Abstract
A new type of packaging that combines food packaging materials with antimicrobial substances to control microbial surface contamination of foods to enhance product microbial safety and to extend shelf-life is attracting interest in the packaging industry. Several antimicrobial compounds can be combined with different types of packaging materials. But in recent years, since consumer demand for natural food ingredients has increased because of safety and availability, these natural compounds are beginning to replace the chemical additives in foods and are perceived to be safer and claimed to alleviate safety concerns. Recent research studies are mainly focused on the application of natural antimicrobials in food packaging system. Biologically derived compounds like bacteriocins, phytochemicals, enzymes can be used in antimicrobial food packaging. The aim of this review is to give an overview of most important knowledge about application of natural antimicrobial packagings with model food systems and their antimicrobial effects on food products.
Keywords: Bacteriocins, Biopolymers, Chitosan, Food packaging, Natural antimicrobials, Phytochemicals
Introduction
There is a growing interest in the development of antimicrobial packaging materials containing natural antimicrobial agents. This interest has been driven by consumer concerns about health-related issues, such as the use of synthetic antimicrobial agents. Incorporating synthetic antimicrobial agents directly into foods can effectively inhibit the growth and survival of various microorganisms, but consumers demand minimally processed, preservative-free food products with a longer shelf life. Antimicrobial packaging provides an additional and final barrier that can prevent the growth of food-borne pathogens (Gould 2000; Han 2000; Appendini and Hotchkiss 2002; Devlieghere et al. 2004; Quintavalla and Vicini 2002; Vermerien et al. 2002; Suppakul et al. 2003a; Coma 2008; Emiroglu et al. 2010; Ibarguren et al. 2010; Guarda et al. 2011). For many food products, antimicrobial packaging other than refrigeration system provides an additional food safety. Most natural antimicrobial agents are biodegradable and degrade readily in the environment. The aim of this review is to provide an overview of the studies concerning natural antimicrobial packaging and efficacy of natural bioactive compounds. We first discussed antimicrobial packaging systems and then described natural antimicrobial agents that are the mostly used in packaging systems. Finally, we provided an overview to polymeric and natural antimicrobial edible packaging films.
Antimicrobial packaging system
An antimicrobial packaging system can be obtained by directly incorporating antimicrobial agents into packaging films, coating packaging films with these antimicrobial substances and developing packaging materials from polymers. Generally, antimicrobial packaging systems are regarded as migrating or non-migrating with the differentiation depending on the antimicrobial agent used and on its interactions with the packaging and food matrix. They can be explained: (1) those that contain an antimicrobial agent that migrates to the surface of the food (migrating film), and (2) those that are effective against surface growth of microorganisms without migration (non-migrating film). (Suppakul et al. 2003a; Kuorwel et al. 2011a; Muriel-Galet et al. 2012a).
The effectiveness of antimicrobial packaging has been demonstrated over the last decade. Antimicrobial packaging increases the shelf life, safety and quality of many food products due to their great potential to reduce microbial growth in non-sterile foods and minimize the hazard of post-contamination of pasteurized products (Hotchkiss 1997), by slow migration of antimicrobial agents from an area of high concentration (packaging material) to an area of low concentration (food) (Han 2000). The concept behind antimicrobial packaging is to enhance the safety and quality measures already used by the food industry. It is not meant as a substitute for solid manufacturing and handling practices, but is meant to serve as an additional hurdle for bacteria to overcome (Cooksey 2005).
The use of antimicrobial films can offer advantages compared with the direct addition of preservatives to the food product, as the preservative agents are applied to the packaging material in a way that only low levels of preservative come into contact with the food. In such an antimicrobial agent delivery mechanism, only the necessary amount of the antimicrobial would be used and it would not be directly added to the food product. Furthermore, the production process can be simplified by combining the packaging step with the addition of preservatives (Vermerien et al. 2002; Cha and Chinnan 2003; Waite 2005; Coma 2008). The use of packaging films containing antimicrobial agents could be more efficient by slow migration of the agents from the packaging material to the surface of the product, thus helping maintain high concentrations where they are needed (Quintavalla and Vicini 2002). Another advantage of antimicrobial packaging is that direct addition of antimicrobials could result in some loss of activity because of leaching into the food matrix and cross-reaction with other food components such as lipids and proteins. Therefore, antimicrobial packaging could be more efficient by a controlled migration of the compound into the food, not only allowing for initial inhibition of undesirable microorganisms, but also residual activity over time, during the transport and storage of food during distribution (Mauriello et al. 2005).
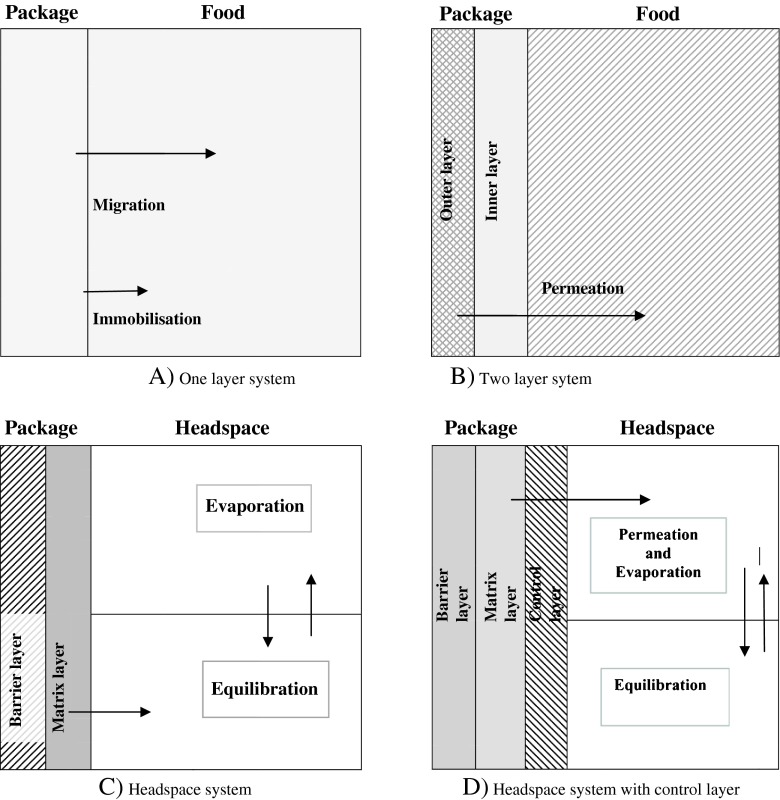
Figure 1 shows antimicrobial systems and their releasing conditions for foods. Food systems (A) and (B) release antimicrobial agents through diffusion and other systems present (C) and (D) release volatile antimicrobial agents by evaporation. In (A) one-layer system, the antimicrobial agent is incorporated into the packaging material; in (B) two-layer system, the antimicrobial agent is coated onto the packaging layer; (C) is a headspace system and volatile antimicrobial compounds are incorporated into the matrix layer released into the headspace; and in (D) headspace system with a control layer, the control layer maintains specific headspace concentration while regulating the permeation of volatile antimicrobial compounds (Han 2003).
Fig. 1.
Food packaging sytems and antimicrobial packaging (Han 2000, 2003; Vartianen 2009)
Other than inherently antimicrobial polymers like chitosan, there are two basic categories to produce antimicrobial films; one involves the incorporation of antimicrobial component into the packaging film, while the other is coating the packaging material with antimicrobial component. The addition of antimicrobial pads and sachets into the packaging is another way to obtain an antimicrobial packaging. Inherent antimicrobial films are not included in this review.
Natural antimicrobial agents
Especially in recent years, consumers prefer natural over synthetic products, and for this reason, naturally-derived antimicrobial agents are becoming increasingly important in antimicrobial packaging, as they present a perceived lower risk to the consumers (Chen et al. 1996; Fernandez 2000; Gould 2000; Suppakul et al. 2003a, b; Kerry et al. 2006; Conte et al. 2007; Guiga et al. 2009; Royo et al. 2010; Ibarguren et al. 2010; Mayachiew et al. 2010; Concha-Meyer et al. 2011) and the use of natural antimicrobial agents might become popular in packaging research (Han 2000). These natural compounds are perceived to be safer and claimed to alleviate safety concerns (Lee et al. 1998; Suhr and Nielsen 2003)
The natural compounds used in antimicrobial packaging can be categorized as biologically-derived components like bacteriocins, enzymes and plant extracts. Some potential natural antimicrobials for food packaging are classified in Table 1.
Table 1.
Examples of potential natural antimicrobial agents and biopolymers for food packaging (Han 2005; Tang et al. 2012; Imran et al. 2010)
| Classification | Antimicrobial agents and biopolymers |
|---|---|
| Plant volatiles and plant/spice extracts | Allyl-isothiocyanate, cinnamaldehyde, eugenol, linalool, terpienol, thymol, carvacrol, pinene, allicin. |
| Grapefruit seed extract, grape seed extract, hop beta acid, Brassica erucic acid oil, rosemary oil, oregano oil, basil oil, other essential oils. | |
| Polysaccharides and derivatives | Starch, chitosan, pullulan, natural gums |
| Cellulose based paper, fatty acids, alginate, carrageenan, chitosan. | |
| Proteins/enzymes/ bacteriocins | Corn-zein, soy-protein isolate, whey-protein isolates, wheat-gluten, peanut-protein, milk- proteins, collagen/gelatin |
| Lysozyme, glucose-oxidase, lactopeoxidase | |
| Nisin, pediocin, subtilin, lacticin | |
| Lipid based coatings | beeswax, carnauba wax, sugar cane wax, rice bran wax, bay berry wax |
| Chelating agents | EDTA |
Bacteriocins
Biologically-derived antimicrobials are attracting increasing interest in recent times, especially for their antilisterial activity. Bacteriocins are peptidic antimicrobial compounds synthesized by different bacteria with bactericidal activity against other generally related species. Generally, most of the bacteriocins are produced by lactic-acid producing bacteria, making their application to control specific bacterial growth in food highly attractive. The use of antimicrobial films containing bacteriocins can improve the quality, safety and prolong the shelf life of food products (Santiago-Silva et al. 2009; Guiga et al. 2009; Divya et al. 2012; Beshkova and Frengova 2012; Nagpal et al. 2012; Perez Espitia et al. 2012; Fucinos et al. 2012; Kumar et al. 2012). The advantages of bacteriocins are that they are thermo-stable, hypoallergenic and easily degraded by proteolytic enzymes in the human gastrointestinal tract (Abreu et al. 2013).
Nisin, a commercially valuable bacteriocin is a protenaceous compound obtained from several Lactococcus lactis strains that occur naturally in raw milk and fermented foods. Nisin has a broad spectrum of antimicrobial activity, which includes some gram-positive bacteria including food-borne pathogenic and spoilage microorganisms such as Listeria monocytogenes, Clostridium botulinum, Staphylococcus aureus and Bacilli. In addition, nisin is heat-stabile, non-toxic and sensitive to digestive proteases. Nisin has been used as a natural food preservative in the world from canned foods to dairy products. The use of nisin as a preservative in foods has been recognized by the Food and Agriculture Organization of the United Nations (FAO), World Health Organization (WHO) and the US Food and Drug Administration (FDA 2004) has given it generally recognized as safe (GRAS) status. It is allowed for use in pasteurized cheese and liquid eggs and commercially used in a range of foods including dairy, eggs, vegetables, meat, fish, beverages and cereal-based products (Padgett et al. 1998; Siragusa et al. 1999; Natrajan and Sheldon 2000a, b; Scannell et al. 2000; Coma et al. 2001; Vermerien et al. 2002; Cha et al. 2003a; Leung et al. 2003; Limjaroen et al. 2003; Suppakul et al. 2003a; Guerra et al. 2005; Guiga et al. 2009; Hoang et al. 2010).
The use of nisin in antimicrobial packaging is attracting intense interest in recent times. There are numerous studies showing that nisin applied in antimicrobial films and packages may control bacterial growth, maintaining food quality, safety and extending the shelf life of food products. Some of the studies about nisin and other related bacteriocins with food packaging can be seen in Table 2. It was indicated that nisin applied in packaging films of plastic, paperboard and edible films has antimicrobial activity against some pathogenic and spoilage microorganisms such as L. monocytogenes and Brochothrix thermosphacta, Micrococcus flavus, Micrococcus luteus, Lactobacillus spp., L. innocua, S. aureus, Salmonella typhimurium; also, these nisin-activated antimicrobial packaging materials have been effective in extending the shelf-life of food products by applying model food systems of meat and meat products, milk, cream milk, cheese and orange juice. Most of the studies on nisin applied packaging materials were about polymeric packaging materials, biopolymers, edible films and paperboard packaging materials. However, some researchers reported that dipping pork meat in a solution of nisin was ineffective against Pseudomonadaceae and increased the growth of Enterobacteriaceae (Barbiroli et al. 2012).
Table 2.
Some researches and results related with the bacteriocin treated food packaging against the microorganisms
| Application | Target microorganisms | Product | Reduction in microbial count | References |
|---|---|---|---|---|
| Pimaricin-loaded nanohydrogels | Saccharomyces cerevisiae | On agar media | 19 cfu/ml, after 15 days at 25 °C, | Fucinos et al. (2012) |
| Poly lactic acid polymer, allyl isothiocyanate, nisin, zinc oxide nanoparticles coated bottle | Salmonella enteric ssp. enterica | Liquid egg white | 3–7 log cfu/ ml, after 7 and 21 days at 10 °C | Jin and Gurtler (2011) |
| Alginate film coated with nisin | Listeria monocytogenes | Smoked salmon | 2.4 log cfu/g, after 28 days at 4 °C | Concha-Meyer et al. (2011) |
| Sodium caseinate film coated with nisin | Listeria innocua | Mini red Babybel cheese surface | 1.1 log cfu/g, after 7 days at 4 °C | Hoang et al. (2010) |
| Edible coatings with nisin | Listeria monocytogenes | Ricotta cheese | 1 log cfu/g, after 28 days at 4 °C | Martins et al. (2010) |
| Bottle coated with nisin and polylactic acid | Listeria monocytogenes | Liquid egg white, skim milk | Completely inactivated, after 48–70 days at 4–10 °C. | Jin (2010) |
| Nisin blend incorporated into cellulose coating | Listeria monocytogenes | Fresh Beef | 1 log cfu/g, after 36 days at 4 °C | Matthews et al. (2010) |
| Polyethylene films coated nisin | Total bacteria count | Soft cheese | 1.1 log cfu/g, after 10 days at 23 °C | Hanusova et al. (2010) |
| Nisin and alginate coated films | Listeria monocytogenes | Ready-to- eat turkey products | 5 log cfu/g, after 8 weeks at 4 °C, | Juck et al. (2010) |
| Nisin/ natamycin treated films | Bacteria, yeast, moulds | Blatacke Zlato cheese | >1 log cfu/g (cheese) | Hanusova et al. (2010) |
| Raw chicken meat | >2 log cfu /g (raw chicken meat), after 28 days at 6–23 °C | |||
| Enterocin EJ97 coated polyethylene films | Bacillus coagulans | Canned corn and canned peas | 0.3 and 1.1 log cfu/g, after 24 h at 4 °C and 20 °C | Viedma et al. (2010) |
| Pediocin containing films | Listeria innocua | Sliced ham | After 15 days at 12 °C, | Santiago-Silva et al. (2009) |
| Salmonella spp. | 2 log cfu/g | |||
| 0.5 log cfu/g |
Nisin is composed of small molecules, and this case causes releasing peptides from packaging films after contact with food or liquid. Generally, nisin is incorporated into coatings together with acids and occasionally with other compounds (Kuorwel et al. 2011b; Abreu et al. 2013). It was also determined that nisin and phenolic extracts such as green tea and grape seed can damage the cytoplasmic membrane of bacterial cells. Theivendran et al. (2006) reported that at 4 °C and 10 °C, the antimicrobial activity of combined nisin (10,000 IU) with either GSE (1 %) or GTE (1 %) was significantly greater than the activity of GTE (1 %) or GSE (1 %) or nisin (10,000 IU) alone against L. monocytogenes. They demonstrated that the use of natural extracts with nisin in an edible coating can provide additional safety and improve the quality of ready-to-eat meat products. Khwaldia et al. (2010) gave place to nisin and chitosan coating with 3 % concentrations, onto paper with a binder medium of a vinyl acetate/ethylene copolymer can provide antimicrobial activity against L. monocytogenes and/or E. coli. Additively applying nisin on the surface of paperboard can be effective inhibition of Micrococcus flavus growth in a model emulsion and in milk cream. Kuorwel et al. (2011b) exemplified coating nisin onto an LDPE film showed effective inhibition against L. monocytogenes on the surface of individually-packed hot dogs, significant antimicrobial activity of nisin-coated PVC, linear low-density polyethylene (LLDPE) and nylon against S. typhimurium on broiler drumstick skin stored at 4 ° C and coated nisin onto LDPE film was found to reduce the microbial growth in packaged fresh oysters and ground beef stored at 3 and 10 °C.
In Abreu et al. (2013) review, they were also stated that other bacteriocins lactocin 705 and lactocin AL705 produced by Lactobacillus curvatus CRL 705, bacteriocin 32Y from Lb. curvatus, enterocin 416 K1 produced from Enterococcus casseliflavus IM 416 K1, enterocins A and B and sakacin K, pediocins produced by Pediococcus sp., bacteriocins lacticin 3147 and Nisaplin are used in the development of antimicrobial packaging systems.
Enzymes
Many enzymes are currently being used in several food processes; however, more recently there are a number of investigations in which these enzymes are also being immobilized in packaging materials. Applying enzyme immobilization systems to food packaging materials, nanostructures offer new, innovative possibilities in this area (Abreu et al. 2013). One of these enzymes is lysozyme, which is a single peptide protein and that possesses enzymatic activity against the beta 1–4 glycosidic linkages between N-acetylmuramic acid and N-acetylglucosamine found in peptidoglycan. Peptidoglycan is the major component of the cell wall of both gram-positive and gram-negative bacteria. Hydrolysis of the cell wall by lysozyme can damage the structural integrity of the cell wall and result in the lysis of bacterial cells. Lysozyme is of interest for use in food systems, as it is a naturally occurring enzyme that is produced by humans and many animals, and has activity against cellular structure specific to bacteria (Mastromatteo et al. 2010; Ntzimani et al. 2010).
The effectiveness of lysozyme-immobilized-polyvinyl-alcohol based films were investigated against Alicyclobacillus acidoterrestris (Conte et al. 2006a) and the films were produced with a new technique by spraying along with a suitable bonding agent onto the surface of the cross-linked polymeric matrix (Conte et al. 2006b). And complete immobilization of the lysozyme onto the polymeric material and acts directly from the film without being the released into the packed foods were also studied (Conte et al. 2007). Buonocore et al. (2005) developed two techniques to control the release of lysozyme from a polymeric material into the foodstuff: a monolayer cross-linked polyvinyl alcohol film and a multilayer structure made of cross-linked polyvinyl alcohol films. Buonocore et al. (2003) developed a mathematical model to predict lysozyme enzyme kinetics from cross-linked polyvinly alcohol (PVOH) into aqueous solution. The inhibitory effect of lysozyme immobilized into polyvinyl alcohol, nylon and cellulose acetate against Micrococcus lysodeikticus cells was also studied and cellulose triacetate yielded the highest antimicrobial activity (Appendini and Hotchkiss 1997). Similarly, Souza et al. (2010) found that caseinate films modified by pH and glyoxal with the release of lysozymes extend food storage and enhance food safety.
Lysozyme enzyme was also investigated for application in biopolymers in antimicrobial packaging. The antimicrobial activities of lysozyme incorporated in to Na-alginate and k-carrageean based biodegradable films against Listeria innocua ATCC 33090, Escherichia coli ATCC 9637, Salmonella enteritidis ATCC 4931, Staphylococcus aureus ATCC 25923, and Micrococcus luteus ATCC 10240 (Cha et al. 2002) and incorporated in soy bean and corn zein biodegradable films against Lactobacillus plantarum (Padgett et al. 1998) were determined. Rodrigues and Han (1993) determined that lysozyme and nisin incorporated into edible films of whey protein isolate were effective at inhibiting B. thermosphacta, but not L. monocytogenes. Antimicrobial nanofilms made of poly- L- glutamic acid (PLGA) with edible protein hen egg white lysozyme inhibited the growth of model microorganism M. luteus in the surrounding liquid medium (Rudra et al. 2006). Unalan et al. (2011) researched antimicrobial activity of zein films incorporated with lysozyme and disodium-ethylene-diamine-tetra-acetic acid (Na2EDTA) on some pathogenic bacteria and refrigerated ground beef patties. The developed films (700 μg/cm2 lysozyme and 300 μg/cm2 Na2EDTA), showed antimicrobial activity against L. monocytogenes, E. coli O157:H7 and Salmonella typhimurium.
Another enzyme and packaging method example is the feasibility of immobilizing naringinase in food contact film of cellulose acetate for the reduction of citrus juice bitterness during storage (Soares and Hotchkiss 1998).
Phytochemicals
Plant extracts have been used for a wide variety of purposes for thousands of years. Antimicrobial properties of essential oils from some plant extracts and spices/herbs have been investigated and used for food preservation since ancient times. Some of the plant extracts and essential oils have strong antimicrobial properties because of their high percentage of phenolic compounds such as carvacrol, thymol and eugenol (Royo et al. 2010; Kechichian et al. 2010; Irkin et al. 2011; Tyagi et al. 2012; Li et al. 2012). Incorporation of plant extracts into the packaging materials or coatings with the extracts instead of plastic films could meet consumer demands for more natural, disposable, recyclable or biodegradable food packaging materials (Emiroglu et al. 2010). In Table 3, some researches about phytochemicals and food packaging systems can be seen.
Table 3.
Some applications of phytochemicals and biopolymers/polymer packaging materials in food systems
| Test Food/ Media | Base materials for edible films/packaging materials | Essential Oil’s /components/plant extracts | Results | References |
|---|---|---|---|---|
| Fresh beef | Sorbitol –plasticized whey protein | Oregano ess.oil | Total viable count, Pseudomonas spp. and lactic acid bacteria were significantly reduced with 1.5 % (w/w) essential oil. | Zinoviadou et al. (2009) |
| Ground beef | Paper in pouch | Horse radish extract | E. coli O157:H7 was inhibited. | Han (2005) |
| Ground beef patties | Soy-protein edible films | Oregano, thyme, oregano + thyme mixture ess. oils | Reductions in coliforms and Pseudomonas spp. counts were observed with 5 % (v/w) essential oils. | Emiroglu et al. (2010) |
| Minced beef | Multilayer PE films | Grape Fruit Seed Extract | Reductions in aerobic bacteria and coliform group bacteria during storage up to 18 days at 3 °C. | Lim et al. (2010a) |
| Beef muscle fillets | Milk-protein based edible film | Oregano, pimento ess. oils 1 % (v/w) | Pseudomonas spp. E. coli O157:H7 levels significantly reduced in meat samples. | Coma (2008) |
| Chicken breast | Apple | Carvacrol | Reductions in E. coli O157:H7 counts were determined. | Du et al. (2011) |
| Chicken breast | Whey Protein Isolate (100 g/kg) | Oregano and clove ess. oils (20 g/ kg films) | Coating extended storage time from 6 to 13 days. Total aerobic mesophilic, Enterobacteriaceae, total aerobic psychrotrophic bacteria, lactic acid bacteria, Pseudomonas levels decreased in coated samples. | Fernandez- Pan et al. (2014) |
| Cod smoke sardine | Gelatin based films | Oregano, rosemary ess. oils | Total viable counts, sulphide-reducing bacteria were inhibited. | Gomez-Estaca et al. (2007) |
| Cod fish fillets | Gelatin-chitosan films | Clove, fennel, cypress, lavender, thyme, herb of the cross pine, rosemary (0.75 ml/ g biopolymer) ess. oils | Some food pathogens and spoilage bacteria can be reduced effectively. Clove essential oil showed the highest inhibitory effect followed by rosemary and lavender. | Gomez- Estaca et al. (2010) |
| Indian oil sardine | Edible coating with chitosan (1 % and 2 % w/v) | – | Effective in reducing the spoilage and extending shelf-life. | Mohan et al. (2012) |
| Salmon | Barley bran protein and gelatin films | Grapefruit Seed Extract | E. coli O157:H7 and L. monocytogenes in packages decreased by 0.53–0.50 log cfu/ g, respectively. | Song et al. (2014) |
| Salmon fillets | PP/EVOH/PP | Carvacrol (6.5 % v/w) | The active package was successfully developed and preserved the salmon samples. | Cerisuelo et al. (2013) |
| Rainbow trout | Chitosan films (2 % w/v) | Cinnamon ess. oil (1.5 % v/v) | Chitosan coating with cinnamon oil extended shelf life of trouts about 16 days at 4 °C. | Ojagh et al. (2010) |
| Rainbow trout fillets | Gelatin films (8 % w/w) | Laurel ess. oil (1 % v/w) | Total viable microorganisms, psychrotrophic microorganisms, Enterobacteriaceae and lactic acid bacteria were inhibited and shelf life of fillets were extended about 5–7 days more comparing with control groups. | Alparslan et al. (2014) |
| Carrot sticks | Chitosan edible films (0.05 ml/ml) under MAP conditions | – | Shelf-life can be extended about 12 days at 4 °C. | Simoes et al. (2009) |
| Cherry tomato | Paraffin-based paper | Oregano ess. oil (3 and 6 % v/w) | Alternaria alternata was fully inhibited with all the concentrations of essential oils paraffin based paper packaging systems. | Rodriguez-Lafuente et al. (2010) |
| Packaged salad | PP/EVOH | Oregano ess. oil, Citral | Citral-based films appeared to be more effective than materials containing oregano essential oil in reducing spoilage flora (Enterobacterias, total viable count, yeast and moulds). | Muriel-Galet et al. (2013) |
| Table grapes | MAP | Thymol, Eugenol | Reductions for yeasts-mould counts (1.7–2.4 log cfu/g) and mesophilic aerobic bacteria (2.2–2.4 log cfu/g) counts were observed. | Tyagi et al. (2012) |
| Fresh cut cantaloupe | Chitosan edible films | Trans-cinnamaldehyde and pectin | 2 g/100 g trans-cinnamaldehyde, 2 g/100 g chitosan and 1 g/100 g pectin helps extend the shelf life of fresh-cut cantaloupe up to 9 days. | Martinon et al. (2014) |
| Strawberries | Chitosan (1 % w/w) | Lemon ess. oil 3 % (v/w) | Chtiosan coatings with lemon essential oil exhibited a high anti- Botyrtis effect. | Perdones et al.(2012) |
| White cheese/sliced bread | Gliadin films | Cinnamaldehyde | No fungi was observed after 26 days of storage at 4 °C for cheese and 27 days at 23 °C for sliced breads. | Balaguer et al. (2013) |
| Sliced breads | Chitosan composite films | Grapefruit Seed Extract (0.5–1.5 % w/w) | Chitosan-based composite films showed antifungal effects. | Tan et al. (2014) |
| Agar media | Alginate or chitosan films | Garlic ess.oil | Garlic oil (0.2 % v/v) in the alginate edible films decreased viable cell counts for Staphylococcus aureus and Bacillus cereus, significantly. | Pranato et al. (2005) |
| Agar media | Whey protein isolate films | Oregano, garlic ess. oils | Oregano essential oil was the most effective at 2 % (v/w) against E. coli O157:H7, S. aureus, S. enteritidis, L. monocytogenes, Lb. plantarum. | Seydim and Sarikus (2006) |
| Agar media | Chitosan-PVA films | Mint extract/pomegranate peel extract | The films show antibacterial activity against gram-positive food pathogens. | Kanatt et al. (2012) |
| Agar media | Agar- Fish Gelatine films | Green Tea extract | Tea extract has antibacterial effects. Antimicrobial activity of the films was not affected by the presence of gelatin. | Gimenez et al. (2013) |
| Agar media | Agar films | Grape Fruit Seed Extract (0.6, 3.3,6.6, 10 and 13.3 μg/mL) | The agar/GSE composite films exhibited strong antimicrobial activity against various gram-positive and gram-negative food-borne pathogens. | Kanmani and Rhim (2014) |
Essential oils
Various studies have marked the antimicrobial activity of plant essential oils including Thymus essential oils, cumin, fennel, laurel, mint, marjoram, oregano, sage, savory, thyme (Ozkan et al. 2003), cinnamon (Manso et al. 2013) onion and garlic oils (Benkeblia 2004), garlic and onion extracts (Satya et al. 2005), Australian native plants oils (Wilkinson and Cavanagh 2005), clove and cinnamon oils (Matan et al. 2006). Their components are becoming increasingly popular as naturally occurring antimicrobial agents. The number of studies showing the possibility of using essential oils and/or some of their components in food systems to prevent the growth of food-borne bacteria/pathogens and to extend the shelf life of the food is very high. Researchers have been investigating the use of essential oils within the packaging materials to extend the shelf life of food.
Because of the antimicrobial properties of the essential oils, they have been the subject of antimicrobial packaging by many researchers.
The antimicrobial packaging showed great efficiency, which supports its likely application as a food packaging material. Seydim and Sarikus (2006) investigated the antimicrobial properties of whey protein isolate films containing 1.0-4.0 % (wt/vol) ratios of oregano, rosemary and garlic essential oils against E. coli O157:H7, S. aureus, Salmonella enteritidis, L. monocytogenes and L. plantarum. In this study, it was found that the film containing oregano essential oil was the most effective against these bacteria at 2 %. Milk protein-based films containing 1 % (w/v) oregano, 1 % (w/v) pimento or 1 % (w/v) oregano pimento (1:1) essential oils mix were applied on beef muscle slices to control the growth of pathogenic bacteria during shelf-life (Oussalah et al. 2004). The film containing oregano was the most effective against E. coli O157:H7 and Pseudomonas spp. Pranato et al. (2005) studied antibacterial alginate-based edible film with incorporation of garlic oil. E. coli, S. typhimurium, S. aureus and B. cereus were affected and the results revealed that garlic oil has potential to be incorporated into alginate to make antimicrobial edible film or coating for various applications. Other examples are E. coli, E. coli O157:H7 and S. aureus, which were significantly reduced by soy edible films, incorporated with thyme and oregano essential oils on fresh ground beef patties (Emiroglu et al. 2010). Allspice, cinnamon and clove bud essential oils in edible apple films were found to be effective against E. coli O157:H7, L. monocytogenes, S. enterica (Du et al. 2009). Similarly, antimicrobial edible films-based on alginate and chitosan were used by incorporating garlic oil gave inhibitory results against Listeria monocytogenes and S. aureus (Pranato et al. 2005).
Mayachiew et al. (2010) determined that an increase in the galangal (Alpinia galanga Linn.) extract concentration in edible films lead to a higher antimicrobial activity against S. aureus. Guarda et al. (2011) determined the antimicrobial properties of plastic flexible films with a coating of microcapsules containing carvacrol and thymol as natural antimicrobial substances, which are the major component of oregano and thyme essential oils and they found that these agents are strong inhibitors of the growth of a broad spectrum of microorganisms such as, E. coli O157:H7, S. aureus, L. innocua, Saccharomyces cerevisia and A. niger.
Muriel-Galet et al. (2012b), Muriel-Galet et al. 2013 researched the antimicrobial effects of PP/EVOH bags incorporating oregano or citral essential oils to be applied in the packaging of ready-to-eat salad and characterized the effects of packaging against E. coli, Salmonella enterica and L. monocytogenes. They found that films showed a significant inhibition of microflora and pathogen flora commonly found on salad. Sensory evaluations suggested that PP/EVOH packaging with 5 % oregano essential oils would be acceptable to consumers.
Basil (Ocimum basilicum L.)’s essential oil contains primarily linalool and methyl chavicol as the active volatile components. Although the antimicrobial properties of basil were investigated by several authors (Elgayyar et al. 2001; Ozcan and Erkmen 2001; Soliman and Badeaa 2002; Suppakul et al. 2002; Edris and Farrag 2003; Bagamboula et al. 2003, 2004; Yano et al. 2006; Yonzon et al. 2005; Kuorwel et al. 2011b) the antimicrobial effect of basil was found in moderate levels in most of the studies.
Suppakul et al. (2003b) expressed that since the principle constituents of basil, namely linalool and methyl chavicol are generally recognized as safe and are stable at high temperatures, they have potential for use in antimicrobial film applications. But the number of studies showing the effectiveness of these compounds in antimicrobial packaging concept is not high. The antimicrobial effect of linalool or methyl chavicol added LDPE films against E. coli was shown by Suppakul et al. (2003b). These compounds might be useful in preparation of antimicrobial packages for extending shelf life of some foods. Suppakul et al. (2008) study demonstrated that the natural antimicrobial components of basil (linalool and methylchavicol) can be successfully incorporated into LDPE-based polymers and retain their inhibitory effect against microbial growth in model (i.e., solid medium) and real (cheddar cheese) systems. They suggested that these kinds of additives might be useful in packaging of some foods by enhancing microbial stability and food safety.
Plant extracts
Grape Fruit Seed Extract (GFSE) has a wide antimicrobial spectrum and has a high heat-stability. It contains naringin, ascorbic acid, hesperidins and various organic acids such as citric acid (Nishina et al. 1991; Lee et al. 1998; Ha et al. 2001; Kim and Cho 2002).
There are numerous studies showing the antimicrobial properties of GFSE. Most of the researchers determined the efficiency of GFSE against L. monocytogenes, E. coli and some important food- borne pathogens and food spoilage bacteria (Jayaprakasha et al. 2003; Baydar et al. 2004; Ahn et al. 2007; Luther et al. 2007; Sivarooban et al. 2008) and fungi (Xu et al. 2007).
Theivendran et al. (2006) determined that GFSE (1 %) was effective in inhibiting L. monocytogenes when incorporated in soy protein edible films. Cha et al. (2002) indicated the potential of application of GFSE with and without EDTA, especially in inhibition of gram-negative bacteria contaminating foods for Na-alginate- and k-carrageenan-based biodegradable films. Antimicrobial efficiency of these films against L. innocua, E. coli, S. aureus, Salmonella enteridis and M. luteus was tested. Na-alginate-based films produced a larger inhibition zone than k-carrageenan-based films when the same levels of antimicrobial agents were present in each film. GFSE-EDTA in both Na-alginate and k-carrageenan based films showed strongest inhibitory effect against all indicator microorganisms.
Sung et al. (2013) showed that a linear low density polyethylene (LLDPE) co-extruded film with 0.5 and 1 % (v/w) GFSE can extended the time of beef stored under 3 °C. GFSE coated on LLDPE film has extended the shelf life of beef from 9 to 14 days.
Ha et al. (2001) tested the antimicrobial activity of GFSE incorporated by a co-extrusion or solution coating process in LDPE film against several spoilage microorganisms and then applied them to the packaging of ground meat. The film co-extruded with 1 % GFSE layer showed antimicrobial activity only against M. flavus, while the film coated with 1 % GFSE showed activity against several microorganisms such as E. coli and S. aureus and B. subtilis. Lim et al. (2010a) showed that an edible film with Gelidium corneum, Cloisite-Na-treated with thymol and GFSE had inhibitory effects on the growth of E. coli O157:H7 and L. monocytogenes.
The review of literature shows that the coating process was more effective than co-extrusion for antimicrobial efficiency of GFSE. However, both types of GFSE-incorporated multilayer polyethylene films contributed to a reduction of the growth rates of aerobic and coliform bacteria on the ground beef when compared to plain PE film.
In Table 3, it can be found some more applications about GFSE extract in the food packaging systems.
Allyl isothiocyanate is the major pungent component of black mustard, brown mustard, wasabi, and is also found in common plants such as broccoli, horseradish, cabbage, cauliflower, kale, turnips and is suggested to be employed in antimicrobial packaging applications (Nielsen and Rios 2000; Suppakul et al. 2003a; Pires et al. 2009; Gonçalves et al. 2009; Jin and Gurtler 2011; Chen and Brody 2013). The antimicrobial efficiency of AITC was determined against E. coli 0157:H7 (Ogawa et al. 2000; Park et al. 2000; Muthukumarasamy et al. 2003; Nadarajah et al. 2005a, b; Chacon et al. 2006a, b) L. monocytogenes, Salmonella montevideo together with E. coli O157:H7 (Lin et al. 2000a, b) and against mesophilic bacteria and coliforms (Inatsu et al. 2005), Penicillium notatum (Tunc et al. 2007). Nielsen and Rios (2000) also determined that antifungal activity of AITC was most efficient when added as volatiles. Lim and Tung (1997) studied the vapor pressure of AITC and its transport in PVDC/PVC copolymer packaging film.
In the area of antimicrobial packaging, there are several studies on the AITC application. Winther and Nielsen (2006) investigated the antimicrobial effect of AITC labels against cheese -related fungi such as Penicillium commune, P. nalgiovense, P. roqueforti, A. flavus, Geotrichum candidum, Debaryomyces hansenii. AITC labels were placed in packaging prior to sealing under modified atmosphere. All inoculated microorganisms were inhibited on the cheese surface and the shelf life of cheese extended and was impacted; AITC was an effective antimicrobial compound in a food matrix such as cheese. Nadarajah et al. (2005a) investigated the antimicrobial activity of AITC, which was placed on ground meat patty packaged in Nylon/EVOH/PE under 100 % N2 against mesophilic bacteria and E. coli. The results of this study showed that AITC can substantially reduce the numbers of E. coli O157:H57 in fresh ground beef during refrigerated and frozen storage, but it was not effective against mesophilic bacteria. Suhr and Nielsen (2003) investigated antimicrobial effect of several essential oils including AITC against rye-bread spoilage fungi and they found that smaller compounds such as AITC and citral were most effective when added as volatiles. Nielsen and Rios (2000) investigated antimicrobial effect of AITC against moulds and yeasts associated with bread. AITC was added in PA/EVOH/PA bags of bread packaged under a modified atmosphere. As a result of the study it was reported that AITC could be fungicidal and fungistatic depending on the concentration of AITC and the number of fungus spores and it was determined that the required shelf-life of rye bread could be achieved by antimicrobial packaging with AITC added in PA/EVOH/PA bags.
Shin et al. (2010) showed that AITC in HDPE film with MAP can together be used to inhibit the growth of fresh poultry-related pathogens such as S. typhimurium and L. monocytogenes in fresh chicken samples.
In addition to these compounds, Kim et al. (2006) investigated the antimicrobial activity of green tea extract (GTE) in antimicrobial packaging which has been evaluated as a good antimicrobial substance against S. aureus food pathogenic bacteria in several studies (Si et al. 2006; Wu et al. 2007). Kim et al. (2006) found that GTE incorporated in to soy protein isolate film exhibited good antimicrobial property against S. aureus and S. mutans. Theivendran et al. (2006) also determined that GTE (1 %) was effective in inhibiting L. monocytogenes when incorporated into soy protein edible films.
Rheum palmatum extracts (Chinese rhubarb) and Coptis chinensis (Chinese goldthread) extracts were the substances evaluated in antimicrobial packaging by incorporating LDPE. (Chung et al. 1998; An et al. 1998). It was determined that the LDPE films retard the growth aerobic bacteria, lactic acid bacteria and yeasts on fruits and fruit decay was significantly lowered but these films did not show any antimicrobial activity against E. coli, S. aureus, Leuconostoc mesenteroides, Saccharomyces cerevisiae, Aspergillus niger, A. oryzae, Penicillum chrysogenum. However, Chana-Thaworn et al. (2011) determined that the antimicrobial activity of edible films incorporated with kiam wood (Cotyleobium lanceotatum) extract against E. coli O157:H7, S. aureus and L. monocytogenes. Ture et al. (2008) found antifungal activity of biopolymers containing rosemary extract against A. niger and P. roquefortii.
Applications of natural antimicrobial polymers
There are several applications for the introduction of antimicrobial activity into polymeric materials. These include incorporating antimicrobial agents directly into polymers, coating antimicrobials onto polymer surfaces, immobilizing antimicrobials by chemical grafting. Bioactive polymers such as, alginate, chitosan, gelatin, etc., can be used for the packaging of food products. Natural biopolymers have the advantage over synthetic polymers in that they are biodegradable and renewable as well as edible (Khan et al. 2014; Rhim and Ng 2007; Shemesh et al. 2015). Some applications about antimicrobial polymers can be seen in the follow.
Polymeric packaging materials
Most of the studies have focused on polymeric packaging materials, especially for low- density polyethylene (LDPE) films since LDPE is commonly used as an inner layer in packaging combinations.
Neetoo et al. (2007) evaluated the antimicrobial effect of nisin- coated plastic films with different chemical compositions and surface properties: low-density polyethylene, ethylene vinly acetate copolymer and three types of ethylene methacrylic acid copolymers against L. monocytogenes. The film type didn’t have any significant effect on the antimicrobial activity of nisin-coated film and commercially available packaging films can be coated with nisin and can be conveniently stored at room temperature without any adverse effect on nisin activity. Patsy et al. (2003) investigated the nisin coating process for similar polymeric packaging films with different hydrophobicity, including low-density polyethylene, linear low-density polyethylene (LLDPE), ethylene acrylic acid copolymer (two compositions with different acrylic acid concentrations), ethylene vinly acetate copolymer and it was found that the highest antimicrobial activity was on the most hydrophobic nisin-coated films. Higher nisin coating concentration and higher solution to film area ratio also exhibited higher antimicrobial activity. Cooksey (2005) investigated the effectiveness of nisin coated on LDPE and the minimum concentration of nisin necessary to inhibit L. monocytogenes. The minimum nisin concentration was found as 156 IU/ml, but levels of 2500 and 7500 IU/ml of nisin concentration significantly reduced the population of L. monocytogenes on hot dogs after 60 days of refrigerated storage. Mauriello et al. (2005) studied the activity of nisin coated in LDPE to inhibit Micrococcus luteus in raw and pasteurized milk. Cha et al. (2003b) also studied the application of nisin on to the polyethylene film coated with methylcellulose (MC) and hydroxyl-propyl-methyl-cellulose (HPMC) and application of this antimicrobial film in packaging of tofu, a nutritional gel-like soy food to prevent the growth of L. monocytogenes. Cutter et al. (2001) studied the efficiency of nisin impregnated in polyethylene and polyethylene oxide blends to exhibit any microbial activity against B. thermosphacta and they impacted nisin-incorporated polymers by controlling the growth of undesirable bacteria, thereby extending the shelf life and possibly enhancing the microbial safety of meats.
Other than LDPE, the effect of nisin coated on polyvinyl chloride packaging film to inhibit L. monocytogenes was studied by Limjaroen et al. (2003). Natrajan and Sheldon (2000a), investigated the efficiency of packaging films (PVC, LLDPE, nylon) coated with one of three liquid formulations (pH 3.5 to 3.8) composed of 100 μg/ml of nisin and varying concentrations of citric acid, EDTA and Tween 80 to reduce Salmonella contamination of fresh broiler drumstick skin and to increase the refrigerated shelf life. They found that S. typhimurium and spoilage microorganism populations on the surface of fresh broiler skin and drumsticks can be significantly reduced by packaging films treated with nisin-containing formulations. Scannell et al. (2000) developed nisin-immobilized PA/PE films and cellulose based packaging paper inserts with nisin. The nisin-immobilized PA/PE films reduced the lactic acid bacteria counts in sliced cheese and ham in MAP at refrigeration temperatures, thus extending shelf-life. Nisin-adsorbed bioactive inserts reduced the levels of L. innocua to below 2 log units in cheese and ham and S. aureus ~1.5 log units in cheese and ~2.8 log units in ham. Guerra et al. (2005) studied the effectiveness of nisin adsorbed in cellophane in reducing total aerobic bacteria and they determined that cellophane activated by nisin could be used for controlling the microbial growth in and for extension of shelf-life of chopped meat under refrigeration temperatures.
Biopolymers
Over the last decade, environmental issues have become increasingly important both to the food industry and the consumers, including materials from non-renewable sources, leading to bio-based packaging materials being used in the food industry. Biopolymers could be prepared from renewable sources such as whey protein, corn protein zein, aliphatic-aromatic copolysters, cellulose, alginates and starch. They provide protection against moisture, gases and vapor. Beside these advantages, they can decompose more readily in the environment. In combination with various antimicrobial agents such as bacteriocins and plant extracts, different types of biopolymers were created. Commercially-available biopolymers in packaging have been used for fruit and vegetables, mainly due to their short shelf life and relatively few studies have reported results for fresh meat or fish products. Combinations of antimicrobial agents enhance the antimicrobial effect of biopolymers if they are compared with individual agents, owing to their synergistic action. It was shown before that wheat gluten (WG) and methyl cellulose (MC) films with natamycin (NA) have antifungal activities against Aspergillus niger and Penicillium roquefortii. Additionally, it was determined that NA- containing casein coatings and cellulose-based films prevented mould spoilage in cheese (Ku and Bin 2007; Ture et al. 2009; Sanchez-Gonzales et al. 2009; Pastor et al. 2010; Pettersen et al. 2011; Kuorwel et al. 2011a).
Cha et al. (2003b) investigated nisin-incorporated biopolymers (MC, hydroxyl-propyle-methyl-cellulose (HPMC), κ-carrageenan and chitosan) films made by heat-press and casting methods. They determined that both heat processed and cast films with nisin had excellent properties. For the heat pressed films, the antimicrobial activity was the most effective in the MC films and cast films.
It was explained that biopolymer coating on paper packaging materials has many advantages for the future improvement of food packaging. The use of such biopackagings will open potential economic benefits to farmers and the food industry because of quality and safety for their products (Khwaldia et al. 2010).
Paper board
In view of cost, acceptance and sustainability properties can be regarded as excellent materials for controlled release of antimicrobials in food packaging (Barbiroli et al. 2012). A new packaging based on the incorporation of natural materials (essential oils) to paraffin used as a coating in paper and board has recently been proposed. Two different paraffin formulations can be used as vehicles to incorporate the essential oils: pure solid paraffin waxes and paraffin emulsions can be applied to paper by a water evaporation process. The shelf life of cherry tomatoes has been shown to be extended with active paraffin-based paper packaging (Rodriguez-Lafuente et al. 2010).
Lee et al. (2004a) studied the effectiveness of nisin incorporated into paperboard coated with vinyl acetate-ethylene copolymer for total aerobic bacteria in milk and yeasts in orange juice and they determined that at the higher temperatures of 20 °C, the degree of microbial suppression was negligible or only marginal. But the microbial inhibition at the low temperatures of 3 °C and 10 °C was significantly important. Lee et al. (2004b) also studied the activity of nisin incorporated into paperboard using a binder medium of vinyl acetate-ethylene copolymer for M. flavus in cream milk. It was determined that nisin coated paperboard was effective for inhibiting microbial growth and it was a potential packaging for preserving the microbial and chemical quality of perishable foods, thus extending their shelf life.
Edible films containing natural antimicrobials
Biopolymers from renewable sources can be used as active packaging for foods (Bitencourt et al. 2014). Carbonhydrates and proteins are the most commonly used materials for edible film productions. Edible films and coatings that inhibit the growth of food-borne pathogens and prevent lipid oxidation can also extend the storage and shelf life of food products. Edible films based on carbonhydrates or protein may contain antimicrobial agents like nisin, lysozyme, chitosan, essential oils or their components (Sanchez-Gonzales et al. 2009; Lim et al. 2010a; Mayachiew et al. 2010; Ibarguren et al. 2010; Atares et al. 2010; Pastor et al. 2010; Moura et al. 2011; Moreira et al. 2011).
Chitosan is a renewable material which is obtained from sources such as; shrimp’s shell, fungi, yeast, protozoa and green microalgae. Chitosan has a good film property as well as it has biodegradability, biocompatibility and non-toxicity properties. Chitosan films can be divided into edible films or coatings, for application directly on food in order to improve food safety and shelf life, films and blends. All of its applications can contribute to food preservation and shelf-life extension. Many authors have investigated chitosan coatings for their potential to enhance the quality and extend the storage life of food products (Dutta et al. 2009; Kanatt et al. 2012; Sung et al. 2013; Pushkala et al. 2013; Gyawali and Ibrahim 2014; Broek et al. 2015).
Among the biopolymers, chitosan and gelatin films are remarkable because of their adequate mechanical properties and excellent gas barrier properties at intermediate and low relative humidity (Pereda et al. 2011). Gelatin has also been reported to be one of the first materials used as carrier of bioactive components (Kavoosi et al. 2014). Elsabee and Abdou (2013) reported that mixing gelatin and chitosan can improve the physico-chemical performance of the films and exhibit antimicrobial activities against pathogen microorganisms.
Theivendran et al. (2006) also demonstrated the use of soy protein film coating containing both nisin and natural extracts (grape seed extract (GSE), green tea extract (GTE), nisin and their combinations against L. monocytogenes for ready-to-eat meat products. The greatest inhibitory effect was observed in medium containing GSE (1 %) and nisin (a 9-log cycle reduction of L. monocytogenes population) and in the meat system L. monocytogenes population was decreased by more than 2 -log cycle in the samples containing nisin combined with either GSE (%1) or GTE (%1). Dawson et al. (2002) studied the activity of nisin added into the film of isolated soy protein and glycerol in turkey bologna inoculated with L. monocytogenes and they determined that nisin films reduced cell numbers on turkey bologna from 106 to 105 after 21 d storage at 4 °C.
Natrajan and Sheldon (2000b) studied the use of protein and polysaccharide-based packaging films containing different nisin formulations for inhibiting Salmonella in fresh broiler skin. The results of the study demonstrated that the inclusion of nisin-based treatments in these films yielded significant S. typhimurium population reductions ranging between 1.8 to 4.6 log cycles after 72 to 96 h of exposure at 4 °C in contaminated broiler drumstick skin. The level of inhibition was affected by film type, gel concentration, exposure time and nisin concentration. Ko et al. (2001) investigated the physical and chemical properties of whey protein and soy protein isolates, egg albumen and wheat gluten-based edible films containing nisin and they found various inhibition activities against L. monocytogenes. In another study Lim et al. (2010b) researched a type of red algae “Gelidium corneum (GC)” edible films containing thymol as a natural antimicrobial agent against Escherichia coli O157:H7 and L. monocytogenes for ham packages. Their study showed application of the film to ham packaging inhibited the microbial growth. Currently, incorporation of natural plant extracts into soy protein isolate film has elicited great interest, because these combinations provide the films with additional nutrients or quality-enhancing materials (Wang et al. 2012). Similarly, Fernandez-Pan et al. (2012) found that whey protein isolate edible films incorporating oregano and clove essential oils had the most intense inhibitory effect on microbial growth of Listeria innocua, Staphylococcus aureus, Salmonella enteritidis and Pseudomonas fragi in their research.
Conclusions
Antimicrobial packaging is an innovative food packaging concept and has been gaining interest among researchers and within the industry due to its potential to provide food quality and safety measures. Antimicrobial agents incorporated into or coated onto packaging materials are demonstrated to have significant inhibition activity against various kinds of microorganisms. Antimicrobial packaging systems can cause eco-friendly and more effective antimicrobial formulations for food packaging. The conscious use of one or more types of antimicrobial agents and evaluation of food matrix, active compounds and target microorganisms in food packaging system will increase shelf life and food safety. Due to their potential to provide quality and safety benefits, antimicrobial packaging systems are expected to grow over the next decade. However acceptance and cost effectiveness of these packaging systems will depend on the industry and consumer preferences. Furthermore, antimicrobial agents to food products organoleptic properties of the packaged food are essential to evaluate.
References
- Abreu DAP, Cruz JM, Losada PP. Active and Intelligent packaging for the food industry. Food Rev Int. 2013;28:146–187. doi: 10.1080/87559129.2011.595022. [DOI] [Google Scholar]
- Ahn J, Grun IU, Mustapha A. Effects of plant extracts on microbial growth, color change and lipid oxidation in cooked beef. Food Microbiol. 2007;24:7–14. doi: 10.1016/j.fm.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Alparslan Y, Baygar T, Baygar T, Hasanhocaoglu H, Metin C. Effects of gelatin-based edible films enriched with laurel essential oil on the quality of rainbow trout (Oncorhynchus mykiss) fillets during refrigerated storage. Food Technol Biotechnol. 2014;52(3):325–333. [Google Scholar]
- An DS, Hwang YI, Cho SH, Lee DS. Packaging of fresh curled lettuce and cucumber by using low density polyethylene films impregnated with antimicrobial agents. J Korean Soc Food Sci Nutr. 1998;27:675–681. [Google Scholar]
- Appendini P, Hotchkiss JH. Immobilization of lysozyme on food contact polymers as potential antimicrobial films. Packag Technol Sci. 1997;10:271–279. doi: 10.1002/(SICI)1099-1522(199709/10)10:5<271::AID-PTS412>3.0.CO;2-R. [DOI] [Google Scholar]
- Appendini P, Hotchkiss JH. Review of antimicrobial food packaging. Innovative Food Sci Emerg Technol. 2002;3:113–126. doi: 10.1016/S1466-8564(02)00012-7. [DOI] [Google Scholar]
- Atares L, Bonilla J, Chiralt A. Characterization of sodium caseinate-based edible films incorporated with cinnamon or ginger essential oils. J Food Eng. 2010;100:678–687. doi: 10.1016/j.jfoodeng.2010.05.018. [DOI] [Google Scholar]
- Bagamboula CF, Uyttendaele M, Debevere J. Antimicrobial effect of spices and herbs on Shigella sonnei and Shigella flexneri. J Food Prot. 2003;66:668–673. doi: 10.4315/0362-028x-66.4.668. [DOI] [PubMed] [Google Scholar]
- Bagamboula CF, Uyttendaele M, Debevere J. Inhibitory effect of thyme and basil essential oils, carvacrol, thymol, estragol, linalool and p-cymene towards Shigella sonnei and S. flexneri. Food Microbiol. 2004;21:33–42. doi: 10.1016/S0740-0020(03)00046-7. [DOI] [Google Scholar]
- Balaguer MP, Lopez-Carballo CR, Gavara R, Hernandez- Munoz P. Antifungal properties of gliadin films incorporating cinnamaldehyde and application in active food packaging of bread and cheese spread foodstuffs. Int J Food Microbiol. 2013;166:369–377. doi: 10.1016/j.ijfoodmicro.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Barbiroli A, Bonomi F, Capretti G, Lametti S, Manzoni M, Piergiovanni L, Rollini M. Antimicrobial activity of lysozyme and lactoferrin incorporated in cellulose-based food packaging. Food Control. 2012;26:387–392. doi: 10.1016/j.foodcont.2012.01.046. [DOI] [Google Scholar]
- Baydar GN, Ozkan G, Sagdıç O. Total phenolic contents and antibacterial activities of grape (Vitis Vinifera L.) Extracts. Food Control. 2004;15:335–339. doi: 10.1016/S0956-7135(03)00083-5. [DOI] [Google Scholar]
- Benkeblia N. Antimicrobial activity of essential oil extracts of various onions (Allium cepa) and garlic (Allium sativum) LWT-Food Sci Technol. 2004;37:263–268. doi: 10.1016/j.lwt.2003.09.001. [DOI] [Google Scholar]
- Beshkova D, Frengova G. Bacteriocins from lactic acid bacteria: microorganisms of potential biotechnological importance for the dairy industry. Eng Life Sci. 2012;4:1–4. [Google Scholar]
- Bitencourt CM, Favaro-Tridade CS, Sobral PJA, Carvalho RA. Gelatin-based films additivated with curcuma ethanol extract: antioxidant activity and physical properties of films. Food Hydrocoll. 2014;40:145–152. doi: 10.1016/j.foodhyd.2014.02.014. [DOI] [Google Scholar]
- Broek LM, Knoop RJI, Kappen FHJ, Boeriu CG. Chitosan films and blends for packaging material. Carbohydr Polym. 2015;116:237–242. doi: 10.1016/j.carbpol.2014.07.039. [DOI] [PubMed] [Google Scholar]
- Buonocore GG, Del Nobile MA, Panizza A, Bove S, Battaglia G, Nicolais L. Modeling the lysozyme release kinetics from antimicrobial films intended for food packaging applications. J Food Sci. 2003;68:1365–1370. doi: 10.1111/j.1365-2621.2003.tb09651.x. [DOI] [Google Scholar]
- Buonocore GG, Conte A, Corbo MR, Sinigaglia M, Del Nobile M. Mono and multilayered active films containing lysozyme as antimicrobial agent. Innovative Food Sci Emerg Technol. 2005;6:459–464. doi: 10.1016/j.ifset.2005.05.006. [DOI] [Google Scholar]
- Cerisuelo JP, Bermudez JM, Aucejo S, Catala R, Gavara R, Hernandez-Munoz P. Describing and modeling the release of an antimicrobial agent from an active PP/EVOH/PP package for salmon. J Food Eng. 2013;116:352–361. doi: 10.1016/j.jfoodeng.2012.12.028. [DOI] [Google Scholar]
- Cha DS, Chinnan MS. Emerging role of nisin in food and packaging systems. Food Sci Biotechnol. 2003;12:1–6. [Google Scholar]
- Cha DS, Choi JH, Manjeet S, Park HJ. Antimicrobial films based on Na-alginate and k-carrageenan. LWT-Food Sci Technol. 2002;35:715–719. doi: 10.1006/fstl.2002.0928. [DOI] [Google Scholar]
- Cha DS, Chen J, Park HJ, Chinnan MS. Inhibition of Listeria monocytogenes in tofu by use of a polyethylene film coated with a cellulosic solution containing nisin. Int J Food Sci Technol. 2003;38:499–503. doi: 10.1046/j.1365-2621.2003.00684.x. [DOI] [Google Scholar]
- Cha DS, Cooksey K, Chinnan MS, Park HJ. Release of nisin from various heat-press and cast films. LWT-Food Sci Technol. 2003;36:209–213. doi: 10.1016/S0023-6438(02)00209-8. [DOI] [Google Scholar]
- Chacon PA, Muthukumarasamy P, Holley RA. Elimination of Escherichia coli O157:H7 from fermented dry sausages at an organoleptically acceptable level of microencapsulated allyl isothiocyanate. Appl Environ Microbiol. 2006;72:3096–4102. doi: 10.1128/AEM.72.5.3096-3102.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacon PA, Buffo RA, Holley RA. Inhibitory effects of microencapsulated allyl isothiocyanate (AIT) against Escherichia coli O157: H7 in refrigerated, nitrogen packed, finely chopped beef. Int J Food Microbiol. 2006;107:231–237. doi: 10.1016/j.ijfoodmicro.2005.08.030. [DOI] [PubMed] [Google Scholar]
- Chana-Thaworn J, Chanthachum S, Wittaya T. Properties and antimicrobial activity of edible films incorporated with kiam wood (Cotyleobium lanceotatum) extract. LWT-Food Sci Technol. 2011;44:284–292. doi: 10.1016/j.lwt.2010.06.020. [DOI] [Google Scholar]
- Chen J, Brody AL. Use of active packaging structures to control the microbial quality of a ready to eat meat product. Food Control. 2013;30:306–310. doi: 10.1016/j.foodcont.2012.07.002. [DOI] [Google Scholar]
- Chen MC, Yeh GHC, Chiang BH. Antimicrobial and physicochemical properties of methylcellulose and chitosan films containing a preservative. J Food Process Preserv. 1996;20:379–390. doi: 10.1111/j.1745-4549.1996.tb00754.x. [DOI] [Google Scholar]
- Chung SK, Cho SH, Lee DS. Modified atmosphere packaging of fresh strawberries by antimicrobial plastic film. Korean J Food Sci Technol. 1998;30:1140–1145. [Google Scholar]
- Coma V. Bioactive packaging technologies for extended shelf-life of meat based products. Meat Sci. 2008;78:90–103. doi: 10.1016/j.meatsci.2007.07.035. [DOI] [PubMed] [Google Scholar]
- Coma V, Sebti I, Pardon P, Deschamps A, Pichauant FH. Antimicrobial edible packaging based on cellulosic ethers, fatty acids and nisin incorporation to inhibit Listeria innocua and S.aureus. J Food Prot. 2001;64:470–475. doi: 10.4315/0362-028x-64.4.470. [DOI] [PubMed] [Google Scholar]
- Concha-Meyer A, Schöbitz R, Brito C, Fuenters R. Lactic acid bacteria in an aliginate film inhibit Listeria monocytogenes growth on smoked salmon. Food Control. 2011;22:485–489. doi: 10.1016/j.foodcont.2010.09.032. [DOI] [Google Scholar]
- Conte A, Sinigaglia M, Del Nobile MA. Antimicrobial effectiveness of lysozyme immobilized on polyvinyl alcohol based film against Alicyclobacillus acidoterrestris. J Food Prot. 2006;69:861–865. doi: 10.4315/0362-028x-69.4.861. [DOI] [PubMed] [Google Scholar]
- Conte A, Buonocore GG, Bevilacqua A, Sinigaglia M, Del Nobile MA. Immobilization of lysozyme on polyvinyl alcohol films for active packaging applications. J Food Prot. 2006;69:866–870. doi: 10.4315/0362-028x-69.4.866. [DOI] [PubMed] [Google Scholar]
- Conte A, Buonocore GG, Sinigaglia M, Del Nobile MA. Development of immobilized lysozyme based active film. J Food Eng. 2007;78:741–745. doi: 10.1016/j.jfoodeng.2005.11.013. [DOI] [Google Scholar]
- Cooksey K. Effectiveness of antimicrobial food packaging materials. Food Addit Contam. 2005;22:980–987. doi: 10.1080/02652030500246164. [DOI] [PubMed] [Google Scholar]
- Cutter CN, Willett JL, Siragusa GR. Improved antimicrobial activity of nisin incorporated polymer films by formulation change and addition of food grade chelator. Lett Appl Microbiol. 2001;33:325–338. doi: 10.1046/j.1472-765X.2001.01005.x. [DOI] [PubMed] [Google Scholar]
- Dawson PL, Carl GD, Acton JC, Han IY. Effect of lauric acid and nisin-impregnated soy-based films on the growth Listeria monocytogenes on turkey bologna. Poult Sci. 2002;81:721–726. doi: 10.1093/ps/81.5.721. [DOI] [PubMed] [Google Scholar]
- Devlieghere F, Vermerien L, Debevere J. New preservation technologies: possibilities and limitations. Int Dairy J. 2004;14:273–285. doi: 10.1016/j.idairyj.2003.07.002. [DOI] [Google Scholar]
- Divya JB, Varsha KK, Madhavan K, Ismail NB, Pandey A. Probiotic fermented foods for health benefits. Eng Life Sci. 2012;12:1–14. doi: 10.1002/elsc.201290001. [DOI] [Google Scholar]
- Du WX, Olsen CW, Avena RJB, Mc Huhg TH, Levin CE, Friedman M. Effects of allspice, cinnamon and clove bud essential oils in edible apple films on physical properties and antimicrobial activities. J Food Sci. 2009;74(7):372–378. doi: 10.1111/j.1750-3841.2009.01282.x. [DOI] [PubMed] [Google Scholar]
- Du W-X, Avena-Bustillos RJ, Hua SST, McHugh TH (2011) Antimicrobial volatile essential oils in edible films for food safety. Formatex 1:1124–1134
- Dutta PK, Tripathi S, Werotra GK, Dutta J. Perspectives for chitosan based antimicrobial films in food applications. Food Chem. 2009;114:1173–1182. doi: 10.1016/j.foodchem.2008.11.047. [DOI] [Google Scholar]
- Edris AE, Farrag ES. Antifungal activity of peppermint and sweet basil essential oils and their major aroma constituents on some plant pathogenic fungi from the vapor phase. Nahrung/Food. 2003;47:117–121. doi: 10.1002/food.200390021. [DOI] [PubMed] [Google Scholar]
- Elgayyar M, Draughon FA, Golden DA, Mount JK. Antimicrobial activity of essential oils from plants against selected pathogenic and saprophytic microorganisms. J Food Prot. 2001;64:1019–1024. doi: 10.4315/0362-028x-64.7.1019. [DOI] [PubMed] [Google Scholar]
- Elsabee MZ, Abdou ES. Chitosan based edible films and coatings: a review. Mater Sci Eng C. 2013;33:1819–1841. doi: 10.1016/j.msec.2013.01.010. [DOI] [PubMed] [Google Scholar]
- Emiroglu ZK, Yemis GP, Coskun BK, Candogan K. Antmicrobial activity of soy edible films incorporated with thyme and oregano essential oils on fresh ground beef patties. Meat Sci. 2010;86:283–288. doi: 10.1016/j.meatsci.2010.04.016. [DOI] [PubMed] [Google Scholar]
- FDA (2004) Food and Drug Administration. Centre for food safety and applied nutrition. Office of food additive safety. Agency response letter. GRAS Notice No: GRN 000133, US. Available at: http://www.cfsan.fda.gov/~rdb/opa-g133.html. Accessed 25. March. 2009
- Fernandez AM. Review: active food packaging. Food Sci Technol Int. 2000;6:97–108. doi: 10.1177/108201320000600203. [DOI] [Google Scholar]
- Fernandez- Pan I, Carrion-Granda X, Mate JI. Antimicrobial efficiency of edible coatings on the preservation of chicken breast fillets. Food Control. 2014;36:69–75. doi: 10.1016/j.foodcont.2013.07.032. [DOI] [Google Scholar]
- Fernandez-Pan I, Royo M, Mate JI. Antimicrobial activity of whey protein isolate edible films with essential oils against food spoilers and fodborne pathogens. J Food Sci. 2012;77(7):383–390. doi: 10.1111/j.1750-3841.2012.02752.x. [DOI] [PubMed] [Google Scholar]
- Fucinos C, Guerra NP, Teijon JM, Pastrana LM, Rua ML, Katime I. Use of poly(n-isopropylacrylamide) nanohydrogels for the controlled release of pimaricin in actice packaging. J Food Sci. 2012;77(7):21–28. doi: 10.1111/j.1750-3841.2012.02781.x. [DOI] [PubMed] [Google Scholar]
- Gimenez B, Lacey AL, Perez-Santin E, Lopez-Caballero ME, Montero P. Release of active compounds from agar and agaregelatin films with green tea extract. Food Hydrocoll. 2013;30:264–271. doi: 10.1016/j.foodhyd.2012.05.014. [DOI] [Google Scholar]
- Gomez- Estaca J, Lacey LA, Lopez-Caballero ME, Gomez- Guillen MC, Montero P. Biodegradable gelatinechitosan films incorporated with essential oils as antimicrobial agents for fish preservation. Food Microbiol. 2010;27:889–896. doi: 10.1016/j.fm.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Gomez-Estaca J, Montero P, Gimenez B, Gomez-Guillen MC. Effect of functional edible films and high pressure processing on microbial and oxidative spoilage in cold-smoked sardine (Sardina pilchardus) Food Chem. 2007;105:511–520. doi: 10.1016/j.foodchem.2007.04.006. [DOI] [Google Scholar]
- Gonçalves MPJC, Pires ACS, Soares FF, Araujo EA. Use of allyl isothiocyanate sachet to preserve cottage cheese. J Food Serv. 2009;20:275–279. [Google Scholar]
- Gould GW. Preservation: past, present and future. Br Med Bull. 2000;56:84–96. doi: 10.1258/0007142001902996. [DOI] [PubMed] [Google Scholar]
- Guarda A, Rubilar JF, Miltz J, Galotto MJ. The antimicrobial activity of microencapsulated thymol and carvacrol. Int J Food Microbiol. 2011;146:144–150. doi: 10.1016/j.ijfoodmicro.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Guerra NP, Macias CL, Agrasar AT, Castro LP. Development of a bioactive packaging cellophane using nisaplin as biopreservative agent. Lett Appl Microbiol. 2005;40:106–110. doi: 10.1111/j.1472-765X.2004.01649.x. [DOI] [PubMed] [Google Scholar]
- Guiga W, Galland S, Peyrol E, Degraeve P, Pantiez AC, Sebti I. Antimicrobial plastic film: physico-chemical characterization and nisin desorption modeling. Innovative Food Sci Emerg Technol. 2009;10:203–207. doi: 10.1016/j.ifset.2008.09.006. [DOI] [Google Scholar]
- Gyawali R, Ibrahim SA. Natural products as antimicrobial agents. Food Control. 2014;46:412–429. doi: 10.1016/j.foodcont.2014.05.047. [DOI] [Google Scholar]
- Ha J, Kim Y, Lee D. Multilayered antimicrobial polyethylene films applied to the packaging of ground beef. Packag Technol Sci. 2001;15:55–62. doi: 10.1002/pts.537. [DOI] [Google Scholar]
- Han JH. Antimicrobial food packaging. Food Technol. 2000;54:56–65. [Google Scholar]
- Han JH. Antimicrobial foof packaging. In: Ahvenainen R, editor. Novel food packaging techniques. Cambridge: CRC Press; 2003. pp. 50–65. [Google Scholar]
- Han JH. Antimicrobial packaging systems: innovations in food packaging. California: Elsevier; 2005. [Google Scholar]
- Hanusova K, Stastna M, Votavova L, Klaudisova K, Dobias J, Voldrich M, Marek M. Polymer films releasing nisin and/or natamycin from polyvinyldichloride lacquer coating: Nisin and natamycin migration, efficiency in cheese packaging. J Food Eng. 2010;99:491–496. doi: 10.1016/j.jfoodeng.2010.01.034. [DOI] [Google Scholar]
- Hoang LC, Chaine A, Gregoire L, Wache Y. Potential of nisin incorporated sodium cseinate films to control Listeria in artificially contaminated cheese. Food Microbiol. 2010;27:940–944. doi: 10.1016/j.fm.2010.05.025. [DOI] [PubMed] [Google Scholar]
- Hotchkiss J. Food packaging interactions influencing quality and safety. Food Addit Contam. 1997;14:601–607. doi: 10.1080/02652039709374572. [DOI] [PubMed] [Google Scholar]
- Ibarguren C, Vivas L, Bertuzzi MA, Apella MC, Carina M. Edible films with anti-Listeria monocytogenes activity. Int J Food Sci Technol. 2010;45:1443–1449. doi: 10.1111/j.1365-2621.2010.02286.x. [DOI] [Google Scholar]
- Imran M, Revol-Junelles AM, Martyn A, Tehrany EA, Jacquot M, Linder M, Desorby S. Active food packaging evolution: transformation from micro-to nanotechnology. Crit Rev Food Sci Nutr. 2010;50:799–821. doi: 10.1080/10408398.2010.503694. [DOI] [PubMed] [Google Scholar]
- Inatsu Y, Bari ML, Kawasaki S, Kawamoto S. Effectiveness of some natural antimicrobial compounds in controlling pathogen or spoilage bacteria in lightly fermented Chinese cabbage. J Food Sci. 2005;70:393–397. doi: 10.1111/j.1365-2621.2005.tb08323.x. [DOI] [Google Scholar]
- Irkin R, Abay S, Aydin F. Inhibitory effects of some plant essential oils against Arcobacter butzleri and potential for rosemary oil as a natural food preservative. J Med Food. 2011;14(3):291–296. doi: 10.1089/jmf.2010.0001. [DOI] [PubMed] [Google Scholar]
- Jayaprakasha GK, Selvi T, Sakariah KK. Antibacterial and antioxidant activities of grape (Vitis Vinifera) seed extracts. Food Res Int. 2003;36:177–182. doi: 10.1016/S0963-9969(02)00116-3. [DOI] [Google Scholar]
- Jin T. Inactivation of Listeria monocytogenes in skim milk and liquid egg white by antimicrobial bottle coating with polylactic acid and nisin. J Food Sci. 2010;75(2):83–88. doi: 10.1111/j.1750-3841.2009.01480.x. [DOI] [PubMed] [Google Scholar]
- Jin T, Gurtler JB. Inactivation of Salmonella in liquid egg albumen by antimicrobial bottle coatings infused with allyl isothiocyanate, nisin and zinc oxide nanoparticles. J Appl Microbiol. 2011;110:704–712. doi: 10.1111/j.1365-2672.2011.04938.x. [DOI] [PubMed] [Google Scholar]
- Juck G, Neetoo H, Chen H. Application of an active alginate coating to control the growth of Listeria monocytogenes on poached and deli turkey products. Int J Food Microbiol. 2010;142:302–308. doi: 10.1016/j.ijfoodmicro.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Kanatt SR, Rao MS, Chawla SP, Sharma A. Active chitosanepolyvinyl alcohol films with natural extracts. Food Hydrocoll. 2012;29:290–297. doi: 10.1016/j.foodhyd.2012.03.005. [DOI] [Google Scholar]
- Kanmani P, Rhim JW. Antimicrobial and physical-mechanical properties of agar-based filmsincorporated with grapefruit seed extract. Carbohydr Polym. 2014;102:708–716. doi: 10.1016/j.carbpol.2013.10.099. [DOI] [PubMed] [Google Scholar]
- Kavoosi G, Rahmatollahi A, Dadfar SMM, Purfard AM. Effects of essential oil on the water binding capacity, physicomechanical properties, antioxidant and antibacterial activity of gelatin films. LWT Food Sci Technol. 2014;57:556–561. doi: 10.1016/j.lwt.2014.02.008. [DOI] [Google Scholar]
- Kechichian V, Ditchfield C, Veiga-Santos P, Tadini CC. Natural antimicrobial ingredients incorporated in biodegradable films based on cassava starch. LWT-Food Sci Technol. 2010;43:1088–1094. doi: 10.1016/j.lwt.2010.02.014. [DOI] [Google Scholar]
- Kerry JP, O’Grady MN, Hogan SA. Past, current and potential utilisation of active and intelligent packaging systems for meat and muscle-based products: A review. Meat Sci. 2006;74:113–130. doi: 10.1016/j.meatsci.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Khan A, Huq T, Khan RA, Riedl B, Lacroix M. Nanocellulose-based composites and bioactive agents for food packaging. Crit Rev Food Sci Nutr. 2014;54:163–174. doi: 10.1080/10408398.2011.578765. [DOI] [PubMed] [Google Scholar]
- Khwaldia K, Arab-Tehrany E, Desorby S. Biopolymer coatings on paper packaging materials. Compr Rev Food Sci Food Saf. 2010;9:82–91. doi: 10.1111/j.1541-4337.2009.00095.x. [DOI] [PubMed] [Google Scholar]
- Kim CH, Cho SH. Development of functional additives and packaging paper for prolonging freshness of cut flowers. Korea Tech Assoc Pulp Paper Ind. 2002;34:32–41. [Google Scholar]
- Kim KM, Lee BY, Kim YT, Choi SG, Lee J, Cho SY, Choi WS. Development of antimicrobial edible film incorporated with green tea extract. Food Sci Biotechnol. 2006;15:478–481. [Google Scholar]
- Ko S, Janes ME, Hettiarachchy NS, Johnson MG. Physical and chemical properties of edible films containing nisin and their action against Listeria Monocytogenes. J Food Sci. 2001;66:1006–1011. doi: 10.1111/j.1365-2621.2001.tb08226.x. [DOI] [Google Scholar]
- Ku K, Bin SK. Physical properties of nisin incorporated gelatin and corn-zein films and antimicrobial activity against Listeria Monocytogenes. J Microbiol Biotechnol. 2007;17:520–523. [PubMed] [Google Scholar]
- Kumar M, Jain AK, Ghosh M, Ganguli A (2012) Potential application of an anti-aeromonas bacteriocin of Lactococcus lactis spp. lactis in the preservation of vegetable salad. J Food Saf 1:1–10
- Kuorwel KK, Cran MJ, Sonneveld K, Miltz J, Bigger SW. Antimicrobial activity of biodegradable polysaccharide and protein based films containing active agents. J Food Sci. 2011;76(3):90–102. doi: 10.1111/j.1750-3841.2011.02102.x. [DOI] [PubMed] [Google Scholar]
- Kuorwel KK, Cran MJ, Sonneveld K, Miltz J, Biger SW. Essential oils and their principal constituents as antimicrobial agents for synthetic packaging films. J Food Sci. 2011;76(9):164–177. doi: 10.1111/j.1750-3841.2011.02384.x. [DOI] [PubMed] [Google Scholar]
- Lee DS, Hwang Y, Cho SH. Developing antimicrobial packaging film for curled lettuce and soybean sprouts. Food Sci Biotechnol. 1998;7:117–121. [Google Scholar]
- Lee CH, Park HJ, Lee DS. Influence of antimicrobial packaging on kinetics of spoilage microbial growth in milk and orange juice. J Food Eng. 2004;65:527–531. doi: 10.1016/j.jfoodeng.2004.02.016. [DOI] [Google Scholar]
- Lee CH, An DS, Lee SC, Park HJ, Lee DS. A coating for use as an antimicrobial and antioxidative packaging material incorporating nisin and ∝-tocopherol. J Food Eng. 2004;62:323–329. doi: 10.1016/S0260-8774(03)00246-2. [DOI] [Google Scholar]
- Leung PP, Yousef AE, Shellhammer TH. Antimicrobial properties of nisin coated polymeric films as influenced by film type and coating conditions. J Food Saf. 2003;23:1–12. doi: 10.1111/j.1745-4565.2003.tb00347.x. [DOI] [Google Scholar]
- Li J, Han Q, Chen W, Ye L. Antimicrobial activity of Chinese bayberry extract for the preservation of surimi. J Sci Food Agric. 2012;92:2358–2365. doi: 10.1002/jsfa.5641. [DOI] [PubMed] [Google Scholar]
- Lim L, Tung MA. Vapor pressure of allyl isothiocyanate and its transport in PVDC/PVC copolymer packaging film. J Food Sci. 1997;62:1061–1066. doi: 10.1111/j.1365-2621.1997.tb15038.x. [DOI] [Google Scholar]
- Lim GO, Jang SA, Song KB. Physical and antimicrobial properties of Gelidium corneum/nano clay composite film containing grapefruit seed extract or thymol. J Food Eng. 2010;98:415–420. doi: 10.1016/j.jfoodeng.2010.01.021. [DOI] [Google Scholar]
- Lim GO, Hong YH, Song KB. Application of Gelidium corneum edible films containing carvacrol for ham packages. J Food Sci. 2010;75(1):90–93. doi: 10.1111/j.1750-3841.2009.01431.x. [DOI] [PubMed] [Google Scholar]
- Limjaroen P, Ryser E, Lockhart H, Harte B. Development of a food packaging coating material with antimicrobial properties. J Plast Film and Sheeting. 2003;19:95–108. doi: 10.1177/8756087903039409. [DOI] [Google Scholar]
- Lin CM, Preston JF, Wei CI. Antibacterial mechanism of allyl isothiocyanate. J Food Prot. 2000;63:727–734. doi: 10.4315/0362-028x-63.6.727. [DOI] [PubMed] [Google Scholar]
- Lin CM, Kim J, Du WX, Wei C. Bactericidal activity of isothiocyanate against pathogens on fresh producer. J Food Prot. 2000;63:25–30. doi: 10.4315/0362-028x-63.1.25. [DOI] [PubMed] [Google Scholar]
- Luther M, Parry J, Moore J, Meng J, Zhang Y, Cheng Z, Yu L. Inhibitory effect of Chardonnay and black raspberry seed extracts on lipid oxidation in fish oil and their radical scavenging and antimicrobial properties. Food Chem. 2007;104:1065–1073. doi: 10.1016/j.foodchem.2007.01.034. [DOI] [Google Scholar]
- Manso S, Cacho-Nerin F, Becerril R, Neric C. Combined analytical and microbiological tools to study the effect on Aspergillus flavus of cinnamon essential oil contained in food packaging. Food Control. 2013;30:370–378. doi: 10.1016/j.foodcont.2012.07.018. [DOI] [Google Scholar]
- Martinon ME, Moreira RG, Castell-Perez ME, Gomes C (2014) Development of a multilayered antimicrobial edible coating for shelf-life extension of fresh-cut cantaloupe (Cucumis melo L.) stored at 4°C. LWT-Food Sci Technol 56:341–350
- Martins JT, Cerquerira MA, Souza BWS, Avides MC, Vicente AA. Shelf life of Ricotta cheese using coatings of galactomannans from nonconventional sources incorporating nisin against Listeria monocytogenes. J Agric Food Chem. 2010;58:1884–1891. doi: 10.1021/jf902774z. [DOI] [PubMed] [Google Scholar]
- Mastromatteo M, Lucera A, Sinigaglia M, Corbo MR. Synergic antimicrobial activity of lysozyme, nisin and EDTA against Listeria monocytogenes in ostrich meat patties. J Food Sci. 2010;75(7):422–429. doi: 10.1111/j.1750-3841.2010.01732.x. [DOI] [PubMed] [Google Scholar]
- Matan N, Rimkeeree H, Mawson AJ, Chompreeda P, Haruthaithanasan V, Parker M. Antimicrobial activity of cinnamon and clove oils under modified atmosphere conditions. Int J Food Microbiol. 2006;107:180–185. doi: 10.1016/j.ijfoodmicro.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Matthews BB, Mangalasary S, Darby D, Cooksey K (2010) Effectiveness of barrier film with a cellulose coating that carries nisin blends for the inhibition of Listeria monocytogenes. Packag Technol Sci 23:267–273
- Mauriello G, De Luca E, La Storia A, Villani F, Ercoloni D. Antimicrobial activity of a nisin activated plastic film for food packaging. Lett Appl Microbiol. 2005;41:464–469. doi: 10.1111/j.1472-765X.2005.01796.x. [DOI] [PubMed] [Google Scholar]
- Mayachiew P, Devahastin S, Mackey BM, Niranjan K. Effects of drying methods and conditions on antimicrobial activity of edible chitosan films enriched with galangal extract. Food Res Int. 2010;43:125–132. doi: 10.1016/j.foodres.2009.09.006. [DOI] [Google Scholar]
- Mohan CO, Ravishankar CN, Lalitha KV, Srinivasa Gopal TK. Effect of chitosan edible coating on the quality of double filleted Indian oil sardine (Sardinella longiceps) during chilled storage. Food Hydrocoll. 2012;26:167–174. doi: 10.1016/j.foodhyd.2011.05.005. [DOI] [Google Scholar]
- Moreira MR, Pereda M, Marcovich NE, Roura SI. Antimicrobial effectiveness of bioactive packaging materials from edible chitosan and casein polymers:assessment on carrot, cheese and salami. J Food Sci. 2011;76:54–63. doi: 10.1111/j.1750-3841.2010.01910.x. [DOI] [PubMed] [Google Scholar]
- Moura MR, Lorevice MV, Mattoso LHC, Zucolotto V. Highly stable, edible cellulose films incorporating chitosan nanoparticles. J Food Sci. 2011;76(2):25–29. doi: 10.1111/j.1750-3841.2010.02013.x. [DOI] [PubMed] [Google Scholar]
- Muriel-Galet V, Lopez-Carballo G, Gavara R, Hernandez-Munoz P. Antimicrobial food packaging film based on the release of LAE from EVOH. Int J Food Microbiol. 2012;157:239–244. doi: 10.1016/j.ijfoodmicro.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Muriel-Galet V, Cerisuelo JP, Lopez-Carballo G, Hernandez-Munoz P. Development of antimicrobial films for microbiological control of packaged salad. Int J Food Microbiol. 2012;157:195–201. doi: 10.1016/j.ijfoodmicro.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Muriel-Galet V, Cerisuelo JP, Lopez-Carballo G, Aucejo S, Gavara R, Hernandez-Munoz P. Evaluation of EVOH-coated PP films with oregano essential oil and citral to improve the shelf-life of packaged salad. Food Control. 2013;20:137–143. doi: 10.1016/j.foodcont.2012.06.032. [DOI] [Google Scholar]
- Muthukumarasamy P, Han JH, Holley RA. Bactericidal effects of Lactobacillus reuteri and allyl isothiocyanate on Escherichia coli O157:H7 in refrigerated ground beef. J Food Prot. 2003;66:2038–2044. doi: 10.4315/0362-028x-66.11.2038. [DOI] [PubMed] [Google Scholar]
- Nadarajah D, Han JH, Holley RA. Inactivation of Escherichia coli 0157: H7 in packaged ground beef by allyl isothiocyanate. Int J Food Microbiol. 2005;99:269–279. doi: 10.1016/j.ijfoodmicro.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Nadarajah D, Han JH, Holley RA. Use of mustard flour to inactivate Escherichia coli O157: H7 in ground beef under nitrogen flushed packaging. Int J Food Microbiol. 2005;99:257–267. doi: 10.1016/j.ijfoodmicro.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Nagpal R, Kumar A, Kumar M, Behare PV, Jain S, Yadav H. Probiotics, their health benefits and applications for developing healthier foods:a review. FEMS Microbiol Lett. 2012;334:1–15. doi: 10.1111/j.1574-6968.2012.02593.x. [DOI] [PubMed] [Google Scholar]
- Natrajan N, Sheldon BW. Efficiency of nisin-coated polymer films to inactivate Salmonella typhimurium on fresh broiler skin. J Food Prot. 2000;63:1189–1196. doi: 10.4315/0362-028x-63.9.1189. [DOI] [PubMed] [Google Scholar]
- Natrajan N, Sheldon BW. Inhibition of Salmonella on poultry skin using protein- and polysaccharide-based films containing a nisin formulation. J Food Prot. 2000;63:1268–1272. doi: 10.4315/0362-028x-63.9.1268. [DOI] [PubMed] [Google Scholar]
- Neetoo H, Ye M, Chen HQ. Effectiveness and stability of plastic films coated with nisin for inhibition of Listeria monocytogenes. J Food Prot. 2007;70:1267–1271. doi: 10.4315/0362-028x-70.5.1267. [DOI] [PubMed] [Google Scholar]
- Nielsen PV, Rios R. Inhibition of fungal growth on bread by volatile components from spices and herbs, and the possible application in active packaging, with special emphasis on mustard essential oil. Int J Food Microbiol. 2000;60:219–229. doi: 10.1016/S0168-1605(00)00343-3. [DOI] [PubMed] [Google Scholar]
- Nishina A, Kihara H, Vehibori T, Oi T. Antimicrobial substances in “DF-100” extract of grape fruits seeds. J Antibacterial Antifungal Agents. 1991;19:401–404. [Google Scholar]
- Ntzimani AG, Giatrakou VI, Savvaidis IN. Combined natural antimicrobial treatments (EDTA, lysozyme, rosemary and oregano oil) on semi cooked coated chicken meat stored in vacuum packages at 4 °C: Microbiological and sensory evaluation. Innovative Food Sci Emerg Technol. 2010;11:187–196. doi: 10.1016/j.ifset.2009.09.004. [DOI] [Google Scholar]
- Ogawa T, Nakatani A, Matsuzaki H, Isobe S, Isshiki KJ. Combined effects of hydrostatic pressure, temperature, and the addition of allyl isothiocyanate on inactivation of Escherichia coli. J Food Prot. 2000;63:884–888. doi: 10.4315/0362-028x-63.7.884. [DOI] [PubMed] [Google Scholar]
- Ojagh SM, Rezaei M, Razavi SH, Hosseini SMH. Effect of chitosan coatings enriched with cinnamon oil on the quality of refrigerated rainbow trout. Food Chem. 2010;120:193–198. doi: 10.1016/j.foodchem.2009.10.006. [DOI] [Google Scholar]
- Oussalah M, Caillet S, Salmieri S, Saucier L, Lacroix M. Antimicrobial and antioxidant effects of the milk protein based film containing essential oils for the preservation of whole beef muscle. J Agric Food Chem. 2004;52:5598–5605. doi: 10.1021/jf049389q. [DOI] [PubMed] [Google Scholar]
- Ozcan M, Erkmen O. Antimicrobial activity of the essential oils of Turkish plant spices. Eur Food Res Technol. 2001;212:658–660. doi: 10.1007/s002170100310. [DOI] [Google Scholar]
- Ozkan G, Sagdıc O, Ozcan M. Inhibition of pathogenic bacteria by essential oils at different concentrations. Food Sci Technol Int. 2003;9:85–88. doi: 10.1177/1082013203009002003. [DOI] [Google Scholar]
- Padgett T, Han IY, Dawson PL. Incorporation of food grade antimicrobial compounds in to biodegradable packaging films. J Food Prot. 1998;61:1330–1335. doi: 10.4315/0362-028x-61.10.1330. [DOI] [PubMed] [Google Scholar]
- Park CM, Taormina PJ, Beuchat LR. Efficacy of allyl isothiocyanate in killing enterohemorrhagic Escherichia coli O157:H7 on alfalfa seeds. Int J Food Microbiol. 2000;56:13–20. doi: 10.1016/S0168-1605(99)00202-0. [DOI] [PubMed] [Google Scholar]
- Pastor C, Sanchez-Gonzalez L, Chafer M, Chiralt A, Martinez CG. Physical and antifungal properties of hydroxypropylmethylcellulose based films containing propolis as affected by moisture content. Carbonhydr Polym. 2010;82:1174–1183. doi: 10.1016/j.carbpol.2010.06.051. [DOI] [Google Scholar]
- Patsy P, Yousef AE, Shellhammer TH. Antimicrobial properties of nisin-coated polymeric films as influenced by film type and coating conditions. J Food Saf. 2003;23:1–12. doi: 10.1111/j.1745-4565.2003.tb00347.x. [DOI] [Google Scholar]
- Perdones A, Sanchez-Gonzalez L, Chiralt A, Vargas M. Effect of chitosan–lemon essential oil coatings on storage-keeping quality of strawberry. Postharvest Biol Technol. 2012;70:32–41. doi: 10.1016/j.postharvbio.2012.04.002. [DOI] [Google Scholar]
- Pereda M, Ponce AG, Marcovich NE, Ruseckaite RA, Martucci JF. Chitosan-gelatin composites and bi-layer films with potential antimicrobial activity. Food Hydrocoll. 2011;25:1372–1381. doi: 10.1016/j.foodhyd.2011.01.001. [DOI] [Google Scholar]
- Perez Espitia PJ, Soares NFF, Coimbra JSR, Andrade NJ, Cruz RS, Medeiros EAA. Bioactive peptides:synthesis, properties and applications in the packaging and preservation of food. Compr Rev Food Sci Food Saf. 2012;11:187–204. doi: 10.1111/j.1541-4337.2011.00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen MK, Bardet S, Nilsen J, Fredriksen SB. Evaluation and suitability of biomaterials for modified atmosphere packaging of fresh salmon fillets. Packag Technol Sci. 2011;24:237–248. doi: 10.1002/pts.931. [DOI] [Google Scholar]
- Pires ACS, Soares NFF, Andrade NJ, Silva LHM, Camilloto GP, Bernardes PC. Increased preservation of sliced mozzarella cheese by antimicrobial sachet incorporated with allyl isothiocyanate. Braz J Microbiol. 2009;40:1002–1008. doi: 10.1590/S1517-83822009000400036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pranato Y, Salokhe VM, Rakshit SK. Physical and antibacterial properties of alginate-based edible film incorporated with garlic oil. Food Res Int. 2005;38:267–272. doi: 10.1016/j.foodres.2004.04.009. [DOI] [Google Scholar]
- Pushkala R, Raghuram PK, Srividya N. Chitosan based powder coating technique to enhance phytochemicalsand shelf life quality of radish shreds. Postharvest Biol Technol. 2013;86:402–408. doi: 10.1016/j.postharvbio.2013.07.025. [DOI] [Google Scholar]
- Quintavalla S, Vicini L. Antimicrobial food packaging in meat industry. Meat Sci. 2002;62:373–380. doi: 10.1016/S0309-1740(02)00121-3. [DOI] [PubMed] [Google Scholar]
- Rhim JW, Ng PKW. Natural biopolymer-based nanocomposite films for packaging applications. Crit Rev Food Sci Nutr. 2007;47:411–433. doi: 10.1080/10408390600846366. [DOI] [PubMed] [Google Scholar]
- Rodrigues ET, Han JH. Antimicrobial whey-protein films against spoilage and pathogenic bacteria : proceedings of the IFT Annual Meeting. Chicago: Institute of Food Technologists; 1993. [Google Scholar]
- Rodriguez-Lafuente A, Nerin C, Batlle R. Active paraffin based paper packaging for extending the shelf life of cherry tomatoes. J Agric Food Chem. 2010;58:6780–6786. doi: 10.1021/jf100728n. [DOI] [PubMed] [Google Scholar]
- Royo M, Fernandez-Pan I, Mate JI. Antimicrobial effectiveess of oregano and sage essential oils incorporated into whey protein films or cellulose based filter paper. J Sci Food Agric. 2010;90:1513–1519. doi: 10.1002/jsfa.3977. [DOI] [PubMed] [Google Scholar]
- Rudra JS, Komal D, Donald T. Antimicrobial polypeptide multilayer nanocoatings. J Biomater Sci Polym Ed. 2006;17:1301–1315. doi: 10.1163/156856206778667433. [DOI] [PubMed] [Google Scholar]
- Sanchez-Gonzales L, Vargas M, Martinez CG, Chiralt A, Chafer M. Characterization of edible films based on hydroxypropylmethylcellulose and tea tree essential oil. Food Hydrocoll. 2009;23:2102–2109. doi: 10.1016/j.foodhyd.2009.05.006. [DOI] [Google Scholar]
- Santiago-Silva P, Soares NFF, Nobrega JE, Junior MAW, Barbosa KBF, Volp ACP, Zerdas ERMA, Würlitzer NJ. Antimicrobial efficiency of film incorporated with pediocin (ALTA 2351) on preservation of sliced ham. Food Control. 2009;20(1):85–89. doi: 10.1016/j.foodcont.2008.02.006. [DOI] [Google Scholar]
- Satya VK, Radhajeyalakshmi R, Kavitha K, Paranidharan V, Bhaskaran R, Velazhahan R. In vitro antimicrobial activity of zimmu (Allium sativum L., Allium cepa L.) leaf extract. Arch Phytopathol Plant Protect. 2005;38:185–192. doi: 10.1080/03235400500094357. [DOI] [Google Scholar]
- Scannell AGM, Hill C, Ross RP, Marx S, Hartmeier W, Arendt AK. Development of bioactive food packaging materials using Immobilised bacteriocins lacticin 3147 and nisaplin. Int J Food Microbiol. 2000;60:241–249. doi: 10.1016/S0168-1605(00)00314-7. [DOI] [PubMed] [Google Scholar]
- Seydim AC, Sarikus G. Antimicrobial activity of whey protein based edible films incorporated with oregano, rosemary and garlic essential oils. Food Res Int. 2006;39:639–644. doi: 10.1016/j.foodres.2006.01.013. [DOI] [Google Scholar]
- Shemesh R, Goldman D, Krepker M, Danin-Poleg Y, Kashi Y, Vaxman A, Segal E (2015) LDPE/Clay/Carvacrol nanocomposites with prolonged antimicrobial activity. J Appl Polym Sci 132. doi:10.1002/app.41261
- Shin J, Harte B, Ryser E, Selke S. Active packaging of fresh chicken breast, with Allyl Isothiocyanate (AITC) in combination with modified atmosphere packaging (MAP) to control the growth of pathogens. J Food Sci. 2010;75(2):65–71. doi: 10.1111/j.1750-3841.2009.01465.x. [DOI] [PubMed] [Google Scholar]
- Si WD, Gong J, Tsao R, Kalab M, Yang R, Yin YL. Bio-assay guided purification and identification of antimicrobial components in Chinese green tea extract. J Chromatogr A. 2006;1125:204–210. doi: 10.1016/j.chroma.2006.05.061. [DOI] [PubMed] [Google Scholar]
- Simoes ADN, Tudela JA, Allende A, Puschmann R, Gil MI. Edible coatings containing chitosan and moderate modified atmospheres maintain quality and enhance phytochemicals of carrot sticks. Postharvest Biol Technol. 2009;51:364–370. doi: 10.1016/j.postharvbio.2008.08.012. [DOI] [Google Scholar]
- Siragusa GR, Cutter CN, Willet JL. Incorporation of bacteriocin in plastic retains activity and inhibits surface growth of bacteria on meat. Food Microbiol. 1999;1:229–235. doi: 10.1006/fmic.1998.0239. [DOI] [Google Scholar]
- Sivarooban T, Hettiarachchy NS, Johnson MG. Physical and antimicrobial properties of grape seed extract, nisin and EDTA incorporated soy protein edible films. Food Res Int. 2008;4:781–785. doi: 10.1016/j.foodres.2008.04.007. [DOI] [Google Scholar]
- Soares NFF, Hotchkiss JH. Nariginase immobilization in packaging films for reducing naringin concentration in grape fruit juice. J Food Sci. 1998;63:61–65. doi: 10.1111/j.1365-2621.1998.tb15676.x. [DOI] [Google Scholar]
- Soliman KM, Badeaa KI. Effect of oil extracted from some medicinal plants on different mycotoxigenic fungi. Food Chem Toxicol. 2002;40:1669–1675. doi: 10.1016/S0278-6915(02)00120-5. [DOI] [PubMed] [Google Scholar]
- Song NB, Lee JH, Mijan MA, Song KB. Development of a chicken feather protein film containing clove oil and its application in smoked salmon packaging. LWT Food Sci Technol. 2014;57:453–460. doi: 10.1016/j.lwt.2014.02.009. [DOI] [Google Scholar]
- Souza PM, Fernandez A, Carballo GL, Gavara R, Munoz PH. Modified sodium caseinate films as releasing carriers of lysozyme. Food Hydrocoll. 2010;24:300–306. doi: 10.1016/j.foodhyd.2009.10.005. [DOI] [Google Scholar]
- Suhr KI, Nielsen PV. Antifungal activity of essential oils evaluated by two different application techniques against rye bread spoilage fungi. J Appl Microbiol. 2003;94:665–674. doi: 10.1046/j.1365-2672.2003.01896.x. [DOI] [PubMed] [Google Scholar]
- Sung SY, Sin LT, Tee TT, Bee ST, Rahmat AR, Rahman WAWA, Tan AC, Vikhraman M. Antimicrobial agents for food packaging applications. Trends Food Sci Technol. 2013;33:110–123. doi: 10.1016/j.tifs.2013.08.001. [DOI] [Google Scholar]
- Suppakul P, Miltz J, Sonneveld K, Bigger SW (2002) Preliminary study of antimicrobial films containing the principal constituents of basil : the Proceedings of 13th IAPRI world conference on packaging, east leasing. Singh SP (Ed) LLC, CRC Press, Boca, Raton, June 23–28
- Suppakul P, Miltz J, Sonneveld K, Bigger SW. Active packaging technologies with an emphasis on antimicrobial packaging and its applications. J Food Sci. 2003;68:408–420. doi: 10.1111/j.1365-2621.2003.tb05687.x. [DOI] [Google Scholar]
- Suppakul P, Miltz J, Sonneveld K, Bigger SW. Antimicrobial properties of basil and its possible application in food packaging. J Agric Food Chem. 2003;51:3197–3207. doi: 10.1021/jf021038t. [DOI] [PubMed] [Google Scholar]
- Suppakul P, Sonneveld K, Bigger SW, Miltz J. Efficacy of polyethylene-based antimicrobial films containing principal constituents of basil. LWT-Food Sci Technol. 2008;41:779–788. doi: 10.1016/j.lwt.2007.06.006. [DOI] [Google Scholar]
- Tan YM, Lim SH, Tay BY, Lee MW, Thian ES (2014) Functional chitosan-based grapefruit seed extract composite films for applications in food packaging technology. Mater Res Bull. doi:10.1016/j.materresbull.2014.11.041
- Tang XZ, Kumar P, Alavi S, Sandeep KP. Recent advances in biopolymers and biopolymer-based nanocomposites for food packaging materials. Crit Rev Food Sci Nutr. 2012;52:426–442. doi: 10.1080/10408398.2010.500508. [DOI] [PubMed] [Google Scholar]
- Theivendran S, Hettiarachchy NS, Johnson MG. Inhibition of Listeria monocytogenes by nisin combined with grape seed extract or green tea extract in soy protein film coated on turkey frankfurters. J Food Sci. 2006;71:39–44. doi: 10.1111/j.1365-2621.2006.tb08905.x. [DOI] [Google Scholar]
- Tunc S, Chollet E, Chalier P, Preziosi-Belloy L, Gontard N. Combined effect of volatile antimicrobial agents on the growth of Penicillum notatum. Int J Food Microbiol. 2007;113:270–273. doi: 10.1016/j.ijfoodmicro.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Ture H, Eroglu E, Soyer F, Ozen B. Antifungal activity of biopolymers containing natamycin and rosemary extract against Aspergillus niger and Penicillium roquefortii. Int J Food Sci Technol. 2008;43:2026–2032. doi: 10.1111/j.1365-2621.2008.01816.x. [DOI] [Google Scholar]
- Ture H, Eroglu E, Ozen B, Soyer F. Physical properties of biopolymers containing natamycin and rosemary extract. Int J Food Sci Technol. 2009;44:402–408. doi: 10.1111/j.1365-2621.2008.01785.x. [DOI] [Google Scholar]
- Tyagi AM, Malik A, Gottardi D, Guerzoni ME. Essential oil vapour and negative air ions: a novel tool for food preservation. Trends Food Sci Technol. 2012;26:99–113. doi: 10.1016/j.tifs.2012.02.004. [DOI] [Google Scholar]
- Unalan IU, Korel F, Yemenicoglu A. Active packaging of ground beef patties by edible zein films incorporated with partially purified lysozyme and Na2EDTA. Int J Food Sci Technol. 2011;46:1289–1295. doi: 10.1111/j.1365-2621.2011.02625.x. [DOI] [Google Scholar]
- Vartianen JV. In: Antimicrobial surface coatings in packaging applications: surface coatings. Rizzo M, Bruno G, editors. New York: Nova Sci. Publishers, Inc; 2009. [Google Scholar]
- Vermerien L, Devlieghere F, Debevere J. Effectiveness of some recent antimicrobial packaging concepts. Food Addit Contam. 2002;19:163–171. doi: 10.1080/02652030110104852. [DOI] [PubMed] [Google Scholar]
- Viedma PM, Ercolini D, Ferrocino I, Abriouel H, Omar NB, Lopez RL, Galvez A. Effect of polyethylene film activated with enterocin EJ97 in combination with EDTA against Bacillus coagulans. LWT-Food Sci Technol. 2010;43:514–518. doi: 10.1016/j.lwt.2009.09.020. [DOI] [Google Scholar]
- Waite N (2005) Antimicrobial packaging. Pira international-profit through innovation (2005) UK. Available at: (http://pira.atalink.co.uk/articles/packaging/54). Accessed: 24/04/2006
- Wang S, Marcone M, Barbut S, Lim L-T. The impact of anthocyanin-rich red raspberry extract (ARRE) on the properties of edible soy protein isolate (SPI) films. J Food Sci. 2012;77(4):497–505. doi: 10.1111/j.1750-3841.2012.02655.x. [DOI] [PubMed] [Google Scholar]
- Wilkinson JM, Cavanagh MA. Antibacterial activity of essential oils from Australian native plants. Phytother Res. 2005;19:643–646. doi: 10.1002/ptr.1716. [DOI] [PubMed] [Google Scholar]
- Winther M, Nielsen PV. Active packaging of cheese with allyl isothiocyanate, an alternative to modified atmosphere packaging. J Food Prot. 2006;69:2430–24355. doi: 10.4315/0362-028x-69.10.2430. [DOI] [PubMed] [Google Scholar]
- Wu SC, Yen GC, Wang BS, Chiu CK, Yen WJ, Chang LW, Duh PD. Antimutagenic and antimicrobial activities of puerh tea. Food SciTechnol. 2007;40:506–512. [Google Scholar]
- Xu WT, Huang KL, Guo F, Qu W, Yang JJ, Liang ZH, Luo YB. Postharvest grape-fruit seed extract and chitosan treatments of table grapes to control Botrytis cinerea. Postharvest Biol Technol. 2007;46:86–94. doi: 10.1016/j.postharvbio.2007.03.019. [DOI] [Google Scholar]
- Yano Y, Satomi M, Oikawa H. Antimicrobial effect of spices and herbs on Vibrio parahaemolyticus. Int J Food Microbiol. 2006;111:6–11. doi: 10.1016/j.ijfoodmicro.2006.04.031. [DOI] [PubMed] [Google Scholar]
- Yonzon M, Lee DJ, Yokochi T, Kawano Y. Antimicrobial activities of essential oils of Nepal. J Essent Oil Res. 2005;17:107–111. doi: 10.1080/10412905.2005.9698846. [DOI] [Google Scholar]
- Zinoviadou KG, Koutsoumanis KP, Biliaderis CG. Physico-chemical properties of whey protein isolate films containing oregano oil and their antimicrobial action against spoilage flora of fresh beef. Meat Sci. 2009;82:338–345. doi: 10.1016/j.meatsci.2009.02.004. [DOI] [PubMed] [Google Scholar]