Abstract
The effect of membrane processing on the functional properties, structural changes, subunit profile and sensory attributes of the groundnut protein concentrate was investigated. Results indicated an increase in the nitrogen solubility and foaming capacity of the protein concentrate over all pH ranges (2–10) compared to acid precipitated protein isolate. Protein concentrate showed higher emulsion stability index compared to control flour and protein isolate. Surface hydrophobicity studies showed that protein concentrate is less hydrophobic (~29 units) compared to acid precipitated protein isolate (~34 units). SDS-PAGE profile of the samples showed similarity in the subunit pattern of groundnut protein concentrate and groundnut flour. Sensory analysis suggested membrane filtration could reduce the groundnut-like nutty and beany notes of the concentrate. Thus, membrane technology could give a protein concentrate with improved functionality and sensory characteristics which will have potential application in the development of food product formulations.
Keywords: Groundnut protein concentrate, Nitrogen solubility, Functional properties, Surface hydrophobicity, Sensory analysis
Introduction
The rapidly growing world protein requirement has directed major attention to plant proteins. Oilseeds are valuable sources of lipid and basically processed for their edible oils leaving behind a lot of protein-rich meal. Proteins are usually recovered from the meals and marketed as food ingredients in developed countries. The most produced oilseeds worldwide are, in decreasing order, soybean, rapeseed, cotton, groundnut and sunflowers, amongst others (FAO 2009).
Groundnut (Arachis hypogae, L.) is the fourth important oilseed in the world. Groundnuts after oil extraction leaves a lot of meal called as defatted groundnut flour which contains 50–55 % of high-quality protein. The development of a new protein product such as groundnut protein concentrate from defatted groundnut flour is necessary to provide the food industries with new high-protein food ingredient for various food product formulations.
Groundnut protein concentrate/isolate are usually made by alkali extraction followed by isoelectric precipitation and well known for their functional properties (Lawal et al. 2007; Adebowale et al. 2011). However, there is either very less or no scientific information available on groundnut protein concentrate preparation employing membrane technology. Therefore, the focus of current study is on the preparation of groundnut protein concentrate preparation using ultrafiltration and characterizing it in terms of the functional properties, protein quality and sensory attributes.
Material and methods
Preparation of defatted groundnut flour
Groundnut seeds were cleaned and dehulled by passing through a plate type mill with an attached air blower. The dehulled seeds were equilibrated at 20 % moisture and passed through a flaking machine to obtain flakes of 0.3 mm thickness and dried to 5 % moisture level. Dried flakes were defatted by repeated extraction with n-hexane until the fat content was less than 1 %. The defatted flakes were dried at 50 °C and passed through a quadrumat mill having standard sieves with pore size below 100 μm.
Preparation of groundnut protein isolate using acid precipitation
Defatted groundnut flour was mixed with water at the ratio of 1:10 (w/v) and pH of the solution was adjusted to 8.5 with 1 N NaOH. Mixture was stirred for 2 h at room temperature and then centrifuged at 6000 rpm for 30 min. Supernatant was collected and adjusted to pH 4.5 with 1 N HCl. The suspension was centrifuged at 6000 rpm for 20 min to recover protein precipitate. Protein precipitate collected and freeze dried. The dry protein isolate powder was stored in the refrigerator until experimental use.
Preparation of membrane processed groundnut protein concentrate
Ten percent aqueous solution of groundnut flour was treated with cellulase (1 % v/v) provided by Genencore (Rochester, New York, USA) for 2 h by mixing at 200 rpm at a pH of 5.0. The temperature of the slurry was maintained between 45 and 50 °C. The slurry was centrifuged at 6000 rpm for 30 min. The clarified aqueous protein solution was passed through an ultrafiltration membrane (MWCO 30 kDa). The initially feed volume was reduced to half to obtain a concentrated protein solution in the retentate stream while the permeate is discarded. Further retentate is diluted back to the original feed volume with water, reduced again to half of its original volume as before and freeze dried to obtain the membrane processed groundnut protein concentrate.
Proximate analysis of groundnut protein samples was carried out according to AOAC (2005) procedure.
Nitrogen solubility
Nitrogen solubilities of defatted groundnut flour, groundnut protein isolate and groundnut protein concentrate were determined according to the method of Adebiyi et al. (2007) with slight modifications. Samples were dispersed in distilled water (1 % w/v), and the pH of the solution was adjusted to the required working pH (2–10) with HCl or NaOH of high normality to limit dilution. After a 30 min equilibrium period at room temperature and readjustment of the pH, if necessary, the samples were centrifuged at 10,000 rpm. Solubility of nitrogen in the supernatant was determined Kjeldahl method. The nitrogen solubility (NS) was calculated according to the formula:
Foaming properties
Foaming capacity and foam stability were determined according to the method of Booma and Prakash (1990). A 2 % aqueous dispersion of the protein sample was mixed thoroughly in a blender and whipped for 3 min at high speed. The contents were transferred immediately to a 250 mL graduated measuring cylinder, and the foam volume measured at different time intervals. Foaming capacity was calculated as the increase in volume (mL) of the protein dispersion upon mixing and expressed as a percentage increase in volume. Foam stability was estimated as the relative volume of foam left after 30 min of standing.
Surface hydrophobicity
Surface hydrophobicity of groundnut flour, groundnut protein isolate and groundnut protein concentrate was determined using the fluorescent probe l-aniline-8-naphthalene-sulfonate (ANS) to estimate aromatic hydrophobicity (Hayakawa and Nakai 1985). Protein samples suspended in 20 ml of 0.01 M-phosphate buffer; pH 7.0 were stirred at 250 rpm on a magnetic stirrer for 60 min. Protein solutions were centrifuged at 10,000 rpm for 20 min. Supernatant was diluted with 0.01 M phosphate buffer (pH 7.0) to obtain protein concentrations ranging from 0.0015 to 0.015 %. ANS (8 mM, 20 ml) prepared in 0.01 M-phosphate buffer; pH 7.0 was added to 4.0 ml of protein solutions. Fluorescence intensity of ANS-protein conjugates was measured with a spectrofluorometer (Shimadzu, RF-5000) using excitation and emission wavelength of 390 and 470 nm respectively. The fluorescence reading was standardized by adjusting the reading of the fluorimeter to 30 % full scale for 8 mM ANS in methanol. Initial slope of fluorescence intensity versus protein concentration plot was used as an index of hydrophobicity.
Water holding capacities
Water holding capacity of the samples was determined by the method of Tomotake et al. (2002) with slight modification. One gram of groundnut protein sample was weighed into a pre-weighed 15 ml centrifuge tube. For each sample, 10 ml of distilled water was added and mixed in a vortex mixer. After the mixture was thoroughly wetted, samples were allowed to stand at room temperature for 30 min., and then centrifuged at 3000 rpm for 20 min. Supernatants were decanted, and the centrifuge tubes containing the sediment were weighed. Water holding capacity (grams of water per gram of protein) was calculated as WHC = (W2-W1)/W0, where W0 was the weight of the dry sample (g), W1 was the weight of the tube plus the dry sample (g), and W2 was the weight of the tube plus the sediment (g).
Oil binding capacity
Oil binding capacity was determined according to the method of Monteiro and Prakash (1994). Ten milliliters of refined groundnut oil was added to 1 g of groundnut protein sample in a graduated centrifuge tube. Tube was agitated for 1 min. and allowed to stand for 30 min. Protein oil mixture was centrifuged for 20 min. at 3000 rpm. Immediately after centrifugation the volume of free oil was read. Oil binding capacity was expressed as the volume of oil absorbed per gram of sample.
Emulsifying properties
Emulsifying properties of defatted groundnut flour, groundnut protein isolate and groundnut protein concentrate were determined by the method of Pearce and Kinsella (1978), with minor modifications. Ten milliliters of refined groundnut oil and 0.1 % protein solution (0.1 M phosphate buffer pH 7.0, 30 mL) were homogenized in a Virtis homogenizer at a speed setting of 6 for 1 min. at room temperature. Emulsions (10 μL) were pipetted out at 0 and 10 min. and diluted with 10 mL of 0.1 % sodium dodecyl sulphate (SDS). Absorbance of the diluted emulsion was measured at 500 nm versus 0.1 % SDS as blank using a spectrophotometer. Emulsifying activity was expressed as the Emulsifying activity index (EAI) and calculated in units of m2/g as follows:
In which the dilution factor is 100, C is the weight of protein per unit volume of the aqueous phase before emulsion formation, A500 is the absorbance at 500 nm and √ is the oil volume fraction (0.025) of the emulsion.
Emulsion stability (ESI) was determined as follows:
Where Ao is the initial absorbance at 0 time, ^A is the change in absorbance A, occurring over the interval t (10 min.) minutes of standing.
Sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE)
Effect of processing on the subunit profile of the samples, defatted groundnut flour, groundnut protein isolate and groundnut protein concentrate was studied by sodium dodecyl sulphate polyacrylamide gel electrophoresis using 0.75 mm thick gels according to the method of Laemmli (1970). A 12 % polyacrylamide gel prepared in 1.5 M Tris–HCl buffer, pH 8.8, containing 1 % SDS was used. Protein samples were prepared by extracting the flours in 0.02 M-phosphate buffer, pH 7.9 for 1 h. Protein extract was centrifuged and freeze dried. Protein samples (2 mg/ml) were mixed 1:1 (v/v) with a solubilisation buffer containing 62 mM Tris, 10 % glycerol, 0.01 % bromophenol blue, pH 6.8 and boiled at 95 °C, for 5 min., before electrophoresis. Protein bands were stained with Coomassie brilliant blue R-250.
Scanning electron microscopy (SEM)
To see the microstructural changes of samples, scanning electron microscopic studies of defatted groundnut flour, groundnut protein isolate and groundnut protein concentrate were carried out using a Scanning Electron Microscope. Sample was coated with gold using Poloron SEM coating system E-5000 before loading it into the system. Average coating time was 2–3 min. Thickness of the coating was 200–300 nm, which was calculated using the standard formula (Radha et al. 2008). The image was captured using 35 mm Ricoh Camera.
Sensory analysis
Panel Training: A group of 6–8 panelists aged 30–50 years participated for odour and flavour profiling of Groundnut protein samples. The members of the panel are from the scientific staff, familiar with sensory analysis techniques and had earlier experience in the sensory evaluation of oil seeds. Samples were evaluated in a sensory booth room maintained at a temperature of 22 ± 2 °C under fluorescent lighting equal to daylight (IV and Wolf 1996).
Odour profiling
Odour profiling of groundnut protein samples were carried out using Quantitative Descriptive Analysis (QDA) method (Stone and Sidel 1998). Samples were prepared by weighing 2 g of samples into 100 ml conical flask with stoppers. Before sniffing by the panelists, enough time was given for accumulation of volatiles in the headspace of the flask. Panelists were trained to sniff the headspace and distinguish various odour notes. In the preliminary session, the panelists were asked to list the odour descriptors perceived by sniffing. The panelists were asked to mark the intensity of the attributes on QDA, which consisted of a 15 cm line scale, wherein 1.25 cm was anchored as low (recognition threshold) and 13.75 cm as high (saturated threshold). Panelists were asked to mark a vertical line on the scale and write the code of the sample closed to the line. In between two evaluations, enough time (15 min.) was given for the accumulation of volatiles in the headspace. Score cards were decoded, and the mean values of the attribute were calculated.
Flavour profiling
Samples for sensory flavour profiling were prepared in 2 % dispersion in hot water and thoroughly stirred to get an uniform dispersion. As a prerequisite for the flavour profiling of Groundnut proteins, the panelists were asked to give as many descriptors as applicable. Following this, open discussion was held to reach an agreement on appropriate descriptors, especially for odour and flavor as per the guideline described by Dravnieks (1985). Panelists were trained with the descriptors and the respective reference compounds. Samples (10 ml) were served in a 25 ml beaker with three-digit codes. Samples were served one by one in random order. Panelists were asked to mark the intensity of the attribute on the QDA scale.
Statistical analysis
Data and Statistical analysis were performed using Scientific Graphic and analysis Computer software OriginPro (version 7) supplied by Origin Lab Corporation, Northampton, MA, USA and data was expressed as Mean ± standard deviation of three experiments.
Results and discussion
Proximate analysis
Proximate composition of all the three protein products is shown in Table 1. Protein content of acid precipitated groundnut protein isolate was the highest ~86 % followed by membrane processed groundnut protein concentrate, ~72 % and defatted groundnut flour, ~51 %. Groundnut flour had the highest moisture content of ~8.4 % and the lowest of ~4.4 % for groundnut protein isolate. Crude fat of groundnut protein isolate reduced to ~0.24 % from ~0.82 % of groundnut flour. Ash content also decreased in groundnut protein isolate compared to groundnut flour and membrane processed protein concentrate. Carbohydrate content was less in both protein isolate, and protein concentrate compared to the control flour. The difference in the chemical composition of groundnut protein isolate and protein concentrate from that of defatted groundnut flour might have been attributed to the difference in extraction methods.
Table 1.
Proximate analysis of groundnut protein samples
| Constituents (%) | Groundnut flour | Groundnut protein concentrate | Groundnut protein isolate |
|---|---|---|---|
| Protein | 51.23 ± 0.92a | 72.01 ± 0.83b | 86.03 ± 1.25c |
| Carbohydrate | 28.64 ± 0.54a | 6.93 ± 0.35b | 3.45 ± 0.47c |
| Ash | 5.36 ± 0.02a | 3.24 ± 0.14b | 2.83 ± 0.08b |
| Moisture | 8.40 ± 0.12a | 5.65 ± 0.10b | 4.43 ± 0.14c |
| Crude fat | 0.82 ± 0.13a | 0.83 ± 0.14a | 0.21 ± 0.13b |
Mean ± S.D. (n = 3 runs)
Different superscripts within the same row indicate significant differences (P < 0.05)
Nitrogen solubility
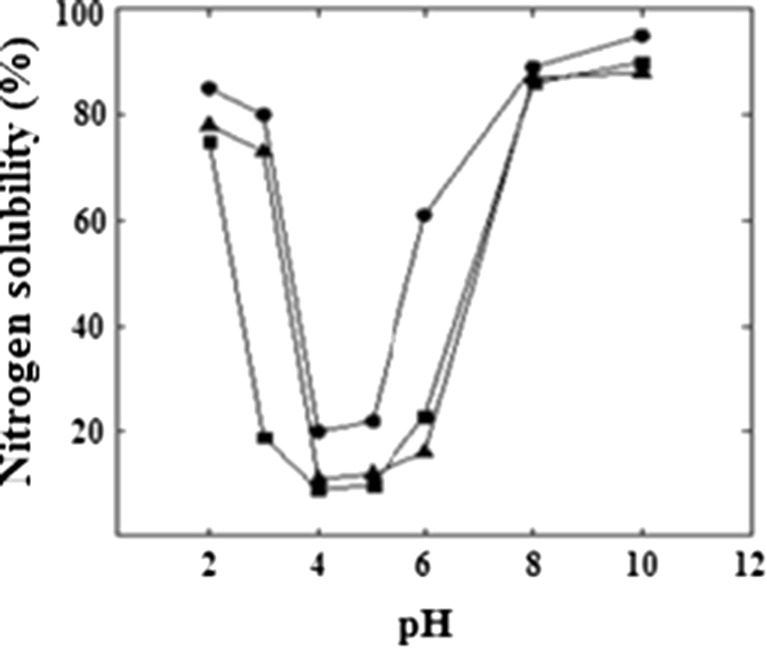
Solubility characteristics of proteins are among the most important functional properties since many functional performances of proteins depend upon their capacity to go into solution initially. Figure 1 shows the nitrogen solubility profile of defatted groundnut flour, groundnut protein isolate and groundnut protein concentrate over a pH range of 2–10. Solubilities were higher in the pH range of 2–3 and were least at pH 4–5 following which steady increases were observed for the protein as pH increased. Lack of electric charge corresponding to the isoelectric point has been known to promote hydrophobic aggregation which translates to the minimum solubility observed between pH 4.0 and 5.0 (Damodaran 1996). Similar observations were reported for soy protein (Zhang et al. 2007) and bambarra groundnut (Lawal et al. 2007). Groundnut protein concentrate showed higher solubility over all pH ranges compared to groundnut protein isolate and defatted groundnut flour. The lesser solubility of groundnut protein isolate may be attributed to the partial extraction of soluble proteins. In the case of groundnut protein concentrate, the processing would have resulted in the formation of smaller, more hydrophilic and solvated polypeptide chains. Structural compactness of the control protein might be responsible for the comparatively lesser solubility. It is reported that membrane processing used to concentrate soy proteins leads to leave the protein molecule intact and in the native state (Rao et al. 2002).
Fig. 1.
Nitrogen solubility profile of groundnut protein samples in water as a function of pH. black square - Defatted groundnut flour; black circle – Groundnut protein concentrate; black triangle – Groundnut protein isolate
Foaming properties
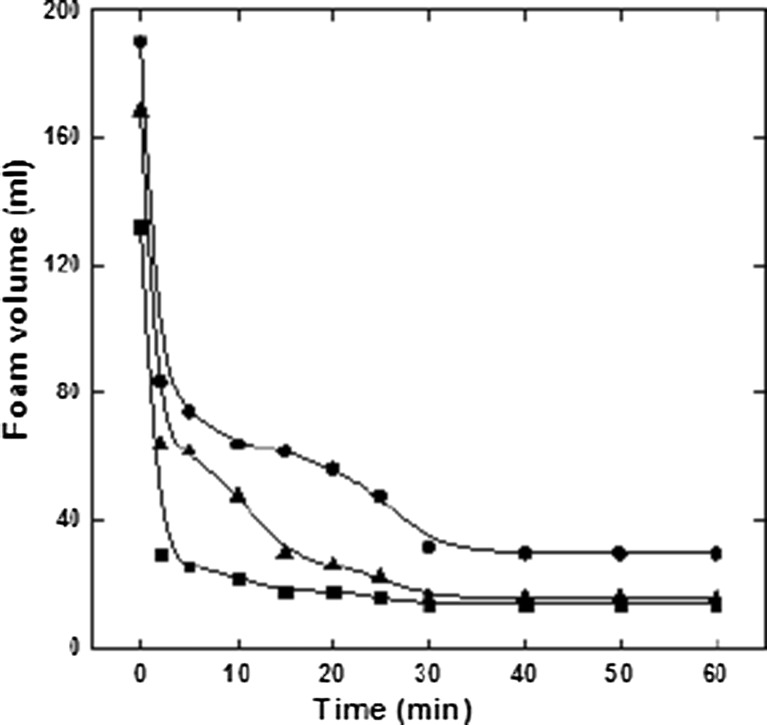
Foam capacities and foam stabilities of defatted groundnut flour, groundnut protein isolate and groundnut protein concentrate are shown in Fig. 2. Results shows an increase in foaming capacity and foam stability of the groundnut protein concentrate as compared to defatted groundnut flour and groundnut protein isolate. Proteins in dispersion cause a lowering of the surface tension at the water air interface thus creating foaming capacity (Surówka and Fik 1994). Foaming capacity is also determined by molecular flexibility and physicochemical properties (hydrophobicity, net charge and charge distribution, hydrodynamic properties) of proteins and to foam efficiently (i.e. to possess high foamability), protein needs to adsorb rapidly during the transient stage of foam formation (Martin et al. 2002; González-Pérez et al. 2005). These results suggest that an increase in surface activity of groundnut protein concentrate may be due to the initially greater number of polypeptide chains (Kuehler and Stine 1974), which are formed and allowing more air to be incorporated as shown in Fig. 4 of Scanning electron microscopy results.
Fig. 2.
Foaming properties of groundnut protein samples. black square - Defatted groundnut flour; black circle – Groundnut protein concentrate; black triangle – Groundnut protein isolate
Fig. 4.
SEM of groundnut protein samples. a: Defatted groundnut flour; b: Groundnut protein concentrate; c: Groundnut protein isolate
Emulsifying properties
Emulsifying properties of defatted groundnut flour, groundnut protein isolate and groundnut protein concentrate are shown in Table 2. Among the three protein products, groundnut flour had significantly higher emulsifying activity indices. However, groundnut protein concentrate showed a significant increase in emulsion stability index compared to groundnut flour and protein isolate. Emulsion stability is a property often evaluated in relation to time and is related to the droplet size wherein, the smaller the size; the greater is the stability. The results suggest that through groundnut protein concentrate may not form an emulsion readily; the stability of the emulsion after its formation is relatively higher. Stability of the protein film formed at the interface of the emulsion is dependent on the interactions of the proteins in the oil and aqueous phases (Damodaran 1996), and it is in this aspect that the higher solubility of the concentrate might have been advantageous to form a stable emulsion. A similar observation was reported by Wu et al. (2009) in which the effect of pH on the emulsifying properties of different groundnut protein products was reported.
Table 2.
Emulsifying properties and surface hydrophobicities of groundnut protein samples
| Parameters | Groundnut flour | Groundnut protein concentrate | Groundnut protein isolate |
|---|---|---|---|
| Emulsion activity index (m2/g) |
7.56 ± 0.11a | 5.43 ± 0.07b | 6.42 ± 0.08c |
| Emulsion stability index (m2/g) |
33.41 ± 0.71a | 56.84 ± 0.42b | 28.62 ± 0.64c |
| Surface hydrophobicity | 25.08 ± 0.83a | 29.06 ± 0.54a | 34.22 ± 0.62b |
Mean ± S.D. (n = 3 runs)
Different superscripts within the same row indicate significant differences (P < 0.05)
The emulsifying activity index of protein is based on the relationship between turbidity and surface area (Pearce and Kinsella 1978). It is reported that emulsifying properties of groundnut proteins have been related to their solubility (Sosulski and Fleming 1977). Protein denaturation usually decreases solubility and then adversely affects protein functionality (Radha and Prakash 2010). Other than solubility, different molecular properties of proteins such as hydrophobicity, flexibility and amino acid composition are responsible for the emulsifying behavior of proteins.
Water holding and oil binding capacity
Water holding capacity of proteins is used to determine the interaction between the protein product and water resulting in some of the water remaining within the product. Evaluation of this hydrophilic interaction is of importance for the formulation of ingredients for products like gravies and sauces. Data obtained for water holding and oil binding capacities of groundnut flour, groundnut protein isolate and groundnut protein concentrate are shown in Table 3. Results shows that isoelectric precipitation of groundnut protein increased both water holding capacity and oil binding capacity while membrane filtration decreased water holding and oil binding capacity of the protein. Arrese et al. (1991) studied the functional properties of several commercial soya isolates and reported that isolates with a higher degree of denaturation showed a greater water holding capacity. They attributed this to the unfolding of the polypeptide chain resulting in a matrix that is capable of trapping the absorbed water. Lesser water holding capacity of the groundnut protein concentrate shows that membrane processing did not disrupt the structures sufficiently to bring about an increase in either water holding or oil binding capacity.
Table 3.
Water hydration and oil binding capacities of groundnut protein samples
| Parameters | Groundnut flour | Groundnut protein concentrate | Groundnut protein isolate |
|---|---|---|---|
| Water holding capacity (g/g) | 2.38 ± 0.12a | 2.86 ± 0.18a | 3.64 ± 0.13b |
| Oil binding capacity (ml/g) | 2.12 ± 0.02a | 1.36 ± 0.08b | 3.48 ± 0.03c |
Mean ± S.D. (n = 3 runs)
Different superscripts within the same row indicate significant differences (P < 0.05)
Surface hydrophobicity
Surface hydrophobicities of defatted groundnut flour, groundnut protein isolate and groundnut protein concentrate are shown in Table 2. Result shows that groundnut protein isolate was the utmost hydrophobic in nature. Defatted groundnut flour and groundnut protein concentrate shown significantly lower surface hydrophobicities index than the groundnut protein isolate which indicate that proteins of these products have more of their hydrophobic residues buried inside. Relatively higher values for surface hydrophobicity measured in the acid precipitated groundnut isolate may decrease the interfacial tension, owing to the presence of nonpolar amino acid residues. This also explains the higher emulsifying activity index of the acid precipitated isolate compared to the membrane concentrate.
Protein subunit profile and its influence on the functionality
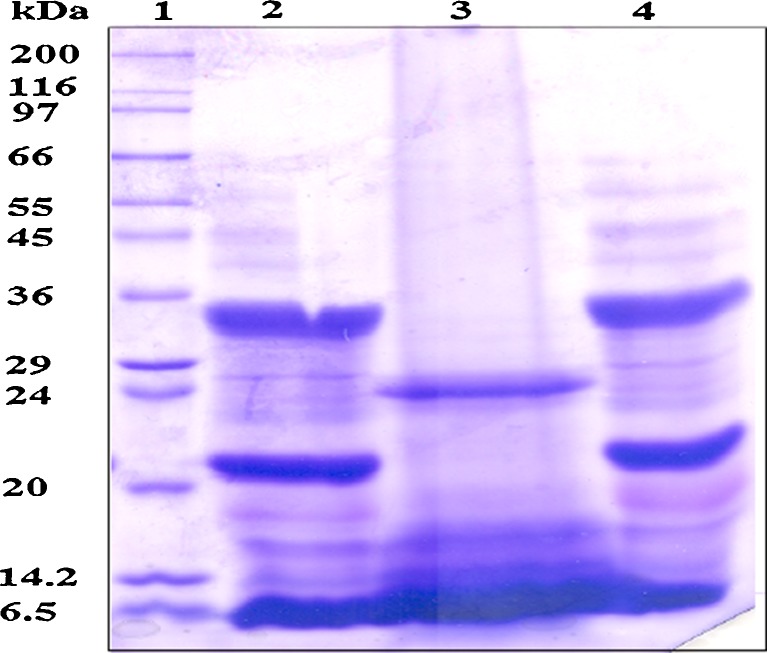
SDS-PAGE of the three proteins was used to compare the subunit profile of proteins (Fig. 3). Lane 1 consists of standard molecular weight marker. Lanes 2, 3 and 4 refer to groundnut protein concentrate, groundnut protein isolate and defatted groundnut flour, respectively. The electrophoretic pattern flour and protein concentrate was comparable showing most of the poly-peptides present in flour are also present in the protein concentrate. Subunit profile of protein isolate was distinctly different from that of flour wherein the protein bands corresponding to the molecular weights of ~34 and ~21 kDa were absent in the former. The absence of protein bands in the protein isolate suggests denaturation of the protein. Membrane processing retained the protein subunit composition in a native state as reflected by the similar subunit profiles of groundnut flour and protein concentrate. The subunits that makeup the protein structure are mainly responsible for its functionality (Rao et al. 2002) and the similarities in functional properties of the groundnut protein concentrate with that of control flour may be due to the similarity in its respective electrophoretic pattern.
Fig. 3.
SDS-PAGE pattern of groundnut protein samples. Lane 1: Molecular weight standards Lane 2: Defatted groundnut flour; Lane 3: Groundnut protein isolate; Lane 4: Groundnut protein concentrate
Scanning electron microscopy
SEM was used to examine the micro-structural changes of protein prepared by two different methods. Figure 4a shows the scanning electron micrographs of the control groundnut flour. The control flour particles are rounded granules separated by air vacuoles. Protein concentrate showed porous type morphology of particles (Fig. 4b) which may be helpful in the faster hydrolysis of the protein which in turn can improve the digestibility of the protein. Protein isolate made by isoelectric precipitation method showed intact flakes like structure (Fig. 4c).
Sensory evaluation
Sensory odour profile for the three samples of groundnut protein shows that groundnut flour and groundnut protein isolate samples exhibited similar profile with high intensity of raw groundnut like, nutty and beany notes (Fig. 5a). Groundnut protein concentrate sample have higher intensity of roasted wheat and caramelised notes and lesser intensity of typical raw groundnut like, nutty and beany notes.
Fig. 5.
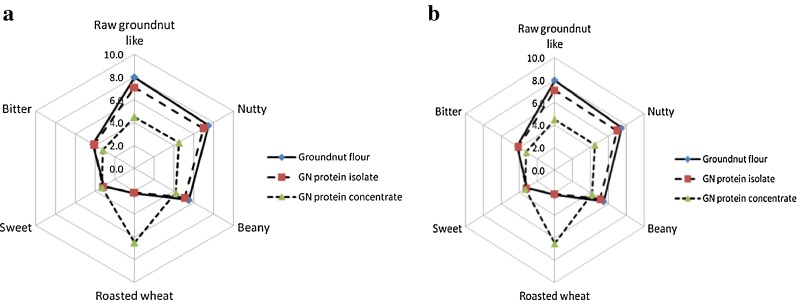
Sensory profile of groundnut proteins. a. Odour profile; b. Flavour profile
The flavour profile of groundnut protein samples showed a distinct difference for typical raw groundnut like, nutty and beany notes between concentrate, groundnut flour and groundnut protein isolate (Fig. 5b). Roasted wheat odour was more pronounced in membrane processed groundnut protein concentrate. The perception of sweet did not differ among the samples while bitterness was slightly less for groundnut protein concentrate.
Based on the results of the descriptive analysis, it would seem prudent to conclude there is a reduction in typical groundnut beany odour and flavour for groundnut protein concentrate. Above characterstics would be of importance in the development of designer products where the odour and flavour have to be either masked or toned down.
Conclusion
In this study, groundnut protein concentrate was prepared by employing membrane technology and its functional properties were compared with the conventionally made isoelectric precipitated protein isolate. Groundnut protein concentrate showed better nitrogen solubility, foaming and emulsifying properties compared to acid precipitated protein isolate. Subunit profile of the groundnut protein concentrate was similar to that of groundnut flour which revealed that the presence of all the proteins of flour in the concentrate. Porous type morphology of the membrane processed protein concentrate compared to the acid precipitated protein isolate can improve the digestibility of protein. Sensory analysis revealed that due to a reduction in typical nutty odor and flavor, membrane processed groundnut protein concentrate is more acceptable compared to the raw groundnut flour or protein isolate. These results indicated that groundnut protein concentrate prepared using membrane technology showed superior functional properties, structural changes, subunit profile and sensory attributes compared to acid precipitated protein isolate. The improved functional properties of the membrane processed groundnut protein concentrate are expected to affect the quality attributes of food products containing it and can find application as a potential source of nutrient supplement for use in specific functional foods.
Acknowledgments
The authors are grateful to the Director, CSIR–CFTRI, Mysore for the encouragement.
Footnotes
Research Highlights • The functionality of groundnut protein concentrate was investigated
• Membrane technology was used for concentrate preparation
• Concentrate showed changes in structure, subunit profile and sensory attributes
• Functionality of concentrate was superior compared to acid precipitated isolate
References
- Adebiyi AP, Adebiyi AO, Ogawa T, Muramoto K. Preparation and characterization of high-quality rice bran proteins. J Sci Food Agric. 2007;87:1219–1227. doi: 10.1002/jsfa.2819. [DOI] [Google Scholar]
- Adebowale YA, Schwarzenbolz U, Henle T. Protein Isolates from Bambara Groundnut (Voandzeia Subterranean L.): Chemical Characterization and Functional Properties. Int J Food Prop. 2011;14:758–775. doi: 10.1080/10942910903420743. [DOI] [Google Scholar]
- AOAC (2005) International Methods 925.10, In official methods of analysis of association of official analytical chemists, 18th ed. Washington
- Arrese EL, Sorgentini DA, Wagner JR, Anon MC. Electrophoretic, solubility and functional properties of commercial soy protein isolates. J Agric Food Chem. 1991;39:1029–1032. doi: 10.1021/jf00006a004. [DOI] [Google Scholar]
- Booma K, Prakash V. Functional properties of the flour and the major protein fraction from sesame seed, sunflower seed and safflower seed. Acta Aliment. 1990;19:163–176. [Google Scholar]
- Damodaran S (1996) Amino acids, peptides and proteins. Food Chem. third Ed. CRC Press, pp 321–329
- Dravnieks A. Atlas of odor character profiles by ASTM Data Series DS 61. American Society for Testing and Materials. Philadelphia: PA; 1985. [Google Scholar]
- FAO (2009) Perspectivas Alimentarias – análisis de los mercados mundiales.
- González-Pérez S, Vereijken JM, van Koningsveld GA, et al. (2005) Formation and stability of foams made with sunflower (Helianthus annuus) proteins. J Agric Food Chem 53:6469–6476. doi:10.1021/jf0501793 [DOI] [PubMed]
- Hayakawa SS, Nakai S. Relationships of Hydrophobicity and Net Charge to the Solubility of Milk and Soy Proteins. J Food Eng. 1985;50:486–491. [Google Scholar]
- IV EC, Wolf MB (1996) Sensory testing methods, 2nd edn. ASTM International, pp. 54–72
- Kuehler CA, Stine CM. Effect of enzymatic hydrolysis on some functional properties of whey protein. J Food Sci. 1974;39:379–382. doi: 10.1111/j.1365-2621.1974.tb02899.x. [DOI] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawal OS, Adebowale KO, Adebowale YA. Functional properties of native and chemically modified protein concentrates from bambarra groundnut. Food Res Int. 2007;40:1003–1011. doi: 10.1016/j.foodres.2007.05.011. [DOI] [Google Scholar]
- Martin A, Grolle K, Bos M, et al. Network Forming Properties of Various Proteins Adsorbed at the Air/Water Interface in Relation to Foam Stability. J Colloid Interface Sci. 2002;254:175–183. doi: 10.1006/jcis.2002.8592. [DOI] [PubMed] [Google Scholar]
- Monteiro PV, Prakash V. Functional properties of homogeneous protein fractions from peanut (Arachis hypogaea L.) J Agric Food Chem. 1994;42:274–278. doi: 10.1021/jf00038a009. [DOI] [Google Scholar]
- Pearce KN, Kinsella JE. Emulsifying properties of proteins: evaluation of a turbidimetric technique. J Agric Food Chem. 1978;26:716–723. doi: 10.1021/jf60217a041. [DOI] [Google Scholar]
- Radha C, Prakash V. Structural and Functional Properties of Heat-processed Soybean Flour: Effect of Proteolytic Modification. Food Sci Technol Int. 2010;15:453–463. doi: 10.1177/1082013209350347. [DOI] [Google Scholar]
- Radha C, Ramesh Kumar P, Prakash V. Enzymatic modification as a tool to improve the functional properties of heat-processed soy flour. J Sci Food Agric. 2008;88:336–343. doi: 10.1002/jsfa.3094. [DOI] [Google Scholar]
- Rao A, Shallo HE, Ericson AP, Thomas RL. Characterization of soy protein concentrate produced by membrane ultrafiltration. J Food Sci. 2002;67:1412–1418. doi: 10.1111/j.1365-2621.2002.tb10299.x. [DOI] [Google Scholar]
- Sosulski F, Fleming SE. Chemical, functional, and nutritional properties of sunflower protein products. J Am Oil Chem Soc. 1977;54:A100–A104. doi: 10.1007/BF02912382. [DOI] [PubMed] [Google Scholar]
- Stone H, Sidel JL (1998) Quantitative descriptive analysis: developments, applications and the future. Food Technol.
- Surówka K, Fik M. Studies on the recovery of proteinaceous substances from chicken heads: II—Application of pepsin to the production of protein hydrolysate. J Sci Food Agric. 1994;65:289–296. doi: 10.1002/jsfa.2740650305. [DOI] [Google Scholar]
- Tomotake H, Shimaoka I, Kayashita J, et al. Physicochemical and functional properties of buckwheat protein product. J Agric Food Chem. 2002;50:2125–2129. doi: 10.1021/jf011248q. [DOI] [PubMed] [Google Scholar]
- Wu H, Wang Q, Ma T, Ren J. Comparative studies on the functional properties of various protein concentrate preparations of peanut protein. Food Res Int. 2009;42:343–348. doi: 10.1016/j.foodres.2008.12.006. [DOI] [Google Scholar]
- Zhang K, Li Y, Ren Y. Research on the phosphorylation of soy protein isolate with sodium tripoly phosphate. J Food Eng. 2007;79:1233–1237. doi: 10.1016/j.jfoodeng.2006.04.009. [DOI] [Google Scholar]