Abstract
A low molecular weight type-II collagenous polypeptide (CIIp) from whale shark (WS) cartilage was prepared by thermolysin digestion; and examined for their physico-functional and antioxidant properties. The purified collagen was composed of an identical (α1)3 chains and was characterized as type-II. After hydrolysis with thermolysin, the α-chain of the WS collagen was degraded into smaller peptides with molecular weight ranging from 70 to 20KDa. CIIp was successfully separated from the hydrolysates with molecular weight of approximately 37 kDa. Amino acid analysis of CII, and CIIp indicated imino acid contents of 155 and 121 amino acid residues per 1000 residues, respectively. Differing Fourier transform infrared (FTIR) spectra of CII and CIIp were observed, which suggested that the hydrolysis process by thermolysin affected the secondary structure and molecular order of collagen, particularly the triple-helical structure. The denaturation temperature of CII (34 °C) was higher than that of CIIp. Low content of glycoprotein was observed in CII than CIIp due to removal of some polypeptides by thermolysin digestion. The antioxidant activity against 1,1-diphenyl-2-picrylhydrazyl radicals and the reducing power of CIIp was greater than that of CII. The results proposed that the purified CIIp from WS cartilage with excellent antioxidant activities could be the suitable biomaterial for therapeutic applications.
Keywords: Collagenous polypeptide, Thermolysin, Glycoprotein content, Amino acid composition, Antioxidant activity
Introduction
Twenty-six types of genetically distinct collagen have now been identified. According to their structure and molecular organization, they are classified into fibril-forming collagens, fibril-associated collagens, network-forming collagens, anchoring fibrils, transmembrane collagens, basement collagens, and others with unique functions (Gelse et al. 2003). There are three major fibrillar collagens in vertebrates: type-I collagens as the biomaterial for bone and dentine; type-II collagen found in mineralized cartilage; type-III collagen found in most of soft tissues. In the current commercial market, most of the collagen is isolated from terrestrial mammalian cartilage due to its high biocompatibility, and it is widely used in the pharmaceutical and biomedical industries. However, the incidences of diseases such as bovine spongiform encephalopathy (BSE) and foot and mouth disease (FMD) have raised concerns about its safety. Alternatively, collagen from fish may be a suitable substitute. Production of fish collagen not only adds significant value to the fish processing sector but also to other pharmacological industries.
Marine collagen from fish skin, bone, fins and scales had been widely investigated to apply as a biomaterial because of its bioactivity properties such as its excellent biocompatibility, low antigenicity and high biodegradability (Jeevithan et al. 2014a; Jongjareonrak et al. 2005; Muyonga et al. 2004). Type-II collagen (CII) is a principal component of the extracellular matrix of articular cartilage. It is a homotrimer comprising of three indistinguishable polypeptide strands (α1 (II) chains). Recently, type-II collagen hydrolysates are used as a popular nutriceuticals in biomedical industries.
To date, there have been some studies on the arthritogenicity and tolerogenicity of low molecular weight polypeptides from CII (Chen et al. 2012; Xi et al. 2009). At first, Schmid and Conrad (1982) identified a new collagenous polypeptide with a molecular weight (MW) of ~ 59 kDa and further they stated that this low molecular weight collagenous polypeptide plays a major role in calcification of the cartilage. Recently, Xi et al. (2009) confirmed that T cells can recognize type-II collagenous polypeptide (CIIp) with the molecular weight of 28 kDa and further they explained that degree of hydroxylation and percentage of glycosylation should markedly affect the ability of CII epitopes to induce immune tolerance. Indeed, a few recent studies have also demonstrated that low molecular weight polypeptides from the T-cell determinant of human CII possess the ability to suppress T-cell activation in rheumatoid arthritis (RA), both in vitro and in the development of collagen induced arthritis (CIA) (Myers et al. 2004). Recently, few studies have been focused on the preparation and biomedical application of type-II collagen peptides from cartilagous fish species (Ehrlich 2015; Xie et al. 2014). To the best of our knowledge, the physicochemical and functional properties of these low molecular weight type-II collagenous polypeptides have not yet been reported.
Few studies have been conducted on the immunohistochemical and intestinal absorption properties of polypeptide derived from teleost fish species (Lundin and Holmgren 1989; Ronnestad et al. 2010). To get a clear idea, previously we performed qualitative investigations of absorption pattern of whale shark type-II collagen in the intestinal tract (“gut-sac” experiment). Interestingly, our data specified that whale shark cartilage CII hydrolysates in a range from 8 kDa to 70 kDa MW were absorbed by intestinal villi and the intestinal absorption of CII was more facilitated with a molecular weight of 37 kDa (Jeevithan et al. 2015). Therefore, in the present study, we aimed to investigate the physicochemical, thermal stability and antioxidant activity of low molecular weight collagenous polypeptide (37 kDa) for further therapeutic applications.
Materials and methods
Materials
The cartilage (cartilaginous skeleton) of the whale shark (Rhincodon typus) was used as the raw material for the isolation of CII and was obtained from a private fish processing plant, M/s. Yueqing Ocean Biological Health Care Product Co., Ltd. Zhejiang, China. All other chemicals and reagents used in this study were analytical grade and acquired from commercial vendors.
Isolation and purification of Pepsin soluble collagen
Type-II pepsin soluble collagen (CII) in the whale shark cartilage was extracted with 0.5 M acetic acid containing 1 % pepsin as per our earlier reported method (Jeevithan et al. 2014b). The purification of extracted collagen was performed using Gel filtration chromatography (AKTA prime plus, GE Healthcare, Tokyo, Japan). In brief, samples (100 mg) were dissolved in 10 ml of a running buffer (20 mM sodium acetate buffer, pH 4.8) and were applied to a Sephadex G-100 (Sigma-Aldrich, Shanghai, China) column (25.0 × 3.0 cm). The column was previously equilibrated with the same buffer until A230 was less than 0.05 to achieve baseline correction. The elution volume was 200 mL with flow rate of 1 mL/min. Protein fractions were identified by UV absorbance spectroscopy and 5 ml fractions were collected. As shown in Fig. 1a, the collagen peak was pooled and salted out by addition of 2 M NaCl. The precipitate was dialyzed against distilled water and freeze dried (Labconco Freezone 2.5, Kansas City, Missouri, USA). The sample hydroxyproline content was determined according to the method of Bergman and Loxley (1963).
Fig. 1.
Elution profile of type-II collagen (a) and hydrolysates (b) prepared with gel filtration chromatography
Preparation of low molecular weight collagenous polypeptide (CIIp)
Purified collagen sample (100 mg) was suspended in 3 ml of 0.5 M acetic acid. The mixtures were pre-incubated at 37 °C for 10 min prior to enzymatic hydrolysis. The enzymatic hydrolysis was carried out using thermolysin from Bacillus thermoproteolyticusrokko (EC Number: 3.4.24.27) (Sigma-Aldrich, Shanghai, China). The protein hydrolysis reaction was initiated by the addition of the enzyme at a level of 1 % (w/w) of the protein content. The enzymatic reaction was performed (30 min) with continuous stirring by maintaining optimum temperature (70 °C) and pH (8.0) for enzyme activity. The enzyme activity was terminated by addition of EDTA (20 mM). After enzyme inactivation, the hydrolysate was processed through a series of centrifugal ultrafiltration (UF) filters with MWCO of 100, and 10 kDa (Millipore, Shanghai, China). By passing the solution sequentially through these two filters with different pore sizes, three hydrolysates (H1: ≥100 kDa; H2:100 ≤ MW ≥ 10 kDa; H3: ≤10 kDa) were obtained. The H2 fraction was freeze dried and stored at −20 °C until further experiments.
Degree of hydrolysis
The degree of hydrolysis (DH) was calculated by the determination of free amino group after the reaction with 2, 4, 6-trinitrobenzenesulfonic acid (TNBS) following the method described by Adler-Nissen (1979) with slight modification. About 0.25 ml of protein hydrolysate was withdrawn after the hydrolysis reaction into 2.0 ml of aqueous 1 % SDS and incubated at 70 °C for 15 min. From this, 0.25 ml was transferred into the test tubes containing 2.0 ml of 0.2 M sodium phosphate buffer (pH 8.2). A blank was set using 0.25 ml of 1 % SDS. Then, 2 ml of TNBS reagent (0.1 % w/v) was added, mixed well, covered with aluminum foil and incubated in dark at 50 °C for 50 min. The reaction was then terminated by the addition of 4.0 ml of 0.1 N HCl and cooled at room temperature for 30 min. The absorbance was read at 420 nm in a UV-spectrophotometer. The amount of free amino group liberated was expressed as L-leucine equivalent.
where Lt is the amount of a specific liberated amino acid at time t, L0 is the amount of the specific amino acid in the original substrate (blank), and Lmax is the maximum amount of the specific amino acid in the substrate obtained after hydrolysis.
Separation of low molecular weight collagenous polypeptide
The low molecular weight collagenous polypeptide (37 kDa) was purified from enzymatic hydrolysate using gel filtration column chromatography. The H2 fraction from MWCO membrane was loaded onto a Sephadex G-50 medium (Sigma-Aldrich, Shanghai, China) column equilibrated with 20 mM sodium acetate buffer, (pH 4.8) and followed the above procedure. The desired collagen peak (fraction no 2) was pooled (Fig. 1b), lyophilized and further referred to as CIIp.
Protein pattern
The protein pattern was analyzed using SDS-PAGE according to the method of Laemmli (1970) with modification. Briefly, the purified collagen samples were dissolved in 5 % SDS, kept in a water bath at 60 °C for 20 min and centrifuged at 1890 g. The supernatant was mixed with a sample buffer (1:1) containing Tris HCl (pH 6.8), 1 % 2-mercaptoethanol, 40 % sucrose, 20 % glycerol, 0.02 % bromophenol blue and 1 % SDS. The mixtures were loaded onto a polyacrylamide gel, composed of 10 % separating gel and 4 % stacking gel, and were subjected to electrophoresis at a constant current of 50 mA. After electrophoresis, gels were fixed with a mixture of methanol and acetic acid (5:1) for 1 h. This was followed by staining with Coomassie blue R-250 in methanol and acetic acid at a ratio of 0.5:150:50 for 30 min. Finally, the gels were destained with a mixture of 3:1 methanol and acetic acid.
Amino acid profile
Collagen samples were hydrolyzed under reduced pressure in 6 M HCl at 110 °C for 24 h. The hydrolysates were neutralized with 3.5 M NaOH and diluted with 0.02 M HCL (pH 2.2). The final solution was filtrated through a 0.45 nm hydrophilic membrane. An aliquot of 0.4 mL was applied to an amino acid analyzer (Hitachi L-8800, Tokyo, Japan). Amino acid content is expressed as the number of residues/1000 residues.
Glycoprotein/carbohydrate estimation
Purified collagen samples were examined for carbohydrate content using the glycoprotein/carbohydrate estimation kit (Thermo Scientific Catalog #23260) (Merly and Smith 2013). All samples were diluted to 0.25 and 2.5 mg/ml protein in glycoprotein assay buffer. Lysozyme (2.5 mg/ml) and bovine serum albumin (2.5 mg/ml) served as the negative control standards. Ovalbumin (2.5 mg/ml), human apotransferrin (2.5 mg/ml), fetuin (0.25 mg/ml), and α1-acid glycoprotein (0.25 mg/ml) were the positive control standards. All samples and standards were tested in triplicate in microtiter wells at 50 μl/well. Blank wells were loaded with the glycoprotein assay buffer. Samples were processed according to the manufacturer’s instructions and absorbance was measured at 550 nm. Carbohydrate content of samples was estimated based on a line of best fit for the standard curve.
UV absorption
UV absorption spectrum of collagen samples was measured using a UV- spectrophotometer. Samples were dissolved in 0.5 M acetic acid and UV spectra were measured between 190 and 400 nm at a scan speed of 2 nm/s with an interval of 1 nm.
Thermal stability
Differential scanning calorimetry (DSC) was conducted following the method of Rochdi et al. (2000), briefly, collagen samples were rehydrated with deionized water at a solid/solution ratio of 1:10 (w/v). DSC was performed using a differential scanning calorimeter (Model- DSC822e, Mettler-Toledo GmbH, Switzerland) and the temperature calibration was conducted using an indium standard. Samples were weighed into aluminum pans and sealed. Subsequently, samples were scanned at 5 °C/min from 20 to 120 °C using ice water as the cooling medium. An empty pan was used as the reference. The denaturation and melting temperatures were estimated from the DSC thermogram.
Fourier transform infrared spectroscopy (FTIR)
FTIR spectra of the samples were obtained using a Nicolet 6700-Fourier transform infrared spectrometer (Thermofisher Scientific Inc., Waltham, Mass., U.S.A.) equipped with a DLaTGS detector. Collagen samples (5 mg) were mixed with dried KBr (100 mg), ground in a mortar and pestle and subjected to a pressure of approximately 5 × 106 Pa in an evacuated die to produce a 13 × 1 mm clear transparent disk. The absorption intensity of the peaks was calculated using the base-line method. The resultant spectra were analyzed using ORIGIN 8.0 software (Thermo Nicolet, USA).
Circular dichroism (CD)
The molecular conformations and denaturation temperature (Td) of collagen were assessed by CD using a spectropolarimeter (Jasco J-810, Shanghai, China) according to the method of Cao et al. (2013) with modification. Briefly, collagen samples were dissolved in 0.1 M acetic acid to obtain a final concentration of 0.1 mg/mL and were stirred for 6 h. Then, the sample was placed in a quartz cell with a path length of 10 mm. The spectra were recorded between 190 and 300 nm at 25 °C. The acetic acid spectrum was used as a reference and CD spectra of the samples were obtained after subtracting the reference spectrum.
Antioxidant activity
DPPH radical scavenging assay
DPPH radical scavenging activity was acquired based on the method of Shimada et al. (1992). Briefly, 500 μLof collagen samples (1 mg) were added to 500 μL of 99.5 % ethanol and 125 μL of 99.5 % ethanol containing 0.02 % DPPH. The mixture was kept in the dark at room temperature for 60 min before measuring absorbance at 517 nm. In the blank, the sample was replaced with distilled water (500 μL) and the above procedure was followed. 2.0 mM butylated hydroxyl toluene (BHT) was used as a positive control. A lower absorbance indicated higher DPPH scavenging activity. Radical scavenging activity was calculated as follows:
Reducing power
Collagen samples (1 mg) were mixed with 2.5 mL of phosphate buffer (0.2 M, pH 6.6) and 2.5 mL of 1.0 % potassium ferricyanide and the mixture was incubated at 50 °C for 20 min. Trichloroacetic acid (2.5 mL of a 10 % solution) was added, and the mixture was centrifuged. The supernatant (2.5 mL) was mixed with water (2.5 mL) and 0.1 % ferric chloride (0.5 mL), and the absorbance was measured at 700 nm. Higher absorbance indicated higher reducing power. BHT was used as a reference antioxidant.
Results and discussion
Molecular weight analysis by SDS-PAGE
Protein pattern of the WS cartilage collagen had (α1)3 chains, with molecular weight of approximately 116 KDa and did not contain high MW component, β-chain (Fig. 2). A similar protein pattern for CII obtained from the articular cartilage of human and chicks has been reported in several studies (Lima et al. 1999; Cao et al. 2013). On the basis of the electrophoretic mobilities, the α-chain of the WS collagens hydrolyzed by thermolysin was degraded into smaller peptides with molecular weight ranging from 70 to 20 KDa. As shown in Fig. 2, thermolysin digestion caused the increased migration of the CIIp (37 kDa). Gibson et al. (1983) synthesized collagenous polypeptides with molecular weight of 45 kDa by pepsin digestion and, 53 and 59 kDa by chymotrypsin digestion. Recently, Xi et al. (2009) successfully separated CIIp (28 kDa) from chicken CII by trypsin digestion. In the present study, the SDS-PAGE pattern of the column fraction no 2 clearly indicated that the CIIp was pure with molecular weight of 37 kDa. Therefore, it has been proven that CIIp could be effectively separated from the hydrolysates under the current experiment conditions.
Fig. 2.
SDS-PAGE pattern of type-II collagen, hydrolysates and purified low molecular weight collagenous polypeptide. Lane M:Marker; lane 1: Hydrolysates; lane 2: purified collagenous polypeptide; lane3: type-II collagen
Degree of hydrolysis
Degree of hydrolysis (DH) is defined as the percentage of peptide bonds cleaved during the enzymatic reaction. CII was hydrolyzed with thermolysin in order to produce CIIp with 37 kDa molecular weight. The percentage of degree of hydrolysis after 30 min was 8.37 % at 70 °C (Table 3). The pattern of DH for the hydrolysis of CII was closely dependent upon the applied enzyme concentration (namely the E/S ratio), time, pH and enzyme type. Gimenez et al. (2009) reported that the degree of hydrolysis of Giant squid gelatin with alcalase was 43–49 % after 3 h. In this study, rapid hydrolysis of CII was observed within 30 min for thermolysin. Varying DH between collagens from different species depends on the type of enzyme used and also steps involved in the hydrolysis process (Klompong et al. 2007).
Table 3.
Antioxidant, maxima absorption, degree of hydrolysis and thermal transition temperature of type-II collagen and low molecular weight collagenous polypeptide
| Samples | Maxima Absorption | DSC | DPPH radical scavenging (%) | Reducing power (absorbance at 700 nm) | Degree of hydrolysis (%) | |
|---|---|---|---|---|---|---|
| Denaturation Temp (°C) | Melting Temp (°C) | |||||
| CII | 239.1 | 34.02 | 69.54 | 19.77 | 0.74 | 8.37 |
| CIIp | 210 | 21.50 | 48.35 | 21.85 | 1.27 | |
| BHT | – | – | – | 68.28 % | 0.91 | |
CII-whale shark type II collagen, CIIp- low molecular weight collagenous polypeptide
Amino acid compositions
The extracted CII from WS had high glycine contents (289 residue/1000 residues), which was the primary amino acid followed by alanine and proline (Table 1). This result was in accordance with other fish collagen studies (Kittiphattanabawon et al. 2010; Veeruraj et al. 2013). The purified CIIp had high glycine contents (202 residue/1000 residues) followed by alanine and leucine. Higher contents of alanine, leucine, valine and isoleucine were observed in CIIp compared to CII. It was reported that thermolysin cleaves the adjacent peptide bonds to hydrophobic and/or aromatic amino acids, such as isoleucine, leucine, phenylalanine, tyrosine, and valine (Hernandez-Ledesma et al. 2006).
Table 1.
Amino acid compositions of type-II collagen and low molecular weight collagenous polypeptide (residues/1000 residues)
| Amino acids | CII | CIIp |
|---|---|---|
| Hydroxyproline (Hyp) | 58.03 | 43.0508 |
| Aspartic acid (Asp) | 49.07 | 42.536 |
| Threonine (Thr) | 26.28 | 19.8168 |
| Serine (Ser) | 32.83 | 28.4541 |
| Glutamic acid (Glu) | 87.43 | 78.1177 |
| Proline (Pro) | 98.83 | 79.5766 |
| Glycine (Gly) | 289.84 | 202.963 |
| Alanine (Ala) | 96.51 | 160.7048 |
| Cysteine (Cys) | 2.36 | 5.51649 |
| Valine (Val) | 31.39 | 47.3144 |
| Methionine (Met) | 18.35 | 10.2 |
| Isoleucine (Ile) | 25.70 | 75.7229 |
| Leucine (Leu) | 81.67 | 97.478 |
| Tyrosine (Tyr) | 8.04 | 9.8195 |
| Phenylalanine (Phe) | 28.06 | 29.159 |
| Lysine (Lys) | 25.40 | 27.4756 |
| Arginine (Arg) | 43.12 | 49.669 |
| Total | 1000 | 1000 |
| Imino acid | 155.22 | 121.17 |
CII-whale shark type II collagen, CIIp- low molecular weight collagenous polypeptide
Imino acid content of CII was in the range of 15.5 %, which was relatively higher than that of CIIp (12.1 %). When compared to the present report, a higher imino acid content was observed in skin collagen from brownstripe redsnapper (22 %) and eel fish (20 %) (Jongjareonrak et al. 2005; Veeruraj et al. 2013). However, the present result was similar to that reported for Alantic cod skin collagen (15.4 %) (Duan et al. 2009). Imino acid content of collagen is associated with the fish species habitat, seasonal variations and their feed intake (Kittiphattanabawon et al. 2005). The removal of small peptide fragments and free amino acids during the hydrolysis and purification process likely resulted in the changes in the amino acid composition between CII and CIIp (Xie et al. 2014).
Glycoprotein/carbohydrate content
Based on the standard curve, CIIp had high carbohydrate content (18–27 %) than CII (Table 2). It was evident that the carbohydrate contents of collagen isolated from commercial shark cartilage capsules were between 5 and 40 % (Merly and Smith 2013). Backlund et al. (2002) opined that glycoprotein content of CII is a key element for the recognition of T cell in collagen-induced arthritis models. Recently, Merly and Smith (2013) studied the effect of oral administration of fetal bovine articular cartilage collagen for the treatment of RA. In the present study, the higher content of glycoprotein showed that the purified CIIp could be used as a novel drug to treat RA. However, in-vivo and in-vitro examination of CIIp is required to prove the effect of their immune response against RA for further practical uses.
Table 2.
Glycoprotein/carbohydrate contents of type-II collagen and low molecular weight collagenous polypeptide
| Standard or sample | Mean absorbance | CHO content (%) |
|---|---|---|
| Lysozyme | 0.112 | 0 |
| Bovine serum albumin | 0.16967 | Trace amounts |
| Ovalbumin | 0.39667 | 3.20 |
| Apo-transferrin | 0.468 | 5.80 |
| Fetuin | 3.0733 (10×) | 22.90 |
| Alpha acid glycoprotein | 2.4733 (10×) | 41.40 |
| CII (0.25 mg/ml and 2.5 mg/ml) | 0.128/ 0.406 | 5.03–15.86 |
| CIIp | 0.3715/1.1305 | 18.92–27.68 |
CII-whale shark type II collagen, CIIp- low molecular weight collagenous polypeptide
Maximum absorbance
Maximum absorptions of CII (239.1 nm) from WS cartilage was different to CIIp (210 nm) (Table 3). Edwards et al. (1997) suggested that the functional groups such as C = O, −COOH, CONH2 present in polypeptides chains of collagen is responsible for the maximum absorbance in UV region. In the present study, the whale shark cartilage collagen and polypeptide did not shown absorption peak at 280 nm. This was due to a low tyrosine content (8.0-9.8 residues per 1000 residues), which absorbs UV light at 280 nm (Duan et al. 2009). Kittiphattanabawon et al. (2010) stated that no absorption peak of the collagen at 280 nm indicates the purity of collagen and efficacy of non-collagenous protein removal. This indicates adequate efficiency of the collagen isolation processes used in this study.
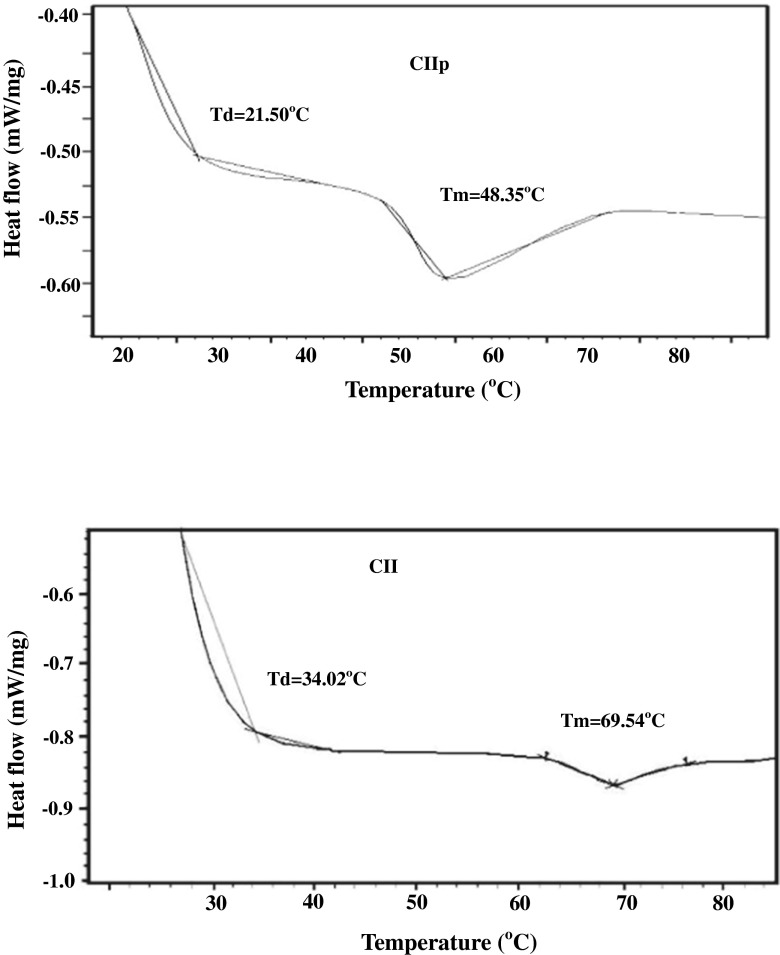
Thermal stability
CII and purified CIIp had two endothermic peaks (Fig. 3). The first one was connected with the thermal denaturation of collagen. The second one was related to the breakage of peptide chains. According to the DSC spectra, the Tm of CIIp and CII were observed at 48 and 69 °C, respectively (Table 3). When compared to the present study, a lower melting temperature (58.07 °C) was observed for type-II collagen isolated from silvertip shark cartilage (Jeevithan et al. 2014b). Hydrolysis may produce a reduced molecular weight collagen and almost certainly reduced the number of inter-chain covalent linkages, leading to reduced Tm of CIIp. Stabilization of collagen helix is more likely to occur primarily by intermolecular rather than intra-molecular association, which thus increases thermal properties (Montero and Gomez-Guillen 2000)
Fig. 3.
Thermal denaturation curves of type-II collagen and low molecular weight collagenous polypeptide. CII- type-II collagen; CIIp-low molecular weight collagenous polypeptide
The Td of CII (34.02 °C) was higher than that of CIIp (21.5 °C). The Td of other fish and calf collagen ranged from 19.4 to 40.8 °C (Merly and Smith 2013; Kittiphattanabawon et al. 2010; Muyonga et al. 2004; Wang et al. 2014). In the present study, the Td of collagen was quite higher than those reported for skin collagen of allied teleosts such as cod (15 °C) and carp (28 °C) (Duan et al. 2009; Sadowska et al. 2003). However, it was lower than that of collagen from eel fish skin (35.0 °C), brownbanded bamboo shark (36.73 °C), porcine skin (37 °C) and chick sternal cartilage (43.8 °C) (Nagai et al. 2008; Veeruraj et al. 2013; Cao et al. 2013; Kittiphattanabawon et al. 2010). Wang et al. (2014) suggested that the difference in thermal stability of collagens from Amur sturgeon skin may be due to the level of hydration and the number and nature of covalent cross-linkages.
The denaturation temperature may be affected by the degree of hydroxylation of the Pro and the Gly-Pro-Hyp sequence in collagen (Kittiphattanabawon et al. 2005). Imino acids content are primarily responsible for the thermal stability and strengthen the triple helix. Wong (1989) opined that the hydroxyl group of the hydroxyproline plays a major role in the stability of the helix through inter-chain hydrogen bonding. Therefore, the high content of hydroxyproline in CII than CIIp also justifies the high Td.
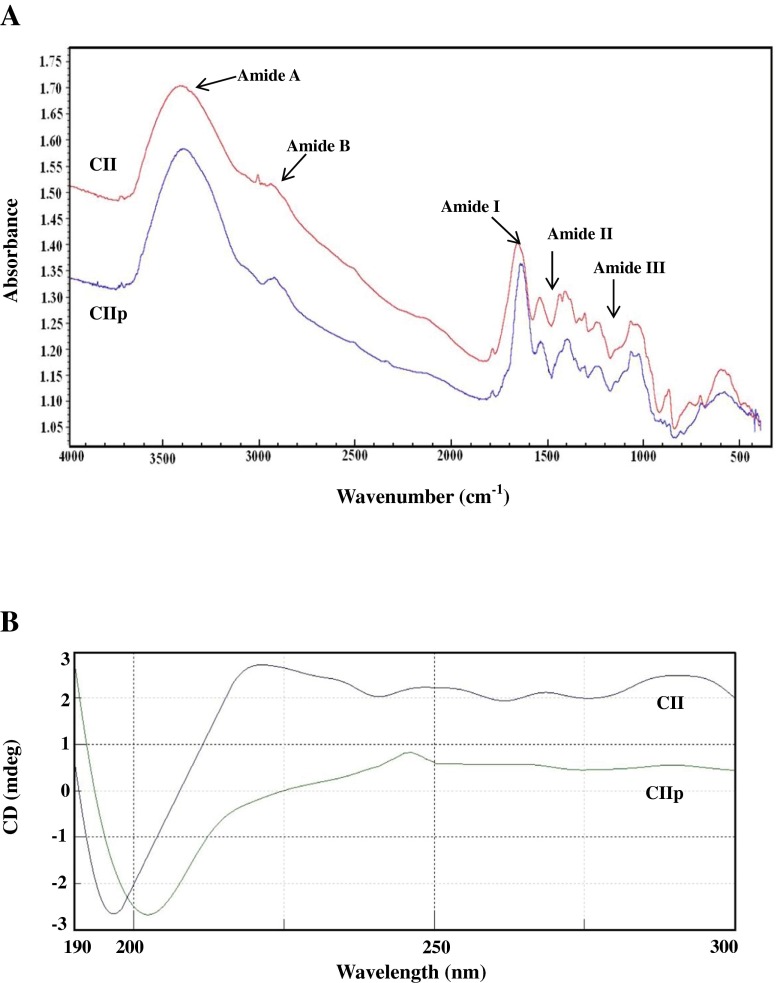
FTIR spectra
The frequencies at which major peaks occurred for CII and CIIp are given in Table 4. Amide-A peak of CII and CIIp was found at 3368.19 and 3393.96 cm−1 respectively (Fig. 4a). This peak is generally associated with N-H stretching coupled with the hydrogen bond of a carbonyl group in a peptide chain. A free N-H stretching vibration occurs in the range of 3400–3440 cm−1 (Veeruraj et al. 2013). Major peaks in the spectra of CII from WS cartilage were similar to others fish collagen (Kittiphattanabawon et al. 2010; Veeruraj et al. 2013). Amide-A peak of CIIp is shifted to lower frequency in CII. When the NH group of a peptide is involved in a hydrogen bond, the position is shifted to lower frequency, usually 3300 cm−1 (Li et al. 2008). Amide-B peak was observed at 2997.15 and 2993.11 cm−1 for CII and CIIp, respectively. This peak represents the asymmetric stretching vibration of = C-H as well as NH3+. Thermolysin hydrolysis had brought major shifts in amide-B peaks of CIIp towards lower frequency due to the changes in the C = O, C-H and NH2 stretching in the collagen molecules.
Table 4.
General peak assignments of the FTIR spectra of type-II collagen and low molecular weight collagenous polypeptide
| Peak wavenumber (cm−1) | ||
|---|---|---|
| CII | CIIp | Assignment |
| 3368.19 | 3393.96 | Amide A: NH stretch coupled with hydrogen bond |
| 2997.15 | 2993.11 | Amide B: CH2 asymmetrical stretch |
| 1796.63 | 1794.30 | Carbonyl C = O stretch: lipids |
| 1666.05 | 1651.14 | Amide I: C = O stretch/hydrogen bond coupled with COO- |
| 1550.78 | 1546.40 | Amide II: NH bend coupled with CN stretch |
| 1448.43 | 1437.78 | CH2 bend |
| 1343.93 | 1316.09 | CH2 wag of proline |
| 1254.45 | 1254.59 | Amide III: NH bend coupled with CN stretch |
| 1132.99 | – | CO O C asymmetric stretch: glycogen and nucleic acids |
| 1117.25 | 1118.05 | O-H bend of glycoprotein |
| 1075.81 | 1074.89 | C-O stretch |
| 877.10 | 875.75 | Skeletal stretch |
| 605.27 | 586.71 | Skeletal stretch |
CII-whale shark type II collagen, CIIp- low molecular weight collagenous polypeptide
Fig. 4.
Fourier transform infrared (a) and Circular dichroism (b) spectra of type-II collagen and low molecular weight collagenous polypeptide. CII- type-II collagen; CIIp-low molecular weight collagenous polypeptide
Amide-I peak was observed at 1666.05 and 1651.14 cm−1 for CII and CIIp, respectively. In the present study, the Amide-I peak of CII was similar to that of other reported fish collagens (Muyonga et al. 2004; Veeruraj et al. 2013; Wang et al. 2014). Amide-I region is mainly used for the analysis of the secondary structure of proteins (Muyonga et al. 2004). The amide-I vibration mode is primarily due to the C = O stretching vibration of the peptide linkages (approximately 80 %). The shift of the amide-I peak to a lower frequency occurred in CIIp compared to CII, which was due to alteration in the triple helical structure by C = O stretching vibration of the peptide linkages. The characteristic peak of the amide-II region of CII and CIIp was observed at 1550.78 and 1546.40 cm−1, respectively. Amide-II vibration modes are attributed to the N-H in-plane bend (40–60 %) and the C-N stretching vibration (18–40 %). Muyonga et al. (2004) also observed the amide II peak at 1540–1558 cm−1 for Nile perch skin collagen. Therefore, FTIR spectra clearly indicated that the triple helix structure, molecular order and intermolecular cross-linkages of CII and CIIp varied based on their amino acid composition (Wang et al. 2014).
CD spectra
CD spectra can be readily used to estimate the fraction of a molecule that is in the alpha-helix conformation, the beta-sheet conformation, the beta turn conformation, or some other (e.g., random coil) conformation (Whitmore and Wallace 2008). CD spectra of CII and CIIp exhibited a rotatory maximum at 222 and 246.5 nm, a minimum at 197 and 202.5 nm and a crossover point at 207 and 218 nm, respectively (Fig. 4b). Generally, native collagen exhibits two typical spectra (the negative spectra is at 198 nm and the positive spectra is at 221 nm, respectively), which is characteristic of triple helical protein conformation (Whitmore and Wallace 2008). The maximum and minimum rotatory CD spectra reported for eel skin collagen were 230 and 204 nm, respectively (Veeruraj et al. 2013). Triple helical structure of collagen molecule is more stable with higher imino acid content as these facilitate intra- and inter-molecular crosslinking. In the present study, CD spectrum with lower intensity was observed for CIIp, which was due to partial or complete denaturation of collagen (He et al. 2012).
Antioxidant activity
In the present study, the protective abilities of CII and CIIp against oxidation were examined with the DPPH radical scavenging capacity and the reducing power. The DPPH radical scavenging rate is 19.77 % and 21.85 % for CII and CIIp, respectively, which is lower than that of BHT (68 %) (Table 3). According to the literature, the DPPH radical scavenging rate of collagenous polypeptide was lower than those of protein hydrolysates from Spanish mackerel skin (47.82 kDa, 35.82 %), hemp (48 kDa, 23 %) and rapeseed (3–10 kDa,45 %), but higher than those of protein hydrolysates from abalone foot muscle (14 %), and scallop adductor muscle (10 %), respectively (Chi et al. 2014; He et al. 2013; Tang et al. 2009; Zhou et al. 2012). In the present study, CIIp exhibited higher scavenging activity than CII. This was due to the peptide group of CIIp, which are electron donors that can react with free radicals to convert them to more stable products and terminate the radical chain reaction (Chi et al. 2014).
The reducing power is determined by the ability of collagen to reduce ferric into ferrous ions. Similar to the DDPH activity, the reducing power was higher in CIIp than in CII, with a value of 1.27 and 0.74 at 700 nm, respectively (Table 3), which was higher than those (0.08–1.24) of the protein hydrolysates from chickpea and hemp (Li et al. 2008; Tang et al. 2009). Interestingly, the reducing power of CIIp was higher than BHT (0.91). From the above results, it was declared that the reducing power of the CIIp obtained by thermolysin was higher than CII. It is thought that collagen can inactivate reactive oxygen species, reduce hydroperoxides, enzymatically eliminate specific oxidants, chelate pro-oxidative transition metals and scavenge free radicals, which may contribute to their antioxidant activities (Zhu et al. 2012). Moreover, certain specific amino acid residues and their sequences are thought to be responsible for antioxidant activities (Mendis et al. 2005). In the present study, higher content of hydrophobic amino acids (valine, leucine and isoleucine) and glycoprotein content in CIIp than that of CII also supports the superior antioxidant activity of CIIp.
Conclusion
In the present study, the relationship between CII and CIIp and their physico-functional properties were examined. The FTIR and CD spectra of CII and CIIp were not similar in term of their primary and secondary structure. The higher content of hydrophobic amino acids and glycoprotein content of purified CIIp were expected to contribute to its antioxidant activity. Based on these results, this new collagen polypeptide (37 kDa) obtained from thermolysin hydrolysis may be considered potential alternative biomaterial to synthetic antioxidants and can find applications in the food and could also be used as a novel drug to treat RA. However, much more work is warranted and will be necessary to clarify the therapeutic effects of CIIp on established CIA, in addition to the suppressive effects.
Acknowledgments
This work received financial support from National High Technology Research and Development Program of China (No. 2011AA09070109) and National Natural Science Foundation of China (No. 81341082).
Contributor Information
Elango Jeevithan, Phone: +86-15216836553, Email: srijeevithan@gmail.com.
Wenhui Wu, Phone: +86-21-61900388, Email: whwu@shou.edu.cn.
References
- Adler-Nissen J. Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid. J Agric Food Chem. 1979;27:1256–1262. doi: 10.1021/jf60226a042. [DOI] [PubMed] [Google Scholar]
- Backlund J, Treschow A, Bockermann R, Holm B, Holm L, Issazadeh-Navikas S. Glycosylation of type-II collagen is of major importance for T cell tolerance and pathology in collagen-induced arthritis. Eur J Immunol. 2002;32:3776–3784. doi: 10.1002/1521-4141(200212)32:12<3776::AID-IMMU3776>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Bergman I, Loxley R. Two improved and simplified methods for the spectrophotometric determination of hydroxyproline. Anal Chem. 1963;35:1961–1965. doi: 10.1021/ac60205a053. [DOI] [Google Scholar]
- Cao H, Shi FX, Xu F, Yu JS. Molecular structure and physicochemical properties of pepsin-solubilized type-II collagen from the chick sternal cartilage. Eur Rev Med Pharmacol Sci. 2013;17:1427–1437. [PubMed] [Google Scholar]
- Chen L, Bao B, Wang N, Xie J, Wu WH. Oral administration of shark type-II collagen suppresses complete Freund’s adjuvant-induced rheumatoid arthritis in rats. Pharm. 2012;5:339–352. doi: 10.3390/ph5040339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi CF, Cao ZH, Wang B, Hu FY, Li ZR, Zhang B. Antioxidant and functional properties of collagen hydrolysates from Spanish mackerel skin as influenced by average molecular weight. Molecules. 2014;19:11211–11230. doi: 10.3390/molecules190811211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan R, Zhang J, Du X, Yao X, Konno K. Properties of collagen from skin scale and bone of carp (Cyprinuscarpio) Food Chem. 2009;112:702–706. doi: 10.1016/j.foodchem.2008.06.020. [DOI] [Google Scholar]
- Edwards HGM, Farwell DW, Holder JM, Lawson EE. Fourier transform Ramanspectroscopy of ivory: II Spectroscopic analysis and assignments. J Mol Str. 1997;435:49–58. doi: 10.1016/S0022-2860(97)00122-1. [DOI] [Google Scholar]
- Ehrlich H. Cartilage of marine vertebrates. Biol Mater Mar Orig. 2015;4:69–89. [Google Scholar]
- Gelse K, Poschl E, Aigner T. Collagens-structure, function, and biosynthesis. Adv Drug Deliv Rev. 2003;55:1531–1546. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Gibson GJ, Kielty CM, Garner C, Schor SL, Grant ME. Identification and partial characterization of three low-molecular-weight collagenous polypeptides synthesized by chondrocytes cultured within collagen gels in the absence and in the presence of fibronectin. Biochem J. 1983;211:417–426. doi: 10.1042/bj2110417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez B, Gomez-Estaca J, Aleman A, Gómez-Guillen MC, Montero MP. Improvement of the antioxidant properties of squid skin gelatin films by the addition of hydrolysates from squid gelatin. Food Hydrocoll. 2009;23:1322–1327. doi: 10.1016/j.foodhyd.2008.09.010. [DOI] [Google Scholar]
- He L, Cai S, Wu B, Mu C, Zhang G, Lin W. Trivalent chromium and aluminum affect the thermostability and conformation of collagen very differently. J Inorg Biochem. 2012;117:124–130. doi: 10.1016/j.jinorgbio.2012.08.017. [DOI] [PubMed] [Google Scholar]
- He R, Girgih AT, Malomo SA, Ju X, Aluko RE. Antioxidant activities of enzymatic rapeseed protein hydrolysates and the membrane ultrafiltration fractions. J Funct Foods. 2013;5:219–227. doi: 10.1016/j.jff.2012.10.008. [DOI] [Google Scholar]
- Hernandez-Ledesma B, Ramos M, Recio I, Amigo L. Effect of β-lactoglobulin hydrolysis with thermolysin under denaturing temperatures on the release of bioactive peptides. J Chromatogr A. 2006;1116(1–2):31–37. doi: 10.1016/j.chroma.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Jeevithan E, Bao B, Bu Y, Zhou Y, Zhao Q, Wu WH (2014a) Type II collagen and gelatin from silvertip shark (carcharhinus albimarginatus) cartilage: isolation purification physicochemical and antioxidant properties. Mar Drugs 12:3852–3873 [DOI] [PMC free article] [PubMed]
- Jeevithan E, Wu WH, Wang N, He L, Bao B. Isolation, purification and characterization of pepsin soluble collagen isolated from silvertip shark (Carcharhinus albimarginatus) skeletal and head bone. Process Biochem. 2014;49:1767–1777. doi: 10.1016/j.procbio.2014.06.011. [DOI] [Google Scholar]
- Jeevithan E, Jingyi Z, Wang N, He L, Bao B, Wu WH (2015) Physico-chemical, antioxidant and intestinal absorption properties of whale shark type-II collagen based on its solubility with acid and pepsin. Process Biochem doi:10.1016/j.procbio.2014.11.015
- Jongjareonrak A, Benjakul S, Visessanguan W, Nagai T, Tanaka M. Isolation and characterisation of acid and pepsin-solubilised collagens from the skin of brownstripe red snapper (Lutjanusvitta) Food Chem. 2005;93:475–484. doi: 10.1016/j.foodchem.2004.10.026. [DOI] [Google Scholar]
- Kittiphattanabawon P, Benjakul S, Visessanguan W, Nagai T, Tanaka M (2005). Characterisation of acid-soluble collagen from skin and bone of bigeyesnapper (Priacanthustayenus). Food Chem 89: 363–372
- Kittiphattanabawon P, Benjakul S, Visessanguan W, Shahidi F. Isolation and characterization of collagen from the cartilages of brownbanded bamboo shark (Chiloscylliumpunctatum) and blacktip shark (Carcharhinuslimbatus) LWT Food Sci Technol. 2010;43:792–800. doi: 10.1016/j.lwt.2010.01.006. [DOI] [Google Scholar]
- Klompong V, Benjakul S, Kantachote D, Shahidi F. Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem. 2007;102:1317–1327. doi: 10.1016/j.foodchem.2006.07.016. [DOI] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li Y, Jiang B, Zhang T, Mu W, Liu J. Antioxidant and free radical-scavenging activities of chickpea protein hydrolysate (CPH) Food Chem. 2008;106:444–450. doi: 10.1016/j.foodchem.2007.04.067. [DOI] [Google Scholar]
- Lima OC, Figueiredo CC, Pereira BAS, Coelho MGP, Morandi V, Lopes-Bezerra LM. Adhesion of the human pathogen Sporothrixschenckii to several extracellular matrix proteins. Braz J Med Biol Res. 1999;32:651–657. doi: 10.1590/S0100-879X1999000500020. [DOI] [PubMed] [Google Scholar]
- Lundin K, Holmgren S. The occurrence and distribution of peptide- or 5-HT-containing nerves in the swimbladder of four different species of teleosts (Gadus morhua, Ctenolabrus rupestris, Anguilla anguilla, Salmo gairdneri) Cell Tissue Res. 1989;257:641–647. doi: 10.1007/BF00221475. [DOI] [Google Scholar]
- Mendis E, Rajapakse N, Byun HG, Kim SK. Investigation of jumbo squid (Dosidicusgigas) skin gelatin peptides for their in vitro antioxidant effects. Life Sci. 2005;77:2166–2178. doi: 10.1016/j.lfs.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Merly L, Smith SL. Collagen type-II alpha 1 protein: a bioactive component of shark cartilage. Int Immunopharmacol. 2013;15:309–315. doi: 10.1016/j.intimp.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Montero P, Gomez-Guillen MC. Extracting conditions for megrim (Lepidorhombus boscii) skin collagen affect functional properties of the resulting. J Food Sci. 2000;65:434–438. doi: 10.1111/j.1365-2621.2000.tb16022.x. [DOI] [Google Scholar]
- Muyonga JH, Cole CGB, Duodu KG. Characterisation of acid soluble collagen from skins of young and adult Nile perch (Latesniloticus) Food Chem. 2004;85:81–89. doi: 10.1016/j.foodchem.2003.06.006. [DOI] [Google Scholar]
- Myers LK, Sakurai Y, Rosloniec EF, Stuart JM, Kang AH. An analog peptide that suppresses collagen-induced arthritis. Am J Med Sci. 2004;327:212–216. doi: 10.1097/00000441-200404000-00007. [DOI] [PubMed] [Google Scholar]
- Nagai T, Suzuki N, Nagashima T. Collagen from common minke whale (Balaenopteraacutorostrata) unesu. Food Chem. 2008;111:296–301. doi: 10.1016/j.foodchem.2008.03.087. [DOI] [PubMed] [Google Scholar]
- Rochdi A, Foucat L, Renou JP. NMR and DSC studies during thermal denaturation of collagen. Food Chem. 2000;69:295–299. doi: 10.1016/S0308-8146(99)00267-8. [DOI] [Google Scholar]
- Ronnestad I, Murashita K, Kottra G, Jordal AE, Narawane S, Jolly C, et al. Molecular cloning and functional expression of atlantic salmon peptide transporter 1 in Xenopus oocytes reveals efficient intestinal uptake of lysine-containing and other bioactive Di- and tripeptides in teleost. Fish J Nutr. 2010;140:893–900. doi: 10.3945/jn.109.118240. [DOI] [PubMed] [Google Scholar]
- Sadowska M, Kolodziejska I, Niecikowska C. Isolation of collagen from the skins of Baltic cod (Gadusmorhua) Food Chem. 2003;81:257–262. doi: 10.1016/S0308-8146(02)00420-X. [DOI] [Google Scholar]
- Schmid TM, Conrad HE. A unique low molecular weight collagen secreted by cultured chick embryo chondrocytes. J Biol Chem. 1982;257:12444–12450. [PubMed] [Google Scholar]
- Shimada K, Fujikawa K, Yahara K, Nakamura T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cycloextrin emulsion. J Agric Food Chem. 1992;40:945–948. doi: 10.1021/jf00018a005. [DOI] [Google Scholar]
- Tang CH, Wang XS, Yang XQ. Enzymatic hydrolysis of hemp (Cannabis sativaL.) protein isolate by various proteases and antioxidant properties of the resulting hydrolysates. Food Chem. 2009;114:1484–1490. doi: 10.1016/j.foodchem.2008.11.049. [DOI] [Google Scholar]
- Veeruraj A, Arumugam M, Balasubramanian T. Isolation and characterization ofthermostable collagen from the marine eel-fish (Evenchelysmacrura) Process Biochem. 2013;48:1592–1602. doi: 10.1016/j.procbio.2013.07.011. [DOI] [Google Scholar]
- Wang L, Liang Q, Chen T, Wang Z, Xu J, Ma H. Characterization of collagen from the skin of Amur sturgeon (Acipenserschrenckii) Food Hydrocolloids. 2014;38:104–109. doi: 10.1016/j.foodhyd.2013.12.002. [DOI] [Google Scholar]
- Whitmore L, Wallace BA. Protein secondary structure analyses from circular dichroism spectroscopy: methods and reference databases. Biopolymer. 2008;89:392–400. doi: 10.1002/bip.20853. [DOI] [PubMed] [Google Scholar]
- Wong DWS. Mechanism and theory in food chemistry. New York: Van Nostrand Reinhold Company Inc; 1989. p. 428. [Google Scholar]
- Xi C, Tan L, Sun Y, Liang F, Liu N, Xue H, et al. A novel recombinant peptide containing only two T-cell tolerance epitopes of chicken type-II collagen that suppresses collagen-induced arthritis. Mol Immunol. 2009;46:729–737. doi: 10.1016/j.molimm.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Xie J, Ye HY, Luo XF. An efficient preparation of chondroitin sulfate and collagen peptides from shark cartilage. Int Food Res J. 2014;21:1171–1175. [Google Scholar]
- Zhou DY, Tang Y, Zhu BW, Qin L, Li DM, Yang JF, et al. Antioxidant activity of hydrolysates obtained from scallop (Patinopectenyessoensis) and abalone (Haliotis discus HannaiIno) muscle. Food Chem. 2012;132:815–822. doi: 10.1016/j.foodchem.2011.11.041. [DOI] [Google Scholar]
- Zhu B, Dong X, Zhou D, Gao Y, Yang J, Li D, et al. Physicochemical properties and radical scavenging capacities of pepsin-solubilized collagen from sea cucumber (Stichopus japonicas) Food Hydrocolloids. 2012;28:182–188. doi: 10.1016/j.foodhyd.2011.12.010. [DOI] [Google Scholar]