Abstract
High-amylose wheat was subjected to various germination conditions and changes in its nutritional values and antioxidant capacity were investigated. Amounts of soluble dietary fiber, total protein and free lipid of germinated high-amylose wheat increased with increased germination times, whereas no significant changes were observed for insoluble dietary fiber and free fatty acids. Total free amino acid contents of high-amylose wheat gradually increased from 129.7 to 314.4 mg/100 g of grain (db) during 48 h of germination. As compared to ungerminated wheat, essential and functional amino acids including isoleucine, leucine, phenylanaline, valine and gamma-amino butyric acid in the 48 h-germinated wheat increased by 3–10 times. Total phenolic contents of both free and bound phenolics and their antioxidant capacities significantly increased after 24 h of germination and were further improved with prolonged germination times. It appears that nutritional values and bioactive compounds of high amylose wheat significantly improved for enhanced food applications.
Keywords: High-amylose wheat, Germination, Nutrition, Antioxidant
Introduction
Whole grains containing the nutritional constituents in bran and germ have been reported to have significant health benefits. The consumption of whole grain foods was found to prevent from several chronic diseases such as coronary cardiovascular disease (Bazzano et al. 2002), colon cancer (Bingham et al. 2003) and diabetes (Anderson et al. 2004). In wheat, bran and germ are rich in dietary fiber, vitamins, minerals and bioactive compounds, which are always removed during milling by the conventional milling methods. Therefore, the consumers are always encouraged to eat whole wheat products such as whole wheat breads, cakes and noodles though the texture and mouthfeel quality of the whole wheat products are reduced as compared to the white wheat products.
In order to improve nutrients and sensory quality of whole wheat foods, germination technologies has been widely employed because the nutrients of whole grains including dietary fiber, free amino acids, phenolic compounds and antioxidant capacity have been reported to increase during germination (Hung et al. 2012; Nelson et al. 2013). Tkachuk (1979) reported that the free amino acid content after 122 h of germination at 10, 16.5 and 25 °C was respectively 4×, 10× and 7× that of ungerminated wheat. An increase in levels of ash and dietary fiber was clearly observed for the 48 h-germinated waxy wheat in the report of Hung et al. (2012). Free phenolic compounds including ferulic acid, vanillic acid and syringic acid as well as total phenolic compounds and antioxidant capacity of germinated wheat significantly increased as compared with ungerminated wheat (Hung et al. 2011, 2012). As a result, sprouted food consumption has enjoyed growing popularity with health conscious consumers (Nelson et al. 2013).
Recently, wheat grains containing starch with various ratios of amylose and amylopectin have been developed widely using genetic techniques. High-amylose wheat (>37 % amylose) was firstly produced in Japan by Dr. Yamamori’s research group (Yamamori et al. 2000). The granular structure and physicochemical properties of the high-amylose wheat starches have been changed as compared to the normal wheat starch due to the difference in the amylose/amylopectin ratios. Hung et al. (2007) reported that the high-amylose wheat starch had a significantly altered structure of amylopectin which did not show any major peaks in the X-ray diffractogram. Yamamori et al. (2000) also found that the short chains (DP 6–10) in amylopectin molecules of the high-amylose wheat starch increased, whereas the level of DP 11–25 chains decreased. The high-amylose wheat was also found to have high values of protein, ash, lipid and dietary fiber as compared to the normal wheat (Morita et al. 2002). The unique structure and characteristics of starch and flour composition of the high-amylose wheat contributed to the new texture and quality of the wheat-base food products such as bread and pasta. The substitution with 50 % of high-amylose wheat flour for 1CW (No. 1 Canada Western Red Spring) flour produced noodle like pasta with the similar textural property to durum flour (Morita et al. 2003). The high-amylose wheat flour was also used to substitute for the normal wheat flour in breadmaking to increase the amount of dietary fiber and resistant starch in the breads (Hung et al. 2005). As a result, the high-amylose wheats have been recently encouraged to be grown and applied for food processing to improve the texture and quality of the end-use products. In order to improve its nutritional values for wide food applications, high-amylose wheat was subjected to germinate and the changes in chemical composition, nutritive values and antioxidant capacity during germination were observed in this study.
Materials and methods
Materials
High-amylose wheat grains (~37.5 % amylose) were obtained from Dr. Yamamori, National Agricultural Research Center for Tohoku Region, Morioka, Japan. High-amylose wheat was bred from Kanto 79/Turkey 116 F2 // Chousen 57 (Yamamori et al. 2000) and their F5 and F6 progeny without SGP-1 were harvested in Nagano Prefecture in Japan in 2004.
The compound 1,1-diphenyl-1-picrylhydrazyl (DPPH), Folin-Ciocalteu phenol reagent and other chemicals were commercially purchased from Wako Chemical Co. (Osaka, Japan). Total dietary fibre assay kit (TDF-100A) was obtained from Sigma-Aldrich Co.
Germination conditions
The procedure of preparation and germination of high-amylose wheat grains were the same procedure to that of waxy wheat grains as previously reported (Hung et al. 2012). Briefly, High-amylose wheat grains (~50 g) were initially rinsed of their surface and soaked in excess distilled water for 30 min at 25 °C before germination in a dark cabinet at 30 °C and a relative humidity of 85 % for 0, 6, 12, 24, 36 and 48 h. After incubation, the samples were washed carefully with distilled water and then freeze-dried after freezing at −84 °C. Freeze-dried samples and un-germinated grains (control) were ground using a Retsch ZM1 milling apparatus (Retsch, Haan, Germany) with a 0.5 mm mesh.
Determination of soluble, insoluble and total dietary fibers
Soluble, insoluble and total dietary fiber contents of the control and germinated high-amylose wheat grains were determined based on the AOAC method 985.29 (AOAC 1997) using a total dietary fibre assay kit (Product code TDF-100A, Sigma-Aldrich Co. Ltd.). Total dietary fiber content was the sum of the soluble and insoluble dietary fiber fractions.
Determination of total protein and free amino acids
Total protein contents of the control and germinated high-amylose wheats were determined according to the standard AACC International Approved method 46–10 (AACC 2000) using a Kjeltec Auto Sampler System 1035 Analyzer (Tecator Ltd., Tokyo).
Free amino acids of the control and germinated high-amylose wheats were determined according to the method of Saikusa et al. (1994). Wheat flour (1.6 g) were homogenized with 4 ml of 8 % trichloroacetic acid solution in test tubes (2 × 16 cm) using a homogenizer for 5 min and then shaken (100 strokes/min; 5-cm amplitude) at 30 °C for 1 h. The suspension was centrifuged at 4 °C and 14,000g for 15 min and the supernatant was recovered by filtration through a 0.45 μm membrane filter (Advantec Co., Ltd., Tokyo, Japan). Free amino acids were analysed using a LC-11 Amino Acid Analyzer (Yanaco Co., Kyoto, Japan).
Determination of free, bound and total lipids and fatty acid composition
Free and bound lipids were extracted using a Soxhlet system according to the Commission des Communautes Europeennes (CEC) standard procedures (Ruibal-Mendieta et al. 2002). Free lipids content was determined by extracting of wheat flour (5 g) with hot diethyl ether (110 ml) using a Soxhlet system for 6 h, recovering by a rotary evaporator under reduced pressure at 35 °C and then drying to constant weight at 105 °C. Bound lipids were extracted from the free lipid-removed residue. The residue was subjected to acid hydrolysis in 100 ml hot 3 N HCl for 1 h, washed with at least 800 ml of distilled water and then dried overnight at 70–75 °C. Finally, the hydrolyzed residue was extracted with diethyl ether as described previously. Total lipids were calculated by adding free to bound lipid. Lipid content value is expressed as percentage of dry matter and is the means of duplicate determinations.
Free fatty acid composition in free and bound lipids of germinated waxy wheat was determined using a gas-liquid chromatography (GLC). Free and bound lipids were extracted with n-hexane as described above, then were used to prepare methylated fatty acids (FAME) using 14 % (w/v) boron trifluoride (BF3) in methanol according to the method of Christie (1982). The fatty acid composition was analysed using an Yanaco GLC apparatus (Model G 3800, Osaka, Japan).
Determination of total phenolic compounds and their antioxidant capacity
The contents of free and bound phenolics in the control and germinated high-amylose wheats were determined according to the method of Liyana-Pathirana and Shahidi (2007) with a slight modification. Free phenolic compounds were extracted from 1 g of wheat flour by shaking with 10 ml of 80 % chilled ethanol for 20 min. The extraction was repeatedly done for 3 times and the combined supernatants were evaporated at 45 °C and reconstituted with methanol to a final volume of 10 ml. The extract were then stored at −40 °C until use. Bound phenolic compounds were extracted 6 times with diethyl ether-ethyl acetate (1:1) after alkaline hydrolysis of the residue from free phenolic compound extraction. The ether-ethyl acetate extracts were evaporated to dryness and bound phenolic compounds were reconstituted in 10 ml of methanol and then stored at −40 °C until use.
The appropriate dilutions of free and bound phenolic extracts (0.5 ml) were oxidized with Folin-Ciocalteu’s reagent (0.5 ml) in a centrifuge tube (50 ml). The reaction was neutralized with saturated sodium carbonate solution (1 ml), followed by adjusting the volume to 10 ml with distilled water. The contents in the tubes were thoroughly mixed and allowed to stand at ambient temperature for 45 min until the characteristic blue color developed. Then the tubes were centrifuged at 4,000g for 5 min. Absorbance of the clear supernatants was measured at 725 nm using a spectrophotometer (UV-160A, Shimadzu, Kyoto, Japan). The content of total phenolics in each extract was calculated based on a standard curve prepared using ferulic acid and expressed as milligrams of ferulic acid equivalent (FAE) per gram of sample. Standard calibration was made from 0, 20, 40, 60, 80 and 100 μg/ml.
Antioxidant capacity of free and bound phenolic extracts were determined using the DPPH radical scavenging method as previously described by Hung et al. (2011). A final concentration of DPPH solution (0.075 mM) was used for wheat phenolic extracts. DPPH solution (3.9 ml) was mixed with sample solution (0.1 ml). The mixture was kept in the dark at ambient temperature. The absorbance of the mixtures was recorded at 515 nm for exactly 30 min. Blank was made from 3.9 ml of DPPH and 0.1 ml methanol and measured absorbance at t = 0. The scavenging of DPPH was calculated according to the following equation (Liyana-Pathirana and Shahidi (2007).
Where: Abs(t=0) = absorbance of DPPH radical + methanol at t = 0 min; Abs(t=30) = absorbance of DPPH radical + phenolic extracts at t = 30 min.
Statistical analysis
All tests were performed at least in duplicate. Analysis of variance (ANOVA) was performed using Duncan’s multiple-range test to compare treatment means at P < 0.05 using SPSS software version 16 (SPSS Inc., USA).
Results and discussion
Soluble, insoluble and total dietary fibers of germinated high-amylose wheats
Changes of soluble, insoluble and total dietary fibers in high-amylose wheat during 48 h of germination are shown in Table 1. Levels of soluble dietary fiber in the high-amylose wheat significantly increased during germination, whereas the insoluble dietary fiber only significantly decreased in the initial 6 h of germination and remained unchanged for a longer time of germination. The total dietary fibers of the germinated high-amylose wheat overall increased although the levels of the total dietary fibers of the 6 and 12 h-germinated wheats were lower than that of the un-germinated wheat. The increase of soluble and insoluble dietary fibers by 25 % in wheat after 4 days of germination was also found by Harmuth-Hoene et al. (1987). However, Koehler et al. (2007) reported that during the first 96 h of germination, the concentration of soluble dietary fiber in wheat did not increase while the amount of insoluble dietary fiber decreased resulting in the concentration of total dietary fiber remained constant or decreased. The decrease in total dietary fibers was clearly observed in wheats germinated at low temperatures (15 and 20 °C) than those germinated at high temperatures (25 and 30 °C). The different results observed were due to the different wheat varieties and the different germination methods used in each research. The wheats containing high amount of dietary fiber such as high-amylose wheat or waxy wheat and germination temperature of 30 °C are considered to be suitable for producing higher amounts of total dietary fiber and soluble dietary fiber in germinated wheats (Hung et al. 2012; Koehler et al. 2007).
Table 1.
Changes in soluble, insoluble and total dietary fibers and total protein of high-amylose wheat grains during germination
| Germination time (h) | Soluble dietary fiber (mg/g sample, db) | Insoluble dietary fiber (mg/g sample, db) | Total dietary fiber (mg/g sample, db) | Total protein (mg/g, db) |
|---|---|---|---|---|
| 0 | 9.1 ± 0.5a | 201.3 ± 2.0b | 210.4 ± 2.0bc | 205.9 ± 2.7a |
| 6 | 11.1 ± 0.4b | 192.1 ± 1.5a | 203.2 ± 1.8a | 208.3 ± 0.7ab |
| 12 | 18.0 ± 0.5c | 188.6 ± 2.5a | 206.6 ± 1.5ab | 209.5 ± 2.6ab |
| 24 | 22.3 ± 0.6d | 191.1 ± 1.2a | 213.3 ± 2.1cd | 209.7 ± 1.7ab |
| 36 | 24.8 ± 0.5e | 191.2 ± 1.0a | 216.0 ± 1.6d | 213.0 ± 1.6b |
| 48 | 25.9 ± 0.4e | 198.4 ± 2.0b | 224.3 ± 2.0e | 213.5 ± 0.6b |
The values are means of triplicate measurements
Means by the same letter in the same column are not significant difference (P < 0.05), n = 3
The significant increase in levels of soluble dietary fiber in this study may have potential nutritional benefits because soluble dietary fibre consumption was found to significantly lower blood cholesterol and help stabilize blood glucose levels (Anderson et al. 1990; Brown et al. 1999).
Total proteins and free amino acids
The changes in total proteins of the high-amylose wheat during germination are given in Table 1. The results indicate that the total proteins in the high-amylose wheat did not change during the first 24 h of germination. However, a substantial increase in the total proteins in the 36 and 48 h-germinated wheats was observed. These results agreed with the results reported by Lemar and Swanson (1976), who has found that the crude protein in all four sprouted wheats significantly increased after germination for 1–3 days to sprout lengths of 0.25 to 1 in.. Other studies also reported that the total proteins in the hard red winter wheat or the waxy wheat appreciably increased during sprouting for 3–5 days (Ranhotra et al. 1977) or germination time longer than 48 h (Hung et al. 2012), respectively.
The profile of free amino acids of the germinated high-amylose wheats is shown in Table 2. The total free amino acids in the 6, 12, 24, 36, and 48 h-germinated high-amylose wheats were 133.9, 182.5, 236.6, 276.0 and 314.4 mg/100 g of grains (db), respectively, which were significantly higher than that in the un-germinated high-amylose wheats (129.7 mg/100 g of grains, db). The results also indicate that the longer the time of germination was, the higher the total free amino acids were. The essential amino acids including isoleucine, leucine, phenylanaline and valine in the 48 h-germinated wheat significantly increased by 3–10 times as compared to those in the un-germinated wheat. In addition, other essential amino acids also increased during germination contributing to high nutritional quality of germinated wheats. These results are consistent with the results reported by Hung et al. (2012) for the germinated waxy wheats. The semi- and non-essential amino acids in the germinated wheat also significantly increased by increasing germination time and reached their highest levels after 48 h of germination. In cereal and pseudocereal grains, gamma-amino butyric acid (GABA) is considered as a functional compound with potential nutritional benefits. The concentration of GABA in the germinated high-amylose wheat significantly increased by 4 times after 48 h of germination, suggesting that the germinated high-amylose wheat may also be a potential functional food with high biological health effects.
Table 2.
Changes in free amino acid composition of high-amylose wheat grains during germination
| Amino acids | Germination time (h) | |||||
|---|---|---|---|---|---|---|
| 0 | 6 | 12 | 24 | 36 | 48 | |
| Essential amino acids | ||||||
| I-Leu | 3.2 | 3.3 | 4.4 | 6.1 | 9.9 | 13.1 |
| Leu | 3.9 | 4.0 | 5.0 | 9.3 | 12.2 | 15.0 |
| Lys | 1.9 | 2.9 | 8.8 | 3.5 | 11.4 | 4.9 |
| Met | 2.2 | 2.9 | 2.8 | 3.3 | 3.9 | 4.7 |
| Phe | 2.9 | 3.2 | 3.9 | 6.2 | 8.9 | 10.4 |
| Thr | 2.0 | 2.6 | 3.1 | 4.7 | 5.9 | 6.1 |
| Val | 3.3 | 3.7 | 15.6 | 22.8 | 17.4 | 34.4 |
| Semi essential amino acids | ||||||
| Arg | 22.0 | 27.7 | 36.3 | 34.2 | 32.8 | 35.2 |
| Gly | 4.2 | 2.7 | 5.4 | 9.3 | 11.2 | 12.1 |
| His | 2.4 | 2.3 | 2.7 | 4.7 | 6.4 | 9.0 |
| Tyr | 3.4 | 3.6 | 4.1 | 6.2 | 9.0 | 9.6 |
| Non essential amino acids | ||||||
| Ala | 9.8 | 8.8 | 11.5 | 16.3 | 18.9 | 20.2 |
| Asn | 20.4 | 16.5 | 17.1 | 16.9 | 17.3 | 21.5 |
| Asp | 3.0 | 1.9 | 2.4 | 3.5 | 4.5 | 5.1 |
| Cys | 3.3 | 3.2 | 3.4 | 4.3 | 3.8 | 4.6 |
| GABA | 3.4 | 3.4 | 4.9 | 7.7 | 11.0 | 16.0 |
| Glu | 8.4 | 10.5 | 16.5 | 28.3 | 32.8 | 35.0 |
| Orn | 2.8 | 1.9 | 2.6 | 1.5 | 2.5 | 2.4 |
| Pro | 24.9 | 26.7 | 29.2 | 43.3 | 51.3 | 46.9 |
| Ser | 2.9 | 2.6 | 3.1 | 4.9 | 5.5 | 8.6 |
| Total | 129.7 | 133.9 | 182.5 | 236.6 | 276.0 | 314.4 |
The values are means of duplicate measurements (mg/100 g, db)
Free, bound and total lipids and fatty acid compositions
Table 3 shows the changes in free and bound lipids of the high-amylose wheat during germination. During germination, amount of free lipids in wheat significantly increased, whereas the amount of bound lipids did not change. The total lipids, a sum of free and bound lipids, in the germinated wheat were found to enhance along with the increased germination time. The results suggest that glycerol and free fatty acids were rapidly released by lipase during germination. Thus, the increased total protein and total lipids in the germinated high-amylose wheat might be due to the loss of starch during germination, resulting in the decrease in the weight of grain and increase in proportion of total protein and lipid.
Table 3.
Changes in free and bound lipids of high-amylose wheat grains during germination
| Free lipid | Bound lipid | Total lipid | |
|---|---|---|---|
| 0 | 2.40 ± 0.08a | 1.26 ± 0.13a | 3.66 ± 0.07a |
| 6 | 2.70 ± 0.01b | 1.16 ± 0.02a | 3.87 ± 0.04a |
| 12 | 2.69 ± 0.09b | 1.06 ± 0.11a | 3.75 ± 0.03a |
| 24 | 2.87 ± 0.07c | 1.11 ± 0.01a | 3.98 ± 0.10b |
| 36 | 2.82 ± 0.09c | 1.23 ± 0.04a | 4.05 ± 0.07b |
| 48 | 2.85 ± 0.22c | 1.23 ± 0.09a | 4.08 ± 0.41b |
The values are means of triplicate measurements
Means by the same letter in the same column are not significant difference (P < 0.05), n = 3
Eleven fatty acids were detected in the high-amylose wheat with concentration of 98.9–99.6 and 93.1–98.6 % of total free and bound lipids, respectively (Table 4). In free lipids, the major components were polyunsaturated fatty acids (57.1–66.0 % of total fatty acids), followed by monounsaturated (24.4–25.1 %) and saturated (16.5–16.8 %) fatty acids. The polyunsaturated fatty acids were also major components in bound lipids (52.8–62.6 % of total fatty acids), followed by saturated (22.6–23.1 %) and monounsaturated (12.9–17.4 %) fatty acids. Three main fatty acids in the both free and bound lipids were linoleic (53.5–55.1 and 45.2–59.8 %, respectively), oleic (22.5–23.3 and 11.8–12.9 %, respectively) and palmitic acid (14.5–14.9 and 16.5–21.4 %, respectively). The fatty acid composition of both free and bound lipids of high-amylose wheat did not change during germination. This result was also found by Hung et al. (2012) for the germinated waxy wheat.
Table 4.
Changes in fatty acids composition (% of total fatty acids) of high-amylose wheat grains during germination
| Fatty acids | Free lipid | Bound lipid | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Germination time (h) | |||||||||||||
| 0 | 6 | 12 | 24 | 36 | 48 | 0 | 6 | 12 | 24 | 36 | 48 | ||
| C14:0 | Myristic | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 | 0.2 | 0.1 |
| C16:0 | Palmitic | 14.9 | 14.5 | 14.8 | 14.9 | 14.8 | 14.9 | 21.1 | 19.4 | 19.7 | 16.5 | 21.4 | 17.1 |
| C16:1 | Palmitoleic | 0.3 | 0.4 | 0.2 | 0.2 | 0.2 | 0.2 | 0.3 | 0.9 | 0.7 | 2.0 | 0.2 | 1.7 |
| C18:0 | Stearic | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 0.7 | 0.7 | 0.7 | 0.6 | 0.7 | 0.6 |
| C18:1 | Oleic | 23.3 | 22.9 | 23.0 | 22.9 | 22.7 | 22.5 | 11.8 | 12.5 | 12.7 | 12.2 | 12.8 | 12.9 |
| C18:2 | Linoleic | 54.6 | 53.5 | 55.0 | 55.1 | 55.0 | 55.0 | 59.8 | 54.0 | 54.8 | 45.2 | 58.9 | 47.2 |
| C18:3 | Linolenic | 3.1 | 3.2 | 3.1 | 3.1 | 3.1 | 3.1 | 2.8 | 3.4 | 3.3 | 4.8 | 3.3 | 5.0 |
| C20:0 | Arachidic | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.4 | 0.4 | 0.4 | 0.3 | 0.4 | 0.3 |
| C20:1 | Eicosenoic | 1.1 | 1.2 | 1.1 | 1.2 | 1.2 | 1.2 | 0.5 | 0.5 | 0.4 | 0.4 | 0.5 | 0.4 |
| C20:3 | Eicosatrienoic | 0.1 | 0.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.9 | 0.8 | 2.8 | 0.0 | 2.3 |
| C20:5 | Eicosapentaenoic | 0.1 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.0 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 |
| Saturated | 16.6 | 16.8 | 16.5 | 16.5 | 16.5 | 16.6 | 23.0 | 22.9 | 23.0 | 22.9 | 23.1 | 22.6 | |
| Monounsaturated | 25.1 | 25.0 | 24.7 | 24.7 | 24.5 | 24.4 | 12.9 | 14.7 | 14.3 | 17.4 | 13.4 | 16.2 | |
| Polyunsaturated | 57.9 | 57.1 | 58.3 | 58.3 | 58.3 | 58.3 | 62.6 | 58.4 | 59.0 | 52.8 | 62.2 | 54.5 | |
| Total | 99.6 | 98.9 | 99.5 | 99.5 | 99.4 | 99.3 | 98.5 | 96.0 | 96.3 | 93.1 | 98.6 | 93.2 | |
The values are an average of duplicate measurements and expressed as percentage of amount of lipids
Total phenolic contents of germinated high-amylose wheat extracts
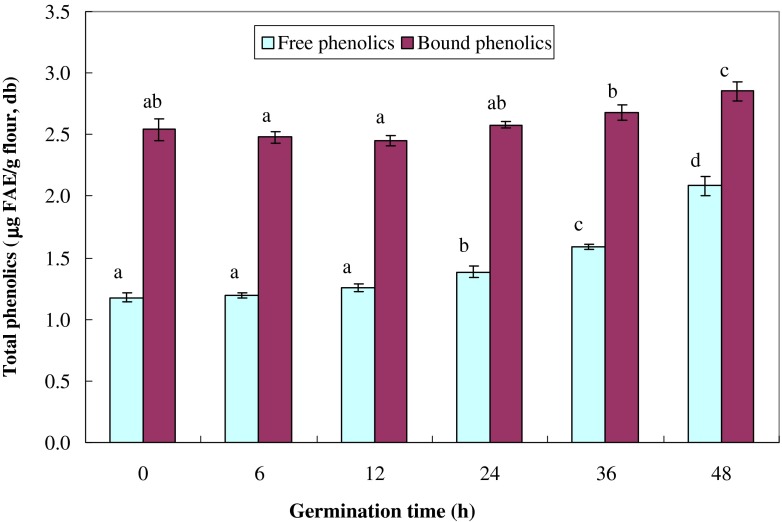
Changes in total free and bound phenolic contents of high-amylose wheat during germination are given in Fig. 1. The results showed that the total phenolics of the bound extracts were significantly higher than those of the free forms in both un-germinated and germinated wheats. The previous studies also reported that phenolic acids in wheat grains are mostly in the bound form and exist in bran associated with cell wall materials (Adom and Liu 2002; Liyana-Pathirana and Shahidi 2006). During the first 12 h of germination, the concentration of total phenolics did not change for both free and bound extracts. The amounts of total phenolics of both free and bound extracts significantly increased after 24 h of germination and prolonged germination times led to a further increase of total phenolics. These results are consistent with the report by Hung et al. (2012) for germination of waxy wheat. The increase in free phenolic content is due to the increase in syringic acid, while ferulic acid accumulation during phenolic biosynthesis contributed to the increase in bound phenolic content after 24 h of germination (Hung et al. 2011).
Fig. 1.
Changes in total free and bound phenolic contents of high-amylose wheats during germination. Values are means of triplicate measurements. The same letters in the same series are not significantly different (P < 0.05)
Antioxidant capacity of germinated high-amylose wheat extracts
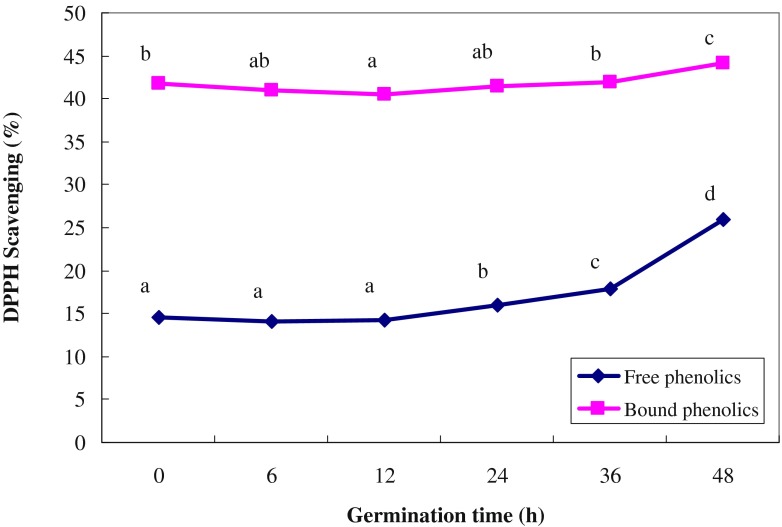
Changes in antioxidant capacity of high-amylose wheat extracts during germination are shown in Fig. 2. The antioxidant capacities of the bound phenolic extracts were significantly higher than those of the free phenolic extracts for both un-germinated and germinated high-amylose wheats. These results are in agreement with the higher amounts of total phenolics of the bound forms than the free forms of the extracts. During the first 12 h of germination, the antioxidant capacities of both free and bound phenolic extracts did not increase but rapidly increased after 36 h of germination. These results are possibly due to the increase in amount of syringic, caffeic and vanillic acids during germination (Hung et al. 2011). In addition, the antioxidant compounds such as vitamin C and tocopherols also increased with the length of germination time, which might also increase the antioxidant activity of the sprouted wheat flours (Yang et al. 2001).
Fig. 2.
DPPH (1,1-diphenyl-2-picrylhydrazyl) radical scavenging capacity of free and bound phenolic extracts from germinated high-amylose wheats. Concentration of DPPH = 0.075 mM. Values are means of triplicate measurements. The same letters in the same line are not significantly different (P < 0.05)
Conclusion
The nutritional properties and antioxidant capacity of the high-amylose wheat were improved through germination. The soluble and total dietary fibers, total proteins and free lipids were found to increase significantly after germination. The prolonged times of germination led to an increased amounts of free amino acids, especially the essential and functional amino acids such as isoleucine, leucine, phenylanaline, valine and GABA. The total phenolics and antioxidant capacities of both free and bound extracts significantly increased after 36 and 48 h of germination. Our results suggested that the germinated high-amylose wheat with significant improvement of nutritional value and bioactive compound content may be used to produce functional foods such as whole grain foods with enhanced nutritional quality.
Contributor Information
Pham Van Hung, Phone: +84-8-37244270, Email: pvhung74@gmail.com.
Naofumi Morita, Phone: +84-8-37244270, Email: moritana2007@yahoo.co.jp.
References
- AACC . Approved methods of the American association of cereal chemists. 10. St Paul: AACC International; 2000. [Google Scholar]
- Adom KK, Liu RH. Antioxidant activity of grains. J Agric Food Chem. 2002;50:6182–6187. doi: 10.1021/jf0205099. [DOI] [PubMed] [Google Scholar]
- Anderson JW, Deakins DA, Floore TL, Smith BM, Whitis SE. Dietary fiber and coronary heart disease. Crit Rev Food Sci Nutr. 1990;29:95–147. doi: 10.1080/10408399009527518. [DOI] [PubMed] [Google Scholar]
- Anderson JW, Randles KM, Kendall CWC, Jenkins DJA. Carbohydrate and fiber recommendations for individuals with diabetes: a quantitative assessment and meta-analysis of the evidence. J Am Coll Nutr. 2004;23:5–17. doi: 10.1080/07315724.2004.10719338. [DOI] [PubMed] [Google Scholar]
- AOAC . Official methods of analysis. 16. Arlington: AOAC International; 1997. [Google Scholar]
- Bazzano LA, He J, Ogden LG, Loria CM, Vupputuri ML, et al. Fruit and vegetable intake and risk of cardiovascular disease in US adults: the first national health and nutrition examination survey epidemiologic Followup study. Am J Clin Nutr. 2002;76:93–99. doi: 10.1093/ajcn/76.1.93. [DOI] [PubMed] [Google Scholar]
- Bingham SA, Day NE, Luben R, Ferrari P, Slimani N, Norat T, et al. Dietary fibre in food and protection against colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC): an observational study. Lancet. 2003;361:1496–1501. doi: 10.1016/S0140-6736(03)13174-1. [DOI] [PubMed] [Google Scholar]
- Brown L, Rosner B, Willett W, Sacks F. Cholesterol-lowering effects of dietary fiber: a meta-analysis. Am J Clin Nutr. 1999;69:30–42. doi: 10.1093/ajcn/69.1.30. [DOI] [PubMed] [Google Scholar]
- Christie WW. Lipid analysis. 2. Oxford: Pergamon; 1982. [Google Scholar]
- Harmuth-Hoene AE, Bognar AE, Kornemann U, Diehl JF. The influence of germination on the nutritional value of wheat, mung beans and chickpeas. Z Lebensm Unters Forsch. 1987;185:386–393. doi: 10.1007/BF01042260. [DOI] [PubMed] [Google Scholar]
- Hung PV, Yamamori M, Morita N. Formation of resistant starch as affected by high-amylose wheat flour substitutions. Cereal Chem. 2005;82:690–694. doi: 10.1094/CC-82-0690. [DOI] [Google Scholar]
- Hung PV, Maeda T, Morita N. Study on physicochemical characteristics of waxy and high-amylose wheat starches in comparison with normal wheat starch. Starch/Staerke. 2007;59:125–131. doi: 10.1002/star.200600577. [DOI] [Google Scholar]
- Hung PV, Hatcher DW, Barker W. Phenolic acid composition of sprouted wheats by ultra-performance liquid chromatography (UPLC) and their antioxidant activities. Food Chem. 2011;126:1896–1901. doi: 10.1016/j.foodchem.2010.12.015. [DOI] [PubMed] [Google Scholar]
- Hung PV, Maeda T, Yamamoto S, Morita N. Effects of germination on nutritional composition of waxy wheat. J Sci Food Agric. 2012;92(3):667–672. doi: 10.1002/jsfa.4628. [DOI] [PubMed] [Google Scholar]
- Koehler P, Hartmann G, Wieser H, Rychlik M. Changes of folates, dietary fiber, and proteins in wheat as affected by germination. J Agric Food Chem. 2007;55:4678–4683. doi: 10.1021/jf0633037. [DOI] [PubMed] [Google Scholar]
- Lemar LE, Swanson B. Nutritive value of sprouted wheat flour. J Food Sci. 1976;41:719–720. doi: 10.1111/j.1365-2621.1976.tb00709_41_3.x. [DOI] [Google Scholar]
- Liyana-Pathirana CM, Shahidi F. Importance of insoluble-bound phenolics to antioxidant properties of wheat. J Agric Food Chem. 2006;54:1256–1264. doi: 10.1021/jf052556h. [DOI] [PubMed] [Google Scholar]
- Liyana-Pathirana CM, Shahidi F. The antioxidant potential of milling fractions from breadwheat and durum. J Cereal Sci. 2007;45:238–247. doi: 10.1016/j.jcs.2006.08.007. [DOI] [Google Scholar]
- Morita N, Maeda T, Miyazaki M, Yamamori M, Miura H, Ohtsuka I. Dough and baking properties of high-amylose and waxy wheat flours. Cereal Chem. 2002;79:491–495. doi: 10.1094/CCHEM.2002.79.4.491. [DOI] [Google Scholar]
- Morita N, Maeda T, Hung PV, Watanabe M, Handoyo T, Yamamori M (2003) Textural properties and microscope observation of noodles made from various novel wheat flours. Proceedings of the 53rd Australian cereal chemistry conference. 153–156.
- Nelson K, Stojanovska L, Vasiljievic T, Mathai M. Germinated grains: a superior whole grain functional food? Can J Physiol Pharmacol. 2013;91(6):429–441. doi: 10.1139/cjpp-2012-0351. [DOI] [PubMed] [Google Scholar]
- Ranhotra GS, Loewe RJ, Lehmann TA. Breadmaking quality and nutritive value of sprouted wheat. J Food Sci. 1977;42:1373–1375. doi: 10.1111/j.1365-2621.1977.tb14501.x. [DOI] [Google Scholar]
- Ruibal-Mendieta NL, Delacroix DL, Meurens M. A comparative analysis of free, bound and total lipid content on spelt and winter wheat wholemeal. J Cereal Sci. 2002;35:337–342. doi: 10.1006/jcrs.2001.0434. [DOI] [Google Scholar]
- Saikusa T, Horino T, Mori Y. Distribution of free amino acids in rice kernel and kernel fraction and the effect of water soaking on the distribution. J Agric Food Chem. 1994;42:1122–1125. doi: 10.1021/jf00041a015. [DOI] [Google Scholar]
- Tkachuk R. Free amino acids in germinated wheat. J Sci Food Agric. 1979;30:53–58. doi: 10.1002/jsfa.2740300110. [DOI] [PubMed] [Google Scholar]
- Yamamori M, Fujita S, Hayakawa K, Matsuki J, Yasui T. Genetic elimination of starch granule protein, SGP-1, of wheat generates and altered starch with apparent high amylase. Theor Appl Genet. 2000;101:21–29. doi: 10.1007/s001220051444. [DOI] [Google Scholar]
- Yang F, Basu TK, Ooraikul B. Studies on germination conditions and antioxidant contents of wheat grain. Int J Food Sci Nutr. 2001;52:319–330. doi: 10.1080/09637480120057567. [DOI] [PubMed] [Google Scholar]