Abstract
The present study has been undertaken to analyze the effect of various processing methods like (i) soaking followed by autoclaving with (a) ash, (b) sodium bicarbonate, (c) sugar and (d) water; (ii) dry heating and (iii) fermentation on nutritional and antinutritional components of under-utilized tree legume Parkia roxburghii. The applied methods were found to enhance the protein (15–36 %) and lipid content (11–69 %) and to decrease the other proximal components. All the methods significantly reduced the antinutrients viz. condensed tannins, phytate, saponins, trypsin inhibitors, chymotrypsin inhibitors and lectins. Exceptionally, increased content was documented on total phenolics (117–207 %) and tannins (171–257 %). These reduced antinutritional loads have led to an increase in protein (9–20 %) and starch digestibility (75–254 %). Fermented kernels, the best processed form showed characteristic leguminous pattern for content and composition of amino acids, fatty acids and minerals. So knowledge gathering and exploration of nutritionally balanced under-utilized legumes would enhance food and nutritional security.
Keywords: Under-utilized legumes, Parkia roxburghii, Processing, Antinutrients, Non-nutrient bioactives
Introduction
Food insecurity, a global issue in the present scenario has occurs due to narrowing of food baskets which in turn caused by demography and household structure changes, pressure from imported species, loss of agrobiodiversity, scarcity of fertile land and farmer’s preference on few numbers of selective crops for uniform and higher yield. This makes the consumer’s to depend on selected cash crops. This further leads to nutritional insecurity and results in 870 million undernourished people and 2 billion people with micronutrient deficiencies around the world during 2010–2012. As a consequence, protein-energy malnutrition is found to be the major cause (35 %) of death in children’s below 5 years of age (FAO, WFP, IFAD 2012). Economic growth alone is not the valid solution for this interlinked problem; a pragmatic approach is reducing the over-reliance on very limited number of major crops and providing awareness/nutrition education on protein and micronutrient rich locally available/neglected/under-utilized crops. This enormous untapped commodity resource can acts as life-saver of millions of poor people for whom, food and nutritional security is a major problem.
Food legumes are nearly worldwide in distribution and one or more of them are consumed regularly in almost every part of the world. Due to their high quality protein (up to 50 %), they occupy an important place in human nutrition, especially for the low-income groups where the usage of animal protein is an economic constraint. Hence it derived the name ‘poor man’s meat’. Other nutritional benefits like high carbohydrate (up to 77 %) (starch as the most abundant carbohydrate), high fibre (up to 47 %), low fat (up to 12 %) (except oilseeds), high minerals and B complex vitamins (Carvalho et al. 2011; Swaminathan 1974) crafting them as an ideal food. In addition, they are rich in dietary antioxidants such as polyphenols, flavonoids, isoflavones, tannins, condensed tannins, anthocyanins, etc. which functions as radical scavengers, reducing agents, potential complexes of pro-oxidant metals and quenchers of singlet oxygen. It is interesting to note the report of Bravo (1998), who states higher phenolic compounds in legumes (78–1710 mg/100 g DM) than the well-known primary sources of phytochemicals such as apples (27–298 mg/100 g DM), berries (4–490 mg/100 g DM) and vegetables (6–180 mg/100 g DM).
Besides the nutritional and nutraceutical potential of legumes, their direct usage is hindered due to the presence of antinutritional factors. However, this can be overwhelmed by household to industrial scale processing methods such as soaking, germination, boiling, autoclaving, microwaving, roasting, fermentation and mirconization. This can eliminate/inactivate the antinutritional factors and increase the digestibility and bioavailability of nutrients (Khattab et al. 2009). Though, the antinutrients were recognized for their negative impacts in the past decades, their residual presence after processing, now have been recognized them as ‘double-edged sword’ because of their registered health-promoting potential in vitro and in vivo on the other end. Hence, consumption of legumes as a nutritional and therapeutic food is recommended by various health organizations (Shahidi 1997).
It is pertinent to note that, of the 20,000 legume species from 650 genera, only a fraction is being used extensively today. Selected cash crops such as soybean, pea and cowpea etc. alone have been exploited with the remaining as unexplored and some become threatened, vulnerable and extinct too. Severe genetic erosion of the legume species is occurring due to anthropogenic activities and also due to the introduction of genetically modified crops. A total of 2206 legume species have been listed in International Union for Conservation of Nature (IUCN) red list (Walters and Gillet 1998). Increasing demand and switching of world’s population to the protein and phytochemicals rich vegetarian diet has created unwarranted scarcity to plant resources. In view of the aforesaid points, the present scenario have necessitated contemporary research efforts geared towards the study of potential utilization of protein from locally available food crops, especially from under-utilized/relatively neglected protein rich oilseeds and legumes as an alternative to conventional legumes.
Parkia, a perennial leguminous tree species commonly known as locust beans consists of nearly 70 species and has centers of distribution in Africa, South East Asia and South America. Parkia roxburghii (Syn: P. timoriana and P. javanica) is one of the under-utilized species, distributed from north east India (Arunachal Pradesh, Nagaland, Manipur, Mizoram, Tripura, Meghalaya and Andaman and Nicobar Islands) to Irian Jaya. It is also known as yongchak in Manipuri and Zong tan in Mizoram with other local names such as sapota, long-chak and kuki tetai. The seeds of P. roxburghii is consumed either as raw/along with condiments or cooked with vegetables/meat. The Manipurians takes this pod as raw in a typical Manipuri salad called ‘singju’ and along with fish as ‘iromba’. The ethnic groups of Mizos, Garos, Kacharis, Nagas and Mikirs mainly consume this vegetable. Pods pounded with water are used for washing of head and face (Hopkins 1983; Longvah and Deosthale 1998; Seal 2011). In view of the above demands, the present study has been designed to explore the nutritional value of P. roxburghii and the effect various cost-effective processing methods on nutritional and antinutritional factors.
Materials
Chemicals
All the chemicals were of analytical grade from Sigma Chemicals and Co (St. Louis, MO, USA), Merck (Darmstadt, Germany) and HiMedia Laboratories (Mumbai, Maharashtra, India) unless otherwise specified. The water was treated by arium 67316 reverse osmosis (Sartorius Stedim Biotech GmbH, Germany). All the spectrophotometric measurements were done using UV 100 (Cyberlab, Westborough, MA, USA).
Legumes seeds
The seeds of P. roxburghii were collected from local market of Chumukedima, Dimapur, Nagaland during the month of April 2009 as a single lot. They were cleaned by removing foreign particles, broken and damaged seeds and stored in air tight containers for further processing.
Physical properties
Seed color was determined subjectively. Seed (n = 30) weight was determined by digital balance (to the nearest 0.001 g) (CPA224S, Sartorious, Germany). The same seeds were measured for their dimensions (cm) such as length, width and thickness using vernier caliper. Proportions of the seed coat and cotyledon were also determined. Physical properties such as (i) seed density (g/mL) – Weight of seeds/volume increased, (ii) seed volume (mL/100 seeds), (iii) hydration capacity – (Weight of soaked seeds-weight of unsoaked seeds/Number of seeds), (iv) hydration index – ((Hydration capacity/seed)/weight of one seed), (v) swelling capacity (Volume of soaked seeds-volume of unsoaked seeds/Number of seeds) and (vi) swelling index ((Swelling capacity/seed)/Seed volume in mL) has been determined (Sood et al. 2002).
Processing methods
The seeds were randomly divided into 7 batches (200 g/batch). The first batch (kernels) was kept as raw without any treatments. The remaining six batches were subjected to various processing methods.
Soaking followed by autoclaving
The legume seeds of IInd – Vth batch were soaked in following soaking solutions in the ratio of 1:10 (seed:soaking solution, w/v) and kept for 12 h. Batch II – 0.2 % ash (prepared from palm tree dried male inflorescence), Batch III – 0.1 % sodium bicarbonate, Batch IV – 1 % palm sugar (hereafter denoted as sugar), Batch V – distilled water. The soaking solutions were discarded at the end of soaking period and the non-edible hard seed coats of P. roxburghii were removed. The kernels obtained were autoclaved with their appropriate fresh soaking solution in 1:3 ratio (w/v) at 121 °C for 15 min.
Fermentation
The VIth Batch seeds were soaked in water (as like Batch V) and the kernels obtained were ground into coarse slurry with 1:3 ratio of water and autoclaved as said above. The slurry was kept for natural fermentation (solid state) at room temperature for 36 h under anaerobic conditions. Later it was dried at 45 ± 2 °C.
Dry heating
The VIIth Batch seeds were mixed with acid washed sand particles and dry heated in a hot air oven at 130 °C/20 min. The seed coats have been removed to obtain dry heated kernels after attaining to room temperature.
Handling of processed seeds
All of the autoclaved kernels were drained from the excess autoclaving solutions and dried at 45 ± 2 °C. The dried, processed kernels and raw kernels were ground into fine powder using a laboratory blender, followed by ball mill MM400 (Retsch, Haan, Germany) and stored in air tight polythene bags at 4 °C until further analysis. Similarly, roasted seeds were ground and stored after attaining the ambient temperature.
Proximate composition
The moisture content of raw and processed samples were determined using Moisture Analyzer MA35 (Sartorius AG, Germany) at 105 °C. Micro-Kjeldahl method was employed to determine the total nitrogen and a nitrogen-protein conversion factor 6.25 is used for crude protein (N × 6.25) determination. Crude lipid (Soxhlet extraction), crude fibre and ash contents (gravimetric) were determined by the methods outlined in Association of Official Analytical Chemists (AOAC) (1990). The nitrogen free extractives (NFE) was estimated by the difference. The ingredients of proximate composition were expressed as g/100 g DM. The gross energy (kJ) was determined by multiplying the percentage of crude protein, crude lipid and NFE by modified Atwater factors 17, 37 and 17 respectively.
Analysis of antinutritional factors
Total phenolic content (TPC) was estimated by Folin-Ciocalteau method (Makkar et al. 1997) and calculated from the tannic acid calibration curve (2–10 μg tannic acid). The results were expressed as g tannic acid equivalents (TAE)/100 g DM. Tannins in the extracts was estimated after the treatment with polyvinyl polypyrrolidone (PVPP), a tannin binding agent as per Makkar et al. (1997). The content of nontannin phenolics was calculated as g TAE/100 g DM and the tannin content of the samples were calculated as, Tannin (%) = Total phenolics (%) - Nontannin phenolics (%). Condensed tannins was estimated by HCl-butanol method (Porter et al. 1986) and expressed as g leucocyanidin equivalents (LE) calculated using the formula: Condensed tannins/100 g DM = (OD value at 550 nm × 78.26 × dilution factor)/(% dry matter). Phytic acid content (g/100 g DM) was estimated by the method of Vaintraub and Lapteva (1988) and calculated using phytate standard graph (32–160 μg phytic acid). Total saponin content was determined by the method of Hiai et al. (1976) using diosgenin calibration graph (20–100 μg diosgenin) and the content was expressed as g diosgenin equivalents (DE)/100 g DM.
Trypsin inhibitor activity was measured using the synthetic substrate benzyl-DL-arginine-para-nitroanilide (BAPNA) according to Kakade et al. (1969) and Smith et al. (1980) and the results were expressed as mg pure trypsin inhibited/g sample. Chymotrypsin inhibitor activity of raw and processed samples was estimated (Kakade et al. 1970) and expressed as chymotrypsin units inhibited (CUI)/g sample using standard chymotrypsin calibration curve (2–8 μg chymotrypsin). α-Amylase inhibitor activity was determined according to Deshpande et al. (1982) by estimating the reducing sugars liberated by α-amylase using DNSA reagent (Sumner 1924) and the result was expressed as α-amylase inhibitor units/g sample. Lectins (phytohemeagglutinins) in legume seeds were determined using their unique feature hemeagglutination with the trypsinized erythrocytes of rat, cow, goat and human (A, B and O) blood (Gordon and Marquardt 1974). Hemagglutination activity is defined as the inverse of the amount of material per mL in the last dilution giving positive agglutination. The result was expressed as hemagglutinating units/g protein.
In vitro protein and in vitro starch digestibility
The in vitro protein digestibility (IVPD) of raw and processed samples was determined by multienzyme technique (Satterlee et al. 1979) using the regression equation Y = 234.84-22.56X and the results were expressed on % basis. The in vitro starch digestibility (IVSD) was determined according to Goni et al. (1997) and the starch digestibility % was calculated as % starch hydrolyzed from the total starch content of the sample.
Amino acid analysis
The raw and fermented kernel flours were hydrolyzed by 6 N HCl at 110 °C for 24 h under vacuum (AOAC 1990). The amino acids were analyzed by HPLC equipped with a fluorescence detector (Agilent, Santa Clara, CA, USA). The analytical column was a reversed phase C18 column. Amino acid standards (Sigma-Aldrich, St. Louis, MO, USA) were used for peak identification and quantification. In addition, 2.5 mM L-α-amino-n-butyric acid was used as an internal standard.
Fatty acid analysis
The oil from raw and fermented samples was extracted with petroleum ether (40–60 °C) in the ratio of 1:20 (w/v) for 8 h at room temperature. After centrifugation, the solvent was removed under a nitrogen atmosphere. Methyl esters were prepared from the total lipids by the method of AOAC (1990). These fatty acid methyl esters were analyzed by gas chromatography (GC-14A, Shimadzu, Japan) equipped with flame ionization detector. The column temperature was set as gradient ranged from 160 to 225 °C and nitrogen, at a flow rate of 1 mL/min was used as a carrier gas. A standard fatty acid methyl ester mixture was run and retention times were used in identifying the sample peaks. Fatty acid contents were estimated from the peak area of standard and sample methyl esters.
Mineral analysis
One g of raw and fermented kernel flours were digested with a mixture of concentrated nitric acid, sulfuric acid and 60 % perchloric acid (9:2:1, v/v). After cooling the digest was diluted with distilled water, filtered (Whatman No. 1 filter paper) and the filtrate was made up to 100 mL with nanopure water. The minerals sodium (Na), potassium (K), calcium (Ca) was determined by flame photometer. Total phosphorous (P) was estimated by the ascorbic acid method after acid digestion and neutralization by phenolphthalein indicator and combined reagent with KH2PO4 as standard (APHA 1995). Magnesium (Mg), iron (Fe), copper (Cu), zinc (Zn) and manganese (Mn) were estimated by atomic absorption spectrophotometer (Perkin-Elmer, Model 5000). Standard calibration curve from pure metal/metal oxide is used for estimations.
Statistical analysis
The data were subjected to a one-way analysis of variance (ANOVA) and the significant difference between mean values was determined by Duncan’s multiple range test (p < 0.05) using SPSS (Statistical Package for the Social Sciences) version 13.0. (SPSS Inc., Illinois, USA). Values expressed were mean of triplicate determinations (n = 3) ± standard deviation (SD).
Results and discussion
Physical characteristics of P. roxburghii seeds
Physical characteristics of P. roxburghii seeds were shown in Table 1. Color of the seeds and cotyledon was found to be black and green respectively. Weight assessment on whole seed, cotyledon and seed coat, revealed that 70 % of seed is constituted by cotyledon fraction (a major reservoir of proteins and carbohydrates) and the remaining 30 % by seed coat. This ratio is similar to P. biglobosa (Urua et al. 2013) and other underutilized legume seeds Canavalia virosa (71 %) and C. gladiata (78 %) (Siddhuraju and Becker 2001a). Water absorption properties measured both gravimetrically (hydration capacity and hydration index) and volumetrically (swelling capacity and swelling index) revealed equivalent values with M. pruriens var. Utilis (Siddhuraju et al. 2000).
Table 1.
Physical properties of P. roxburghii seeds
| Parameters | P. roxburghii |
|---|---|
| Seed coat color | Black |
| Cotyledon color | Green |
| Seed weight (g) a | 0.53 ± 0.04(100) |
| Cotyledon weight (g) a | 0.37 ± 0.01(69.8*) |
| Seed coat weight (g) a | 0.16 ± 0.01(30.2*) |
| Length (cm seed−1) a | 1.48 ± 0.68 |
| Width (cm seed−1) a | 0.79 ± 0.11 |
| Thickness (cm seed−1) a | 1.40 ± 0.02 |
| Seed density (g mL−1) b | 0.45 ± 0.05 |
| Seed volume (mL 100 seeds−1) b | 45.0 ± 5.0 |
| Hydration capacity (g seed−1) b | 0.74 ± 0.03 |
| Hydration index b | 1.50 ± 0.04 |
| Swelling capacity (mL seed−1) b | 0.33 ± 0.06 |
| Swelling index b | 0.74 ± 0.07 |
Values are mean ± SD. a - (n = 30); b - (n = 3). * - Ratio of cotyledon/seed coat to the whole seeds.
Nutrient distribution of P. roxburghii kernels
Proximate composition of P. roxburghii raw kernels has been summarized in Table 2. Moisture content observed in the present study (4.2 %) showed variation with the previous report on the same seeds by 1.7–2.2 fold lower values. However, other proximal components such as protein (29.7 g/100 g DM), crude fibre (6.1 g/100 g DM) and ash (5.3 g/100 g DM) were agree with the previous reports on the same seeds (Mohan and Janardhanan 1993; Longvah and Deosthale 1998). Though the protein content is lower than soybean (40 %) and lupin (42 %), it is comparable to other legumes cowpea, chickpea, horse gram, kidney bean and peas (Khattab et al. 2009; Sreerama et al. 2012). The lipid content (22.7 g/100 g DM) observed in the present study disagree with the both of the above mentioned earlier reports which showed 17 and 34 %. Besides this, either one or multiple proximal components of P. roxburghii in the present study have showed similarity with other Parkia sp. such as P. biglandulosa (Prakash et al. 2001) and P. biglobosa (Elemo et al. 2011).
Table 2.
Proximate composition of raw and differentially processed P. roxburghii
| Samples: Raw/Processed kernels | % Dry matter basis | ||||||
|---|---|---|---|---|---|---|---|
| Moisture | Protein | Lipid | Crude fibre | Ash | NFE | Gross energy‡ | |
| Raw | 4.2v ± 0.6 | 29.7y ± 0.9 | 22.7z ± 0.4 | 6.1v ± 0.4 | 5.3v ± 0.3 | 36.1 | 1,955.5 |
| Ash soaked and autoclaved | 4.0v ± 0.6(-12) | 36.6w ± 0.5(23) | 37.6vw ± 0.5(65) | 4.6w ± 0.4(−24) | 2.9x ± 0.1(−45) | 18.3(−49) | 2,332.4 |
| NaHCO3 soaked and autoclaved | 4.2v ± 0.8(−7) | 34.0wx ± 1.2(15) | 38.4v ± 0.2(69) | 3.9wx ± 0.2(−35) | 2.8xy ± 0.1(−48) | 20.8(−42) | 2,363.0 |
| Sugar soaked and autoclaved | 4.7v ± 0.1(5) | 31.8xy±1.0(7) | 36.8w ± 0.7(62) | 4.3wx ± 0.6(−30) | 2.8xy ± 0.2(−47) | 24.3(−33) | 2,323.0 |
| Water soaked and autoclaved | 3.9v ± 0.1(−13) | 32.2xy ± 2.8(8) | 37.9v ± 0.5(67) | 1.9z ± 0.3(−70) | 2.2y ± 0.2(−59) | 25.8(−29) | 2,400.0 |
| Dry heated | 4.4v ± 0.4(−2) | 31.9xy±0.7(7) | 25.1y ± 0.5(11) | 2.9y ± 0.5(−52) | 4.3w ± 0.8(−20) | 35.8(−1) | 2,076.8 |
| Fermented | 4.5v ± 0.7(1) | 40.0v ± 2.4(36) | 32.5x ± 1.2(43) | 3.4xy ± 0.9(−45) | 4.7vw ± 0.3(−12) | 19.4(−47) | 2,216.4 |
Values are mean ± SD (n = 3). Mean values followed by different superscript lower case letters in the same column are significantly different (p < 0.05). Values in the parenthesis indicate percent increase (positive numerical values) or decrease (negative numerical values) estimated for the proximal components of processed kernels over the raw kernels. NFE - Nitrogen Free Extractives calculated as: 100 - (protein % DM + lipid % DM + crude fibre % DM + ash % DM); ‡ - kJ/100 g DM
Effect of processing on chemical composition
As a result of various processing methods, redistribution of chemical composition has occurred (Table 2). Insignificant moisture gain (1–5 %)/loss (2–13 %) registered by the processed P. roxburghii kernels is similar to the tendency registered on field pea, black gram, chickpea and lentil by autoclaving (Kumaraguru vasagam and Rajkumar 2010). Significantly increased protein content of 15–36 % was indexed on ash and NaHCO3 soaked followed by autoclaved kernels and fermented kernels. Other processing methods registered insignificant protein gain. Similar results were noticed on P. biglobosa on fermentation and autoclaving (Oboh et al. 2008; Sadiku 2010). Higher protein content observed in fermented samples might be attributed to the decrease in carbon ratio in the total mass, since carbohydrates are the prime energy source for microorganisms. This leads to increased concentration of nitrogen in the fermented slurry and thus the proportion of protein in the total mass.
All the processing methods registered significantly enhanced lipid content (11–69 %). The very farmer report of Ibiyemi et al. (1988) supports this trend by the lipid gain of 46–123 % in P. filicoideae on boiling and roasting. On the other hand, crude fibre, ash and carbohydrate content were found to be decreased significantly upon all of the processing methods employed by 24–70 %, 12–59 % and 29–49 % respectively. All these changes are suspected to the so called phenomenon ‘concentration effect’ i.e., removal of soluble carbohydrates, fibre, tannins, minerals and proteins (especially albumin) results in redistribution of nutrient percentages. All the processed forms indexed higher energy values (2076.8–2400.0 kJ/100 g DM) than the raw kernels, which might be due anonymously increased lipid content (the high energy yielding molecules) in all the processed samples.
Effect of processing on antinutritional factors of P. roxburghii
Phenolics, tannins and condensed tannins
Polyphenols, the ubiquitous groups of plant secondary metabolites and hence acts an integral part of human and animal diets. Traditionally, they have been considered as antinutrients due to their adverse effects on protein digestibility especially by tannins. However, recent interest in food phenolics has increased greatly, owing to their antioxidant capacity (free radical scavengers and metal chelators) and their key roles in the treatment and prevention of cancer, cardiovascular disease and other pathological scenario (Bravo 1998). Content of phenolics (0.3 g TAE/100 g DM) and tannins (0.07 g TAE/100 g DM) of raw P. roxburghii kernels (Table 3) is lower than the previous report on the same seeds (0.6 g/100 g DM) (Mohan and Janardhanan 1993). Dry heated and fermented P. roxburghii kernels have registered significant gain on phenolics (117 and 207 %) and tannins (171 and 257 %). Other processed forms registered either insignificant gain (phenolics)/loss (tannins). Corroborated results have been given by Urua et al. (2013) on boiled and fermented P. biglobosa. Condensed tannins marginally present in P. roxburghii kernels (0.03 g LE/100 g DM) have been obliterated to 100 %, indifferently by all of the processing methods. This might be due to the use of kernels alone because condensed tannins predominantly present in seed coat/testa rather than cotyledons. In supporting this, 1.9–3.6 g equ cat kg DM of condensed tannins observed in Vicia faba and Phaselous vulgaris has been lost up to 93 % by dehulling (Alonso et al. 2000).
Table 3.
Effect of different processing methods on anti-nutrients of non-protein and protein origin of P. roxburghii
| Samples: Raw/processed kernels | Anti-nutrients of non-protein origin | ||||||
|---|---|---|---|---|---|---|---|
| TPC ‼ | Tannins ‼ | CT † | Phytate ¶ | Saponins § | |||
| Raw | 0.30x ± 0.02 | 0.07x ± 0.02 | 0.03 ± 0.02 | 1.72v ± 0.06 | 1.05v ± 0.01 | ||
| Ash soaked and autoclaved | 0.34x ± 0.04(13) | 0.05x ± 0.02(−29) | ND(−100) | 0.78y±0.01(−55) | 0.49y ± 0.01(−53) | ||
| NaHCO3 soaked and autoclaved | 0.32x ± 0.01(7) | 0.04x ± 0.01(−43) | ND(−100) | 0.80y ± 0.01(−53) | 0.63x ± 0.02(−40) | ||
| Sugar soaked and autoclaved | 0.34x ± 0.01(13) | 0.04x ± 0.01(−43) | ND(−100) | 0.79y ± 0.01(−54) | 0.53y ± 0.02(−50) | ||
| Water soaked and autoclaved | 0.33x ± 0.01(10) | 0.03x ± 0.01(−57) | ND(−100) | 0.77y ± 0.00(−55) | 0.49y ± 0.01(−53) | ||
| Dry heated | 0.65w ± 0.03(117) | 0.19w ± 0.07(171) | ND(−100) | 0.86x ± 0.02(−50) | 0.69w ± 0.01(−34) | ||
| Fermented | 0.92v ± 0.05(207) | 0.25v ± 0.03(257) | ND(−100) | 0.65z ± 0.03(−62) | 0.52y ± 0.08(−50) | ||
| Samples: Raw/processed kernels | Anti-nutrients of protein origin | ||||||
| TIU" | CUI * | AIU! | Lectins (HU) ‡ | ||||
| Source of RBC | |||||||
| Cow | Goat | Rat | Human (A,B,O) | ||||
| Raw | 97.0a ± 4.6 | 16.1 ± 1.7 | ND | ND | ND | 75.3 | ND |
| Ash soaked and autoclaved | 2.2b ± 0.2(98) | ND(100) | ND | ND | ND | ND(100) | ND |
| NaHCO3 soaked and autoclaved | 1.9b ± 0.4(98) | ND(100) | ND | ND | ND | ND(100) | ND |
| Sugar soaked and autoclaved | 1.6b ± 0.1(98) | ND(100) | ND | ND | ND | ND(100) | ND |
| Water soaked and autoclaved | 1.7b ± 0.1(98) | ND(100) | ND | ND | ND | ND(100) | ND |
| Dry heated | 2.7b ± 0.2(97) | ND(100) | ND | ND | ND | ND(100) | ND |
| Fermented | 0.9b ± 0.1(99) | ND(100) | ND | ND | ND | ND(100) | ND |
Values are mean ± SD (n = 3). Mean values followed by different superscript lower case letters (v-z/a-b) in the same column are significantly different (p < 0.05). %I/D - Percent increase (positive numerical values) or decrease (negative numerical values) estimated for phytoconstituents of processed kernels over the raw kernels. ‼ - g tannic acid equivalents/100 g DM; † - g leucocyanidin equivalents/100 g DM; ¶ - g/100 g DM; § - g diosgenin equivalents/100 g DM; TIU - trypsin inhibitor units; " - mg pure trypsin inhibited/g sample; CUI - chymotrypsin units inhibited; *- chymotrypsin units inhibited/g sample; AIU - amylase inhibitor units; ! - α-amylase inhibitor units/g sample; HU - hemagglutinating units; ‡ - hemagglutinating units/g protein; ND - Not detected
Phytate
Phytate, a major reservoir of phosphate and inositol in plant seeds is popularly known for its chelating effects on cations such as Ca, Mg, Zn, Cu, Fe and K and forms unavailable/insoluble salts. Besides their negative effects on mineral absorption, now research trend has been moved to augment its proficient role as antioxidants, anticancer and cholesterol lowering agents (Shahidi 1997). Phytate content estimated for raw P. roxburghii kernels (1.7 g/100 g DM) is higher than other legume seeds faba beans, peas, chickpeas and kidney beans (6–11 mg/g) (Abd El-Hady and Habiba 2003). However, significant phytate loss (50–62 %) has been addressed by all the processed forms irrespective of the processing mode (Table 3). Suspected reasons for the losses are (i) lixiviation of compounds with low molecular weight and ionic character during soaking in either acidic/neutral/alkaline medium, and (ii) activation of endogenous phytase or microbial phytase during soaking/fermentation process (Azeke et al. 2005).
Saponins
One of the complex and chemically diverse group of plant secondary compounds are saponins which have a carbohydrate moiety attached to a triterpenoid/steroid aglycon. Though, they have been evaded for hemolytic/membranolytic/reduced protein digestibility properties, currently they have been recognized for their cholesterol lowering effects, anticancer activity and biocidal effect on dreadful pathogens (Shahidi 1997). The raw kernels of P. roxburghii have been documented with the saponin content of 1.05 g DE/100 g DM (Table 3), which is fairly comparable to red and brown varieties of C. gladiata, C. virosa (0.8-1 g DE/100 g DM) and higher than C. ensiformis (0.5 g DE/100 g DM) (Siddhuraju and Becker 2001a). The processing regimens caused significant saponin loss of 34–53 % in P. roxburghii. Ruiz et al. (1996) reported a detailed vision on saponin loss of chickpeas and lentils by various soaking medium (water, citric acid and NaHCO3) and cooking times and found that neither the content nor the composition of saponins was affected by soaking regardless of the pH of the soaking solution. Saponin loss on natural (60–76 %) and controlled fermentation (100 %) of P. vulgaris varieties has been registered by Shimelis and Rakshit (2008).
Enzyme inhibitors
Typical inhibitors of digestive enzymes, present in legume seeds are protein substances. However, phenolic compounds are also known for this inhibition activity. We determined the proteinaceous inhibitors of legumes (seed’s buffer extracts consists mainly proteinaceous inhibitors) against protein (trypsin and chymotrypsin) and starch digestive enzymes (α-amylase).
Trypsin and chymotrypsin inhibitor activity
Trypsin inhibitors, major protein digestive inhibitors instigate reduction in protein digestibility, absorption of amino acids and growth depression. Besides this, the protease inhibitors with both trypsin and chymotrypsin inhibition activity/Bowman-Birk inhibitors now have been recognized as small molecule protein suppressors of carcinogenesis in vitro and in vivo against an array of cancers (Clemente et al. 2010). Table 3 values documented, 97 mg trypsin inhibited/g sample for raw kernels of P. roxburghii. Most of the legumes (peas, chickpeas, kidney beans, faba beans) have been identified with the trypsin inhibitors (Abd El-Hady and Habiba 2003). However, each report has different expression units. Hence precise comparison could not be possible. As these inhibitors are heat labile, significant reductions (97–99 %) have been found invariably in all of the processed P. roxburghii kernels. Similarly, autoclaving (field pea, chickpea, black gram and lentils: 87–90 %) (Kumaraguru vasagam and Rajkumar 2010) and fermentation (cow pea: 100 %) (Egounlety and Aworh 2003) on other legumes has also been registered with loss of inhibitors.
P. roxburghii have been detected with the chymotrypsin inhibitor activity of 16.1 CUI/g sample in raw kernels. It was invariably reduced to 100 % upon all of the processing methods (Table 3). Chymotrypsin inhibitors have also been detected in V. faba and P. vulgaris (4 IU/mg DM) (Alonso et al. 2000). Report of Siddhuraju et al. (2000) on M. pruriens var. Utilis revealed the germplasm differences for chymotrypsin inhibitors and also the existence of higher inhibitory activity in decorticated seeds (14–16 CIU/mg) than the whole seeds (11–13 CIU/mg).
Amylase inhibitor activity
Amylase inhibitors, the other heat labile protein inhibitor, form complexes with α-amylase of diverse origin i.e., human/animal/bacteria/insect. It can inhibit either salivary/pancreatic amylase, and leads to reduced starch digestion and pancreatic hypertrophy. Besides this, now they have been recognized as anti-diabetic/hypoglycemic agents (Shahidi 1997). However, these inhibitors have not been detected in P. roxburghii kernels (Table 3). This is contradictory to the results of Singh et al. (2010) who found the presence of amylase inhibitors in P. roxburghii raw seeds and 72 % decreased inhibitor activity in processed seeds. This variation might be due the influence of genetic composition and agroclimatic conditions (Gonzalez et al. 2012; Stoughton-Ens et al. 2010).
Lectins
Lectins are proteins/glycoproteins, which agglutinate animal and/or human erythrocytes and stimulate mitogeny in resting lymphocytes. Their distribution in various plant family leads to appreciable consumption by human and animals. Its subsistence against digestion by gastro intestinal tract of the recipients makes them to exert their toxic effect by disruption of intestinal mucosa, brush border of duodenal and jejunal erythrocytes. Hence, digestion and absorption of nutrients is interrupted (Shahidi 1997). Raw kernels of P. roxburghii showed agglutination against rat RBC’s (75.3 HU/g protein) alone (Table 3); neither cow/goat nor human A, B and O RBC’s have been agglutinated. The results obtained are parallel to the agglutination pattern of earlier reports, (i) Utarabhand and Akkayanont (1995), who showed agglutination on the same seeds P. javanica against rat (267 HU/mg protein) and rabbit (68267 HU/mg protein) and not on human A, B and O blood group RBC’s, and (ii) Suvachittanont and Peutpaiboon (1992) and Cavada et al. (2000) who showed agglutination against rabbit and rat RBC’s respectively, rather than human RBC’s by P. discolor and P. speciosa. In contrast, Mohan and Janardhanan (1993) reported the presence of agglutinating activity against human A, B and O blood group RBC’s by the same legume seeds. All the processing regimens in the current study revealed complete abolishment (100 %) of agglutination on rat RBC’s. Cooking of V. faba and P. vulgaris has also been noticed with complete loss of lectins (Alonso et al. 2000).
In vitro protein digestibility
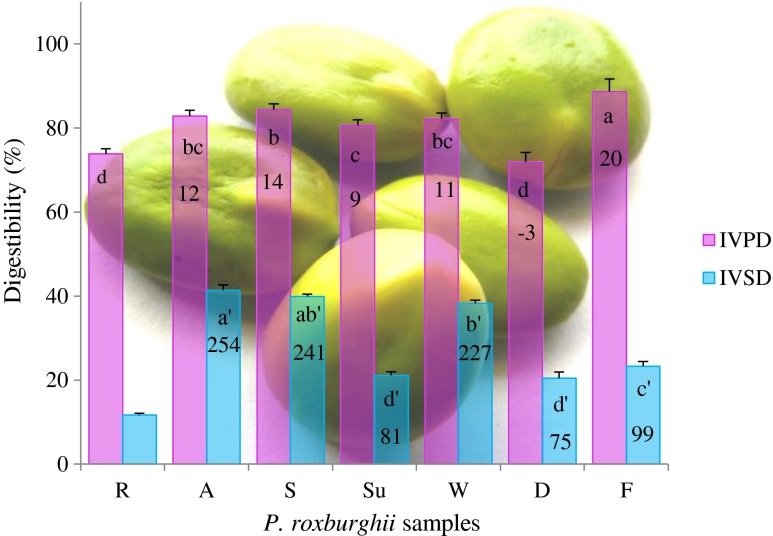
The observed IVPD of 73.9 % in P. roxburghii raw kernels (Fig. 1) is on par with the IVPD of other legumes viz. peas (77 %), kidney beans (78 %), faba beans (79 %) and chickpea (80 %) (Abd El-Hady and Habiba 2003). Reduced digestibility of legume seeds is attributed by the antinutritional factors in the albumin fraction, higher resistance of globulin fraction against enzymatic attacks due to its native structure and the presence of others molecules such as phytic acid and polyphenolics, specifically tannins, the partial protein precipitants (Park et al. 2010). Significantly increased digestibility rate from 9 to 20 % with the IVPD values of 81–89 % was invariably noticed on all of the moist cooking methods. On the other hand, dry heated kernels (72 % IVPD) registered insignificantly decreased digestibility rate of 3 %. Our results are corroborated with Khattab et al. (2009) on cow pea, kidney bean and pea upon various processing methods in which, increased digestibility rate was dictated by boiling (19–25 %), microwaving (13–16 %), autoclaving (10–12 %), soaking (6–8 %) and fermentation (3–4 %) while, dry heating (6–8 %) and mirconization (3–4 %) registered reduced the digestibility rate.
Fig. 1.
in vitro protein digestibility and in vitro starch digestibility of raw and differentially processed P. roxburghii kernels. Values are mean ± SD (n = 3). Bars with similar lowercase letter of a-d and a’-d’ are not significantly different (p < 0.05). Numerical values on the bars indicate the percent increase (positive numerical values) or decrease (negative numerical values) estimated for protein/starch digestibility of processed kernels over the values of raw kernels
The improvement of IVPD after heat treatment is most likely ascribed to the destruction of heat labile protease inhibitors, and denaturation of proteins especially globulins which commands to open up their structure, increases the chain flexibility and hence less resistance against digestive proteases. Diminution of phytic acid and tannins are the other aids (Mubarak 2005). Proteolytic enzymes produced by microflora during the fermentation are also equal contributors. However, the decreased digestibility observed on dry heated P. roxburghii seeds might be due to toughening of cotyledons, buildup of disulphide bridges between sulphur containing amino acids (the high heat resistant bonds which defend against digestive enzyme attack) and non-enzymatic browning (Maillard reaction) by thermal cross linking between sugar and amino acids occurred at high temperatures (Fagbemi et al. 2005).
In vitro starch digestibility
The tightly packed granule form of legume starch restricts the attack of hydrolytic enzymes and leads to reduced digestion rates. Source of native starch, granule size, extent of molecular association between starch components, amylose/amylopectin ratio, degree of crystallinity, physical distribution of starch in relation to dietary fibre components and presence of antinutritional factors are the interfering factors of digestibility process. In the present study, 11.7 % IVSD indexed by raw kernels of P. roxburghii (Fig. 1) is higher than the under-utilized legume M. pruriens var. Utilis germplasm (6–7 %) (Siddhuraju and Becker 2001b). Significantly increased digestibility rate was noticed on the all the processed kernels from 75 to 254 % with the IVSD values of 21–41 %. These results were fairly supported by Siddhuraju and Becker (2001b) who found increased IVSD of 22–485 % on M. pruriens var. Utilis on soaking followed by cooking/autoclaving in water, tamarind extract, NaHCO3 and citric acid; dry heating and germination. The process of hydrothermal cooking gelatinizes the intact starch granules and makes them sensitive to enzyme attacks and thus increases the digestibility. Diminution of antinutritional factors, especially the amylase inhibitors is another contributing factor (Alonso et al. 2000).
Analysis of raw and fermented P. roxburghii kernels
Fermented samples were selected for the analysis of amino acid, fatty acid and mineral profile by considering their enhanced nutritional value especially protein, significant diminution of antinutrients and enhanced digestibility of protein and starch.
Amino acid profile
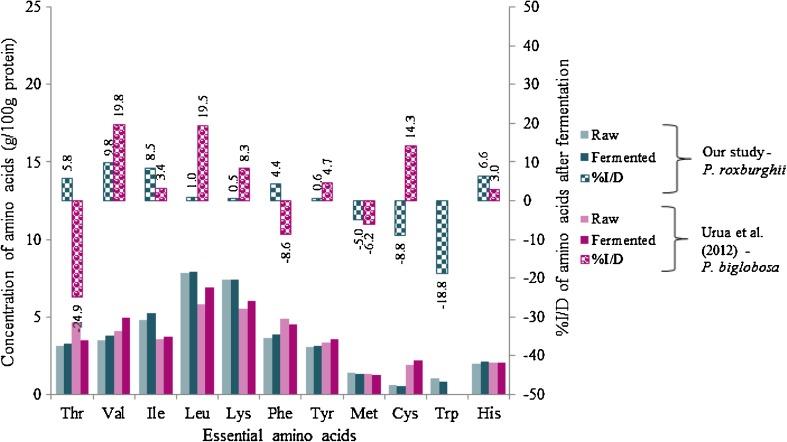
Table 4 shows the amino acid composition of raw and fermented P. roxburghii kernels. Both of the samples virtually met the FAO recommendations for amino acids except the limiting amino acids of legumes i.e., Trp and sulphur amino acids (Met and Cys). Among the non-essential group, Arg is found to be the highly concentrated amino acids followed by Asp and Glu which might be for their function as storage forms of nitrogen and precursors for the backbone of other amino acids (Onwuliri and Anekwe 1993). Hence, P. roxburghii has exhibited the characteristic amino acid pattern of legumes and found to be comparable with the report of Mohan and Janardhanan (1993) and Longvah and Deosthale (1998) on the same seeds, and Carvalho et al. (2011) on P. platycephala. Fermentated P. roxburghii kernels has recorded negligible changes in Leu, Lys and Tyr content and the highest loss of 18.8 % was noticed on Trp followed by Cys (8.8 %) and Met (5 %) (Fig. 2). The minimal losses of amino acids observed in the present study are in accordance with Chau et al. (1997) who reported the higher content of essential amino acids than the FAO/WHO pattern (except cysteine and methionine) besides the increased cooking times adopted for Phaseolus angularis, P. calcaratus and Dolichos lablab. Effect of various processing methods on amino acids content was revealed by Khattab et al. (2009) on peas, cowpea and kidney bean with the magnitude as, autoclaving > boiling > microwave cooking > soaking > roasting > fermentation > micronization.
Table 4.
Essential and non-essential amino acid composition (g/16 g N) and essential amino acid score of raw and fermented P. roxburghii kernels compared with standard plant and animal protein and FAO recommendations§
| Samples | Amino acids (g/16 g N) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Essential amino acids (EAA) | Non-essential amino acids (NEAA) | |||||||||||||||||
| Thr | Val | Ile | Leu | Lys | Phe | Tyr | Met | Cys | Trp | His | Asp | Glu | Ser | Gly | Ala | Arg | Pro | |
| P. roxburghii | Our study | |||||||||||||||||
| Raw | 3.1 | 3.5 | 4.8 | 7.8 | 7.4 | 3.7 | 3.1 | 1.4 | 0.6 | 1.0 | 2.0 | 11.5 | 10.5 | 4.7 | 4.6 | 5.0 | 14.7 | 5.2 |
| Fermented | 3.3 | 3.8 | 5.2 | 7.9 | 7.4 | 3.8 | 3.1 | 1.3 | 0.5 | 0.8 | 2.1 | 11.4 | 11.2 | 4.8 | 5.1 | 5.2 | 13.9 | 4.2 |
| Mohan and Janardhanan (1993) | ||||||||||||||||||
| Raw | 2.3 | 4.8 | 6.7 | 9.1 | 4.1 | 3.1 | 3.0 | 0.6 | trace | ND | 0.8 | 11.0 | 13.6 | 3.2 | 3.1 | 3.6 | 2.3 | 4.2 |
| Longvah and Deosthale (1998) | ||||||||||||||||||
| Raw | 3.4 | 4.4 | 4.2 | 8.0 | 6.5 | 5.5 | 4.6 | 1.8 | 1.0 | 2.6 | 8.2 | 16.2 | 4.8 | 4.2 | 5.6 | 8.6 | 4.8 | |
| Other Parkia sp. | P. platycephala (Carvalho et al. 2011) | |||||||||||||||||
| Raw | 3.9 | 4.9 | 3.8 | 6.8 | 15.5 | 4.5 | 3.5 | 1.1 | 3.4 | 0.4 | 4.1 | 8.5 | 13.1 | 5.5 | 4.7 | 5.5 | 9.2 | 2.1 |
| Standards | ||||||||||||||||||
| Soybean ‡ | 3.81 | 4.66 | 3.79 | 7.68 | 6.54 | 6.05 | 5.02 | 1.38 | 1.63 | 0.54 | 3.04 | 11.14 | 18.24 | 4.22 | 3.87 | 4.26 | 8.51 | 5.33 |
| Ovalbumin ¶ | 4.93 | 5.54 | 5.78 | 7.71 | 11.02 | 8.94 | 4.40 | 5.23 | 2.38 | 1.46 | 2.34 | 6.11 | 8.61 | 5.42 | 3.26 | 5.81 | 10.40 | 2.91 |
| Amino acid content meeting FAO recommendations § | ||||||||||||||||||
| 3.4 | 3.5 | 2.8 | 6.6 | 5.8 | 6.3† | 2.5‼ | 1.1 | 1.9 | ||||||||||
| Essential amino acid score of raw and fermented P. roxburghii from our study | ||||||||||||||||||
| Raw | 91.47 | 99.12 | 172.14 | 118.64 | 127.18 | 107.30 | 79.21 | 91.82 | 104.21 | |||||||||
| Limiting amino acids: I - Methionine + Cysteine; II – Tryptophan | ||||||||||||||||||
| Fermented | 96.76 | 108.86 | 186.79 | 119.85 | 127.93 | 110.15 | 87.60 | 74.55 | 111.05 | |||||||||
| Limiting amino acids: I - Tryptophan; II - Methionine + Cysteine | ||||||||||||||||||
Fig. 2.
Comparative essential aminoacid composition of raw and fermented P. roxburghii kernels with previous study on raw and feremented P. biglobosa. Solid bars ( ) are the concentration of essential aminoacids on left axis and patterned bars (
) are the concentration of essential aminoacids on left axis and patterned bars ( ) are the %I/D of essential aminoacids of the processed samples (calculated over the raw samples) on right axis
) are the %I/D of essential aminoacids of the processed samples (calculated over the raw samples) on right axis
Fatty acid profile
Fatty acids acts as double-edged swords due to their role as major energy source, structural components of cell membranes, precursors for bioactive molecules, regulators of enzyme activities and gene expression on the positive side; ischaemic/reperfusion injury and heart failures on the negative side via the imbalance in their homeostasis. This depends upon the dietary fatty acid supplied to the body. Table 5 values indicate the fatty acids of raw and fermented P. roxburghii kernels and marginally increased fatty acid content in fermented kernels. This is supported by study of Fapojuwo et al. (1986) who studied the fatty acid profile of P. filicoideae up on cooking and fermentation. The increased fatty acid content might be due to the lipase/esterase activity aided by the microflora under the fermenting conditions (Azokpota et al. 2006). Though P. roxburghii have higher saturated fatty acids, it is predominated by stearic acid (28 %), a saturated fatty acid which has negative effect on raising LDL-C (low density lipoprotein-cholesterol) rather than palmitic acid (19 %). α-linolenic acid, an essential fatty acid and precursor of ω3 polyunsaturated fatty acids has been retained in fermented kernels and comparable with green peas and white beans (Kalogeropoulos et al. 2010). The P/S ratio and essential fatty acids of P. roxburghii indicates that consumption of this seed oil will deliver healthy fatty acids and can overcome the negative effects associated with fatty acids.
Table 5.
Fatty acid composition of raw and fermented P. roxburghii kernels (% of total fatty acids)
| Fatty acids | Raw | Fermented |
|---|---|---|
| C14:0 (Myristic acid) | – | – |
| C16:0 (Palmitic acid) | 18.1 | 19.6(9) |
| C16:1 (Palmitolic acid) | – | – |
| C18:0 (Stearic acid) | 25.2 | 28.3(12) |
| C18:1 (Oleic acid) | 23.7 | 28.0(19) |
| C18:2 (Linoleic acid) | 49.7 | 51.6(4) |
| C18:3 (Linolenic acid) | 2.3 | 2.7(14) |
| C20:0 (Arachidic acid) | 2.8 | 2.9(2) |
| C22:0 (Behenic acid) | – | – |
| C22:1 (Erucic acid) | 4.6 | 4.8(5) |
| Unknown fatty acids | 0.6 | 1.6 |
| Total fatty acids | 126.3 | 139.5 |
| Total saturated fatty acids | 46.1 | 50.8 |
| Total unsaturated fatty acids | 80.2 | 87.1 |
| P/S ratio¶ | 1.2 | 1.1 |
¶ - P/S ratio: (C18:2 + C18:3)/(C14:0 + C16:0 + C18:0). Values in the parenthesis are the percent increase estimated for fatty acids of fermented kernels over the raw kernels
Mineral profile
Minerals constitute a small proportion of plant materials but have great physiological and nutritional importance in human metabolisms. Minerals of raw and fermented P. roxburghii kernels have been shown in Table 6. Presence of high K (990 mg/100 g) and low Na (91 mg/100 g) in the major element group; and high Fe (7.4 mg/100 g) and low Cu (0.8 mg/100 g) in the micro element group observed in the present study corroborates with P. platycephala (Carvalho et al. 2011) and also with other underutilized legumes Brachystegia eurycoma, Tamarindus indica and Mucuna flagellipes (Ajayi et al. 2006). Minimal loss (5–16 %)/gain (7–21 %) of mineral elements has been noticed on fermented kernels. Still both raw and fermented forms can meet RDI (Reference Daily Intake) recommendations of FDA up to 56 %. Literature survey indicates that minerals of processed seeds have higher bioavailability than the raw seeds despite their low concentrations which is due to the suppression of mineral binders such as phytates and phenolics upon processing.
Table 6.
Mineral composition of raw and fermented P. roxburghii compared with FDA (2010)‡
| Minerals (mg/100 g) | P. roxburghii | RDI (mg/day) as per FDA | |||
|---|---|---|---|---|---|
| Raw kernels | % RDI met | Fermented kernels | % RDI met | ||
| Macro elements | |||||
| Sodium | 91.6 | 3 | 111.1 (21) | 3 | 3500 |
| Potassium | 990.8 | 41 | 827.8 (−16) | 34 | 2400 |
| Phosphorous | 291.6 | 24 | 276.5 (−5) | 23 | 1200 |
| Calcium | 306.8 | 31 | 327.6 (7) | 33 | 1000 |
| Magnesium | 199.4 | 50 | 225.8 (13) | 56 | 400 |
| Micro elements | |||||
| Iron | 7.4 | 41 | 8.5 (16) | 47 | 18 |
| Copper | 0.8 | 44 | 1.0 (15) | 51 | 2 |
| Zinc | 2.7 | 18 | 3.1 (17) | 21 | 15 |
| Manganese | 1.1 | 56 | 0.9 (−16) | 47 | 2 |
‡ - Patterns of mineral requirement for adults and children four or more years of age based on a 2000 kcal (8368 kJ) intake; RDI - Reference daily intake in 101.9 (c) (8) iv. FDA (2010). %RDI met is the ratio of the individual mineral element to the recommended RDI by FDA calculated as; (estimated mineral content × 100)/RDI of FDA. Values in the parenthesis are the percent increase estimated for fatty acids of fermented kernels over the raw kernels
Conclusions
Result of our study suggests that, the various processing regimens applied in this work have drastically reduced the antinutritional load and enhanced the digestibility values for protein and starch. Fermentation, being the most effective processed form has showed significant composition and content of amino acids, fatty acids and trace elements on par with the recommendation of FAO and FDA. Since, all the regimens are economically viable, they can be advocated for enhancing the safety and quality of legume seeds. The practical application of this study would be the use of this nutritionally balanced under-utilized legume P. roxburghii in formulation of nutraceutical foods, fortification purposes and also in feed formulations as an ingredient.
Acknowledgments
The authors wish to thank University Grants Commission (UGC), New Delhi, India (F.No. 34-259\2008) for financial assistance.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Abd El-Hady EA, Habiba RA. Effect of soaking and extrusion conditions on antinutrients and protein digestibility of legume seeds. LWT Food Sci Technol. 2003;36:285–293. doi: 10.1016/S0023-6438(02)00217-7. [DOI] [Google Scholar]
- Ajayi IA, Oderinde RA, Kajogbola DO, Uponi JI. Oil content and fatty acid composition of some underutilized legumes from Nigeria. Food Chem. 2006;99:115–120. doi: 10.1016/j.foodchem.2005.06.045. [DOI] [Google Scholar]
- Alonso R, Aguirre A, Marzo F. Effect of extrusion and traditional processing methods on antinutrients and in vitro digestibility of protein and starch in faba and kidney beans. Food Chem. 2000;68:159–165. doi: 10.1016/S0308-8146(99)00169-7. [DOI] [Google Scholar]
- AOAC (1990) Official methods of analysis of the Association of Official Analytical Chemists (15th ed.). Association of Official Analytical Chemists, Washington, DC, USA
- APHA (1995) Standard methods for examination of water and waste water (19th ed.) American Public Health Association, Washington, DC, USA
- Azeke MA, Fretzdorff B, Buening-Pfaue H, Holzapfel W, Betsche T. Nutritional value of African yambean (Sphenostylis stenocarpa L): improvement by lactic acid fermentation. J Sci Food Agric. 2005;85:963–970. doi: 10.1002/jsfa.2052. [DOI] [Google Scholar]
- Azokpota P, Hounhouigan DJ, Nago MF, Jakobsen M. Esterase and protease activities of Bacillus spp. from afitin, iru and sonru; three African locust bean (Parkia biglobosa) condiments from Benin. Afr J Biotechnol. 2006;5:265–272. [Google Scholar]
- Bravo L. Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev. 1998;56:317–333. doi: 10.1111/j.1753-4887.1998.tb01670.x. [DOI] [PubMed] [Google Scholar]
- Carvalho AFU, Farias DF, da Rocha-Bezerra LCB, de Sousa MN, Cavalheiro MG, Fernandes GS, Brasil ICF, Maia AAB, de Sousa DOB, Vasconcelos IM, Gouveia ST, Machado OLT. Preliminary assessment of the nutritional composition of underexploited wild legumes from semi-arid Caatinga and moist forest environments of northeastern Brazil. J Food Compost Anal. 2011;24:487–493. doi: 10.1016/j.jfca.2011.01.013. [DOI] [Google Scholar]
- Cavada BS, Madeira SVF, Calvete JJ, Souza LAG, Bomfim LR, Dantas AR, Lopes MC, Grangeiro TB, Freitas BT, Pinto VPT, Leite KB, Ramos MV. Purification, chemical and immunochemical properties of a new lectin from Mimosoideae (Parkia discolor) Prep Biochem Biotechnol. 2000;30:271–280. doi: 10.1080/10826060008544966. [DOI] [PubMed] [Google Scholar]
- Chau C, Cheung PCK, Wong Y. Effects of cooking on content of amino acids and antinutrients in three Chinese indigenous legume seeds. J Sci Food Agric. 1997;75:447–452. doi: 10.1002/(SICI)1097-0010(199712)75:4<447::AID-JSFA896>3.0.CO;2-5. [DOI] [Google Scholar]
- Clemente A, Moreno FJ, Marín-Manzano Mdel C, Jiménez E, Domoney C. The cytotoxic effect of Bowman-Birk isoinhibitors, IBB1 and IBBD2, from soybean (Glycine max) on HT29 human colorectal cancer cells is related to their intrinsic ability to inhibit serine proteases. Mol Nutr Food Res. 2010;54:396–405. doi: 10.1002/mnfr.200900122. [DOI] [PubMed] [Google Scholar]
- Deshpande SS, Sathe SK, Salunkhe DK, Cornforth DP (1982) Effects of dehulling on phytic acid, polyphenols, and enzyme inhibitors of dry beans (Phaseolus vulgaris L.). J Food Sci 47:1846-1850
- Egounlety M, Aworh OC. Effect of soaking, dehulling, cooking and fermentation with Rhizopus oligosporus on the oligosaccharides, trypsin inhibitor, phytic acid and tannins of soybean (Glycine max Merr.), cowpea (Vigna unguiculata L. Walp) and groundbean (Macrotyloma geocarpa Harms) J Food Eng. 2003;56:249–254. doi: 10.1016/S0260-8774(02)00262-5. [DOI] [Google Scholar]
- Elemo GN, Elemo BO, Oladunmoye OO, Erukainure OL. Comprehensive investigation into the nutritional composition of dehulled and defatted African locust bean seed (Parkia biglobosa) Afr J Plant Sci. 2011;5:291–295. [Google Scholar]
- Fagbemi TN, Oshodi AA, Ipinmoroti KO. Processing effects on some antinutritional factors and in vitro multienzyme protein digestibility (IVPD) of three tropical seeds: breadnut (Artocarpus altilis), cashewnut (Anacardium occidentale) and fluted pumpkin (Telfairia occidentalis) Pak J Nutr. 2005;4:250–256. doi: 10.3923/pjn.2005.250.256. [DOI] [Google Scholar]
- FAO, WFP, IFAD (2012) The State of Food Insecurity in the World 2012. Economic growth is necessary but not sufficient to accelerate reduction of hunger and malnutrition. Rome, FAO [DOI] [PMC free article] [PubMed]
- FAO/WHO (1989) Protein quality evaluation. Report of a joint FAO/WHO expert consultation. Food and nutrition paper no. 51
- Fapojuwo OO, Afolabi OA, Iheanacho KE, Maga JA. Nature of lipids in African locust beans (Parkia filicoidea Welw.) and changes occurring during processing and storage. J Agric Food Chem. 1986;34:246–248. doi: 10.1021/jf00068a022. [DOI] [Google Scholar]
- FDA (2010) Nutritional Labeling and Education Act (NLEA) Requirements (8/94-2/95). Revised by 2010
- Goni I, Garcia-Diz L, Manas E, Saura-Calixto FA. A starch hydrolysis procedure to estimate the glycemic index. Nutr Res. 1997;17:427–437. doi: 10.1016/S0271-5317(97)00010-9. [DOI] [Google Scholar]
- Gonzalez JA, Konishi Y, Bruno M, Valoy M, Prado FE. Interrelationships among seed yield, total protein and amino acid composition of ten quinoa (Chenopodium quinoa) cultivars from two different agroecological regions. J Sci Food Agric. 2012;92:1222–1229. doi: 10.1002/jsfa.4686. [DOI] [PubMed] [Google Scholar]
- Gordon JA, Marquardt MD. Factors affecting haemagglutination by concanavalin A and soybean agglutinin. Biochim Biophys Acta. 1974;332:136–144. doi: 10.1016/0005-2736(74)90368-X. [DOI] [Google Scholar]
- Hiai S, Oura H, Nakajima T. Color reaction of some sapogenins and saponins with vanillin sulfuric acid. Planta Med. 1976;29:116–122. doi: 10.1055/s-0028-1097639. [DOI] [PubMed] [Google Scholar]
- Hopkins HC. The taxonomy, reproductive biology and economic potential of Parkia (Leguminosae: Mimosoideae) in Africa and Madagascar. Bot J Linn Soc. 1983;87:135–167. doi: 10.1111/j.1095-8339.1983.tb00987.x. [DOI] [Google Scholar]
- Ibiyemi SA, Abiodun A, Akanji SA. Andasonia digitata Bombax and Parkia filicoideae Welw: fruit pulp for the soft drink industry. Food Chem. 1988;28:111–116. doi: 10.1016/0308-8146(88)90140-9. [DOI] [Google Scholar]
- Kakade ML, Simons N, Liener IE. An evaluation of natural vs synthetic substrates for measuring the antitryptic activity of soybean samples. Cereal Chem. 1969;46:518–526. [Google Scholar]
- Kakade ML, Swenson DH, Liener IE. Note on the determination of chymotrypsin and chymotrypsin inhibitor activity using casein. Anal Biochem. 1970;33:255–258. doi: 10.1016/0003-2697(70)90294-0. [DOI] [PubMed] [Google Scholar]
- Kalogeropoulos N, Chiou A, Ioannou M, Karathanos VT, Hassapidou M, Andrikopoulos NK. Nutritional evaluation and bioactive microconstituents (phytosterols, tocopherols, polyphenols, triterpenic acids) in cooked dry legumes usually consumed in the Mediterranean countries. Food Chem. 2010;121:682–690. doi: 10.1016/j.foodchem.2010.01.005. [DOI] [Google Scholar]
- Khattab RY, Arntfield SD, Nyachoti CM. Nutritional quality of legume seeds as affected by some physical treatments, Part 1: protein quality evaluation. LWT Food Sci Technol. 2009;42:1107–1112. doi: 10.1016/j.lwt.2009.02.008. [DOI] [Google Scholar]
- Kumaraguru Vasagam KP, Rajkumar M. Beneficial influences of germination and subsequent autoclaving of grain legumes on proximate composition, antinutritional factors and apparent digestibility in black tiger shrimp, Penaeus monodon Fabricius. Aquacult Nutr. 2010;17:e188–e195. doi: 10.1111/j.1365-2095.2009.00748.x. [DOI] [Google Scholar]
- Longvah T, Deosthale YG. Nutrient composition and food potential of Parkia roxburghii, a less known tree legume from northeast India. Food Chem. 1998;62:477–481. doi: 10.1016/S0308-8146(97)00179-9. [DOI] [Google Scholar]
- Makkar HPS, Becker K, Abel H, Pawelzik E. Nutrient contents, rumen protein degradability and antinutritional factors in some colour and white flowering cultivars of Vicia faba beans. J Sci Food Agric. 1997;75:511–520. doi: 10.1002/(SICI)1097-0010(199712)75:4<511::AID-JSFA907>3.0.CO;2-M. [DOI] [Google Scholar]
- Mohan VR, Janardhanan K. Chemical and nutritional evaluation of raw seeds of the tribal pulses Parkia roxburghii G. Don. and Entada phaseoloides (L.) Merr. Int J Food Sci Nutr. 1993;44:47–53. doi: 10.3109/09637489309017422. [DOI] [Google Scholar]
- Mubarak AE. Nutritional composition and antinutritional factors of mung bean seeds (Phaseolus aureus) as affected by some home traditional processes. Food Chem. 2005;89:489–495. doi: 10.1016/j.foodchem.2004.01.007. [DOI] [Google Scholar]
- Oboh G, Alabi KB, Akindahunsi AA. Fermentation changes the nutritive value, polyphenol distribution and antioxidant properties of Parkia biglobosa seeds (African locust beans) Food Biotechnol. 2008;22:363–376. doi: 10.1080/08905430802463404. [DOI] [Google Scholar]
- Onwuliri VA, Anekwe GE. Amino acid composition of B. pinnatum (Lim) Med Sci Res. 1993;21:507–508. [Google Scholar]
- Park SJ, Kim TW, Baik B. Relationship between proportion and composition of albumins, and in vitro protein digestibility of raw and cooked pea seeds (Pisum sativum L.) J Sci Food Agric. 2010;90:1719–1725. doi: 10.1002/jsfa.4007. [DOI] [PubMed] [Google Scholar]
- Porter LJ, Hrstich LN, Chan BC. The conversion of procyanidines and prodelphinidins to cyanidin and delphinidin. Phytochemistry. 1986;25:223–230. doi: 10.1016/S0031-9422(00)94533-3. [DOI] [Google Scholar]
- Prakash D, Niranjan A, Tewari SK, Pushpangadan P. Underutilised legumes: potential sources for low-cost protein. Int J Food Sci Nutr. 2001;52:337–341. doi: 10.1080/09637480120057521. [DOI] [PubMed] [Google Scholar]
- Ruiz RG, Price KR, Arthur AE, Rose ME, Rhodes MJC, Fenwick RG. Effect of soaking and cooking on the saponin content and composition of chickpeas (Cicer arietinum) and lentils (Lens culinaris) J Agric Food Chem. 1996;44:1526–1530. doi: 10.1021/jf950721v. [DOI] [Google Scholar]
- Sadiku OA. Processing methods influence the quality of fermented African locust bean (iru/ogiri/dadawa) Parkia biglobosa. J Appl Sci Res. 2010;6:1656–1661. [Google Scholar]
- Satterlee LD, Marshall HF, Tennyson JM. Measuring protein quality. J Am Oil Chem Soc. 1979;56:103–109. doi: 10.1007/BF02671431. [DOI] [PubMed] [Google Scholar]
- Seal T. Nutritional composition of wild edible fruits in Meghalaya state of India and their ethano-botanical importance. Res J Bot. 2011;6:58–67. doi: 10.3923/rjb.2011.58.67. [DOI] [Google Scholar]
- Shahidi F. Beneficial health effects and drawbacks of antinutrients and phytochemicals in foods. In: Shahidi F, editor. Antinutrients and phytochemicals in food. Washington: American Chemical Society; 1997. pp. 1–9. [Google Scholar]
- Shimelis EA, Rakshit SK. Influence of natural and controlled fermentations on α-galactosides, antinutrients and protein digestibility of beans (Phaseolus vulgaris L.) Int J Food Sci Technol. 2008;43:658–665. doi: 10.1111/j.1365-2621.2006.01506.x. [DOI] [Google Scholar]
- Siddhuraju P, Becker K. Effect of various domestic processing methods on antinutrients and in vitro protein and starch digestibility of two indigenous varieties of Indian tribal pulse, Mucuna pruriens var. Utilis. J Agric Food Chem. 2001;49:3058–3067. doi: 10.1021/jf001453q. [DOI] [PubMed] [Google Scholar]
- Siddhuraju P, Becker K. Species/variety differences in biochemical composition and nutritional value of Indian tribal legumes of the genus Canavalia. Die Nahrung. 2001;45:224–233. doi: 10.1002/1521-3803(20010801)45:4<224::AID-FOOD224>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Siddhuraju P, Becker K, Makkar HPS. Studies on the nutritional composition and antinutritional factors of three different germplasm seed materials of an under-utilized tropical legume, Mucuna pruriens var. Utilis. J Agric Food Chem. 2000;48:6048–6060. doi: 10.1021/jf0006630. [DOI] [PubMed] [Google Scholar]
- Singh SJ, Singh PK, Dutta BK, Sahoo UK. Effect of processing and cooking methods on some anti-nutritive, toxic components and nutritional constituents in stink bean (Parkia roxburghii G. Don) seeds. Indian J Agric Biochem. 2010;23:97–102. [Google Scholar]
- Smith C, Megen WV, Twaalfhoven L, Hitchcock C. The determination of trypsin inhibitor levels in foodstuffs. J Sci Food Agric. 1980;31:341–350. doi: 10.1002/jsfa.2740310403. [DOI] [PubMed] [Google Scholar]
- Sood M, Malhotra SR, Sood BC. Effect of processing and cooking on proximate composition of chickpea (Cicer arietinum) varieties. J Food Sci Technol. 2002;39:69–71. [Google Scholar]
- Sreerama YN, Sashikala VB, Pratape VM, Singh V. Nutrients and antinutrients in cowpea and horse gram flours in comparison to chickpea flour: evaluation of their flour functionality. Food Chem. 2012;131:462–468. doi: 10.1016/j.foodchem.2011.09.008. [DOI] [Google Scholar]
- Stoughton-Ens MD, Hatcher DW, Wang N, Warkentin TD. Influence of genotype and environment on the dietary fiber content of field pea (Pisum sativum L.) grown in Canada. Food Res Int. 2010;43:547–552. doi: 10.1016/j.foodres.2009.07.011. [DOI] [Google Scholar]
- Sumner JB. The estimation of sugar in diabetic urine, using dinitrosalicylic acid. J Biol Chem. 1924;62:287–290. [Google Scholar]
- Suvachittanont W, Peutpaiboon A. Lectin from Parkia speciosa seeds. Phytochemistry. 1992;31:4065–4070. doi: 10.1016/0031-9422(92)80415-B. [DOI] [Google Scholar]
- Swaminathan M. Pulses - Essentials of food and nutrition. Madras: Ganesh and Co; 1974. [Google Scholar]
- Urua IS, Uyoh EA, Ntui VO, Okpako EC. Effect of processing on proximate composition, anti-nutrient status and amino acid content in three accessions of African locust bean (Parkia biglobosa (jacq.) benth. Int J Food Sci Nutr. 2013;64:94–102. doi: 10.3109/09637486.2012.704903. [DOI] [PubMed] [Google Scholar]
- Utarabhand P, Akkayanont P. Purification of a lectin from Parkia javanica beans. Phytochemistry. 1995;38:281–285. doi: 10.1016/0031-9422(94)00550-D. [DOI] [Google Scholar]
- Vaintraub IA, Lapteva NA. Colorometric determination of phytate in unpurified extracts of seeds and the products of their processing. Anal Biochem. 1988;175:227–230. doi: 10.1016/0003-2697(88)90382-X. [DOI] [PubMed] [Google Scholar]
- Vasconcelos IM, Maia AA, Siebra EA, Oliveira JTA, Carvalho AFU, Melo VMM, Carlini CR, Castellar LIM. Nutritional study of two Brazilian soybean (Glycine max) cultivars differing in the contents of antinutritional and toxic proteins. J Nutr Biochem. 2001;12:55–62. doi: 10.1016/S0955-2863(00)00148-0. [DOI] [PubMed] [Google Scholar]
- Walters KS, Gillet HJ. 1997 IUCN Red list of threatened plants. Compiled by the world conservation monitoring centre. Switzerland: IUCN - The World Conservation Union; 1998. [Google Scholar]