Abstract
This study aimed at improving the mechanical properties and water solubility of peanut protein isolate (PPI) films by glycosylating with xylose (X). The modification process of glycosylation was optimized by using response surface methodology (RSM). The effects of pH, temperature and time on degrees of glycosylation (DG), tensile strength (TS), elongation (E), solubility and microstructure of xylose glycosylated PPI films (PPI-XF) were determined. The changes of DG in different conditions indicated that crosslinking should occur between PPI and xylose during the modification. Optimum glycosylation conditions were found to be pH 9.5, 91.5 °C and 95 min. Under these conditions, TS and E values of PPI-XF were 10.37 MPa and 96.47 %, respectively. Due to glycosylation, solubility of PPI-XF decreased from 96.64 to 35.94 % and these films remained intact in water for 24 h. The microstructure of PPI-XF was denser and more compact than the unmodified PPI films. These results suggest that the xylose glycosylated PPI films have potentiality of being used as biodegradable films in food packaging application.
Keywords: Peanut protein isolate, Xylose, Glycosylation, Biodegradable film, Properties, RSM
Introduction
There is an increasing interest in biodegradable materials produced from natural biopolymers in an attempt to replace non-biodegradable plastics (Vilaseca et al. 2007). Biopolymers such as proteins, polysaccharides, lipids and their combinations have been widely investigated and used to develop biodegradable films (Ma et al. 2013; Tang et al. 2009). Protein-based biodegradable films have attracted more attention due to their superior barrier and mechanical properties which come from strong intermolecular cross-linking of protein. The film forming behavior and film characteristics of biodegradable films prepared from soy protein (Denavi et al. 2009), pea protein (Kowalczyk and Baraniak 2011), peanut protein (Reddy et al. 2013) have been studied and reported.
Peanut meal is a by-product of peanut oil industry and it contains about 45 % proteins. However, it is still primarily used as a feed ingredient for animals. In previously research, peanut meal was used to produce biodegradable films (Reddy et al. 2013). However, due to their hydrophilic nature, the strength, elongation and water barrier properties of protein based films are poor compared to those of the polyolefin based synthetic films. Because of this reason, the commercial application of peanut protein film has not taken place in food applications. Therefore, the modification of functionality of proteins is essential to improve the properties of peanut protein films.
Physicochemical modification of proteins and/or addition of saccharides have been successfully employed to improve the mechanical properties of protein films (Al-Hassan and Zorziah 2012; Arabestani et al. 2013). Saccharides with small molecular weight are usually considered to be good plasticizers to improve the mechanical properties of the protein films, particularly the elongation. Soininen et al. (2013) found that a mixture of fructose and glucose in fructose-to-glucose ratio of 1.67:1 improved the appearance, mechanical properties and water barrier properties of the protein films. On the other hand, Maillard reaction occurred between proteins and saccharides were found to be beneficial for the improvement of mechanical properties and water resistance of protein films (Su et al. 2010). Glycosylation of peanut protein and soy protein by respectively grafting with arabic gum (Li et al. 2014) and glucomanna (Zhang et al. 2014) which were used to improve the properties of protein film have been reported.
Despite the application of various carbohydrates to improve the properties of protein based films, the application of xylose is not reported. As xylose is 5-carbon sugar which can be abundantly produced from natural wood, the use of this sugar in peanut protein films for improving the film properties is of practical significance. Our exploratory work has shown that the application of xylose in peanut protein films not only increases the strength and elongation of peanut protein films, but also decreases their solubility in water quite remarkably.
Hence, the objectives of this study were to investigate the glycosylation effect of xylose in peanut protein with the aim of improving the mechanical properties of biodegradable peanut protein films. In order to analyze the changes of interactions between PPI and xylose and to identify the effect of glycosylation modification on the properties of the modified protein films, degrees of glycosylation (DG) of PPI under different modification conditions were measured. And the effects of this modification on the tensile strength and elongation, solubility in water and microstructure of peanut protein films were evaluated. The findings can broaden the application of biodegradable peanut protein films in food packaging industry.
Materials and methods
Materials and chemicals
Peanut meal was obtained from Gaotang Lanshan Co., Ltd. (Shandong Province, China). It contained 47.81 % peanut proteins. All other reagents used in the experiments such as xylose, glycerol, NaOH and NaBr were of analytical grade and were used as received.
Glycosylation of peanut protein and preparation of protein films
Preparation of peanut protein isolate (PPI)
Peanut protein isolate (PPI) was prepared from the peanut meal by using the alkaline extraction and acid precipitation method described by He et al. (2014) with slight modification. The obtained PPI contained 90.93 % of protein, 4.04 % of carbohydrate, 0.72 % of crude fat and 3.86 % of ash.
Optimization of glycosylation of peanut protein using xylose
A 5 % (w/w) PPI solution was prepared by dispersing PPI powders in distilled water. And the initial pH of this PPI solution was 6.86. Xylose was added to the PPI solution to obtain the protein to xylose ratio of 10:1, because poor mechanical properties and solubility were found to be attributed to a lower or higher ratio of protein to xylose according to our exploratory work. The pH values of the mixed solutions were adjusted to 3.00 to 11.00 using 1 N HCl and 1 N NaOH (Jangchud and Chinnan 1999; Kowalczyk and Baraniak 2011; Popović et al. 2011). These pH adjusted solutions were heated to 30–90 °C for a certain time (30–180 min) to obtain the xylose glycosylated protein (PPI-X) solutions.
Response surface methodology (RSM) was used to optimize the pH, temperature and time. The effects of three independent variables X1 (pH), X2 (temperature) and X3 (time) at five levels on the mechanical properties including the tensile strength (TS) and elongation at break (E) of the glycosylated peanut protein films were investigated using central composite design (CCD). The data were analyzed by multiple regressions to fit the following quadratic polynomial model.
| 1 |
Yi represents the response functions (TS and E), βk0 is an intercept, and βki, βkii, βkij are the coefficients of the linear, quadratic and interactive terms, respectively. Xi and Xj represent the coded independent variables (pH, temperature and time).
Preparation of the films
Films of PPI-X (PPI-XF) were prepared by casting. Glycerol was added as a plasticizer at protein: glycerol ratio of 4:1 to the PPI-X solutions mentioned in the preceding section. All solutions were degased by filtrating through nylon filters and 15 mL solutions were poured onto nonstick plates (15 cm diameter) to cast into films. The films (PPI-XF) were prepared by drying off the water at 60 °C for 1 h and were peeled off after cooling. These films were conditioned at 58 % RH (25 °C) for 48 h by placing them in a desiccator containing saturated NaBr solution.
PPI films (PPI-F) were prepared as followed: A 5 % (w/w) PPI solution was prepared by dispersing PPI powders in distilled water. The pH of the film forming solution was adjusted to 9.5 and heated at 91.5 °C for 95 min. The glycerol was added to this protein solution. This modified solution was filtered, cast and dried to produced films. These films were also conditioned in the same condition as mentioned above.
Determining the properties of peanut protein films
Degrees of glycosylation (DG)
Degrees of glycosylation (DG) of PPI under different modification conditions were determined by employing the method based on a spectrophotometric assay using OPA (o-phthalaldehyde) as described by Zhang and Chi (2011).
Mechanical properties
Tensile strength (TS) and elongation at break (E) of films were measured on a TA-TX2i texture analyzer (Stable Micro System Ltd., Godalming, UK) following an ASTM standard method D882-01 (ASTM 2001). Each film strip (1.0 × 7.0 cm) was mounted between the grips of the texture analyzer and initial grip separation and cross-head speed were set at 50 mm and 0.5 mm/s, respectively. TS (MPa) was calculated by dividing the maximum force by the cross-sectional area of the film (width × thickness). E was calculated as the percentage of change in the length of the specimen compared to the original length of the film strip. TS and E measurements were repeated for each type of films at least five times and the average values were reported.
Moisture content
Film samples (2 × 2 cm) were weight and dried in an air-circulating oven at 105 °C for 24 h. The moisture content (MC) values were determined by using the mass loss data of film during drying.
Solubility of film in water
The solubility of films in water was expressed in terms of total soluble matter (TSM), which was calculated as the ratio of soluble dry matter to the initial dry matter of the film. Film specimens (2 × 2 cm) were placed into test tubes containing 10 ml of distilled water. These test tubes were capped and stored at room temperature for 24 h with occasional gentle shaking. After allowing the water soluble content to dissolve in water for 24 h, the content of the tube was centrifuged at 3000 rpm for 10 min. The remaining portion of the film was gently rinsed with distilled water and dried in an air-circulating oven at 105 °C for 24 h. The mass of TSM was calculated by subtracting the mass of insoluble solid matter from the initial dry mass of the film. Initial dry mass and TSM were not determined on the same film specimen in order to avoid heating the film sample before the solubility tests which would increase the water resistance of protein films (Rhim et al. 1998, 2002). The initial dry mass values of the films were obtained from MC measurements for different replicates of the same film sample.
Water vapor permeability
Water vapor permeability of the films was measured using the ASTM method (ASTM 1995). The sample film was cut into a circle of 7 cm diameter and placed on a circular cup (3 cm in diameter and 5 cm in depth) with 3 g of CaCl2 in each cup. Sealed cups were placed in a desiccator in which the RH was maintained at 100 % and temperature kept at 25 °C. Then, the cups were weighed every 12 h up to a week. The WVP of films was measured from the weight gain of the cups. The WVP (g mm/m2 h kPa) was calculated as: WVP = (WVTR /Δp)×d, where WVTR was the measured water vapor transmission rate (g/m2 h) through the film specimen; d was the mean film thickness (mm); Δp was the partial water vapor pressure difference (kPa) across the two sides of the film specimen.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)
PPI and PPI-X films were analyzed by SDS-PAGE to examine the molecular weight distribution according to Feng et al. (2014). The discontinuous system consisted of 5 % (w/v) acrylamide stacking gel and 13 % (w/v) acrylamide separating gel. About 5 mg of film samples were suspended in 0.5 mL buffer before boiling for 5 min. 4.0 μL of supernatant was loaded into each well. Finally the gels were stained with 0.1 % (w/v) coomassie brilliant blue for 30 min and destained in acetic acid solution (fermentation alcohol:acetic acid:water ratio = 2:1:17) for 12 h.
Observation of microstructure
Film microstructure was observed by using Hitachi S570 scanning electron microscope. Film samples (5 × 5 mm) were mounted on the stub with double-sided adhesive tape at an angle of 90° to the surface and coated with a thin layer of gold. Film cross-section was observed at a magnification of × 3000 and using an accelerating voltage of 12 kV.
Statistical analysis
The data were analyzed with SAS software (SAS Institute Inc., Cary, NC, USA). Turkey’s multiple comparison method was used to determine the significant difference between mean values at 95 % confidence level (p < 0.05).
Results and discussion
Effect of pH on DG and mechanical properties of PPI-XF
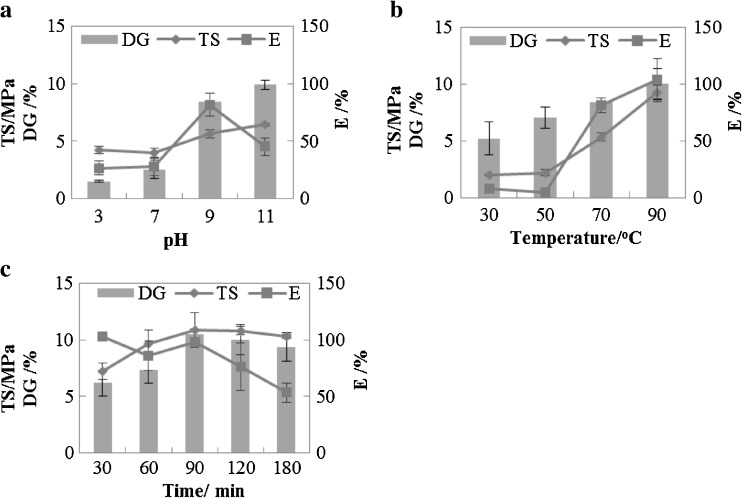
Effect of pH on degrees of glycosylation (DG) and mechanical properties of PPI-XF is shown in Fig. 1a. Films were not formed around pH 5.0 due to the fact that the isoelectric point (IEP) of peanut protein is about 5.0. Similar result was reported earlier in the case of pumpkin protein isolate that the formation of film did not take place near its IEP (Popović et al. 2012). Figure 1a shows that the DG were sharply increased from 1.49 to 9.89 % with increasing pH from 3.0 to 11.0, which indicated that glycosylation between free amino groups and xylose has occurred as pH increased gradually (Lertittikul et al. 2007). On the other hand, tensile strength of PPI-XF increased from 4.20 to 6.42 MPa with the increase of pH when the glycosylation was carried out at 70 °C for 120 min. At alkaline pH TS of PPI-XF was significantly higher than at acidic pH. The elongation of the films also increased with the increase of pH from 3.00 to 9.00, and the highest value (81.50 %) was observed at pH value 9.00. However, when the pH was further increased from 9.00 to 11.00, the elongation decreased quite rapidly. Researchers (Jangchud and Chinnan 1999) have reported that mechanical properties of peanut protein films were improved when the pH was varied from 6.0 to 9.0 as observed in this study. The increase of tensile strength with the increase in pH can be attributed to the increased exposure of sulfhydryl and hydrophobic groups in alkaline condition and the reformation of such groups during drying. In addition, much greater extent of new bonds is formed between protein and carbohydrate due to Maillard reaction at alkaline pH (Lertittikul et al. 2007; Wang et al. 2013). These new bonds increase or enhance the mechanical properties (Su et al. 2010). However, at very high pH values (>9.00) excessive intermolecular cross-linking occurs and this restricts the flexibility and movement of the protein molecules which leads to a decrease in elongation (Reddy et al. 2012). Thus, pH 9.0 was considered to be the optimal pH value in this study.
Fig. 1.
Effects of (a) pH, (b) modification temperature, (c) modification time on DG, TS and E of xylose glycosylated peanut protein isolate films. DG represents degrees of glycosylation, while TS and E represent tensile strength and elongation at break, respectively
Effect of modification temperature on mechanical properties of PPI-XF
When the temperature varied from 30 to 90 °C, DG increased significantly (p < 0.05) from 5.19 to 9.99 %, while TS increased from 1.95 to 9.30 MPa and E from 7.87 to 103.67 % (Fig. 1b). It has been suggested that heat-denaturing would disrupt hydrogen bonds and hydrophobic groups of protein molecules, which exposes the amino acid groups to the solvent and produces a more open structure (Wihodo and Moraru 2013), enhancing the possibility of crosslinking between PPI and xylose through Maillard reaction. As protein unfolds after heat-denaturing, the covalent disulfide bonding of the denatured protein produces stronger films and enables the films to withstand higher deformations (Perez-Gago and Krochta 2001). Cao et al. (2007) observed that heating induced protein-protein cross-linking by denaturing (unfolding) the protein structure and exposing the sulfhydryl and hydrophobic groups. Consequently, denaturation of protein improved the mechanical properties of SPI/gelatin composite films. Studies also showed that heating improved the tensile strength and elongation of protein films made of whey protein-mesquite gum (Osés et al. 2009), pumpkin protein (Popović et al. 2011) and soy protein (Jiang et al. 2012). Our preliminary trials showed that the xylose glycosylated PPI tended to form large aggregates when heated above 90 °C at alkaline pH and no films could be formed. Similar results were reported in the case of soy protein isolate (Jiang et al. 2012). Therefore, 90 °C was chosen as the optimum temperature for glycosylating PPI with xylose and no testing was conducted at higher than this temperature.
Effect of modification time on mechanical properties of PPI-XF
As shown in Fig. 1c, DG exhibited an increasing tendency with increasing modification time ranged from 30 to 90 min. As the time extended, no significant increase (p > 0.05) in DG was observed. According to Li et al., as the glycosylation continued, the DG of PPI-saccharides conjugates increased gradually, but reached an equilibrium level after a certain time (Li et al. 2014). At the same time, the tensile strength of PPI-XF was increased by 51.88 % when the modification time was increased from 30 to 90 min. No significant increase (p > 0.05) in tensile strength was observed when the modification time was >90 min. It has been pointed out that interactions between saccharides and proteins can be increased when the modification time is increased (Lertittikul et al. 2007; Shih 1994). On the other hand, a longer modification time negatively affected the elongation of PPI-XF as a sharp decrease of elongation from 97.99 to 53.04 % occurred when the time was increased from 90 to 180 min. Excessive interactions between peanut proteins and xylose restricted the mobility of the protein molecules and resulted into decrease in elongation. Based on these results, a 90 min long modification time was used in this work.
Fitting of the response surface polynomial models
The modification process parameters and mechanical properties (TS and E) of PPI-XF obtained according to the central composite design are shown in Table 1. The results were fitted to a second order polynomial equation. The regression equations obtained for the TS (Y1) and E (Y2) of the PPI-XF were found to be as follows:
| 2 |
| 3 |
Table 1.
Central composite design for pH, temperature and time and the corresponding experimental tensile strength (TS) and elongation (E) values
| No. | X1 /pH | X 2 / (°C) | X3 / (min) | TS/MPa | E/% |
|---|---|---|---|---|---|
| 1 | 1 (10) | 1 (95) | 1 (105) | 10.31 | 74.89 |
| 2 | 1 (10) | 1 (95) | −1 (75) | 9.10 | 92.90 |
| 3 | 1 (10) | −1 (85) | 1 (105) | 9.68 | 67.18 |
| 4 | 1 (10) | −1 (85) | −1 (75) | 8.83 | 74.02 |
| 5 | −1 (8) | 1 (95) | 1 (105) | 9.54 | 32.37 |
| 6 | −1 (8) | 1 (95) | −1 (75) | 9.46 | 47.58 |
| 7 | −1 (8) | −1 (85) | 1 (105) | 9.37 | 29.00 |
| 8 | −1 (8) | −1 (85) | −1 (75) | 7.60 | 45.30 |
| 9 | −1.6818 (7.32) | 0 (90) | 0 (90) | 7.76 | 35.24 |
| 10 | 1.6818 (10.68) | 0 (90) | 0 (90) | 8.97 | 90.36 |
| 11 | 0 (9) | −1.6818 (81.59) | 0 (90) | 7.34 | 35.97 |
| 12 | 0 (9) | 1.6818 (98.41) | 0 (90) | 9.29 | 66.22 |
| 13 | 0 (9) | 0 (90) | −1.6818 (64.77) | 8.62 | 100.18 |
| 14 | 0 (9) | 0 (90) | 1.6818 (115.23) | 9.68 | 62.38 |
| 15 | 0 (9) | 0 (90) | 0 (90) | 10.02 | 90.53 |
| 16 | 0 (9) | 0 (90) | 0 (90) | 10.05 | 91.85 |
| 17 | 0 (9) | 0 (90) | 0 (90) | 9.05 | 104.39 |
| 18 | 0 (9) | 0 (90) | 0 (90) | 9.48 | 96.46 |
| 19 | 0 (9) | 0 (90) | 0 (90) | 9.45 | 95.81 |
| 20 | 0 (9) | 0 (90) | 0 (90) | 10.11 | 91.25 |
| 21 | 0 (9) | 0 (90) | 0 (90) | 10.81 | 85.90 |
| 22 | 0 (9) | 0 (90) | 0 (90) | 10.00 | 87.22 |
| 23 | 0 (9) | 0 (90) | 0 (90) | 9.50 | 88.55 |
The statistical significance of the regression model was checked by F-test and p-value, and the analysis of variance (ANOVA) for TS and E of PPI-XF are presented in Table 2. The p-values were used to determine the significance of each coefficient. When the p-value becomes smaller, the corresponding coefficient became more significant or important (Hong et al. 2013). It could be seen in Table 2, p-values of the polynomial models applied to TS and E were 0.0291 and <0.0001, respectively, indicating that these models suitably represented both dependent variables. The lack of fit test was a measure of failure of the models to represent data in the experimental domain at points which were not included in the regression (Zhong and Wang 2010). No significant lack of fit was found as the p-values for TS and E were 0.1862 and 0.1943, respectively, which indicated that the response surface models were adequate for predicting the TS and E of PPI-XF at the suggested combination of variables.
Table 2.
Analysis of variance of the fitted quadratic polynomial model for tensile strength (TS) and elongation (E) in the case of xylose glycosylated PPI film (PPI-XF)
| Terms | Source | SS | DF | MS | F-value | p-value |
|---|---|---|---|---|---|---|
| TS | X1 | 1.1573 | 1 | 1.1573 | 3.3199 | 0.0915 |
| X 2 | 2.8177 | 1 | 2.8177 | 8.0829 | 0.0138 | |
| X3 | 2.3604 | 1 | 2.3604 | 6.7712 | 0.0219 | |
| X1X1 | 2.201 | 1 | 2.201 | 6.3139 | 0.026 | |
| X2X2 | 2.4295 | 1 | 2.4295 | 6.9694 | 0.0204 | |
| X3X3 | 0.1448 | 1 | 0.1448 | 0.4155 | 0.5304 | |
| X1X2 | 0.1617 | 1 | 0.1617 | 0.464 | 0.5077 | |
| X1X3 | 0.0055 | 1 | 0.0055 | 0.0158 | 0.9018 | |
| X2X3 | 0.2149 | 1 | 0.2149 | 0.6165 | 0.4464 | |
| Model | 11.4458 | 9 | 1.2718 | 3.64817 | 0.0291 | |
| Residual | 4.5318 | 13 | 0.3486 | |||
| Lack of Fit | 2.3867 | 5 | 0.4773 | 1.78016 | 0.1862 | |
| Pure error | 2.1451 | 8 | 0.2681 | |||
| Cor Total | 15.9776 | 22 | ||||
| E | X1 | 4483.049 | 1 | 4483.049 | 106.9502 | <0.0001 |
| X 2 | 505.7772 | 1 | 505.7772 | 12.0661 | 0.0041 | |
| X3 | 1053.249 | 1 | 1053.249 | 25.1269 | 0.0002 | |
| X1X1 | 2144.747 | 1 | 2144.747 | 51.1663 | <0.0001 | |
| X2X2 | 3945.497 | 1 | 3945.497 | 94.1261 | <0.0001 | |
| X3X3 | 410.8074 | 1 | 410.8074 | 9.8005 | 0.008 | |
| X1X2 | 54.8097 | 1 | 54.8097 | 1.3076 | 0.2735 | |
| X1X3 | 5.5414 | 1 | 5.5414 | 0.1322 | 0.722 | |
| X2X3 | 12.6669 | 1 | 12.6669 | 0.3022 | 0.5918 | |
| Model | 12546.43 | 9 | 12546.43 | 33.25721 | <0.0001 | |
| Residual | 544.9231 | 13 | 544.9231 | |||
| Lack of Fit | 284.0643 | 5 | 284.0643 | 1.74233 | 0.1943 | |
| Pure error | 260.8588 | 8 | 260.8588 | |||
| Cor Total | 13091.35 | 22 |
Significance of each coefficient was also determined and is presented in Table 2. In the case of TS, two linear coefficients (X2, X3) and two quadratic term coefficients (X12, X22) were found to be significant with p-values being lower than 0.05. The coefficients of other terms (X1, X32, X1X2, X1X3, X2X3) were not found to be significant (p > 0.05). In the case of E, the linear coefficients (X1, X2, X3) and quadratic term coefficients (X12, X22, X32) were found to be significant with p-values being lower than 0.05. The coefficients of the interaction terms (X1X2, X1X3, X2X3) were not found to be significant (p > 0.05).
Optimization of the process parameters
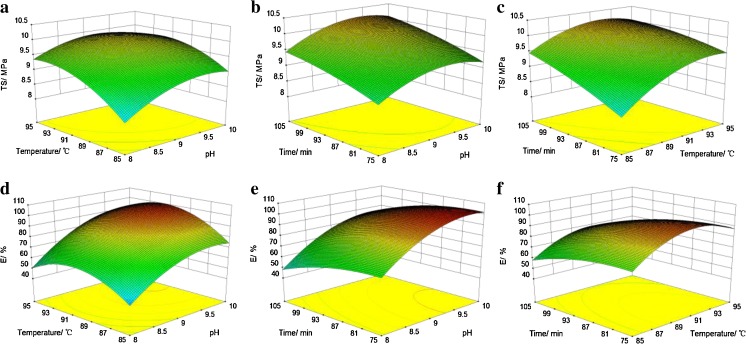
The 3-D response surface plots (Fig. 2) and contour plots (Fig. 3) were used to illustrate the effects of pH, modification temperature and modification time on the tensile strength (Figs. 2 and 3a–c) and elongation (Figs. 2 and 3d–f) in the case of PPI-XF. In the response surface plots and contour plots, TS and E were plotted together with two continuous variables, while the third variable was kept at level zero.
Fig. 2.
Response surfaces showing the effect of pH, modification temperature and modification time on tensile strength (a–c) and elongation (d–f) of peanut protein isolate films glycosylated with xylose (PPI-XF)
Fig. 3.
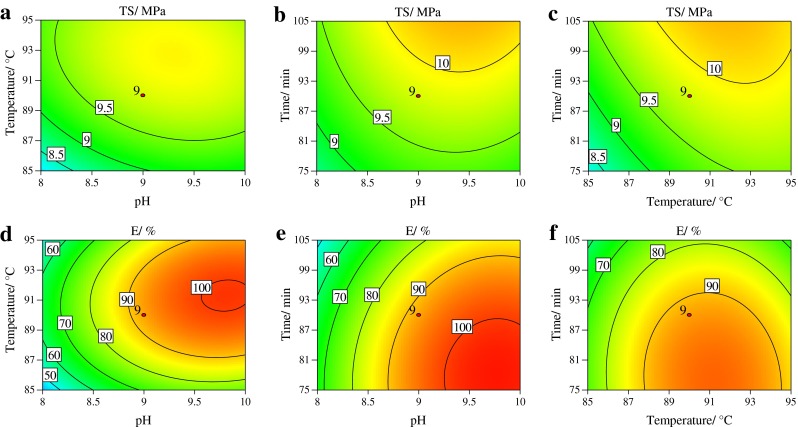
Contour plots showing the effect of pH, modification temperature and modification time on tensile strength (a–c) and elongation (d–f) of peanut protein isolate films glycosylated with xylose (PPI-XF)
The results showed that modification temperature and time significantly affected the TS of the films. It can be observed that the TS of films increased with the increase in the modification time. Among the three process parameters (pH, temperature, time) studied, the modification temperature was the most significant factor affecting the strength of PPI-XF, followed by modification pH and time as indicated by the slopes of the 3-D response surface plots. However, no significant effect of interaction among these 3 parameters was observed on the TS of the films. From the ANOVA and surface plots it can be shown that the best modification process parameters for TS in the case of PPI-XF the modification pH, temperature and time were found to be 9.0, 95 °C and 115 min, respectively.
The modification time negatively affected the elongation of the films. The modification pH, time and temperature affected the film elongation in decreasing order. The interaction terms of these parameters did not affect the elongation significantly (p > 0.05). The optimal modification pH, temperature and time for film elongation were 10.0, 90 °C and 75 min, respectively.
Simultaneous optimization for the modification of peanut protein isolate with xylose was performed to obtain maximum TS and E of the films, and the optimal modification conditions were pH 9.5, modification temperature 91.5 °C and modification time 95 min. Under these conditions, predicted TS and E of the films were 10.11 MPa and 95.63 % respectively.
Adequacy of developed mathematical models
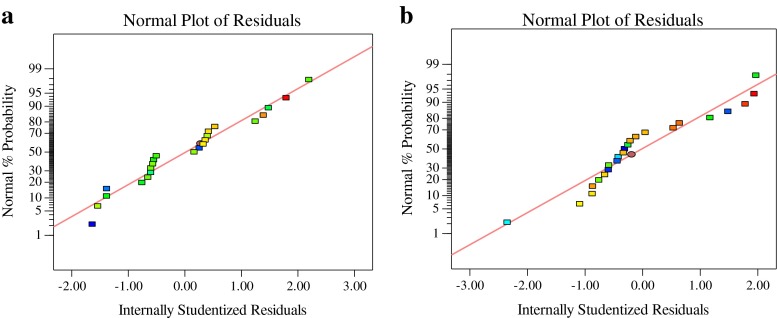
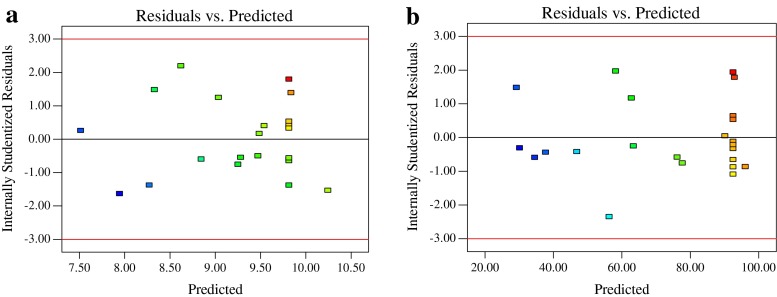
Normal probability plot and plot of residuals versus the predicted response were used to evaluate the adequacy of developed mathematical models. The residual plots indicated a normal distribution as they lay approximated along a straight line and there were no apparent problems with normality in all cases (Fig. 4a–b). As shown in the plot of residuals versus the predicted response (Fig. 5a–b), the residuals scattered randomly on the display, suggesting that the variance of the original observation was constant for all values of response variables. Both of the plots (Figs. 4 and 5) are satisfactory, and this result can confirm the adequacy of the developed models.
Fig. 4.
Normal probability of internally studentized residuals of the response variables. a Tensile strength; b Elongation
Fig. 5.
Plots of internally studentized residuals vs. predicted response variables. a Tensile strength; b Elongation
Validation of response surface models
Experiments were performed to validate the adequacy of the response surface based model in predicting the optimum condition for glycosylation. TS and E of PPI-XF were 10.37 MPa and 96.47 % respectively under the optimized condition (Table 3). No significant differences (p > 0.05) between predicted TS and E values and the experimental values were found indicating that the response surface based optimization can be confidently used to predict the optimum glycosylation condition to produce PPI-XF films.
Table 3.
Predicted and experimental values of the responses at optimum conditions
| Optimum condition | TS (MPa) | E (%) | ||||
|---|---|---|---|---|---|---|
| pH | Temperature (°C) | Time (min) | Predicted | Experimentala | Predicted | Experimentala |
| 9.5 | 91.5 | 95 | 10.11b | 10.37 ± 0.70b | 95.63a | 96.47 ± 5.59a |
a Mean value of three replicates ± standard deviation. Different letters in superscript indicate significant (p < 0.05) difference within a column
Physical properties of peanut protein films
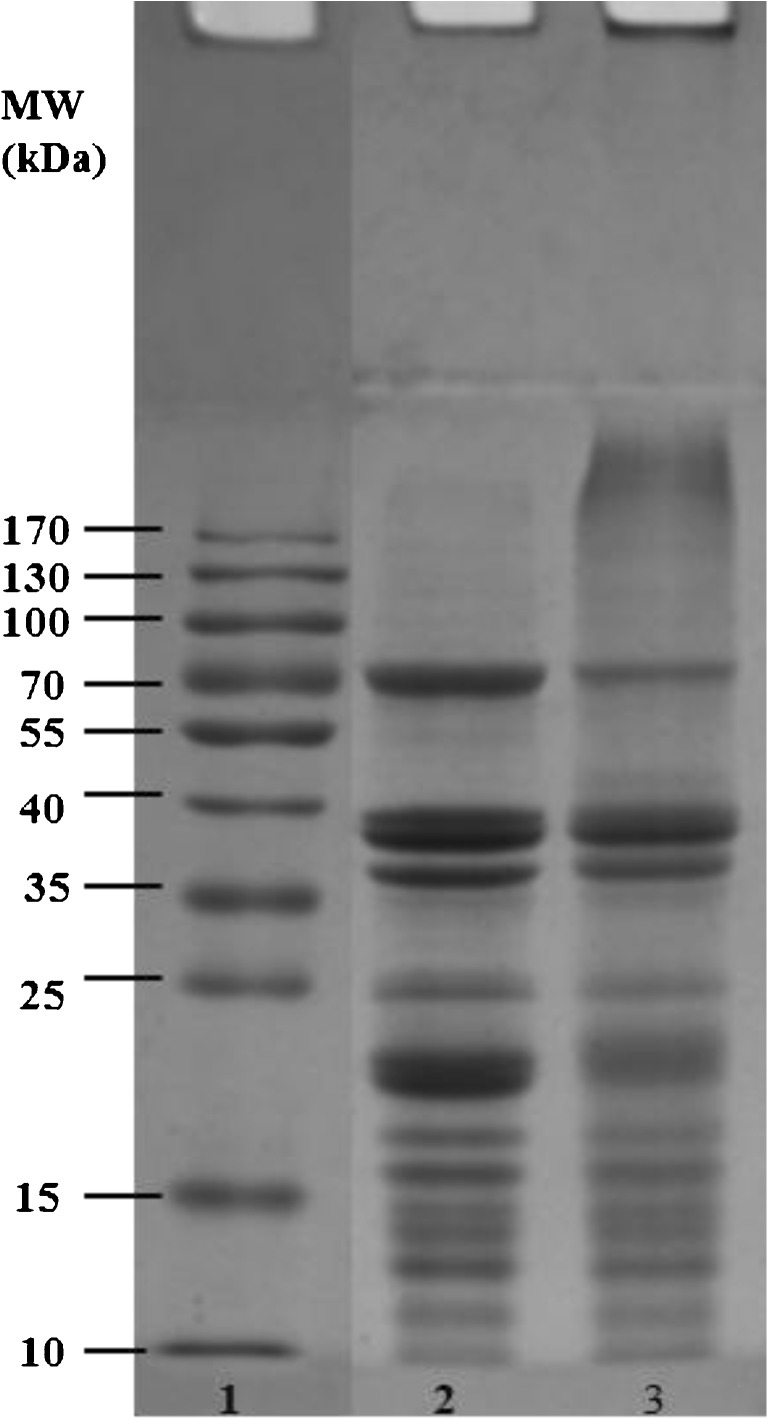
PPI and PPI-X films were analyzed by SDS-PAGE to examine the molecular weight distribution. The results (Fig. 6) showed that the two protein films were comprised of similar protein subunits, but the molecular weight distributions were different. The intensity of the bands of protein decreased after glycosylating with xylose (lane 3) and high molecular weight aggregates (higher than 170 kDa) were observed in PPI-X due to cross-linking.
Fig. 6.
SDS-PAGE analysis of PPI-XF and PPI-F. The lane 1, 2 and 3 indicated standard protein marker, PPI-F and PPI-XF. PPI-XF and PPI-F represent films produced from peanut protein isolate glycosylated with xylose and films produced from peanut protein isolate without xylose, respectively
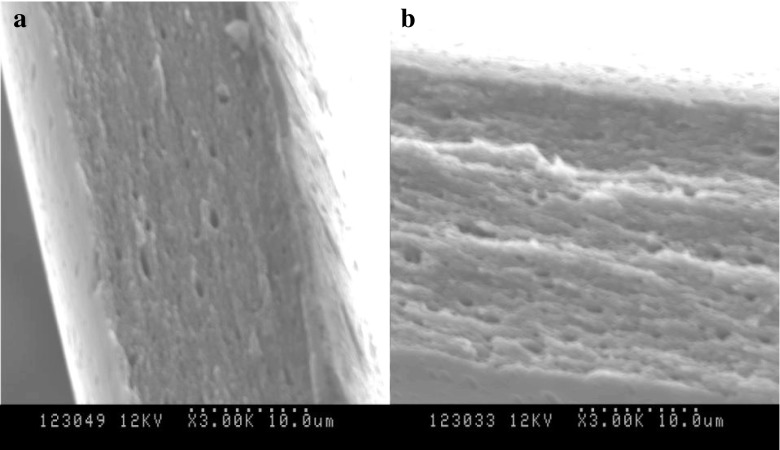
Glycosylation of PPI with xylose significantly improved the mechanical properties and solubility of PPI films. Small molecular weight carbohydrates are usually considered to be good plasticizers for film formation (Muscat et al. 2012). The Maillard reaction that occurs between proteins and saccharides was also found to be beneficial for improving the mechanical and water barrier properties of protein films (Su et al. 2010). As shown in Table 4, the tensile strength and the elongation both have increased significantly (p < 0.05) due to the glycosylation of PPI with xylose. The tensile strength of the modified peanut protein films developed in this study was much higher than the tensile strength of peanut protein films produced by crosslinking with citric acid (Reddy et al. 2012) and plasticizing with glycerin (Reddy et al. 2013; Liu et al. 2004; Jangchud and Chinnan 1999). The elongation of the xylose glycosylated peanut protein films developed in this study was higher than or very close to the values reported in the literature (Table 4). Meanwhile, the cited literature showed that films with higher TS exhibited lower E, while films with higher E exhibited lower TS. However, films made from xylose glycosylated peanut protein displayed much better mechanical properties with both high strength and high elongation. The higher tensile strength of the films developed in this study compared to the similar peanut protein films reported in the cited literature is due to the formation of high molecular weight biopolymers (Fig. 6) and compact network after glycosylation with xylose. As shown in Fig. 7, the cross sectional microstructure of the glycosylated film (Fig. 7a) shows a homogenous and more compact structure compared to that of the PPI-F film (Fig. 7b). The SDS-PAGE and microscopic images explain why the tensile strength of the xylose glycosylated film was much higher than the unmodified one.
Table 4.
Comparison of the tensile strength (TS), elongation (E), total soluble matter (TSM) and water vapor permeability (WVP) of peanut protein films
| Films | TS (MPa) | E (%) | TSM (%) | WVP (g mm/m2 h kPa) | Reference |
|---|---|---|---|---|---|
| PPI-XFa | 10.37 ± 0.91a | 96.47 ± 5.95a | 35.94 ± 3.44b | 0.16 ± 0.01b | This paper |
| PPI-Fa | 7.49 ± 0.51b | 88.53 ± 16.20a | 96.64 ± 2.11a | 0.19 ± 0.01a | This paper |
| Peanut protein film | 8.0 ± 0.6 | 63.0 ± 13.5 | – | – | Reddy et al. 2013 |
| Peanut protein film | 6.1 ± 0.6 | 38 ± 10 | – | – | Reddy et al. 2012 |
| Peanut protein film | 1.27 ± 0.23 | 98.26 ± 32.25 | 42.74 ± 1.04 | 0.94 | Liu et al. 2004 |
| Peanut protein film | 4.35 ± 0.12 | 105.00 ± 39.55 | – | 0.38 | Jangchud and Chinnan 1999 |
a Mean value of three replicates ± standard deviation. Different letters in superscript indicate significant (p < 0.05) difference within a column. PPI-XF represents films produced from peanut protein isolate glycosylated with xylose. PPI-F represents films prepared from peanut protein isolate without xylose
Fig. 7.
SEM micrographs of the cross-section of (a) PPI-XF, (b) and PPI-F. PPI-XF and PPI-F represent films produced from peanut protein isolate glycosylated with xylose and films produced from peanut protein isolate without xylose, respectively
Liu et al. (2004) reported the TSM of peanut protein film to be 42.74 %. However, these authors determined the solubility after the films were dried at 105 °C for 24 h. This drying step could increase the resistance of protein films to water and hence the solubility measured in this way is expected to be lower (Tang et al. 2005). Hence, we determined the solubility of the films by immersing the films into water immediately after they were obtained from film casting step. Interestingly the solubility of the xylose glycosylated PPI films was significantly (p < 0.05) lower (Table 4), than those without xylose. The xylose glycosylated film remained intact after immersion in water (Fig. 8a) and an intact structure could be observed after 12 h (Fig. 8b) and 24 h (Fig. 8c) of immersion. On the contrary, the PPI-F dissolved immediately (Fig. 8d) after immersion in water and was disintegrated into small pieces (Fig. 8e), and fully dissolved in water within 30 min (Fig. 8f). On the other hand, the water vapor permeability of peanut protein film was also decreased significantly (p < 0.05) due to glycosylation with xylose (Table 4). It is reported that the tertiary structure of protein can be significantly affected by Maillard reaction (Huang et al. 2012). Glycation-induced cross-linking increases the interactions of protein chains and exposes the free sulfydryl (SH) groups and hydrophobic side chains. The formation of new disulfide cross-linking takes place and intermolecular hydrophobic interaction increases due to the evaporation of the solvent during drying of the film-forming solution (Liu et al. 2004). The disulfide bonds and intermolecular hydrophobic interactions give rise to the tighter and denser protein network, which improves the mechanical properties and decreases solubility of films in water.
Fig. 8.
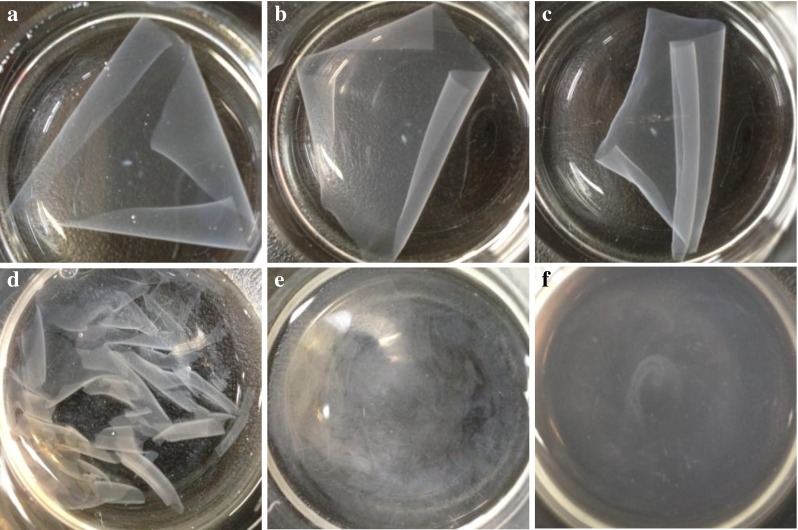
Pictorial view of dissolution (solubility) of PPI-XF (a–c) and PPI-F (d–f). PPI-XF and PPI-F represent films prepared from peanut protein isolate glycosylated with xylose and films prepared from peanut protein without xylose, respectively. a, b and c show solubility of PPI-XF when immersed in water for 1 min, 12 and 24 h, respectively. d, e and f show solubility of PPI-F when immersed in water for 1, 10 and 30 min, respectively
Conclusions
The glycosylation and film forming process of peanut protein isolate using xylose was optimized. The differences in degrees of glycosylation suggested that during the modification process the structure of peanut protein underwent some changes and crosslinking should occur between PPI and xylose due to Maillard reaction. On the other hand, the response surface based optimization methodology was suitable to optimize the glycosylation process in terms of pH, temperature and time. The optimum modification pH, temperature and time were found to be 9.5, 91.5 °C and 95 min, respectively. Under these conditions, the tensile strength and elongation of the films produced from the xylose glycosylated PPI were 10.37 MPa and 96.47 %, respectively. The glycosylation of PPI with xylose was found to significantly (p < 0.05) improve the properties of films by increasing the TS and E values and decreasing the solubility significantly. The solubility of PPI films decreased from 96.64 to 35.94 % due to glycosylation with xylose and these films remained intact even when they were immersed in water for 24 h. The interior structure of these modified films was denser and more compact than the PPI-only films. This could broaden the application scope of biodegradable PPI films in food packaging sector.
Acknowledgments
We acknowledge the financial support from National Key Technology Research and Development Program of the Ministry of Science and Technology of China (2012BAD29B00) and the Agricultural Science and Technology Innovation Program. The authors also wish to thank the Key Laboratory of Agro-Products Processing within Ministry of Agriculture for allowing us to use its laboratory facility and providing us with some technical assistance.
Footnotes
Highlights:
• Glycosylation of peanut protein isolate (PPI) with xylose (X) was proposed.
• Effects of glycosylation on the properties of PPI films were evaluated.
• The optimum modification parameters were obtained by using RSM.
• Glycosylation improved the mechanical properties of PPI-X films significantly.
• Water solubility of PPI-X films was significantly lower than PPI-only films.
Contributor Information
Wei-Jing Lin, Email: llwjing@foxmail.com.
Hong-Zhi Liu, Email: lhz0416@126.com.
Ai-Min Shi, Email: sam_0912@163.com.
Li Liu, Email: liuli02@caas.cn.
Qiang Wang, Phone: +86 10 62815837, Email: wangqiangcaas@126.com.
Benu Adhikari, Email: benu.adhikari@rmit.edu.au.
References
- Al-Hassan AA, Zorziah MH. Starch-gelatin edible films: water vapor permeability and mechanical properties as affected by plasticizers. Food Hydrocoll. 2012;26:108–117. doi: 10.1016/j.foodhyd.2011.04.015. [DOI] [Google Scholar]
- Arabestani A, Kadivar M, Shahedi M, Goli SAH, Porta R. Properties of a new protein film from bitter vetch (Viciaervilia) and effect of CaCl2 on its hydrophobicity. Int J Biol Macromol. 2013;57:118–123. doi: 10.1016/j.ijbiomac.2013.02.020. [DOI] [PubMed] [Google Scholar]
- ASTM (1995) Standard test method for water vapor transmission of materials. Philadelphia
- ASTM (2001) Standard test method for tensile properties of thin plastic sheeting. Philadelphia
- Cao N, Fu Y, He J. Preparation and physical properties of soy protein isolate and gelatin composite films. Food Hydrocoll. 2007;21:1153–1162. doi: 10.1016/j.foodhyd.2006.09.001. [DOI] [Google Scholar]
- Denavi G, Tapia-Blácido DR, Añón MC, Sobral PJA, Mauri AN, Menegalli FC. Effects of drying conditions on some physical properties of soy protein films. J Food Eng. 2009;90:341–349. doi: 10.1016/j.jfoodeng.2008.07.001. [DOI] [Google Scholar]
- Feng X, Liu H, Shi A, Liu L, Wang Q, Adhikari B. Effects of transglutaminase catalyzed crosslinking on physicochemical characteristics of arachin and conarachin-rich peanut protein fractions. Food Res Int. 2014;62:84–90. doi: 10.1016/j.foodres.2014.02.022. [DOI] [Google Scholar]
- He X, Liu H, Liu L, Zhao G, Wang Q, Chen Q. Effect of high pressure on the physicochemical and functional properties of peanut protein isolate. Food Hydrocoll. 2014;36:123–129. doi: 10.1016/j.foodhyd.2013.08.031. [DOI] [Google Scholar]
- Hong Y, Liu W, Tong L, She S. Optimization of extraction of Eucommiaulmoides polysaccharides by response surface methodology. Carbohydr Polym. 2013;92:1761–1766. doi: 10.1016/j.carbpol.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Huang X, Tu Z, Xiao H, Wang H, Zhang L, Hu Y, Zhang Q, Niu P. Characteristics and antioxidant activities of ovalbumin glycated with different saccharides under heat moisture treatment. Food Res Int. 2012;48:866–872. doi: 10.1016/j.foodres.2012.06.036. [DOI] [Google Scholar]
- Jangchud A, Chinnan MS. Peanut protein film as affected by drying temperature and pH of film forming solution. J Food Sci. 1999;64:153–157. doi: 10.1111/j.1365-2621.1999.tb09881.x. [DOI] [Google Scholar]
- Jiang J, Xiong YL, Newman MC, Rentfrow GK. Structure-modifying alkaline and acidic pH-shifting processes promote film formation of soy proteins. Food Chem. 2012;132:1944–1950. doi: 10.1016/j.foodchem.2011.12.030. [DOI] [Google Scholar]
- Kowalczyk D, Baraniak B. Effects of plasticizers pH and heating of film-forming solution on the properties of pea protein isolate films. J Food Eng. 2011;105:295–305. doi: 10.1016/j.jfoodeng.2011.02.037. [DOI] [Google Scholar]
- Lertittikul W, Benjakul S, Tanaka M. Characteristics and antioxidative activity of Maillard reaction products from a porcine plasma protein-glucose model system as influenced by pH. Food Chem. 2007;100:669–677. doi: 10.1016/j.foodchem.2005.09.085. [DOI] [Google Scholar]
- Li C, Zhu W, Xue H, Chen Z, Chen Y, Wang X. Physical and structural properties of peanut protein isolate-gum Arabic films prepared by various glycation time. Food Hydrocoll. 2014 [Google Scholar]
- Liu CC, Tellez-Garay AM, Castell-Perez ME. Physical and mechanical properties of peanut protein films. LWT - Food Sci Technol. 2004;37:731–738. doi: 10.1016/j.lwt.2004.02.012. [DOI] [Google Scholar]
- Ma W, Tang C, Yang X, Yin S. Fabrication and characterization of kidney bean (Phaseolus vulgaris L.) protein isolate-chitosan composite films at acidic pH. Food Hydrocoll. 2013;31:237–247. doi: 10.1016/j.foodhyd.2012.10.007. [DOI] [Google Scholar]
- Muscat D, Adhikari B, Adhikari R, Chaudhary DS. Comparative study of film forming behaviour of low and high amylose starches using glycerol and xylitol as plasticizers. J Food Eng. 2012;109:189–201. doi: 10.1016/j.jfoodeng.2011.10.019. [DOI] [Google Scholar]
- Osés J, Fabregat-Vázquez M, Pedroza-Islas R, Tomás SA, Cruz-Orea A, Maté JI. Development and characterization of composite edible films based on whey protein isolate and mesquite gum. J Food Eng. 2009;92:56–62. doi: 10.1016/j.jfoodeng.2008.10.029. [DOI] [Google Scholar]
- Perez-Gago MB, Krochta JM. Denaturation time and temperature effects on solubility tensile properties and oxygen permeability of whey protein edible films. J Food Sci. 2001;66:705–710. doi: 10.1111/j.1365-2621.2001.tb04625.x. [DOI] [Google Scholar]
- Popović S, Perićčin D, Vaštag Z, Popović L, Lazić V. Evaluation of edible film-forming ability of pumpkin oil cake: effect of pH and temperature. Food Hydrocoll. 2011;25:470–476. doi: 10.1016/j.foodhyd.2010.07.022. [DOI] [Google Scholar]
- Popović S, Perićčin D, Vaštag Z, Lazić V, Popović L. Pumpkin oil cake protein isolate films as potential gas barrier coating. J Food Eng. 2012;110:374–379. doi: 10.1016/j.jfoodeng.2011.12.035. [DOI] [Google Scholar]
- Reddy N, Jiang Q, Yang Y. Preparation and properties of peanut protein films crosslinked with citric acid. Ind Crop Prod. 2012;39:26–30. doi: 10.1016/j.indcrop.2012.02.004. [DOI] [Google Scholar]
- Reddy N, Chen L, Yang Y. Thermoplastic films from peanut proteins extracted from peanut meal. Ind Crop Prod. 2013;43:159–164. doi: 10.1016/j.indcrop.2012.06.051. [DOI] [Google Scholar]
- Rhim JW, Gennadios A, Weller CL, Cezeirat C, Hanna MA. Soy protein isolate-dialdehyde starch films. Ind Crop Prod. 1998;8:195–203. doi: 10.1016/S0926-6690(98)00003-X. [DOI] [Google Scholar]
- Rhim JW, Gennadios A, Weller CL, Hanna MA. Sodium dodecyl sulfate treatment improves properties of cast films from soy protein isolate. Ind Crop Prod. 2002;15:199–205. doi: 10.1016/S0926-6690(01)00114-5. [DOI] [Google Scholar]
- Shih FF. Interaction of soy isolate with polysaccharide and its effect on film properties. J Am Oil Chem Soc. 1994;71:1281–1285. doi: 10.1007/BF02540552. [DOI] [Google Scholar]
- Soininen JT, Heinämäkia J, Yliruusi J. From acacia honey monosaccharide content to a new external binary plasticizer applicable in aqueous whey protein films. Food Bioprod Process. 2013;91:440–446. doi: 10.1016/j.fbp.2013.03.002. [DOI] [Google Scholar]
- Su J, Huang Z, Yuan X, Wang X, Li M. Structure and properties of carboxymethyl cellulose/soy protein isolate blend edible films crosslinked by Maillard reactions. Carbohydr Polym. 2010;79:145–153. doi: 10.1016/j.carbpol.2009.07.035. [DOI] [Google Scholar]
- Tang C, Jiang Y, Wen Q, Yang X. Effect of transglutaminase treatment on the propertiesof cast films of soy protein isolates. J Biotechnol. 2005;120:296–307. doi: 10.1016/j.jbiotec.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Tang C, Xiao M, Chen Z, Yang X, Yin S. Properties of cast films of vicilin-rich protein isolates from Phaseolus legumes: influence of heat curing. LWT - Food Sci Technol. 2009;42:1659–1666. doi: 10.1016/j.lwt.2009.05.020. [DOI] [Google Scholar]
- Vilaseca F, Mendez JA, Pèlach A, Llop M, Cañigueral N, Gironès J, Turon X, Mutjé P. Composite materials derived from biodegradable starch polymer and jute strands. Process Biochem. 2007;42:329–334. doi: 10.1016/j.procbio.2006.09.004. [DOI] [Google Scholar]
- Wang W, Bao Y, Chen Y. Characteristics and antioxidant activity of water-soluble Maillard reaction products from interactions in a whey protein isolate and sugars system. Food Chem. 2013;139:355–361. doi: 10.1016/j.foodchem.2013.01.072. [DOI] [PubMed] [Google Scholar]
- Wihodo M, Moraru CI. Physical and chemical methods used to enhance the structure and mechanical properties of protein films: a review. J Food Eng. 2013;114:292–302. doi: 10.1016/j.jfoodeng.2012.08.021. [DOI] [Google Scholar]
- Zhang H, Chi Y. Modified soy protein isolate with improved gelling stability by glycosylation under the conditions of ocean shipping. Int J Food Sci Technol. 2011;46:14–22. doi: 10.1111/j.1365-2621.2010.02355.x. [DOI] [Google Scholar]
- Zhang H, Wang S, Zheng X, Jiang L, Lv X, Shi Y, Li L. Effect of glycosylation on the mechanical properties of edible soy protein packaging film. Eur Food Res Technol. 2014;238:1049–1055. doi: 10.1007/s00217-014-2190-3. [DOI] [Google Scholar]
- Zhong K, Wang Q. Optimization of ultrasonic extraction of polysaccharides from dried longan pulp using response surface methodology. Carbohydr Polym. 2010;80:19–25. doi: 10.1016/j.carbpol.2009.10.066. [DOI] [Google Scholar]