Abstract
α-Mangostin, the major xanthone constituent of mangoteen fruit pericarp, has several important pharmaceutical application but its bioavailability is restricted due to its insolubility in water. Herein, we synthesized water soluble α-mangostin-D-glucoside by glycosylation of α-mangostin at hydroxyl group; using amyloglucosidase (3.2.1.3) catalyzed reaction in supercritical carbon dioxide (SC-CO2) media. Response surface methodology (RSM) based on a five-variable central composite rotatable design involving 32 experiments was used to determine the effect of pressure (80–160 bar), temperature (35–75 °C), enzyme concentration (15–45 mg), buffer pH (4.0–8.0) and buffer volume (1.0–5.0 mL). Experimental data fitted the second-order polynomial equation as indicated by R2 value of 0.94. The optimal enzymatic conversion within the experimental range of the variables reached 20.3 % at a pressure of 120 bar, temperature of 55 °C, enzyme concentration of 30 mg, buffer volume of 3 mL and pH 6.0 which is well matched with the predictive yield.
Keywords: Supercritical carbon dioxide media, Amyloglucosidase, α-Mangostin-D-glucoside, Optimization, Central composite rotatable design
Introduction
α–Mangostin is the major constituent of Garcinia mangostana. The biological activities of α–mangostin have been confirmed to consist of a competitive antagonism of the histamine H1 receptor (Chairungsrilerd et al. 1996; Iikubo et al. 2002), antibacterial activity against Helicobacter pylori, anti-inflammatory activity, inhibition of oxidative damage by human low-density lipoproteins (LDL) (Iikubo et al. 2002), antimicrobial activity against methicillin-resistant Staphylococcus aureus, vancomycin resistant enterococci (VRE) (Iinuma et al. 1996; Sakagamia et al. 2005) and antioxidant activity (Devi Sampath and Vijayaraghavan 2007). Its poor solubility in water limits its bioavailability in pharmacological applications. Glucosylation is a useful tool to improve the conversion of water-insoluble compounds into water-soluble derivatives to enhance the bioavailability and pharmacological properties (Parvathy et al. 2009).
Enzymatic glucosylation in supercritical fluid (SCF) media confers many advantages, such as environmental compatibility; zero chemical residues in the synthesized product and considerable processing flexibility (Jessop and Leitner 1999). Among SCFs, supercritical carbon dioxide (SC-CO2) is ideal for heat-sensitive substances treatment and bio-catalytic conversions because of its relatively mild critical temperature (31.1 °C) and pressure (73.8 bar). Its unique transport properties such as gas like diffusivities and low viscosities enhance the mass transfer rates of substrates to active sites of enzymes. The adjustable solvent power of the fluid allows the design of a production process integrating biochemical reaction and separation into a single step. Syntheses of carbohydrate derivatives such as glycosides by chemical methods involve protection and deprotection (Konstantinovic et al. 2001) whereas enzymatic methods do not involve such tedious procedures. One advantage of using SCFs as enzymatic reaction media is separation of products from the reaction mixture by changing the density (pressure) of SCF. As can be assessed from the foregoing discussions, the solvent power of SCFs can indeed be adjusted to conduct the reactions. The products can then be easily removed from the reactor and precipitated in a separator by adjusting the pressure (Knez 2009).
The present study is aimed at synthesing water-soluble α–mangostin glucoside using amyloglucosidase and to optimize the process by response surface methodology. A central composite rotatable design (CCRD) was employed with five variables, namely: pressure, temperature, enzyme concentration, pH and buffer volume for the synthesis of α–mangostin–D–glucoside (1).
Materials and methods
Enzyme and chemicals
Amyloglucosidase from Aspergillus niger (67.4 U/mg) was obtained from Sigma (St. Louis, MO, USA), HPLC grade water, acetonitrile, D-glucose was from Merck (Mumbai, India). Dimethyl formamide (DMF) was from Qualigens Fine Chemicals (Mumbai, India). α–mangostin was extracted from the pericarp of mangosteen fruits (Mahabusarakam et al. 2000) and isolated by preparative HPLC.
Glucosylation at SC-CO2 conditions
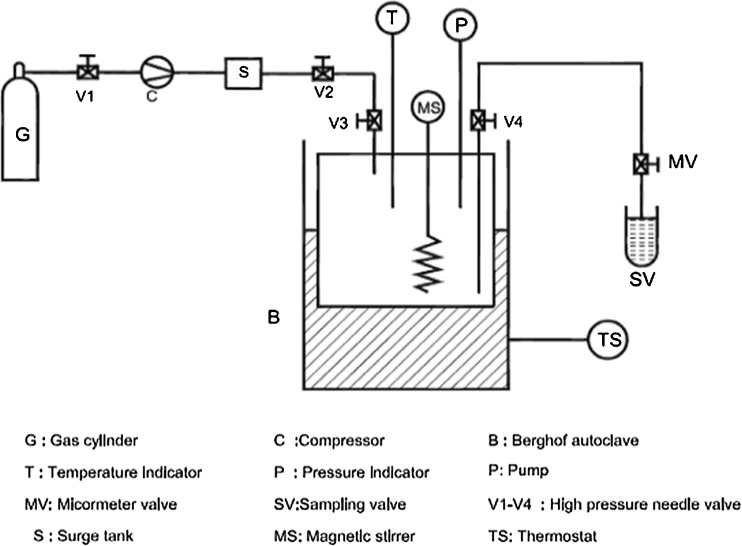
Synthesis of (1) was carried out in SC-CO2 reactor (Berghof, Germany). The schematic flow diagram of the reactor vessel is shown in Fig. 1. It consists of a reactor of 120 mL capacity with a magnetic stirrer for continuous mixing the substrates and a recirculating fluid loop by a pressure differential for sampling through a rheodyne valve with 0.5 mL sampling loop. Pumping CO2 into the reactor pressurized the equipment and the pressure were controlled by a backpressure regulator and read from calibrated pressure indicator. The Berghof autoclave was electrically heated with a heating jacket. Reaction conditions employed were: α–mangostin (0.25 mmol) and carbohydrate D–glucose (0.75 mmol). The variables studied were namely, pressure (80–160 bar), temperature (35–75 °C), enzyme concentration (15–45 mg), buffer pH (4.0–8.0) and buffer volume (1.0–5.0 mL). Buffers employed were sodium acetate buffer for pH 4.0 and 5.0, sodium phosphate buffer for pH 6.0 and 7.0 and sodium borate buffer for pH 8.0 with 24 h incubation period in 10–15 mL of DMF. CO2 was slowly released and the reaction mixture was held in a boiling water bath for 5–10 min to denature the enzyme and 15–20 mL of water was added to dissolve the unreacted carbohydrate and the product glycoside. General format for glycosylation is shown in Format 1. The aqueous layer was evaporated to dryness to get the unreacted carbohydrate and the product glycoside. The glycosides formed were separated through size exclusion chromatography using Sephadex G-10 eluting with water.
Fig 1.
Schematic diagram of SC-CO2 reactor
Format 1.
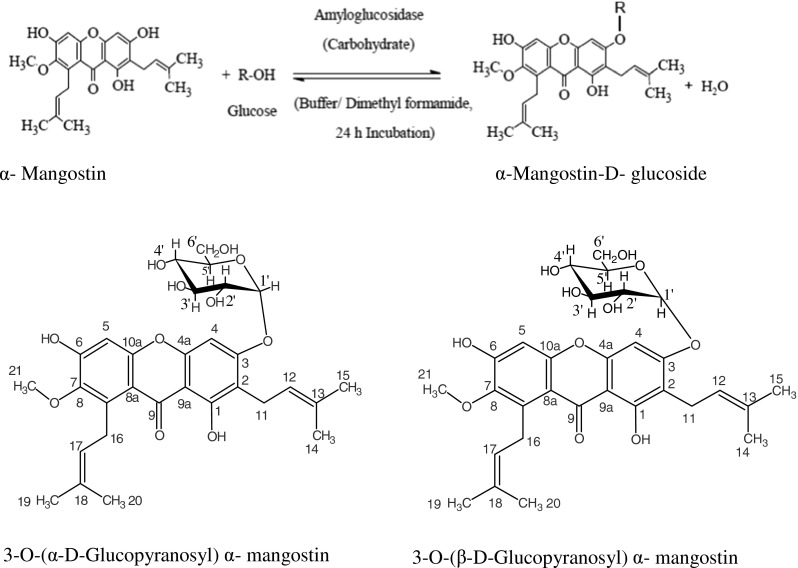
Synthesis of α–mangostin glucosides
HPLC analysis
The reaction mixtures were analyzed by HPLC (Shimadzu LC10AT) using amino-propyl column (10 μm particle size, 3.9 × 300 mm length, μ Bondapak, Ireland) and acetonitrile: water in 80:20 (v/v) as the mobile phase at a flow rate of 1 mL/min with refractive index detector. Conversion yields were determined from HPLC peak areas of the glycoside and free carbohydrate with respect to the free carbohydrate employed. The retention times (RT) were 7.5 min for standard D-glucose and 10.8 min for α-mangostin-D glucoside (Fig.2a).
Fig 2.
a HPLC and MS of α–mangostin–D–glucoside. b FTIR of α–mangostin–D–glucoside
Product characterization
The isolated glycosides were characterized by UV, IR, MS and 2D-NMR (HSQCT) which provided good information on the nature and proportions of the products formed. A Shimadzu UV–160 spectrophotometer was used for recording UV spectra of the isolated glycosides. A Nicolet 5700 FTIR instrument was used for recording the IR spectra. Mass spectra of the isolated glycosides and esters were recorded using a Q-TOF Waters Ultima instrument (No. Q-TOF GAA 082, Waters Corporation, Manchester, UK) fitted with an electron spray ionization (ESI) source. 1H and 13C NMR spectra were recorded on a Bruker Avance 500 MHz spectrometer (500.13 MHz for 1H and 125 MHz 13C). Proton and carbon 90° pulse widths were 10.5 and 12.25 μs, respectively. In NMR data only resolvable signals are shown. Chemical shift values were expressed in ppm relative to internal tetramethylsilane. Since the compounds were viscous and gave broad signals individual coupling constant values could not be determined.
3 − O−(D − glucopyranosyl) α − mangostin
Semi solid viscous; UV (H2O, λmax): 314 nm (n → π*, logε 314 − 18.85 M−1), 207 nm (σ → σ *, logε 207 − 797.14 M−1); IR (DMSO): 1024 cm−1 (glycosidic aryl alkyl, C-O-C), 3433 cm−1 (phenol group, OH), 1654 cm−1 (chelated carbonyl group, C = O) (Fig.2b); MS (m/z) 595 [M + Na]+; 2D-HSQCT (DMSO-d6): C1α-glucoside:1H NMR δ ppm:(500.13 MHz) Glu: 4.94 (H-1′α), 3.82 (H-2′α), 3.44 (H-3′α), 3.09 (H-4′α), 3.62 (H-5′α), 3.74(H-6′α); α − mangostin: 7.86 (H-4), 3.17 (H-11), 2.93 (H-16), 3.27 (H-21); 13C NMR δ ppm :(125 MHz) Glu: 93.9 (C-1′α), 72.9 (C-2′α),72.0 (C-3′α), 71.2 (C-4′α), 71.7(C-5′α), 62.05 (C-6′α); α − mangostin: 177.7 (C-1), 168.9 (C-3), 168.5 (C-4a), 101.80 (C-9a), 178.2 (C-9), 24.4 (C-11); C1β-glucoside: 1H NMR Glu: 4.30 (H-1′β), 2.69 (H-2′β), 13C NMR Glu: 98.0 (C-1′β), 75.0 (C-2′β), 76.3 (C-3′β), 76.1 (C-5′β), 62.7 (C-6′β). NMR data confirmed the formation of 3-O-(α and β–D–glucopyranosyl) α − mangostin (Format 1).
Response surface methodology
A response surface method is a practical technique used in modeling the relationship between several quantitative factors and one or more response variables and in locating the combination of the factor levels that give the optimum expected response (Trinca and Gilmour 2000). CCRD was employed to optimize the glucosylation reaction which consisted of 32 experiments comprising five variables at −2, −1, 0, +1, +2 levels. The coded and corresponding uncoded independent variables used in the RSM design are listed in Table 1. The design composed of 24 factorial with 16 factorial points, 10 star points (star distance is 0), and 6 central points as shown in Table 2. A multiple regression model with second-order polynomial equation was designed to analyze the effect of variables on the glucosylation yields in terms of linear, quadratic and cross product terms. The equation is of the general form
Table 1.
Treatment levels and coded values for each of the independent variables used in the design of experiments
| Codes | Factors | Levels | ||||
|---|---|---|---|---|---|---|
| −2 | −1 | 0 | 1 | 2 | ||
| X 1 | Pressure (bar) | 80 | 100 | 120 | 140 | 160 |
| X 2 | Temperature (°C) | 35 | 45 | 55 | 65 | 75 |
| X 3 | Enzyme (mg) | 15 | 22.5 | 30 | 37.5 | 45 |
| X 4 | pH | 4.0 | 5.0 | 6.0 | 7.0 | 8.0 |
| X 5 | Buffer volume (ml) | 1 | 2 | 3 | 4 | 5 |
Table 2.
CCRD design with experimental and predicted yields of glucosylation based on response surface methodology
| Run | X1 | X 2 | X3 | X4 | X5 | *Y- Experimental | Y-Predicted | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | −1 | (100) | −1 | (45) | −1 | (22.5) | −1 | (5) | 1 | (4) | 5.41 | 5.22 |
| 2 | −1 | (100) | −1 | (45) | −1 | (22.5) | 1 | (7) | −1 | (2) | 5.34 | 3.93 |
| 3 | −1 | (100) | −1 | (45) | 1 | (37.5) | −1 | (5) | −1 | (2) | 10.84 | 8.61 |
| 4 | −1 | (100) | −1 | (45) | 1 | (37.5) | 1 | (7) | 1 | (4) | 3.61 | 3.46 |
| 5 | −1 | (100) | 1 | (65) | −1 | (22.5) | −1 | (5) | −1 | (2) | 1.46 | 2.46 |
| 6 | −1 | (100) | 1 | (65) | −1 | (22.5) | 1 | (7) | 1 | (4) | 3.61 | 5.30 |
| 7 | −1 | (100) | 1 | (65) | 1 | (37.5) | −1 | (5) | 1 | (4) | 1.18 | 2.06 |
| 8 | −1 | (100) | 1 | (65) | 1 | (37.5) | 1 | (7) | −1 | (2) | 11.30 | 10.95 |
| 9 | 1 | (140) | −1 | (45) | −1 | (22.5) | −1 | (5) | −1 | (2) | 1.60 | 0.00 |
| 10 | 1 | (140) | −1 | (45) | −1 | (22.5) | 1 | (7) | 1 | (4) | 2.03 | 0.64 |
| 11 | 1 | (140) | −1 | (45) | 1 | (37.5) | −1 | (5) | 1 | (4) | 7.33 | 5.64 |
| 12 | 1 | (140) | −1 | (45) | 1 | (37.5) | 1 | (7) | −1 | (2) | 2.73 | 0.69 |
| 13 | 1 | (140) | 1 | (65) | −1 | (22.5) | −1 | (5) | 1 | (4) | 3.68 | 3.83 |
| 14 | 1 | (140) | 1 | (65) | −1 | (22.5) | 1 | (7) | −1 | (2) | 3.01 | 2.80 |
| 15 | 1 | (140) | 1 | (65) | 1 | (37.5) | −1 | (5) | −1 | (2) | 3.23 | 1.33 |
| 16 | 1 | (140) | 1 | (65) | 1 | (37.5) | 1 | (7) | 1 | (4) | 6.27 | 6.46 |
| 17 | 0 | (120) | 0 | (55) | 0 | (30) | −2 | (4) | 0 | (3) | 4.35 | 6.16 |
| 18 | 0 | (120) | 0 | (55) | 0 | (30) | 2 | (8) | 0 | (3) | 8.62 | 9.24 |
| 19 | 0 | (120) | 0 | (55) | −2 | (15) | 0 | (6) | 0 | (3) | 3.35 | 3.35 |
| 20 | 0 | (120) | 0 | (55) | 2 | (45) | 0 | (6) | 0 | (3) | 3.10 | 5.53 |
| 21 | 0 | (120) | −2 | (35) | 0 | (30) | 0 | (6) | 0 | (3) | 1.60 | 5.97 |
| 22 | 0 | (120) | 2 | (75) | 0 | (30) | 0 | (6) | 0 | (3) | 8.08 | 6.14 |
| 23 | −2 | (80) | 0 | (55) | 0 | (30) | 0 | (6) | 0 | (3) | 12.30 | 11.46 |
| 24 | 2 | (160) | 0 | (55) | 0 | (30) | 0 | (6) | 0 | (3) | 4.62 | 7.89 |
| 25 | 0 | (120) | 0 | (55) | 0 | (30) | 0 | (6) | −2 | (1) | 2.27 | 5.66 |
| 26 | 0 | (120) | 0 | (55) | 0 | (30) | 0 | (6) | 2 | (5) | 3.62 | 2.66 |
| 27 | 0 | (120) | 0 | (55) | 0 | (30) | 0 | (6) | 0 | (3) | 20.05 | 19.89 |
| 28 | 0 | (120) | 0 | (55) | 0 | (30) | 0 | (6) | 0 | (3) | 20.12 | 19.89 |
| 29 | 0 | (120) | 0 | (55) | 0 | (30) | 0 | (6) | 0 | (3) | 20.77 | 19.89 |
| 30 | 0 | (120) | 0 | (55) | 0 | (30) | 0 | (6) | 0 | (3) | 20.18 | 19.89 |
| 31 | 0 | (120) | 0 | (55) | 0 | (30) | 0 | (6) | 0 | (3) | 20.35 | 19.89 |
| 32 | 0 | (120) | 0 | (55) | 0 | (30) | 0 | (6) | 0 | (3) | 20.30 | 19.89 |
*conversion yields were obtained from HPLC with respect to 0.75 mmoles D-glucose,the experimental yields are an average from two experiments
values in parenthesis indicate actual level
x1: pressure; bar, x2: temperature; °C, x3: enzyme; mg, x4: pH; x5: buffer volume; ml, Y: experimental glucosylation yield
where Y is the glucosylation yield (per cent), β0 is a constant term, βi is the coefficient of the linear terms, βii is the coefficient of the quadratic terms, βij is the coefficient of the cross product terms and N is the number of variables. Xi and Xj are independent variables (pressure, temperature, enzyme concentration, pH and buffer volume). Coefficients for the above equation were determined by employing Kyplot Software Version 2.0 Beta. Analysis of variance (ANOVA) and contour plots were obtained using Kyplot software by varying any two of the variables and maintaining the other variable at 0 coded value. The statistical analysis of the model was performed as analysis of variance (ANOVA). This analysis includes the Fisher’s F-test, its associated probability (P), determination coefficient R2 that measures the goodness of fit of the regression model. The test of statistical significance was based on the total error criteria with a confidence level of 95.0 %.
Test for ferrous ion chelating ability
Ferrous ion chelating activity was measured according to the method of Suter and Richter (2000) with minor modifications. The reaction mixture containing ferrous chloride (200 μM) and potassium ferricyanide (400 μM) without or with extracts was made upto 1 mL with water and the reaction mixture was incubated at 20 °C for 10 min. Formation of the potassium hexacyanoferrate complex was measured at 700 nm. Lower absorbance of the reaction mixture indicated higher iron chelating capacity. The control was without any chelating compound or test sample. The per cent ferrous iron chelating effect was calculated from the following equation.
Results and discussion
Table 3 summarizes the ANOVA, F-test and p-value used as a means to check the significance of each coefficient and to indicate the interaction strength of each parameter. The p-values of this model were 0.001 which indicated that the model fitness was significant. The plot predicted versus observed yield showed R2 = 0.93, a measure of the goodness of fit of the model was very much significant at the level of 93 % meaning the model was unable to explain only 7 % of the total variations. The adjusted R2 = 0.86 indicated the significance of the model to describe the experimental observations, which indicate the accuracy and general availability of the polynomial model is adequate.
Table 3.
Analysis of variance (ANOVA) of the response surface model
| Regression statistics | |||||
|---|---|---|---|---|---|
| Multiple R | 0.969 | ||||
| R 2 | 0.939 | ||||
| Adjusted R2 | 0.828 | ||||
| Standard Error | 2.801 | ||||
| Observations | 32 | ||||
| Degree of freedom | Sum of the square | Mean sum of the square | F ratio | Significance F | |
| Regression | 20 | 1335.01 | 66.75 | 8.50 | (P < =0.001) *** |
| Residual | 11 | 86.32 | 7.84 | ||
| Total | 31 | 1421.33 | |||
From Table 2 it was observed the conversion yields of the glucoside formed under SC-CO2 conditions were in the range from 1.18 to 20.34 %. To visualize the relationship between the responses and experimental levels for each of the factors and to deduce the optimum conditions, the significant coefficients of Table 4 was considered. The fitted polynomial equation is as given below
Table 4.
Regression coefficient for main factors and their interaction
| Terms | Factors/Interaction | Regression coefficient | Standard Error | t-Stat |
|---|---|---|---|---|
| β o | Mean/Intercept | 19.889 | 1.125 | 17.686 |
| β 1 | Pressure (X 1) | −0.892 | 0.597 | −1.495 |
| β 2 | Temperature (X 2) | 0.042 | 0.597 | 0.070 |
| β 3 | Enzyme concentration (X 3) | 0.543 | 0.597 | 0.910 |
| β 4 | pH (X 4) | 0.771 | 0.597 | 1.291 |
| β 5 | Buffer (X 5) | −0.749 | 0.737 | −1.017 |
| β 11 | Pressure (X 21) | −2.553 | 0.533 | −4.794 |
| β 22 | Temperature (X 22) | −3.458 | 0.533 | −6.493 |
| β 33 | Enzyme concentration (X 23) | −3.861 | 0.533 | −7.251 |
| β 44 | pH (X 24) | −3.046 | 0.533 | −5.721 |
| β 55 | Buffer (X 25) | −3.931 | 0.533 | −7.383 |
| β 12 | X 1 X 2 | 1.418 | 1.062 | 1.335 |
| β 13 | X 1 X 3 | 0.841 | 1.118 | 0.753 |
| β 14 | X 1 X 4 | −1.207 | 1.062 | −1.137 |
| β 15 | X 1 X 5 | 1.807 | 0.804 | 2.247 |
| β 23 | X 2 X 3 | −1.062 | 1.062 | −1.000 |
| β 24 | X 2 X 4 | 2.525 | 0.989 | 2.554 |
| β 25 | X 2 X 5 | −0.558 | 0.747 | −0.748 |
| β 34 | X 3 X 4 | 1.036 | 1.062 | 0.975 |
| β 35 | X 3 X 5 | −1.065 | 0.804 | −1.324 |
| β 45 | X 4 X 5 | −0.033 | 0.747 | −0.044 |
where X1 is the pressure, X2 is the temperature, X3 amount of amyloglucosidase, X4 is the pH and X5 is buffer volume.
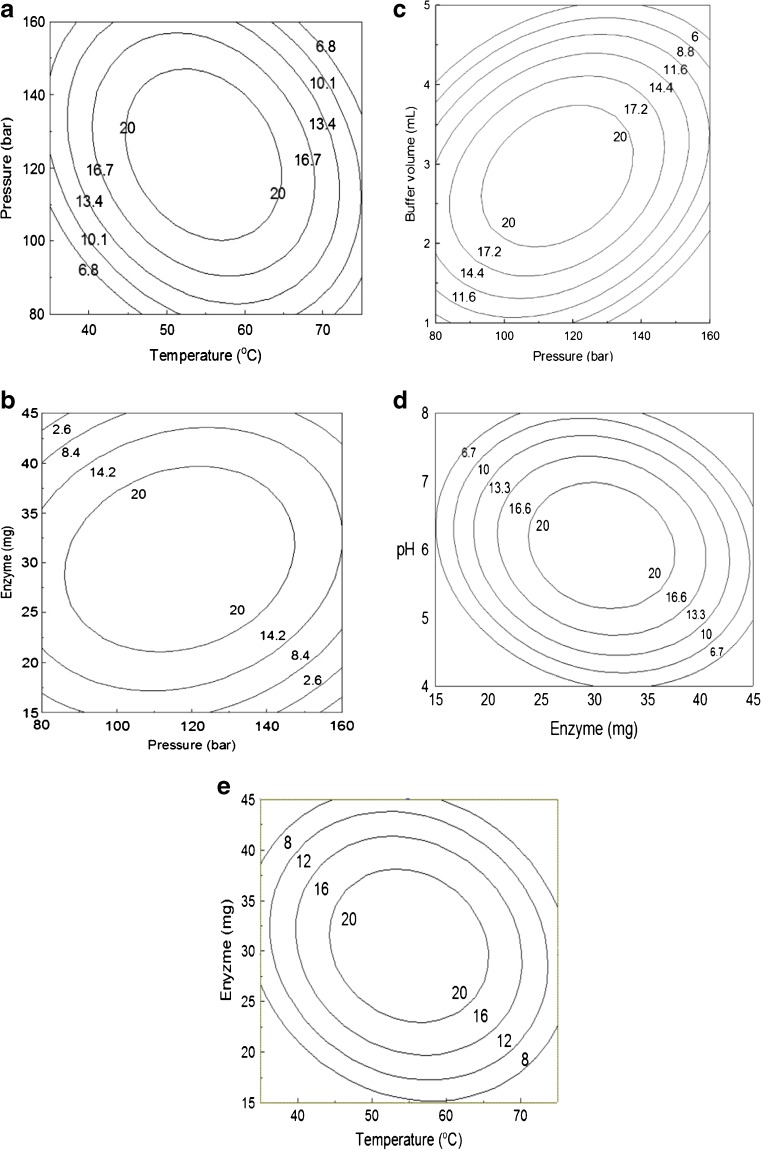
Since SC-CO2 conditions are mild they are used as an ideal condition for the formation of glucosides. 2D contour plots generated describe the relationship between operating variables and the conversion yields. Experiments at different temperature (35–75 °C) and pressure (80–160 bar) combinations in SC-CO2 showed that higher glucosylation yield of (1) were obtained at 120 bar. Increase in pressure from 80 to 140 bar resulted in higher conversion yields but further increase in pressure lowered the glucosylation conversion (Fig. 3a). Temperature significantly affected the conversion yields, maximum glucosylation yield was obtained at 55 °C and it decreased at temperature below or above this value. The combined effect of pressure and temperature influence the solubility of substrates and products. At temperature above 65 °C and pressure above 150 bar the conversion yield decreased and the probable reason is higher pressure and temperature may lead to enzyme denaturation and loss of enzyme activity.
Fig 3.
a Contour plot for yield of α–mangostin–D–glucoside as influenced by pressure and temperature. b Contour plot for yield of α-mangostin-D-glucoside as influenced by pressure and enzyme concentration. c Contour plot for yield of α-mangostin-D-glucoside as influenced by buffer volume and pressure. d Contour plot for yield of α-mangostin-D-glucoside as influenced by pH and enzyme concentration. e Contour plot for yield of α-mangostin-D-glucoside as influenced by enzyme concentration and temperature
The contour plot on the effect of pressure and enzyme concentration (Fig. 3b) on the glucosylation of (1), indicates a maximum conversion yield of 20 % is obtained with increase in pressure from 80 to 140 bar and enzyme concentration from 15 to 37 mg. With further increase in pressure above 140 bar and enzyme concentration above 35 mg the yield decreased, similarly at lower enzyme concentration and pressure the yield decreased. The increase in conversion yield with an increase in pressure in the range from 80 to 140 bar is due to the solubility of substrate (α-mangostin) in enzyme-catalyzed reaction at the applied conditions in supercritical carbon dioxide and increasing substrate aggregation. At lower enzyme concentration the conversion was lower as the enzyme was not able to convert effectively α-mangostin into glycosides.
Figure 3c shows the glucosylation with respect to pressure and buffer volume and Fig. 3d shows the effect of enzyme concentration and pH. Increase in buffer volume from 2 to 3.5 mL along with increase in pressure from 80 to 130 bar showed significant influence on glucosylation yield. Addition of buffers of certain pH values gave better conversions since buffers stabilized the enzyme against deactivation from the applied pressure and temperature (Ponrasu et al. 2009). At buffer volume around 2 to 3.5 mL an effective pH memory is rendered for catalytic glucosylation (Manohar and Divakar 2002). With increasing pressure and buffer volume there was a decrease in conversion yield, due to the formation of inter-phase solubility of buffer in carbon dioxide. Usually a very low-water content in the reaction mixture is required for optimal enzyme action in SCFs. At too high a water content in the reaction mixture a decrease in the reaction rates may occur.
Higher conversion yield of 20 % was obtained within the studied range of enzyme concentration of 20–37 mg and at pH from 5.0 to 7.0. Addition of buffers retains the pH value of the system and to some extent salt hydrate pairs can control water activity and have a beneficial effect on both initial rates and conversion yields (Peres et al. 2005). At pH lower than 5.0 and above 7.0 the conversion yield decreased irrespective of the concentration of the enzyme used. The extent of conversion yield in the presence of sodium acetate and phosphate buffer solutions of three different pH values pH 5, pH 6 and pH 7.0 showed positive effects on extent of glucosylation yield. Thus buffer volume, pH and enzyme effectively indicate the role of the concentration buffer salts on importing pH memory to amyloglucosidase.
The contour plot (Fig. 3e) for the effect of temperature and enzyme concentration indicates higher conversion yield of 20 % can be obtained at temperature around 45–65 °C and enzyme concentration of 24–37 mg indicating that the enzyme is highly stable at these applied temperatures. Higher temperature of around 55 to 65 °C gives faster reaction rates by enhancing mass transfer of the analyte to the active site of the enzyme at the specified concentration. The study indicates at the above mentioned enzyme concentration there is sufficient amount of α-mangostin available for the transfer of D-glucose to α-mangostin-D-glucoside resulting in increase in glucosylation.
The optimum level of parameters for a higher conversion yield (20.3 %) was a pressure of 120 bar, temperature of 55 °C, enzyme concentration of 30 mg, pH of 6.0 and a buffer volume of 3 mL. These results clearly demonstrated that the conjugation of α − mangostin at the phenloic position with glucose, led to the improvement of its water soluble property.
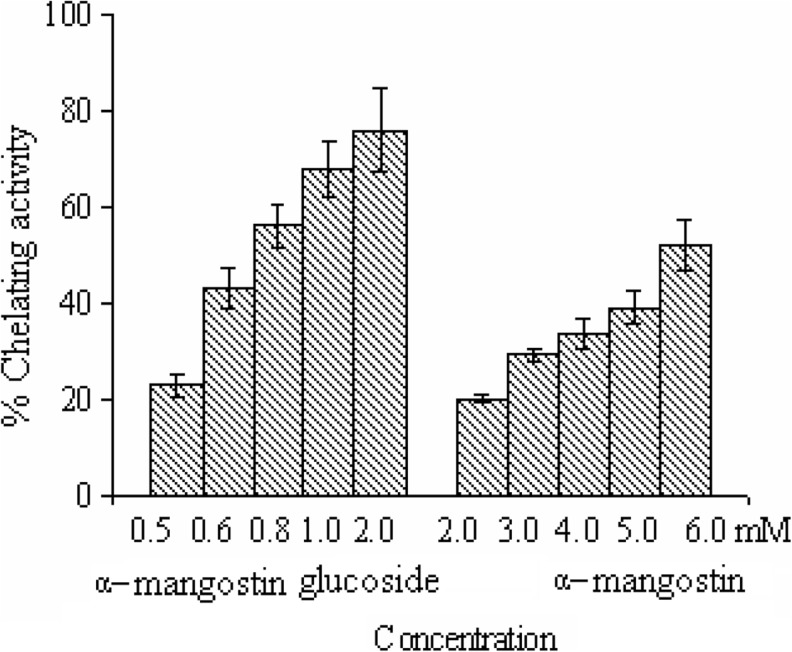
Chelating assay was done by taking α − mangostin as a reference for comparison with the water-soluble α-mangostin-D-glucoside. The chelating assay showed 52 % and 76 % activity at 6 mM α − mangostin and 2 mM of α-mangostin-D-glucoside respectively (Fig. 4). These results clearly showed that α-mangostin-D-glucoside had higher antioxidant activity than had α − mangostin at all concentrations. The glucoside exhibited chelating effect on ferrous ions, suggesting that they minimize the concentration of ferrous ions in the Fenton reaction after its modifications at the phenolic hydroxylic positions.
Fig 4.
Chelating activities of α-mangostin and α-mangostin-D- glycoside
Conclusion
Glucosylation of α-mangostin-D-glucoside has not been reported hitherto. This work deals with the utilization of SC-CO2 as a medium for the enzymatic glucosylation of α-mangostin. The preliminary studies indicated the possibility of enzymatic reactions in SCFs as the enzymes studied were able to retain their activity and stability in non-aqueous media. The study emphasized on the importance of optimizing and controlling the reaction procedure. The results show that the modification carried out with α-mangostin at its hydroxyl positions renders the α-mangostin molecule water-soluble and enhances its antioxidant properties.
References
- Chairungsrilerd N, Takeuchi K, Ohizumi Y, Nozoe S, Ohta T. Garcinol, a new prenyl xanthone from Garcinia mangostana. Phytochemistry. 1996;43:1099–1102. doi: 10.1016/S0031-9422(96)00410-4. [DOI] [Google Scholar]
- Devi Sampath P, Vijayaraghavan K. Cardioprotective effect of α-mangostin, a xanthone derivative from mangosteen on tissue defense system against isoproterenol-induced myocardial infarction in rats. J Biochem Mol Toxicol. 2007;21:336–339. doi: 10.1002/jbt.20199. [DOI] [PubMed] [Google Scholar]
- Iikubo K, Ishikawa Y, Ando N, Umezawa K, Nishiyama S. The first direct synthesis of α-mangostin, a potent inhibitor of the acidic sphingomyelinase. Tetrahedron Lett. 2002;43:291–293. doi: 10.1016/S0040-4039(01)02137-2. [DOI] [Google Scholar]
- Iinuma M, Tosa H, Tanaka T, Asai F, Kobayashi Y, Shimano R, Miyauchi K. Antibacterial activity of xanthones from guttiferaeous plants against methicillin-resistant Staphylococcus aureus. J Pharm Pharmacol. 1996;48:861–865. doi: 10.1111/j.2042-7158.1996.tb03988.x. [DOI] [PubMed] [Google Scholar]
- Jessop P, Leitner W. Chemical synthesis using supercritical fluids. Weinheim: Wiley-VCH; 1999. p. 478. [Google Scholar]
- Knez Z. Enzymatic reactions in dense gases. J Supercrit Fluids. 2009;47:357–372. doi: 10.1016/j.supflu.2008.11.012. [DOI] [Google Scholar]
- Konstantinovic S, Predojevic J, Gojkovic S, Ratkovic Z, Mojsilovic B, Pavlovic V. Synthesis of C7-C16 alkyl 2,3 dideoxy glucosides from glucose and fatty acids. Indian J Chem. 2001;40:1242–1244. [Google Scholar]
- Mahabusarakam W, Proudfoot J, Taylor W, Croft K. Inhibition of lipoprotein oxidation by prenylated xanthones derived from mangostin. Free Radic Res. 2000;33:643–659. doi: 10.1080/10715760000301161. [DOI] [PubMed] [Google Scholar]
- Manohar B, Divakar S. Application of central composite rotatable design to lipase catalysed synthesis of m-cresyl acetate. World J Microbiol Biotechnol. 2002;18:745–751. doi: 10.1023/A:1020446105194. [DOI] [Google Scholar]
- Parvathy KS, Negi PS, Srinivas P. Antioxidant, antimutagenic and antibacterial activities of curcumin-β-diglucoside. Food Chem. 2009;115:265–271. doi: 10.1016/j.foodchem.2008.12.036. [DOI] [Google Scholar]
- Peres C, Harper N, Da Silva MDRG, Barreiros S. Effect of zeolites on lipase catalyzed esterification in nonaqueous media. Enzym Microb Tech. 2005;37:145–149. doi: 10.1016/j.enzmictec.2005.02.003. [DOI] [Google Scholar]
- Ponrasu T, Manohar B, Divakar S. A response surface methodological study on prediction of glucosylation yields of thiamin using immobilized β-glucosidase. Process Biochem. 2009;44:251–255. doi: 10.1016/j.procbio.2008.10.017. [DOI] [Google Scholar]
- Sakagamia Y, Iinumab M, Piyasenac KGNP, Dharmaratne HRW. Antibacterial activity of α-mangostin against vancomycin resistant Enterococci (VRE) and synergism with antibiotics. Phytomedicine. 2005;12:203–208. doi: 10.1016/j.phymed.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Suter M, Richter C. Anti- and pro-oxidative properties of PADMA 28, a Tibetan herbal formula. Redox Rep. 2000;5:17–22. doi: 10.1179/rer.2000.5.1.17. [DOI] [PubMed] [Google Scholar]
- Trinca LA, Gilmour SG. An algorithm for arranging response surface designs in small blocks. Comput Stat Data Anal. 2000;33:25–43. doi: 10.1016/S0167-9473(99)00033-X. [DOI] [Google Scholar]