Abstract
Adipose tissue plays a central role in the control of energy balance as well as in the maintenance of metabolic homeostasis. It was not until recently that the first evidences of the role of heat shock protein (Hsp) 90 and high molecular weight immunophilin FKBP51 have been described in the process of adipocyte differentiation. Recent reports describe their role in the regulation of PPARγ, a key transcription factor in the control of adipogenesis and the maintenance of the adipocyte phenotype. In addition, novel roles have been uncovered for FKBP51 in the organization of the architecture of the nucleus through its participation in the reorganization of the nuclear lamina. Therefore, the aim of this review is to integrate and discuss the recent advances in the field, with special emphasis on the roles of Hsp90 and FKBP51 in the process of adipocyte differentiation.
Keywords: adipogenesis, FKBP51, Hsp90, nuclear lamina, PPARγ
Abbreviations
- ALDO
aldosterone
- Gelda
geldanamycin
- CCNB1
cyclin B1
- CyP
cyclophilin
- DEXA
dexamethasone
- EPAC
exchange proteins activated by cAMP
- FKBP
FK506 binding protein
- GR
glucocorticoid receptor
- Hsp
heat shock protein
- IBMX
3-isobutyl-1-methylxanthine
- IMMs
immunophilins
- LMNA
lamin A/C gene
- MEF-51 KO
mouse embryonic fibroblasts null for FKBP51
- MR
mineralocorticoid receptor
- NE
nuclear envelope
- NL
nuclear lamina
- NRs
nuclear receptors
- PP5
protein phosphatase 5
- PHLPP
PH domain leucine-rich repeat protein phosphatase
- PML
promyelocytic bodies
- PPARs
peroxisome proliferator-activated receptors
- PPIase
peptidyl-prolylisomerase
- RelA
NF-κB subunit p65
- RelB
NF-κB subunit p68
- TPR
tetratricopeptide repeat motif
- WISp
WAF-1/CIP1 stabilizing protein
- XAP2/ARA9
hepatitis virus B X-associated protein 2 /AhR-associated protein 9
Introduction
There is no doubt that adipose tissue plays a central role not only in the regulation of energy balance and lipid homeostasis but also in the homeostasis of whole body metabolism through the release of active molecules, generically called adipokines that signal to key organs such as the brain, liver, skeletal muscle, and the immune system.1-3 Therefore, the adipose tissue is not just a mere deposit of lipids but an active endocrine organ. Different aspects of adipose tissue functions appear to be modulated by the location of the adipose depot (visceral vs. subcutaneous vs. bone marrow);4,5 by the size of the average adipocyte in the tissue;6 by cross-talks between adipocytes and other cell types present in this tissue, such as macrophages;7,8 as well as by adipocyte metabolism of glucose9 and corticosteroids.10-12 In obese individuals, the secretion of adipokines is deregulated13 and adipose tissue is generally hypertrophic and infiltrated by a higher number of macrophages compared to normal tissue,7 events that correlate with measures of adiposity and insulin resistance,14-16 and the establishment of a state of chronic inflammation.13 However, a very recent report shows that up to a certain level, proinflammatory signaling is necessary in the adipocyte for the adequate remodeling and expansion of the adipose tissue.17 Conversely, lipodystrophy, a disorder characterized by selective total or partial loss of body fat, is also accompanied by similar metabolic consequences as seen in obesity, including insulin resistance, dyslipidemia, hepatic and myocellular steatosis, and increased risk for diabetes and atherosclerosis,18,19 reinforcing the notion that adipose tissue plays a key role in the control of whole body metabolism homeostasis.
Great effort has been done to uncover the factors that control not only adipogenesis but also those that exert control in the function of the adipose cell itself. It is well established that glucocorticoids and mineralocorticoids are key regulators not only of fat distribution, but also of adipocyte differentiation,20-24 the induction of lipogenic genes and lypolisis in adipocytes25,26 and are potent inhibitors of adipose tissue inflammatory response.27 Corticosteroids exert their action by binding to their receptors, the glucocorticoid- and mineralocorticoid receptor (GR and MR, respectively) that are present in the cytoplasm (Fig. 1C). For proper steroid hormone action, GR and MR need to be part of a heterocomplex with the 90-kDa and 70-kDa heat shock proteins, Hsp90 and Hsp70, respectively, the acidic protein p23, and a protein that belongs to the conserved and large family known as immunophilins (IMMs).28,29 Among the members of the IMMs family, FK506 binding protein (FKBP)52, FKBP51, Cyclophilin (CyP) 40, and 3 IMM-like proteins, protein phosphatase 5 (PP5), hepatitis virus B X-associated protein 2 /AhR-associated protein 9 (XAP2/ARA9), and WAF-1/CIP1 stabilizing protein (WISp) 39 have been recovered to date in steroid receptor•Hsp90 complexes.28,30 A great body of evidence sustains the role of glucocorticoids and mineralocorticoids actions through their binding to GR and MR in adipose tissue biology.11,12 However, it was not until recently that studies started to appear demonstrating the role of the chaperones and co-chaperones of the nuclear receptors (NRs) in the process of adipogenesis, and the aim of this review is to discuss these new findings.
Hsp90 participates in the control of PPARγ
Hsp90 accounts for 1–2% of the total soluble proteins in resting cells, ∼6–7% in cancer cells and up to 10% in stressed cells.31–33 There are 2 major cytoplasmic isoforms, Hsp90α (inducible form) and Hsp90β (constitutive form); Hsp90N, which is associated with cellular transformation; and Hsp90 analogs that include Grp94 (94-kDa glucose-regulated protein) in the endoplasmic reticulum and Hsp75/TRAP1 (tumor necrosis factor-associated protein1) in the mitochondrial matrix.34,35 Genome analysis revealed that the human Hsp90 family includes 17 genes34,36. In most cells, Hsp90α expression is lower compared to Hsp90β, and its inducible transcription is tightly regulated by the 5´upstream promoter sequences containing several heat shock elements (HSE).34 The heat shock responsive transcription factor HSF binds to HSE to control Hsp90 expression.34,37 In addition, members of the signal transducers and activators of transcription family, STAT1 and STAT3, in complex with HSF1 also participate in the control of the heat shock induction of Hsp90α gene transcription.38 In regard to adipogenesis, we have recently shown that no change is observed in the protein expression level of Hsp90 during the differentiation of 3T3-L1 preadipocytes.39 It remains to be explored whether there are differences in the expression of the different Hsp90s during adipogenesis and whether they are deregulated in obesity due to the functional importance of this chaperone in response to cell stress.
Hsp90 is a molecular chaperone that associates with numerous substrate proteins called “clients” in order to modulate their folding and function, among them protein kinases and transcription factors, including GR and MR already mentioned.40-42 In this manner, Hsp90 controls metastable proteins that are regulatory hubs in biological networks. Peroxisome proliferator-activated receptors (PPARs) are members of the NR superfamily of ligand-dependent transcription factors. Three subtypes of this receptor have been found, PPARα, -β/δ and -γ, controlling target genes involved in cell growth, differentiation and apoptosis in a variety of cells. Of these NRs, PPARγ has been proven to be a master regulator of adipogenesis.43,44 PPARγ as well as PPARβ/δ interact with Hsp90, albeit to a lesser extent than PPARα.45 Hsp90 inhibition by geldanamycin leads to the increase of PPARα and –β/δ transcriptional capacity, being proposed that Hsp90 is a repressor of both transcription factors.45 PPARα•Hsp90 complexes interact with XAP2, and XAP2 appears to function as a repressor based on the observation that expression of XAP2 inhibits PPARα transcriptional capacity in reporter gene assays.46
As already mentioned, PPARγ is an Hsp90 client protein.45,47 Inhibition of Hsp90 by treatment of 3T3-L1 cells with geldanamycin or its analogs at early time points of the adipogenic process has been shown to prevent the cells from differentiating properly.47,48 In fact, disruption of the PPARγ•Hsp90 complex by geldanamycin targets PPARγ to degradation by the proteasome, being thus proposed that the anti-adipogenic effect of geldanamycin may result from the destabilization of PPARγ (Fig. 1D).47 Since Hsp90 is indispensable for proper GR and MR function, inhibition of Hsp90 also inhibits proper adipogenesis by interfering with GR and MR actions.48 Hsp90 is essential for a wide spectrum of cellular processes such as protein folding, protein degradation, and signal transduction cascades,40,49 having been recently shown that Hsp90 also participates in the maintenance of RNA polymerase II pausing, function required for adequate gene expression when cells have to respond to environmental stimuli.50 Therefore, the blockade of the adipogenic program upon Hsp90 inhibition could be the resultant of a wider disruption of signaling pathways as well as nuclear events dependent on Hsp90 surveillance that need to be further explored.
Figure 1.
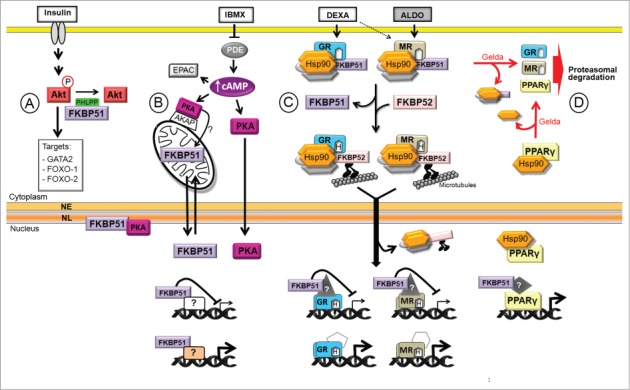
Model of Hsp90 and FKBP51 functions in adipogenesis. The adipogenic media contains insulin, 3-isobutyl-1-methylxanthine (IBMX), dexamethasone (DEXA), and is supplemented with fetal bovine serum that contains aldosterone (ALDO) among many other hormones. (A) Insulin activates many signaling pathways, among them Akt that phosphorylates GATA2 and FOXO-1 and -2, transcription factors that are excluded from the nucleus. (B) IBMX increases cAMP level leading to PKA activation that triggers the translocation of FKBP51 from mitochondria to the nucleus, possibly upon changes in its phosphorylation status. FKBP51 interacts with lamin B in the NL (nuclear lamina) modulating NL reorganization at the onset of adipogenesis. In addition, FKBP51 regulates GR-, MR- and PPARγ-target genes, and possibly other genes. (C) Upon DEXA and ALDO binding to GR and/or MR, FKBP51 is exchanged for FKBP52 facilitating the retrograde movement of the NRs toward the nucleus where they bind to target genes and control their expression. (D) Hsp90 protects PPARγ from degradation. Gelda: geldanamycin, an Hsp90 inhibitor; NE: nuclear envelope.
High molecular weight immunophilins in adipocyte differentiation
IMMs comprise a family of proteins classified by their ability to bind immunosuppressant drugs in which cyclophilins bind cyclosporine A, whereas FKBPs bind FK506. The high molecular weight IMMs FKBP51 and FKBP52 do not play a role in immunosuppression, but rather have been related to steroid receptor regulation.51 The FKBPs are modular proteins that possess FKBP12-like peptidyl-prolyl isomerase (PPIase) domains 1 and 2 (FK1 and FK2) and a tetratricopeptide repeat motif (TPR). The FK1 domain is required for the binding of the immunosuppressive drug FK506, it confers PPIase activity, and it is also the primary domain required for steroid hormone receptor regulation.51–53 The FK2 domain links the FK1 with the TPR domain, lacks detectable PPIase activity and is required in FKBP51 but not in FKBP52 for their interaction with the progesterone receptor heterocomplexes.54 The TPR domain contains sequences of 34 amino acids repeated in tandem through which FKBPs interact with Hsp90. FKBP51 and FKBP52 share 60% identity and 70% similarity; however the former has been so far mainly reported to be a negative regulator of steroid hormone receptors while the latter is a positive one.51,53,55-58 When differentiation of 3T3-L1 preadipocytes is induced, it was reported that FKBP51 had a transient expression at very early time points (day 1 up to day 4 of differentiation) and then its expression decreased to undetectable protein levels.59 More recent studies demonstrate that FKBP51 and FKBP52 exhibit opposite changes in their level of expression during the process of adipocyte differentiation. FKBP51 expression progressively increases whereas FKBP52 decreases as adipogenesis proceeds.39,60 The differences observed between these studies may possibly depend on the development of highly sensitive and specific antibodies now available for the study of these IMMs. Importantly, the changes in level of expression of both IMMs during 3T3-L1 preadipocyte differentiation are in agreement with the high expression of FKBP51 and low levels of FKBP52 in white adipose tissue (J.T. and GPP unpublished results, and61. The organization of the Fkbp51 and Fkbp52 genes has been described, showing that hormone regulatory elements lie within intronic sequences distal to the promoter.62-64 Expression of FKBP51 is strongly enhanced by glucocorticoids,65-67 progestins,68,69 and androgens,70,71 while FKBP52 mRNA increases in response to estrogen and heat stress.72,73 It remains to be further explored how their expression is modulated in the adipose tissue, and whether they are differentially expressed in pathophysiological conditions like metabolic syndrome or obesity.
FKBP51 shuttles from mitochondria to the nucleus in a PKA-dependent manner at the onset of adipocyte differentiation
FKBP51 is present in the cytoplasm and mitochondria,74 and upon oxidative stress the mitochondrial fraction of this IMM rapidly translocates to the nucleus protecting cells from apoptosis.74 When 3T3-L1 preadipocytes are induced to differentiate, FKBP51 also rapidly and transiently translocates from mitochondria to the nucleus.39 Adipogenesis is controlled by many signaling pathways that coordinately modulate the sequential activation of transcription factors required for cells to differentiate.75 We found that IBMX (3-isobutyl-1-methylxanthine), a phosphodiesterase inhibitor that increases intracellular cAMP, and to a lesser extent DEXA, are responsible for the rapid relocalization of mitochondrial FKBP51 to the nucleus (Fig. 1B).39 Several reports have shown that the second messenger cAMP is associated with immediate events of adipogenesis by the classic PKA signaling pathway, as well as by the non-classical pathway of the exchange proteins activated by cAMP (EPAC), which function as guanine nucleotide exchange factor for the Ras-like small GTPases Rap1 and Rap2.76-79 FKBP51 nuclear translocation depends on PKA but not on EPAC pathway activation, demonstrating a differential role of PKA and EPAC/Rap during adipogenesis.39
FKBP51 interacts with PKA-cα as shown by immunoprecipitation assays, and when PKA signaling is blocked dramatic changes in the electrophoretic pattern of migration of FKBP51 are observed, supporting the notion that FKBP51 is a PKA substrate.39 By using NetPhosk 1.0, we found that Serine 312 located in the TPR domain of FKBP51, is a candidate PKA phospho-acceptor site. The TPR domain confers to the IMM the ability to bind Hsp90 through the EEVD motif present in the extreme C terminus of the chaperone. FKBP51 localization in mitochondria depends on TPR integrity, since FKBP51 TPR-deficient mutants are constitutively nuclear.74 Therefore, changes in phosphorylation of Serine 312 present in the TPR domain of FKBP51 may possibly regulate its interaction with Hsp90 and consequently its subcellular localization, possibility that is under current investigation. Interestingly, when the interaction of FKBP51 with Hsp90 is disrupted by Hsp90 inhibitors such as radicicol, FKBP51 is no longer in mitochondria and concentrates in the nucleus.74 As mentioned already, geldanamycin and radicicol inhibit 3T3-L1 preadipocytes differentiation;47,48 therefore it is possible that the Hsp90 inhibitors not only affect PPARγ, GR and MR function, but they may also alter the dynamic mitochondrial-nuclear shuttling of FKBP51 at the onset of the differentiation process required for adipogenesis to proceed, resulting in the inhibition of adipogenesis.
During the past few years, several studies revealed a dramatic and dynamic modulation of the chromatin landscape during the first hours of adipocyte differentiation.80-84 These changes coincide with cooperative binding of early adipogenic transcription factors, including GR, to enhancers and promoters of many genes.82,83 However, genes such as PPARγ are not transcriptionally activated until later in adipogenesis, and it has been proposed that the activation of additional factors and/or signals is required for their later activation.83 It can be speculated that, in spite of chromatin relaxation and the increased binding of transcription factors at the early stages of adipogenesis, gene expression is kept controlled by factors that restrain the transcriptional capacity of complexes already bound to those sites. When adipogenesis is triggered, FKBP51 translocates to the nucleus and its interaction with GR progressively increases, rendering a GR less transcriptionally active.39 It is possible that the presence of FKBP51 in the nucleus at the onset of adipogenesis may be critical for the control not only of GR but also for MR. It has recently been shown FKBP51 impairs both the nuclear translocation rate of NF-κB and its transcriptional activity.58 Interestingly, NF-κB subunits p65 (RelA), p68 (RelB) and IκB increase their level of expression during the process of adipocyte differentiation.85 It was reported that endotoxin sensitivity of the classical NF-κB pathway is substantially delayed and attenuated despite increased overall inflammatory response in adipocytes.85 Thus, we hypothesize that FKBP51, whose level of expression increases as adipogenesis proceeds, may also modulate NF-κB pathway in mature adipocytes. Future studies will demonstrate the existence of other transcription factors that need to be repressed or activated by nuclear FKBP51, at a step of the adipogenic program in which high level of chromatin remodeling takes place and transcription needs to be kept controlled.
Role of FKBP51 in the control of PPARγ
It has been recently demonstrated that FKBP51 interacts with over-expressed PPARγ in COS7 cells, and reporter gene assays shows that FKBP51 is a positive regulator of this NR.60 PPARγ like other NRs can be regulated by changes in its phosphorylation status. MAPK ERK1/2, and JNK are able to phosphorylate PPARγ at Serine 112 reducing its transcriptional capacity.86-88 Furthermore, inhibition of p38MAPK increases PPARγ expression and its transcriptional activity.89 GR is also a substrate of p38MAPK, post-translational modification that increases GR transcriptional capacity.90 Then, MAPK–dependent phosphorylation of PPARγ and GR has opposite effects on the transcriptional capacities of these NRs: PPARγ transactivation decreases while GR transactivation increases. FKBP51 is a scaffold protein for the interaction between the protein kinase Akt and the PH domain leucine-rich repeat protein phosphatase (PHLPP) that specifically dephosphorylates the hydrophobic motif of Akt (Serine 473 in Akt1), thus inhibiting the kinase activity (Fig. 1A).91 Stechschulte et al. showed that in mouse embryonic fibroblasts null for FKBP51 (MEF-51KO) elevated Akt activity leads to increased activation of p38MAPK that is able to phosphorylate GR and PPARγ, promoting transcriptional activation of the former and inhibition of the latter.60 Moreover, they show that knock down of FKBP51 in 3T3-L1 preadipocytes makes cells resistant to differentiation and MEF-51KO have impaired differentiation.60 The authors proposed a model, in which FKBP51 restrains Akt activation by scaffolding PHLPP, favoring the inactive state of p38MAPK that prevents PPARγ phosphorylation and keeps this NR in a transcriptionally active state to induce the expression of the adipogenic genes.60 While the role of p38MAPK in adipogenesis is still rather controversial,89,92-94 several lines of evidence demonstrate that Akt is required for proper adipogenesis. Akt is a key component of insulin signaling and is required for PPARγ expression.75,95 Over-expression of constitutively active Akt induces spontaneous differentiation of 3T3-L1 preadipocytes,96 and mice null for Akt1 and Akt2 have impaired adipogenesis.95 Akt is responsible for phosphorylation and nuclear exclusion of anti-adipogenic factors such as the forkhead proteins FOXO-197 and FOXO-2,98 and the transcription factor GATA2.99 Therefore, proper activation of Akt is required for normal adipogenesis and it could be speculated that Akt inhibition by the FKBP51-PHLPP could have a negative effect on this process. In line with this possibility, Toneatto et al. showed that knock down of FKBP51 favors the process of adipogenesis and its overexpression blocks 3T3-L1 preadipocyte differentiation, based on the fact that this IMM also restrains the adipogenic potential of GR,39 and possibly the pro-adipogenic action of MR. It is possible that the discrepancies between these studies could result, in part, from differences in the protocol of adipogenesis used in each case, as well as differences in the level of expression of FKBP51 (knock out vs. knock down), thus more research work is required to shed light on this conundrum.
FKBP51 and the nuclear lamina reorganization at the onset of adipogenesis
The nucleus is organized in highly dynamic nuclear compartments which correspond to the nuclear lamina that lies below the nuclear envelope, the nuclear matrix or nucleoskeleton, the chromosome territories that comprise the volume of the nucleus in interphase occupied by each chromosome, the interchromatin domain, and nuclear bodies that include the nucleolus, spliceosomes or nuclear speckles, paraspeckles, the Cajal bodies, the promyelocytic (PML) bodies, and transcription factories, among others.100-103 A great body of evidence demonstrates that dynamic changes in the nuclear compartments take place during the process of cell differentiation, including adipogenesis.104-107 It has been shown that the repositioning of genes from repressive to transcriptionally favorable nuclear compartments and vice versa plays a key role for their proper expression or repression.108-115 In other words, we need to understand how the architecture of the nucleus is delineated to uncover how the cell modifies the pattern of gene expression required for the acquisition and maintenance of the final phenotype.
The nuclear lamina (NL) is a filamentous protein mesh-work that lines the nucleoplasmic surface of the nuclear envelope (NE) interacting with inner nuclear membrane proteins and the nuclear pores116,117 (reviewed in118,119.) It consists of a polymeric assembly of lamins, members of the type V intermediate filament protein family120 that correspond to the A-type (LA and LC) and the B-type lamins (LB1 and LB2), respectively. LA and LC are derived from a single gene by alternative splicing and are expressed only in differentiated cells. The NL is thought to provide a structural framework for the NE contributing to the size, shape and mechanical stability of the nucleus. It also provides anchoring site for interphase chromosomes at the nuclear periphery, and plays important roles in DNA replication and repair, RNA polymerase II transcription, and the epigenetic control of chromatin remodeling.121,122 The functional importance of the NL is demonstrated by the fact mutations in the lamin A/C (LMNA) gene or in the FACE-1 gene that affects the correct posttranslational processing of prelamin A are responsible for a group of genetic diseases known as laminopathies.122-124 It has been proposed that mutations that affect lamins might disrupt their binding to yet unidentified tissue-specific partner proteins to generate pathology in a particular tissue (reviewed in125.) Laminopathies affecting the adipose tissue are characterized by lipodystrophies with selective and variable loss of adipose tissue, accompanied by metabolic complications including insulin resistance, type 2 diabetes, hypertriglyceridemia, and liver steatosis. These laminopathies include Dunnigan-type familial partial lipodystrophy and partial lipodystrophy with mandibuloacral dysplasia, both associated with mutations in LMNA gene; congenital generalized lipodystrophy, also known as Berardinelli-Seip syndrome; and some cases of Barraquer-Simons syndrome with acquired partial lipodystrophy associated with mutations in lamin B2.126 Lipodystrophy can also be acquired, as occurs with the lipodystrophy associated with the use of anti-viral drugs in patients infected with human immunodeficiency virus.127
Analysis of the expression level of lamin A and the NE transmembrane protein emerin at the onset of differentiation of 3T3F442A preadipocytes showed that while lamin A expression progressively decreases, emerin expression increases.128 Emerin participates in the control of β-catenin129 whose sustained activation inhibits the process of adipogenesis.130 Increased expression of emerin could control the efficient redistribution of β-catenin from the nucleus to the cytoplasm facilitating its proteasomal degradation and consequently allowing the process of adipocyte differentiation to proceed.128 Interestingly, it was demonstrated that the NL is fragmented at the early stages of adipogenesis, event that is accompanied by the loss not only of lamin A, but also C, B1, and emerin at the nuclear rim.131 Later on upon maturation of the adipose cell (day 18 post-induction of adipogenesis) lamins A, C and B1 increase at the nuclear rim independently of the low levels of lamins A/C protein.131 In contrast, lamin B2 remained constant at the NL throughout the process of adipogenesis.131 Since the NL participates in the control of many aspects of nuclear events as already described, it was proposed that the decreased presence of most lamin subtypes at the nuclear rim and the fragmentation of the NL results in enhanced plasticity of the nucleus as adipogenesis proceeds.131 FKBP51 translocates from mitochondria to the nucleus at the onset of adipogenesis and, not only co-localizes with lamin B in the fragmented pattern of the lamina, but also interacts with lamin B.39 Interestingly, PKA-cα also translocates to the nucleus, and concentrates in the NL possibly through its interaction with FKBP51.39 Several phosphorylation sites, including those for the cyclin B1-(CCNB1)-CDC2 complex, PKC and PKA are important in nuclear lamina disassembly.132,133 Therefore, we propose that enrichment of PKA-cα in the NL may facilitate its reorganization by phosphorylation of lamins during the process of adipogenesis. It can be speculated that the accumulation of PKA-cα in the NL may be also involved in the control of gene expression at the onset of adipogenesis possibly by regulating the phosphorylation of transcription factors enriched in this nuclear compartment as shown for the control of AP-1 transcriptional activity upon the sequestration of c-fos in the NL in an ERK1/2 dependent manner.134
FKBP51 and FKBP51 knock out animal models
To uncover the functional importance of these IMM, knockout mice were generated.51 Fkbp51-deficient mice were initially observed to display no overt phenotype, but these mice are less vulnerable to the detrimental effects of stress.135-137 Interestingly, Fkbp51 knockout mice showed reduced body weight compared to wild type littermates; however, upon expose to chronic stress, these animals exhibited a significant increase in body weight,137 results that suggest that the process of adipogenesis might not be impaired in the absence of FKBP51. It has been recently reported by Balsevich et al. a differential spatial pattern of Fkbp51 gene induction in different areas of the brain dependent on either diet or stress conditions. In mice exposed to high-fat diet, Fkbp51 is induced in the ventromedial hypothalamic nuclei, in accordance with the hypothalamus being involved in the control of energy balance.138 In contrast, under conditions of chronic stress, the expression of this IMM increases in the hippocampus, area of the brain involved in the response to stress.138 Inasmuch as environmental stress is another risk factor for the development of obesity,139 future studies are needed to uncover the role of FKBP51 in different areas of the brain, whether this IMM plays a role in the control of appetite and energy balance, and whether FKBP51 is implicated in the relationship between control of energy, metabolic homeostasis and stress response. On the other hand, Fkbp52-deficient male mice display phenotypes related to partial androgen insensitivity syndrome.140,141 Heterozygous Fkbp52-deficient mice show increased susceptibility to high fat-diet-induced hyperglycemia and hyperinsulinemia that correlates with reduced insulin clearance, hepatic steatosis and glucocorticoid resistance.61 Fkbp51-Fkbp52 double knockout results in embryonic lethality,51 indicating that these IMMs have some physiological functional redundancies that need to be uncovered by tissue-specific conditional double knockouts.
Final remarks
Undoubtedly during the last decade, great progress has been accomplished in the understanding of the complex biology of the adipose tissue, the pathophysiology of obesity and its role in metabolic syndrome. However, many aspects of the physiology of the adipocyte need to be explored further in depth, including how chaperones, such as Hsp90 and Hsp70, and co-chaperones, such as FKBP51 and FKBP52, may directly or indirectly coordinate the action of signaling pathways and transcription factor complexes function. Their study will not only enrich our basic knowledge but also will possibly be crucial for the design of new therapeutic strategies for the treatment of obesity, lipodystrophies and metabolic problems associated with these pathologies.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are very grateful to Cecilia Galigniana for her assistance in graphics design.
Funding
This work was supported by a grant from Agencia Nacional de Promoción Científica y Tecnológica (PICT2012–2612 to GPP, and PICT2013–1745 to JT). NLC is a recipient of a doctoral fellowship from CONICET.
References
- 1.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 2004; 89:2548-56; PMID:15181022; http://dx.doi.org/ 10.1210/jc.2004-0395 [DOI] [PubMed] [Google Scholar]
- 2.Schwartz MW, Porte D Jr. Diabetes, obesity, and the brain. Science 2005; 307:375-9; PMID:15662002; http://dx.doi.org/ 10.1126/science.1104344 [DOI] [PubMed] [Google Scholar]
- 3.Olefsky JM. Fat talks, liver and muscle listen. Cell 2008; 134:914-6; PMID:18805083; http://dx.doi.org/ 10.1016/j.cell.2008.09.001 [DOI] [PubMed] [Google Scholar]
- 4.Das M, Gabriely I, Barzilai N. Caloric restriction, body fat and ageing in experimental models. Obes Rev 2004; 5:13-9; PMID:14969503; http://dx.doi.org/ 10.1111/j.1467-789X.2004.00115.x [DOI] [PubMed] [Google Scholar]
- 5.Cawthorn WP, Scheller EL, Learman BS, Parlee SD, Simon BR, Mori H, Ning X, Bree AJ, Schell B, Broome DT, et al.. Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab 2014; 20:368-75; PMID:24998914; http://dx.doi.org/ 10.1016/j.cmet.2014.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weyer C, Wolford JK, Hanson RL, Foley JE, Tataranni PA, Bogardus C, Pratley RE. Subcutaneous abdominal adipocyte size, a predictor of type 2 diabetes, is linked to chromosome 1q21–q23 and is associated with a common polymorphism in LMNA in Pima Indians. Mol Genet Metab 2001; 72:231-8; PMID:11243729; http://dx.doi.org/ 10.1006/mgme.2001.3147 [DOI] [PubMed] [Google Scholar]
- 7.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003; 112:1796-808; PMID:14679176; http://dx.doi.org/ 10.1172/JCI200319246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, Mukundan L, Brombacher F, Locksley RM, Chawla A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature 2011; 480:104-8; PMID:22101429; http://dx.doi.org/ 10.1038/nature10653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abel ED, Peroni O, Kim JK, Kim YB, Boss O, Hadro E, Minnemann T, Shulman GI, Kahn BB. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature 2001; 409:729-33; PMID:11217863; http://dx.doi.org/ 10.1038/35055575 [DOI] [PubMed] [Google Scholar]
- 10.Masuzaki H, Paterson J, Shinyama H, Morton NM, Mullins JJ, Seckl JR, Flier JS. A transgenic model of visceral obesity and the metabolic syndrome. Science 2001; 294:2166-70; PMID:11739957; http://dx.doi.org/ 10.1126/science.1066285 [DOI] [PubMed] [Google Scholar]
- 11.Lee MJ, Pramyothin P, Karastergiou K, Fried SK. Deconstructing the roles of glucocorticoids in adipose tissue biology and the development of central obesity. Biochim Biophys Acta 2014; 1842:473-81; PMID:23735216; http://dx.doi.org/ 10.1016/j.bbadis.2013.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marzolla V, Armani A, Zennaro MC, Cinti F, Mammi C, Fabbri A, Rosano GM, Caprio M. The role of the mineralocorticoid receptor in adipocyte biology and fat metabolism. Mol Cell Endocrinol 2012; 350:281-8; PMID:21945603; http://dx.doi.org/ 10.1016/j.mce.2011.09.011 [DOI] [PubMed] [Google Scholar]
- 13.Cao H. Adipocytokines in obesity and metabolic disease. J Endocrinol 2014; 220:T47-59; PMID:24403378; http://dx.doi.org/ 10.1530/JOE-13-0339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes 2007; 56:16-23; PMID:17192460; http://dx.doi.org/ 10.2337/db06-1076 [DOI] [PubMed] [Google Scholar]
- 15.Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes 2008; 57:3239-46; PMID:18829989; http://dx.doi.org/ 10.2337/db08-0872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest 2011; 121:2111-7; PMID:21633179; http://dx.doi.org/ 10.1172/JCI57132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wernstedt Asterholm I, Tao C, Morley TS, Wang QA, Delgado-Lopez F, Wang ZV, Scherer PE. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab 2014; 20:103-18; PMID:24930973; http://dx.doi.org/ 10.1016/j.cmet.2014.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garg A, Agarwal AK. Lipodystrophies: disorders of adipose tissue biology. Biochim Biophys Acta 2009; 1791:507-13; PMID:19162222; http://dx.doi.org/ 10.1016/j.bbalip.2008.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krahmer N, Farese RV Jr., Walther TC. Balancing the fat: lipid droplets and human disease. EMBO Mol Med 2013; 5:905-15; PMID:23740690; http://dx.doi.org/ 10.1002/emmm.201100671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hauner H, Entenmann G, Wabitsch M, Gaillard D, Ailhaud G, Negrel R, Pfeiffer EF. Promoting effect of glucocorticoids on the differentiation of human adipocyte precursor cells cultured in a chemically defined medium. J Clin Invest 1989; 84:1663-70; PMID:2681273; http://dx.doi.org/ 10.1172/JCI114345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaillard D, Wabitsch M, Pipy B, Negrel R. Control of terminal differentiation of adipose precursor cells by glucocorticoids. J Lipid Res 1991; 32:569-79; PMID:1649886 [PubMed] [Google Scholar]
- 22.Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev 1998; 78:783-809; PMID:9674695 [DOI] [PubMed] [Google Scholar]
- 23.Rondinone CM, Rodbard D, Baker ME. Aldosterone stimulated differentiation of mouse 3T3-L1 cells into adipocytes. Endocrinology 1993; 132:2421-6; PMID:8504747 [DOI] [PubMed] [Google Scholar]
- 24.Caprio M, Feve B, Claes A, Viengchareun S, Lombes M, Zennaro MC. Pivotal role of the mineralocorticoid receptor in corticosteroid-induced adipogenesis. Faseb J 2007; 21:2185-94; PMID:17384139; http://dx.doi.org/ 10.1096/fj.06-7970com [DOI] [PubMed] [Google Scholar]
- 25.Pedersen SB, Jonler M, Richelsen B. Characterization of regional and gender differences in glucocorticoid receptors and lipoprotein lipase activity in human adipose tissue. J Clin Endocrinol Metab 1994; 78:1354-9; PMID:8200937 [DOI] [PubMed] [Google Scholar]
- 26.Peckett AJ, Wright DC, Riddell MC. The effects of glucocorticoids on adipose tissue lipid metabolism. Metabolism 2011; 60:1500-10; PMID:21864867; http://dx.doi.org/ 10.1016/j.metabol.2011.06.012 [DOI] [PubMed] [Google Scholar]
- 27.Patsouris D, Neels JG, Fan W, Li PP, Nguyen MT, Olefsky JM. Glucocorticoids and thiazolidinediones interfere with adipocyte-mediated macrophage chemotaxis and recruitment. J Biol Chem 2009; 284:31223-35; PMID:19740750; http://dx.doi.org/ 10.1074/jbc.M109.041665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pratt WB, Galigniana MD, Morishima Y, Murphy PJ. Role of molecular chaperones in steroid receptor action. Essays Biochem 2004; 40:41-58; PMID:15242338 [DOI] [PubMed] [Google Scholar]
- 29.Smith DF, Toft DO. Minireview: the intersection of steroid receptors with molecular chaperones: observations and questions. Mol Endocrinol 2008; 22:2229-40; PMID:18451092; http://dx.doi.org/ 10.1210/me.2008-0089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKeen HD, McAlpine K, Valentine A, Quinn DJ, McClelland K, Byrne C, O'Rourke M, Young S, Scott CJ, McCarthy HO, et al.. A novel FK506-like binding protein interacts with the glucocorticoid receptor and regulates steroid receptor signaling. Endocrinology 2008; 149:5724-34; PMID:18669603; http://dx.doi.org/ 10.1210/en.2008-0168 [DOI] [PubMed] [Google Scholar]
- 31.Welch WJ, Feramisco JR. Purification of the major mammalian heat shock proteins. J Biol Chem 1982; 257:14949-59; PMID:7174676 [PubMed] [Google Scholar]
- 32.Lai BT, Chin NW, Stanek AE, Keh W, Lanks KW. Quantitation and intracellular localization of the 85K heat shock protein by using monoclonal and polyclonal antibodies. Mol Cell Biol 1984; 4:2802-10; PMID:6396506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nollen EA, Morimoto RI. Chaperoning signaling pathways: molecular chaperones as stress-sensing ‘heat shock’ proteins. J Cell Sci 2002; 115:2809-16; PMID:12082142 [DOI] [PubMed] [Google Scholar]
- 34.Sreedhar AS, Kalmar E, Csermely P, Shen YF. Hsp90 isoforms: functions, expression and clinical importance. FEBS Lett 2004; 562:11-5; PMID:15069952; http://dx.doi.org/ 10.1016/S0014-5793(04)00229-7 [DOI] [PubMed] [Google Scholar]
- 35.Johnson JL. Evolution and function of diverse Hsp90 homologs and cochaperone proteins. Biochim Biophys Acta 2012; 1823:607-13; PMID:22008467; http://dx.doi.org/ 10.1016/j.bbamcr.2011.09.020 [DOI] [PubMed] [Google Scholar]
- 36.Chen B, Piel WH, Gui L, Bruford E, Monteiro A. The HSP90 family of genes in the human genome: insights into their divergence and evolution. Genomics 2005; 86:627-37; PMID:16269234; http://dx.doi.org/ 10.1016/j.ygeno.2005.08.012 [DOI] [PubMed] [Google Scholar]
- 37.Shen Y, Liu J, Wang X, Cheng X, Wang Y, Wu N. Essential role of the first intron in the transcription of hsp90beta gene. FEBS Lett 1997; 413:92-8; PMID:9287123; http://dx.doi.org/ 10.1016/S0014-5793(97)00883-1 [DOI] [PubMed] [Google Scholar]
- 38.Chen XS, Zhang Y, Wang JS, Li XY, Cheng XK, Zhang Y, Wu NH, Shen YF. Diverse effects of Stat1 on the regulation of hsp90alpha gene under heat shock. J Cell Biochem 2007; 102:1059-66; PMID:17427945; http://dx.doi.org/ 10.1002/jcb.21342 [DOI] [PubMed] [Google Scholar]
- 39.Toneatto J, Guber S, Charo NL, Susperreguy S, Schwartz J, Galigniana MD, Piwien-Pilipuk G. Dynamic mitochondrial-nuclear redistribution of the immunophilin FKBP51 is regulated by the PKA signaling pathway to control gene expression during adipocyte differentiation. J Cell Sci 2013; 126:5357-68; PMID:24101724; http://dx.doi.org/ 10.1242/jcs.125799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taipale M, Jarosz DF, Lindquist S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat Rev Mol Cell Biol 2010; 11:515-28; PMID:20531426; http://dx.doi.org/ 10.1038/nrm2918 [DOI] [PubMed] [Google Scholar]
- 41.Taipale M, Krykbaeva I, Koeva M, Kayatekin C, Westover KD, Karras GI, Lindquist S. Quantitative analysis of HSP90-client interactions reveals principles of substrate recognition. Cell 2012; 150:987-1001; PMID:22939624; http://dx.doi.org/ 10.1016/j.cell.2012.06.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Erlejman AG, Lagadari M, Toneatto J, Piwien-Pilipuk G, Galigniana MD. Regulatory role of the 90-kDa-heat-shock protein (Hsp90) and associated factors on gene expression. Biochim Biophys Acta 2014; 1839:71-87; PMID:24389346; http://dx.doi.org/ 10.1016/j.bbagrm.2013.12.006 [DOI] [PubMed] [Google Scholar]
- 43.Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem 2008; 77:289-312; PMID:18518822; http://dx.doi.org/ 10.1146/annurev.biochem.77.061307.091829 [DOI] [PubMed] [Google Scholar]
- 44.Lefterova MI, Haakonsson AK, Lazar MA, Mandrup S. PPARgamma and the global map of adipogenesis and beyond. Trends Endocrinol Metab 2014; 25:293-302; PMID:24793638; http://dx.doi.org/ 10.1016/j.tem.2014.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sumanasekera WK, Tien ES, Davis JW 2nd, Turpey R, Perdew GH, Vanden Heuvel JP. Heat shock protein-90 (Hsp90) acts as a repressor of peroxisome proliferator-activated receptor-α (PPARalpha) and PPARbeta activity. Biochemistry 2003; 42:10726-35; PMID:12962497; http://dx.doi.org/ 10.1021/bi0347353 [DOI] [PubMed] [Google Scholar]
- 46.Sumanasekera WK, Tien ES, Turpey R, Vanden Heuvel JP, Perdew GH. Evidence that peroxisome proliferator-activated receptor α is complexed with the 90-kDa heat shock protein and the hepatitis virus B X-associated protein 2. J Biol Chem 2003; 278:4467-73; PMID:12482853; http://dx.doi.org/ 10.1074/jbc.M211261200 [DOI] [PubMed] [Google Scholar]
- 47.Nguyen MT, Csermely P, Soti C. Hsp90 chaperones PPARgamma and regulates differentiation and survival of 3T3-L1 adipocytes. Cell Death Differ 2013; 20:1654-63; PMID:24096869; http://dx.doi.org/ 10.1038/cdd.2013.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Desarzens S, Liao WH, Mammi C, Caprio M, Faresse N. Hsp90 blockers inhibit adipocyte differentiation and fat mass accumulation. PLoS One 2014; 9:e94127; PMID:24705830; http://dx.doi.org/ 10.1371/journal.pone.0094127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.da Silva VC, Ramos CH. The network interaction of the human cytosolic 90 kDa heat shock protein Hsp90: A target for cancer therapeutics. J Proteomics 2012; 75:2790-802; PMID:22236519; http://dx.doi.org/ 10.1016/j.jprot.2011.12.028 [DOI] [PubMed] [Google Scholar]
- 50.Sawarkar R, Sievers C, Paro R. Hsp90 globally targets paused RNA polymerase to regulate gene expression in response to environmental stimuli. Cell 2012; 149:807-18; PMID:22579285; http://dx.doi.org/ 10.1016/j.cell.2012.02.061 [DOI] [PubMed] [Google Scholar]
- 51.Sivils JC, Storer CL, Galigniana MD, Cox MB. Regulation of steroid hormone receptor function by the 52-kDa FK506-binding protein (FKBP52). Curr Opin Pharmacol 2011; 11:314-9; PMID:21511531; http://dx.doi.org/ 10.1016/j.coph.2011.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pirkl F, Buchner J. Functional analysis of the Hsp90-associated human peptidyl prolyl cis/trans isomerases FKBP51, FKBP52 and Cyp40. J Mol Biol 2001; 308:795-806; PMID:11350175; http://dx.doi.org/ 10.1006/jmbi.2001.4595 [DOI] [PubMed] [Google Scholar]
- 53.Riggs DL, Roberts PJ, Chirillo SC, Cheung-Flynn J, Prapapanich V, Ratajczak T, Gaber R, Picard D, Smith DF. The Hsp90-binding peptidylprolyl isomerase FKBP52 potentiates glucocorticoid signaling in vivo. Embo J 2003; 22:1158-67; PMID:12606580; http://dx.doi.org/ 10.1093/emboj/cdg108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sinars CR, Cheung-Flynn J, Rimerman RA, Scammell JG, Smith DF, Clardy J. Structure of the large FK506-binding protein FKBP51, an Hsp90-binding protein and a component of steroid receptor complexes. Proc Natl Acad Sci U S A 2003; 100:868-73; PMID:12538866; http://dx.doi.org/ 10.1073/pnas.0231020100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wochnik GM, Ruegg J, Abel GA, Schmidt U, Holsboer F, Rein T. FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. J Biol Chem 2005; 280:4609-16; PMID:15591061; http://dx.doi.org/ 10.1074/jbc.M407498200 [DOI] [PubMed] [Google Scholar]
- 56.Davies TH, Ning YM, Sanchez ER. A new first step in activation of steroid receptors: hormone-induced switching of FKBP51 and FKBP52 immunophilins. J Biol Chem 2002; 277:4597-600; PMID:11751894; http://dx.doi.org/ 10.1074/jbc.C100531200 [DOI] [PubMed] [Google Scholar]
- 57.Gallo LI, Ghini AA, Piwien Pilipuk G, Galigniana MD. Differential recruitment of tetratricorpeptide repeat domain immunophilins to the mineralocorticoid receptor influences both heat-shock protein 90-dependent retrotransport and hormone-dependent transcriptional activity. Biochemistry 2007; 46:14044-57; PMID:18001136; http://dx.doi.org/ 10.1021/bi701372c [DOI] [PubMed] [Google Scholar]
- 58.Erlejman AG, De Leo SA, Mazaira GI, Molinari AM, Camisay MF, Fontana V, Cox MB, Piwien-Pilipuk G, Galigniana MD. NF-kappaB transcriptional activity is modulated by FK506-binding proteins FKBP51 and FKBP52: a role for peptidyl-prolyl isomerase activity. J Biol Chem 2014; 289:26263-76; PMID:25104352; http://dx.doi.org/ 10.1074/jbc.M114.582882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yeh WC, Li TK, Bierer BE, McKnight SL. Identification and characterization of an immunophilin expressed during the clonal expansion phase of adipocyte differentiation. Proc Natl Acad Sci U S A 1995; 92:11081-5; PMID:7479941; http://dx.doi.org/ 10.1073/pnas.92.24.11081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stechschulte LA, Hinds TD Jr., Ghanem SS, Shou W, Najjar SM, Sanchez ER. FKBP51 reciprocally regulates GRalpha and PPARgamma activation via the Akt-p38 pathway. Mol Endocrinol 2014; 28:1254-64; PMID:24933248; http://dx.doi.org/ 10.1210/me.2014-1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Warrier M, Hinds TD Jr., Ledford KJ, Cash HA, Patel PR, Bowman TA, Stechschulte LA, Yong W, Shou W, Najjar SM, et al.. Susceptibility to diet-induced hepatic steatosis and glucocorticoid resistance in FK506-binding protein 52-deficient mice. Endocrinology 2010; 151:3225-36; PMID:20427484; http://dx.doi.org/ 10.1210/en.2009-1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hubler TR, Denny WB, Valentine DL, Cheung-Flynn J, Smith DF, Scammell JG. The FK506-binding immunophilin FKBP51 is transcriptionally regulated by progestin and attenuates progestin responsiveness. Endocrinology 2003; 144:2380-7; PMID:12746298; http://dx.doi.org/ 10.1210/en.2003-0092 [DOI] [PubMed] [Google Scholar]
- 63.Hubler TR, Scammell JG. Intronic hormone response elements mediate regulation of FKBP5 by progestins and glucocorticoids. Cell Stress Chaperones 2004; 9:243-52; PMID:15544162; http://dx.doi.org/ 10.1379/CSC-32R.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scammell JG, Hubler TR, Denny WB, Valentine DL. Organization of the human FK506-binding immunophilin FKBP52 protein gene (FKBP4). Genomics 2003; 81:640-3; PMID:12782134; http://dx.doi.org/ 10.1016/S0888-7543(03)00090-9 [DOI] [PubMed] [Google Scholar]
- 65.Baughman G, Wiederrecht GJ, Chang F, Martin MM, Bourgeois S. Tissue distribution and abundance of human FKBP51, and FK506-binding protein that can mediate calcineurin inhibition. Biochem Biophys Res Commun 1997; 232:437-43; PMID:9125197; http://dx.doi.org/ 10.1006/bbrc.1997.6307 [DOI] [PubMed] [Google Scholar]
- 66.Reynolds PD, Roveda KP, Tucker JA, Moore CM, Valentine DL, Scammell JG. Glucocorticoid-resistant B-lymphoblast cell line derived from the Bolivian squirrel monkey (Saimiri boliviensis boliviensis). Lab Anim Sci 1998; 48:364-70; PMID:10090044 [PubMed] [Google Scholar]
- 67.Vermeer H, Hendriks-Stegeman BI, van der Burg B, van Buul-Offers SC, Jansen M. Glucocorticoid-induced increase in lymphocytic FKBP51 messenger ribonucleic acid expression: a potential marker for glucocorticoid sensitivity, potency, and bioavailability. J Clin Endocrinol Metab 2003; 88:277-84; PMID:12519866; http://dx.doi.org/ 10.1210/jc.2002-020354 [DOI] [PubMed] [Google Scholar]
- 68.Kester HA, van der Leede BM, van der Saag PT, van der Burg B. Novel progesterone target genes identified by an improved differential display technique suggest that progestin-induced growth inhibition of breast cancer cells coincides with enhancement of differentiation. J Biol Chem 1997; 272:16637-43; PMID:9195978; http://dx.doi.org/ 10.1074/jbc.272.26.16637 [DOI] [PubMed] [Google Scholar]
- 69.Richer JK, Jacobsen BM, Manning NG, Abel MG, Wolf DM, Horwitz KB. Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J Biol Chem 2002; 277:5209-18; PMID:11717311; http://dx.doi.org/ 10.1074/jbc.M110090200 [DOI] [PubMed] [Google Scholar]
- 70.Amler LC, Agus DB, LeDuc C, Sapinoso ML, Fox WD, Kern S, Lee D, Wang V, Leysens M, Higgins B, et al.. Dysregulated expression of androgen-responsive and nonresponsive genes in the androgen-independent prostate cancer xenograft model CWR22-R1. Cancer Res 2000; 60:6134-41; PMID:11085537 [PubMed] [Google Scholar]
- 71.Mousses S, Wagner U, Chen Y, Kim JW, Bubendorf L, Bittner M, Pretlow T, Elkahloun AG, Trepel JB, Kallioniemi OP. Failure of hormone therapy in prostate cancer involves systematic restoration of androgen responsive genes and activation of rapamycin sensitive signaling. Oncogene 2001; 20:6718-23; PMID:11709706; http://dx.doi.org/ 10.1038/sj.onc.1204889 [DOI] [PubMed] [Google Scholar]
- 72.Kumar P, Mark PJ, Ward BK, Minchin RF, Ratajczak T. Estradiol-regulated expression of the immunophilins cyclophilin 40 and FKBP52 in MCF-7 breast cancer cells. Biochem Biophys Res Commun 2001; 284:219-25; PMID:11374893; http://dx.doi.org/ 10.1006/bbrc.2001.4952 [DOI] [PubMed] [Google Scholar]
- 73.Mark PJ, Ward BK, Kumar P, Lahooti H, Minchin RF, Ratajczak T. Human cyclophilin 40 is a heat shock protein that exhibits altered intracellular localization following heat shock. Cell Stress Chaperones 2001; 6:59-70; PMID:11525244; http://dx.doi.org/ 10.1379/1466-1268(2001)006%3c0059:HCIAHS%3e2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gallo LI, Lagadari M, Piwien-Pilipuk G, Galigniana MD. The 90-kDa Heat-shock Protein (Hsp90)-binding Immunophilin FKBP51 Is a Mitochondrial Protein That Translocates to the Nucleus to Protect Cells against Oxidative Stress. J Biol Chem 2011; 286:30152-60; PMID:21730050; http://dx.doi.org/ 10.1074/jbc.M111.256610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol 2006; 7:885-96; PMID:17139329; http://dx.doi.org/ 10.1038/nrm2066 [DOI] [PubMed] [Google Scholar]
- 76.Reusch JE, Colton LA, Klemm DJ. CREB activation induces adipogenesis in 3T3-L1 cells. Mol Cell Biol 2000; 20:1008-20; PMID:10629058; http://dx.doi.org/ 10.1128/MCB.20.3.1008-1020.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Petersen RK, Madsen L, Pedersen LM, Hallenborg P, Hagland H, Viste K, Døskeland SO, Kristiansen K. Cyclic AMP (cAMP)-mediated stimulation of adipocyte differentiation requires the synergistic action of Epac- and cAMP-dependent protein kinase-dependent processes. Mol Cell Biol 2008; 28:3804-16; PMID:18391018; http://dx.doi.org/ 10.1128/MCB.00709-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Martini CN, Plaza MV, Vila Mdel C. PKA-dependent and independent cAMP signaling in 3T3-L1 fibroblasts differentiation. Mol Cell Endocrinol 2009; 298:42-7; PMID:19010385; http://dx.doi.org/ 10.1016/j.mce.2008.10.023 [DOI] [PubMed] [Google Scholar]
- 79.Xiao H, Leblanc SE, Wu Q, Konda S, Salma N, Marfella CG, Ohkawa Y, Imbalzano AN. Chromatin accessibility and transcription factor binding at the PPARgamma2 promoter during adipogenesis is protein kinase A-dependent. J Cell Physiol 2011; 226:86-93; PMID:20625991; http://dx.doi.org/ 10.1002/jcp.22308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nielsen R, Pedersen TA, Hagenbeek D, Moulos P, Siersbaek R, Megens E, Denissov S, Børgesen M, Francoijs KJ, Mandrup S, et al.. Genome-wide profiling of PPARgamma:RXR and RNA polymerase II occupancy reveals temporal activation of distinct metabolic pathways and changes in RXR dimer composition during adipogenesis. Genes Dev 2008; 22:2953-67; PMID:18981474; http://dx.doi.org/ 10.1101/gad.501108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lefterova MI, Zhang Y, Steger DJ, Schupp M, Schug J, Cristancho A, Feng D, Zhuo D, Stoeckert CJ Jr, Liu XS, et al.. PPARgamma and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes Dev 2008; 22:2941-52; PMID:18981473; http://dx.doi.org/ 10.1101/gad.1709008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Steger DJ, Grant GR, Schupp M, Tomaru T, Lefterova MI, Schug J, Manduchi E, Stoeckert CJ Jr, Lazar MA. Propagation of adipogenic signals through an epigenomic transition state. Genes Dev 2010; 24:1035-44; PMID:20478996; http://dx.doi.org/ 10.1101/gad.1907110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Siersbaek R, Nielsen R, John S, Sung MH, Baek S, Loft A, Hager GL, Mandrup S. Extensive chromatin remodelling and establishment of transcription factor ‘hotspots’ during early adipogenesis. Embo J 2011; 30:1459-72; PMID:21427703; http://dx.doi.org/ 10.1038/emboj.2011.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Susperreguy S, Prendes LP, Desbats MA, Charo NL, Brown K, MacDougald OA, Kerppola T, Schwartz J, Piwien-Pilipuk G. Visualization by BiFC of different C/EBPbeta dimers and their interaction with HP1alpha reveals a differential subnuclear distribution of complexes in living cells. Exp Cell Res 2011; 317:706-23; PMID:21122806; http://dx.doi.org/ 10.1016/j.yexcr.2010.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Berg AH, Lin Y, Lisanti MP, Scherer PE. Adipocyte differentiation induces dynamic changes in NF-kappaB expression and activity. Am J Physiol Endocrinol Metab 2004; 287:E1178-88; PMID:15251865; http://dx.doi.org/ 10.1152/ajpendo.00002.2004 [DOI] [PubMed] [Google Scholar]
- 86.Hu E, Kim JB, Sarraf P, Spiegelman BM. Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARgamma. Science 1996; 274:2100-3; PMID:8953045; http://dx.doi.org/ 10.1126/science.274.5295.2100 [DOI] [PubMed] [Google Scholar]
- 87.Adams M, Reginato MJ, Shao D, Lazar MA, Chatterjee VK. Transcriptional activation by peroxisome proliferator-activated receptor gamma is inhibited by phosphorylation at a consensus mitogen-activated protein kinase site. J Biol Chem 1997; 272:5128-32; PMID:9030579; http://dx.doi.org/ 10.1074/jbc.272.8.5128 [DOI] [PubMed] [Google Scholar]
- 88.Camp HS, Tafuri SR. Regulation of peroxisome proliferator-activated receptor gamma activity by mitogen-activated protein kinase. J Biol Chem 1997; 272:10811-6; PMID:9099735; http://dx.doi.org/ 10.1074/jbc.272.16.10811 [DOI] [PubMed] [Google Scholar]
- 89.Aouadi M, Laurent K, Prot M, Le Marchand-Brustel Y, Binetruy B, Bost F. Inhibition of p38MAPK increases adipogenesis from embryonic to adult stages. Diabetes 2006; 55:281-9; PMID:16443758; http://dx.doi.org/ 10.2337/diabetes.55.02.06.db05-0963 [DOI] [PubMed] [Google Scholar]
- 90.Miller AL, Webb MS, Copik AJ, Wang Y, Johnson BH, Kumar R, Thompson EB. p38 Mitogen-activated protein kinase (MAPK) is a key mediator in glucocorticoid-induced apoptosis of lymphoid cells: correlation between p38 MAPK activation and site-specific phosphorylation of the human glucocorticoid receptor at serine 211. Mol Endocrinol 2005; 19:1569-83; PMID:15817653; http://dx.doi.org/ 10.1210/me.2004-0528 [DOI] [PubMed] [Google Scholar]
- 91.Pei H, Li L, Fridley BL, Jenkins GD, Kalari KR, Lingle W, Petersen G, Lou Z, Wang L. FKBP51 affects cancer cell response to chemotherapy by negatively regulating Akt. Cancer Cell 2009; 16:259-66; PMID:19732725; http://dx.doi.org/ 10.1016/j.ccr.2009.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Engelman JA, Lisanti MP, Scherer PE. Specific inhibitors of p38 mitogen-activated protein kinase block 3T3-L1 adipogenesis. J Biol Chem 1998; 273:32111-20; PMID:9822687; http://dx.doi.org/ 10.1074/jbc.273.48.32111 [DOI] [PubMed] [Google Scholar]
- 93.Engelman JA, Berg AH, Lewis RY, Lin A, Lisanti MP, Scherer PE. Constitutively active mitogen-activated protein kinase kinase 6 (MKK6) or salicylate induces spontaneous 3T3-L1 adipogenesis. J Biol Chem 1999; 274:35630-8; PMID:10585441; http://dx.doi.org/ 10.1074/jbc.274.50.35630 [DOI] [PubMed] [Google Scholar]
- 94.Hata K, Nishimura R, Ikeda F, Yamashita K, Matsubara T, Nokubi T, Yoneda T. Differential roles of Smad1 and p38 kinase in regulation of peroxisome proliferator-activating receptor gamma during bone morphogenetic protein 2-induced adipogenesis. Mol Biol Cell 2003; 14:545-55; PMID:12589053; http://dx.doi.org/ 10.1091/mbc.E02-06-0356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Peng XD, Xu PZ, Chen ML, Hahn-Windgassen A, Skeen J, Jacobs J, Sundararajan D, Chen WS, Crawford SE, Coleman KG, et al.. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev 2003; 17:1352-65; PMID:12782654; http://dx.doi.org/ 10.1101/gad.1089403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Magun R, Burgering BM, Coffer PJ, Pardasani D, Lin Y, Chabot J, Sorisky A. Expression of a constitutively activated form of protein kinase B (c-Akt) in 3T3-L1 preadipose cells causes spontaneous differentiation. Endocrinology 1996; 137:3590-3; PMID:8754791 [DOI] [PubMed] [Google Scholar]
- 97.Nakae J, Kitamura T, Kitamura Y, Biggs WH 3rd, Arden KC, Accili D. The forkhead transcription factor Foxo1 regulates adipocyte differentiation. Dev Cell 2003; 4:119-29; PMID:12530968; http://dx.doi.org/ 10.1016/S1534-5807(02)00401-X [DOI] [PubMed] [Google Scholar]
- 98.Wolfrum C, Shih DQ, Kuwajima S, Norris AW, Kahn CR, Stoffel M. Role of Foxa-2 in adipocyte metabolism and differentiation. J Clin Invest 2003; 112:345-56; PMID:12865419; http://dx.doi.org/ 10.1172/JCI18698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Menghini R, Marchetti V, Cardellini M, Hribal ML, Mauriello A, Lauro D, Sbraccia P, Lauro R, Federici M. Phosphorylation of GATA2 by Akt increases adipose tissue differentiation and reduces adipose tissue-related inflammation: a novel pathway linking obesity to atherosclerosis. Circulation 2005; 111:1946-53; PMID:15837948; http://dx.doi.org/ 10.1161/01.CIR.0000161814.02942.B2 [DOI] [PubMed] [Google Scholar]
- 100.Ho CY, Lammerding J. Lamins at a glance. J Cell Sci 2012; 125:2087-93; PMID:22669459; http://dx.doi.org/ 10.1242/jcs.087288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nickerson J. Experimental observations of a nuclear matrix. J Cell Sci 2001; 114:463-74; PMID:11171316 [DOI] [PubMed] [Google Scholar]
- 102.Cremer T, Zakhartchenko V. Nuclear architecture in developmental biology and cell specialisation. Reprod Fertil Dev 2011; 23:94-106; PMID:21366985; http://dx.doi.org/ 10.1071/RD10249 [DOI] [PubMed] [Google Scholar]
- 103.Chung I, Osterwald S, Deeg KI, Rippe K. PML body meets telomere: the beginning of an ALTernate ending? Nucleus 2012; 3:263-75; PMID:22572954; http://dx.doi.org/ 10.4161/nucl.20326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Koehler D, Zakhartchenko V, Froenicke L, Stone G, Stanyon R, Wolf E, Cremer T, Brero A. Changes of higher order chromatin arrangements during major genome activation in bovine preimplantation embryos. Exp Cell Res 2009; 315:2053-63; PMID:19254712; http://dx.doi.org/ 10.1016/j.yexcr.2009.02.016 [DOI] [PubMed] [Google Scholar]
- 105.Solovei I, Kreysing M, Lanctot C, Kosem S, Peichl L, Cremer T, Guck J, Joffe B. Nuclear architecture of rod photoreceptor cells adapts to vision in mammalian evolution. Cell 2009; 137:356-68; PMID:19379699; http://dx.doi.org/ 10.1016/j.cell.2009.01.052 [DOI] [PubMed] [Google Scholar]
- 106.Kuroda M, Tanabe H, Yoshida K, Oikawa K, Saito A, Kiyuna T, Mizusawa H, Mukai K. Alteration of chromosome positioning during adipocyte differentiation. J Cell Sci 2004; 117:5897-903; PMID:15537832; http://dx.doi.org/ 10.1242/jcs.01508 [DOI] [PubMed] [Google Scholar]
- 107.Szczerbal I, Foster HA, Bridger JM. The spatial repositioning of adipogenesis genes is correlated with their expression status in a porcine mesenchymal stem cell adipogenesis model system. Chromosoma 2009; 118:647-63; PMID:19585140; http://dx.doi.org/ 10.1007/s00412-009-0225-5 [DOI] [PubMed] [Google Scholar]
- 108.Brown KE, Guest SS, Smale ST, Hahm K, Merkenschlager M, Fisher AG. Association of transcriptionally silent genes with Ikaros complexes at centromeric heterochromatin. Cell 1997; 91:845-54; PMID:9413993; http://dx.doi.org/ 10.1016/S0092-8674(00)80472-9 [DOI] [PubMed] [Google Scholar]
- 109.Brown KE, Baxter J, Graf D, Merkenschlager M, Fisher AG. Dynamic repositioning of genes in the nucleus of lymphocytes preparing for cell division. Mol Cell 1999; 3:207-17; PMID:10078203; http://dx.doi.org/ 10.1016/S1097-2765(00)80311-1 [DOI] [PubMed] [Google Scholar]
- 110.Volpi EV, Chevret E, Jones T, Vatcheva R, Williamson J, Beck S, Campbell RD, Goldsworthy M, Powis SH, Ragoussis J, et al.. Large-scale chromatin organization of the major histocompatibility complex and other regions of human chromosome 6 and its response to interferon in interphase nuclei. J Cell Sci 2000; 113 (Pt 9):1565-76; PMID:10751148 [DOI] [PubMed] [Google Scholar]
- 111.Kosak ST, Skok JA, Medina KL, Riblet R, Le Beau MM, Fisher AG, Singh H. Subnuclear compartmentalization of immunoglobulin loci during lymphocyte development. Science 2002; 296:158-62; PMID:11935030; http://dx.doi.org/ 10.1126/science.1068768 [DOI] [PubMed] [Google Scholar]
- 112.Taddei A, Van Houwe G, Hediger F, Kalck V, Cubizolles F, Schober H, Gasser SM. Nuclear pore association confers optimal expression levels for an inducible yeast gene. Nature 2006; 441:774-8; PMID:16760983; http://dx.doi.org/ 10.1038/nature04845 [DOI] [PubMed] [Google Scholar]
- 113.Sexton T, Schober H, Fraser P, Gasser SM. Gene regulation through nuclear organization. Nat Struct Mol Biol 2007; 14:1049-55; PMID:17984967; http://dx.doi.org/ 10.1038/nsmb1324 [DOI] [PubMed] [Google Scholar]
- 114.Meister P, Towbin BD, Pike BL, Ponti A, Gasser SM. The spatial dynamics of tissue-specific promoters during C. elegans development. Genes Dev 2010; 24:766-82; PMID:20395364; http://dx.doi.org/ 10.1101/gad.559610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bickmore WA, van Steensel B. Genome architecture: domain organization of interphase chromosomes. Cell 2013; 152:1270-84; PMID:23498936; http://dx.doi.org/ 10.1016/j.cell.2013.02.001 [DOI] [PubMed] [Google Scholar]
- 116.Fawcett DW. On the occurrence of a fibrous lamina on the inner aspect of the nuclear envelope in certain cells of vertebrates. Am J Anat 1966; 119:129-45; PMID:6007824; http://dx.doi.org/ 10.1002/aja.1001190108 [DOI] [PubMed] [Google Scholar]
- 117.Patrizi G, Poger M. The ultrastructure of the nuclear periphery. The zonula nucleum limitans. J Ultrastruct Res 1967; 17:127-36; PMID:6017352; http://dx.doi.org/ 10.1016/S0022-5320(67)80025-X [DOI] [PubMed] [Google Scholar]
- 118.Wilson KL, Berk JM. The nuclear envelope at a glance. J Cell Sci 2010; 123:1973-8; PMID:20519579; http://dx.doi.org/ 10.1242/jcs.019042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hirata A, Maeda N, Nakatsuji H, Hiuge-Shimizu A, Okada T, Funahashi T, Shimomura I. Contribution of glucocorticoid-mineralocorticoid receptor pathway on the obesity-related adipocyte dysfunction. Biochem Biophys Res Commun 2012; 419:182-7; PMID:22326264; http://dx.doi.org/ 10.1016/j.bbrc.2012.01.139 [DOI] [PubMed] [Google Scholar]
- 120.McKeon FD, Kirschner MW, Caput D. Homologies in both primary and secondary structure between nuclear envelope and intermediate filament proteins. Nature 1986; 319:463-8; PMID:3453101; http://dx.doi.org/ 10.1038/319463a0 [DOI] [PubMed] [Google Scholar]
- 121.Shimi T, Pfleghaar K, Kojima S, Pack CG, Solovei I, Goldman AE, Adam SA, Shumaker DK, Kinjo M, Cremer T, et al.. The A- and B-type nuclear lamin networks: microdomains involved in chromatin organization and transcription. Genes Dev 2008; 22:3409-21; PMID:19141474; http://dx.doi.org/ 10.1101/gad.1735208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shimi T, Butin-Israeli V, Goldman RD. The functions of the nuclear envelope in mediating the molecular crosstalk between the nucleus and the cytoplasm. Curr Opin Cell Biol 2012; 24:71-8; PMID:22192274; http://dx.doi.org/ 10.1016/j.ceb.2011.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Worman HJ, Bonne G. “Laminopathies:” a wide spectrum of human diseases. Exp Cell Res 2007; 313:2121-33; PMID:17467691; http://dx.doi.org/ 10.1016/j.yexcr.2007.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shimi T, Butin-Israeli V, Adam SA, Goldman RD. Nuclear lamins in cell regulation and disease. Cold Spring Harb Symp Quant Biol 2010; 75:525-31; PMID:21467145; http://dx.doi.org/ 10.1101/sqb.2010.75.045 [DOI] [PubMed] [Google Scholar]
- 125.de Las Heras JI, Meinke P, Batrakou DG, Srsen V, Zuleger N, Kerr AR, Schirmer EC. Tissue specificity in the nuclear envelope supports its functional complexity. Nucleus 2014; 4:460-77; PMID:24213376; http://dx.doi.org/ 10.4161/nucl.26872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hegele RA, Joy TR, Al-Attar SA, Rutt BK. Thematic review series: Adipocyte Biology. Lipodystrophies: windows on adipose biology and metabolism. J Lipid Res 2007; 48:1433-44; PMID:17374881; http://dx.doi.org/ 10.1194/jlr.R700004-JLR200 [DOI] [PubMed] [Google Scholar]
- 127.Garg A. Acquired and inherited lipodystrophies. N Engl J Med 2004; 350:1220-34; PMID:15028826; http://dx.doi.org/ 10.1056/NEJMra025261 [DOI] [PubMed] [Google Scholar]
- 128.Tilgner K, Wojciechowicz K, Jahoda C, Hutchison C, Markiewicz E. Dynamic complexes of A-type lamins and emerin influence adipogenic capacity of the cell via nucleocytoplasmic distribution of β-catenin. J Cell Sci 2009; 122:401-13; PMID:19126678; http://dx.doi.org/ 10.1242/jcs.026179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Markiewicz E, Tilgner K, Barker N, van de Wetering M, Clevers H, Dorobek M, Hausmanowa-Petrusewicz I, Ramaekers FC, Broers JL, Blankesteijn WM, et al.. The inner nuclear membrane protein emerin regulates β-catenin activity by restricting its accumulation in the nucleus. Embo J 2006; 25:3275-85; PMID:16858403; http://dx.doi.org/ 10.1038/sj.emboj.7601230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, MacDougald OA. Inhibition of adipogenesis by Wnt signaling. Science 2000; 289:950-3; PMID:10937998; http://dx.doi.org/ 10.1126/science.289.5481.950 [DOI] [PubMed] [Google Scholar]
- 131.Verstraeten VL, Renes J, Ramaekers FC, Kamps M, Kuijpers HJ, Verheyen F, Wabitsch M, Steijlen PM, van Steensel MA, Broers JL. Reorganization of the nuclear lamina and cytoskeleton in adipogenesis. Histochem Cell Biol 2011; 135:251-61; PMID:21350821; http://dx.doi.org/ 10.1007/s00418-011-0792-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.D'Angelo MA, Hetzer MW. The role of the nuclear envelope in cellular organization. Cell Mol Life Sci 2006; 63:316-32; PMID:16389459; http://dx.doi.org/ 10.1007/s00018-005-5361-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Stuurman N. Identification of a conserved phosphorylation site modulating nuclear lamin polymerization. FEBS Lett 1997; 401:171-4; PMID:9013881; http://dx.doi.org/ 10.1016/S0014-5793(96)01464-0 [DOI] [PubMed] [Google Scholar]
- 134.Gonzalez JM, Navarro-Puche A, Casar B, Crespo P, Andres V. Fast regulation of AP-1 activity through interaction of lamin A/C, ERK1/2, and c-Fos at the nuclear envelope. J Cell Biol 2008; 183:653-66; PMID:19015316; http://dx.doi.org/ 10.1083/jcb.200805049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.O'Leary JC 3rd, Dharia S, Blair LJ, Brady S, Johnson AG, Peters M, Cheung-Flynn J, Cox MB, de Erausquin G, Weeber EJ, et al.. A new anti-depressive strategy for the elderly: ablation of FKBP5/FKBP51. PLoS One 2011; 6:e24840; PMID:21935478; http://dx.doi.org/ 10.1371/journal.pone.0024840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Touma C, Gassen NC, Herrmann L, Cheung-Flynn J, Bull DR, Ionescu IA, Heinzmann JM, Knapman A, Siebertz A, Depping AM, et al.. FK506 binding protein 5 shapes stress responsiveness: modulation of neuroendocrine reactivity and coping behavior. Biol Psychiatry 2011; 70:928-36; PMID:21907973; http://dx.doi.org/ 10.1016/j.biopsych.2011.07.023 [DOI] [PubMed] [Google Scholar]
- 137.Hartmann J, Wagner KV, Liebl C, Scharf SH, Wang XD, Wolf M, Hausch F, Rein T, Schmidt U, Touma C, et al.. The involvement of FK506-binding protein 51 (FKBP5) in the behavioral and neuroendocrine effects of chronic social defeat stress. Neuropharmacology 2012; 62:332-9; PMID:21839098; http://dx.doi.org/ 10.1016/j.neuropharm.2011.07.041 [DOI] [PubMed] [Google Scholar]
- 138.Balsevich G, Uribe A, Wagner KV, Hartmann J, Santarelli S, Labermaier C, Schmidt MV. Interplay between diet-induced obesity and chronic stress in mice: potential role of FKBP51. J Endocrinol 2014; 222:15-26; PMID:24781256; http://dx.doi.org/ 10.1530/JOE-14-0129 [DOI] [PubMed] [Google Scholar]
- 139.Tamashiro KL. Metabolic syndrome: links to social stress and socioeconomic status. Ann N Y Acad Sci 2011; 1231:46-55; PMID:21884160; http://dx.doi.org/ 10.1111/j.1749-6632.2011.06134.x [DOI] [PubMed] [Google Scholar]
- 140.Cheung-Flynn J, Prapapanich V, Cox MB, Riggs DL, Suarez-Quian C, Smith DF. Physiological role for the cochaperone FKBP52 in androgen receptor signaling. Mol Endocrinol 2005; 19:1654-66; PMID:15831525; http://dx.doi.org/ 10.1210/me.2005-0071 [DOI] [PubMed] [Google Scholar]
- 141.Yong W, Yang Z, Periyasamy S, Chen H, Yucel S, Li W, Lin LY, Wolf IM, Cohn MJ, Baskin LS, et al.. Essential role for Co-chaperone Fkbp52 but not Fkbp51 in androgen receptor-mediated signaling and physiology. J Biol Chem 2007; 282:5026-36; PMID:17142810; http://dx.doi.org/ 10.1074/jbc.M609360200 [DOI] [PMC free article] [PubMed] [Google Scholar]


