Abstract
Buckwheat flour was incorporated into wheat flour at different levels (0, 20, 40, 60, 80, and 100 %) and the physicochemical, functional and antioxidant properties of the blended flour were studied. This study also investigated the effect of buckwheat on the retention of antioxidant properties of cookies during baking. The results showed significant variation in physicochemical and functional properties of the blended flour. The addition of buckwheat flour into wheat flour also increased the antioxidant properties of blended flour proportionally, but metal chelating properties decreased. The incorporation of buckwheat in wheat flour helped in better retention of antioxidant potential of cookies during baking process as buckwheat cookies (100 % buckwheat) showed greater percentage increase in antioxidant properties than control (100 % wheat). Quality characteristics of cookies such as hardness and spread ratio decreased, while as non-enzymatic browning (NEB) increased significantly with increase in the proportion of buckwheat flour in wheat flour. The Overall acceptability of cookies by sensory analysis was highest at 40 % level of blending. This study concluded that addition of buckwheat in wheat flour, may not only improve the physico-chemical and functional properties of the blended flour but may also enhance the nutraceutical potential of the product prepared from it.
Keywords: Buckwheat, Wheat flour, Antioxidant properties, Functional properties
Introduction
Various underutilized pseudo-cereals such as buckwheat found in high Himalayan regions are known for their tremendous health benefits. Buckwheat is ubiquitous almost found everywhere but grows mainly in the northern hemisphere (Li and Zhang 2001). Many of the health benefits of buckwheat have been due to its high levels of phenolic compounds and antioxidant activity. Whole buckwheat contains 2–5 times more phenolic compounds than oats or barley, while buckwheat bran and hulls have 2–7 times higher antioxidant activity than barley, triticale, and oats (Holasova et al. 2002; Zdunczyk et al. 2006). Rutin and quercetin are the main polyphenols with antioxidant activity present in buckwheat. This pseudo cereal not only contains proteins, starch and vitamins but also contain large amounts of flavonoids and polyphenols (Sakac et al. 2011). The proteins of buckwheat have a high biological value (Eggum et al. 1981). Buckwheat proteins also act similarly to dietary fiber by exhibiting cholesterol lowering, anti-hypertentive effects, reducing constipation and obesity (Ikeda 2002; Li and Zhang 2001).
Although buckwheat is a rich source of nutrients in general and antioxidants in particular, the effect of baking on the antioxidant activity of buckwheat is very rarely reported. Since buckwheat flour is gluten free, thus replacing wheat flour with buckwheat flour will definitely dilute the wheat gluten proteins and thereby help in preventing celiac disorders, and hence act as a good replacement for wheat flour in cookie making. Several researchers have incorporated various natural components (like whey prorein, mango peel powder, green tea powder, etc.) in wheat flour, to improve functional & nutraceutical properties of products prepared from it (Gani et al. 2014a; Ajila et al. 2010; Ahmad et al. 2015). However, limited studies have been reported on cookie making behavior of buckwheat-wheat flour blend. The objective of the present study was to utilize the buckwheat at different levels for cookie making and to study its effect on the retention of antioxidants properties during baking.
Materials and methods
Procurement of buckwheat flour and wheat flour
Whole buckwheat grains were procured from Ladakh division of Jammu and Kashmir. Grains were milled by laboratory hand mill. Refined wheat flour was procured from local market of Srinagar, Kashmir. Whole buckwheat flour was sieved through 40 mm sieve to obtain required refined flour from it.
Preparation of flour blend
Blends of buckwheat flour were made by taking the wheat flour as control. Blends were prepared by mixing buckwheat flour (BWF) with wheat flour (WF) in the proportions of 00:100, 100:20, 100:40, 100:60, 100:80, 100:00, which correspond to 0, 20, 40,60,80, 100 % blends.
Proximate composition of BWF, WF and their blends
The proximate composition of flours and blends, including moisture, crude ash, crude fat, crude fiber and crude protein, were determined according to the methods of AOAC 14.091, 14.103, 14.093, 14.111 and 14.108, respectively (AOAC 1990). The nitrogen conversion factor used for crude protein calculation was 5.70. The carbohydrate content (%) was calculated by subtracting the contents of crude ash, fat, fiber and protein from 100 % of dry matter.
Functional properties of BWF and WF blends
Water and oil absorption capacity (WAC and OAC)
The water and oil absorption capacities of flour samples were determined by the method described by Soluski et al. (1976) with modifications. The flour samples (1.0g each) were mixed with 10 ml distilled water or refined mustard oil and were kept at ambient temperature for 30 min and then centrifuged for 10 min at 2000 g. The aqueous supernatant or clear oil obtained after centrifuging was decanted and the test tubes were inverted and allowed to drain for 5 min on a paper towel. By weighing the residue, WAC and OAC were calculated and expressed as percentage of water or oil absorbed per gram of sample, respectively.
Foaming capacity (FC) and foam stability (FS)
The method described by Narayana and Narasinga Rao (1982) was used for the determination of foaming capacity (FC) and foaming stability (FS). Two gram of flour samples were mixed with 50 ml of distilled water at 30 ± 2 °C in a 100 ml measuring cylinder. The suspension was shaken properly by whipping for 3 min in an electrical blender (Double-M, Germany) to form foam. The content in the blender was transferred into the measuring cylinder and the volume of the foam after 30 s was recorded. The FC was expressed as a percentage increase in volume:
The foam volume was recorded 1 h after whipping to determine the Foam Stability (FS) as a percentage of the initial foam volume.
Swelling power (SP)
The swelling power was determined according to the method described by Gani et al., (2013) with modification for small samples. Briefly, 3 gm. of each sample was mixed with 30 ml distilled water in a centrifuge tube and heated at 70 °C in a water bath for 30 minutes and then centrifuged at 1000 rpm for 15 minutes After heating, the suspension was centrifuged at 1000 rpm for 15 min. The supernatant was decanted and the weight of the paste was taken. The swelling power was calculated as:
Bulk density (BD)
The flour samples (100 gm each) were gently filled in 500 ml graduated cylinder, previously tarred. The bottom of the cylinder was gently tapped on a laboratory bench several times until there was no further diminution of the sample level after filling to 500 ml mark. Bulk density was calculated as weight of sample per unit volume of sample (g/ml).
Antioxidant properties of BWF, WF and their blends
Preparation of extracts
Each sample (0.3 g) were dissolved in 20 ml of 70 % methanol then stirred for 2 hours on a magnetic stirrer followed by the centrifugation for 10 minutes at 3500 rpm. The supernatant was evaporated at 40 °C and supernatant was stored at -18 °C.
Total phenolic content
Total phenolic content (TPC) was determined by Follin-Ciocateu spectrophotometric method Shah et al., (2014), with some modifications. The results were expressed as Gallic acid equivalents (nmolGAE/μl) of sample.
DPPH radical scavenging activity
DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging activity of the extract solutions (Methanolic) of each sample was determined according to the method described by shah et al 2015), with some modifications with slight modifications. The absorbance at 517 nm was measured after each sample solution had incubated in dark for 30 minutes. Lower absorbance of the reaction mixture indicates higher free radical scavenging activity. Percentage inhibition was calculated by using the formulae
Where Acontrol517 is the absorbance of the control and Asample517 is the absorbance of the extract.
Reducing power
Reducing power of methanol extracts of wheat flour and buckwheat flour and their blends were determined by the method described by Gani et al., (2014b) with some modifications. Different volumes of extracts of six samples were mixed with 0.2 M sodium phosphate buffer pH 6.6 (2.5 ml) and 1 % (w/v) of aqueous potassium ferricyanide (2.5 ml). The mixture was incubated at 50 °C for 20 min. 10 % (w/v) trichloroacetic acid (2.5 ml) was added to the mixture, which was then centrifuged at 3000 g for 10 minutes. After centrifugation, 2.5 ml supernatant was collected and diluted with 2.5 ml of deionised water and 0.1 % (w/v) ferric chloride 0.5 ml was added. The absorbance was taken at 700 nm against methanol and compared to ascorbic acid that was used as a standard. A higher absorbance indicates a higher reducing power.
Where,
- AC
absorbance of standard
- AS
absorbance of sample
FRAP value (Ferric reducing antioxidant power)
FRAP Value was determined according to the method described by Benzie and Strain (1999). The 3 ml of the FRAP reagent [10 ml of acetate buffer (300 mM, PH 3.6), 1 ml TPTZ (10 mM) in hydrochloric acid solution (40 mM) and 1 ml FeCl3 solution (20 mM)] was added to 0.1 ml of each sample extract. Absorbance was taken at 593 nm immediately after 4 minutes of incubation time at room temperature and the results were expressed as μM Fe2+ /L
Where 2 is FRAP value of ascorbic acid.
Metal chelating activity
The metal chelating activity of samples was measured as reported by Dinis et al., (1994). The chelating activity of the extract for Fe2+ was calculated as follows:
Where,
- AS562
Absorbance of sample at 562 nm
- AC562
Absorbance of control at 562 nm
Cookie, preparation & evaluation
The cookies were prepared according to formula described by Tyagi et al. (2007). The cookies’ formula based on flour weight was: 100 g flour, 53 g sugar, 26.5 g shortening, 1.1 g sodium bicarbonate, 0.89 g sodium chloride and 12 cm3 water. Prepared dough was then sheeted to a thickness of 1 cm with a rolling pin. The cookies were cut round in shape with a cookie die of diameter 5.5 cm and transferred to a tray lined with aluminum foil and were baked at 180 °C for 15 min in an electric oven. The baked cookies were cooled to room temperature and packed in airtight containers for further analysis.
Proximate composition of cookies
Proximate composition of cookies was determined by same method as in flour blends described above.
Physical characters of cookies
The thickness of the cookies was measured by a Vernier Caliper & the diameter was determined using the scale placing them edge-to-edge. From the measurements taken, the spread ratio (W/T) was calculated, where W is the diameter & T is the thickness of cookies.
Texture analysis of cookies
Textural properties of the final products were investigated using Texture analyzer (TA XP plus Exponent Stable Micro System, Haslemere, U.K.). The fracture strength was measured using a 3-point Bending Rig & 5 kg load cell. The distance between two beams was 7 mm. Another identical beam was brought down from above at a pre-test speed of 1.0 mm/s, test speed of 3.0 mm/s, post-test speed of 10.0 mm/s. The downward movement was continued until the cookie broke. The peak force was reported as fracture strength.
Color analysis of cookies
Color was measured using a Hunter Lab, (Hunter Lab, Mini Scan XE Plus, and Reston, Virginia, USA). Color values L*, a* and b*, were recorded, each value being the average of four measurements at different points of the cookies. L* value is the lightness variable from 100 for perfect white to zero for black, while as a* and b* values are the chromaticity values that indicate + redness/ - greenness, respectively.
Non-enzymatic browning (NEB) index
Non-enzymatic browning index (NEB) of the samples was determined as previously reported by Sharma and Gujral (2011). The browning index (∆A) was calculated as follows:
Antioxidant properties of cookies
Antioxidant activity of the cookies was again determined using assays like DPPH, reducing power, FRAP and TPC, as described above.
Sensory evaluation of cookies
Cookies prepared from WF, BWF and their blends were subjected to sensory evaluation by a sensory panel of ten persons. Before the sensory evaluation was conducted, the panels were trained by using commercial cookies to get familiar with the use of rating method, terminology for each attribute and sensory characteristics. The judges rated the quality characteristics of each sample on a nine-point hedonic rating scale where, 9 = like extremely, 8 = like very much, 7 = like moderately, 6 = like slightly, 5 = neither like nor dislike, 4 = dislike slightly, 3 = dislike moderately, 2 = dislike very much, 1 = dislike extremely. The judges evaluated randomly coded cookies in terms of color, appearance, flavor, texture, taste and overall acceptability.
Statistical analysis
Experiments were performed in triplicates. The data was analyzed using one way analysis of variance (ANOVA) and Duncan test by SPSS (version 16.1.)
Results and discussion
Proximate composition of flours
The proximate composition as given in Table 1 shows that fat content of buckwheat and refined wheat flour does not differ significantly. However, a significant difference was observed in ash, crude fiber, carbohydrate and protein content of buckwheat and refined wheat flour. Buckwheat flour showed lower protein content (8.68 %) and higher crude fiber content (0.71 %) in comparison to refined wheat flour. The average protein content of buckwheat is 12.94 % (Guo et al 2007).
Table 1.
Proximate composition and antioxidant properties of blended flour and cookies
| Sample | Proximate composition | Antioxidant properties | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| % Moisture content | % Ash | % Fibre | % Fat | % Protein | TPC (nmolGAE/μl) | (μM Fe2+ /L) | % Inhibition of DPPH | % Reducing power | % Metal chelating activity | |
| A | 11.5a ± 0.45 | 1.41h ± 0.01 | 0.71b ± 0.01 | 1.81a ± 0.01 | 8.68g ± 0.07 | 12.07a ± 0.14 | 0.88d ± 0.01 | 73a ± 0.03 | 47.4a ± 11.14 | 3.35a ± 1.92 |
| B | 11.92f ± 0.01 | 1.39h ± 0.01 | 0.72b ± 0.05 | 1.79a ± 0.005 | 9.57h ± 0.03 | 10.99b ± 0.04 | 0.69c ± 0.18 | 66b ± 0.02 | 44.48b ± 13.97 | 15.34b ± 12.45 |
| C | 12.18g ± 0.08 | 1.34g ± 0.01 | 1.34g ± 0.01 | 1.78a ± 0.01 | 10.25i ± 0.22 | 9.75c ± 0.01 | 0.30b ± 0.06 | 59c ± 0.07 | 43.18c ± 14.56 | 23.23c ± 13.00 |
| D | 12.6h ± 0.08 | 1.34g ± 0.02 | 0.66ab ± 0.01 | 1.77a ± 0.01 | 11.23j ± 0.04 | 8.63c ± 0.02 | 0.23a ± 0.23 | 48d ± 0.04 | 41.07d ± 16.48 | 28.28d ± 17.20 |
| E | 12.97i ± 0.02 | 1.33g ± 0.01 | 0.62a ± 0.01 | 1.76a ± 0.01 | 12.15k ± 0.15 | 8.01d ± 0.01 | 0.08f ± 0.01 | 44e ± 0.05 | 37.30e ± 17.6 | 35.93e ± 11.63 |
| F | 13.20i ± 0.096 | 1.29f ± 0.01 | 0.62a ± 0.006 | 1.75a ± 0.01 | 13.14l ± 0.15 | 7.67e ± 0.01 | 0.08e ± 0.1 | 38 ± 0.01 | 33.15f ± 17.03 | 62.73f ± 18.5 |
| A1 | 1.90a ± 0.08 | 0.81b ± 0.01 | 3.12a ± 0.01 | 26.20c ± 0.18 | 4.17a ± 0.29 | 9.47a ± 0.14 | 1.87a ± .011 | 96.30a ± 0.01 | 73.73a ± 2.30 | 5.81f ±1.97 |
| B1 | 2.23b ± 0.02 | 0.72a ± 0.01 | 2.78b ± 0.01 | 24.17c ± 0.17 | 4.79b ± 0.15 | 7.65b ± 0.01 | 1.70 b ± .040 | 75.17b ± 0.10 | 68.09b ± 1.39 | 23.01e ± 15.00 |
| C1 | 2.30c ± 0.01 | 0.69c ± 0.01 | 2.6c ± 0.1 | 24.92b ± 0.12 | 5.22c ± 0.19 | 7.53c ± 0.01 | 0.62c ± 0.043 | 67.79c ± 0.06 | 63.41c ± 2.53 | 33.21d ± 20.86 |
| D1 | 2.47d ± 0.04 | 0.67c ± 0.01 | 2.10d ± 0.04 | 23.11b ± 0.14 | 5.71d ± 0.10 | 7.21d ± 0.00 | 0.61c ± 0.058 | 61.65d ± 0.06 | 49.82d ± 4.14 | 52.47c ± 12.87 |
| E1 | 3.07e ± 0.06 | 0.61d ± 0.01 | 1.94d ± 0.04 | 21.91a ± 0.1 | 6.22e ± 0.16 | 6.85d ± 0.01 | 0.16d ± .006 | 57.18e ± 0.10 | 45.5e ± 6.91 | 59.08b ± 10.69 |
| F1 | 3.34f ± 0.06 | 0.53e ± 0.005 | 1.30e ± 0.03 | 20.9a ± 0.32 | 7.30f ±0.1 | 6.71e ± 0.00 | 0.09d ± 0.001 | 55.53e ± 0.08 | 27.16f ± 18.91 | 72.19a ±9.44 |
The values with different superscript- small letters in a column differ significantly (p ≤ 0.05).The values are mean ± SD of three independent determinations. A,B,C,D,E,F, refer to the 100,80,60,40,20,0 -% BWF blends respectively and A1, B1, C1, D1, E1 and F1 refer to the cookies made from 100, 80, 60, 40, 20, and 0 % buckwheat flour respectively
Functional properties of flours
The functional properties of flours play an important role in the product development. Table 2 shows various functional properties of flours. The water absorption capacity (WAC) of buckwheat flour (BWF) was found to be significantly (p ≤ 0.05) lower than that of wheat flour and this property decreased as the proportion of BWF increased in the flour blends. Difference in protein structure and the presence of different hydrophilic carbohydrates might be responsible for variations in the WAC between both the flours (Kaur et al. 2014). The oil absorption capacity (OAC) of BWF was significantly higher than that of refined WF and this property of corresponding flour blends increased as the BWF proportion increased in the blend. The oil absorption capacity (OAC) of flour is equally important as it improves the mouth feel and retains the flavor. Variations in the presence of non-polar side chains, which might bind the hydrocarbon side chains of oil among the flours, possibly explain difference in the oil binding capacity of the flours (Kaur et al. 2014). The swelling capacities of BWF were lower than that of refined WF. Foaming capacity and foaming stability of 100 % BWF were found significantly higher and lower respectively than that of 100 % WF. Results of swelling power and foaming capacity are similar to those found by Kaur et al. (2014) and Yadav et al. (2010). Foaming capacity is assumed to be dependent on the configuration of protein molecules. Flexible proteins have good foaming capacity but highly ordered globular molecule gives low foam ability (Baljeet et al. 2010; Graham and Philips 1976).
Table 2.
Functional properties of blended flour
| WAC | OAC | FC (%) | FS (%) | SP | TD | STD D | |
|---|---|---|---|---|---|---|---|
| A | 127.17a ± 6.38 | 169.89c ± 2.88 | 31.65d ± 0.34 | 74.37a ± 0.94 | 3.89f ± 0.03 | 0.84e ± 0.04 | 0.64e ± 0.01 |
| B | 126a ± 3.04 | 160.36b ± 0.34 | 20.78c ± 0.37 | 66.74b ± 0.31 | 4.38e ± 0.06 | 0.63d ± 0.03 | 0.43d ± 0.01 |
| C | 131.1ab ± 0.95 | 157.96b ± 1.15 | 14.90b ± 0.45 | 64.13c ± 0.72 | 4.93d ± 0.04 | 0.54c ± 0.03 | 0.41c ± 0.00 |
| D | 131.53ab ± 0.5 | 151.36a ± 2.02 | 8.85a ± 0.09 | 62.13d ± 0.43 | 5.13c ± 0.01 | 0.51bc ± 0.01 | 0.40c ± 0.01 |
| E | 134.7b ± 0.58 | 151.24a ± 2.31 | 8.41a ± 0.11 | 56.43e ± 0.63 | 5.31b ± 0.04 | 0.46b ± 0.05 | 0.34b ± 0.01 |
| F | 147.33c ± 1.83 | 147.87a ± 1.22 | 8.4a ± 0.29 | 54.12f ± 0.94 | 5.78a ± 0.09 | 0.33a ± 0.02 | 0.26a ± 0.01 |
The values with different superscript- small letters in a column differ significantly (p ≤ 0.05).The values are mean ± SD of three independent determinations. A,B,C,D,E,F, refer to the 100,80,60,40,20,0 -% BWF blends respectively
Bulk density
Bulk density of flour blends was increased with increase in BWF content. True/compact and standard bulk densities of six samples of blends were analyzed and it was seen that compact density of each sample was higher than standard bulk density (Table 2). Usually compacted densities are higher than the standard densities.
Antioxidant properties of BWF, WF and their blends
Total phenolic content
The total phenolic content of each flour extract is given in Table 1. Phenolic content in 100 % wheat flour was found to be 7.67 nmol GAE/μl extract, while in buckwheat flours it was found to be (12.07 nmol GAE/μl extract). Comparing the results among flour types, higher content of phenolics was found in 100 % Buckwheat flour and the phenolic content decreased significantly(p ≤ 0.05) in the corresponding blends, as the BWF proportion decreased in the blend. The total phenolic content in the blends 20, 40, 60, 80 % BWFs were found to be 8.01, 8.63, 9.75 & 10.99 (nmol GAE/μl) respectively. Zielinski and Kozlowska (2000) reported significant correlation between antioxidative activities and total phenolics of cereals and their fractions. A correlation between antioxidative activity and rutin content or total flavonoids content in buckwheat cultivars has already been reported (Jiang et al. 2007).
DPPH scavenging potential
DPPH radical scavenging activity were higher in buckwheat flour and BWF blends than in wheat flour (Table 1) as the consequence of higher polyphenolic content in buckwheat flour. A high correlation coefficient (R2 = 0.96) was seen between TPC and DPPH radical scavenging activity of samples. Reduction of DPPH radicals reveals that examined extracts possess radical inhibitors or scavengers with possibility to act as primary antioxidants. Similar results were found by Sedej et al., (2011b) for milling fractions of wheat and buckwheat. They might react with free radicals, particularly with the peroxy radicals, which are the major propagators of the auto-oxidation chain of fat, thereby terminating the chain reaction (Sharma and Gujral 2014).
Reducing power
The values of reduction power (%) of each sample are given in Table 1. Reducing power assay showed high differences in antioxidant activity (AOA) of wheat and buckwheat flour and their blend extracts. Better antioxidant activity was found in 100 % buckwheat than in 100 % wheat flour. Strong antioxidant activity of buckwheat flour extract might be due to the presence of polyphenols, especially rutin, quercetin, and quercetin (Zhang et al. 2012). Rutin possesses all structural features, which have been demonstrated to increase the antioxidative activity of flavonoids and their O-glycosides (Sedej et al. 2011b). A progressive increase in the level of BW flour in the blends increased reducing power significantly which is attributed to the higher reducing power of the BW flour as compared to refined wheat flour. Lin et al. (2009) found similar results in BW enhanced bread. Reducing power of flour blends were correlated with total phenolic content which showed the positive correlation (R2 = 0.89) among them.
FRAP value
FRAP value of WF-BWF blends increased with the incorporation of buckwheat (Table 1). FRAP was found to be highest for whole buckwheat flour and it decreased as the proportion of BWF decreased in the blends. These results are due to higher reducing power of buckwheat than wheat flour (Sedej et al. 2011b). Poly phenolic content in buckwheat especially rutin and quercetin possess the reducing ability (Zhang et al. 2012). The correlation between FRAP and TPC showed the value of R2 = 0.98.
Metal chelating activity
The chelating activity on Fe2+ were significantly higher for wheat flours extracts than for buckwheat flours extracts (Table 1). Since ferrous and cupric ions are the most effective pro-oxidant in food systems (Sedej et al. 2011b) and ferrous ions are commonly found in food systems, high chelating activity of investigated extracts would be beneficial in retarding metal-catalyzed oxidation. The possibility of complexing metal ions depends on structure of the molecule, in case of polyphenols (predominant flavonoids from buckwheat and phenolic acids from wheat), it is depends on the number and position of OH- and CH3O- groups. Although rutin possesses more structural features than ferulic acid for complexing metal ions, the methanolic extracts of wheat flours exhibited more potent chelating activity on Fe2+ than the buckwheat extracts. Lower antioxidative capacity of rutin as metal chelator in comparison to ferulic acid (and other-hydroxycinnamic acids from wheat flour) might be due to steric hindrance of the sugar moiety of rutin (Sedej et al. 2011b). The presence of some higher potent chelating component(s) in wheat flour extract like ferulic acid might be responsible for its higher chelating capacity than buckwheat flour extract
Proximate composition of cookies
Proximate composition of cookies made from BWF-WF blend is shown in Table 1. The ash content of cookies increased with the addition of BWF. The increase in ash content may be due to the high mineral content in the BWF i.e., iron, copper and magnesium (Yadav et al. 2010). The moisture content ranged from 2.43 % (100 % BWF cookies) to 3.37 % (0 % BWF cookies). The decrease in moisture content may be due to the decrease in protein content. Mustafa et al. (1986) reported an increase in moisture content of bakery products with increase in protein content. The fat content of 0 % BWF cookie was found to be 20.9 % and it increased to 26.20 % in 100 % BWF cookies. This was probably due to the oil retention ability of buckwheat flour during baking process (Kaur et al. 2014; Baljeet et al. 2010). No definite trend in increase or decrease in crude fiber contents was observed. The protein content of cookies ranged from 4.17 to 7.30 %. The cookies showed decrease in protein content when BWF concentration was increased.
Physical properties of cookies made from BWF-WF blends
The physical properties of cookies prepared from BWF and refined WF are shown in Table 3. The diameters of cookies made from 20, 40, 60, 80, 100 % BWF were found significantly lower than 100 % wheat flour. The thickness of cookies ranged from 0.11 to 0.73 cm. It increased with the incorporation of BWF. Increase in thickness may be due to the decrease in diameter on addition of BWF content in cookie dough. The changes in diameter and thickness were reflected in spread ratio. Spread ratio decreased with the addition of buckwheat flour. Sharma and Gujral (2014) obtained similar results in barley blend cookies. Reduced spread ratio of cookies made from wheat flour with substituted fenugreek and buckwheat flour has been reported (Yadav et al. 2010; Kaur et al 2014). Reduced spread ratios of BWF fortified cookies were attributed to the fact that composite flours apparently form aggregates with increased numbers of hydrophilic sites available that compete for the limited free water in biscuit dough (Yadav et al 2010; McWatters 1978). The weight of cookies increased as the concentration of BWF increased in the blends. The range of cookie weight was 24gm to 27gm with maximum value in 100 % BWF cookies. The increase in cookie weight was probably due to the ability of buckwheat flour to retain oil during baking process (Baljeet et al 2010).
Table 3.
Physical properties of cookies
| Sample | Colour analysis | Spread ratio | NEB | ||
|---|---|---|---|---|---|
| l* | a* | b* | |||
| A1 | 38.13a ±0.44 | 10.1a ± 0.51 | 20.54a ±0.38 | 07.30a ± 0.01 | 0.024f ± 0.1 |
| B1 | 46.51b ±0.40 | 7.91b ± 1.45 | 27.44b ± 0.37 | 07.30a ± 0.01 | 0.019e ± 0.1 |
| C1 | 46.94b ±0.35 | 7.71a ± 1.29 | 28.56c ± 0.51 | 12.11b ± 0.01 | 0.016d ± 0.1 |
| D1 | 48.56c ±0.61 | 6.83d ± 1.73 | 31.04d ± 0.86 | 14.52c ± 0.01 | 0.015c ± 0.1 |
| E1 | 51.92d ±0.88 | 6.49e ±1.83 | 33.19e ± 0.43 | 15.65d ± 0.01 | 0.014b ± 0.1 |
| F1 | 72.55e ±0.71 | 6.00f ± 0.94 | 35.39f ± 0.75 | 72.42e ± 0.01 | 0.008a ± 0.1 |
The values with different superscript- small letters in a column differ significantly (p ≤ 0.05).The values are mean ± SD of three independent determinations. A1, B1, C1, D1, E1 and F1 refer to the cookies made from 100, 80, 60, 40, 20, and 0 % buckwheat flour respectively
Texture profile analysis
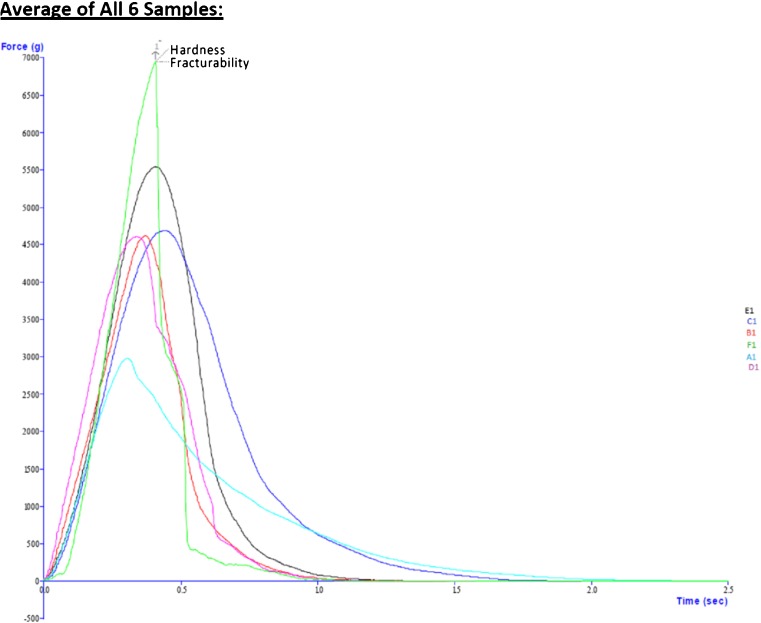
The hardness of the cookies (of six samples) is determined from curve shown in Fig. 1. The cookies made from 100 % BWF were found to be the least hard and most fragile. The hardness of cookies decreased with the incorporation of BWF. The hardness of WF cookies (100 %) and 100 % BWF cookie was 6952.48 g and 2987.25 g, respectively. The order of the hardness was found to be as follows; A1 < B1͠=C1͠=D1 < E1 < F1, where A1,B1,C1,D1,E1 and F1 refer to cookies made from 100, 80, 60, 40, 20 and 0 % BWF. The values of hardness and fracturability are given in the Table 4. Baljeet et al. (2010) for buckwheat biscuits, found similar results. The dilution of gluten content in the flour blends due to addition of less gluten component (buckwheat) to it, may be responsible for the softer cookies with increasing BWF content.
Fig. 1.
Where A1, B1,C1,D1, E1 and F1 refers to the cookies made from 100, 80,60,40,20,0-% BWF blends. OBSERVATIONS: Once the trigger force is attained the force is seen to increase until such time as the biscuit/cookie fractures and falls into two pieces. This is observed as the maximum force and can be referred to as the ‘hardness’ of the sample. The distance at the point of break is the resistance of the sample to bend and so relates to the ‘fracturability’ of the sample i.e., a sample that breaks at a very short distance has a high fracturability
Table 4.
Sensory analysis and textural analysis of cookies
| Quality parameter | Appearance | Colour | Flavour | texture | Overall acceptability | Hardness(g) force-1 | Fracturability(mm) distance -1 |
|---|---|---|---|---|---|---|---|
| A1 | 6.5 ± 0.75 | 6.37 ± 0.91 | 5.87 ± 1.55 | 5.00 ± 1.69 | 5.25 ± 1.48 | 2987.25 | 0.92 |
| B1 | 6.75 ± 0.70 | 6.00 ± 0.75 | 6.37 ± 1.18 | 5.62 ± 1.18 | 6.00 ± 1.30 | 4614.29 | 1.01 |
| C1 | 7.62 ± 0.51 | 7.37 ± 0.11 | 6.75 ± 0.70 | 6.75 ± 0.70 | 7.00 ± 0.53 | 4625.25 | 1.11 |
| D1 | 8.5 ± 0.53 | 8.37 ± 0.74 | 7.37 ± 0.74 | 7.5 ± 0.75 | 7.75 ± 0.46 | 4700.88 | 1.31 |
| E1 | 8.75 ± 0.16 | 8.75 ± 0.46 | 8.12 ± 0.64 | 7.75 ± 0.46 | 8.125 ± 0.35 | 5551.84 | 1.22 |
| F1 | 9.00 ± 0 | 8.85 ± 0.00 | 8.62 ± 0.51 | 8.62 ± 0.51 | 8.37 ± 0.51 | 6952.48 | 1.22 |
A1, B1, C1, D1, E1 and F1 refer to the cookies made from 100, 80, 60, 40, 20, and 0 % buckwheat flour respectively
Color analysis
The surface color of the cookie decreased for lightness (a*) and yellowness (b*) as the BWF content increased in the blends (Table 3). While as, Increase in a* (redness) was observed with increase in BWF content in the cookies. Similar results were found in buckwheat (15 % level) enhanced wheat bread by Lin et al., (2009). This may be due to the browning reactions caused during baking. Maillard browning and caramelization of sugar is considered to produce brown pigments during baking (Laguna et al 2011). These browning reactions are influenced by many factors such as water activity, pH, temperature, sugars, type and ratio of amino compounds (Sharma and Gujral 2013; Stojceska et al 2009).
Non-enzymatic browning index
Incorporation of BW flour to wheat flour affected significantly (P ≤ 0.05) on non-enzymatic browning index of cookies. Non-enzymatic browning index of pure wheat and pure buckwheat cookies was observed to be 0.008 and 0.024 respectively (Table 3). Baking of cookies leads to a significant increase in non-enzymatic browning index with increasing order of incorporation of BWF. Ramirez-Jimenez et al., (2000) also reported a significant increase in browning upon baking of bread. Zielinski et al., (2009) reported that raw buckwheat had browning index of 0.24 (absorbance at 420 nm) while after roasting it was increased significantly up to 0.34. Increase in browning is associated with Maillard browning occurring during baking of cookies. It has been widely accepted that Maillard browning is influenced by many factors which further influences the intensity of brown pigment produced (Michalska et al 2008). Besides Maillard reactions, browning may also be contributed by caramelization of sugar, as cookie formulation is high in sugar (Sharma and Gujral 2014; Ajandouz et al 2001). The non-enzymatic browning index of the cookies decreased (Table 3) as the levels of WF flour increased in the blends, which may be due to possible dilution of the sugars and proteins of the wheat flour upon incorporation of whole BW flour.
Antioxidant properties of cookies (prepared from BWF, WF, and their blends
BWF showed greater antioxidant activity than wheat flour due to greater amount of polyphenols. Baking resulted in greater increase in antioxidant activity in case of buckwheat cookies, which can be due to higher generation of Maillard compounds which was also supported by higher NEB values for buckwheat cookies than wheat flour cookies.
Total phenolic content
Total phenolic content was found to be significantly (P ≤ 0.05) different in 100 % BWF cookies and 100 % WF cookies (Table 3). This is due to the presence of flavonoids in buckwheat. Baking leads to a decrease in total phenolic content as compared to raw flour blends. A decrease of 21.54 & 12.5 % was seen in case of blends made of 100 & 0 % BWF; it suggests that antioxidants of buckwheat are more susceptible to changes during baking. Despite greater decrease in TPC of buckwheat during baking, the TPC value in buckwheat cookies was still higher than that of wheat cookies. Sharma et al. (2012) also found decrease in TPC in barley cultivars by giving them temperature of 150–180 °C. Sompong et al., (2011) and Holtekjolen et al., (2008) reported that the baking of bread containing barley flour exhibited decrease in TPC. The decrease in TPC may be due to alteration in the chemical structure of the phenolic compounds, possible polymerization leading to reduced extractability and oxidation (Alton et al. 2009; Sharma and Gujral 2011).
FRAP value
Ferric reducing power of cookies was found to increase with increase in BWF proportion in cookies (Table 1). This is due due to the presence of polyphenols, especially rutin, it is main antioxidative component found in buckwheat (Zhang et al 2012). Rutin possesses all structural features, which have been demonstrated to increase antioxidative activity of flavonoids, and their O-glycosides (Sedej et al. 2011b). Baking increased the ferric reducing power of cookies. After baking, an increase of 112.5 & 12.5 % was seen in blends containing 100 & 0 % of BWF. The baking of cookies significantly increase the ferrous reducing power due to formation of maillard reaction products, different factor such as glucose content, baking temperature and baking time significantly affect the antioxidant properties (Morales et al 2009).
DPPH radical scavenging activity
The percentage inhibition of DPPH radicals for each sample was found to increase with increase in BWF content in the cookies (Table 1). This is attributed to highest antioxidant activity of BWF (Sedej et al 2011b). Baking resulted in increase in scavenging activity of radicals as compared to raw flour blends. Baking resulted in 31.51 & 28.94 % increase in blends containing 100 and 0 % of buckwheat respectively. Increase in antioxidant activity because of processing such as baking (Sharma and Gujral 2014) and microwave roasting (Baba et al 2014) has earlier been reported. This may be attributed due to the formation of brown pigments melanoidins, which are the products of maillard reaction (a non-enzymatic browning reaction), which takes place during baking (Manzocco et al 2000; Xu and Chang 2008).
Reducing power
Reducing power was increased in the cookies with increase in BWF content in the blends (Table 1). The reducing power of an antioxidant compound is associated with the presence of reductones and their antioxidant capacity is based on the breaking of the free radical chain reaction by donating a hydrogen atom, and preventing peroxide formation. Baking of cookies lead to a significant increase in reducing power, the highest reducing power was observed for cookies prepared from only buckwheat flour. Because of baking, an increase of 55.54 & 12.09 % was seen in blends containing 100 & 0 % buckwheat flour respectively. Increase in reducing power upon baking has been reported by Filipcev et al., (2011) for bread prepared by incorporation of buckwheat and rye flour to wheat flour. Lin et al., (2009) reported that incorporation of husked and unhusked buckwheat flour in wheat flour at 15 % levels increased the reducing power significantly. The Maillard reaction products, which were generated during the baking of cookies, might contribute in increasing the reducing power (Sharma and Gujral 2014).
Metal chelating activity of cookies
The cookies with higher proportion of WF showed higher metal chelating activity (Table 1). Baking of BWF-WF blend dough leads to a significant increase in metal chelating activity. Filipcev et al., (2011) reported similar results for cookies prepared by incorporating buckwheat and rye flour to wheat flour. Similar results were found in the wheat and barley flour blend cookies (Sharma and Gujral 2014). An increase of 73.43 & 15.08 % was seen in blends containing 100 & 0 % of BWF. The increase may be due to Maillard browning; the soluble part of these compounds is known to have metal chelating activity (Sharma and Gujral 2014) and the formation of these compounds depends upon different factors such as chemical composition of raw material, process conditions and water activity. It has been widely reported that thermal processing of food material generates maillard browning pigments, which are known to have greater antioxidant activity than their precursors (Sharma and Gujral 2014; Nicoli et al 1997).
Sensory analysis of cookies
The cookies prepared from WF, BWF and their blends viz. 0, 20, 40, 60, 80 and 100 BWF were evaluated for their color, appearance, flavor, taste and overall acceptability using 9-point hedonic scale (Table 4). Panelists rated the cookies prepared from WF with best quality whereas those prepared from BWF had the lowest sensorial scores. Among blends of 20,40, 60 80, 100, and 0 % BWF, the panelists rated the highest score for the cookies made from 40 %BWF in terms of color, appearance, flavor, taste and overall acceptability. Cookies prepared from BWF were darker in color as compared to those made from WF. Yadav et al., (2010) found similar results by incorporating BWF to WF at the level of 40 g/100 g. The low score for taste of BWF cookies may be due to the greater number of phenolic compounds (rutin, quercetin and protocatechuic acid) present in BWF than wheat cookies (Sedej et al. 2011a).
Conclusion
From our study, it can be concluded that addition of Buckwheat flour in wheat flour enhances the crude fiber, fat, mineral, total phenolic content and antioxidant properties of cookies. There was decrease in protein content in wheat flour cookies upon addition of buckwheat flour, due to dilution of gluten content in cookies. Functional properties were also significantly affected. Buckwheat flour could be a potential raw material for cookies with improved bioactivity potential. Baking decreased total phenolic content but an increase in overall antioxidant activity was observed, greater increase in antioxidant activity of buckwheat cookies than wheat cookies can be attributed to greater generation of melanoidins in the former which is supported by higher NEB values of buckwheat cookies than wheat cookies. The Overall acceptability of cookies by sensory analysis was highest at 40 % level of blending. This study may provide scope for utilization of buckwheat in other food products.
Footnotes
The author Waqas N. Baba is the mentor of the article.
Contributor Information
Adil Gani, Phone: +918715022903, Email: adil.gani@gmail.com.
Mudasir Ahmad, Email: mudasirahmad63@yahoo.in.
Umar Shah, Email: umarzahoorshah@yahoo.com.
Waqas N. Baba, Email: waqasbaba7@gmail.com
F. A. Masoodi, Email: masoodi_fa@yahoo.co.in
Sajid Maqsood, Email: sajid.m@uaeu.ac.ae.
Asir Gani, Email: asir.gani@gmail.com.
Idress Ahmed Wani, Email: idwani07@gmail.com.
S. M. Wani, Email: wanisajad82@gmail.com
References
- Ajandouz EH, Tchiakpe LS, Ore FD, Benajiba A, Puigserver A. Effects of pH on caramelization and Maillard reaction kinetics in fructose elysine model systems. J Food Sci. 2001;66:926–931. doi: 10.1111/j.1365-2621.2001.tb08213.x. [DOI] [Google Scholar]
- Ajila CM, Aalami M, Leelavathi K, Prasada Rao UJS (2010) Mango peel powder: a potential source of antioxidant and dietary fiber in maca roni preparations. Innov Food Sci Emerg Technol 11:219–224
- Ahmad M, Gani A, Baba WN, Wani TA, Gani A, Shah U, Wani SM, Masoodi FA (2015) Effect of green tea powder on thermal, rheological & functional properties of wheat flour and physical, nutraceutical & sensory analysis of cookies. J Food Sci Technol. doi:10.1007/s13197-014-1701-3 [DOI] [PMC free article] [PubMed]
- Alton A, McCarthy KL, Maskan M. Effect of extrusion process on antioxidant activity, total phenolics and β-glucan content of extrudates developed from barley– fruit and vegetable by-products. Int J Food Sci Technol. 2009;44:1263–1271. doi: 10.1111/j.1365-2621.2009.01956.x. [DOI] [Google Scholar]
- AOAC. (1990) Washington, DC, USA: Association of Official Analytical Chemists. Official methods of analysis (15th ed.)
- Baba N, Rashid I, Shah A, Ahmad M, Gani A, Masoodi FA. Effect of microwave roasting on antioxidant and anti-cancerous activities of barley flour. J Saudi Soc Agric Sci. 2014;27:143–154. [Google Scholar]
- Baljeet SY, Ritika BY, Roshan LY. Studies on functional properties and incorporation of buckwheat flour for biscuit making. Int Food Res J. 2010;17:1067–1076. [Google Scholar]
- Benzie IIF, Strain JJ. Ferric reducing antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration, oxidants and antioxidants. Methods Enzymol. 1999;299:15–27. doi: 10.1016/S0076-6879(99)99005-5. [DOI] [PubMed] [Google Scholar]
- Dinis TCP, Madeira VMC, Almeidam LM. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and peroxyl radical scavengers. Arch Biochem Biophys. 1994;315:161–169. doi: 10.1006/abbi.1994.1485. [DOI] [PubMed] [Google Scholar]
- Eggum BO, Kreft I, Javornik B. Chemical composition and protein quality of buckwheat (Fagopyrum esculentumMoench) Plant Foods Hum Nutr. 1981;30:175–179. doi: 10.1007/BF01094020. [DOI] [Google Scholar]
- Filipcev B, Smurina O, Sakac M, Sedej I, Jovanov P, Pestoric M, et al. Feasibility of use of buckwheat flour as an ingredient in ginger nut biscuit formulation. Food Chem. 2011;125:164–170. doi: 10.1016/j.foodchem.2010.08.055. [DOI] [Google Scholar]
- Gani A, Wani SM, Masoodi FA, Salim R. Characterization of rice starches extracted from Indian cultivars. Food Sci Technol Inter. 2013;19(2):143–152. doi: 10.1177/1082013212442189. [DOI] [PubMed] [Google Scholar]
- Gani A, Broadway AA, Ahmad M, Ashwar BA, Wani AA, Wani SM, Masoodi FA, Khatkar S (2014a) Effect of whey and casein protein hydrolysates on rheological, textural and sensory properties of cookies. J Food Sci Technol. doi:10.1007/s13197-014-1649-3 [DOI] [PMC free article] [PubMed]
- Gani A, Rasool N, Shah A, Ahmad M, Gani A, Masoodia FA. DNA scission inhibition, antioxidant, and antiproliferative activities of water chestnut (Trapa natans) extracted in different solvents. Cyta-J Food. 2014 [Google Scholar]
- Graham DE, Philips MC. The conformation of proteins at the air-water interface and their role instabilizing foam. In: Akers RJ, editor. Foams. New York: Academic; 1976. pp. 237–255. [Google Scholar]
- Guo YZ, Chen QF, Yang LY, Huang YH. Analyses of the seed protein contents on the cultivated and wild buckwheat (Fagopyrum esculentum) resources. Genet Resour Crop Evol. 2007;54:1465–1472. doi: 10.1007/s10722-006-9135-z. [DOI] [Google Scholar]
- Holasova M, Fiedlerova V, Smrcinova H, Orsak M, Lachman J, Vavreinova S. Buckwheat—the source of antioxidant activity in functional foods. Food Res Int. 2002;35:207–211. doi: 10.1016/S0963-9969(01)00185-5. [DOI] [Google Scholar]
- Holtekjolen AK, Baevere AB, Rodbotten M, Berg H, Knutsen SH. Antioxidant properties and sensory profiles of breads containing barley flour. Food Chem. 2008;110:414–421. doi: 10.1016/j.foodchem.2008.02.054. [DOI] [PubMed] [Google Scholar]
- Ikeda K. Buckwheat: composition, chemistry and processing. Adv Food Nutr Res. 2002;44:395–434. doi: 10.1016/S1043-4526(02)44008-9. [DOI] [PubMed] [Google Scholar]
- Jiang P, Campbell FCB, Pierce GJ, Austria A, Briggs CJ. Rutin and Flavonoid contents in three buckwheat species Fagopyrum esculentum, F. tataricum, and F. homotropicum and their protective effects against lipid peroxidation. Food Res Int. 2007;3:356–364. doi: 10.1016/j.foodres.2006.10.009. [DOI] [Google Scholar]
- Kaur M, Singh KS, Arora AP, Sharma A. A gluten free cookies prepared from buckwheat flour by incorporation of various gums: physicochemical and sensory properties. LWT Food Sci Technol. 2014 [Google Scholar]
- Laguna L, Salvador A, Sanz T, Fiszman SM. Performance of a resistant starch rich ingredient in the baking and eating quality of short-dough biscuits. LWT Food Sci Technol. 2011;44:737–746. doi: 10.1016/j.lwt.2010.05.034. [DOI] [Google Scholar]
- Li SQ, Zhang QH. Advances in the development of functional foods from buckwheat. Crit Rev Food Sci Nutr. 2001;41:451–464. doi: 10.1080/20014091091887. [DOI] [PubMed] [Google Scholar]
- Lin L, Liu H, Yu Y, Mau J. Quality and antioxidant property of buckwheat enhanced wheat bread. Food Chem. 2009;112:987–991. doi: 10.1016/j.foodchem.2008.07.022. [DOI] [Google Scholar]
- Manzocco LS, Calligaris S, Mastrocola D, Nicoli MC, Lerici CR. Review of non-enzymatic browning and antioxidant capacity in processed foods. Trends Food Sci Technol. 2000;11:340–346. doi: 10.1016/S0924-2244(01)00014-0. [DOI] [Google Scholar]
- McWatters KH. Cookie baking properties of defatted peanuts, soya bean and field pea flours. Cereal Chem. 1978;55:853–863. [Google Scholar]
- Michalska A, Amigo-Benavent M, Zielinski H, Del-Castillo MD. Effect of bread making on formation of maillard reaction products contributing to the overall antioxidant activity of rye bread. J Cereal Sci. 2008;48:123–132. doi: 10.1016/j.jcs.2007.08.012. [DOI] [Google Scholar]
- Morales FJ, Martin S, Acar OC, Arribas-Lorenzo G, Gokmen V. Antioxidant activity of cookies and its relationship with heat-processing contaminants: a risk/benefit approach. Eur Food Res Technol. 2009;228:345–354. doi: 10.1007/s00217-008-0940-9. [DOI] [Google Scholar]
- Mustafa AI, Alwessali MS, SI-Busha OM, Al-Amia RH. Utilization of cowpea flour and protein isolate in bakery products. Cereals Food World. 1986;31:756–759. [Google Scholar]
- Narayana K, Narasinga Rao MS. Functional properties of raw and heat processed winged bean flour. J Food Sci. 1982;42:534–538. [Google Scholar]
- Nicoli MC, Anese M, Parpinel M, Franceschi S, Lerici CR. Loss and/or formation of antioxidants during food processing and storage. Cancer Lett. 1997;114:71–74. doi: 10.1016/S0304-3835(97)04628-4. [DOI] [PubMed] [Google Scholar]
- Ramirez-Jimenez A, Guerra-Hernandez E, GarciaVillanova B. Browningindicators in bread. J Agric Food Chem. 2000;48:4176–4181. doi: 10.1021/jf9907687. [DOI] [PubMed] [Google Scholar]
- Sakac M, Torbica A, Sedej I, Hadnadev M. Influence of bread making on antioxidant capacity of gluten free breads based on rice and buckwheat flours. Food Res Int. 2011;44:2806–2813. doi: 10.1016/j.foodres.2011.06.026. [DOI] [Google Scholar]
- Sedej I, Sakak M, Mandic A, Misan A, Tumbas V. Assessment of antioxidant activity and rheological properties of wheat and buckwheat milling fractions. J Cereal Sci. 2011;54:347–353. doi: 10.1016/j.jcs.2011.07.001. [DOI] [Google Scholar]
- Sedej I, Sakac M, Mandic A, Misan A, Pestoric M, Simurina O. Quality assessment of gluten-free crackers based on buckwheat flour. LWT Food Sci Technol. 2011;44:694–699. doi: 10.1016/j.lwt.2010.11.010. [DOI] [Google Scholar]
- Shah U, Baba WN, Ahmad M, Shah A, Gani A, Masoodi FA, Gani A. Invitro antioxidant and antiproliferative activities of seed extracts of Nympheae Mexicana in different solvents and GC-MS analysis. Int J Drug Dev Res. 2014;6(4):280–285. [Google Scholar]
- Shah A, Ahmad M, Ashwar BA, Gani A, Masoodi FA, et al. Effect of γ-irradiation on structure and nutraceutical potential of β-d-glucan from barley (Hordeum vulgare) Int J Biol Macromol. 2015;72:1168–1175. doi: 10.1016/j.ijbiomac.2014.08.056. [DOI] [PubMed] [Google Scholar]
- Sharma P, Gujral HS. Effect of sand roasting and microwave cooking on antioxidant activity of barley. Food Res Int. 2011;44:235–240. doi: 10.1016/j.foodres.2010.10.030. [DOI] [Google Scholar]
- Sharma P, Gujral HS. Extrusion of hulled barley affecting b-glucan and properties of extrudates. Food Bioprocess Technol. 2013;6:1374–1389. doi: 10.1007/s11947-011-0777-2. [DOI] [Google Scholar]
- Sharma P, Gujral HS. Cookie making behavior of wheat-barley flour blends and effects on antioxidant properties. LWT Food Sci Technol. 2014;55:301–307. doi: 10.1016/j.lwt.2013.08.019. [DOI] [Google Scholar]
- Sharma P, Gujral HS, Singh B. Antioxidant activity of barley as affected by extrusion coking. Food Chem. 2012;131:1406–1413. doi: 10.1016/j.foodchem.2011.10.009. [DOI] [Google Scholar]
- Soluski FW, Garratt MO, Slinkard AE. Functional properties of ten legume flours. Int J Food Sci Technol. 1976;9:66–69. [Google Scholar]
- Sompong R, Siebenhandel-Ehn S, Berghofer E, Schoenlechner R. Extrusion cooking properties of white and coloured rice varieties. Starch/Stärke. 2011;63:55–63. doi: 10.1002/star.201000086. [DOI] [Google Scholar]
- Stojceska V, Ainsworth P, Plunkett A, Ibanoglu S. The effect of extrusion cooking using different water feed rates on the quality of ready-to-eat snacks made from food by products. Food Chem. 2009;114:226–232. doi: 10.1016/j.foodchem.2008.09.043. [DOI] [Google Scholar]
- Tyagi SK, Manikantan MR, Oberoi HS, Kaur G. Effect of mustard flour incorporation on nutritional, textural, and organoleptic characteristics of biscuits. J Food Eng. 2007;80(4):1043–1050. doi: 10.1016/j.jfoodeng.2006.08.016. [DOI] [Google Scholar]
- Xu B, Chang SKC. Total phenolics, phenolic acids, isoflavones, and anthocyanins and antioxidant properties of yellow and black soyabeansas affected by thermal processing. J Agric Food Chem. 2008;56:7165–7175. doi: 10.1021/jf8012234. [DOI] [PubMed] [Google Scholar]
- Yadav BS, Ritika BY, Roshan LY. Studies on functional properties and incorporation of buckwheat flour for biscuit making. Int Food Res J. 2010;17:1067–1076. [Google Scholar]
- Zdunczyk Z, Flis M, Zielinski H, Wroblewska M, Antoszkiewicz Z, Juskiewicz J. In vitro antioxidant activities of barley, husked oat, naked oat, triticale, and buckwheat wastes and their influence on the growth and biomarkers antioxidant status in rats. J Agric Food Chem. 2006;54:4168–4175. doi: 10.1021/jf060224m. [DOI] [PubMed] [Google Scholar]
- Zhang ZL, Zhou ML, Tang LFL, Tang YX, Shao JR, Xue WT, Wu YM. Bioactive compounds in functional buckwheat food. Food Res Int. 2012;49:389–395. doi: 10.1016/j.foodres.2012.07.035. [DOI] [Google Scholar]
- Zielinski H, Kozlowska H. Antioxidant activity and total phenolics in selected cereal grains and their different morphological fractions. J Agric Food Chem. 2000;48(6):2008–2016. doi: 10.1021/jf990619o. [DOI] [PubMed] [Google Scholar]
- Zielinski H, Michalska A, Amigo-Benavent M, Dolores Del Castillo M, Piskula MK. Changes in protein quality and antioxidant properties of buckwheat seeds and groats induced by roasting. J Agric Food Chem. 2009;57:4771–4776. doi: 10.1021/jf900313e. [DOI] [PubMed] [Google Scholar]