Abstract
Effect of isolated astaxanthin (ASX) and astaxanthin esters (ASXEs) from green microalga-Haematococcus pluvialis on hepatotoxicity and antioxidant activity against carbon tetrachloride (CCl4) induced toxicity in rats was compared with synthetic astaxanthin (SASX). ASX, ASXEs, and SASX, all dissolved in olive oil, fed to rats with 100 and 250 μg/kg b.w for 14 days. They were evaluated for their hepatoprotective and antioxidant activity by measuring appropriate enzymes. Among the treated groups, the SGPT, SGOT and ALP levels were decreased by 2, 2.4, and 1.5 fold in ASXEs treated group at 250 μg/Kg b.w. when compared to toxin group. Further, antioxidant enzymes catalase, glutathione, superoxide dismutase and lipid peroxidase levels were estimated in treated groups, their levels were reduced by 30–50 % in the toxin group, however these levels restored by 136.95 and 238.48 % in ASXEs treated group at 250 μg/kg. The lipid peroxidation was restored by 5.2 and 2.8 fold in ASXEs and ASX treated groups at 250 μg/kg. The total protein, albumin and bilirubin contents were decreased in toxin group, whereas normalized in ASXEs treated group. These results indicates that ASX and ASXEs have better hepatoprotection and antioxidant activity, therefore can be used in pharmaceutical and nutraceutical applications and also extended to use as food colorant.
Keywords: H. pluvialis; ASX; ASXEs; CCl4; Antioxidants; Hepatoprotection; SGPT, SGOT, ALP
Introduction
The imbalance of reactive oxygen species concentration to the antioxidative defense mechanism is said to be oxidative stress (Uttara et al. 2009), which causes various diseases such as immune inflammatory lesions, nervous system, cardiovascular, and cancer (Lee et al. 2012). Carbon tetrachloride is well-known toxicant, supposed to generate free radicals in various tissues. Free radicals can destroy proteins, cell membranes, and nucleic acids (Kadiiska et al. 2005). Carotenoids have shown potential antioxidant properties which inhibit free radical formation in various diseases (Chatterjee et al. 2012). Microalgae are known to produce various bio-active compounds such as astaxanthin, β-carotene, lutein, phycocyanin, and phycoerythrin (Takaichi 2011; Ranga Rao et al. 2009, 2014). β- carotene bioavailability was lower than that of astaxanthin and xanthophyll’s (Yeum and Russell 2002). As the natural carotenoids are associated with oxygenated functional group they are absorbed better than synthetic ones (Yeum and Russell 2002).
H. pluvialis is a green microalga, known to produce carotenoids under stress, which contain 70 % mono-ester, 15–20 % di-ester and 4–5 % free form of ASX (Ranga Rao et al. 2010, 2013a). It is approved as an antioxidant food supplement by the Swedish Health Food Council Advisory Board, and permitted as a food colorant in salmon feed, by food and Drug Administration in United States (Yuan et al. 2011) for imparting pigmentation. Astaxanthin is used as pigmentation source in trout, farmed salmon, and poultry (Lorenz and Cysewski 2000). Nutraceutical and medical applications of astaxanthin were reported against diseases such as age-related macular degeneration, inflammation, cancer, Helicobacter pylori infection (Fassett and Combes 2011). Natural astaxanthin has claimed its importance in functional foods. H. pluvialis has been cultivated at large scale in various systems (Wang et al. 2013). In CFTRI laboratory many studies have been conducted on H. pluvialis, including its production, characterization of astaxanthin (ASX), astaxanthin esters (ASXEs) etc. Recently our research group reported that the potency of ASXEs on anti-ulcer and anti-skin cancer activity in rat models (Ranga Rao et al. 2013a, b; Kamath et al. 2008). The present study is about the effect of isolated astaxanthin (ASX) and astaxanthin esters (ASXEs) from H. pluvialis on CCl4 induced hepatotoxicity in rats. The current results showed that ASXEs showed better protection than ASX on hepatic biochemical markers and antioxidant enzymes in CCl4 induced hepatotoxicity in rats.
Materials and methods
Chemicals and reagents
Solvents HPLC grade- methanol, acetonitrile, dichloromethane were purchased from Rankem Chemicals Ltd (Mumbai, India). Analytical grade-hexane, acetone, methanol, chloroform, and petroleum ether were procured from Sisco Chemicals Laboratorary (Mumbai, India). The serum glutamate transaminase (SGPT), serum glutamate oxaloacetate (SGOT), alkaline phosphatase (ALP), bilirubin and albumin kits were obtained from Kumar Diagnostics Ltd., Mysore, Karnataka, India. Synthetic astaxanthin procured from Sigma Chemicals, Co (St. Louis, Mo).
Batch culture of H. pluvialis and its carotenoid accumulation
H. pluvialis (34-1a) was procured from Sammlung von Algenkulturen, Pflanzen Physiologisches Institut, Universitat Gottingen, Gottingen, Germany, and grown in bold basal medium. Carotenoids accumulation under stress conditions were reported (Sarada et al. 2002). The encysted algal biomass said to be rich in astaxanthin (ASX) and astaxanthin esters (ASXEs) was harvested and kept at 4 °C until further use.
Extraction of ASX and ASXEs from H. pluvialis
Carotenoid from encysted algal biomass was extracted with acetone, followed by centrifugation at 4000×g (C24; Remi Instruments Ltd, Mumbai, India). ASX and ASXEs were isolated from the carotenoids as earlier reported by Ranga Rao et al. (2013a). Briefly, carotenoid extract was separated on silica gel thin layer chromatography with the solvent ratio of hexane: acetone (7:3, v/v) and the bands were scraped and re-dissolved in acetone. Further acetone was evaporated in the fractions using rota-vapor. The above extraction methods were repeated for 2–3 times in the dark condition to avoid degradation. These fractions were flushed with nitrogen, stored at 0 °C temperature, used it in further experiments.
Characterization of ASX and ASXEs by high performance liquid chromatography (HPLC) and liquid chromatography-mass spectra (LC-MS)
Both, HPLC (Shimadzu 10AS, Kyoto, Japan) reverse phase 25 × 4.6 mm, 5 μm, C18 column (Wakosil 11 5C 18RS) and Waters 2996 modular HPLC system (auto-sampler, gradient pump, thermo-regulator and DAD), coupled to a Q-Tof Ultima (UK) mass spectrometer were used for the identification and characterization of ASX and ASXEs. HPLC and LC-MS conditions used as per the details given in our earlier reports (Ranga Rao et al. 2010, 2013a).
Animals
The institutional animal ethics committee (IAEC No. 116/08) was approved for the animal experiments. Albino Wistar rats [Out B - Wistar, IND-Cft (2C), 200–220 ± 2 g] were individually maintained at room temperature (28 ± 2 °C), provided 12 h light/dark cycle in the animal house. Fresh pellet diet obtained from Amrut feeds, Sangli, India and tap water were given to rats on daily basis.
Carbon tetrachloride (CCl4) treatment
Rats were divided into seven groups, each group consisted of six animals, details are provided in Table 1. Group-1 served as normal, group-II served as toxin, group-III-VII were given single dose of CCl4 2.0 g/kg b.w, dissolved in equal volume of liquid paraffin to produce hepatotoxicity. Administration of ASX, ASXEs and SASX was started 2 weeks prior to CCl4 treatment. The animals were sacrificed after 24 h of CCl4 treatment, collected blood from the heart and liver tissues by the method of Chidambara Murthy et al. (2005a, b).
Table 1.
Experimental groups for carbon tetrachloride treatment
| Group-I | Without any treatment (normal) |
| Group-II | CCl4+olive oil (toxin) |
| Group-III | CCl4+ASX100a |
| Group-IV | CCl4+ASX250a |
| Group-V | CCl4+ASXEs100a |
| Group-VI | CCl4+ASXEs250a |
| Group-VII | CCl4+SASX100a |
a μg/kg b.w Body weight, CCl 4, Carbon tetrachloride, ASX Astaxanthin, ASXEs Astaxanthin esters from H. pluvialis, SASX Synthetic astxanthin
Analysis of hepatic injury
After 24 h of hepatotoxin administration, rats were anesthetized with diethyl ether. The blood was collected by cardiac puncture, allowed to clot for 1–2 h at room temperature and serum was separated by centrifugation at 2500 rpm for 15 min and used to determine the activities of SGPT (Bergmeyer and Horder 1980), SGOT (Bergmeyer et al. 1976), ALP (Szasz et al. 1974), albumin (Wooton 1964) and bilirubin (Malloy and Evelyn 1937) in normal, toxin and sample treated groups using commercially available enzyme kits.
Analysis of antioxidant enzymes of liver tissue
Activities of catalase was measured by the method of Aebi (1984) and superoxide dismutase by the menthod of Flohe and Otting (1984), Glutathione peroxidase and glutathione were measured as per the protocols described earlier (Ranga Rao et al. 2013a, b).
Protein estimation, and lipid peroxidation assay
Protein was determined by the procedure of Lowry et al. (1951). Lipid peroxidation activity was determined by the procedure of Buege and Aust (1978), a pink chromogen, a diadduct, formed by the reaction of thiobarbituric acid (TBA) with malondialdehyde (MDA) which can be detected spectrophotometrically at 532 nm.
Histopathological studies
The liver samples were kept for 24 h in 10 % buffered formalin. The tissue sections were made in parafilm blocks, strained with hematoxylin and eosin dye, and were observed in light microscope (Leitz, Germany) at 60x magnification.
Statistical analysis
The values shown as averages of mean ± standard deviation of six replicates. The values were evaluated by one-way ANOVA followed by Duncan’s multiple-range test (post-hoc) using Microsoft Excel XP (Microsoft Corp., Redmond, WA) software.
Results and discussion
ASX and ASXEs in batch culture of H. pluvialis
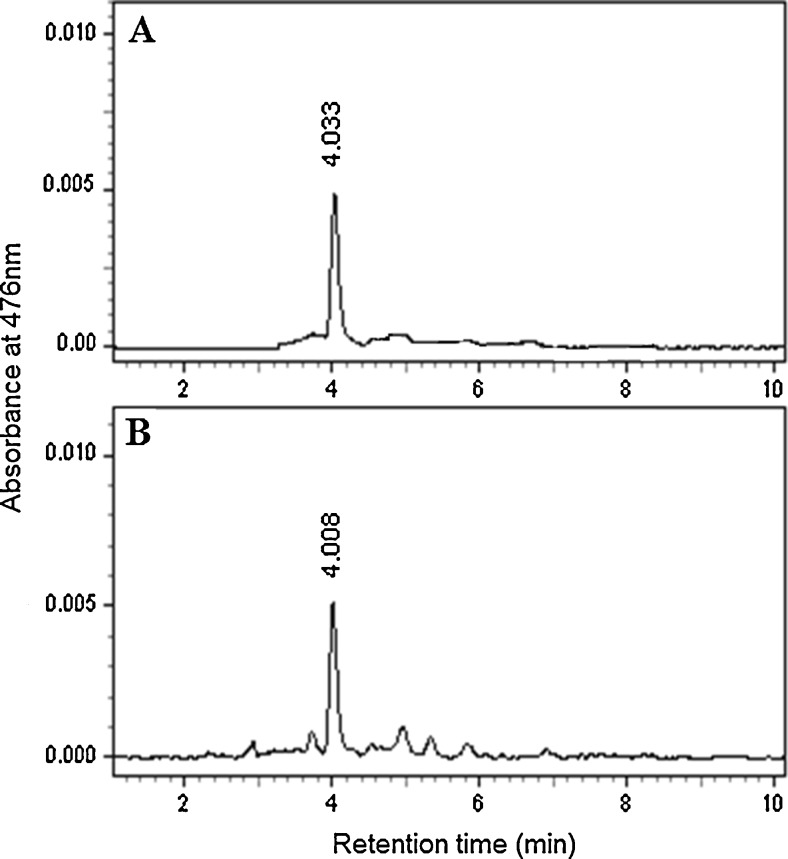
Alga was grown in bold basal medium for 3 weeks and the biomass contained 2.35 % (w/w) total carotenoid and 0.20 % (w/w) chlorophyll. ASX and ASXEs were found to be ~2 and 78 % in total carotenoid on dry weight basis. ASX and ASXEs were identified by absorption spectra at 470–476 nm. HPLC profile of isolated ASX from H. pluvialis is shown in Fig. 1. As ASX and ASXEs showed better resolution on thin layer chromatography those portions were scraped and pure components were isolated by preparative thin layer chromatography after reconfirming their mobility with the same chromatographic system. Results were characterized by mass spectra. ASXEs-C16:0, C17:2, C17:1, C17:0, C18:4, C18:3, C18:2, C18:1, C16:0/C16:0, C16:0/C18:2, C18:1/C18:3, C18:1/C18:2, C18:1/C18:1 were identified by mass spectra. ASXEs fragmentation pattern was interpreted due to breaking of fatty acid and water molecules. Further these molecules were evaluated for their effects on hepatoprotection and antioxidant activity in rats.
Fig. 1.
High performance liquid chromatography (HPLC) profile of standard astaxanthin (a) and isolated astaxanthin (b) from H. pluvialis
Influence of ASX and ASXEs on serum marker enzymes in normal and experimental rats
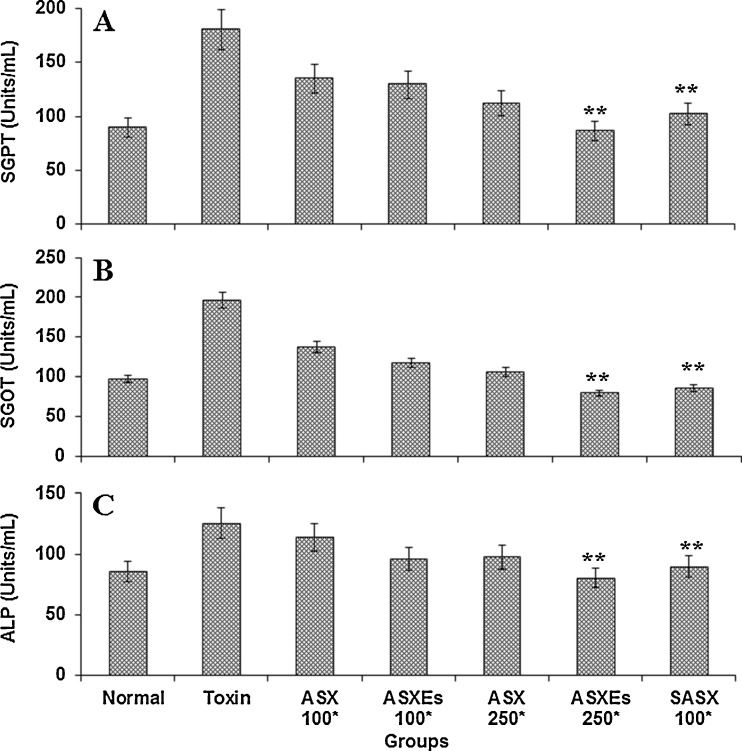
The levels of serum SGPT, SGOT, and ALP were significantly elevated in CCl4 treated group of hepatic damage. Treatment of rats along with ASX, ASXEs and SASX at 100 and 250 μg/kg b.w noticeably prevented the CCl4 induced elevation of SGPT, SGOT and ALP (Fig. 2). The levels of serum enzymes were increased by 1.5–2 folds in CCl4 as compared to control group. The enzyme activity was increased in CCl4 toxin administered group and the enzyme activity of SGPT, SGOT and ALP were 180.39, 196.34 and 125.41 Units/mL respectively. Levels of these enzymes were significantly less in the tested group animals. SGPT activity was 134.85, 112.23 Units/mL in ASX, 129.31, 86.45 Units/mL in ASXEs and 102.28 Units/mL respectively in SASX after their treatment at 100 and 250 μg/kg b.w; whereas for SGOT it was 137.45, 117.45 Units/L in ASX, 117.45, 79.67 Units/L in ASXEs and 85.23 Units/mL in SASX respectively (Fig. 2a and b). The hepatoprotective activity of ASX, ASXEs and SASX on ALP are shown in Fig. 2c. ALP activity was 85.41 Units/mL in normal group, whereas in treated groups it registerd 113.45 Units/mL (ASX), 95.78 Units/mL (ASXEs), 89.75 Units/mL (SASX) Units/mL in treatemts at 100 μg/kg b.w; and 97.45 (ASX), 80.45 (ASXEs) Units/mL in treatments at 250 μg/kg b.w .
Fig. 2.
Influence of ASX, ASXEs and SASX administration on serum parameters in carbon tetrachloride treated rats. ASX, ASXEs and SASX treated for 14 days, followed by CCl4 treatment, and sacrifice 24 h later. Each value represents the average of mean ± SD (n = 6), **P < 0.001 compared to toxin treated group. *μg/kg b.w; body weight; SGPT, serum glutamate pyruvate transaminase; SGOT, serum glutamate oxaloacetate transaminase; ALP, alkaline phosphatases; toxin, carbon tetrachloride; ASX, astaxanthin, ASXEs, astaxanthin esters; SASX, synthetic astxanthin
Influence of ASX and ASXEs on protein, albumin, bilirubin contents in normal and experimental rats
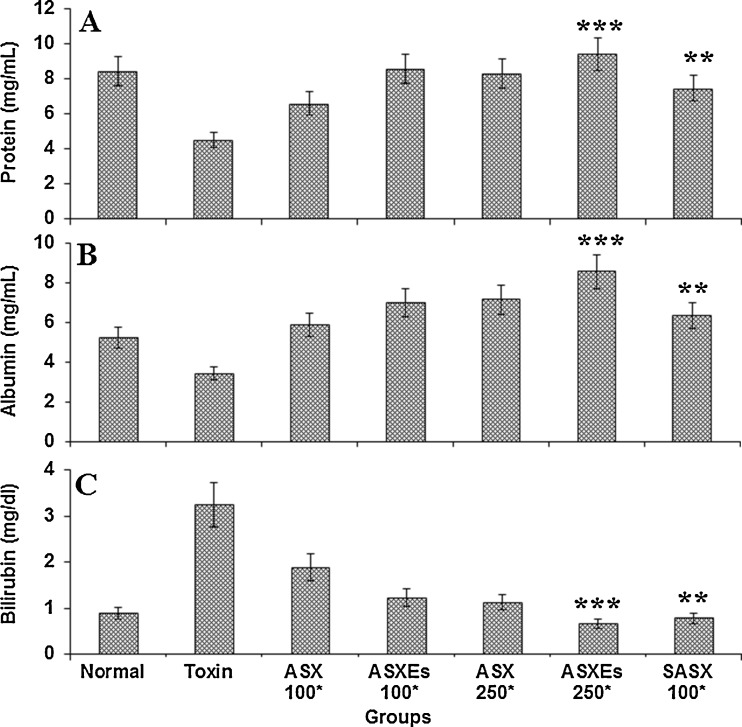
The total protein, albumin and bilirubin levels were measured in normal, in experimental rats treated with toxin, ASX, ASXEs and SASX after the administration of CCl4 (Fig. 3). The protein and albumin levels decreased in toxin group, whereas these levels increased in ASX, ASXEs and SASX treated groups (Fig. 3a and b). The increased serum bilirubin levels by treatment with toxin, were significantly lowered by treating with ASX, ASXEs and SASX in a dose depend manner (Fig. 3c).
Fig. 3.
Influence of ASX, ASXEs and SASX administration on protein, albumin and bilirubin in carbon tetrachloride treated rats. ASX, ASXEs and SASX treated for 14 days, followed by CCl4 treatment, and sacrifice 24 h later. *μg/kg b.w, each value represents the mean ± SD (n = 6), **P < 0.01, ***P < 0.001 compared to toxin treated group. *μg/kg b.w, body weight; toxin, carbon tetrachloride; ASX, astaxanthin, ASXEs, astaxanthin esters; SASX, synthetic astxanthin
Influence of ASX and ASXEs on antioxidant enzymes, glutathione levels in normal and experimental rats
The liver antioxidant enzymes activity noticeably decreased in the toxin (CCl4) treated groups and elevated in the ASX, SASX and ASXEs treated groups when compared to normal animals (Table 2). The group treated with ASXEs at 250 μg/kg was more active i.e. protective when compared to toxin and SASX treated group. ASXEs showed hepato-protection which was measured in terms of level of hepatic enzymes namely, catalase, glutathione peroxidase, superoxide dismutase and anti-lipid peroxidation. Rats treated with at 2.0 g/kg b.w. toxin showed the decrease in levels of catalase, glutathione peroxidase and superoxide dismutase by 31.65, 35.17 and 51.70 %, whereas, lipid peroxidation activity increased by 2.4 folds as compared to normal group. However, antioxidant enzymes superoxide dismutase, catalase and peroxidase enzyme activities were preserved in the pretreatment of rats with 250 μg/kg of ASX, SASX and ASXEs treated groups. Catalase restoration was 136.95 % and 238.48 % higher compared to toxin groups respectively at 250 μg/kg of ASX and ASXEs whereas treated with 100 μg/kg SASX treated group was 179.61 %. The peroxidase and superoxide dismutase enzymes also showed similar trend (Table 2). This shows the protection provided by feeding ASX and ASXEs and its ability to maintain these enzyme levels even after toxin treatment. Similarly, the level of GSH was low in toxin treated groups whereas in ASX, ASXEs and SASX treated groups their levels were significantly increased (Table 2). The lipid peroxidation was retained by 5.2 and 2.8 folds in 250 μg/kg b.w of ASX and ASXEs treated groups and 1.9 fold in SASX treated group at 100 μg/kg b.w.
Table 2.
Influence of ASX, ASXEs and SASX on liver antioxidant enzymes and glutathione in carbon tetrachloride treated rats
| Groups | Catalase (U/mg protein) | Glutathione peroxidase (U/mg protein) | Superoxide dismutase (U/mg protein) | % Anti-lipid peroxidase | Glutathione (nmol/mg protein) |
|---|---|---|---|---|---|
| Normal | 457.23 ± 6.98 | 10.12 ± 1.42 | 20.81 ± 1.75 | 22.45 ± 2.61 | 11.23 ± 1.15 |
| Toxin | 312.51 ± 10.37 | 6.56 ± 1.74 | 10.05 ± 2.38 | 53.78 ± 3.80 | 4.25 ± 0.93 |
| ASX100a | 428 ± 12. 61** | 11.98 ± 4.63** | 12.13 ± 2.66** | 18.91 ± 2.13** | 8.16 ± 1.84** |
| ASX250* | 543.01 ± 9.61** | 15.98 ± 2.30** | 15.02 ± 1.73** | 26.46 ± 1.09** | 10.47 ± 1.06** |
| ASXEs100* | 623. 84 ± 8.25*** | 21.79 ± 3.06*** | 26.13 ± 3.67*** | 21.81 ± 2.74*** | 15.03 ± 1.09*** |
| ASXEs250* | 745.28 ± 6.05*** | 23.65 ± 1.65*** | 32.98 ± 4.01*** | 10.33 ± 1.12*** | 18.69 ± 2.78** |
| SASX100* | 561.31 ± 11.82** | 11.24 ± 2.38** | 16.81 ± 3.22** | 27. 58 ± 2.91** | 13.52 ± 2.13** |
* μg/kg b.w Body weight, toxin Carbon tetrachloride (CCl4), ASX Astaxanthin, ASXEs Astaxanthin esters, SASX Synthetic astxanthin. Each value represents the average of mean ± SD (n = 6), **P < 0.01, ***P < 0.001 compared to toxin treated group
Influence of ASX and ASXEs on liver histopathology in normal and experimental rats
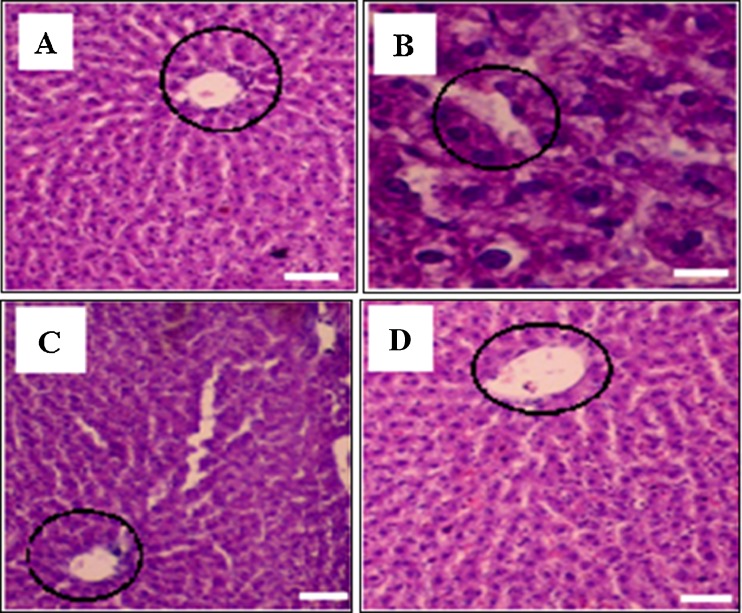
Liver histopathology of normal, toxin, ASX and ASXEs treated groups is shown in Fig. 4. According to histopathology studies, hepatocytes with normal architecture, portal triad, portal veins, hepatic artery and vein were found to be visible in normal group animals, whereas in toxin treated group they were significantly changed. The liver has retained the normal hepatic architecture with minor hemorrhage at 250 μg/kg b.w in ASXEs treated group (Fig. 4) followed by ASX and SASX treated groups.
Fig. 4.
Histopathological observation of liver of different treated groups (60x). Section through the liver of normal rats showing central vein and hepatocytes (a), section through the liver of CCl4-treated rats showing central vein and hepatocytes (b) and section through the liver of ASX, ASXEs (250 μg/kg b.w) treated rats (c and d) showing the central vein (round marking) and hepatocytes
Liver is highly affected by toxic agents as being vital organ to metabolise xenobiotics. The free radicals deactivate liver detoxification enzymes during the catalytic cycle. Carbon tetrachloride has been widely studied as a liver toxicant, and its metabolites formed as trichloromethyl peroxyl radical and trichloromethyl radical which are involved in the pathogenesis of liver and kidney damage. The increased lipid peroxidation in liver was triggered by the enormously generated free radicals in the toxic group. The massive free radical generation help the cytotoxicity effect to propagate intracellularly, increasing the interaction of these radicals with phospholipids structure and inducing a peroxidation process that destroys structure of organ (Jaeschke et al. 2012). The interaction of free radical with polyunsaturated fatty acids of the membrane lipids initiate lipid peroxidation, leads to oxidative stress, further forms malondialdehyde (MDA), which is end product of this process (Negre‐Salvayre et al. 2008).
This study has shown that the isolated ASX and ASXEs of H. pluvialis offered better protection to the toxin induced rats and also maintained their enzyme levels in both serum and liver tissue (Fig. 2 and Table 2). Antioxidant enzymes and glutathione levels were significantly increased in liver tissue by the treatment of ASX and ASXEs, effectively demonstrating their protective effect (Table 2). Biochemical parameters protein, albumin contents declined in toxin treated groups while bilirubin levels were increased. However, these values were significantly protected in the both ASX and ASXEs treated groups (Fig. 3a, b and c). These results indicate that ASX and ASXEs from H. pluvialis have a elevated anti-hepatotoxic effect than SASX, these current observations have been shown the potential biological activity of ASXEs. Earlier studies from our laboratory conducted on the bioavailability, antioxidant effect, gastro-protective effect and anti-cancer activity of ASX and ASXEs from H. pluvialis in the rat models, results showed that the antioxidant enzymes levels were significantly increased by ASX and ASXEs treatment (Ranga Rao et al. 2013a, b; Kamath et al. 2008). Risk of various disorders can be reduced by taking carotenoids like astaxanthin, lutein and zeaxanthin (Sanda et al. 2008). Scientific reports indicate that astaxanthin has shown beneficial effects on cardiovasucalr disease, blood pressure in in-vitro and in-vivo model, this may be due to its higher antioxidant activity (Fassett and Combes 2011). Recently our research group has shown that skin tumors and tyrosinase enzyme activity significantly decreased, whereas antioxidant enzyme levels increased by ASX and ASXEs treatment in skin carcinogenesis rats (Ranga Rao et al. 2013a). Similar results were observed by astaxanthin treatment against 2, 3, 7, 8-tetrachloridebenzo-p-dioxin and methylnitrosourea induced toxicity in rats by stimulating the cellular antioxidant enzymes, hindering lipid peroxidation, and protein oxidation (Sanda et al. 2008; Turkez et al. 2012).
Carotenoids pro-antioxidant ability was well defined, in membrane protection ability mostly by preventing lipid peroxidation and restoring various antioxidant enzymes like superoxide dismutase, catalase, and peroxidases (González-Burgos and Gómez-Serranillos 2012). However, protection abilities in ASX and ASXEs treated groups were less exposed. In humans, dietary astaxanthin boosted immune response, reduced DNA oxidative damage and inflammation (Park et al. 2010). Astaxanthin showed various pharmacological activities, including anti-inflammatory, anti-diabetic activities as well as antioxidant properties (Ranga Rao et al. 2010, 2013a, b; Maoka et al. 2012). Lipid peroxidation in the serum and liver of astaxanthin-fed rats treated with CCl4 was significantly inhibited relative to rats fed a control diet (Kang et al. 2001). Micro algal carotenoids β-carotene from Dunaliella, Spirulina biomass were enhanced antioxidant enzyme activity and hepatoprotective property on carbon tetra chloride induced toxicity in rats (Chidambara murthy et al. 2005a, b; Vanitha et al. 2007). Lutein producing microalga-Botryococcus braunii enhanced bioavailability and antioxidant activity in experimental rats by inhibition of lipid peroxidation (Ranga Rao et al. 2006). Recently Sindhu et al. (2010) reported the carotenoid lutein isolated from marigold flowers (Tagetes erecta L.) protected the liver damage in paracetamol, carbon tetrachloride, ethanol induced hepatotoxic rats by increasing liver antioxidant enzyme activity. In another study, astaxanthin showed anti-hypertensive activity in rats (Hussein et al. (2006) which have been used as a model to study the mechanism, pathophysiology, and management of hypertension.
Conclusion
In summary, the current results revealed that ASX and ASXEs prevented hepatic damage induced by carbon tetra chloride with the enhancement of antioxidant enzyme activities. ASX and ASXEs treatments showed potent anti-hepatoprotective and anti-oxidant activity in rats when compared to SASX treated ones. As H. pluvialis accumulate ASX and ASXEs which has a higher degree of hepatoprotective and antioxidant activity, they can be used in various formulations and functional foods, which have high demand in the market. Therefore, ASX and ASXEs can be considered for various health applications in food, feed, pharmaceutical and nutraceutical formulations.
Acknowledgments
The award of Senior Research Fellowship to ARR by the Indian Council of Medical Research (ICMR), New Delhi, is gratefully acknowledged.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Aebi H. Catalase in vitro methods of assays. Methods Enzymol. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Bergmeyer HU, Horder M. International federation of clinical chemistry methods for the measurement of catalytic concentrations of enzymes. Clin Chem Acta. 1980;105:147–172. doi: 10.1016/0009-8981(80)90105-9. [DOI] [PubMed] [Google Scholar]
- Bergmeyer HU, Bowers JR, Hörder M, Moss DW. Provisional recommendations on IFCC Methods for the measurement of catalytic concentrations of enzymes. Part 2. IFCC method for aspartat aminotransferase. Clin Chim Acta. 1976;70:19–42. doi: 10.1016/0009-8981(76)90437-X. [DOI] [PubMed] [Google Scholar]
- Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/S0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- Chatterjee M, Roy K, Janarthan M, Das S, Chatterjee M. Biological activity of carotenoids: its implications in cancer risk and prevention. Curr Pharm Biotechnol. 2012;13:180–190. doi: 10.2174/138920112798868683. [DOI] [PubMed] [Google Scholar]
- Chidambara Murthy KN, Rajesha J, Vanitha A, Swamy MM, Ravishankar GA. Protective effect of Dunaliella salina- A marine micro alga, against carbon tetrachloride hepatotoxicity in rats. Hepatol Res. 2005;33:313–319. doi: 10.1016/j.hepres.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Chidambara Murthy KN, Rajesha J, Swamy MM, Ravishankar GA. Comparative evaluation of hepatoprotective activity of carotenoids of microalgae. J Med Food. 2005;8:523–528. doi: 10.1089/jmf.2005.8.523. [DOI] [PubMed] [Google Scholar]
- Fassett RG, Combes JS. Astaxanthin: a potential therapeutic agent in cardiovascular disease. Mar Drugs. 2011;9:447–465. doi: 10.3390/md9030447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flohe L, Otting F. Superoxide dismutase assays. Methods Enzymol. 1984;105:93–104. doi: 10.1016/S0076-6879(84)05013-8. [DOI] [PubMed] [Google Scholar]
- González-Burgos E, Gómez-Serranillos MP. Terpene compounds in nature: a review of their potential antioxidant activity. Curr Med Chem. 2012;19:5319–5341. doi: 10.2174/092986712803833335. [DOI] [PubMed] [Google Scholar]
- Hussein G, Goto H, Oda S, Sankawa U, Matsumoto K, Watanabe H. Antihypertensive potential and mechanism of action of astaxanthin: III. Antioxidant and histopathological effects in spontaneously hypertensive rats. Biol Pharm Bull. 2006;29:684–688. doi: 10.1248/bpb.29.684. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, McGill MR, Ramachandran A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity. Drug Metab Rev. 2012;44:88–106. doi: 10.3109/03602532.2011.602688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadiiska MB, Gladena BC, Bairda DD, Germoleca D. Biomarkers of oxidative stress study II. Are oxidation products of lipids, proteins and DNA markers of CCl4 poisoning? Free Radic Biol Med. 2005;38:698–710. doi: 10.1016/j.freeradbiomed.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Kamath BS, Srikanta BM, Dharmesh SM, Sarada R, Ravishankar GA. Ulcer preventive and antioxidative properties of astaxanthin from Haematococcus pluvialis. Eur J Pharmacol. 2008;590:387–395. doi: 10.1016/j.ejphar.2008.06.042. [DOI] [PubMed] [Google Scholar]
- Kang JO, Kim SJ, Kim H. Effect of astaxanthin on the hepatotoxicity, lipid peroxidation and antioxidative enzymes in the liver of CCl4-treated rats. Methods Find Exp Clin Pharmacol. 2001;23:79–84. doi: 10.1358/mf.2001.23.2.627931. [DOI] [PubMed] [Google Scholar]
- Lee R, Margaritis M, Channon KM, Antoniades C. Evaluating oxidative stress in human cardiovascular disease: methodological aspects and considerations. Curr Med Chem. 2012;19:2504–2520. doi: 10.2174/092986712800493057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz RT, Cysewski GR. Commercial potential for Haematococcus microalgae as a natural source of astaxanthin. Trends Biotechnol. 2000;18:160–167. doi: 10.1016/S0167-7799(00)01433-5. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall JL. Protein measurement with Folin-phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Malloy HT, Evelyn KA. The determination of bilirubin by photoelectric colorimeter. J Biol Chem. 1937;119:481–487. [Google Scholar]
- Maoka T, Tokuda H, Suzuki N, Kato H, Etoh H. Anti-oxidative, anti-tumor-promoting, and anti-carcinogensis activities of nitro astaxanthin and nitrolutein, the reaction products of astaxanthin and lutein with peroxynitrite. Mar Drugs. 2012;10:1391–1399. doi: 10.3390/md10061391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negre‐Salvayre A, Coatrieux C, Ingueneau C, Salvayre R. Advanced lipid peroxidation end products in oxidative damage to proteins. Potential role in diseases and therapeutic prospects for the inhibitors. Br J Pharmacol. 2008;153:6–20. doi: 10.1038/sj.bjp.0707395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Jong HC, Yoo KK, Larry LL, Boon PC. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr Metab. 2010;7:18. doi: 10.1186/1743-7075-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranga Rao A, Sarada R, Baskaran V, Ravishankar GA. Antioxidant activity of Botryococcus braunii extract elucidated in vitro models. J Agric Food Chem. 2006;54:4593–4599. doi: 10.1021/jf060799j. [DOI] [PubMed] [Google Scholar]
- Ranga Rao A, Sarada, Baskaran V, Ravishankar GA. Identification of carotenoids from green alga Haematococcus pluvialis by HPLC and LC-MS (APCI) and their antioxidant properties. J Microbiol Biotechnol. 2009;19:1333–1341. [PubMed] [Google Scholar]
- Ranga Rao A, Raghunath Reddy RL, Baskaran V, Sarada R, Ravishankar GA. Characterization of microalgal carotenoids by mass spectrometry and their bioavailability and antioxidant properties elucidated in rat model. J Agric Food Chem. 2010;58:8553–8559. doi: 10.1021/jf101187k. [DOI] [PubMed] [Google Scholar]
- Ranga Rao A, Baskaran V, Sarada R, Ravishankar GA. In vivo bioavailability and antioxidant activity of carotenoids from microalgal biomass—a repeated dose study. Food Res Int. 2013;54:711–717. doi: 10.1016/j.foodres.2013.07.067. [DOI] [Google Scholar]
- Ranga Rao A, Sindhuja HN, Dharmesh SM, Sankar KU, Sarada R, Ravishankar GA. Effective inhibition of skin cancer, tyrosinase, and antioxidative properties by astaxanthin and astaxanthin esters from the green alga Haematococcus pluvialis. J Agric Food Chem. 2013;61:384–3851. doi: 10.1021/jf304609j. [DOI] [PubMed] [Google Scholar]
- Ranga Rao A, Phang SM, Sarada R, Ravishankar GA. Astaxanthin: sources, extraction, stability, biological activities and its commercial applications—a review. Mar Drugs. 2014;12:128–152. doi: 10.3390/md12010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanda A, Gal A, Pintea A, Bedecean I, Arion A, Baba AI. Influence of astaxanthin administration on hepatic oxidative stress markers in rats injected with methylnitrosurea. Bull UASVM Vet Med. 2008;65(1):pISS 1843–pISS 5270. [Google Scholar]
- Sarada R, Usha T, Ravishankar GA. Influence of stress on astaxanthin production in Haematococcus pluvialis grown under different culture conditions. Process Biochem. 2002;37:623–627. doi: 10.1016/S0032-9592(01)00246-1. [DOI] [Google Scholar]
- Sindhu ER, Firdous AP, Preethi KC, Kuttan R. Carotenoid lutein protects rats from paracetamol-, carbon tetrachloride- and ethanol-induced hepatic damage. J Pharm Pharmacol. 2010;62:1054–1060. doi: 10.1111/j.2042-7158.2010.01123.x. [DOI] [PubMed] [Google Scholar]
- Szasz G, Weimann G, Stahler F, Wahlefeld AW, Persijn JP. New substrates for measuring gamma glutamyl transpeptidase activity. Z Klin Chem Klin Biochem. 1974;12:228. [PubMed] [Google Scholar]
- Takaichi S. Carotenoids in algae: distributions, biosynthesis and functions. Mar Drugs. 2011;9:1101–1118. doi: 10.3390/md9061101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkez H, Geyikoglu F, Yousef MI. Beneficial effect of astaxanthin on 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin-induced liver injury in rats. Toxicol Ind Health. 2012;29:591–599. doi: 10.1177/0748233711434959. [DOI] [PubMed] [Google Scholar]
- Uttara B, Singh AV, Zamboni P, Mahajan RT. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol. 2009;7:65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanitha A, Chidambara Murhty KN, Kumar V, Sakthivelu G, Veigas JM, Saibaba P, Ravishankar GA. Effect of the carotenoid producing alga, Dunaliella bardawil, on CCl4 induced toxicity in rats. Int J Toxicol. 2007;26:159–167. doi: 10.1080/10915810701224748. [DOI] [PubMed] [Google Scholar]
- Wang J, Han D, Sommerfeld MR, Lu C, Hu Q. Effect of initial biomass density on growth and astaxanthin production of Haematococcus pluvialis in an outdoor photobioreactor. J Appl Phycol. 2013;25:253–260. doi: 10.1007/s10811-012-9859-4. [DOI] [Google Scholar]
- Wooton IDP. Microanalysis in medical biochemistry. 4. London: J. and A. Churchill Ltd; 1964. pp. 138–140. [Google Scholar]
- Yeum KJ, Russell RM. Carotenoid bioavailability and bioconversion. Annu Rev Nutr. 2002;22:483–504. doi: 10.1146/annurev.nutr.22.010402.102834. [DOI] [PubMed] [Google Scholar]
- Yuan JP, Peng J, Yin K, Wang JH. Potential health-promoting effects of astaxanthin: a high-value carotenoid mostly from microalgae. Mol Nutr Food Res. 2011;55:150–165. doi: 10.1002/mnfr.201000414. [DOI] [PubMed] [Google Scholar]