Abstract
The total phenolic and flavonoid contents, antioxidant and antibacterial properties of flavonoid- (water, ethyl acetate and hexane fractions), polyphenol- and anthocyanin-rich extracts of Thymus kotschyanus aerial parts were investigated. All the extracts showed significant amounts of phenolic and flavonoid compounds and exhibited strong antioxidant activity. Among the extracts, water fraction contained the highest phenolic and flavonoid contents (881.06 ± 16.52 mg GAE/g of extract and 74.60 ± 3.05 mg QE/g of extract, respectively). It also presented the highest DPPH• scavenging activity with an IC50 of 14.21 ± 0.53 μg mL−1, and the highest reducing power at 400 μg mL−1 by A700 = 2.46 ± 0.04. The extracts were found to exert moderate antibacterial activity against both Gram-negative and Gram-positive bacteria. These findings highlighted a scientific basis to the traditional usage of T. kotschyanus, also showed its potential as a rich source of natural antioxidant and antibacterial compounds.
Keywords: Thymus kotschyanus, Total phenolic content, Flavonoid-rich, Antibacterial activity
Introduction
Consumers desire to reduce the risk for chronic diseases or to manage a specific health condition through improved diet. Antioxidants have been widely used as food additives to provide protection against oxidative degradation of foods by free radicals. In order to prolong the storage stability of foods, synthetic antioxidants are used for industrial processing. However, the commonly used synthetic antioxidants such as butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT) are restricted by legislative rules, related to their potential health risks and toxicity (Imaida et al. 1983; Kahl and Kappus 1993). Therefore, scientists are now searching for naturally occurring antioxidants in plant sources for food or medicinal materials as alternative for synthetic antioxidants (Proestos et al. 2005; D’Abrosca et al. 2007; Annegowda et al. 2013). Natural antioxidants such as phenolic compounds, flavonoids, anthocyanins and other phytochemicals can function as free radical scavengers, reducing agents, chelators of prooxidant metals, or as quenchers of singlet oxygen and thus delay the lipid oxidation process in food products (Rice-Evans et al. 1997; Kahkonen et al. 1999; Miraliakbari and Shahidi 2008; Sun et al. 2009; Amarowicz et al. 2010). A number of antioxidant sources based on natural origin have been explored so far, but still there exists need for the search of newer sources, which may be safer, more economical and preferably from dietary sources.
The genus Thymus L., known as “Avishan” in Persian, is a well known aromatic perennial herb originated from Mediterranean region. Among 215 species of this genus grown in the world, 14 species are distributed in Iranian flora (Jalas 1982; Stahl-Biskup and Saez 2002). Thymus species are well known as medicinal plants due to their biological and pharmacological properties including antispasmodic, antiviral, antibacterial, antifungal, anti-parasite, and antioxidant activities (Stahl-Biskup and Saez 2002). In traditional medicine, leaves and flowering parts of Thymus species are widely used as tonic and herbal tea, antiseptic, antitussive and carminative as well as treating colds (Zargari 1990).
The chemical composition and biological activities of Thymus kotschyanus have been previously investigated (Rasooli and Mirmostafa 2003; Bagci and Baser 2005; Nickavar et al. 2005; Morteza-Semnani et al. 2007; Mohammed and Al-Bayati 2009). Moreover, hydroalcoholic extract of this whole plant has exhibited strong antioxidant activity (Nickavar and Esbati 2012). Thus, the aim of the present work is to evaluate the total phenolic and flavonid contents, antioxidant and antibacterial activities of flavonoid-, polyphenol- and anthocyanin-rich extracts from T. kotschyanus aerial parts for their possible use as source of antioxidants and also as antibacterial agents that can be used to prevent food spoilage.
Materials and methods
Chemicals
Trichloroacetic acid (TCA), Folin–Ciocalteau, gallic acid, quercetin, ascorbic acid, butylated hydroxytoluene (BHT) and 1,1-diphenyl-2-picrylhydrazyl (DPPH) were purchased from Sigma-Aldrich Chemical Company. Other chemicals and reagents were of analytical grade.
Plant material
The aerial parts of T. kotschyanus were collected from the central Alborz Mountains, Veresk area, north of Iran, in June 2012. The plant was identified and authenticated by Dr. A. Naqinezhad at Department of Biology, University of Mazandran, Babolsar, Iran. A specimen is deposited in the Herbarium of this Institution, under the voucher number 4026.
Preparation of flavonoid-rich extracts (water, ethyl acetate and hexane fractions)
Separation of the flavonoid-rich fractions was done as described previously by Nabavi et al. (2012). Accordingly, 100 g of dried sample was defatted twice with 100 mL of CHCl3 and extracted twice with 100 mL of 60 % acetone for 12 h at room temperature. The extract was then separated from the sample residue by filtration through Whatman No.1 filter paper. The solvent in the combined filtrates was removed at 35 °C using a rotary evaporator, leaving the crude acetone extract (12 g). After preparation of 10 % methanol slurry, the acetone extract was separately fractionated sequentially with hexane, ethyl acetate, and finally with water. All the extracts were dried at 40 °C using a rotary evaporator. The percentage yield of extracts was expressed in terms of dried weight of plant material.
Extraction of polyphenols and anthocyanins
Polyphenols and anthocyanins were extracted from crushed aerial part tissues (100 g) of T. kotschyanus, according to the modified method of Zhang et al. (2000). The extraction was performed twice at 20 °C in a shaking incubator (ZHWY-200B, Zhicheng Analytical Co., Shanghai, China). Extracting time was 30 min, and extracting solvents were 100 mL of methanol/acetone/water (3.5:3.5:3, v/v/v) containing 1 % formic acid. The extract was then separated from the sample residue by filtration through Whatman No.1 filter paper. The combined filtrates were evaporated under vacuum at 40 °C to remove methanol and acetone. Lipophilic pigments were then eliminated from the aqueous phase by two successive extractions in a separatory funnel with a twofold volume of petroleum ether. The aqueous phase was collected and further extracted three times by ethyl acetate (ethyl acetate: aqueous phase = 1:1, v/v) in the separatory funnel. Three ethyl acetate phases were collected, evaporated and dried under vacuum at 35 °C to obtain polyphenol sample. The aqueous phase was also dried under vacuum at 50 °C and used as an anthocyanin sample. All samples were stored at −20 °C prior to further assays.
Determination of total phenolic content (TPC)
Total phenolic content was determined by the Folin-Ciocalteau method (Meda et al. 2005). Accordingly, aliquots of 4 mg lyophilized powder of extracts were separately dissolved in 50 mL deionized water. Folin-Ciocalteau reagent (0.2 N, 2.5 mL) for 5 min and then 2.0 mL of 75 g/L sodium carbonate were added to extract solution (0.5 mL). The absorbance of reaction was measured spectrophotometrically (UV-Visible EZ201, Perkin Elmer: Norwalk, CA, USA) at 760 nm after 30 min of incubation at room temperature. The results were expressed as gallic acid equivalent (GAE)/g of the dry extract.
Determination of total flavonoid content (TFC)
The total flavonoids content was evaluated by the aluminum chloride colorimetric method (Willet 2002). Accordingly, aliquots of 4 mg lyophilized powder of extracts were separately dissolved in 50 mL deionized water. This solution (0.5 mL) was mixed with 1.5 mL of methanol, 0.1 mL of 10 % aluminum chloride, 0.1 mL of 1 M potassium acetate, and 2.8 mL of distilled water and left at room temperature for 30 min. The absorbance of the reaction mixture was measured at 415 nm on a spectrophotometer (UV-Visible EZ201, Perkin Elmer: Norwalk, CA, USA). Total flavonoid content was calculated as quercetin equivalent (QE)/g of the dry extract.
DPPH radical-scavenging activity
The stable 1,1-diphenyl-2-picryl hydrazyl radical (DPPH) was used for determination of free radical-scavenging activity of the extracts (Brand-Williams et al. 1995). Two milliliters of different concentrations of extracts (5–80 μg mL−1, in water) were added to 2 mL of methanol solution of DPPH (100 μM). After 15 min at room temperature, the absorbance was recorded at 517 nm. The experiment was repeated for three times. Ascorbic acid and BHT were used as standard controls for comparison. IC50 values denote the concentration of sample, which is required to scavenge 50 % of DPPH free radicals.
Reducing power
The reducing power of fractions was determined according to the method of Yen and Chen (1995). Accordingly, 2.5 mL of sample with different concentrations (25–400 μg mL−1, in water) was mixed with phosphate buffer (2.5 mL, 0.2 M, pH 6.6) and potassium ferricyanide [K3Fe(CN)6] (2.5 mL, 1 %). The mixture was incubated at 50 °C for 20 min. A portion (2.5 mL) of trichloroacetic acid (10 %) was added to the mixture to stop the reaction, which was then centrifuged at 3000 rpm for 10 min. The upper layer of solution (2.5 mL) was mixed with distilled water (2.5 mL) and FeCl3 (0.5 mL, 0.1 %), and the absorbance was measured at 700 nm. Ascorbic acid was used as positive control.
Antimicrobial activity (disk diffusion assay)
In vitro antibacterial activity of the extracts was evaluated by disc diffusion method using Mueller-Hinton Agar with determination of inhibition zones (IZ) (Kirby-Bauer Method; Drago et al. 1999). The Gram-negative and Gram-positive bacteria were as follows: Escherichia coli PTCC 1330, Pseudomonas aeruginosa PTCC 1074, Staphylococcus aureus ATCC 35923 and Bacillus subtilis PTCC 1023. 20 μL of the extracts (20 mg/ml in DMSO) was applied on the paper discs (the disc diameter was 6 mm). The paper discs were placed on nutrient agar plates that had been previously seeded with inoculum containing indicator microorganisms. After incubation of the plates at 37 °C for 24 h, the diameter of growth inhibition zones were measured. The antibacterial activity of the extracts was compared with known antibiotics gentamicin (10 μg/disc) and chloramphenicol (30 μg/disc) as positive controls and DMSO (20 μL/disc) as negative control.
Statistical analyses
Experimental results were mean ± kS.D. of three parallel measurements and analyzed by SPSS 18. Differences between means were determined using ANOVA and Tukey multiple comparisons and least significant difference (LSD). Correlations were obtained by Pearson correlation coefficient in bivariate correlations. p values ≤ 0.05 were regarded significant.
Results and discussion
Extraction yields, total phenolic and flavonoid contents
Content of phenolic and flavonoid compounds, and extraction yields of T. kotschyanus aerial parts were listed in Table 1. Yields varied from 0.43 to 7.52 % among the extracts. The highest yield was obtained for water fraction (7.52 %) followed by anthocyanin fraction (6.41 %), polyphenols fraction (2.57 %), ethyl acetate fraction (0.58 %), and hexane fraction (0.43 %), respectively; differences being significant (p < 0.05) among them.
Table 1.
Extraction yields, total phenolic and flavonoid contents of water, ethyl acetate, hexane, polyphenol, and anthocyanin extracts of T. kotschyanus aerial parts
| Extract | Extraction yields (%) | Total phenolic contenta | Total flavonoid contentb |
|---|---|---|---|
| Flavonoid-rich (Water fraction) | 7.52 ± 0.16 | 881.06 ± 16.52 | 74.60 ± 3.05 |
| Flavonoid-rich (Ethyl acetate fraction) | 0.58 ± 0.03 | 751.56 ± 6.91 | 59.52 ± 2.31 |
| Flavonoid-rich (Hexane fraction) | 0.43 ± 0.04 | 555.72 ± 5.23 | 42.45 ± 3.54 |
| Polyphenols fraction | 2.57 ± 0.09 | 391.14 ± 10.82 | 47.61 ± 1.92 |
| Anthocyanin fraction | 6.41 ± 0.14 | 309.31 ± 15.84 | 32.04 ± 0.53 |
The data are expressed as mean ± S.D. (n = 3). ANOVA test for data in the same column indicates significant difference (p < 0.05)
a mg GAE/g of dry extract
b mg QE/g of dry extract
The TPC of extracts was determined spectrophotometrically by Folin-Ciocalteu reagent and calculated in terms of gallic acid equivalent with reference to standard curve (y = 0.0054x + 0.0628, R2 = 0.987). The high phenolic content was observed in all extracts which varied over a wide range, 309.31–881.06 mg GAE/g of dry extract (Table 1). The results revealed that water fraction possessed the highest phenolic content (881.06 ± 16.52) mg GAE/g followed by ethyl acetate fraction (751.56 ± 6.91) mg GAE/g, hexane fraction (555.72 ± 5.23) mg GAE/g, polyphenols fraction (391.14 ± 10.82) mg GAE/g, and anthocyanin fraction (309.31 ± 15.84) mg GAE/g; with significant difference between them (p < 0.05).
The total flavonoid contents of various extracts were recorded spectrophotometrically using quercetin as calibration standard (y = 0.0063x, R2 = 0.999). The TFC ranged from 32.04 to 74.60 mg QE/g of dry extract (Table 1). Highest TFC was recorded for water extract, while the lowest was for the anthocyanin extract; differences again being significant for TFC among the extracts (p < 0.05). Variations in the TPC and TFC of T. kotschyanus extracts might be attributed to the different polarities of the extraction solvents as well as the chemical nature of the endogenous extractable compounds (Jaffery et al. 2003).
Free-radical scavenging activity
The DPPH radical-scavenging assay is a widely used and comparably facile method to evaluate antioxidant activity. Antioxidant molecules scavenge DPPH radicals by the process of either hydrogen or electron donation and the purple color from the DPPH• assay solution becomes light yellow which can be quantified by its decrease of absorbance at a wavelength 517 nm (Blois 1985). Free radical scavenging is one of the known mechanisms by which antioxidants inhibit lipid oxidation.
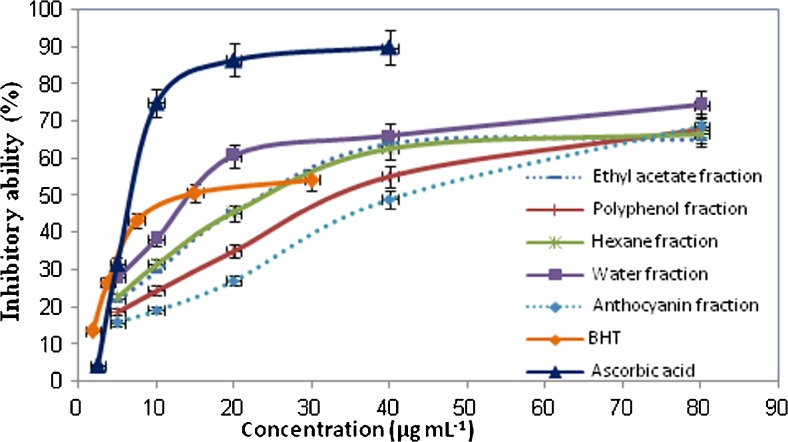
Figure 1 illustrates the percent inhibition of DPPH radical by the various extracts and the standard antioxidants at different concentrations. The descending order of IC50, the effective concentration of the sample required to scavenge 50 % of DPPH•, was as follows: anthocyanin extract (IC50: 41.13 ± 5.94 μg mL−1) > polyphenol extract (IC50: 32.66 ± 3.16 μg mL−1) > hexane extract (IC50: 23.71 ± 3.68 μg mL−1) ≈ ethyl acetate extract (IC50: 23.71 ± 1.21 μg mL−1) > water extract (IC50: 14.21 ± 0.53 μg mL−1) > BHT (13.18 ± 3.11 μg mL−1) > ascorbic acid (6.56 ± 0.12 μg mL−1); with significant difference between them (p < 0.05). However, the tested extracts presented a remarkable capacity to scavenge DPPH-radical with IC50 values being found at the μg mL−1 level. It is interesting to mention that water fraction, which contains the highest phenolic and flavonoid contents, showed better DPPH• scavenging activity than other extracts (p < 0.05), while it exhibited a slightly greater IC50 value than that of ascorbic acid and BHT (p > 0.05). Interestingly, according to Fig. 1, when sample concentration was above 20 μg mL−1, the radical scavenging activity of water fraction was higher than that of BHT.
Fig. 1.
DPPH radical scavenging activity of flavonoid- (water, ethyl acetate and hexane fractions), polyphenol- and anthocyanin- rich extracts of T. kotschyanus
In the present study, DPPH radical scavenging capacity of the flavonoid- rich extracts (water, ethyl acetate and hexane fractions) of T. kotschyanus was found to be greater than that reported for hydroalcoholic extracts of T. pubescens (IC50: 31.47 μg/mL), T. kotschyanus (IC50: 47.22 μg/mL), and T. daenensis (IC50: 48.68 μg/mL) (Nickavar and Esbati 2012).
Relation between measured antioxidant capacity and phenolic/flavonoid contents
Phenolic compounds are a class of antioxidant agents due to their redox properties, which allow them to act as reducing agents, hydrogen donors, and singlet oxygen quenchers (Chang et al.2001). Flavonoids are a large group of phenolic plant constituents, which show antioxidant activity through scavenging or chelating processes.
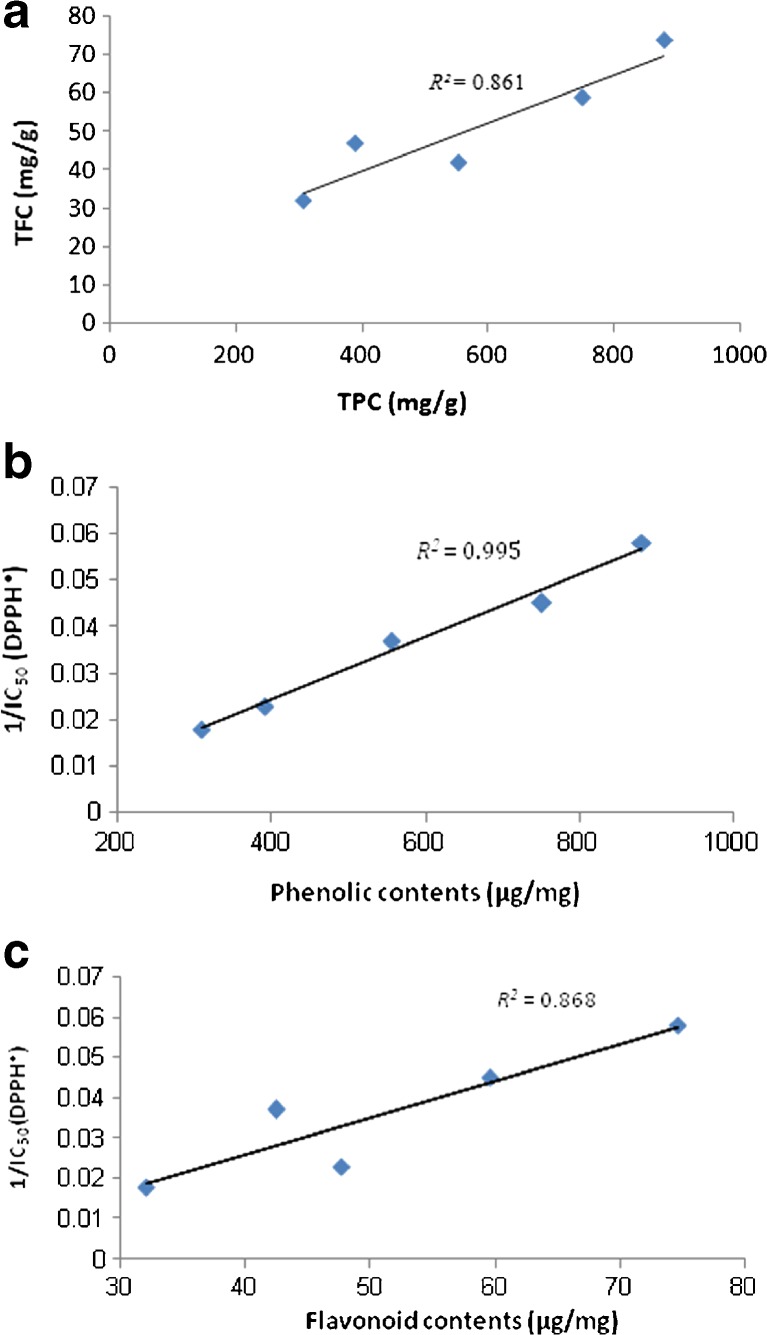
In our experiments, it was observed that the TFC of extracts correlated well (R2 = 0.86) with the data obtained for the TPC. This indicates that flavonoids might be the major contributors towards the phenolic compounds count for T. kotschyanus aerial parts (Fig. 2a). In addition, a significant linear relationship was found between both phenolic and flavonoid contents, and DPPH• scavenging capacity (expressed as the reciprocal of the calculated IC50 values) of different extracts (R2 = 0.99, p < 0.05; R2 = 0.86, p < 0.05, respectively) (Fig. 2b and c). The positive correlation indicates that the higher phenolic and flavonoid contents resulted in a stronger antioxidant activity. Moreover, these correlation coefficients suggest that the phenolic and flavonoid compounds of T. kotschyanus extracts contributed by 99 and 86 %, respectively, to their antioxidant capacities. This observation has also been reported by other researchers (Bhatt and Negi 2012; Yusri et al. 2012; Turumtay et al. 2014).
Fig. 2.
a Relationship between the TFC and TPC of T. kotschyanus extracts. b Relationship between the antioxidant capacity of T. kotschyanus extracts measured using the DPPH assay and their total phenolic content. c Relationship between the antioxidant capacity of T. kotschyanus extracts measured using the DPPH assay and their total flavonoid content
Reducing power
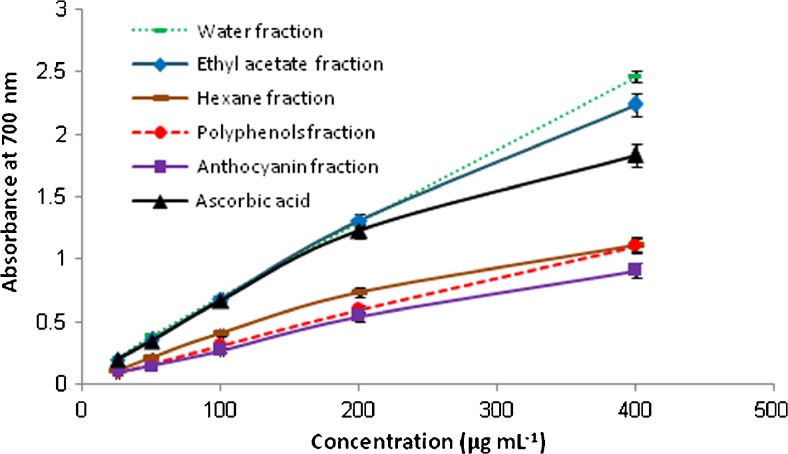
Reducing power is another mechanism for determination of electron donating ability of extracts. The presence of electron donor in the extract would result in the reducing of Fe3+ to Fe2+. The amount of Fe2+ complex can be then monitored by measuring the formation of Perl’s Prussian blue at 700 nm. Increasing absorbance at 700 nm indicates an increase in reductive ability. Figure 3 shows the dose–response curves for the reducing power of studied extracts. It is found that, the reducing power of sample increases with the increase of its concentration. Moreover, in a concentration of 400 μg mL−1 of tested extracts the descending order of reducing power was as follows: water fraction (A700 = 2.46 ± 0.04) > ethyl acetate fraction (A700 = 2.23 ± 0.06) > hexane fraction (A700 = 1.11 ± 0.03) ≈ polyphenols fraction (A700 = 1.10 ± 0.02) > anthocyanin fraction (A700 = 0.91 ± 0.01) with significant difference between them (P = 0.000). The water and ethyl acetate fractions exhibited higher reducing power than ascorbic acid (A700 = 1.83 ± 0.03) (p < 0.05).
Fig. 3.
Reducing power of flavonoid- (water, ethyl acetate and hexane fractions), polyphenol- and anthocyanin- rich extracts of T. kotschyanus
Antibacterial activity
The antibacterial activity of T. kotschyanus extracts against Gram-positive (S. aureus and B. subtilis) and Gram-negative (E. coli and P. aeruginosa) bacteria were recorded by the disc diffusion method (see Table 2). These bacteria are commonly known to cause food poisoning or food spoilage. In addition, the finding towards inhibition of bacterial strains was compared with that of positive controls, gentamicin and chloramphenicol. As shown in Table 2, the extracts exhibited moderate to good growth inhibitory effect against the tested microorganisms. Maximum activity was observed against P. aeruginosa, whereas B. subtilis was the most resistant to these extracts. Interestingly, chloramphenicol as a standard antibacterial agent remained inactive against P. aeruginosa.
Table 2.
Antibacterial activity of T. kotschyanus extracts using Kirby–Bauer disk diffusion method
| Sample | Zone of growth inhibition (mm) | |||
|---|---|---|---|---|
| E. coli | P. aeruginosa | S. aureus | B. subtilis | |
| Flavonoid-rich (Water fraction) | 10.5 ± 0.7 | 15.0 ± 1.1 | 11.0 ± 1.3 | 11.5 ± 1.4 |
| Flavonoid-rich (Ethyl acetate fraction) | 11.5 ± 0.7 | 13.5 ± 0.7 | 14.0 ± 1.4 | 16.5 ± 0.7 |
| Flavonoid-rich (Hexane fraction) | 8.5 ± 0.7 | 11.0 ± 1.4 | 12.5 ± 0.7 | 10.5 ± 0.7 |
| Polyphenols fraction | 10.5 ± 0.6 | 14.0 ± 1.4 | 10.5 ± 0.7 | 12.0 ± 1.4 |
| Anthocyanin fraction | 13.0 ± 1.4 | 12.5 ± 0.5 | 11.5 ± 0.7 | 10.5 ± 0.4 |
| Gentamicin (10 μg/disc) | 19.6 ± 1.1 | 15.6 ± 0.5 | 20.3 ± 1.5 | 26.0 ± 1.7 |
| Chloramphenicol (30 μg/disc) | 20.7 ± 1.5 | NE a | 21.7 ± 0.6 | 22.3 ± 1.2 |
The data are expressed as mean ± S.D. (n = 3). ANOVA test for data in the same column indicates significant difference (p = 0.000)
Concentration of extract: 20 mg/ mL
a No Effect
The antibacterial activity of the plant extracts could be attributed to the presence of bioactive compounds such as tannins, terpenoids, polyphenols, and flavonoids (Fernandez et al. 1996; Ouattara et al. 2011). Literature review shows the presence of the two main groups of secondary metabolites in the genus of Thymus, volatile terpenoids and polyphenolic compounds. Both of them are mainly responsible for the biological effects of this genus. The various polyphenolic constituents, especially flavonoids and phenolic acids, have been reported in Thymus plants. Among the flavonoids, the most widespread skeletons are flavones (luteolin, apigenin, and scutellarin) and then flavanones (eriodictyol and naringin). Besides, different phenolic acids like caffeic acid and rosmarinic acid have been more frequently found in the Thymus species (Marin et al. 2003; Jordan et al. 2009).
Conclusions
In this study, flavonoid-, polyphenol- and anthocyanin-rich extracts of T. kotschyanus aerial parts were evaluated for their antioxidant capacity, phenolic and flavonoid contents, and antibacterial activity. The results indicated that all of the samples possess high potent free radical scavenging and antioxidant activity. They were also rich in phenolic and flavonoid compounds. Interestingly, the antioxidant activity of various extracts positively correlated with their phenolic and flavonoid contents. Water extract which had the highest phenolic and flavonoid content, showed an appreciable DPPH radical scavenging activity as well as reducing power as compared with other extracts. On the basis of the obtained results from the antibacterial test, the extracts contained moderate to good growth inhibitory effect against both Gram-negative and Gram-positive bacteria. Therefore, the tested Thymus extracts might be valuable antibacterial and antioxidant natural sources and seem to be applicable in both medicine and food industry.
Acknowledgments
We are thankful to the Research Council of Mazandaran University for the financial support of this work.
Conflict of interest
The authors declare that they have no conflict of interest including any financial, personal or other relationships with other people or organizations that could inappropriately influence the present work.
References
- Amarowicz R, Estrella I, Hernández T, Robredo S, Troszynska A, Kosinska A, Pegg RB. Free raical-scavenging capacity, antioxidant activity, and phenolic composition of green lentil (Lens culinaris) Food Chem. 2010;121:705–711. doi: 10.1016/j.foodchem.2010.01.009. [DOI] [Google Scholar]
- Annegowda HV, Bhat R, Tze LM, Karim AA, Mansor SM. The free radical scavenging and antioxidant activities of pod and seed extract of Clitoria fairchildiana (Howard)- an underutilized legume. J Food Sci Techol. 2013;50:535–541. doi: 10.1007/s13197-011-0370-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagci E, Baser KHC. Study of the essential oils of Thymus haussknechtii Velen and Thymus kotschyanus Boiss. et Hohen var. kotschyanus (Lamiaceae) taxa from the eastern Anatolian region in Turkey. Flavour Fragr J. 2005;20:199–202. doi: 10.1002/ffj.1397. [DOI] [Google Scholar]
- Bhatt P, Negi PS. Antioxidant and antibacterial activities in the leaf extracts of Indian Borage (Plectranthus amboinicus) Food Nutr Sci. 2012;3:146–152. doi: 10.4236/fns.2012.32022. [DOI] [Google Scholar]
- Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1985;181:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol 28:25–30
- Chang ST, Wu JH, Wang SY, Kang PL, Yang NS, Shyur LF. Antioxidant activity of extracts from Acacia confusa bark and heartwood. J Agric Food Chem. 2001;49:3420–3424. doi: 10.1021/jf0100907. [DOI] [PubMed] [Google Scholar]
- D’Abrosca B, Pacifico S, Cefarelli G, Mastellone C, Fiorentino A. Limoncella’ apple, an Italian apple cultivar: phenolic and flavonoid contents and antioxidant activity. Food Chem. 2007;104:1333–1337. doi: 10.1016/j.foodchem.2007.01.073. [DOI] [Google Scholar]
- Drago L, Mombelli B, Ciardo G, De Vecchi E, Gismondo MR. Effects of three different fish oil formulations on Helicobacter pylori growth and viability: in vitro study. J Chemother. 1999;11:207–210. doi: 10.1179/joc.1999.11.3.207. [DOI] [PubMed] [Google Scholar]
- Fernandez MA, Garcia MD, Saenz MT. Antibacterial activity of the phenolic acids fractions of Scrophularia frutescens and Scrophularia sambucifolia. J Ethnopharmacol. 1996;53:11–14. doi: 10.1016/0378-8741(96)01419-5. [DOI] [PubMed] [Google Scholar]
- Imaida K, Fukushima S, Shirui T, Ohtani M, Nakanishi K, Ito N. Promoting actions of butylated hydroxy anisole and butylated hydroxy toluene on 2-stageurinary bladder carcinogenesis and ihibition of c-glutamyl transpeptidase-positive foci development in the liver of rats. Carcinogenesis. 1983;4:895–899. doi: 10.1093/carcin/4.7.895. [DOI] [PubMed] [Google Scholar]
- Jaffery EH, Brown AF, Kurilich AC, Keek AS, Matusheski N, Klein BP. Variation in content of bioactive components in broccoli. J Food Compos Anal. 2003;16:323–330. doi: 10.1016/S0889-1575(03)00045-0. [DOI] [Google Scholar]
- Jalas J. Thymus. In: Rechinger KH, editor. Flora Iranica. New York: Springer; 1982. pp. 536–538. [Google Scholar]
- Jordan MJ, Martınez RM, Martınez C, Monino I, Sotomayor JA. Polyphenolic extract and essential oil quality of Thymus zygis ssp. gracilis shrubs cultivated under different watering levels. Ind Crop Prod. 2009;29:145–153. doi: 10.1016/j.indcrop.2008.04.021. [DOI] [Google Scholar]
- Kahkonen MP, Hopia AI, Heikki JV, Rauha JP, Pihlaja K, Kujala TS, Heinonen M. Antioxidant activity of plant extracts containing phenolic compounds. J Agric Food Chem. 1999;47:3954–3962. doi: 10.1021/jf990146l. [DOI] [PubMed] [Google Scholar]
- Kahl R, Kappus H (1993) Toxicology of the synthetic antioxidants BHA and BHT in comparison with the natural antioxidant vitamin E. Z Lebensm Unters Forsch 196:329–338 [DOI] [PubMed]
- Marin PD, Grayer RJ, Kite GC, Matevski V. External leaf flavonoids of Thymus species from Macedonia. Biochem Syst Ecol. 2003;31:1291–1307. doi: 10.1016/S0305-1978(03)00040-1. [DOI] [Google Scholar]
- Meda A, Lamien CE, Romito M, Millogo J, Nacoulma OG. Determination of the total phenolic, flavonoid and praline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005;91:571–577. doi: 10.1016/j.foodchem.2004.10.006. [DOI] [Google Scholar]
- Miraliakbari H, Shahidi F. Antioxidant activity of minor components of tree nut oils. Food Chem. 2008;111:421–427. doi: 10.1016/j.foodchem.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Mohammed MJ, Al-Bayati FA. Isolation and identification of antibacterial compounds from Thymus kotschyanus aerial parts and Dianthus caryophyllus flower buds. Phytomedicine. 2009;16:632–637. doi: 10.1016/j.phymed.2008.12.026. [DOI] [PubMed] [Google Scholar]
- Morteza-Semnani K, Mahmoudi M, Riahi G. Effects of essential oils and extracts from certain Thymus species on swimming performance in mice. Pharm Biol. 2007;45:464–467. doi: 10.1080/13880200701389177. [DOI] [Google Scholar]
- Nabavi SM, Nabavi SF, Alinezhad H, Zare M, Azimi R. Biological activities of flavonoid-rich fraction of Eryngium caucasicum Trautv. Eu Rev Med Pharmacol Sci. 2012;16:81–87. [PubMed] [Google Scholar]
- Nickavar B, Esbati N. Evaluation of the antioxidant capacity and phenolic content of three Thymus Species. J Acupunct Meridian Stud. 2012;5:119–125. doi: 10.1016/j.jams.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Nickavar B, Mojab F, Dolat-Abadi R. Analysis of the essential oils of two Thymus species from Iran. Food Chem. 2005;90:609–611. doi: 10.1016/j.foodchem.2004.04.020. [DOI] [Google Scholar]
- Ouattara L, Koudou J, Zongo C, Barro N, Savadogo A, Bassole IHN, Ouattara AS, Traore AS. J Appl Sci. 2011;11:157–162. doi: 10.3923/jas.2011.157.162. [DOI] [Google Scholar]
- Proestos C, Chorianopoulos N, Nychas GJE, Komaitis M. RP-HPLC analysis of the phenolic compounds of plant extracts. Investigation of their antioxidant capacity and antimicrobial activity. J Agric Food Chem. 2005;53:1190–1195. doi: 10.1021/jf040083t. [DOI] [PubMed] [Google Scholar]
- Rasooli I, Mirmostafa S. Bacterial susceptibility to and chemical composition of essential oils from Thymus kotschyanus and Thymus persicus. J Agric Food Chem. 2003;51:2200–2205. doi: 10.1021/jf0261755. [DOI] [PubMed] [Google Scholar]
- Rice-Evans CA, Miller NJ, Paganga G. Antioxidant properties of phenolic compounds. Trends Plants Sci. 1997;2:152–159. doi: 10.1016/S1360-1385(97)01018-2. [DOI] [Google Scholar]
- Stahl-Biskup E, Saez F (2002) Thyme, The genus Thymus. Taylor and Francis, p 331
- Sun J, Yao J, Huang S, Long X, Wang J, García-García E. Antioxidant activity of polyphenol and anthocyanin extracts from fruits of Kadsura coccinea (Lem.) A.C. Smith. Food Chem. 2009;117:276–281. doi: 10.1016/j.foodchem.2009.04.001. [DOI] [Google Scholar]
- Turumtay EA, Islamoglu F, Cavus D, Sahin H, Turumtay H, Vanholme B. Correlation between phenolic compounds and antioxidant activity of Anzer tea (Thymus praecox Opiz subsp. caucasicus var. caucasicus) Ind Crop Prod. 2014;52:687–694. doi: 10.1016/j.indcrop.2013.11.042. [DOI] [Google Scholar]
- Willet WC. Balancing life-style and genomics research for disease prevention. Science. 2002;296:695–698. doi: 10.1126/science.1071055. [DOI] [PubMed] [Google Scholar]
- Yen GC, Chen HY. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J Agric Food Chem. 1995;43:27–32. doi: 10.1021/jf00049a007. [DOI] [Google Scholar]
- Yusri NM, Chan KW, Iqbal S, Ismail M. Phenolic content and antioxidant activity of Hibiscus cannabinus L. seed extracts after sequential solvent extraction. Molecules. 2012;17:12612–12621. doi: 10.3390/molecules171112612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zargari A. Medicinal plants. Tehran: Tehran University Press; 1990. pp. 28–42. [Google Scholar]
- Zhang DL, Quantick PC, Grigor JM. Changes in phenolic compounds in litchi (Litchi chinensis Sonn.) fruit during postharvest storage. Postharvest Biol Technol. 2000;19:165–172. doi: 10.1016/S0925-5214(00)00084-3. [DOI] [Google Scholar]