between them (p > 0.05).
In the case of Lactic acid bacteria (Fig. 4), as occur with mesophilic bacteria, at day 0, no statistically differences were found (p > 0.05) between uncoated and coated CCH samples. On day 7, the CCH sample coated with CH+TPEO 2 % showed the lowest (p < 0.05) counts with 2.16 log cycle reduction with regard to uncoated CCH sample. In CCH samples coated with CH+TMEO 1 %, CH+TMEO 2 % and CH+TPEO 1 % no significant difference between them (p > 0.05) were found. The reduction of the counts, when compared with the uncoated CCH sample, was 1.89, 1.91 and 1.93 log cycles, respectively. The same behavior was observed at day 14. At the end of the analysis, day 21, no statistically differences (p > 0.05) were found between uncoated CCH sample and the coated CCH sample with chitosan. Again, the CCH samples coated with CH+TPEO 2 % showed the lowest (p < 0.05) counts for lactic acid bacteria followed by the coated CCH sample in which CH+TMEO 2 % was employed, with a reduction in counts, when compared with the uncoated CCH sample of 1.14 and 0.84 log cycles. In coated CCH samples in which CH+TMEO 1 % and CH+TP 1 % was used no significant differences (p > 0.05) between them were found, However, significant differences (p < 0.05) were observed between these samples and uncoated CCH sample.
The use of chitosan edible films or chitosan edible films added with essential oils in meat, meat product and RTE meat products helps to maintain product quality, improve product safety, and increase the shelf life. The purpose of incorporating antimicrobial compounds into an edible film instead of applying them directly onto the meat surface by spraying or dipping was to extend delivery of the antimicrobials during meat storage rather than delivering them in a single massive dose (Ouattara et al. 2000). The scientific literature describes how chitosan film added or not with essential oils improves the shelf-life of RTE meat products. Therefore, Giatrakou, et al. (2010) informed that a 5-day microbiological shelf-life extension was obtained for a poultry product (ready to cook chicken-pepper kebab) treated with either thyme oil (0.2 % v/w) or chitosan (1.5 % w/v). The chitosan films and chitosan-oregano EO films were applied on inoculated bologna samples and stored 5 days at 10 °C were analyzed by Zivanovic et al. (2005). They reported that pure chitosan films reduced Listeria monocytogenes by 2 log cycles, whereas the films with 1 % and 2 % oregano EO decreased the numbers of L. monocytogenes by 3.6 to 4 log cycles, respectively, and Escherichia coli by 3 log cycles. Siripatrawan and Noipha (2012) analyzed chitosan films incorporating green tea for improve the shelf life extension of pork sausages. These authors reported that incorporation of chitosan film with green tea enhanced the antimicrobial properties of the film, as total aerobic counts, yeasts, and molds in the sausages wrapped with chitosan-tea film were lower than those wrapped with chitosan film. Moradi et al. (2011) studied the effectiveness of chitosan films containing Z. multiflora Boiss essential oil (ZEO) and grape seed extract (GSE) on microbial (lactic acid bacteria, aerobic mesophiles and inoculated L. monocytogenes) characteristics of mortadella sausage. They informed that the growth of L. monocytogenes was significantly inhibited by ZEO-GSE containing films especially during storage of the sausages for 6 days. Aerobic mesophiles and lactic acid bacteria were the most sensitive and resistant groups to films by 0.1–1.1 and 0.1–0.7 log cycles reduction, respectively. Zinoviadou et al. (2009) used antimicrobial films that were prepared by incorporating different levels of oregano EO into whey protein isolate films. These authors observed that Total viable count and Pseudomonas were significantly reduced while the growth of lactic acid bacteria was completely inhibited.
The antibacterial activity of chitosan films incorporated with EOs can be attributed to the EOs. The antibacterial activity could be due to one sole component, such as carvacrol, camphor or thymol. However, it is a more widely held point of view that the action is due to a synergistic effect between various components, whether major or minor ones (Daferera et al. 2003). The EOs affect microbial cells by various antimicrobial mechanisms, including attacking the phospholipid bilayer of the cell membrane, disrupting enzyme systems, compromising the genetic material of bacteria, and forming fatty acid hydroperoxidase caused by oxygenation of unsaturated fatty acids (Burt et al. 2007; Arques et al. 2008).
Release of phenolic compounds from the film into cooked cured hams
Figure 5 shows the release of phenolic compounds from chitosan films formulated with TMEO and TPEO essential oils into cooked cured ham storage at 4 °C during 21 days. In the first 7 days of storage, a pronounced release of phenolic compounds from the film to the meat product shown in all samples analyzed. This release was higher in the CH films added with 2 % TMEO or TPEO (7.27 and 8.34 mg GAE/g film, respectively) than CH films added with 1 % TMEO or TPEO (0.87 and 1.46 mg GAE/g film, respectively). In this way, Contini (2013) informed that antioxidant components are released from the coating films into the meat as soon as the meat comes in contact with the films. From day 7 until the end of storage time (21 days) the release of phenolic compounds is more gradual, where all samples, except for CH+TPEO2%, had values comprised between 0.13 and 0.49 mg GAE/g film. For Cagri et al. (2004) the migration of bioactive compounds from edible film is dependent on many factors, including food composition, electrostatic interactions between the bioactive compounds and polymer chains, ionic osmosis and structural changes induced by the presence of antimicrobial and environmental conditions (pH, aw and storage temperature).
Fig. 5.
Release of phenolic compounds from chitosan films formulated with Thymus moroderi (TMEO) and Thymus piperella (TPEO) essential oils into cooked cured ham stored at 4 °C for 21 days
Correlations between different variables
To identify variables with a great correlation, an 8 × 8 matrix was constructed in which the TPC, lipid oxidation (MA), pH, aerobic mesophilic bacteria (AMB), lactic acid bacteria (LAB), lightness (L*), redness (a*) and yellowness (b*) were included (Fig. 6), where the matrix is depicted with squares for greater clarity. Each square has a different color, indicating the correlation value of the two associated variables. The red color indicates maximum positive correlation and the blue maximum negative correlation. This figure illustrates how AMB and LAB show a greater degree of positive correlation with the lipid oxidation with values of 0.93 and 0.94 respectively in the different samples assayed. Lipid oxidation also shows a considerable negative correlation with the other variables, such as TPC, pH and color coordinate redness with values of −0.45, −0.44 and −0.44. These results indicated that higher values of TPC, pH and redness lower values of lipid oxidation. As regards color coordinates (L*, a* and b*) there was a lower correlation between them with values close to zero.
Fig. 6.
Correlation matrix for different variables
Conclusions
The results of this study clearly demonstrated that addition of Thymus moroderi and Thymus piperella essential oil in active chitosan films used as coated in cooked cured ham improve their shelf life, due to a decreased in Aerobic Mesophilic Bacteria, Lactic Acid Bacteria and lipid oxidation compared to uncoated control samples. This increase in shelf life can be attributed to the protective effects of bioactive compounds release from chitosan films into cooked cured ham.
Acknowledgments
The authors would like to thank the project CYTED-IBEROFUN (Codec: 110 AC0386) and CajaMurcia for supporting the doctoral grant of one of the authors.
References
- Alves-Silva JM, Dias dos Santos SM, Pintado ME, Pérez-Álvarez JA, Fernández-López J, Viuda-Martos M. Chemical composition and in vitro antimicrobial, antifungal and antioxidant properties of essential oils obtained from some herbs widely used in Portugal. Food Control. 2013;32:371–378. doi: 10.1016/j.foodcont.2012.12.022. [DOI] [Google Scholar]
- AMSA . Meat color measurement guidelines. Champaign: American Meat Science Association; 2012. [Google Scholar]
- Arques JL, Rodriguez E, Nunez M, Medina M. Inactivation of Gram-negative pathogens in refrigerated milk by reuterin in combination with nisin or the lactoperoxidase system. Eur Food Res Technol. 2008;227(1):77–82. doi: 10.1007/s00217-007-0695-8. [DOI] [Google Scholar]
- Beverlya RL, Janes ME, Prinyawiwatkula W, No HK. Edible chitosan films on ready-to-eat roast beef for the control of Listeria monocytogenes. Food Microbiol. 2008;25:534–537. doi: 10.1016/j.fm.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Bravin B, Peressini D, Sensidoni A. Development and application of polysaccharide-lipid edible coating to extend shelf-life of dry bakery products. J Food Eng. 2006;76:280–290. doi: 10.1016/j.jfoodeng.2005.05.021. [DOI] [Google Scholar]
- Buege JA, Aust SD. Microsomal lipid peroxidation. Met Enzymol. 1978;52:302–304. doi: 10.1016/S0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- Burt SA, Der Zee RV, Koets AP, De Graaff AM, Van Knapen F, Gaastra W, et al. Carvacrol induces heat shock protein 60 and inhibits synthesis of flagellin in Escherichia coli O157:H7. Appl Environ Microbiol. 2007;73:4484–4490. doi: 10.1128/AEM.00340-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagri A, Ustunol Z, Ryser ET. Antimicrobial edible films and coatings. J Food Prot. 2004;67:833–848. doi: 10.4315/0362-028x-67.4.833. [DOI] [PubMed] [Google Scholar]
- Cassens RG, Demeyer D, Eilelemboom G, Honikel KO, Johansson G, Nielsen T et al (1995) Recommendations of reference methods for assessment of meat color. In proceesing 41st International Congress of Meat Science and Technology (pp. 86:410–86:411). San Antonio, Texas. USA
- Cofrades S, Serrano A, Ayo J, Solas MT, Carballo J, Jiménez-Colmenero F. Restructured beef with different proportions of walnut as affected by meat particle size. Eur Food Res Technol. 2004;218:230–236. doi: 10.1007/s00217-003-0808-y. [DOI] [Google Scholar]
- Contini C (2013) Characterisation of citrus extract-based active packaging to reduce lipid oxidation in cooked meats. (PhD Thesis). Ireland: School of Agriculture and Food Science, University College Dublin
- Daferera DJ, Ziogas BN, Polissiou MG. The effectiveness of plant essential oils on the growth of Botrytis cinerea, Fusarium sp. and Clavibacter michiganensis subsp. Michiganensis. Crop Prot. 2003;22:39–44. doi: 10.1016/S0261-2194(02)00095-9. [DOI] [Google Scholar]
- Dorman HJD, Peltoketo A, Hiltunen R, Tikkanen MJ. Characterization of the antioxidant properties of de-odourised aqueous extracts from selected Lamiaceae herbs. Food Chem. 2003;83:255–262. doi: 10.1016/S0308-8146(03)00088-8. [DOI] [Google Scholar]
- Dutta PK, Tripathi S, Mehrotra GK, Dutta J. Perspectives for chitosan based antimicrobial films in food applications. Food Chem. 2009;114:1173–1182. doi: 10.1016/j.foodchem.2008.11.047. [DOI] [Google Scholar]
- Emiroğlu ZK, Yemiş GP, Coşkun BK, Candoğan K. Antimicrobial activity of soy edible films incorporated with thyme and oregano essential oils on fresh ground beef patties. Meat Sci. 2010;86:283–288. doi: 10.1016/j.meatsci.2010.04.016. [DOI] [PubMed] [Google Scholar]
- Falguera V, Quintero JP, Jiménez A, Muñoz JA, Ibarz A. Edible films and coatings: structures, active functions and trends in their use. Trends Food Sci Technol. 2011;22:292–303. doi: 10.1016/j.tifs.2011.02.004. [DOI] [Google Scholar]
- Fernández-López J, Pérez-Alvarez JA, Aranda-Catalá V. Effect of mincing degree on color properties in pork meat. Res Appl. 2000;25:376–380. [Google Scholar]
- Fernández-López J, Sayas-Barberá ME, Navarro C, Sendra E, Pérez-Álvarez JA. Antioxidant and antibacterial activities of natural extracts: application on cooked meat balls. Meat Sci. 2005;69:371–380. doi: 10.1016/j.meatsci.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Fernández-López J, Sayas-Barberá ME, Sendra E, Pérez-Alvarez JA. Shelf life of ostrich (Struthio camelus) liver stores under different packaging conditions. J Food Prot. 2006;69(8):1920–1927. doi: 10.4315/0362-028x-69.8.1920. [DOI] [PubMed] [Google Scholar]
- Georgantelis D, Blekas G, Katikou P, Ambrosiadis I, Fletouris DJ. Effect of rosemary extract, chitosan and α-tocopherol on lipid oxidation and color stability during frozen storage of beef burgers. Meat Sci. 2007;75:256–264. doi: 10.1016/j.meatsci.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Giatrakou V, Ntzimani A, Zwietering M, Savvaidis IN. Combined chitosanthyme treatments with modified atmosphere packaging on a Greek Ready-to-Cook (RTC) poultry product. J Food Prot. 2010;73:663–669. doi: 10.4315/0362-028x-73.4.663. [DOI] [PubMed] [Google Scholar]
- Gil-Díaz M, Santos-Delgado MJ, Rubio-Barroso S, Polo-Díez LM. Free D-amino acids determination in ready-to-eat cooked ham irradiated with electron-beam by indirect chiral HPLC. Meat Sci. 2009;82:24–29. doi: 10.1016/j.meatsci.2008.11.019. [DOI] [PubMed] [Google Scholar]
- Juck G, Neetoo H, Chen H. Application of an active alginate coating to control the growth of Listeria monocytogenes on poached and deli turkey products. Int J Food Microbiol. 2010;142:302–308. doi: 10.1016/j.ijfoodmicro.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Kanatt SR, Chander R, Sharma A. Chitosan and mint mixture: a new preservative for meat and meat products. Food Chem. 2008;107:845–852. doi: 10.1016/j.foodchem.2007.08.088. [DOI] [Google Scholar]
- Moradi M, Tajik H, Rohani SMR, Oromiehie AR. Effectiveness of Zataria multiflora Boiss essential oil and grape seed extract impregnated chitosan film on ready-to-eat mortadella-type sausages during refrigerated storage. J Sci Food Agric. 2011;91:2850–2857. doi: 10.1002/jsfa.4531. [DOI] [PubMed] [Google Scholar]
- Ojagh SM, Rezaei M, Razavi SH, Hosseini SMH. Development and evaluation of a novel biodegradable film made from chitosan and cinnamon essential oil with low affinity toward water. Food Chem. 2010;122:161–166. doi: 10.1016/j.foodchem.2010.02.033. [DOI] [Google Scholar]
- Ouattara B, Ronald E, Simard RE, Piette G, Begin A, Holley RA. Inhibition of surface spoilage bacteria in processed meats by application of antimicrobial films prepared with chitosan. Int J Food Microbiol. 2000;62:139–148. doi: 10.1016/S0168-1605(00)00407-4. [DOI] [PubMed] [Google Scholar]
- Ruiz-Navajas Y, Viuda-Martos M, Sendra E, Pérez-Álvarez JA, Fernández-López J. Chemical characterization and antibacterial activity of Thymus moroderi and Thymus piperella essential oils, two Thymus endemic species from southeast of Spain. Food Control. 2012;27:294–299. doi: 10.1016/j.foodcont.2012.04.005. [DOI] [Google Scholar]
- Ruiz-Navajas Y, Viuda-Martos M, Sendra E, Perez-Alvarez JA, Fernández-López J. In Vitro antioxidant and antifungal properties of essential oils obtained from aromatic herbs endemic to the southeast of Spain. J Food Prot. 2013;76:1218–1225. doi: 10.4315/0362-028X.JFP-12-554. [DOI] [PubMed] [Google Scholar]
- Sánchez-González L, González-Martínez C, Chiralt A, Cháfer M. Physical and antimicrobial properties of chitosan-tea tree essential oil composite films. J Food Eng. 2010;98:443–452. doi: 10.1016/j.jfoodeng.2010.01.026. [DOI] [Google Scholar]
- Sayas-Barberá E, Quesada J, Sánchez-Zapata E, Viuda-Martos M, Fernández-López J, Pérez-Alvarez JA, Sendra E. Effect of the molecular weight and concentration of chitosan in pork model burgers. Meat Sci. 2011;88:740–749. doi: 10.1016/j.meatsci.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Seydim AC, Sarikus G. Antimicrobial activity of whey protein based edible films incorporated with oregano, rosemary and garlic essential oils. Food Res Int. 2006;39:639–644. doi: 10.1016/j.foodres.2006.01.013. [DOI] [Google Scholar]
- Shahidi F, Arachchi JKV, Jeon YJ. Food applications of chitin and chitosans. Trends Food Sci Technol. 1999;10:37–51. doi: 10.1016/S0924-2244(99)00017-5. [DOI] [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- Siripatrawan U, Noipha S. Active film from chitosan incorporating green tea extract for shelf-life extension of pork sausages. Food Hydrocol. 2012;27:102–108. doi: 10.1016/j.foodhyd.2011.08.011. [DOI] [Google Scholar]
- Suman SP, Mancini RA, Joseph P, Ramanathan R, Konda MKR, Dady G, et al. Packaging-specific influence of chitosan on color stability and lipid oxidation in refrigerated ground beef. Meat Sci. 2010;86:994–998. doi: 10.1016/j.meatsci.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Tharanathan RN, Kittur F. Chitin e the undisputed biomolecule of great potential. Crit Rev Food Sci Nutr. 2003;43(1):61–87. doi: 10.1080/10408690390826455. [DOI] [PubMed] [Google Scholar]
- Viuda-Martos M, El Gendy NGS, Sendra E, Fernández-López J, El-Razik KAA, El-Sayed A, Perez-Alvarez JA. Chemical composition and antioxidant and anti-listeria activities of essential oils obtained from some Egyptian plants. J Agric Food Chem. 2010;58:9063–9070. doi: 10.1021/jf101620c. [DOI] [PubMed] [Google Scholar]
- Viuda-Martos M, Ruiz-Navajas Y, Fernández-López J, Pérez-Álvarez JA. Effect of added citrus fibre and spice essential oils on quality characteristics and shelf-life of mortadella. Meat Sci. 2010;85:568–576. doi: 10.1016/j.meatsci.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Wood SN. Generalized additive models: an introduction with R. Boca Raton: Chapman and Hall/CRC; 2006. [Google Scholar]
- Yanishlieva NV, Marinova E, Pokorny J. Natural antioxidants from herbs and spices. Eur J Lipid Sci Technol. 2006;108:776–793. doi: 10.1002/ejlt.200600127. [DOI] [Google Scholar]
- Zinoviadou KG, Koutsoumanis KP, Biliaderis CG. Physico-chemical properties of whey protein isolate films containing oregano oil and their antimicrobial action against spoilage flora of fresh beef. Meat Sci. 2009;82:338–345. doi: 10.1016/j.meatsci.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Zivanovic S, Chi S, Draughon AF. Antimicrobial activity of chitosan films enriched with essential oils. J Food Sci. 2005;70:45–51. doi: 10.1111/j.1365-2621.2005.tb09045.x. [DOI] [Google Scholar]
- Zouari N, Fakhfakh N, Zouari S, Bougatef A, Karray A, Neffati M, Ayadi MA. Chemical composition, angiotensin I-converting enzyme inhibitory, antioxidant and antimicrobial activities of essential oil of Tunisian Thymus algeriensis Boiss. et Reut. (Lamiaceae) Food Bioprod Process. 2011;89:257–265. doi: 10.1016/j.fbp.2010.11.006. [DOI] [Google Scholar]