Abstract
Maintaining natural feeding rhythms with time-restricted feeding (TRF), without altering nutritional intake, prevents and reverses diet-induced obesity (DIO) and its associated metabolic disorders in mice. TRF has a direct effect on animal adiposity, causes an alteration of adipokine signaling, and diminishes white adipose tissue inflammation. Many genes involved in lipid metabolism are normally circadian, but their expression is perturbed with DIO; TRF restores their cyclical expression. One mechanism through which TRF could affect host metabolism is by altering the gut microbiome. Changes in the gut microbiome are coupled with an altered stool bile acid profile. Hence, TRF could affect lipid metabolism by altering bile acid signaling. TRF introduces many new possibilities in treating obesity and its associated metabolic disorders. However, further studies are needed to show whether these physiological findings in mice translate to humans.
Keywords: circadian rhythm, diabesity, diabetes, dyslipidemia, dysmetabolism, fasting, metabolism, steatohepatitis, steatosis
Over the last decade, studies in mice and in humans have shown that the disruption of circadian rhythms contributes significantly to obesity.1,2 Strong evidence from recent publications shows that the preservation of natural feeding rhythms can prevent and reverse obesity and dysmetabolism associated with diet-induced obesity (DIO) in mice.3-6 In this commentary, we discuss the physiological effects of time-restricted feeding (TRF), including its effects on adipose tissue, adipokine signaling, lipid metabolism, gut microbiome, and stool metabolome. Furthermore, we speculate on the potential role it can play in the treatment of diabesity in human populations.
Time-Restricted Feeding (TRF) Prevents and Reverses Obesity
Numerous metabolic genes show a diurnal pattern of expression driven by both the circadian clock and feeding/fasting cycles.7 When fed a normal chow diet ad libitum, mice consume about 80% of their calories during the dark/active phase. However, when the diet is changed to one enriched in fat (high-fat diet, HFD) as in the DIO model, the timing of their caloric intake changes. DIO mice spread their caloric intake throughout the day, eventually leading to a 50/50 distribution of the food consumption between the dark/active and the light/inactive phase.3,4,8 DIO leads to the development of obesity and its associated metabolic disorders such as hepatic steatosis, insulin and leptin resistance, and dyslipidemia.3-5,8,9 Furthermore, the change in the feeding pattern, where calories are consumed at suboptimal times, leads to the dampening, and even the obliteration of the cyclical variation of many metabolic genes in the DIO model.4
The consequences of the altered feeding behavior in DIO mice, in particular the increased food consumption during the light/inactive phase, was unclear. TRF can be used to preserve a more natural feeding rhythm while also maintaining the fasting state in the light/inactive phase. With TRF, feeding is consolidated to the dark/active phase and there is no access to food during the light/inactive phase. Limiting access to a HFD for 8–12 hour in the dark/active phase, does not reduce caloric intake nor the level of activity compared to that of DIO mice.3,4 Nevertheless, TRF protects mice from developing obesity and its associated comorbidities (Table 1).3-5 Furthermore, TRF restores the circadian expression of many metabolic genes to the levels observed in a normal chow fed mice.4 Our most recent studies show that TRF can reverse obesity in DIO.3 Furthermore, it prevents the adverse metabolic consequences of other nutritional challenges such as a high-fructose diet, or a high-sucrose/high-fat diet.3
Table 1.
Physiological Effects of Feeding a High-Fat Diet between Diet Induced Obesity (ad libitum access to food) and Time Restricted Feeding (8–12 hour access to food during active phase)
| Diet Induced Obesity* | Time Restricted Feeding* | |
|---|---|---|
| Body weight | Greatly Increased | Same |
| Activity | Same | Same |
| Calories consumed | Same or Slightly greater | Same |
| Diurnal feeding pattern | Dampened | Strengthened (as part of protocol) |
| Whole body energy expenditure (VO2) | Same | Increased |
| Diurnal rhythms of Circadian Oscillators (e.g., Per2, Bmal1, Rev-erbα) | Dampened | Same |
| Insulin sensitivity | Decreased | Same |
| Leptin sensitivity | Decreased | Same |
| Motor coordination | Decreased | Increased |
| Body composition from fat (Adiposity) | Increased | Same |
| Adipose tissue | Hypertrophied | Same |
| Macrophage infiltration of white adipose tissue | Present | Absent |
| Pro-inflammatory cytokines in white adipose tissue | Greatly Increased | Same |
| Hepatic steatosis | Present | Absent |
| Brown adipose tissue steatosis | Present | Absent |
| Fatty acid synthase activity | Slightly Increased | Decreased |
| Hepatic unsaturated fats | Greatly Increased | Mildly Increased |
| Stool bile acids | Same | Increased |
| Serum bile acids | Decreased diversity | Increased diversity |
Note: * When compared to mice fed a normal chow diet ad libitum.
TRF Affects Adiposity and Adipokine Levels, Adipose Tissue Inflammation, and Lipid Metabolism
For mice receiving a HFD, those on TRF are leaner than mice fed ad libitum and almost indistinguishable from the control mice fed a normal chow diet. The body composition analysis of TRF mice reveals that they have less body fat than their DIO counterparts.3,4 Accordingly, adiponectin and leptin were increased and decreased, respectively, in TRF mice.3,4 The observed decrease in fat was generalized to the whole body, affecting both canonical and non-canonical fat storing organs (e.g. liver, brown adipose tissue). In particular, adipocytes of the white adipose tissue (WAT) were much smaller in TRF mice. Furthermore, the brown adipose tissue (BAT), was protected from the “whitening” that usually occurs in DIO mice where large unicolor droplets resembling white adipocytes are observed.4
Hepatic lipid metabolism has been analyzed extensively in TRF and DIO mice. TRF mice have reduced expression of fatty acid synthase (Fasn), a key lipogenic gene that is controlled by the transcriptional repressor Rev-erbα, which is also a circadian oscillator component.4,10 In addition, TRF mice have reduced expression of peroxisome proliferator-activated receptor gamma (Pparγ), hence diminishing Pparγ-driven lipogenic gene expression, and its targets including stearoyl coA desaturase 1 (Scd1, an enzyme mediating fatty acid desaturation) and fatty acid elongase (Elov5). These changes coincide with significant decline in hepatic unsaturated fatty acids.4
Adipocyte hypertrophy in DIO is often accompanied with the accumulation of pro-inflammatory macrophages in the tissue and the development of an inflammatory environment.3,4 Both histological examinations of the tissue and quantification of pro-inflammatory cytokines revealed an absence of inflammation in the adipose tissue of TRF mice.3 Reduced inflammation in both WAT and BAT likely preserves their proper functioning, allowing appropriate trafficking of fat through the WAT and adequate energy burning in the BAT, thus maintaining whole-body lipid homeostasis.
The reduction of fat accumulation in adipose tissue of TRF mice could be due to reduced synthesis of fatty acids or increased fat oxidation. mRNA quantification of enzymes involved in lipid metabolism revealed that the expression of both synthesis and degradation enzymes increased in the WAT of TRF mice compared to their ad libitum counterparts, suggesting a higher turnover of fat in the tissue.3 This plasticity of the adipose tissue is essential in the regulation of lipid homeostasis. Indeed, adipose tissue regulates lipids trafficking by switching from fat storing in the anabolic state to fat release for subsequent fat oxidation in peripheral tissue in times of energy demand.
TRF Alters Gut Microbiome
Another potential mechanism through which TRF can affect host metabolism is by altering the gut microbiome to one that is less obesogenic. Over the last decade, studies have uncovered profound changes in the composition and metabolic contribution of gastrointestinal microflora in the obese.11-13 However the mechanism underlying the microbiome's contribution to obesity remains unclear. Earlier studies suggest that DIO causes a dysbiosis that could affect host metabolism by altering bile acid (BA) signaling, disrupting intestinal homeostasis, altering nutritional absorption, and/or activating the gut inflammatory cascade.14-17 However, most gut microbiome studies in mouse models either alter the nutritional quality of the diet or utilize transgenic strains. These are potential factors that could obscure specific changes in the microflora that may be protective against obesity and metabolic diseases, thereby hindering our ability to find specific mechanisms that explain how dysbiosis contributes to obesity. TRF provides an ideal backdrop to study intestinal microflora since the factors that have confounded previous studies do not apply to this model.
Measuring the cecal gut microbiome at different time points in mice fed a normal chow diet reveals cyclical variations in many members of the microflora.5 This was apparent at the phylum level with Firmicutes levels rising during the dark/active phase when the animal feeds, and decreasing during the light/inactive phase with relative fasting. Likewise, the Bacteriodetes species and Verrucomicrobia both increased during the light/inactive phase and decreased during the dark/active phase. In DIO mice, where caloric intake is spread throughout the day and night, these cyclical variations observed at the phylum level were obliterated, with Firmicutes phylum dominating the gut microbiome at all of the time points. The TRF protocol did not restore the cyclical variations at the level of the phylum.5
Nevertheless, TRF did restore cyclical variation in many families of bacteria that are thought to be involved in metabolism.5 For example, TRF restored cyclical variation in the Lactobacillus family, which is also cyclical in normal chow mice, but not in DIO mice. Several Lactobacillus species have been associated with diabesity.15,18-21 Members of the Lactobacillus species express bile salt hydrolases which conjugate gut luminal BAs and can affect BA signaling.15 In addition, TRF restored the Ruminococcacea family, including those of the genus Oscillibacter, which are hypothesized to be protective against the metabolic consequences of obesity.21
Previous studies have suggested that the Firmicutes phyla was a contributor to obesity, and that a higher proportion of Firmicutes species in the gut microbiome corresponds to increased adiposity. However, measuring the microbiome at multiple time points in normal mice, and in the TRF mice shows that the level of Firmicutes species is related to the diet and feeding pattern, rather than obesity or dysmetabolism itself. This is supported by previous literature that showed that the Firmicutes phyla, as a whole, is not obesogenic and that it can change within 24 hours after a change in diet.22-24 Furthermore, a low gut microbiome α-diversity (i.e. the types and relative amounts of species within a sample) was also hypothesized to contribute to obesity. However, when the α-diversity was averaged between all time points, there was no difference among normal chow ad libitum, TRF, and DIO mice. Fluctuations in α-diversity were related to diet and feeding time as opposed to the metabolic phenotype.5
TRF Alters Stool Metabolome
The alterations observed in the gut microbiome of the TRF mice corresponded to shifts observed in the stool metabolome.5 These changes were observed mostly in compounds that only gut microbiome can affect: (1) breakdown products of complex sugars and (2) BAs. These changes in the stool metabolome could hint at the mechanisms through which the gut microbiome affects host metabolism.
One potential manner the gut microbiome can affect host metabolism is that dysbiosis affects the host's nutritional absorption. In particular, the digestion of complex sugars such as hemicellulose can only be done with the aide of gut microflora, since there are no innate host enzymes that can break them down. TRF mice excreted far more breakdown products of hemicellulose (i.e., xylose and galactose), suggesting that the fermentation occurs in a region of the gut where they cannot be easily absorbed (e.g., distal colon). However, DIO mice excreted far less xylose and galactose suggesting that these animals are more efficient in absorbing them.
TRF and Bile Acids
TRF fed mice had significant changes in primary and secondary BA levels and composition in multiple compartments (i.e. the serum, the liver and the feces) compared to DIO and mice fed normal chow ad libitum.3-5 Primary BAs are produced by the liver and released during meals to facilitate triglyceride and cholesterol absorption. In the gut, they are modified by gut microbiota that express a variety of deconjugation, dehydrogenation, and dehydroxylation enzymes, leading to the formation of secondary BAs.25 The chemical diversity of BAs is vast and properties of individual BAs only recently being understood. Aside from their essential role in fat absorption, they act as signaling molecules, through interactions with receptors including farnesoid X receptor α (FXRα) or the G protein-coupled BA receptor 1 (TGR5). In hepatocytes, FXRα signaling modulates BA, lipid, cholesterol and glucose metabolism.26-28 Hence, BAs could act as general metabolic integrators and an agent for gut-liver signaling. Different BAs can act as either agonist or antagonist of the same receptor. For example, hydrophobic chenodeoxycholic acid is the most potent FXRα agonist while hydrophilic muricholic acid has been shown to be an FXRα antagonist.29
The luminal BA profile and diversity is affected by the gut microbiome. This is a means by which the gut microbiome can affect host metabolism. For example, Lactobacillus species increase FXRα activity by conjugating tauro-β muricholic acid, an FXRα antagonist.15 Hence different amount of Lactobacillus species in the TRF and DIO gut microbiome could mediate their affects through altered BA signaling. TRF mice have stool that is highly enriched in both primary and secondary BAs.5
Primary BA composition in the liver and serum are quite different between TRF and DIO mice.3,4 For instance, cholate is more abundant in the serum of DIO mice than TRF. These proportions were inverted in the liver and feces where cholate levels are higher in TRF than DIO mice. Cholate can induce the expression of key genes involved in energy expenditure in the BAT by activating TGR5. Increased energy expenditure can lead to weight loss and fat reduction by increasing fat burning.
In sum, the diversity of the BA pool can mediate the effects of TRF. The ratio of certain BAs, some of which are FXRα agonists and antagonists (e.g. chenodeoxycholic acid v. muricholic acid v. tauro-β muricholic acid) could affect host lipid metabolism. These pathways are quite altered in TRF and DIO mice and could explain how the former's benefits are mediated (Fig. 1).
Figure 1.
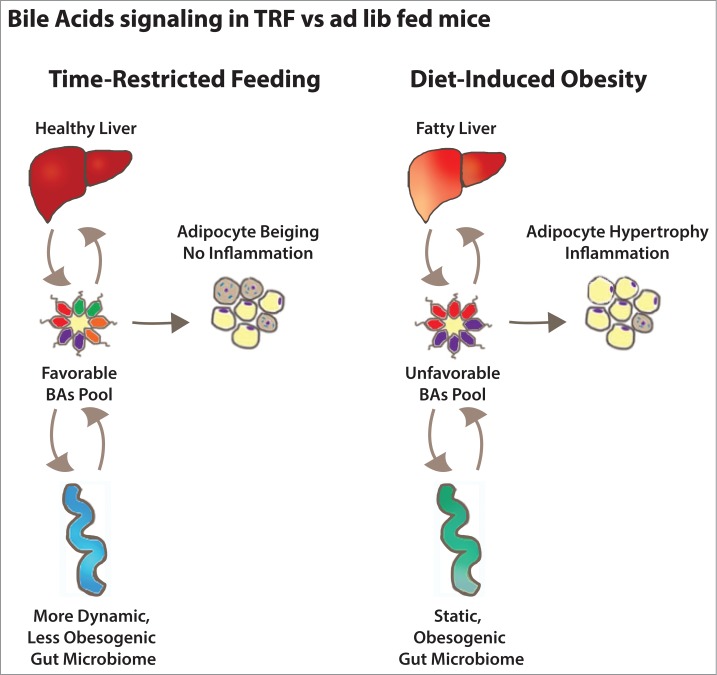
Bile acid signaling in mice fed a high-fat diet ad libitum or time-restricted feeding. The composition of the total bile acid pool is determined by the action of the liver (synthesis), the gut (reabsorption and excretion) and the gut microbiota (bile acids modification). Bile acids feedback on the liver and the gut regulate bile acids homeostasis and also influence their metabolic functions. They also act on the adipose tissue to regulate fat storage and utilization.
TRF and Treating Obesity in Humans
More than one third (34.9%) of the US adult population is obese,30 and it is estimated to cost the healthcare system $147 billion dollars annually.31 Obesity is associated with multiple morbidities including diabetes, heart disease and cancer. However, there is a lack of effective, sustainable, non-surgical treatments of obesity.32 Although light:dark cycle is known to affect the activity of the central clock, current research has shown daily cycle of eating-fasting rhythm in humans is a major determinant of daily metabolic rhythms.7 Multiple studies have shown shift-workers are particularly susceptible to metabolic syndrome and obesity.33 Even brief disturbances in sleep and feeding cycle in healthy individuals can affect insulin sensitivity.34 Furthermore, after controlling for diet and lifestyle, an aberrant eating pattern over the course of a 24 hour day such as late night caloric intake, is a significant risk factor for developing coronary heart disease (CHD) by increasing CHD risk by as much as 55%.35 Hence, TRF introduces multiple potential therapeutic methods to address this growing healthcare problem.
Part of the appeal of TRF is that it works with multiple dietary challenges (e.g., high-fat diet, high-fructose diet). Hence the behavioral intervention to treat obesity could be for patients to restrict the time that they feed, which may be easier to adopt than monitoring their caloric or macronutrient intake. Furthermore, individuals with a lower socioeconomic status, and disadvantaged minorities (e.g. Hispanics, Native Americans) are especially vulnerable to obesity and its associated metabolic disorders. These individuals are more likely to live in food deserts, where access to fresh and nutritious food remains poor. Hence, TRF may be an easier behavioral modification for these particular at-risk populations.
TRF has also identified potential obesity-protective bacteria (e.g., Oscillibacter, Ruminococcacea) which can be administered with probiotics or promoted with prebiotics as a potential treatment. Causing a more dynamic gut microbiome with timed antibiotics that are highly specific and non-absorbable could help reduce obesogenic bacteria (e.g. in the Lactobacillus family). Further studies of the metatranscriptome (i.e., the genes transcribed at a given time by the gut microflora) can help develop therapies that induce obesity-protective bacterial genes, or silence obesogenic bacterial genes. Investigations are already underway to evaluate the role of transgenic bacteria with obesity-protective genes. Preliminary studies with fecal transplantation have been promising, further signifying that targeting gut microbiome to treat obesity and metabolic disease is an appropriate therapeutic strategy that should be investigated more thoroughly.
Potential therapies also exist in making the expression of metabolic genes more robustly cyclical with higher amplitude fluctuations. Though circadian fluctuations and their effects on metabolism have been thoroughly investigated in murine models, they remain poorly understood in primates, including humans. Nevertheless, lab-induced jet lag, as well as long-standing evidence from shift-workers show the negative consequences of discombobulating the normal circadian metabolic machinery.
One way that cyclical changes in the host metabolism can be induced is with BA mimetics that target FXRα and TGR5 receptors. These compounds are currently being actively investigated by multiple pharmaceutical companies and newly discovered ones have received tremendous press from scientific and lay audience.36 Other compounds that signal feeding from gut to liver or gut to brain that can affect metabolism or appetite, respectively, are also actively being investigated.
Still, TRF tests in humans are preliminary and its effects in a clinical population, at this time, remains purely speculative. Currently there are multiple active clinical studies investigating whether its effects hold in particular vulnerable populations. With further studies, it will be interesting to see if this fascinating metabolic phenomenon translates to humans.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank Satchidananda Panda and Hiep Le for critical review of our manuscript.
Funding
AC received salary support from an American Diabetes Association Mentor-Based Postdoctoral Fellowship (7–12-MN-64). A.Z. received support from NIH T32 DK07202, P50 GM085764, KL2 TR00099, and R24 DK080506; AASLD Liver Scholar Award; and the American Gastroenterological Association Research Foundation Elsevier Pilot Research Award and Microbiome Junior Investigator Research Award.
References
- 1.Huang W, Ramsey KM, Marcheva B, Bass J. Circadian rhythms, sleep, and metabolism. J Clin Invest 2011; 121:2133-41; PMID:21633182; http://dx.doi.org/ 10.1172/JCI46043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science 2010; 330:1349-54; PMID:21127246; http://dx.doi.org/ 10.1126/science.1195027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaix A, Zarrinpar A, Miu P, Panda S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab 2014; 20:991-1005; PMID:25470547; http://dx.doi.org/ 10.1016/j.cmet.2014.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JA, et al.. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab 2012; 15:848-60; PMID:22608008; http://dx.doi.org/ 10.1016/j.cmet.2012.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zarrinpar A, Chaix A, Yooseph S, Panda S. Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab 2014; 20:1006-17; PMID:25470548; http://dx.doi.org/ 10.1016/j.cmet.2014.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sherman H, Genzer Y, Cohen R, Chapnik N, Madar Z, Froy O. Timed high-fat diet resets circadian metabolism and prevents obesity. FASEB J 2012; 26:3493-502; PMID:22593546; http://dx.doi.org/ 10.1096/fj.12-208868 [DOI] [PubMed] [Google Scholar]
- 7.Vollmers C, Gill S, DiTacchio L, Pulivarthy SR, Le HD, Panda S. Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proc Natl Acad Sci U S A 2009; 106:21453-8; PMID:19940241; http://dx.doi.org/ 10.1073/pnas.0909591106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, Turek FW, Bass J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab 2007; 6:414-21; PMID:17983587; http://dx.doi.org/ 10.1016/j.cmet.2007.09.006 [DOI] [PubMed] [Google Scholar]
- 9.West DB, York B. Dietary fat, genetic predisposition, and obesity: lessons from animal models. Am J Clin Nutr 1998; 67:505S-12S; PMID:9497161 [DOI] [PubMed] [Google Scholar]
- 10.Cho H, Zhao X, Hatori M, Yu RT, Barish GD, Lam MT, Chong LW, DiTacchio L, Atkins AR, Glass CK, et al.. Regulation of circadian behaviour and metabolism by REV-ERB-alpha and REV-ERB-beta. Nature 2012; 485:123-7; PMID:22460952; http://dx.doi.org/ 10.1038/nature11048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greiner T, Backhed F. Effects of the gut microbiota on obesity and glucose homeostasis. Trends Endocrinol Metab 2011; 22:117-23; PMID:21353592; http://dx.doi.org/ 10.1016/j.tem.2011.01.002 [DOI] [PubMed] [Google Scholar]
- 12.Tilg H, Kaser A. Gut microbiome, obesity, and metabolic dysfunction. J Clin Invest 2011; 121:2126-32; PMID:21633181; http://dx.doi.org/ 10.1172/JCI58109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature 2012; 489:242-9; PMID:22972297; http://dx.doi.org/ 10.1038/nature11552 [DOI] [PubMed] [Google Scholar]
- 14.Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ, et al.. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 2012; 482:179-85; PMID:22297845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li F, Jiang C, Krausz KW, Li Y, Albert I, Hao H, Fabre KM, Mitchell JB, Patterson AD, Gonzalez FJ. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat Commun 2013; 4:2384; PMID:24064762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mukherji A, Kobiita A, Ye T, Chambon P. Homeostasis in intestinal epithelium is orchestrated by the circadian clock and microbiota cues transduced by TLRs. Cell 2013; 153:812-27; PMID:23663780; http://dx.doi.org/ 10.1016/j.cell.2013.04.020 [DOI] [PubMed] [Google Scholar]
- 17.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006; 444:1027-31; PMID:17183312; http://dx.doi.org/ 10.1038/nature05414 [DOI] [PubMed] [Google Scholar]
- 18.Joyce SA, MacSharry J, Casey PG, Kinsella M, Murphy EF, Shanahan F, Hill C, Gahan CG. Regulation of host weight gain and lipid metabolism by bacterial bile acid modification in the gut. Proc Natl Acad Sci U S A 2014; 111:7421-6; PMID:24799697; http://dx.doi.org/ 10.1073/pnas.1323599111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Million M, Angelakis E, Maraninchi M, Henry M, Giorgi R, Valero R, Vialettes B, Raoult D. Correlation between body mass index and gut concentrations of Lactobacillus reuteri, Bifidobacterium animalis, Methanobrevibacter smithii and Escherichia coli. Int J Obesity 2013; 37:1460-6; PMID:23459324; http://dx.doi.org/ 10.1038/ijo.2013.20 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Million M, Maraninchi M, Henry M, Armougom F, Richet H, Carrieri P, Valero R, Raccah D, Vialettes B, Raoult D. Obesity-associated gut microbiota is enriched in Lactobacillus reuteri and depleted in Bifidobacterium animalis and Methanobrevibacter smithii. Int J Obesity 2012; 36:817-25; PMID:21829158; http://dx.doi.org/ 10.1038/ijo.2011.153 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Raman M, Ahmed I, Gillevet PM, Probert CS, Ratcliffe NM, Smith S, Greenwood R, Sikaroodi M, Lam V, Crotty P, et al.. Fecal microbiome and volatile organic compound metabolome in obese humans with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2013; 11:868-75 e1-3; PMID:23454028; http://dx.doi.org/ 10.1016/j.cgh.2013.02.015 [DOI] [PubMed] [Google Scholar]
- 22.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, et al.. Diet rapidly alters the human gut microbiome. Nature 2014; 505:559-63; ; http://dx.doi.org/ 10.1038/nature12820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duncan SH, Lobley GE, Holtrop G, Ince J, Johnstone AM, Louis P, Flint HJ. Human colonic microbiota associated with diet, obesity and weight loss. Int J Obesity 2008; 32:1720-4; PMID:18779823; http://dx.doi.org/ 10.1038/ijo.2008.155 [DOI] [PubMed] [Google Scholar]
- 24.Hildebrandt MA, Hoffmann C, Sherrill-Mix SA, Keilbaugh SA, Hamady M, Chen YY, Knight R, Ahima RS, Bushman F, Wu GD. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology 2009; 137:1716-24 e1-2; PMID:19706296; http://dx.doi.org/ 10.1053/j.gastro.2009.08.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hagey LR, Krasowski MD. Microbial biotransformations of bile acids as detected by electrospray mass spectrometry. Adv Nutr 2013; 4:29-35; PMID:23319120; http://dx.doi.org/ 10.3945/an.112.003061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calkin AC, Tontonoz P. Transcriptional integration of metabolism by the nuclear sterol-activated receptors LXR and FXR. Nat Rev Mol Cell Biol 2012; 13:213-24; PMID:22414897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cariou B, van Harmelen K, Duran-Sandoval D, van Dijk TH, Grefhorst A, Abdelkarim M, Caron S, Torpier G, Fruchart JC, Gonzalez FJ, et al.. The farnesoid X receptor modulates adiposity and peripheral insulin sensitivity in mice. J Biol Chem 2006; 281:11039-49; PMID:16446356; http://dx.doi.org/ 10.1074/jbc.M510258200 [DOI] [PubMed] [Google Scholar]
- 28.Duran-Sandoval D, Cariou B, Percevault F, Hennuyer N, Grefhorst A, van Dijk TH, Gonzalez FJ, Fruchart JC, Kuipers F, Staels B, et al.. The farnesoid X receptor modulates hepatic carbohydrate metabolism during the fasting-refeeding transition. J Biol Chem 2005; 280:29971-9; PMID:15899888; http://dx.doi.org/ 10.1074/jbc.M501931200 [DOI] [PubMed] [Google Scholar]
- 29.Kuipers F, Bloks VW, Groen AK. Beyond intestinal soap–bile acids in metabolic control. Nat Rev Endocrinol 2014; 10:488-98; PMID:24821328; http://dx.doi.org/ 10.1038/nrendo.2014.60 [DOI] [PubMed] [Google Scholar]
- 30.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA 2014; 311:806-14; http://dx.doi.org/ 10.1001/jama.2014.732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff 2009; 28:w822-31; PMID:19635784; http://dx.doi.org/ 10.1377/hlthaff.28.5.w822 [DOI] [PubMed] [Google Scholar]
- 32.Picot J, Jones J, Colquitt JL, Gospodarevskaya E, Loveman E, Baxter L, Clegg AJ. The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation. Health Technol Assess 2009; 13:1-190, 215-357, iii-iv; PMID:19726018; http://dx.doi.org/ 10.3310/hta13410 [DOI] [PubMed] [Google Scholar]
- 33.Karlsson B, Knutsson A, Lindahl B. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people. Occup Environ Med 2001; 58:747-52; PMID:11600731; http://dx.doi.org/ 10.1136/oem.58.11.747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reutrakul S, Hood MM, Crowley SJ, Morgan MK, Teodori M, Knutson KL, Van Cauter E. Chronotype is independently associated with glycemic control in type 2 diabetes. Diabetes Care 2013; 36:2523-9; PMID:23637357; http://dx.doi.org/ 10.2337/dc12-2697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cahill LE, Chiuve SE, Mekary RA, Jensen MK, Flint AJ, Hu FB, Rimm EB. Prospective study of breakfast eating and incident coronary heart disease in a cohort of male US health professionals. Circulation 2013; 128:337-43; PMID:23877060; http://dx.doi.org/ 10.1161/CIRCULATIONAHA.113.001474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fang S, Suh JM, Reilly SM, Yu E, Osborn O, Lackey D, Yoshihara E, Perino A, Jacinto S, Lukasheva Y, et al.. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nature Med 2015; 21(2):159-65; PMID:25559344; http://dx.doi.org/ 10.1038/nm.3760 [DOI] [PMC free article] [PubMed] [Google Scholar]


