Abstract
Adipogenesis is regulated by a complex cascade of transcriptional factors, among them KLF4. This factor was previously shown to be necessary for adipose differentiation. We found that GSK3β activity was required for Klf4 and Klf5 expression during adipogenesis. In addition, retinoic acid inhibited Klf4 and Klf5 expression but not that of Cebpb. Protein synthesis inhibition showed that the transient expression of Klf4, Cebpb and Klf5 during early adipogenesis seemed to require a yet unknown protein for their repression. We also found that Klf4 forced expression in 3T3-F442A cells cultured under non-adipogenic conditions did not induce adipogenesis, nor the expression of Cebpb or Klf5, a Cebpb target gene, showing that KLF4 was not sufficient for adipose differentiation to take place. This would suggest that a more complex combination of molecular pathways not yet understood, is involved during early adipogenesis.
Keywords: adipogenesis, Cebpb, differentiation, GSK3, Klf4, Klf5, retinoic acid
Abbreviations
- SREBP1a
sterol regulatory element binding transcription factor 1a protein
- PPARγ/Pparg2
peroxisome proliferator activated receptor gamma protein/gene
- SREBP1c
sterol regulatory element binding transcription factor 1 protein
- C/EBPα/Cebpa
CCAAT/enhancer binding protein α protein/gene
- C/EBPβ/Cebpb
CCAAT/enhancer binding protein β protein/gene
- KLF4/Klf4
Krüppel-like factor 4 protein/gene
- KFL5/Klf5
Krüppel-like factor 5 protein/gene
- MAPK
mitogen-activated protein kinase (MAPK)
- GSK3β
glycogen synthase kinase 3 β protein
- St
Staurosporine
- Dex
Dexamethasone
- Mix/Dex
methyl isobutyl xanthine/dexamethasone
- St-Dex
Staurosporine-Dexamethasone
- N-Ad
non-adipogenic medium
- CHX
Cycloheximide
- pS-empty
pCMVSport6 empty vector
- Ps-Klf4
pCMVSport6Klf4
- RA
all-trans retinoic acid
Introduction
Differentiation into adipocytes is controlled through the activation of signaling pathways leading to a transcriptional cascade that controls adipose differentiation. The transcription factors, SREBP1a1 and PPARγ regulate the expression of the genes involved in adipose phenotype, whereas SREBP1c regulates that of lipogenic enzymes.2 C/EBPα regulates the expression of Pparg2 and the genes related to glucose uptake.2,3
The adipogenic program is comprised of commitment, clonal expansion, and phenotype expression.4 An important transcription factor during adipose commitment is C/EBPβ, whose activity depends on specific phosphorylation at Thr188 by either GSK3β or MEK1.5 A loss in the activity of GSK3β blocks the adipogenic program.4,6 C/EBPβ is involved in the clonal expansion of committed cells7 and in the expression of Pparg2 and adiponectin gene.1,5 The expression of Pparg2 is delayed several hours after the expression and activation of C/EBPβ.1,7This delay raises the possibility that other genes could participate in the regulation of Pparg2 and the rest of the transcriptional cascade. One of those transcription factors that we reported recently is SREBP1a.1
Members of the Krüppel-like transcription factors (KLF) family have been associated with the adipogenic response of 3T3-L1 cells. The transcription factors KLF4 and KLF5 participate in the initial stages of adipose differentiation in 3T3-L1 cells induced with methyl isobutyl xanthine/dexamethasone (Mix/Dex) and insulin, in medium supplemented with adipogenic bovine serum.8,9 Knockdown of either of these transcription factors impaired adipose differentiation.8,9 KLF4 is able to activate C/EBPβ promoter,8 and Klf5 expression depends on C/EBPβ and C/EBPδ transcriptional activity.9 Pparg2 is activated by KLF5 through a direct interaction with its promoter.9 Klf5 forced expression induced the expression of Pparg2 and adipose phenotype in 3T3-L1 cells cultured with calf serum, which by itself is adipogenic.9 KLF4 is necessary for the 3T3-L1 adipose differentiation, however, it is unknown if its expression is sufficient to induce adipogenesis.
We have already demonstrated that in non-adipogenic conditions, Staurosporine (St) at low concentrations, rapidly induced adipogenesis of 3T3-F442A cells,4 while Dexamethasone (Dex) enhanced St-induced adipose conversion; however, Dex alone, without St, did not induce adipose conversion under the non-adipogenic conditions.10 It was reported that treatment with staurosporine leads to GSK3β activation as shown by its reduced phosphorylation on Ser21/9.11 This adipogenesis cellular model induced with St-Dex has the advantage of not requiring adipogenic serum interfering factors which can affect data interpretation, and it allows the study of some of the early events during induction and stabilization of adipose commitment.1,4 The main transcription factors C/EBPβ, SREBP1a, PPARγ2, C/EBPα, and SREBP1c also participate in adipose differentiation in this cellular model.1,12 There are several factors that affect adipogenesis during commitment,4,13,14 among them, all-trans retinoic acid (RA) that inhibits adipose differentiation.4,15-18
In this paper, we studied the KLF4-C/EBPβ-KLF5 part of the transcriptional cascade during early adipogenesis of 3T3-F442A cells induced by St-Dex. We found that Klf4 forced expression was not enough to promote adipose differentiation without the adipogenic stimuli, nor it was able to induce C/EBPβ expression. Also, we found that both Klf4 and Klf5 expression depends on GSK3β activity, and RA inhibits their expression.
Results
Klf4 and Klf5 are induced early during adipogenesis of 3T3-F442A cells
We attempted to study more about the early stages of 3T3-F442A adipogenesis induced by St-Dex, and the participation of KLF4 and KLF5. We induced 2 day post-confluent cultures of 3T3-F442A cells with St-Dex for 4 h and analyzed gene expression at different time points from 0 to 144 h, when adipose conversion was complete. At the end of experiment, cultures were stained with Oil Red O for lipid accumulation. Adipose conversion of 3T3-F442A cells exhibited the characteristic formation of adipose clusters with intracytoplasmic lipid droplets (Fig. 1A). Cebpb showed an early and transient increased expression with a maximum at 4–8 h (Fig. 1B). Pparg2 expression increased slightly at around 8 h and reached its highest expression at about 48 h, remaining high until the end of adipose conversion at 144 h (Fig. 1B). Cebpa expression increased by 48 h and remained high thereafter (Fig. 1B). The kinetic expression of these adipogenic genes coincide with data we previously reported in this adipogenic model.1,12 When we assessed Klf4 and Klf5, both genes increased their expression as early as 0.5 h after addition of St-Dex. Klf4 expression had a 2- to 3-fold increase, with a maximum at 2 h; while Klf5 expression had a larger increase, about 8- to 14-fold, with a maximum level at 3 h after St-Dex induction (Fig. 1B). Thereafter, expression of both genes decreased to basal levels or lower after 4 h and 30 h, respectively (Fig. 1B). The highest expression levels of Klf4 and Klf5 occurred at similar time points as described for 3T3-L1 cells.8,9 These results demonstrated that Klf4 and Klf5 expression took place very early during adipogenesis of 3T3-F442A cells induced with St-Dex, and it preceded that of Cebpb expression an all the other adipogenic genes.
Figure 1.
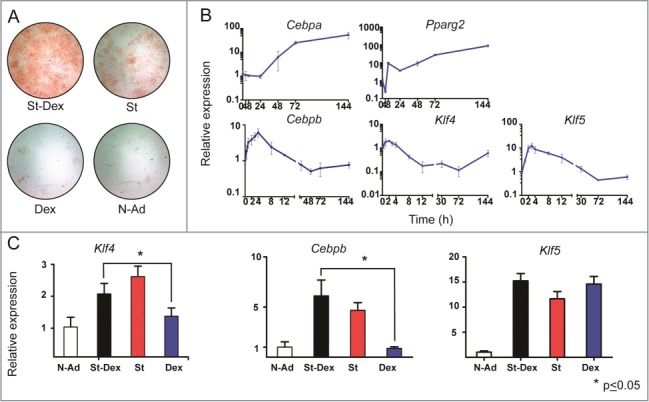
Gene expression during adipose differentiation of 3T3-F442A cells. (A) Adipose conversion shown by lipid staining with Oil Red O. Staurosporine (St) at low concentrations [10–12 nM] induced adipose differentiation in 3T3-F442A cells and this effect was augmented by Dexamethasone (Dex) at [250 nM]. Dex alone did not induce adipose differentiation; (N-Ad, non-adipogenic medium). (B) mRNA expression of adipogenic genes Cebpb, Pparg2, Cepba, Klf4 and Klf5 during adipose differentiation of 3T3-F442A cells treated with St-Dex. (C) Expression of Klf4 at 2h, Cebpb at 4h,and Klf5 at 3h, which are the times of their highest expression.
In another experiment, post-confluent cultures of 3T3-F442A cells were treated with St alone, or with Dex alone, for 4 h. St alone effectively induced adipogenesis; whereas Dex alone did not and it was similar to that found in cultures incubated with non-adipogenic medium. Dex enhanced the adipogenic effect of St when it was incubated together (Fig. 1A). Since we determined that the highest increase of Klf4, Cebpb, and Klf5 expression took place during the first 12 h, we used this time frame to analyze these genes. We found that Cebpb and Klf4 expression was dependent on induction of adipogenesis by St, but not by Dex (Fig. 1C); whereas that of Klf5 was St and Dex dependent (Fig. 1C). These results showed that Klf4, Cebpb, and Klf5 expression followed induction by St, and particularly that of Klf5 was also stimulated by Dex alone, even in the absence of adipose differentiation. Klf5 expression seems to be regulated by Dex and this glucocorticoid could be contributing to augment adipose differentiation through KLF5.
Forced expression of Klf4 does not induce adipose differentiation nor increase Cebpb expression
Our results showed that St induced an early and transient increase of Klf4 expression. Considering that silencing Klf4 blocked adipose differentiation of 3T3-L1 cells cultured in a medium supplemented with Mix/Dex and fetal bovine serum, which are highly adipogenic conditions,8 we decided to evaluate if Klf4 forced expression alone is sufficient to induce adipogenesis in the absence of any adipogenic stimuli. We transfected 3T3-F442A cells with a plasmid harboring Klf4 under the control of a constitutive CMV promoter; as a negative control we also transfected cells with an empty vector. We assessed for adipose conversion under adipogenic or non-adipogenic conditions, and we evaluated gene expression. The results showed that forced expression of Klf4 in 3T3-F442A cells reached up to 100-fold increase (Fig. 2B). Adipogenesis did not take place in cells cultured in non-adipogenic conditions, in comparison with control cultures treated with St-Dex, the adipogenic stimuli (Fig. 2A).
Figure 2.
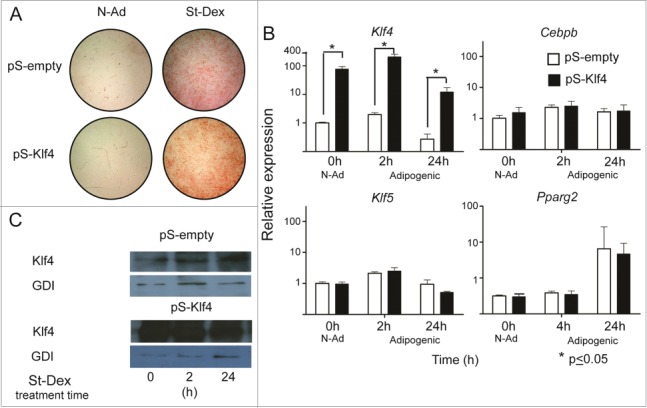
Forced expression of Klf4 does not increase Cebpb and Klf5 expression. (A) Adipose conversion shown by lipid staining with Oil Red O. (B) Expression of the adipogenic genes Klf4, Cebpb, Klf5 and Pparg2 in cultures transfected with pCMVSport6 empty vector (pS-empty) and pCMVSport6Klf4 (pS-Klf4) in cells cultured in non-adipogenic conditions. (C) Representative western blot showing the amounts of KLF4 and using GDI protein as loading control.
The increase in Klf4 expression was also reflected in an increase of KLF4 protein, as detected by immunoblotting (Fig. 2C). Notwithstanding the proposed role of KLF4 to promote Cebpb transcription, Klf4 forced expression in cells cultured under adipogenic conditions did not result in an increase of Cebpb, Pparg2 or Klf5 expression in comparison with cells transfected with empty vector (Fig. 2B). These results showed that 100-fold increase in the expression of KLF4 gene is not sufficient to induce adipogenesis, or to induce the expression of the adipogenic genes.
GSK3β activity is necessary for Klf4 and Klf5 expression during adipogenesis
Phosphorylation of GSK3β at Tyr216 activates the kinase, but phosphorylation at Ser9 inactivates it, even if Tyr216 is also phosphorylated.25 We previously described that GSK3β phosphorylated at Tyr216 was the predominant form during induction of 3T3-F442A cells with St-Dex, showing that this kinase is activated during adipogenesis; its activity was inhibited by its well reported selective inhibitor SB415286, blocking adipogenesis, as we showed in previously published experiments.1 Therefore, we evaluated the participation of GSK3β over the KLF4-C/EBPβ-KLF5 transcriptional cascade. Post-confluent cultures of 3T3-F442A cells in non-adipogenic medium were induced to adipose differentiation with St-Dex for 4 h and treated for 12 h with SB415286. Treatment of cells with SB415286 blocked adipogenesis and the expression of Klf4, Cebpb and Klf5, and their down-stream adipogenic genes Pparg2 and Cepba (Fig. 3A–C). These results showed that the expression of Klf4, Cebpb, and Klf5 should be down-stream and regulated by GSK3β activity. We previously reported that C/EBPβ activation depends on specific phosphorylation at Thr188 by GSK3β.1
Figure 4.
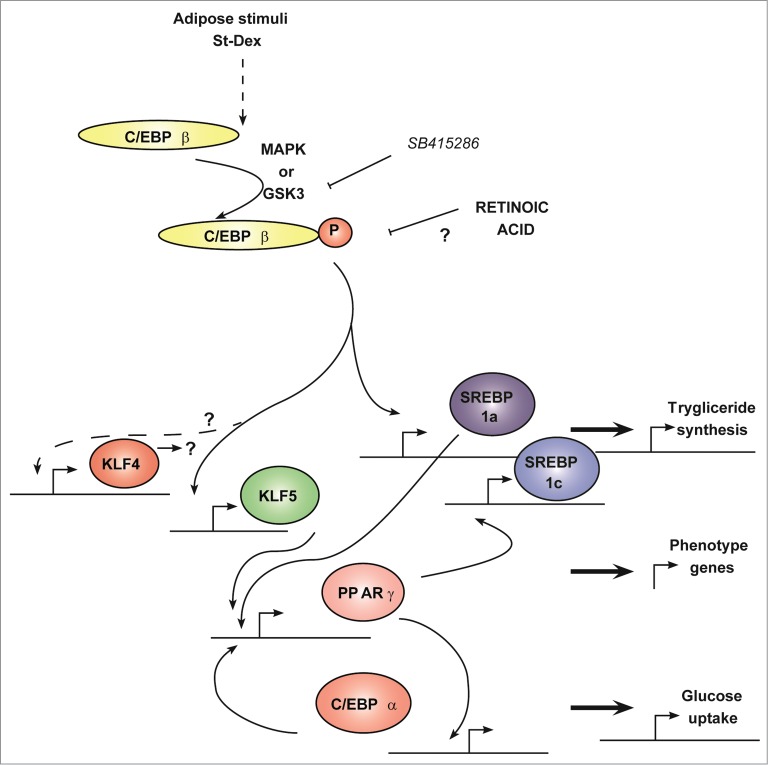
Transcriptional cascade showing Klf4 and Klf5 expression during adipogenesis. The adipogenic program involves a transcriptional cascade with early participation of KLF4 and KLF5, which depends on GSK3β activity. Forced expression of Klf4 showed that Cebpb expression was not dependent on KLF4. All-trans retinoic acid impairs the adipogenic program after Cebpb expression and up-stream of Klf4 and Klf5 expression. Solid line means direct relationship and dashed line means an indirect or multistep involved relationship. This schematic was constructed from cited references 1 and 3.
Figure 3.
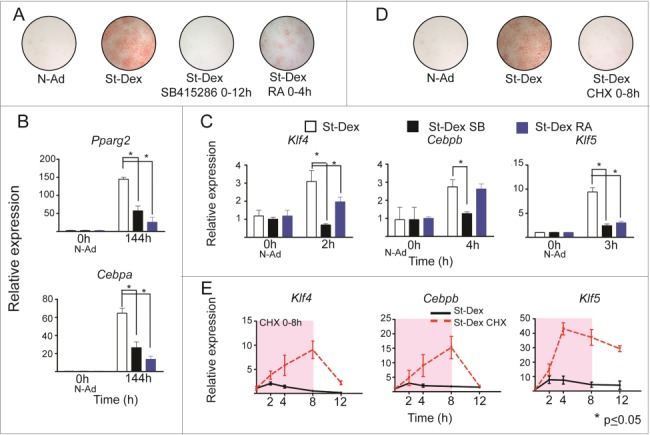
GSK3β activity and retinoic acid affect gene expression during adipose differentiation. (A) Adipose conversion shown by lipid staining with Oil Red O. GSK3β inhibitor (SB415286) at [100μM] or retinoic acid at [10μM]. (B and C) Expression of Pparg2, Cebpa, Klf4, Cebpb and Klf5 in 3T3F442A St-Dex cultures treated with SB415286 (black) or retinoic acid (blue). (D) Adipose conversion shown by lipid staining with Oil Red O in cultures treated with Cycloheximide (CHX) at [15μM]. (E) Expression of Klf4, Cebpb, and Klf5 in cultures treated with or without CHX for 8 h in St-Dex induced cultures.
Another inhibitor of adipose differentiation is all-trans retinoic acid.15-18 Its effect blocked adipogenesis through affecting MAPK signaling only when added to cultures during commitment.4,18,26 RA action was described down-stream of Cebpb,16 inhibiting transcription of Pparg2 and Cebpa. Since it is not known whether retinoic acid inhibits adipose differentiation up-stream or down-stream of Klf4 or Klf5 expression we carried out the following experiment. We induced adipogenesis of 3T3-F442A cells with St-Dex in presence of RA. RA inhibited adipose differentiation (Fig. 3A) as well as the expression of the adipogenic genes Pparg2 and Cebpa (Fig. 3B). Expression of Klf4 and Klf5 was also blocked by RA, whereas expression of Cebpb remained unaltered (Fig. 3C). Therefore, the action of RA preceded the expression of Klf4 and Klf5, but not that of Cebpb.
The transient expression of Klf4, Cebpb, and Klf5 seems to be regulated through a protein repressor
With the goal of determining if protein synthesis is necessary to regulate the transient expression of Klf4, Cebpb, and Klf5 during adipogenesis, we incubated the 3T3-F442A cultures induced with St-Dex with cycloheximide (protein synthesis inhibitor) for 8 h. As expected, cycloheximide blocked adipogenesis, but expression of Klf4, Cebpb or Klf5 was not blocked and it increased further with cycloheximide treatment (Fig. 3D and E). Remarkably, the expression of these genes did not decrease as occurs during adipogenesis, unless cycloheximide was removed from the cultures (Fig. 3E). These results showed that de novo protein synthesis is not required for the expression of Klf4, Cebpb, and Klf5, but it is necessary for turning off their expression, suggesting that a still unknown protein or proteins should be necessary to down-regulate Klf4, Cebpb, and Klf5 expression during early adipogenesis.
Discussion
Adipogenic differentiation is regulated by sequential activation of transcription factors. This is called the main adipogenic transcriptional cascade. Some of these factors, like Cebpa, Klf5, and Pparg2 have shown a key participation in this process. Adipogenic differentiation does not take place without their expression, but forced expression of Cebpa in NIH-3T3 cells, and Klf5 or Pparg2 in 3T3-L1 cells induced adipose differentiation.9,27,28
Knockdown of Klf4 in 3T3-L1 cells cultured under adipogenic conditions blocked adipose differentiation, suggesting that this transcription factor is necessary for cells to undergo adipogenesis.8 However, the necessity of this transcription factor does not prove if its sole expression under non-adipogenic conditions is enough to initiate adipogenesis. In our experiments, we forced the expression, up to 100 fold, of Klf4 under non-adipogenic conditions and showed that 3T3-F442A cells did not undergo adipogenesis, demonstrating thatKLF4 is not sufficient for adipose differentiation to take place. CEBPβ is essential during adipogenesis,16 its gene expression precedes those for Pparg2 and Cebpa.1,12 Since, in non-adipogenic medium adipogenesis did not take place even with the 100-fold forced expression of Klf4, and since that of Cebpb did not increase by the over-expression of Klf4, we suggest that other factors must be required to trigger expression of Cebpb and the adipogenic response in susceptible cells. One of these factors could be Krox20, since it cooperatively transactivates a Cebpb reporter.8 This finding was also reinforced by our results that the expression of Klf5, an established target of Cebpb action, did not increase in those conditions, either. Our experiments also demonstrated that up to 200-fold overexpression of Klf4 in adipogenic conditions did not enhance Cebpb expression, and hence that of Klf5 and Pparg2. This is a surprising result since Cebpb promoter-luciferase reporter plasmid into 293T cells and CHIP assays showed that KLF4 binds directly to the promoter of Cebpb.8 Our results suggested that, in whole cells, the augmented intracellular concentration of KLF4 did not enhance the expression of the genes comprising the adipogenic transcriptional cascade, and we hypothesize that some other limiting proteins must act to regulate adipogenesis.
We previously reported that the activity of GSK3β is necessary for cells to undergo adipogenesis and it preceded the expression of the genes encoding the adipogenic transcriptional cascade.1,4 Our experiments with SB415286, the selective inhibitor of GSK3β, showed that the activity of this kinase is up-stream and it is required for Klf4 transient expression, which peaked at the first 3 h from adipogenic induction.
Our experiments in which we inhibited protein synthesis with cycloheximide showed that the expression of Klf4, Cebpb, and Klf5 that are transiently expressed during early adipogenesis, continued for several hours reaching many more folds as compared with the non-inhibited cells. These results strongly suggested that the expression of these 3 genes should be regulated by a still unknown de novo-synthesized protein or proteins, probably acting as repressor. This raises the possibility that the expression of these transcription factors is subjected to a transient increase and turn-off as an additional point of regulation during commitment. These possibilities warrant more investigation into this subject.
RA is well established as an inhibitor of adipose differentiation.15–18 RA action was described down-stream of Cebpb.16 Our results showed that RA did not affect Cebpb expression, but inhibited the expression of both Klf4 and Klf5, reinforcing previous results that the expression of these Kruppel transcription factors are down stream of Cebpb transcription. Since previous experiments have suggested that KLF4 activates the promoter of Cebpb,8 inhibition of the expression of Klf4 by RA would suggest that the transcription of Cebpb should be regulated by other proteins in the adipogenic pathway that are not related to KLF4 or KLF5.
Since these results and since the forced expression of Klf4 alone did not increase the transient expression of Cebpb and Klf5, we can hypothesize that KLF4-CEBPβ-KLF5 early part of the adipogenic transcriptional cascade must be regulated by important proteins that are not yet identified, and that these proteins should exert their function at a stage located downstream of the activation of GSK3β as depicted in Figure 4. Therefore, other molecular pathways are required and might converge to continue adipogenesis after induction.
Previously, we showed that St induced adipose differentiation in the absence of adipogenic conditions4 and, that Dex enhanced differentiation of 3T3-F442A cells induced with St.11 This conveyed the idea that early adipogenic pathway could be comprised by 2 main stages: a first stage of induction (0–4 h), where St induces progenitor cells to differentiate; a second stage of stabilization (4–44 h), where differentiation continues in the absence of the inducer but it can still be reversed by anti-adipogenic substances or cytokines, such as RA4,18 or TNFα.13Therefore, it seems clear that the second stage of stabilization during commitment can be subjected to regulation by several factors, and early adipogenesis could be reversed. Since Klf4 and Cebpb are expressed during the early period that we called induction, we can hypothesize that the expression of these factors is not enough to lock the cells into the adipogenic pathway. Some other factors should be activated to lock the cells traversing into commitment; one of these might be DNA synthesis that takes place at the end of commitment preceding clonal expansion, as we described in an earlier work.29 The identification of other factors involved in regulating adipogenesis that could affect the main transcriptional cascade is important in understanding adipose biology.
Materials and Methods
Cell culture
The 3T3-F442A cells were seeded at 1.25×103 cell/cm2 in growing medium consisting of DMEM (GIBCO, 12100–061) supplemented with 4% adult cat serum (in-house production in observance of the NIH guidelines on the welfare of research animals and using protocols approved by the Internal Committee for the Care and Use of Laboratory Animals of CINVESTAV-IPN),19 5 μg/mL insulin (Serological, 1003551), 1 μM d-biotin (Sigma, B-4501) and 0.1% calf serum (Hyclone, SH30042–03). Two days post- confluent cultures were switched to non-adipogenic medium (N-Ad) consisting of DMEM supplemented with 2.5% adult cat serum, 0.1% calf serum, 5 μg/mL insulin, 5 μg/mL apo-transferrin (Sigma, B-4501), 1 μM d-biotin, 2 nM triiodothyronine (Sigma, T-2752), 40 μM β-mercaptoethanol (BIO-RAD, 161–0710) and 0.01 ng/mL epidermal growth factor (Upstate, 01–107) (modified from 20). In this medium cells do not undergo adipose differentiation.19 Adipogenesis was induced in post-confluent cultures by adding 10–12 nM St (Sigma, S4400) and 250 nM Dex (Sigma, D-1756) for 4 h in the non-adipogenic medium as previously described.11 All cultures were incubated at 37ºC in a 10% CO2 atmosphere. For experiments, cultures were taken at different time points as described in each experiment. Adipose conversion was complete at 144 h post-adipogenic induction. At the end of experiment, cultures were washed with PBS-1X and fixed overnight in 4% formalin (J.T.Baker, 2106–02) and stained for lipid accumulation with Oil Red O (Sigma, O-0625) for 4 h. After thorough washing with tap water, dishes were allowed to dry.21 Treatment with Cycloheximide [15μM] (Sigma, C-6255), SB415286 [100μM] (Sigma, S3567) and all-trans-retinoic acid [10μM] (Sigma, R-2625) were added to the cultures at the same time of induction of adipogenesis as specified in the text and figures.
Quantitative RT-PCR
Total RNA extraction was carried out with TRIzol® Reagent (Life Technologies, 15596018). cDNA synthesis was carried out with 0.5 μg total RNA and SuperScript II reverse transcriptase kit (Invitrogen, 18064–014), according to manufacturer′s instructions. Gene relative expression was performed with UniversalFastStart DNA Master PLUS SybrGreen I (Rox) kit (Roche Applied Science, 14387600) and real-time PCR were carried out on Thermal cycler C1000 Real Time Thermal Cycler CFX96 detection module (Bio-Rad). We used regions spanning 2 different exons from each gene to design the primers for this work, and they were: Klf4, forward 5′-gtccttctccacgttcgc-3′ and reverse 5′-ccaggaggtcgttgaactc-3′ (Ta 63°C, 251pb, Efficiency (E) = 1.95); Klf5, forward 5′-gccaactctcccacctgtca-3′ and reverse 5′-gtgcacttgtagggcttctcg-3′ (Ta 60°C, 165bp, E = 1.84); Cebpb, forward 5′-ccgcgcaccacgacttcccct-3′ and reverse 5′-cgctcgcgccgcatcttgta-3′ (Ta 61°C, 451bp, E = 1.86);12 Cebpa, forward 5′- gagtcggccgacttctacg-3′ and reverse 5′- gtctcgtgctcgcagatgc -3′ (Ta 62°C, 178bp, E = 1.87);12 Rplp0, forward 5′-aggccctgcactctcgctttctgg-3′ and reverse 5′-tggttgctttggcgggattagtcg-3′ (Ta 60–63°C, 347bp, E = 1.96);4 Pparg2, fwd 5′-tcgctgatgcactgcctatg-3′, and rev 5′-gagaggtccacagagctgatt-3′ (Ta 60°C, 102pb, E = 1.87),.22 We run negative controls without RT or without sample for the RT-PCR reaction to assure absence of unspecific amplification. Relative expression values, were calculated by the formula 2-ΔΔCt,22 using the expression of the Rplp0 gene as the normalizing factor and expressed relative to time zero.
Western-blot
Protein extraction was obtained from cultures, washed 2 times with ice-cold PBS. Extraction buffer was 50 mM Tris (BIO-RAD, 161–0719) pH 8, 137 mM NaCl (J.T.Baker, 3624–01), 10% glycerol (J.T. Baker, 2136–62), 1% Nonidet P-40 (Sigma, N-3516), 2 mM EDTA (J.T. Baker, 8993), 2.5 mM sodium pyrophosphate (Sigma, 221368), 100 mM β-glycerolphosphate(Sigma, 50020), 1 mM Na3VO4(Fisher Scientific, S-454), and Complete protease inhibitor cocktail 2X (Roche, 1697498). Protein was quantified by Lowry method24 before electrophoresis in SDS/PAGE. Then, protein was blotted onto nitrocellulose filter 0.45 μm membranes (Millipore, HATF00010). Immunodetection was carried out with primary antibodies: anti-KLF4 (1:1000 dilution) (Santa Cruz Biotechnology, sc-20691), and anti-Rab Guanine Nucleotide Dissociation Inhibitor GDI (1:2000 dilution)(Invitrogen, 71–0300). As secondary antibody we used a goat HRP-conjugated anti-rabbit IgG (1:10000 dilution) (ZyMax-Invitrogen, 81–6120). Immune complexes were developed with Western Blotting Chemiluminescence Luminol Reagent (Santa Cruz Biotechnology, sc-2048).
Constitutive expression of Klf4
Cells were transfected with the plasmid pCMVSport6Klf4, which harbours the Klf4 mRNA (Invitrogen, ID3156339), with a Nucleofector. The insert was verified by sequencing. Cells were transfected with Nucleofector II equipment (Lonza; 10700371) and following the manufacturer′s protocols (Solution V, Program T-030). Cells were seeded at confluence in non-adipogenic medium and induced to adipogenesis with St-Dex 16h later. As negative control we used the empty vector.
Statistical analysis
Quantitative results are the mean, +/− standard deviation, of 6 independent cultures as 2 sets of experiments (n = 6). Qualitative data corresponds to one representative experiment by triplicate. Data were analyzed by Student′s t-test and statistical differences were set when P value was lower than 0.05.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Juan Pedro Arias Luna, Nicolás Villegas Sepúlveda, and Alejandra Aguilar Martinez for their advice. We also thank Raúl Bonilla Moreno and Manuel Flores Cano for their excellent technical assistance; Victor Hugo Rosales from UCF for flow cytometry service, María Guadalupe Aguilar González for sequencing service, Alberto Rodriguez for his technical assistance and the secretarial assistance of Maria Elena Rojano.
Funding
This study was supported by CONACYT scholarship 203665 and grant 104350.
References
- 1. Ayala-Sumuano J-T, Velez-del Valle C, Beltran-Langarica A, Marsch-Moreno M, Cerbon-Solorzano J, Kuri-Harcuch W. Srebf1a is a key regulator of transcriptional control for adipogenesis. Sci Rep 2011; 1:178; PMID:22355693; http://dx.doi.org/ 10.1038/srep00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Morrison RF, Farmer SR. Insights into the transcriptional control of adipocyte differentiation. J Cell Biochem 1999; 75:Suppl 32-33:59-67; PMID:10629104; http://dx.doi.org/. [DOI] [PubMed] [Google Scholar]
- 3. Farmer SR. Transcriptional control of adipocyte formation. Cell Metab 2006; 4:263-73; PMID:17011499; http://dx.doi.org/ 10.1016/j.cmet.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Diaz-Velasquez CE, Castro-Munozledo F, Kuri-Harcuch W. Staurosporine rapidly commits 3T3-F442A cells to the formation of adipocytes by activation of GSK-3beta and mobilization of calcium. J Cell Biochem 2008; 105:147-57; PMID:18543255; http://dx.doi.org/ 10.1002/jcb.21810. [DOI] [PubMed] [Google Scholar]
- 5. Park BH, Qiang L, Farmer SR. Phosphorylation of C/EBPbeta at a consensus extracellular signal-regulated kinase/glycogen synthase kinase 3 site is required for the induction of adiponectin gene expression during the differentiation of mouse fibroblasts into adipocytes. Mol Cell Biol 2004; 24:8671-80; PMID:15367685; http://dx.doi.org/ 10.1128/MCB.24.19.8671-8680.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ross SE, Erickson RL, Hemati N, MacDougald OA. Glycogen Synthase Kinase 3 Is an Insulin-Regulated C/EBPalpha kinase. Mol Cell Biol 1999; 19:8433-41; PMID:10567568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tang QQ, Lane MD. Activation and centromeric localization of CCAAT/enhancer-binding proteins during the mitotic clonal expansion of adipocyte differentiation. Genes Dev 1999; 13:2231-41; PMID:10485846; http://dx.doi.org/ 10.1101/gad.13.17.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Birsoy K, Chen Z, Friedman J. Transcriptional regulation of adipogenesis by KLF4. Cell Metab 2008; 7:339-47; PMID:18396140; http://dx.doi.org/ 10.1016/j.cmet.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oishi Y, Manabe I, Tobe K, Tsushima K, Shindo T, Fujiu K, Nishimura G, Maemura K, Yamauchi T, Kubota N, et al. Kruppel-like transcription factor KLF5 is a key regulator of adipocyte differentiation. Cell Metab 2005; 1:27-39; PMID:16054042; http://dx.doi.org/ 10.1016/j.cmet.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 10. Ayala-Sumuano JT, Velez-delValle C, Beltran-Langarica A, Marsch-Moreno M, Hernandez-Mosqueira C, Kuri-Harcuch W. Glucocorticoid paradoxically recruits adipose progenitors and impairs lipid homeostasis and glucose transport in mature adipocytes. Sci Rep 2013; 3:2573; PMID:23999235; http://dx.doi.org/ 10.1038/srep02573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koivisto L, Alavian K, Hakkinen L, Pelech S, McCulloch CA, Larjava H. Glycogen synthase kinase-3 regulates formation of long lamellipodia in human keratinocytes. J Cell Sci 2003; 116:3749-60; PMID:12890758; http://dx.doi.org/ 10.1242/jcs.00693. [DOI] [PubMed] [Google Scholar]
- 12. Ayala-Sumuano JT, Velez-Del Valle C, Beltran-Langarica A, Hernandez JM, Kuri-Harcuch W. Adipogenic genes on induction and stabilization of commitment to adipose conversion. Biochem Biophys Res Commun 2008; 374:720-4; http://dx.doi.org/ 10.1016/j.bbrc.2008.07.127. [DOI] [PubMed] [Google Scholar]
- 13. Castro-Munozledo F, Beltran-Langarica A, Kuri-Harcuch W. Commitment of 3T3-F442A cells to adipocyte differentiation takes place during the first 24-36 h after adipogenic stimulation: TNF-α inhibits commitment. Exp Cell Res 2003; 284:163-72; PMID:12651150; http://dx.doi.org/ 10.1016/S0014-4827(02)00036-8. [DOI] [PubMed] [Google Scholar]
- 14. Xing H, Northrop JP, Grove JR, Kilpatrick KE, Su JL, Ringold GM. TNF α-mediated inhibition and reversal of adipocyte differentiation is accompanied by suppressed expression of PPARgamma without effects on Pref-1 expression. Endocrinology 1997; 138:2776-83; PMID:9202217. [DOI] [PubMed] [Google Scholar]
- 15. Sato M, Hiragun A, Mitsui H. Preadipocytes possess cellular retinoid binding proteins and their differentiation is inhibited by retinoids. Biochem Biophys Res Commun 1980; 95:1839-45; PMID:6998485; http://dx.doi.org/ 10.1016/S0006-291X(80)80113-6. [DOI] [PubMed] [Google Scholar]
- 16. Schwarz EJ, Reginato MJ, Shao D, Krakow SL, Lazar MA. Retinoic acid blocks adipogenesis by inhibiting C/EBPbeta-mediated transcription. Mol Cell Biol 1997; 17:1552-61; PMID:9032283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Murray T, Russell TR. Inhibition of adipose conversion in 3T3-L2 cells by retinoic acid. J Supramol Struct 1980; 14:255-66; PMID:6164877; http://dx.doi.org/ 10.1002/jss.400140214. [DOI] [PubMed] [Google Scholar]
- 18. Kuri-Harcuch W. Differentiation of 3T3-F442A cells into adipocytes is inhibited by retinoic acid. Differentiation 1982; 23:164-9; PMID:7166214; http://dx.doi.org/ 10.1111/j.1432-0436.1982.tb01279.x. [DOI] [PubMed] [Google Scholar]
- 19. Kuri-Harcuch W, Green H. Adipose conversion of 3T3 cells depends on a serum factor. Proc Natl Acad Sci U S A 1978; 75:6107-9; http://dx.doi.org/ 10.1073/pnas.75.12.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Morikawa M, Green H, Lewis UJ. Activity of human growth hormone and related polypeptides on the adipose conversion of 3T3 cells. Mol Cell Biol 1984; 4:228-31; PMID:6700589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ramirez-Zacarias JL, Castro-Munozledo F, Kuri-Harcuch W. Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with Oil red O. Histochemistry 1992; 97:493-7; PMID:1385366; http://dx.doi.org/ 10.1007/BF00316069. [DOI] [PubMed] [Google Scholar]
- 22. Wang X, Seed B. A PCR primer bank for quantitative gene expression analysis. Nucleic Acids Res 2003; 31:e154; PMID:14654707; http://dx.doi.org/ 10.1093/nar/gng154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25:402-8; PMID:11846609; http://dx.doi.org/ 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951; 193:265-75. [PubMed] [Google Scholar]
- 25. Forde JE, Dale TC. Glycogen synthase kinase 3: a key regulator of cellular fate. Cell Mol Life Sci 2007; 64:1930-44; PMID:17530463; http://dx.doi.org/ 10.1007/s00018-007-7045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee JS, Park JH, Kwon IK, Lim JY. Retinoic acid inhibits BMP4-induced C3H10T1/2 stem cell commitment to adipocyte via downregulating Smad/p38MAPK signaling. Biochem Biophys Res Commun 2011; 409:550-5; PMID:21605549; http://dx.doi.org/ 10.1016/j.bbrc.2011.05.042. [DOI] [PubMed] [Google Scholar]
- 27. Freytag SO, Paielli DL, Gilbert JD. Ectopic expression of the CCAAT/enhancer-binding protein α promotes the adipogenic program in a variety of mouse fibroblastic cells. Genes Dev 1994; 8:1654-63; PMID:7958846; http://dx.doi.org/ 10.1101/gad.8.14.1654. [DOI] [PubMed] [Google Scholar]
- 28. Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell 1994; 79:1147-56; PMID:8001151; http://dx.doi.org/ 10.1016/0092-8674(94)90006-X. [DOI] [PubMed] [Google Scholar]
- 29. Kuri-Harcuch W, Marsch-Moreno M. DNA synthesis and cell division related to adipose differentiation of 3T3 cells. J Cell Physiol 1983; 114:39-44; PMID:6826660; http://dx.doi.org/ 10.1002/jcp.1041140107. [DOI] [PubMed] [Google Scholar]


