Abstract
Cold pressed rice bran oil (CPRBO) is used in foods, cosmetics, and pharmaceuticals due to its desirable health and functional attributes. The purpose of this work was to study the formation, antioxidant property and oxidative stability of oil-in-water emulsion of CPRBO. The influence of oil (10–40 % CPRBO) and surfactant (1–5 % glyceryl monostearate (GMS)) concentration on the properties of emulsions were studied. The lightness (L*) and yellowness (b*) of CPRBO emulsions decreased as GMS concentration increased, which was attributed to a decrease in droplet size after homogenization. The CPRBO emulsion was stable during storage at room temperature for 30 days. Increasing the oil concentration in the CPRBO emulsions increased their antioxidant activity, which can be attributed to the corresponding increase in phytochemical content. However, GMS concentration had little impact on the antioxidant activity of CPRBO emulsions. The storage of CPRBO emulsion at room temperature showed that lipid oxidation markers gradually increased after 30 days of storage, which was correlated to a decrease in gamma oryzanol content and antioxidant activity. These results have important implications for the utilization of rice bran oil (RBO) as a function ingredient in food, cosmetic, and pharmaceutical products.
Keywords: Rice bran oil, Cold pressed, Emulsion, Quality, Antioxidant
Introduction
Consumption of reactive cell-damaging free radicals has been associated with the increasing rate of chronic health problems such as cancer and cardiovascular disease in many countries (Halpin et al. 2010). Therefore, there has been an increasing interest in the identification and utilization of natural sources of antioxidants that can be used to reduce the adverse health effects of these free radicals (Devasagayam et al. 2004). RBO contains a range of natural antioxidants that make it suitable for application in food, cosmetic and pharmaceutical applications. It is rich in vitamin E complex, phytosterols, polyphenols, and squalene and contains a potent antioxidant known as gamma oryzanol (Vieno et al. 2000; Juliano et al. 2005; Van Hoed et al. 2006). The high levels of vitamin E in RBO have been reported to be responsible for its hypocholesterolemic, anticancer, and neuroprotective properties (Sen et al. 2007). The gamma oryzanol in RBO has been reported to regulate antioxidant and stress genes in rats (Ismail et al. 2010). Gamma oryzanol is a mixture of sterol esters of ferulic acid and triterpene alcohols that have high antioxidant activity. This compound has also been reported to affect blood glucose levels and serum lipid parameters, which is attributed to the fact that the ferulic acid groups have a similar structure to that found in cholesterol (Sen et al. 2007). Gamma oryzanol has also been reported to protect the skin from ultra-violet radiation and to increase skin moisture (Vorarat et al. 2010).
Consequently, there is increasing interest in incorporating RBO into a wide variety of health products in order to benefit from its desirable functional and nutritional characteristics. Recently, cold pressing of RBO has been used in small and medium factories for producing RBO commercially in Thailand. The cold pressing of plant oils offers a valuable alternative to solvent extraction because of consumers’ desire for natural, sustainable, and safe food products. Over the last few years, increasing interest in cold pressed plant oils has been observed as these oils have better nutritive properties than those after refining. Cold pressing is simple, ecologically friendly, and does not require much energy. The cold pressing method has successfully produced virgin olive oil, avocado oil, and hempseed oil (Moreau and Kamal-Elddin 2008). Cold pressed oils typically retain higher levels of natural antioxidants than conventional oil-processing procedures, and exhibit acceptable shelf stability and improved safety without added synthetic antioxidants. In addition, cold pressing involves no organic solvent, which results in a product that is chemically contaminant free (Singer et al. 2008). Cold pressed rice bran oil (CPRBO) contains endogenous antioxidants, and they may have the potential for applications in the promotion of health and prevention of oxidative damages caused by radicals.
Emulsion-based delivery systems have been proven to be particularly suitable for encapsulating lipophilic components (McClements 2005; McClements et al. 2007). These systems can be fabricated entirely from natural food-grade ingredients using relatively simple processing operations, such as blending and homogenization. The composition and microstructure of emulsions can be controlled to create desirable functional attributes such as protection against chemical degradation, ease of handling, and ease of dispersion intocommercial products. Increased consumption of these bioactive lipids may reduce the incidences of human diseases, such as heart disease, hypertension, diabetes, and cancer (Gogus and Smith 2010). The availability of new delivery systems for bioactive lipids could therefore have major benefits in improving public health, as well as in reducing the economic costs associated with chronic diseases. In these systems, it is important that the oil droplets remain both physically and chemically stable throughout the shelf life of the product. The relatively high levels of functional and nutraceutical components present within CPRBO may mean that it behaves differently in emulsions than other edible oils. The presence of minor components within edible oils may has an effect on emulsion performance, due to their ability to impact the physical or chemical stability of these systems (Khan and Shahidi 2000; McClements 2005; Arima et al. 2009). Therefore, the purpose of this work was to study the formation, antioxidant property and oxidative stability of CPRBO emulsions.
Materials and methods
Chemicals
Standard gamma oryzanol, α,α-diphenyl-β-picrylhydrazyl (DPPH), ferriccyanide, bythylated hydroxyanisole (BHA), ferulic acid, sodium azide, sodium hydrogen phosphate, and sodium dihydrogen phosphate were purchased from Sigma-Aldrich Chemical Co., (St. Louis, Mo, USA). Hexane, heptane, dimethyl sulfoxide (DMSO) were obtained from Fisher Scientific (Pittsburgh, PA, USA). All chemicals and reagents were analytical grade. Food grade glyceryl monostearate (flakes) were purchased from Foreverest Resources Development Co., Ltd. (China). The remaining reagents and solvents were procured from Fluka or Merck unless stated otherwise. Double-distilled and deionized water was used for the preparation of all solutions.
Preparation of CPRBO
Rice bran samples (Sangyod Maung Phatthalung Rice) were obtained by milling rice grain (Oryza sativa L.) in a local grinding mill: Khao Klang Village, Pantae, Khunkanun, Phatthalung, Thailand. Freshly milled bran samples were directly collected from the milling system in polyethylene bags. The grains were separated from rice bran by sieving with 177–297 μm sieves. Within 2 h after milling, rice bran samples were heated at 150 °C for 10 min to inactivate endogenous lipase, lipoxygenase, and microbes (Juliano 1985). The resulting rice bran was then stored at 4 °C in a cooler immediately and transported within 2 h for further processing (Ban Thai Herbs Co. Ltd, Tanhodduan, Khunkanun, Phatthalung, Thailand). RBO was obtained by pressing stabilized rice bran with a screw type expeller (1 horse power motor, Oriental Motor, Gear Head DY9 97575, Japan). Fine particles in the expressed oil were separated by vacuum filtration with a double layer of Whatman filter paper no.4. CPRBO samples were stored at −80 °C until used.
Preparation of CPRBO emulsion
The effect of CPRBO and emulsifier (GMS) concentration on the formation of oil-in-water emulsions was studied. GMS was used as an emulsifier in this study because it is widely used in the cosmetics, pharmaceutical, and food industries. Oil-in-water emulsions were prepared using different concentrations of CPRBO (10 to 40 wt%) and GMS (1 to 5 wt%). The ratio of GMS and CPRBO of emulsion was kept constant at 1: 10, which is a typical surfactant-to-oil ratio used to form emulsions from small molecule surfactants (McClements 2005). CPRBO samples and aqueous buffer solutions (10 mM phosphate buffer, pH 7.0) were separately heated in a water bath at 70 °C. GMS was added to the heated CPRBO and mixed until it completely dissolved in CPRBO (a clear oil solution was formed). Heated buffer solution was then added to the heated oil phase solution. Emulsification was carried out by a two-step homogenization process. First, the oil and aqueous phases were blended using a high-shear mixer (Tissue-Tearor, Biospec Products, Bartlesville, OK) operated at 20,000 rpm for 2 min to produce a coarse oil-in-water emulsion. Second, the coarse emulsion was immediately passed through a high-pressure homogenizer (Microfluidizer M-110 L processor, Microfluidics Inc., Newton, MA) three times at 12,000 psi to produce a fine oil-in-water emulsion. During the homogenization process, the temperature was controlled by circulating hot water (70–80 °C) through the device. CPRBO emulsion samples were kept at room temperature for 24 h before further analysis.
Particle size measurement
The particle size distribution (PSD) of the emulsions was measured using a laser light scattering instrument (MasterSizer 2000, Malvern Instruments Ltd., Worcestershire, U.K.). This instrument measures the intensity of laser light scattered from a dilute emulsion, and then reports the PSD that gives the closest fit between theoretical calculations (Mie theory) and experimental measurements of intensity versus scattering angle. To avoid multiple scattering effects emulsions were diluted with the same buffer as the continuous phase. Particle size measurements are reported as volume-surface mean diameters (d32) or volume-weighted mean diameters (d43). The refractive indices of the dispersed and continuous phases used in the calculations of the PSD were 1.464 and 1.330, respectively. We assumed that the imaginary part of the refractive index of the RBO was zero. It should be noted that dilution and stirring are likely to disrupt any weakly flocculated droplets, and so the particle size data on highly aggregated emulsions should be interpreted with caution.
Zeta potential measurement
The electrical charge (zeta potential) on the lipid droplets in the emulsions was determined using a particle electrophoresis instrument (ZEN3600, Nano-series, Zetasizer, Malvern Instruments). Emulsions were diluted until they gave an instrument attenuation factor of approximately 6 using buffer solution at the same pH as the initial sample. The emulsions were agitated prior to analysis to ensure that they were homogeneous. Zeta potential of each individual sample was calculated from the average of 2 freshly prepared samples with at least 2 replications per sample. The instrument used the Smoluchowski approximation to calculate the zeta potential from the measured electrophoretic mobility of the particles.
Creaming index measurement
Emulsion samples (10 mL) were placed in glass test tubes (16 × 150 mm) and then stored at ambient temperature for 30 days before analysis. The susceptibility of the emulsions to creaming was ascertained by measuring the height of the boundary layer between the opaque droplet-rich layer at the top and the transparent or turbid droplet-depleted layer at the bottom of the test tubes. Creaming results are reported as the Creaming Index (CI) = 100 × (height of interface)/(height of total emulsion) (Demetriades and McClements 2000). Pictures of emulsion were using a digital camera (Sony, Cyber shot, Exmor R 10.2 mega pixels, Japan).
Color measurement
Color of the emulsions was measured using an instrumental colorimeter (X-rite, Color Munki Design, China), and recorded using the CIE color system as L*, a* and b* values.
Analysis of phytochemical contents and antioxidant activity
Phytochemicals in CPRBO emulsion were extracted by mixing 1.5 mL of emulsion with 7.5 mL of isooctane/ 2-propanol (3:1) by mixing (10 s, 3 times). The organic solvent phase was isolated by centrifugation at 1000×g for 2 min (Beckman Centrifuge model J2-21, Beckman Instruments Inc., Fullerton, CA). The supernatant was collected for measurement of gamma oryzanol content and DPPH• radical scavenging activity. Methanol was used instead of isooctane/ 2-propanol for extraction of phenolic compounds from the emulsion. The phenolic content was then determined using the method described below.
The gamma oryzanol content in CPRBO emulsions extracted was determined using a UV/visible spectrophotometer (Model DU-530, Beckman Instruments, Fullerton, CA) following the method of Mezouari and Eichner (2007), slightly modified. Briefly, CPRBO emulsion extracted samples were diluted in heptane. The content of gamma oryzanol in unknown samples was determined by measuring the absorbance at 315 nm using a standard curve. The calibration curve was obtained by dissolving known amounts of gamma oryzanol in heptane over a concentration range of 0–200 mg/L. The concentration of gamma oryzanol was expressed as g/100 g sample.
The total phenolic content of CPRBO emulsions was measured according to the method reported by Lai et al. (2009) using the Folin–Ciocalteu reagent, with some modification. Briefly, a 0.1 mL of CPRBO solution (1.0 mg/mL DMSO) was sampled into 2 mL of 2 % Na2CO3 and mixed for 3 min. After adding 0.1 mL of Folin–Ciocalteau reagent, the final mixture was left for 30 min before reading the absorbance at 750 nm (Genesys 20, Thermo Scientific, Waltham, MA). All measurements were conducted in triplicate and the data were expressed as mg ferulic acid equivalent (FAE) per g of the oil (mg FAE/g oil), based on a calibration curve using ferulic acid.
DPPH• radical scavenging activity was determined using the method originally developed by (Blois 1958) with slight modifications by Lai et al. (2009). A portion (0.1 mL) of CPRBO (1.0 mg/mL DMSO) in a test tube was mixed with 3.9 ml of methanol and 1.0 mL of α,α-diphenyl-β-picrylhydrazyl (DPPH•) solution (1.0 mM in methanol). The mixture was kept at ambient temperature for 30 min prior to measurement of the absorbance at 517 nm (Genesys 20, Thermo Scientific, Waltham, MA). BHA was used as the reference standard. All measurements were done in triplicate. The radical scavenging effect was derived using the following equation:
Measurements of oxidative stability
CPRBO emulsion samples (50 mL) were placed in 100 mL Duran glass bottles, and then incubated at room temperature (about 25 °C) in the dark. The samples were kept for 90 days. Samples were taken at 0, 30, 60 and 90 days for study of oxidative stability and changes of phytochemical content and antioxidant activity of CPRBO emulsions.
Lipid hydroperoxides of CPRBO emulsions were measured with a modified ferric thiocyanate method (Alamed et al. 2009) by mixing 0.3 mL of emulsion with 1.5 mL of isooctane/ 2-propanol (3:1) by mixing (10 s, 3 times) and isolation of the organic solvent phase by centrifugation at 1000×g for 2 min (Beckman Centrifuge model J2-21, Beckman Instruments Inc., Fullerton, CA). The organic solvent phase (200 μL) was added to 2.8 mL of methanol/n-butanol (2:1), followed by 15 μL of 3.97 M ammonium thiocyanate and 15 μL of a ferrous iron solution (prepared by mixing 0.132 M BaCl2 and 0.144 M FeSO4.7H2O). After 20 min of incubation at room temperature, absorbance was measured at 510 nm (Genesys 20, Thermo Scientific, Waltham, MA). Hydroperoxide concentrations were determined using a standard curve from cumene hydroperoxide.
Thiobarbituric acid reactive substances (TBARS) (McDonald and Hultin 1987) were determined by mixing 1.0 mL of emulsion with 2.0 mL of TBA reagent in 10 mL test tubes. TBA reagent was prepared by combining 100 mL of TCA-TBA-HCl solution [15 % w/v trichloroacetic acid (TCA) and 0.375 % w/v thiobarbituric acid (TBA) in 0.25 M HCl] with 3.0 mL of ethanol (containing 2 % butylated hydroxytoluene (BHT)). Samples were heated in a boiling water bath for 15 min, cooled to room temperature (10 min), and then centrifuged for 15 min at 3400×g (Beckman Centrifuge model J2-21, Beckman Instruments Inc., Fullerton, CA). Samples were held at room temperature for 10 min before the absorbance of each sample was measured at 532 nm (Genesys 20, Thermo Scientific, Waltham, MA). TBARS concentrations were determined using a standard curve from 1,1,3,3-tetraethoxypropane.
Changes of phytochemical content and antioxidant activity of CPRBO emulsions were measured the gamma oryzanol content, total phenolic content and DPPH• scavenging effect (following the methods as described above).
Statistical analysis
The means and standard deviations of the formation, phytochemical content, antioxidant activity and oxidative stability of CPRBO emulsion were reported in triplicate determinations for each sample. Analysis of variance (ANOVA) in completely randomized design(CRD). Multiple comparison tests were performed using Duncan multiple range tests to determine the significant difference between treatments (Steel and Torrie 1980). Statistical significance was declared at P < 0.05.
Results and discussion
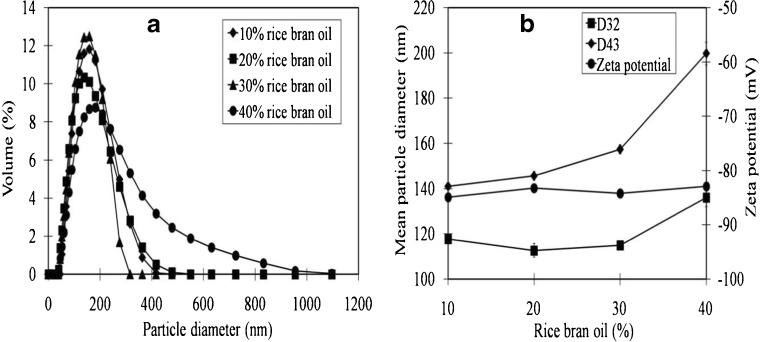
The influence of CPRBO and GMS concentration on the particle size distribution, mean particle diameter, zeta potential, creaming index, phytochemical content, antioxidant activity, and color were studied. For the study of the effect of oil concentration on emulsion properties, the ratio of surfactant-to-oil was fixed at 1:10 and the total concentrations of oil were 10, 20, 30 and 40 wt% (Fig. 1). The particle size distribution of all the emulsions was monomodal (Fig. 1a), however, the mean droplet diameter (Fig. 1b) increased as the oil content increased (at 40 % CPRBO). This result suggests that the efficiency of droplet disruption and/or coalescence within the homogenizer changed with increasing oil content. It is possible that increasing the oil content reduced the efficiency of droplet disruption, or that it increased the efficiency of droplet coalescence. At high droplet concentrations, one would expect the frequency of droplet-droplet collisions within the homogenizer to increase, which may have resulted in larger droplets being produced at the end of the homogenization process. Zeta potential is a key factor to evaluate the stability of colloidal dispersion through the electrostatic repulsion between the particles. High absolute value of zeta potential indicates high surface chare of particles, which lead to strong repellent interaction among the particles in the dispersion and thus high stability (Dinda et al. 2013). Stabilization of the emulsions by GMS (nonionic surfactant) is due to steric repulsion. However, zeta potential value of CPRBO emulsion droplets was highly negative and independent of droplet concentration for all emulsions (Fig. 1b). In principle, GMS should be a non-ionic surfactant that gives little surface charge to oil droplets. In practice, the surfactant and/or oil may have contained some anionic impurities (such as free fatty acids or phospholipids) that contributed to the negative charge on the droplets. The fact that the droplets have a high negative charge is important for certain applications, since it affects the physical and chemical stability of emulsions (Karraker and Radke 2002; McClements 2005). The CPRBO emulsions stabilized by GMS had larger negative zeta potential than RBO emulsions stabilized by whey protein, gum Arabic, or modified starch (Charoen et al. 2011) or by Tween 80 and Span 80 (Nguyen et al. 2013). This may have been due to differences in the type of ionized species adsorbed at the oil–water interface, or due to differences in solution properties (such as pH and ionic strength). For the study of the influence of surfactant concentration on emulsion properties, the oil content was kept constant (30 % CPRBO) while the surfactant content was varied from 1 to 5 wt% GMS (Fig. 2). All of the emulsions had monomodal distributions (Fig. 2a), but the mean droplet diameter decreased as the GMS concentration increased (Fig. 2b). This effect can be attributed to the fact that there was more surfactant available to cover the newly formed oil–water interfaces created during homogenization, as well as to the fact that the interfaces become saturated more rapidly at higher emulsifier concentrations (Walstra 1993, 2003; Jafari et al. 2008). Again, all of the emulsions were highly negatively charged, suggesting the presence of anionic impurities in the oil or surfactant ingredients. Zeta potential of CPRBO emulsions increased as the GMS content increased. Our results were in agreement with Kotikalapudi et al. (2012), they found that the increases of GMS concentration between 50 and 200 mg in solid lipid nanoparticles could increase the values of zeta potential. In general, particles could be dispersed stably when absolute value of zeta potential was above −30 mV due to the electric repulsion between the particles (Kotikalapudi et al. 2012). In the present work, the values of zeta potential of CPRBO ranged from −82.93 to 91.13 (Figs. 1 and 2) were sufficient to keep the particle stable.
Fig. 1.
Influence of oil concentration on particle size distribution, mean particle diameter, and zeta potential of CPRBO emulsions (the ratio of GMS and CPRBO of emulsion was kept constant at 1: 10)
Fig. 2.
Influence of surfactant (GMS) concentration on the particle size distribution, mean particle diameter and zeta potential of 30 % CPRBO emulsions
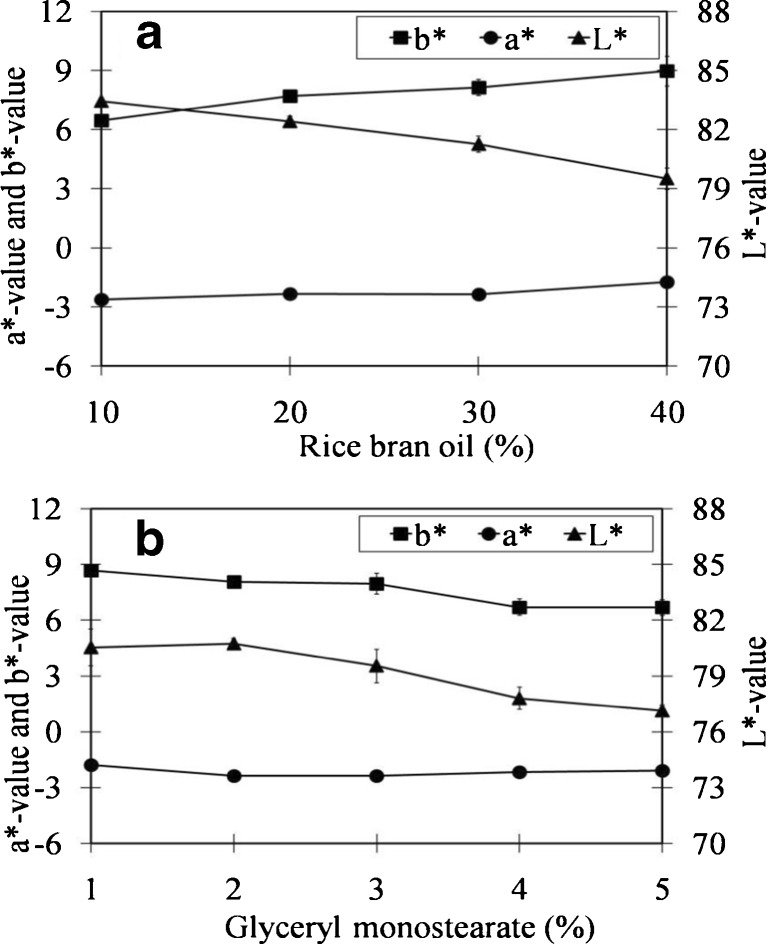
The susceptibility of the emulsions to creaming was studied by measured the creaming index. CPRBO emulsion samples were kept in glass test tubes at ambient temperature for 30 days before analysis. We found that the CPRBO emulsions were stabled at ambient temperature for 30 days (data not show). Color is one of the major attributes that affects consumer perception of product quality. The effect of oil and surfactant concentration on the color of rice bran oil-in-water emulsions therefore determined. The a* and b* values increased, while the L* value decreased, as the oil content was increased at constant surfactant-to-oil ratio (Fig. 3a). The increase in the color intensity (a* and b*) can be attributed to the increase in the amount of chromophores present within the emulsion, since the CPRBO naturally has a yellowish color. The decrease in L* with increasing oil concentration occurred because less light was reflected from the surface of the emulsions due to absorption by the chromophores. The lightness (L*) and yellowness (b*) of the emulsions tended to decrease as the surfactant concentration was increased at a constant oil concentration (Fig. 3b). This effect may be attributed to changes in the particle size as the surfactant concentration changes, since this influences the light scattering efficiency of the emulsion (Chantrapornchai et al. 1998; McClements 1998).
Fig. 3.
Influence of oil and surfactant concentration on the color coordinates of CPRBO emulsions
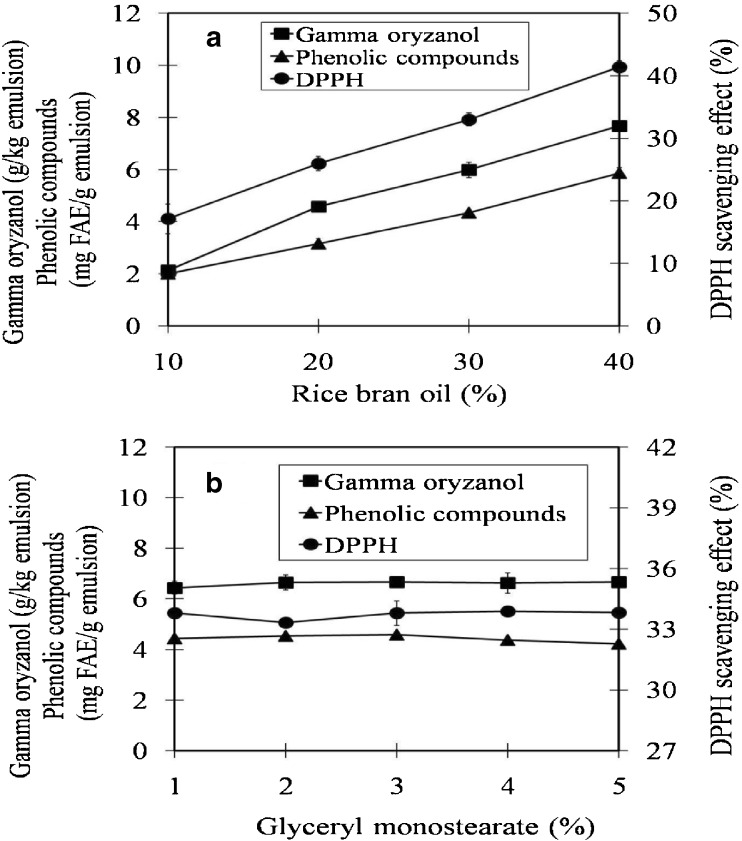
Rice bran oil contains a significant amount of natural phytochemicals such as oryzanols, tocopherols, and tocotrienols that have been reported to be strong antioxidants (Godber and Wells 1994; Lai et al. 2009; Orthoefer and Eastman 2004). Our previous work found that CPRBO contained approximately 11.5 mg FAE/g oil of total phenolics and approximately 2.2 g/100 g oil of gamma oryzanol (Thanonkaew et al. 2012). In this work the effects of oil and surfactant concentration on the phytochemical contents and antioxidant activity of CPRBO emulsions were studied (Fig. 4). The antioxidant activities of the emulsions were evaluated using the DPPH• assay. Increasing the oil content in the CPRBO emulsions increased their antioxidant activity, which can be attributed to the corresponding increase in phytochemical content. On the other hand, increasing the surfactant concentration in the emulsions had little impact on their antioxidant activity, which can be attributed to the fact that the level of antioxidants (gamma oryzanol and phenolics) remained constant. These results suggest that increasing the total amount of phytochemicals present improves the antioxidant activity of CPRBO emulsions.
Fig. 4.
Influence oil and surfactant concentration on phytochemical content (phenolic compounds and oryzanol) and antioxidant activity of CPRBO emulsions
Food manufacturers have been interested in utilization of rice bran oil as a healthy source of lipids in foods because of its well-balanced level of saturated and unsaturated fats, and because it provides a good source of natural antioxidants and nutraceuticals. However, food applications of rice bran oil are limited because of its susceptibility to lipid oxidation. It is therefore important to design emulsion-based delivery systems that are able to protect CPRBO against oxidation, while maintaining good physical stability and oral bioavailability. We therefore examined the oxidative stability of emulsions prepared using 30 % CPRBO and 3 % GMS (10 mM phosphate buffer, pH 7) during storage at 25 °C for 90 days (Fig. 5). The oxidative stability was monitored by measuring hydroperoxide and TBARS concentrations over time. Hydroperoxide is an indicator of the formation of primary reaction products and TBARS is an indicator of the formation of secondary reaction products. These lipid oxidation markers remained relatively low during the first 30 days of storage but increased steadily after this time, indicating that lipid oxidation was occurring (Fig. 5b). There was a corresponding decrease in the gamma oryzanol content and antioxidant activity of the system at a similar time, suggesting that oxidation proceeded after the endogenous antioxidants in the rice bran oil had been used up. The susceptibility of the systems to lipid oxidation may have been due to the absorption of cationic iron ions to the surfaces of the anionic lipid droplets, since this transition metal is known to be a potent pro-oxidant (Yoshida and Niki 1992; Mei et al. 1998; Mancuso et al. 1999).
Fig. 5.
Changes of phytochemical content (phenolic compounds and oryzanol) and lipid oxidation markers (hydroperoxides and TBARS) of CPRBO emulsions during storage at 25 °C for 90 days
Conclusions
In summary, this study has shown that oil-in-water emulsion can be formed from CPRBO that contain relatively small droplets (d < 200 nm). Lipid oxidation occurs in these emulsions after prolonged storage, which can be attributed to the depletion of endogenous antioxidants. This study has important implications for the utilization of rice bran oil in food, cosmetic, and pharmaceutical products.
Acknowledgments
This research was supported by a grant from Thailand Research Fund with a grant number of MRG5380124, grants from Institute of Research and Development of Thaksin University and Thailand Toray Science Foundation. We would also like to give a special thank you to Mr. Brian J. Moore for kindly editing the manuscript.
References
- Alamed J, Chaiyasit W, McClements DJ, Decker EA. Relationships between free radical scavenging and antioxidant activity in foods. J Agric Food Chem. 2009;57:2969–2976. doi: 10.1021/jf803436c. [DOI] [PubMed] [Google Scholar]
- Arima S, Ogawa A, Ueno S, Sato K. Sucrose fatty acid esters on emulsion stability at chilled temperatures of oil-in-water emulsion. J Jpn Soc Food Sci Technol. 2009;56:236–243. doi: 10.3136/nskkk.56.236. [DOI] [Google Scholar]
- Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- Chantrapornchai W, Clydesdale FM, McClement DJ. Influence of droplet size and concentration on the color of oil-in-water emulsions. J Agric Food Chem. 1998;46:2914–2920. doi: 10.1021/jf980278z. [DOI] [Google Scholar]
- Charoen R, Jangchud A, Jangchud K, Harnsilawat T, Naivikul O, McClements DJ. Influence of biopolymer emulsifier type on formation and stability of rice bran oil-in-water emulsions: whey protein, gum Arabic, and modified starch. J Food Sci. 2011;76:165–172. doi: 10.1111/j.1750-3841.2010.01959.x. [DOI] [PubMed] [Google Scholar]
- Demetriades K, McClements DJ. Influence of sodium dodecyl sulfate on the physicochemical properties of whey protein-stabilized emulsions. Colloids Surf A Physicochem Eng Asp. 2000;161:39–400. doi: 10.1016/S0927-7757(99)00210-1. [DOI] [Google Scholar]
- Devasagayam TPA, Tilak JC, Boloor KK, Sane KS, Ghaskadbi SS, Lele RD (2004) Free radicals and antioxidants in human health: current status and future prospects. J Assoc Physician I 52:794–804 [PubMed]
- Dinda A, Biswal I, Chowdhury P, Mohapatra R. Formulation development and evaluation of paclitaxel loaded solid lipid nanoparticles using glyceryl monostearate. J Appl Pharm Sci. 2013;3:133–138. [Google Scholar]
- Godber JS, Wells JH. Rice bran: as a viable source of high value chemicals. Louis Agric. 1994;37:13–17. [Google Scholar]
- Gogus U, Smith C. n-3 Omega fatty acids: a review of current knowledge. Int J Food Sci Technol. 2010;45:417–436. doi: 10.1111/j.1365-2621.2009.02151.x. [DOI] [Google Scholar]
- Halpin HA, Morales-Suarez-Varela MM, Martin-Moreno JM. Chronic disease prevention and the new public health. Public Health Rev. 2010;32:120–154. [Google Scholar]
- Ismail M, Al-Naqeeb G, Mamat WA, Ahmad Z. Gamma-oryzanol rich fraction regulates the expression of antioxidant and oxidative stress related genes in stressed rat’s liver. Nutr Metab (Lond) 2010;7:1–13. doi: 10.1186/1743-7075-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafari SM, Assadpoor E, He Y, Bhandari B. Re-coalescence of emulsion droplets during high-energy emulsification. Food Hydrocoll. 2008;22:1191–1202. doi: 10.1016/j.foodhyd.2007.09.006. [DOI] [Google Scholar]
- Juliano BO. Rice bran. In: Juliano BO, editor. Rice chemistry and technology. 2. St. Paul: The American Association of Cereal Chemist; 1985. p. 659. [Google Scholar]
- Juliano C, Cossu M, Alamanni MC, Piu L. Antioxidant activity of gamma-oryzanol: mechanism of action and its effect on oxidative stability of pharmaceutical oils. Int J Pharm. 2005;299:146–154. doi: 10.1016/j.ijpharm.2005.05.018. [DOI] [PubMed] [Google Scholar]
- Karraker KA, Radke CJ. Disjoining pressures, zeta potentials and surface tensions of aqueous non-ionic surfactant/electrolyte solutions: theory and comparison to experiment. Adv Colloid Interf Sci. 2002;96:231–264. doi: 10.1016/S0001-8686(01)00083-5. [DOI] [PubMed] [Google Scholar]
- Khan MA, Shahidi F. Oxidative stability of stripped and nonstripped borage and evening primrose oils and their emulsions in water. J Am Oil Chem Soc. 2000;77:963–968. doi: 10.1007/s11746-000-0152-z. [DOI] [Google Scholar]
- Kotikalapudi LS, Adepu L, VijayaRatna J, Diwan PV. Formulation and in vitro characterization of domperidone loaded solid lipid nanoparticles. Int J Pharm Biomed Res. 2012;3:22–29. [Google Scholar]
- Lai P, Li KY, Lu S, Chen HH. Phytochemicals and antioxidant properties of solvent extracts from Japonica rice bran. Food Chem. 2009;117:538–544. doi: 10.1016/j.foodchem.2009.04.031. [DOI] [Google Scholar]
- Mancuso JR, McClements DJ, Decker EA. The effects of surfactant type, pH, and chelators on the oxidation of Salmon oil-in-water emulsions. J Agric Food Chem. 1999;47:4112–4116. doi: 10.1021/jf990203a. [DOI] [PubMed] [Google Scholar]
- McClements DJ. Analysis of food emulsions. In: Nielsen SS, editor. Food analysis. 2. Gaithersburg: Aspen Aspen Publishers; 1998. pp. 571–585. [Google Scholar]
- McClements DJ. Food emulsions: principles, practice, and techniques. 2. Boca Raton: CRC Press; 2005. [Google Scholar]
- McClements DJ, Decker EA, Weiss J. Emulsion-based delivery systems for lipophilic bioactive components. J Food Sci. 2007;72:109–124. doi: 10.1111/j.1750-3841.2007.00507.x. [DOI] [PubMed] [Google Scholar]
- McDonald RE, Hultin HO (1987) Some characteristics of the enzymic lipid peroxidation system in the microsomal fraction of flounder skeletal muscle. J Food Sci 52:15–21
- Mei L, McClements DJ, Wu J, Decker EA. Iron-catalyzed lipid oxidation in emulsion as affected by surfactant, pH and NaCl. Food Chem. 1998;61:307–312. doi: 10.1016/S0308-8146(97)00058-7. [DOI] [Google Scholar]
- Mezouari M, Eichner K. Comparative study on the stability of crude and refined rice bran oil during long-term storage at room temperature. Eur J Lipid Sci Technol. 2007;109:198–205. doi: 10.1002/ejlt.200600154. [DOI] [Google Scholar]
- Moreau RA, Kamal-Elddin A. Introduction. In: Moreau RA, Kamal-Elddin A, editors. Gourmet and health-promoting specialty oils. Illinois: AOCS Press; 2008. pp. 1–32. [Google Scholar]
- Nguyen HH, Choi K, Kim DE, Kang W, Ko S. Improvement of oxidative stability of rice bran oil emulsion by controlling droplet size. J Food Process Preserv. 2013;37:139–151. doi: 10.1111/j.1745-4549.2011.00633.x. [DOI] [Google Scholar]
- Orthoefer FT, Eastman J. Rice bran and oil. In: Champagne ET, editor. Rice chemistry and technology. St. Paul: AACC; 2004. pp. 569–593. [Google Scholar]
- Sen CK, Khanna S, Roy S. Tocotrienols in health and disease: the other half of the natural vitamin E family. Mol Aspects Med. 2007;28:692–728. doi: 10.1016/j.mam.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer A, Nogala-Kalucka M, Lampart-Szczap E. The content and antioxidant activity of phenolic compounds in cold-pressed plant oils. J Food Lipids. 2008;15:137–149. doi: 10.1111/j.1745-4522.2007.00107.x. [DOI] [Google Scholar]
- Steel RGD, Torrie JH. Principles and procedures of statistics: a biometrical approach. 2. New York: McGraw-Hill; 1980. [Google Scholar]
- Thanonkaew A, Wongyai S, McClements DJ, Decker EA. Effect of stabilization of rice bran by domestic heating on mechanical extraction yield, quality, and antioxidant properties of cold-pressed rice bran oil (Oryzasaltiva L.) LWT Food Sci Technol. 2012;48:231–236. doi: 10.1016/j.lwt.2012.03.018. [DOI] [Google Scholar]
- Van Hoed V, Depaemelaere G, Vila Ayala J, Santiwattana P, Verhe R, De Greyt W. Influence of chemical refining on the major and minor components of rice brain oil. J Am Oil Chem Soc. 2006;83:315–321. doi: 10.1007/s11746-006-1206-y. [DOI] [Google Scholar]
- Vieno P, David GL, Tatu AM, Jari T, Anna-Maija L. Plant sterols: biosynthesis, biologicalfunction and their importance to human nutrition. J Sci Food Agric. 2000;80:939–966. doi: 10.1002/(SICI)1097-0010(20000515)80:7<939::AID-JSFA644>3.0.CO;2-C. [DOI] [Google Scholar]
- Vorarat S, Managit C, Iamthanakul L, Soparat W, Kamkaen N. Examination of antioxidant activity and development of rice bran oil and gamma-oryzanol microemulsion. J Health Res. 2010;24:67–72. [Google Scholar]
- Walstra P. Principles of emulsion formation. Chem Eng Sci. 1993;48:397–346. doi: 10.1016/0009-2509(93)80021-H. [DOI] [Google Scholar]
- Walstra P. Physical chemistry of foods. New York: Marcel Decker; 2003. [Google Scholar]
- Yoshida Y, Niki E. Oxidation of methyl linoleate in aqueous dispersions induced by copper and iron. Arch Biochem Biophys. 1992;295:107–114. doi: 10.1016/0003-9861(92)90494-H. [DOI] [PubMed] [Google Scholar]