Abstract
Since the publication of the 1998 special issue of Hormones and Behavior on estrogens and cognition, substantial progress has been made towards understanding the molecular mechanisms through which 17β-estradiol (E2) regulates hippocampal plasticity and memory. Recent research has demonstrated that rapid effects of E2 on hippocampal cell signaling, epigenetic processes, and local protein synthesis are necessary for E2 to facilitate the consolidation of object recognition and spatial memories in ovariectomized female rodents. These effects appear to be mediated by non-classical actions of the intracellular estrogen receptors ERα and ERβ, and possibly by membrane-bound ERs such as the G-protein-coupled estrogen receptor (GPER). New findings also suggest a key role of hippocampally-synthesized E2 in regulating hippocampal memory formation. The present review discusses these findings in detail and suggests avenues for future study.
Keywords: estrogen, hippocampus, estrogen receptor, cell signaling, epigenetic, ERK, PI3K, mTOR, histone acetylation, DNA methylation, GPER
Graphical abstract

Introduction
In 1998, Hormones and Behavior published a special issue entitled, “Estrogen Effects on Cognition across the Lifespan” (Volume 34(2), October). Guest edited by Christina Williams, the special issue featured papers from leaders in the fledgling field of “hormones and cognition”. The articles of the special issue deftly summarized the progress made in the relatively short time since estrogens were found to regulate dendritic spine density on pyramidal neurons in the hippocampus (Gould et al., 1990; Woolley et al., 1990; Woolley and McEwen, 1992, 1993). At the time, I was a postdoctoral fellow studying the relationship between age-related memory loss and biochemical alterations in the hippocampus and basal forebrain in mice. Our findings led me to learn about how sex steroid hormones influence the septo-hippocampal system and hippocampal memory. As such, the 1998 volume became a bible of sorts for me. I marked it up, referred to it often, and carried it with me on faculty job interviews as a sort of security blanket when I wanted to make sure I had my facts straight. Needless to say, my copy is well worn and I can still find it in my office at a moment’s notice. Although there remains much work to do, we have learned an enormous amount in the past 17 years about how estrogens regulate cognitive function. Given the tremendous advances made since 1998, it seems high time for another special issue that can serve to inspire young scientists in the way that the previous special issue inspired me.
In recent years, laboratories including my own have made progress towards elucidating the molecular mechanisms through which the potent estrogen 17β-estradiol (E2) regulates hippocampal memory consolidation in female mice. These mechanisms underlie the so-called “rapid” effects of E2 on hippocampal functioning, which encompass those that occur within minutes of E2 exposure. In vivo, E2 activates numerous cell-signaling cascades and alters epigenetic processes in the dorsal hippocampus within 5–30 minutes of treatment, and these actions are necessary for E2 to enhance the consolidation of hippocampal memories (Bi et al., 2000; Bi et al., 2001; Fan et al., 2010; Fernandez et al., 2008; Fortress et al., 2013; Lewis et al., 2008; Zhao and Brinton, 2007; Zhao et al., 2012; Zhao et al., 2010). In vitro studies report that rapid E2-induced activation of some of these same cell-signaling pathways promotes dendritic spine remodeling (Hasegawa et al., in press; Kramár et al., 2009; Srivastava et al., 2008), thus linking estrogenic regulation of spinogenesis to memory formation. Moreover, the discovery that E2 is synthesized and released within the hippocampus (Hojo et al., 2004; Kretz et al., 2004; Prange-Kiel et al., 2006) raises the exciting possibility that learning-induced endogenous E2 synthesis by hippocampal neurons may stimulate the rapid molecular alterations that are necessary for memory formation. Given the emerging importance of rapid E2 effects for hippocampal memory, this review will focus largely on findings detailing the rapid cell signaling, epigenetic, and receptor mechanisms necessary for E2 to enhance hippocampal memory consolidation.
E2 and the hippocampus
Spinogenesis, neurogenesis, and long-term potentiation
Although they were controversial at the time of their publication, the groundbreaking findings showing that exogenous E2 and progesterone increase dendritic spine density on CA1 pyramidal neurons (Woolley and McEwen, 1993) provided incontrovertible evidence that so-called “ovarian” hormones influence hippocampal morphology. Numerous labs have since replicated these findings (e.g., (Frick et al., 2004; Inagaki et al., 2012; MacLusky et al., 2005; Murphy and Segal, 1996; Segal and Murphy, 2001)). Newer data show that E2 also regulates dendritic spine density on neurons in the medial prefrontal cortex, somatosensory cortex, and amygdala (de Castilhos et al., 2008; Hao et al., 2006; Inagaki et al., 2012; Khan et al., 2013; Srivastava et al., 2008), as well as dendritic length in the basal forebrain (Saenz et al., 2006). As such, E2 clearly promotes spinogenesis in multiple regions of the brain that regulate cognitive function. However, much less is known about the role of E2 in mediating the function of brain regions other than the hippocampus. Within the hippocampus, dendritic spinogenesis is accompanied by the E2-induced facilitation of synaptic plasticity. For example, E2 increases glutamate binding to hippocampal NMDA receptors and increases several measures of intrinsic excitability, leading to enhanced sensitivity of CA1 pyramidal neurons to NMDA-receptor mediated synaptic inputs (Carrer et al., 2003; Kumar and Foster, 2002; Wong and Moss, 1992; Woolley et al., 1997). E2 also enhances long-term potentiation (LTP) at CA3-CA1 synapses (Bi et al., 2000; Foy et al., 1999; Kramár et al., 2009; Smith and McMahon, 2005; Vedder et al., 2013; Woolley et al., 1997), which is important because LTP is thought to underlie hippocampal memory formation. It was recently found that E2-induced enhancements in both object recognition and LTP occurred within a similar time frame and required a functional increase in NR2B-containing NMDA receptors (Vedder et al., 2013), linking E2-induced changes in LTP with hippocampal memory formation. Interestingly, the induction of LTP and spinogenesis by E2 in hippocampal slices is blocked by inhibitors of several cell-signaling pathways, including extracellular signal-regulated kinase (ERK), protein kinase A (PKA), protein kinase C (PKC), phosphatidylinositol 3-kinase (PI3K), and calcium calmodulin kinase II (CaMKII) (Hasegawa et al., in press). These data suggest that the effects of E2 on hippocampal synaptic plasticity are regulated by many of the rapid molecular alterations that are also necessary for E2 to enhance memory consolidation (see Cell-signaling mechanisms necessary for E2-induced memory enhancement below).
A substantial literature shows that E2 also regulates neurogenesis in the dentate gyrus of the hippocampus (e.g., (Galea et al., 2013; Ormerod et al., 2003; Tanapat et al., 1999)), generally facilitating the incorporation of new neurons into the established neural circuitry. In particular, numerous studies have investigated the role of hormones in mediating the effects of reproductive experience, stress, depression, and aging on hippocampal neurogenesis in both female and male rodents (Galea et al., 2013; Glasper and Gould, 2013). Interestingly, the effects of E2 on hippocampal neurogenesis appear to depend on timing of treatment, as E2 given prior to the labeling of new neurons increases neuron proliferation and survival (McClure et al., 2013), whereas E2 given after cell labeling decreases new neuron survival (Chan et al., 2014). These data suggest that E2 can enhance neurogenesis when levels are elevated at the time that new neurons are born, but perhaps not afterwards.
Types and distribution of estrogen receptors
How might E2 regulate hippocampal morphology and synaptic plasticity? The canonical intracellular estrogen receptors, ERα and ERβ, are distributed throughout the dorsal-ventral extent of the hippocampus (Mitra et al., 2003; Mitterling et al., 2010; Shughrue et al., 1997a; Shughrue et al., 1997b; Shughrue and Merchenthaler, 2000). Within hippocampal neurons, ERα and ERβ are located within the nucleus, dendritic spines, and axon terminals of pyramidal neurons and interneurons (Milner et al., 2005; Milner et al., 2001; Mitra et al., 2003; Mitterling et al., 2010; Waters et al., 2011). In the nucleus, ERα and ERβ mediate the classical “genomic” effects of estrogens, in which an estrogen-ER complex binds to an estrogen response element on the DNA to promote gene transcription (Fig. 1). However, the localization of these receptors to dendritic spines and axon terminals suggests an additional mechanism of action for ERα and ERβ at these more distal sites; this mechanism is commonly referred to as “non-genomic” or “non-classical” (Fig. 1). Indeed, E2 causes ERβ to translocate to the plasma membrane in hippocampal-derived cell lines and rat primary cortical neurons (Sheldahl et al., 2008). Moreover, both ERα and ERβ interact with metabotropic glutamate receptor 1 (mGluR1) to rapidly activate hippocampal ERK signaling and promote the phosphorylation of cAMP response element binding protein (CREB) (Boulware et al., 2013; Boulware et al., 2005). The ability of each ER to associate with mGluRs and phosphorylate CREB is dependent on S-palmitoylation (Meitzen et al., 2013), a post-translational modification associated with intracellular protein trafficking (Fukata and Fukata, 2010). This finding helps to explain how ERα and ERβ can be shuttled to the plasma membrane to trigger rapid cell-signaling processes. Other data implicate NMDA receptor activation in E2-induced activation of hippocampal ERK and facilitation of object recognition memory consolidation (Lewis et al., 2008), suggesting the involvement of multiple neurotransmitter receptors in the memory-enhancing effects of E2. As will be discussed below, E2 activates numerous other cell-signaling cascades in hippocampal and cortical neurons within 5 minutes of intracranial infusion (Fan et al., 2010; Fortress et al., 2013; Manella and Brinton, 2006; Yokomaku et al., 2003). Although these rapid effects on cell signaling are necessary for E2 to enhance memory consolidation (Fan et al., 2010; Fernandez et al., 2008; Fortress et al., 2013; Lewis et al., 2008), the potential role of classical genomic effects in memory formation should not be discounted, as classical and non-classical receptor mechanisms may interact to regulate memory.
Figure 1.
Classical (genomic) and non-classical (non-genomic) mechanisms of E2 action. In the classical mechanism (left), E2 binds to ERα and ERβ in the cytoplasm, and then the E2-ER complex translocates into the nucleus and binds to an estrogen response element (ERE) on the DNA. Together with histone acetyltransferases (HAT) and other co-regulators (Co), the ERs facilitate gene transcription. Non-classical mechanisms (right) involve action at or near the plasma membrane. ERα and ERβ in the dorsal hippocampus interact with metabotropic glutamate receptor 1a (mGluR1a) to rapidly activate extracellular signal-regulated kinase (ERK) cell signaling, which triggers epigenetic alterations such as histone acetylation, local protein synthesis via the mammalian target of rapamycin (mTOR) cell-signaling pathway, and gene expression via the transcription factor cAMP response element binding protein (CREB). NMDA receptor activation in the dorsal hippocampus is also necessary for E2 to activate ERK. G-protein-coupled estrogen receptor (GPER) rapidly activates c-Jun N-terminal kinase (JNK) cell signaling in the dorsal hippocampus, although E2 does not appear to mediate this effect. Effects of GPER activation on epigenetic processes, gene expression, and protein translation are not yet known.
In addition to ERα and ERβ, which are generally thought to be located intracellularly, the effects of estrogens may also be mediated by ERs located within the plasma membrane. Several putative receptors have been identified, including the recently renamed G-protein-coupled ER (GPER or GPER1, formerly GPR30), ER-X, and Gq-ER (Filardo et al., 2000; Qiu et al., 2003; Toran-Allerand et al., 2002). It is thought that these receptors bind E2 and then transduce its signal by triggering second messenger cascades, which then lead to epigenetic alterations, gene transcription, and protein translation (Fig. 1). However, the existence of plasma membrane ERs has been the matter of extensive debate, largely because they have been difficult to clone (Levin, 1999). Nevertheless, studies using a bovine serum albumin-conjugated form of E2 (BSA-E2) that cannot traverse the plasma membrane have demonstrated that BSA-E2 rapidly activates signaling cascades including ERK, and induces similar ERK-dependent enhancement of object recognition memory consolidation to that observed with free E2 (Fernandez et al., 2008; Wade et al., 2001; Watters et al., 1997). Although these findings suggest a role for membrane ERs in the mnemonic effects of E2, the specific receptors mediating these effects remain unclear. Currently, the best candidate is GPER, which has been shown in ovariectomized rats to mediate spatial working memory acquisition (Hammond et al., 2009; Hammond et al., 2012). GPER has can be found in all lamina within the hippocampus, exclusively at extranuclear sites within pyramidal neurons, interneurons, and glia (Brailoiu et al., 2007; Waters et al., 2015). Within these cells, GPER has been observed within dendrites, dendritic spines, axons, terminals, and cell bodies, where it can generally be found at or near the plasma membrane in association with post-synaptic scaffolding proteins (Akama et al., 2013; Waters et al., 2015). Interestingly, GPER immunoreactivity in dendrites, spines, terminals, and axons differs in CA1, CA3, and the dentate gyrus during estrus relative to proestrus (Waters et al., 2015), suggesting that GPER expression is regulated by estrogens and/or progestins. However, as shall be discussed later, the molecular mechanisms through which dorsal hippocampal GPER regulates object memory consolidation differ from those of E2.
Hippocampal E2 synthesis
As indicated above, it is important to note that E2 and other sex steroid hormones are synthesized within the hippocampus (Hojo et al., 2004; Ikeda et al., 2015; Kretz et al., 2004; Prange-Kiel et al., 2006). In fact, E2 levels are substantially higher in the hippocampus than in the plasma among both male and female rats (Hojo et al., 2009; Kato et al., 2013). Among female rats, hippocampal E2 levels are significantly higher than plasma levels at every stage of the estrous cycle and after ovariectomy (Kato et al., 2013). Hippocampal E2 levels in ovariectomized female rats are similar to those of gonadally-intact females during diestrus, metestrus, and estrus (Kato et al., 2013), suggesting that ovariectomy may not radically alter hippocampal E2 levels during most of the estrous cycle. These findings suggest that the primary endogenous source of E2 for hippocampal neurons may be hippocampal neurons or glia (Azcoitia et al., 2003; Garcia-Segura et al., 1999), rather than the ovaries.
The aromatase inhibitor letrozole decreases levels of E2 and its stereoisomer 17α-estradiol in the hippocampus (Ikeda et al., 2015; Kretz et al., 2004), and has been used in numerous studies to examine the role of hippocampally-synthesized E2 on hippocampal spine morphology and physiology. For example, in rat hippocampal slice cultures, letrozole reduced dendritic spine density, presynaptic bouton number, and synaptic protein levels (Kretz et al., 2004; Prange-Kiel et al., 2006). Letrozole treatment in hippocampal cultures also revealed divergent effects of ER agonists on dendritic spine density, such that an ERα agonist increased, whereas an ERβ agonist decreased, spine density in the presence of letrozole (Zhou et al., 2014). Further, systemic injections of letrozole have been associated with impaired LTP and transient dephosphorylation of the actin binding protein cofilin in gonadally-intact male rats and both intact and ovariectomized female rats (Vierk et al., 2012). Letrozole also reduced the numbers of mature spines, thin spines, and spine synapses in females, but only decreased thin spines in males (Vierk et al., 2012), suggesting a potential sex difference in the role of local E2 in regulating hippocampal spinogenesis.
Clues to the behavioral significance of de novo hippocampal E2 synthesis come from studies of zebra finches, which report that E2 is rapidly synthesized in the auditory caudo-medial nidopallium (NCM) of males during behavioral experiences such as exposure to female conspecifics or conspecific song (Remage-Healey et al., 2008). Our own preliminary data in female mice indicate that object learning stimulates dorsal hippocampal E2 release within 30 minutes (Tuscher et al., 2013). Importantly, studies using aromatase inhibitors show that blocking hippocampal E2 synthesis impairs hippocampal-dependent memory. For example, infusion of the aromatase inhibitor fadrozole into the male zebra finch hippocampus impairs spatial memory in a food-finding task (Bailey et al., 2013). In ovariectomized female mice, our preliminary data indicate that letrozole infusion into the dorsal hippocampus prevents memory consolidation in both the object recognition and object placement tasks (Tuscher et al., 2013). Object training also appears to transiently increase dorsal hippocampal E2 levels 30 minutes after training, an effect that is blocked by letrozole (Tuscher et al., 2013). A role for local E2 in memory among rodents is supported by another recent report that systemic injection of fadrozole prior to or immediately after fear extinction training in male rats significantly impaired fear recall during testing (Graham and Milad, 2014). Combined, these data suggest the intriguing possibility that the hippocampus synthesizes E2 in response to a learning event, and that this synthesis is necessary for hippocampal memory formation.
How might a role for hippocampally-synthesized E2 be reconciled with the literature demonstrating that ovarian-synthesized E2 influences hippocampal memory? At this point, the relative contributions of these two sources of E2 to memory formation are unclear. Ovarian-derived steroids may prime the hippocampus and other brain regions to respond to locally-synthesized E2. As such, E2 synthesized in the hippocampus in response to a learning event or other stimulus may produce greater synaptic potentiation and/or morphological alterations when circulating E2 levels are elevated. In this scenario, one might predict that aromatase inhibition would be most disruptive to memory consolidation during proestrus, when circulating E2 levels are the highest. Alternatively, circulating estrogens may simply be necessary for maintaining the general health of hippocampal neurons, but have little to do with memory formation per se. Some support for this idea comes from the well-established role of E2 as a neuroprotective factor in the adult and aging hippocampus (Brinton, 2001; Garcia-Segura et al., 2001; Wise et al., 2001). The loss of this neuroprotection after ovariectomy or during aging may render hippocampal neurons less responsive to hippocampally-synthesized E2 or more vulnerable to dysfunction due to aging, cellular damage, or ischemia. Future studies will need to test these possibilities and others to determine the unique roles of ovarian- and hippocampally-synthesized E2 to memory formation. In reading the remainder of this review, it is important to note that the rodent subjects used were bilaterally ovariectomized, which eliminates ovarian-synthesized E2. Although ovariectomy reduces one potential interaction with hippocampal-derived E2, it is unknown how exogenous E2 treatments might affect the synthesis of E2 within the hippocampus. Addressing these potential interactions will be an important issue for future study.
E2 and hippocampal memory
Given the ample evidence demonstrating that E2 regulates hippocampal morphology and physiology, it should be no surprise that E2 also influences hippocampal memory. The effects of E2 on hippocampal memory in rodents have been most commonly assessed in spatial tasks (e.g., Morris water maze, radial arm maze, delayed non-match to position, object location/placement) and in object recognition tasks. These effects have been enumerated in hundreds of empirical papers and many outstanding reviews (e.g., see the following reviews: (Choleris et al., 2012; Conrad and Bimonte-Nelson, 2010; Daniel, 2006; Frick, 2009; Gibbs, 2010; Luine, 2014, 2015; Luine and Frankfurt, 2012; Tuscher et al., 2015)), so they will not be discussed extensively here. In brief, most studies of female rodents report that systemic or intracranial E2 administration enhances memory acquisition and consolidation in spatial and object recognition tasks, although some studies found no such beneficial effects (Galea et al., 2001; Marriott et al., 2002). E2 also enhances hippocampal memory in male rodents (Luine and Rodriguez, 1994; Packard et al., 1996), indicating that estrogenic regulation of hippocampal memory is not unique to females. In general, the effects of E2 on hippocampal memory depend on many factors including dose, task type and difficulty, timing of injection relative to training, duration of treatment, duration of ovariectomy prior to treatment, presence of ovaries, number of prior pregnancies, and age at treatment (Frick, 2009; Tuscher et al., 2015; Workman et al., 2012). Nevertheless, the balance of studies supports the broad conclusion that E2 is beneficial for hippocampal memory in both female and male rodents.
Post-training E2 treatment
In recent years, numerous laboratories have reported that a single post-training administration of E2 systemically or intracranially enhances spatial and object recognition memory consolidation (see (Luine, 2015; Luine and Frankfurt, 2012; Tuscher et al., 2015) for reviews). For example, post-training administration of E2 systemically or into the dorsal hippocampus enhances spatial reference memory consolidation in the Morris water maze in male rats, ovariectomized female rats, and ovariectomized female mice (Gresack and Frick, 2006; Packard et al., 1996; Packard and Teather, 1997a, b). In female rats and mice, post-training E2 administered systemically or into the dorsal hippocampus or dorsal third ventricle also enhances spatial memory consolidation tested in an object placement (a.k.a., object location) task and object recognition memory consolidation tested in an object recognition task (Boulware et al., 2013; Gresack and Frick, 2006; Luine et al., 2003; Pereira et al., 2014; Walf et al., 2006). The beneficial effects of post-training E2 on object recognition and/or object placement in young and middle-aged females have been reported in at least two-dozen papers from multiple laboratories in both rats and mice (see (Tuscher et al., 2015) for review), suggesting a broad effect that generalizes across species, dose, route of administration, testing protocol, and types of hippocampal memory.
Because post-training E2 so consistently enhances hippocampal memory consolidation, my laboratory has used the post-training approach as a tool to understand the molecular mechanisms underlying the memory-enhancing effects of E2. In particular, we administer dorsal hippocampal or dorsal third ventricle infusions of a water-soluble form of E2 immediately after training in one-trial object recognition and object placement tasks to pinpoint the molecular mechanisms within the dorsal hippocampus through which E2 regulates memory consolidation. The benefits of this approach have been described in detail elsewhere (Frick, 2012; Frick et al., 2010), so will not be belabored here. However, it is important to note that the near simultaneous infusion of E2 into the dorsal third ventricle (adjacent to the dorsal hippocampus) and of receptor antagonists or enzyme inhibitors into the dorsal hippocampus has allowed us to discern which receptors, cell-signaling pathways, and epigenetic processes are necessary for E2 to enhance hippocampal memory consolidation. This approach requires the implantation of triple cannulae, which allows us to determine if blocking receptor activation or cellular processes bilaterally in the dorsal hippocampus prevents the memory-enhancing effects of an intracerebroventricular (ICV) E2 infusion. Infusing E2 just outside of the dorsal hippocampus prevents tissue damage in the dorsal hippocampus that could result from multiple sequential infusions. Importantly, this method permits identification of specific proteins and enzymatic events in the dorsal hippocampus that are essential for E2 to enhance hippocampal memory consolidation.
Our own efforts have focused on three realms of cellular functioning: cell signaling, epigenetics, and estrogen receptor mechanisms. Therefore, these processes will be discussed in detail in the remainder of this review. Figure 2 presents a schematic synthesis of our laboratory’s findings to provide a guide for the discussion below.
Figure 2.
Schematic illustration of the non-classical mechanisms required for E2 and ERs to enhance hippocampal memory consolidation. Phosphorylation of the p42 isoform of ERK is necessary for E2 to enhance object recognition memory consolidation. This phosphorylation is triggered by numerous upstream events including interactions between mGluR1a and the canonical ERs (ERα and ERβ), and activation of NMDA receptors, protein kinase A (PKA), and phosphatidylinositol-3-kinase (PI3K). E2-induced phosphorylation of ERK, PI3K, and Akt elicits mTOR signaling, promoting local protein synthesis. E2-activated ERK also transduces into the nucleus to phosphorylate the transcription factor CREB. Activation of ERK is also necessary for E2 to increase histone H3 acetylation; E2 increases H3 acetylation at the pII and pIV promoters of the Bdnf gene. DNA methylation is also necessary for E2 to enhance memory consolidation, although the specific genes methylated are unknown. Finally, GPER enhances memory consolidation by activating JNK, which facilitates gene expression via transcription factors such as ATF2.
Cell-signaling mechanisms necessary for E2-induced memory enhancement
ERK
Our initial studies focused on ERK signaling because ERK phosphorylation is necessary for rodents to form long-term hippocampal memories, including spatial memories, contextual fear memories, and object recognition memories (Atkins et al., 1998; Blum et al., 1999; Bozon et al., 2003; Kelly et al., 2003). Interestingly, early in vitro studies indicated that E2 or BSA-E2 increased ERK phosphorylation within 15 minutes of exposure in a variety of cell types including hippocampal neurons (Wade and Dorsa, 2003; Wade et al., 2001; Watters et al., 1997; Yokomaku et al., 2003). This activation was blocked by inhibitors of the enzyme mitogen activated protein kinase kinase (MAPKK or MEK), which is the exclusive upstream activator of ERK (Nilsen and Brinton, 2003; Yokomaku et al., 2003). Subsequent in vivo work demonstrated that an ICV infusion of E2 or BSA-E2 increased ERK phosphorylation in the hippocampus within 5 minutes, and that the classical nuclear estrogen receptor antagonist ICI 182-780 did not block this effect (Kuroki et al., 2000). These in vivo data suggested that E2 activates ERK via a non-genomic mechanism, which together with evidence demonstrating that hippocampal ERK activation is necessary for hippocampal memory formation, led us to hypothesize that hippocampal ERK activation was also necessary for E2 to enhance hippocampal memory consolidation.
Using ovariectomized 8–12 week-old C57BL/6 mice, we found that systemic injection of 0.2 mg/kg E2 increased phosphorylation of the p42, but not p44, isoform of ERK in the dorsal hippocampus 60 minutes later (Fernandez et al., 2008; Lewis et al., 2008). Similarly, bilateral dorsal hippocampal infusion of 5 μg/hemisphere or ICV infusion of 10 μg E2 increased phosphorylation of p42, but not p44, ERK, although on a much more rapid time scale; both intracranial infusions increased phospho-p42 levels within 5 minutes of infusion (Fernandez et al., 2008; Fortress et al., 2013; Zhao et al., 2012; Zhao et al., 2010). Although these data were consistent with previous in vitro work, the key question was whether this E2-induced ERK activation contributed to E2-induced memory consolidation.
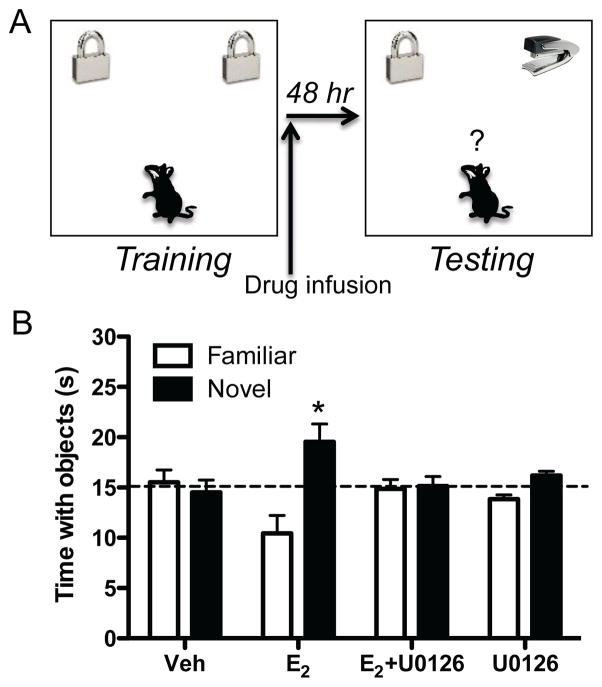
To address this issue, we allowed mice to accumulate 30 seconds exploring two identical objects in a square open field and then infused the MEK inhibitor U0126 into the dorsal hippocampus immediately before an ICV infusion of E2. We used a dose of U0126 that has no effect on memory on its own (Fernandez et al., 2008) to ensure that any potential blockade of E2’s effect on memory was due to an interaction between the two drugs and not a nonspecific effect of U0126 on general memory formation. Memory was then tested 48 hours later by measuring the amount of time spent with a novel object and an object identical to that explored during training (familiar) (Fig. 3A). At this 48-hour delay, vehicle-infused ovariectomized female mice do not remember the familiar object (Gresack et al., 2007), which allows us to observe potential memory-enhancing effects of E2 and related compounds. In contrast to vehicle-treated mice, mice receiving post-training dorsal hippocampal or ICV infusions of E2 spent more time exploring the novel object than chance (Fernandez et al., 2008; Fortress et al., 2013; Zhao et al., 2012; Zhao et al., 2010), indicating that E2 enhances object recognition memory consolidation (Fig. 3B). Consistent with our hypothesis, U0126 prevented E2 administered ICV (Fig. 3B) or systemically from enhancing object recognition (Fernandez et al., 2008; Fortress et al., 2013; Zhao et al., 2010), demonstrating that dorsal hippocampal ERK activation is necessary for E2 to enhance hippocampal memory consolidation. These data were the first to identify a specific molecular pathway through which E2 facilitates memory formation.
Figure 3.
Dorsal hippocampal ERK activation is necessary for E2 to enhance object recognition memory consolidation. (A) Illustration of the object recognition protocol used in our laboratory. During training, mice accumulate 30 seconds of time exploring two identical objects. Immediately after training, mice receive an infusion of E2 or other drugs into the dorsal hippocampus or dorsal third ventricle. Forty-eight hours later, mice accumulate 30 seconds with a novel object and an object identical to that explored during testing (familiar). Mice that remember the familiar object spend significantly more time than chance (15 sec) with the novel object. Adapted with permission from (Fortress and Frick, 2014; Frick, 2013). (B) ERK activation is necessary for E2 to enhance object recognition memory consolidation in ovariectomized 8–12 week-old C57BL/6 mice. Forty-eight hours after training, mice infused with 10 μg E2, but not vehicle, into the dorsal third ventricle spend significantly more time than chance (dashed line at 15 sec) with the novel object (*p < 0.05), demonstrating enhanced object recognition. This effect is blocked by dorsal hippocampal infusion of the ERK activation inhibitor U0126 (0.5 μg/hemisphere), which has no detrimental effect on memory on its own at this dose (Fernandez et al., 2008). Error bars represent the mean ± standard error of the mean (SEM). Reprinted with permission from (Zhao et al., 2010).
PKA and PI3K
We next sought to build on these findings by examining the contributions of signaling pathways upstream and downstream from ERK. Numerous upstream cell-signaling molecules, including PKA, PI3K, and Akt, activate ERK (Fig. 2) (Adams and Sweatt, 2002). These molecules also play key roles in hippocampal memory formation, and are activated in the hippocampus by E2 (Adams and Sweatt, 2002; Horwood et al., 2006; Kelly and Lynch, 2000; Lin et al., 2001; Manella and Brinton, 2006; Nguyen and Woo, 2003; Shingo and Kito, 2005; Yokomaku et al., 2003). Therefore, it was of interest to determine whether these enzymes were involved in E2’s regulation of hippocampal ERK and memory consolidation.
We first found that infusion of the PKA inhibitor Rp-cAMPS into the dorsal hippocampus prevented a post-training systemic injection of E2 from enhancing object recognition memory in ovariectomized mice (Lewis et al., 2008). Next, we showed that E2 significantly increased phosphorylation of PI3K and Akt in the dorsal hippocampus of young and middle-aged ovariectomized mice within 5 minutes of dorsal hippocampal or ICV infusion. In mice of both ages, dorsal hippocampal infusion of the PI3K inhibitor LY298002 prevented ICV-infused E2 from phosphorylating p42 ERK and enhancing object recognition memory consolidation (Fan et al., 2010; Fortress et al., 2013). These data demonstrate not only that dorsal hippocampal PI3K activation is necessary for E2 to facilitate object recognition memory consolidation (Fan et al., 2010; Fortress et al., 2013), but also that E2 first activates PI3K before activating ERK.
mTOR
Interestingly, both PI3K and ERK activate the mammalian target of rapamycin (mTOR) cell-signaling pathway (Fig. 2) (Hoeffer and Klann, 2010; Laplante and Sabatini, 2012; Richter and Klann, 2009). mTOR stimulates local protein synthesis by phosphorylating key components of the protein synthesis machinery, such as p70 ribosomal S6 kinase (S6K) and eukaryotic initiation factor 4E-binding proteins (4E-BPs) (Hoeffer and Klann, 2010). Stimuli that induce long-term potentiation activate mTOR signaling in hippocampal dendrites (Cammalleri et al., 2003; Tsokas et al., 2005), and hippocampal infusions of the mTOR inhibitor rapamycin impair consolidation of object recognition, contextual fear, and spatial memories (Bekinschtein et al., 2007; Dash et al., 2006; Myskiw et al., 2008; Parsons et al., 2006).
Given the important role of mTOR in hippocampal memory consolidation, we reasoned that mTOR activation might contribute to E2-induced memory consolidation. Although dorsal hippocampal infusion of E2 did not increase phosphorylation of mTOR itself, it did increase the phosphorylation of the downstream proteins S6K and 4E-BP1 (Fig. 4A,B) (Fortress et al., 2013), suggesting that E2 activates mTOR signaling. These effects were blocked by dorsal hippocampal infusion of U0126, LY298002, and rapamycin (Fig. 4A,B) (Fortress et al., 2013), as would be expected given that PI3K, ERK, and mTOR lie upstream of S6K and 4E-BP1. Likewise, all three inhibitors prevented E2 from increasing dorsal hippocampal p42 ERK phosphorylation (Fig. 4C) (Fortress et al., 2013). Although we expected the PI3K and ERK inhibitors to block E2-induced ERK phosphorylation given our previous data to this effect (Fan et al., 2010), it was surprising that rapamycin prevented E2 from activating ERK. This interaction may be mediated by one of the two mTOR complexes that provide feedback regulating ERK and PI3K activity (Hoeffer and Klann, 2010; Klann and Dever, 2004). More importantly, rapamycin also prevented E2 from enhancing object recognition memory consolidation (Fig. 4D) (Fortress et al., 2013), suggesting that dorsal hippocampal mTOR activation is necessary for E2 to facilitate hippocampal memory formation. We have yet to determine exactly how mTOR regulates memory consolidation after E2 treatment, but suspect that local protein synthesis within dendrites is involved. This hypothesis remains to be tested in future studies.
Figure 4.
Dorsal hippocampal mTOR activation is necessary for E2 to enhance object recognition memory consolidation. The phosphorylation of S6K (A), 4E-BP1 (B), and p42 ERK (C) were significantly increased in young ovariectomized mice 5 minutes after bilateral dorsal hippocampal infusion of 5 μg/hemisphere E2 (*p < 0.05 relative to vehicle). This effect was blocked by the ERK inhibitor U0126 (0.5 μg/hemisphere), the PI3K inhibitor LY298002 (0.005 μg/hemisphere), or the mTOR inhibitor rapamycin (0.25 μg/hemisphere) (A–C). (D) All three inhibitors also prevented E2 from enhancing object recognition memory consolidation, as indicated by the fact that only ice infused with E2 + vehicle spent more time than chance with the novel object (*p < 0.05). Error bars in all panels represent the mean ± standard error of the mean (SEM). Phosphorylated proteins were normalized to total protein or β-actin. Insets are representative Western blots of phosphorylated and total protein. Adapted with permission from (Fortress et al., 2013).
ERK-driven epigenetic alterations
Before leaving the subject of cell signaling, it is worth noting that ERK activation results in numerous cellular effects beyond triggering mTOR signaling. As mentioned previously, ERK translocates into the nucleus to phosphorylate the transcription factor CREB, and E2 facilitates this interaction in hippocampal neurons (Boulware et al., 2005) (Fig. 2). Thus, the E2-induced activation of ERK in the hippocampus likely leads to gene transcription. Numerous studies have shown that gene expression is altered in the hippocampus after E2 treatment (Aenlle and Foster, 2010; Aenlle et al., 2009; Noriega et al., 2010; Pechenino and Frick, 2009), although it is unclear if this is a direct result of CREB phosphorylation.
Alterations in gene transcription may arise not only from manipulating activity of transcription factors but also by altering the epigenetic mechanisms that regulate access to DNA. Epigenetic processes, such as histone acetylation and DNA methylation, are now well-accepted regulators of memory formation in numerous brain regions including the hippocampus (for recent reviews, see (Day and Sweatt, 2010, 2011; Fischer et al., 2010; Gräff and Tsai, 2013; Jarome and Lubin, 2014; Peixoto and Abel, 2013; Sweatt, 2009)). In brief, DNA is tightly coiled around a nucleosome complex consisting of four histone proteins (H2A, H2B, H3, H4; Fig. 2). Histone proteins can be altered by numerous post-translational modifications that either relax the bond between the DNA and histone proteins (thereby permitting access to the DNA and increasing gene transcription) or tighten the bond (thereby reducing access to the DNA and decreasing gene transcription) (Fortress and Frick, 2014). Histone acetylation generally increases gene transcription. Acetyl groups are added to histone proteins by histone acetyltransferases (HATs) and removed by histone deacetylases (HDACs). Therefore, histone acetylation state is the result of a dynamic balance between the activities of these two classes of enzymes.
Early evidence suggested that either contextual fear conditioning or ERK activation increased acetylation of the H3 histone protein in the hippocampus (Levenson et al., 2004). ERK activation was also found to be necessary for other protein kinases to increase hippocampal H3 acetylation (Levenson et al., 2004), suggesting a key role for ERK in regulating hippocampal histone acetylation. Given the importance of ERK in mediating the effects of E2 on hippocampal memory consolidation, we thought that H3 acetylation might also be involved. In brief, we found that a dorsal hippocampal infusion of E2 in ovariectomized mice significantly increases H3 acetylation in the dorsal hippocampus within 30 minutes, and this increase was blocked by U0126 (Zhao et al., 2010). These data suggest that the E2-induced increase in H3 acetylation depends on dorsal hippocampal ERK activation. We also found that E2 decreases protein levels of histone deacetylase 2 (HDAC2) in the dorsal hippocampus (Zhao et al., 2010), which is consistent with the role of this enzyme as a negative modulator of hippocampal plasticity and memory formation (Guan et al., 2009). In a follow-up study, we used a histone acetyltransferase inhibitor to show that dorsal hippocampal histone acetylation is necessary for E2 to enhance object recognition memory consolidation (Zhao et al., 2012). Collectively, these findings demonstrate the central importance of histone acetylation to the memory-enhancing effects of E2.
However, the specific genes regulated by E2-induced histone acetylation remain a mystery. Our most recent work has begun to shed some light on this subject (Fig. 2). Consistent with our findings from young ovariectomized females, we found in middle-aged ovariectomized female mice that E2 selectively increased H3 acetylation (Fig. 5A) and decreased protein levels of HDAC2 (Fig. 5B) and another negative regulator of hippocampal memory, HDAC3 (Fig. 5C) (Fortress et al., 2014; McQuown et al., 2011). We then examined H3 acetylation of specific promoters of the gene for brain derived neurotrophic factor (BDNF), a trophic factor essential for hippocampal memory formation (Bekinschtein et al., 2014; Heldt et al., 2007). Of the nine Bdnf promoters, we measured H3 acetylation in promoters I, II, and IV because hippocampal learning and/or aging regulate H3 acetylation of these promoters (Fuchikami et al., 2010; Lubin et al., 2008; Perovic et al., 2013). We found that dorsal hippocampal infusion of E2 increased H3 acetylation of Bdnf pII and pIV in the dorsal hippocampus of both young and middle-aged females relative to vehicle-treated age-matched controls (Fig. 5E) (Fortress et al., 2014). For pII, the magnitude of the increase was greater in young females (~3.5 fold) than in middle-aged females (~1.5 fold), but the increase in pIV acetylation was similar in both ages (~0.5 fold). Accordingly, dorsal hippocampal protein levels of both BDNF (Fig. 5D) and Pro-BDNF were increased by E2 infusion in middle-aged females (Fortress et al., 2014), indicating that E2-induced H3 acetylation stimulated an increase in BDNF protein translation. Although these data suggest that epigenetic regulation of Bdnf could be an important mechanism underlying the beneficial effects of E2 on hippocampal memory, much more work needs to be done to better understand the myriad other genes that are likely involved.
Figure 5.
E2 facilitates dorsal hippocampal histone acetylation and BDNF protein expression in middle-aged ovariectomized mice. (A) Bilateral dorsal hippocampal infusion of 5 μg/hemisphere E2 increased H3 acetylation 30 and 60 min later (***p < 0.001, **p < 0.01 relative to vehicle). Acetylated H3 protein was normalized to total H3 protein. Insets are representative Western blots of acetylated and total histone protein. (B,C) Hippocampal infusion of E2 decreased protein levels of HDAC2 (B) four hours after infusion, and of HDAC3 (C) four and six hours after infusion (**p < 0.01, *p < 0.05 relative to vehicle). (D) Conversely, BDNF protein levels were increased in the dorsal hippocampus four and six hours after bilateral dorsal hippocampal infusion of E2 (*p < 0.05 relative to vehicle). HDAC and BDNF proteins in panels B–D were normalized to β-actin protein. Insets are representative Western blots of protein and β-actin. (E) Chromatin immunoprecipitation analysis showed that hippocampal E2 infusion increased acetylation of Bdnf promoters pII and pIV in both young and middle-aged ovariectomized mice (*p < 0.05 relative to age-matched controls). Among vehicle-infused controls, acetylation of pI and pIV was significantly lower in middle-aged females than in young females (#p < 0.05 relative to young vehicle-infused controls), indicating an age-related reduction in acetylation of these promoters. Data were normalized to LINE1 for each sample and then normalized to young vehicle-infused mice for each promoter region and represented as fold of control. For all panels, each bar represents the mean ± SEM. Adapted with permission from (Fortress et al., 2014).
Although our data suggest that histone acetylation is important for estrogenic regulation of memory, it is far from the only epigenetic process involved. Early evidence from our laboratory indicates that DNA methylation also plays a role. DNA methylation involves the preferential addition of methyl groups to cytosine nucleotides located adjacent to guanine nucleotides (so-called CpG islands). DNA methylation typically silences gene transcription, but has been reported to increase transcription (Chahrour et al., 2008). DNA methylation is catalyzed by three enzymes called DNA methyltransferases (DNMTs). DNMT1 is a maintenance methyltransferase that transfers existing methyl marks during DNA replication. DNMT3A and DNMT3B are de novo methyltransferases that add methyl marks to previously unmethylated cytosines. Initial studies showed that contextual fear conditioning increases DNMT3A and DNMT3B, but not DNMT1, gene expression in the male rat hippocampus (Miller and Sweatt, 2007), suggesting that hippocampal learning stimulates de novo DNA methylation. Accordingly, dorsal hippocampal infusion of the DNMT inhibitor 5-AZA blocks contextual fear memory (Miller et al., 2008; Miller and Sweatt, 2007), indicating an important role for DNA methylation in hippocampal memory formation. But if methylation suppresses gene expression, then how can it facilitate memory? The key is the specific genes that are methylated. For example, 5-AZA prevents methylation of the memory suppressor gene PP1 (Miller and Sweatt, 2007), indicating that methylation of genes that negatively regulate memory promotes memory formation. Conversely, contextual fear conditioning demethylates the memory promoter gene reelin (Miller and Sweatt, 2007), suggesting that demethylation of genes that positively regulate memory also promotes memory formation. Together, these data support the notion that hippocampal memory can be facilitated via a combination of methylation of memory suppressing genes and demethylation of memory promoting genes.
With respect to E2 and memory, we were interested in DNA methylation because of its involvement in hippocampal memory and because histone acetylation and DNA methylation are interactive processes (Miller et al., 2008; Zhao et al., 2012). We first asked whether E2 regulated levels of the DNMT enzymes. We found in ovariectomized female mice that E2 transiently increased mRNA for DNMT3A and DNMT3B 45 minutes after dorsal hippocampal infusion, whereas DNMT1 mRNA levels were unchanged up to 180 minutes after infusion (Zhao et al., 2012; Zhao et al., 2010). Moreover, DNMT3B protein levels were significantly increased 4 hours after dorsal hippocampal infusion of E2 (Zhao et al., 2010), suggesting that E2 increases de novo DNA methylation. To determine if this methylation was involved in estrogenic regulation of memory, we infused 5-AZA into the dorsal hippocampus and found that it prevented E2 infused ICV from enhancing object recognition memory consolidation. This effect was limited to the 1–3 hour memory consolidation window, as delaying infusion of 5-AZA for three hours after training had no effect on memory consolidation. Together, these data demonstrate that DNA methylation is essential for E2 to facilitate object recognition memory consolidation. At this point, however, we do not know which genes are methylated or demethylated by E2, which is critical for better understanding how E2 affects gene expression. Additional studies using more sophisticated analyses (e.g., bisulfite sequencing) will be necessary to address this issue.
Estrogen receptors
Absent from the aforementioned discussion of E2’s effects on memory, cell signaling, and epigenetics has been any mention of the receptors that might mediate these effects. This omission results largely because this information remains unclear. Therefore, pinpointing the receptor mechanisms underlying E2’s effects on memory and hippocampal function is an important subject for future study. Numerous investigators have examined the role of ERs in regulating hippocampal memory (for recent reviews, see (Bean et al., 2014; Ervin et al., 2013; Tuscher et al., 2015)), and some of these data will be discussed here. But the picture has gotten more complex in recent years, as data have shown roles not only for ERα, ERβ, and GPER in mediating the mnemonic effects of E2, but also for several neurotransmitter receptors (see below).
Genetic manipulations
The contributions of specific ERs to cellular functioning have been examined using numerous methods, including ER-specific knockouts, siRNAs, and viral vector-mediated delivery of ERs. Studies using ERα and ERβ knockouts (ERαKO, ERβKO) suggest a role for both classical ERs in hippocampal memory. In the absence of exogenous E2 treatment, ERαKO, but not ERβKO, mice exhibit impaired spatial memory, suggesting a key role for ERα in mediating baseline memory function (Bean et al., 2014). This role is supported by data showing that lentiviral delivery of ERα to the hippocampus improved spatial memory in the Morris water maze among female ERαKO mice (Foster et al., 2008). However, E2 can enhance spatial memory, inhibitory avoidance, and object recognition in ERαKO mice, but cannot enhance object recognition or object placement in ERβKO mice (Frick et al., 2010; Fugger et al., 2000; Liu et al., 2008; Walf et al., 2008), suggesting an important role for ERβ in mediating at least some of the effects of exogenous E2 treatment. Interestingly, lentiviral delivery of ERβ to the hippocampus of ERβKO mice has been shown to impair spatial memory, possibly through interactions with ERα (Han et al., 2013).
With respect to membrane ERs, GPER knockouts exist and siRNA has been used to knock down GPER receptor levels in the hippocampus (Kajta et al., 2013; Langer et al., 2010; Ruiz-Palmero et al., 2013). However, the effects of these manipulations on memory have not yet been tested. One recent in vitro study used GPER siRNA to show that GPER is necessary for E2 to induce neuritogenesis and PI3K/Akt activation in primary hippocampal cultures (Ruiz-Palmero et al., 2013), suggesting that GPER may regulate some effects of E2 in the hippocampus. Another in vitro study found that siRNAs for ERβ and GPER blocked the neuroprotective effects of a specific phytoestrogen in primary hippocampal cultures (Kajta et al., 2013). Although these data provide support for the notion that GPER mediates the effects of E2 in the hippocampus, it is not clear whether GPER fulfills such a role in vivo.
Pharmacological manipulations
Because relatively few studies have been conducted to assess the effects of the genetic ER manipulations described above on memory, conclusions to be drawn from these studies are limited. Fortunately, a more substantial in vivo pharmacological literature exists from which to base hypotheses about the role of individual ERs in hippocampal memory. These studies describe the effects of selective ER agonists and antagonists on hippocampal memory (Bean et al., 2014; Ervin et al., 2013; Tuscher et al., 2015). Of these compounds, the most commonly used agonists for ERα and ERβ are propyl pyrazole triol (PPT) and diarylpropionitrile (DPN), respectively (Meyers et al., 2001; Stauffer et al., 2000). Membrane ERs can be targeted generally with BSA-E2. Potential involvement of intracellular ERs in the effects of BSA-E2 is often reduced by co-administration the ER antagonist ICI 182-780 (Wakeling and Bowler, 1992). However, ICI 182-780 can also act a GPER agonist (Thomas et al., 2005), so its effects can be difficult to interpret. GPER is targeted more specifically with the agonist G-1 and antagonists G-15 or G-36 (Bologa et al., 2006; Dennis et al., 2009; Dennis et al., 2011). It is important to note, however, that all ER drugs are relatively, not absolutely, specific for their targeted receptor, so care must be taken to use doses that are low enough to prevent non-specific binding to other ERs.
Classical ERs: Behavioral effects and molecular mechanisms
The vast majority of studies using PPT and DPN have administered these compounds systemically, making it difficult to pinpoint their effects to the hippocampus. Moreover, effects appear to depend on dose, type of memory tested, and task difficulty (Phan et al., 2011; Tuscher et al., 2015). In general, systemic DPN administered pre- or post-training enhances object recognition and object placement memory in ovariectomized rats and mice (Frick et al., 2010; Jacome et al., 2010; Phan et al., 2011; Walf et al., 2008; Walf et al., 2006), although one study found no effect of systemic DPN on object placement in ovariectomized rats (Frye et al., 2007). Although some studies report that systemic PPT does not affect object recognition and object placement memory (Frick et al., 2010; Jacome et al., 2010), others report beneficial effects in these tasks and in a social recognition task (Frye et al., 2007; Phan et al., 2011; Walf et al., 2006).
Only two studies have thus far infused PPT and DPN into the brain, and the results appear to be strain dependent. In ovariectomized Swiss mice, post-training dorsal hippocampal infusion of PPT, but not DPN, enhanced object recognition memory (Pereira et al., 2014). Yet in the same study, either PPT or DPN enhanced object recognition memory in ovariectomized C57BL/6 mice (Pereira et al., 2014). Our own work in ovariectomized C57BL/6 mice has shown that post-training infusion of PPT or DPN into the dorsal hippocampus or dorsal third ventricle at picrogram doses enhanced both object recognition and object placement memory consolidation (Boulware et al., 2013). Additional support for the role of both classical ERs in C57BL/6 mice comes from preliminary studies in our laboratory showing that post-training dorsal hippocampal infusion of the ERα antagonist MPP (1,3-Bis(4-hydroxyphenyl)-4methyl-5-[4-(2-piperidinylethoxy) phenol]-1H-pyrazole) or ERβ antagonist PHTPP (4-[2-Phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]pyrimidin-3-yl]phenol) dose-dependently impairs object recognition and object placement memory consolidation (Kim et al., 2014). As such, there is some agreement among studies for involvement by dorsal hippocampal ERα and ERβ in mediating object and spatial memory consolidation, at least in C57BL/6 mice.
Given these findings, we sought to identify the molecular mechanisms through which PPT and DPN may enhance object recognition and object placement memory in C57BL/6 mice. Of chief interest was whether these mechanisms were similar to those underlying E2’s effects on memory. Because intracranial infusion of E2 so reliably increases dorsal hippocampal ERK activation (Fan et al., 2010; Fernandez et al., 2008; Fortress et al., 2013; Zhao et al., 2012; Zhao et al., 2010), we first measured the effects of intracranial PPT or DPN infusion on dorsal hippocampal ERK phosphorylation. As with E2, infusion of PPT or DPN into the dorsal hippocampus (0.1 pg/hemisphere and 10 pg/hemisphere, respectively) or dorsal third ventricle (0.2 pg and 20 pg, respectively) increased levels of phospho-p42 ERK, but not phospho-p44 ERK, in the dorsal hippocampus within 5 minutes of infusion (Boulware et al., 2013). The ERK activation inhibitor U0126 blocked this increase in p42 ERK phosphorylation, as well as the enhanced object recognition and object placement memory consolidation induced by PPT and DPN (Boulware et al., 2013). These findings demonstrate that either ERα or ERβ can regulate object and spatial memory consolidation by rapidly activating dorsal hippocampal ERK signaling. As such, these data mirror the effects of dorsal hippocampal E2 infusion (Fernandez et al., 2008).
The importance of ERK in mediating the effects of ERα and ERβ in vivo were also consistent with findings from cultured hippocampal primary neurons showing that membrane-localized ERα and ERβ regulate CREB by activating ERK and metabotropic glutamate receptors (mGluRs), including mGluR1a (Boulware et al., 2005). Therefore, we hypothesized that mGluR1a might play a role in the effects of both E2 and the ER agonists. Indeed, we found that dorsal hippocampal infusion of the mGluR1a antagonist LY367385 prevented ICV-infused E2, PPT, or DPN from increasing p42 ERK phosphorylation in the dorsal hippocampus and from enhancing object recognition and object placement memory consolidation in ovariectomized C57BL/6 females (Fig. 6) (Boulware et al., 2013). Moreover, sucrose fractionation and coimmunoprecipitation experiments showed that ERα, ERβ, mGluR1, and ERK physically interact within hippocampal detergent-resistant membranes in vivo (Boulware et al., 2013). These data suggest that E2-induced hippocampal memory consolidation is regulated by interactions among ERα, ERβ, and mGluR1a at the membrane, which then rapidly trigger activation of ERK signaling. These data provide a putative mechanism for how intracellular ERs could rapidly activate cell-signaling pathways that are typically associated with G-protein-coupled receptors, and raise the intriguing possibility that ERα and ERβ may interact with many other receptors, including other neurotransmitter and G-protein-coupled receptors, to regulate memory.
Figure 6.
Dorsal hippocampal mGluR1a activation is necessary for ERα and ERβ to enhance object recognition and object placement memory consolidation, and to increase dorsal hippocampal ERK phosphorylation. (A,B) Immediate post-training infusion of PPT (0.2 pg) or DPN (20 pg) into the dorsal third ventricle significantly increased the time spent with the novel object (A) and moved object (B) relative to chance (dashed line at 15 s, **p < 0.01); these effects were blocked by dorsal hippocampal infusion of the mGluR1a antagonist LY367385 (10 pg/hemisphere). Bars represent the mean ± SEM time spent with each object. (C) Five min after infusion, PPT and DPN significantly increased phospho-p42 ERK levels (*p < 0.05; **p < 0.01 relative to vehicle); this effect was completely abolished by dorsal hippocampal infusion of LY367385. Bars represent mean ± SEM % change from vehicle. Reprinted with permission from (Boulware et al., 2013).
Membrane ERs: Behavioral effects and molecular mechanisms
The contributions of membrane-bound ERs to estrogenic regulation of memory have been difficult to test because these receptors have not been clearly identified. As mentioned earlier, these receptors can be targeted in a very general way by conjugating the large bovine serum albumin molecule to E2, which restricts its activity to the plasma membrane. In vitro, BSA-E2 promotes synaptic plasticity, ERK translocation into the nucleus, and neuroprotection in hippocampal CA1 pyramidal neurons (Carrer et al., 2003; Gu and Moss, 1998; Huang et al., 2004; Tang et al., 2014; Wu et al., 2011; Yang et al., 2010). In ovariectomized C57BL/6 mice, we found that dorsal hippocampal infusion of BSA-E2 mimicked exactly the effects of dorsal hippocampal E2 infusion. That is, dorsal hippocampal or ICV infusion of BSA-E2 increased dorsal hippocampal p42 ERK phosphorylation within 5 minutes and enhanced object recognition memory consolidation (Fernandez et al., 2008). Furthermore, as with E2, ERK activation was necessary for BSA-E2 to enhance object recognition memory consolidation (Fernandez et al., 2008). These data suggested that membrane-bound ERs might mediate the effects of E2 on memory and cell signaling independent of intracellular ERs. However, the identity of the receptor(s) underlying these effects remains unclear.
Interestingly, emerging data suggest that the membrane ER GPER may not play a role in the memory-enhancing effects of E2. GPER does appear to influence hippocampal memory formation and its effects on hippocampal memory are similar to those of E2, PPT, and DPN (Hammond et al., 2009). Studies investigating the role of GPER in spatial working memory have shown that systemic administration of the GPER agonist G-1 enhances, whereas the GPER antagonist G-15 impairs, acquisition of a delayed match-to-position task (Hammond et al., 2009; Hammond et al., 2012). Similarly, our own preliminary data indicate that post-training dorsal hippocampal infusion of G-1 enhances, whereas G-15 impairs, object recognition and object placement memory consolidation in ovariectomized mice (Kim et al., 2013). However, the molecular mechanisms through which this regulation occurs appear to differ from those of E2. Whereas E2 enhances memory by activating ERK, G-1 did not (Kim et al., 2013). Rather, the ability of G-1 to enhance memory consolidation in both object tasks depended instead on rapid activation of c-Jun N-terminal kinase (JNK) signaling (Kim et al., 2013). Moreover, the ability of E2 to activate ERK and enhance object recognition and object placement memory consolidation was not dependent on JNK or G-1 activation, as dorsal hippocampal infusion of G-15 or the JNK inhibitor SP600125 did not block the effects of ICV-infused E2 (Kim et al., 2013). These data are consistent with a new report showing that the ability of E2 to increase dendritic spine density in hippocampal slices from male rats is blocked by inhibitors of many cell-signaling pathways (including ERK, PI3K, and PKA), but not by JNK inhibitors (Hasegawa et al., in press). Together, these data suggest that GPER facilitates memory consolidation in the dorsal hippocampus via a different cell-signaling mechanism than E2. This conclusion is supported by other work showing that G-1 does not rapidly increase Akt phosphorylation in the dorsal hippocampus, unlike E2, which activates Akt between 5 and 30 minutes after treatment (Akama and McEwen, 2003; Fortress et al., 2013; Waters et al., 2015). These findings beg the question of whether GPER really functions as an ER in the dorsal hippocampus. Whether GPER is a true ER has been the subject of intensive debate (Langer et al., 2010; Levin, 2009), and studies such as these are likely to fuel continued conversation. But if E2 is not the endogenous ligand for GPER in the dorsal hippocampus, then what is? At this point, the answer is unknown, which highlights the need for much more research to better understand the molecular mechanisms through which GPER regulates hippocampal memory.
Conclusions and future directions
The field has made considerable progress since the 1998 special issue of Hormones and Behavior on estrogens and cognition (Volume 34(2), October). We have learned a considerable amount in recent years about the molecular and cellular mechanisms through which E2 regulates hippocampal morphology, synaptic plasticity, and memory. Many of these findings have challenged long held dogmas about how sex steroid hormones regulate neural and cellular function. For example, the discovery that E2 and other sex steroid hormones are made within the hippocampus raises important questions about how de novo hormone synthesis regulates acute behavioral events such as a learning episode. In addition, the identification of non-classical effects of E2 and other hormones has allowed memory researchers to tap into the vast neurobiology of memory literature for clues about the rapid molecular mechanisms through which these hormones might regulate memory formation. Of course, much more work is necessary to fully understand how a simple hormonal signal leads to a memory representation in the brain, but the work of the past two decades has given us a great start.
As we look to the future, many issues of importance emerge as areas for further study. For instance, hippocampally-synthesized E2 is likely a major player in memory formation, so we must better understand the role of de novo E2 synthesis in regulating memory. In addition, future work should look beyond E2 and the hippocampus to examine interactions with other brain regions and other hormones. In particular, examining the effects of E2 on memory mediated by other brain regions (e.g., prefrontal cortex, amygdala, perirhinal cortex, entorhinal cortex, and striatum) will provide a considerably more comprehensive view of how E2 regulates learning and memory than has been provided to date by the field’s primary focus on hippocampal-dependent memory tasks.
Finally, expanding our knowledge about the receptor, cell-signaling, and epigenetic mechanisms that mediate the gene expression and protein synthesis alterations that underlie the morphological, physiological, and behavioral effects of E2 will also be essential to understanding why women are at greater risk than men of developing psychiatric and neurodegenerative diseases such as mood and anxiety disorders, and Alzheimer’s disease (Kessler et al., 2005; Yaffe et al., 2007; Zandi et al., 2002). Although low estrogen levels have been implicated in elevated risks of these conditions among women (Graham and Milad, 2013; Meinhard et al., 2013; Milad et al., 2010; Yaffe et al., 2007), estrogen therapies are not attractive treatment options because of harmful side effects associated with such treatment, particularly breast cancer, heart disease, and stroke in women over age 65 (Rossouw et al., 2002). Because ERs are so widely distributed in tissues throughout female body, drugs that target ERs are almost certain to bind ERs in peripheral tissues and produce unintended consequences. Therefore, new avenues of research are needed to provide novel drugs that specifically target the cognition-enhancing mechanisms that lie downstream from ERs (Frick, 2012; Frick et al., 2010). For example, by identifying the cellular and molecular mechanisms downstream from ERs through which E2 enhances memory, we may uncover new molecular targets for drug development that provide the mnemonic benefits of E2 without detrimental side effects (Frick, 2012; Frick et al., 2010). Indeed, shifting the focus of drug discovery from finding better selective ER modulators to identifying novel compounds that target rapid events downstream from ERs could lead to a new generation of drugs for reducing cognitive dysfunction in women.
Although we have much more work to do to fully understand how E2 and other sex steroid hormones regulate cognition, recent advances pinpointing the neural mechanisms underlying the memory-enhancing effects of E2 provide the hope that such drugs might become available in the near future. Fortunately, there are many investigators interested in studying these mechanisms at various levels of analysis, and so the coming years should provide a wealth of extraordinary new data. Undoubtedly, this work will make for another exceptional special issue of Hormones and Behavior in the future.
Highlights.
17β-Estradiol (E2) rapidly enhances hippocampal memory consolidation.
Numerous molecular changes are necessary for E2 to enhance hippocampal memory.
The receptors mediating E2’s effects on memory are not well understood.
This review discusses the molecular mechanisms through which E2 enhances memory.
Acknowledgments
The University of Wisconsin-Milwaukee supported the writing of this manuscript. The empirical work from my laboratory described herein was supported by R01AG022525, R03MH065460, the American Federation for Aging Research, a University of Wisconsin-Milwaukee Research Growth Initiative Award, the University of Wisconsin-Milwaukee, and Yale University. I would like to thank all of the students, postdoctoral fellows, and research staff from my laboratory whose work is reviewed in this article, and to Dr. Ashley Fortress, Jennifer Tuscher, and Jaekyoon Kim for critical comments on this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams JP, Sweatt JD. Molecular psychology: Roles for the ERK MAP kinase cascade in memory. Annu Rev Pharmacol Toxicol. 2002;42:135–163. doi: 10.1146/annurev.pharmtox.42.082701.145401. [DOI] [PubMed] [Google Scholar]
- Aenlle KK, Foster TC. Aging alters the expression of genes for neuroprotection and synaptic function following acute estradiol treatment. Hippocampus. 2010;20:1047–1060. doi: 10.1002/hipo.20703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aenlle KK, Kumar A, Cui L, Jackson TC, Foster TC. Estrogen effects on cognition and hippocampal transcription in middle-aged mice. Neurobiol Aging. 2009;30:932–945. doi: 10.1016/j.neurobiolaging.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akama KT, McEwen BS. Estrogen stimulates postsynaptic density-95 rapid protein synthesis via the Akt/protein kinase B pathway. J Neurosci. 2003;23:2333–2339. doi: 10.1523/JNEUROSCI.23-06-02333.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akama KT, Thompson LI, Milner TA, McEwen BS. Post-synaptic density-95 (PSD-95) binding capacity of G-protein-coupled Receptor 30 (GPR30), an estrogen receptor that can be identified in hippocampal dendritic spines. J Biol Chem. 2013;288:6438–6450. doi: 10.1074/jbc.M112.412478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci. 1998;1:602–609. doi: 10.1038/2836. [DOI] [PubMed] [Google Scholar]
- Azcoitia I, Sierra A, Veiga S, Garcia-Segura LM. Aromatase expression by reactive astroglia is neuroprotective. Ann N Y Acad Sci. 2003;1007:298–305. doi: 10.1196/annals.1286.028. [DOI] [PubMed] [Google Scholar]
- Bailey DJ, Ma C, Soma KK, Saldanha CJ. Inhibition of hippocampal aromatization impairs spatial memory performance in a male songbird. Endocrinology. 2013;154:4707–4714. doi: 10.1210/en.2013-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean LA, Ianov L, Foster TC. Estrogen receptors, the hippocampus, and memory. Neuroscientist. 2014;20:534–545. doi: 10.1177/1073858413519865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekinschtein P, Cammarota M, Medina JH. BDNF and memory processing. Neuropharmacology. 2014;76(Pt C):677–683. doi: 10.1016/j.neuropharm.2013.04.024. [DOI] [PubMed] [Google Scholar]
- Bekinschtein P, Katche C, Slipczuk LN, Igaz LM, Cammarota M, Izquierdo I, Medina JH. mTOR signaling in the hippocampus is necessary for memory formation. Neurobiol Learn Mem. 2007;87:303–307. doi: 10.1016/j.nlm.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Bi R, Broutman G, Foy MR, Thompson RF, Baudry M. The tyrosine kinase and mitogen-activated protein kinase pathways mediate multiple effects of estrogen in hippocampus. Proc Natl Acad Sci USA. 2000;97:3602–3607. doi: 10.1073/pnas.060034497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi R, Foy MR, Vouimba RM, Thompson RF, Baudry M. Cyclic changes in estradiol regulate synaptic plasticity through the MAP kinase pathway. Proc Natl Acad Sci USA. 2001;98:13391–13395. doi: 10.1073/pnas.241507698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum S, Moore AN, Adams F, Dash PK. A mitogen-activated protein kinase cascade in the CA1/CA2 subfield of the dorsal hippocampus is essential for long-term spatial memory. J Neurosci. 1999;19:3535–3544. doi: 10.1523/JNEUROSCI.19-09-03535.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, Oprea TI, Prossnitz ER. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol. 2006;2:207–212. doi: 10.1038/nchembio775. [DOI] [PubMed] [Google Scholar]
- Boulware MI, Heisler JD, Frick KM. The memory-enhancing effects of hippocampal estrogen receptor activation involve metabotropic glutamate receptor signaling. J Neurosci. 2013;33:15184–15194. doi: 10.1523/JNEUROSCI.1716-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci. 2005;25:5066–5078. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozon B, Kelly A, Josselyn SA, Silva AJ, Davis S, Laroche S. MAPK, CREB and zif268 are all required for the consolidation of recognition memory. Philos Trans R Soc Lond, B, Biol Sci. 2003;358:805–814. doi: 10.1098/rstb.2002.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brailoiu E, Dun SL, Brailoiu GC, Mizuo K, Sklar LA, Oprea TI, Prossnitz ER, Dun NJ. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J Endocrinol. 2007:311–321. doi: 10.1677/JOE-07-0017. [DOI] [PubMed] [Google Scholar]
- Brinton RD. Cellular and molecular mechanisms of estrogen regulation of memory function and neuroprotection against Alzheimer’s disease: Recent insights and remaining challenges. Learn Mem. 2001;8:121–133. doi: 10.1101/lm.39601. [DOI] [PubMed] [Google Scholar]
- Cammalleri M, Lütjens R, Berton F, King AR, Simpson C, Francesconi W, Sanna PP. Time-restricted role for dendritic activation of the mTOR-p70S6K pathway in the induction of late-phase long-term potentiation in the CA1. Proc Natl Acad Sci USA. 2003;100:14368–14373. doi: 10.1073/pnas.2336098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrer HF, Araque A, Buño W. Estradiol regulates the slow Ca2+-activated K+ current in hippocampal pyramidal neurons. J Neurosci. 2003;23:6338–6344. doi: 10.1523/JNEUROSCI.23-15-06338.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahrour M, Jung SY, Shaw C, Zhao X, Wong STC, Qin J, Zoghbi HY. MeCP2, a key contributor to neurological disease, activates and repressed transcription. Science. 2008;320:1224–1229. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan M, Chow C, Hamson DK, Lieblich SE, Galea LA. Effects of chronic oestradiol, progesterone and medroxyprogesterone acetate on hippocampal neurogenesis and adrenal mass in adult female rats. J Neuroendocrinol. 2014;26:386–399. doi: 10.1111/jne.12159. [DOI] [PubMed] [Google Scholar]
- Choleris E, Clipperton-Allen AE, Phan A, Valsecchi P, Kavaliers M. Estrogenic involvement in social learning, social recognition and pathogen avoidance. Front Neuroendocrinol. 2012;33:140–159. doi: 10.1016/j.yfrne.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Bimonte-Nelson HA. Impact of the hypothalamic–pituitary–adrenal/gonadal axes on trajectory of age-related cognitive decline. Prog Brain Res. 2010;182:31–76. doi: 10.1016/S0079-6123(10)82002-3. [DOI] [PubMed] [Google Scholar]
- Daniel JM. Effects of oestrogen on cognition: What have we learned from basic research? J Neuroendocrinol. 2006;18:787–795. doi: 10.1111/j.1365-2826.2006.01471.x. [DOI] [PubMed] [Google Scholar]
- Dash PK, Orsi SA, Moore AN. Spatial memory formation and memory-enhancing effect of glucose involves activation of the tuberous sclerosis complex-Mammalian target of rapamycin pathway. J Neurosci. 2006;26:8048–8056. doi: 10.1523/JNEUROSCI.0671-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JJ, Sweatt JD. DNA methylation and memory formation. Nat Neurosci. 2010;11:1319–1323. doi: 10.1038/nn.2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JJ, Sweatt JD. Cognitive neuroepigenetics: A role for epigenetic mechanisms in learning and memory. Neurobiol Learn Mem. 2011;96:2–12. doi: 10.1016/j.nlm.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castilhos J, Forti CD, Achaval M, Rasia-Filho AA. Dendritic spine density of posterodorsal medial amygdala neurons can be affected by gonadectomy and sex steroid manipulations in adult rats: A Golgi study. Brain Res. 2008;1240:73–81. doi: 10.1016/j.brainres.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Dennis MK, Burai R, Ramesh C, Petrie WK, Alcon SN, Nayak TK, Bologa CG, Leitao A, Brailoiu E, Deliu E, Dun NJ, Sklar LA, Hathaway HJ, Arterburn JB, Oprea TI, Prossnitz ER. In vivo effects of a GPR30 antagonist. Nat Chem Biol. 2009;5:421–427. doi: 10.1038/nchembio.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis MK, Field AS, Burai R, Ramesh C, Petrie WK, Bologa CG, Oprea TI, Yamaguchi Y, Hayashi SI, Sklar LA, Hathaway HJ, Arterburn JB, Prossnitz ER. Identification of a GPER/GPR30 antagonist with improved estrogen receptor counterselectivity. J Steroid Biochem Mol Biol. 2011;127:358–366. doi: 10.1016/j.jsbmb.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ervin KS, Phan A, Gabor CS, Choleris E. Rapid oestrogenic regulation of social and nonsocial learning. J Neuroendocrinol. 2013;25:1116–1132. doi: 10.1111/jne.12079. [DOI] [PubMed] [Google Scholar]
- Fan L, Zhao Z, Orr PT, Chambers CH, Lewis MC, Frick KM. Estradiol-induced object memory consolidation in middle-aged female mice requires dorsal hippocampal extracellular signal-regulated kinase and phosphatidylinositol 3-kinase activation. J Neurosci. 2010;30:4390–4400. doi: 10.1523/JNEUROSCI.4333-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM. Estradiol-induced enhancement of object memory consolidation involves hippocampal ERK activation and membrane-bound estrogen receptors. J Neurosci. 2008;28:8660–8667. doi: 10.1523/JNEUROSCI.1968-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Bland KI, Frackelton ARJ. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Mungenast A, Tsai LH. Targeting the correct HDAC(s) to treat cognitive disorders. Trends Pharmacol Sci. 2010;31:605–617. doi: 10.1016/j.tips.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Fortress AM, Fan L, Orr PT, Zhao Z, Frick KM. Estradiol-induced object recognition memory consolidation is dependent on activation on mTOR signaling in the dorsal hippocampus. Learn Mem. 2013;20:147–155. doi: 10.1101/lm.026732.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortress AM, Frick KM. Epigenetic regulation of estrogen-dependent memory. Front Neuroendocrinol. 2014;35:530–549. doi: 10.1016/j.yfrne.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortress AM, Kim J, Poole RL, Gould TJ, Frick KM. 17β-Estradiol regulates histone alterations associated with memory consolidation and increases Bdnf promoter acetylation in middle-aged female mice. Learn Mem. 2014;21:457–467. doi: 10.1101/lm.034033.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC, Rani A, Kumar A, Cui L, Semple-Rowland SL. Viral vector-mediated delivery of estrogen receptor-alpha to the hippocampus improves spatial learning in estrogen receptor-alpha knockout mice. Mol Ther. 2008;16:1587–1593. doi: 10.1038/mt.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foy MR, Xu J, Xie X, Brinton RD, Thompson RF, Berger TW. 17β-estradiol enhances NMDA receptor-mediated EPSPs and long-term potentiation. J Neurophysiol. 1999;81:925–929. doi: 10.1152/jn.1999.81.2.925. [DOI] [PubMed] [Google Scholar]
- Frick KM. Estrogens and age-related memory decline in rodents: What have we learned and where do we go from here? Horm Behav. 2009;55:2–23. doi: 10.1016/j.yhbeh.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM. Building a better hormone therapy?: How understanding the rapid effects of sex steroid hormones could lead to novel therapeutics for age-related memory decline. Behav Neurosci. 2012;126:29–53. doi: 10.1037/a0026660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM. Epigenetics, oestradiol and hippocampal memory consolidation. J Neuroendocrinol. 2013;25:1151–1162. doi: 10.1111/jne.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Fernandez SM, Bennett JC, Prange-Kiel J, MacLusky NJ, Leranth C. Behavioral training interferes with the ability of gonadal hormones to increase CA1 spine synapse density in ovariectomized female rats. Eur J Neurosci. 2004;19:3026–3032. doi: 10.1111/j.1460-9568.2004.03427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Fernandez SM, Harburger LL. A new approach to understanding the molecular mechanisms through which estrogens affect cognition. Biochim Biophys Acta Gen Subj. 2010;1800:1045–1055. doi: 10.1016/j.bbagen.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Duffy CK, Walf AA. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol Learn Mem. 2007;88:208–216. doi: 10.1016/j.nlm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchikami M, Yamamoto S, Morinobu S, Takei S, Yamawaki S. Epigenetic regulation of BDNF gene in response to stress. Psychiatry Investig. 2010;7:251–256. doi: 10.4306/pi.2010.7.4.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugger HN, Foster TC, Gustafsson JA, Rissman EF. Novel effects of estradiol and estrogen receptor α and β on cognitive function. Brain Res. 2000;883:258–264. doi: 10.1016/s0006-8993(00)02993-0. [DOI] [PubMed] [Google Scholar]
- Fukata Y, Fukata M. Protein palmitoylation in neuronal development and synaptic plasticity. Nat Rev Neurosci. 2010;11:161–175. doi: 10.1038/nrn2788. [DOI] [PubMed] [Google Scholar]
- Galea LA, Wainwright SR, Roes MM, Duarte-Guterman P, Chow C, Hamson DK. Sex, hormones, and neurogenesis in the hippocampus: Hormonal modulation of neurogenesis and potential functional implications. J Neuroendocrinol. 2013;25:1039–1061. doi: 10.1111/jne.12070. [DOI] [PubMed] [Google Scholar]
- Galea LAM, Wide JK, Paine TA, Holmes MM, Ormerod BK, Floresco SB. High levels of estradiol disrupt conditioned place preference learning, stimulus response learning and reference memory but have limited effects on working memory. Behav Brain Res. 2001;126:115–126. doi: 10.1016/s0166-4328(01)00255-8. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Azcoitia I, DonCarlos LL. Neuroprotection by estradiol. Prog Neurobiol. 2001:63. doi: 10.1016/s0301-0082(00)00025-3. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Wozniak A, Azcoitia I, Rodriguez JR, Hutchison RE, Hutchison JB. Aromatase expression by astrocytes after brain injury: Implications for local estrogen formation in brain repair. Neuroscience. 1999;89:567–578. doi: 10.1016/s0306-4522(98)00340-6. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Estrogen therapy and cognition: A review of the cholinergic hypothesis. Endrocr Rev. 2010;31:224–253. doi: 10.1210/er.2009-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasper ER, Gould E. Sexual experience restores age-related decline in adult neurogenesis and hippocampal function. Hippocampus. 2013;23:303–312. doi: 10.1002/hipo.22090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gräff J, Tsai LH. Histone acetylation: Molecular mnemonics on the chromatin. Nat Rev Neurosci. 2013;14:97–111. doi: 10.1038/nrn3427. [DOI] [PubMed] [Google Scholar]
- Graham BM, Milad MR. Blockade of estrogen by hormonal contraceptives impairs fear extinction in female rats and women. Biol Psychiatry. 2013;73:371–378. doi: 10.1016/j.biopsych.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BM, Milad MR. Inhibition of estradiol synthesis impairs fear extinction in male rats. Learn Mem. 2014;21:347–350. doi: 10.1101/lm.034926.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresack JE, Frick KM. Post-training estrogen enhances spatial and object memory consolidation in female mice. Pharmacol Biochem Behav. 2006;84:112–119. doi: 10.1016/j.pbb.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Gresack JE, Kerr KM, Frick KM. Life-long environmental enrichment differentially affects the mnemonic response to estrogen in young, middle-aged, and aged female mice. Neurobiol Learn Mem. 2007;88:393–408. doi: 10.1016/j.nlm.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q, Moss RL. Novel mechanism for non-genomic action of 17 beta-oestradiol on kainate-induced currents in isolated rat CA1 hippocampal neurones. J Physiol. 1998;506:745–754. doi: 10.1111/j.1469-7793.1998.745bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan JS, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, Gao J, Nieland TJ, Zhou Y, Wang X, Mazitschek R, Bradner JE, DePinho RA, Jaenisch R, Tsai LH. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 2009;459:55–60. doi: 10.1038/nature07925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond R, Mauk R, Ninaci D, Nelson D, Gibbs RB. Chronic treatment with estrogen receptor agonists restores acquisition of a spatial learning task in young ovariectomized rats. Horm Behav. 2009;56:309–314. doi: 10.1016/j.yhbeh.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond R, Nelson D, Kline E, Gibbs RB. Chronic treatment with a GPR30 antagonist impairs acquisition of a spatial learning task in young female rats. Horm Behav. 2012;62:367–374. doi: 10.1016/j.yhbeh.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Aenlle KK, Bean LA, Rani A, Semple-Rowland SL, Kumar A, Foster TC. Role of estrogen receptor α and β in preserving hippocampal function during aging. J Neurosci. 2013;33:2671–2683. doi: 10.1523/JNEUROSCI.4937-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Leffler AE, Leffler SR, Janssen WG, Lou W, McKay H, Roberts JA, Wearne SL, Hof PR, Morrison JH. Estrogen alters spine number and morphology in prefrontal cortex of aged female rhesus monkeys. J Neurosci. 2006;26:2571–2578. doi: 10.1523/JNEUROSCI.3440-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa Y, Hojo Y, Kojima H, Ikeda M, Hotta K, Sato R, Ooishi Y, Yoshiya M, Chung B-C, Yamazaki T, Kawato S. Estradiol rapidly modulates synaptic plasticity of hippocampal neurons: Involvement of kinase networks. Brain Res. doi: 10.1016/j.brainres.2014.1012.1056. in press. [DOI] [PubMed] [Google Scholar]
- Heldt SA, Stanek L, Chhatwal JP, Ressler KJ. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatry. 2007;12:656–670. doi: 10.1038/sj.mp.4001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffer CA, Klann E. mTOR signaling: At the crossroads of plasticity, memory and disease. Trends Neurosci. 2010;33:67–75. doi: 10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojo Y, Hattori TA, Enami T, Furukawa A, Suzuki K, Ishii HT, Mukai H, Morrison JH, Janssen WG, Kominami S, Harada N, Kimoto T, Kawato S. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P4. 5017alpha and P450 aromatase localized in neurons. Proc Natl Acad Sci USA. 2004;101:865–870. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojo Y, Higo S, Ishii H, Ooishi Y, Mukai H, Murakami G, Kominami T, Kimoto T, Honma S, Poirer D, Kawato S. Comparison between hippocampus-synthesized and circulation-derived sex steroids in the hippocampus. Endocrinology. 2009;150:5106–5112. doi: 10.1210/en.2009-0305. [DOI] [PubMed] [Google Scholar]
- Horwood JM, Dufour F, Laroche S, Davis S. Signaling mechanisms mediated by the phosphoinositide 3-kinase/Akt cascade in synaptic plasticity and memory in the rat. Eur J Neurosci. 2006;23:3375–3384. doi: 10.1111/j.1460-9568.2006.04859.x. [DOI] [PubMed] [Google Scholar]
- Huang Y, Huang YL, Zhang S, Zhu YC, Yao T. Estradiol acutely attenuates glutamate-induced calcium overload in primarily cultured rat hippocampal neurons through a membrane receptor-dependent mechanism. Brain Res. 2004;1026:254–260. doi: 10.1016/j.brainres.2004.08.038. [DOI] [PubMed] [Google Scholar]
- Ikeda T, Makino Y, Yamada K. 17α-estradiol is generated locally in the male rat brain and can regulate GAD65 expression and anxiety. Neuropharmacology. 2015;90:9–14. doi: 10.1016/j.neuropharm.2014.10.019. [DOI] [PubMed] [Google Scholar]
- Inagaki T, Frankfurt M, Luine V. Estrogen-induced memory enhancements are blocked by acute bisphenol A in adult female rats: Role of dendritic spines. Endocrinology. 2012;1534:3357–3367. doi: 10.1210/en.2012-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacome LF, Gautreaux C, Inagaki T, Mohan G, Alves S, Lubbers LS, Luine V. Estradiol and ERβ agonists enhance recognition memory, and DPN, an ERβ agonist, alters brain monoamines. Neurobiol Learn Mem. 2010;94:488–498. doi: 10.1016/j.nlm.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarome TJ, Lubin FD. Epigenetic mechanisms of memory formation and reconsolidation. Neurobiol Learn Mem. 2014;115:116–127. doi: 10.1016/j.nlm.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajta M, Rzemieniec J, Litwa E, Lason W, Lenartowicz M, Krzeptowski W, Wojtowicz AK. The key involvement of estrogen receptor β and G-protein-coupled receptor 30 in the neuroprotective action of daidzein. Neuroscience. 2013;238:345–360. doi: 10.1016/j.neuroscience.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Kato A, Hojo Y, Higo S, Komatsuzaki Y, Murakami G, Yoshino H, Uebayashi M, Kawato S. Female hippocampal estrogens have a significant correlation with cyclic fluctuation of hippocampal spines. Front Neural Circuits. 2013;7:149. doi: 10.3389/fncir.2013.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly A, Laroche S, Davis S. Activation of mitogen-activated protein kinase/extracellular signal-regulated kinase in hippocampal circuitry is required for consolidation and reconsolidation of recognition memory. J Neurosci. 2003;12:5354–5360. doi: 10.1523/JNEUROSCI.23-12-05354.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly A, Lynch MA. Long-term potentiation in dentate gyrus of the rat is inhibited by the phosphoinositide 3-kinase inhibitor, wortmannin. Neuropharmacology. 2000;39:643–651. doi: 10.1016/s0028-3908(99)00169-0. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MM, Dhandapani KM, Zhang QG, Brann DW. Estrogen regulation of spine density and excitatory synapses in rat prefrontal and somatosensory cerebral cortex. Steroids. 2013;78:614–623. doi: 10.1016/j.steroids.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Boulware MI, Frick KM. Soc Neurosci Abstr, Poster 376.305. 2013. Role of G-protein-coupled estrogen receptor (GPER/GPR30) in hippocampal memory and cell signaling in female mice. [Google Scholar]
- Kim J, Szinte JS, Frick KM. Soc Neurosci Abstr Poster 451.11. 2014. Distinct effects of estrogen receptor inhibition on novel object recognition and spatial memory consolidation in ovariectomized mice. [DOI] [PubMed] [Google Scholar]
- Klann E, Dever TE. Biochemical mechanisms for translational regulation in synaptic plasticity. Nat Rev Neurosci. 2004;5:931–942. doi: 10.1038/nrn1557. [DOI] [PubMed] [Google Scholar]
- Kramár EA, Chen LY, Brandon NJ, Rex CS, Liu F, Gall CM, Lynch G. Cytoskeletal changes underlie estrogen’s acute effects on synaptic transmission and plasticity. J Neurosci. 2009;29:12982–12993. doi: 10.1523/JNEUROSCI.3059-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretz O, Fester L, Wehrenberg U, Zhou L, Brauckmann S, Zhao S, Prange-Kiel J, Naumann T, Jarry H, Frotscher M, Rune GM. Hippocampal synapses depend on hippocampal estrogen synthesis. J Neurosci. 2004;24:5913–5921. doi: 10.1523/JNEUROSCI.5186-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Foster TC. 17β-estradiol benzoate decreases the AHP amplitude in CA1 pyramidal neurons. J Neurophysiol. 2002;88:621–626. doi: 10.1152/jn.2002.88.2.621. [DOI] [PubMed] [Google Scholar]
- Kuroki Y, Fukushima K, Kanda Y, Mizuno K, Watanabe Y. Putative membrane-bound estrogen receptors possibly stimulate mitogen-activated protein kinase in the rat hippocampus. Eur J Pharmacol. 2000;400:205–209. doi: 10.1016/s0014-2999(00)00425-8. [DOI] [PubMed] [Google Scholar]
- Langer G, Bader B, Meoli L, Isensee J, Delbeck M, Noppinger PR, Otto C. A critical review of fundamental controversies in the field of GPR30 research. Steroids. 2010;75:603–610. doi: 10.1016/j.steroids.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson JM, O’Riordan KJ, Brown KD, Trinh MA, Molfese DL, Sweatt JD. Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem. 2004;279:40545–40559. doi: 10.1074/jbc.M402229200. [DOI] [PubMed] [Google Scholar]
- Levin ER. Cellular functions of the plasma membrane estrogen receptor. Trends Endocrinol Metab. 1999;10:374–377. doi: 10.1016/s1043-2760(99)00192-7. [DOI] [PubMed] [Google Scholar]
- Levin ER. G protein-coupled receptor 30: Estrogen receptor or collaborator? Endocrinology. 2009;150:1563–1565. doi: 10.1210/en.2008-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MC, Kerr KM, Orr PT, Frick KM. Estradiol-induced enhancement of object memory consolidation involves NMDA receptors and protein kinase A in the dorsal hippocampus of female C57BL/6 mice. Behav Neurosci. 2008;122:716–721. doi: 10.1037/0735-7044.122.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Yeh SH, Lin CH, Lu KT, Leu TH, Chang WC, Gean PW. A role for the PI-3 kinase signaling pathway in fear conditioning and synaptic plasticity in the amygdala. Neuron. 2001;31:841–851. doi: 10.1016/s0896-6273(01)00433-0. [DOI] [PubMed] [Google Scholar]
- Liu F, Day M, Muniz LC, Bitran D, Arias R, Revilla-Sanchez R, Grauer S, Zhang G, Kelley C, Pulito V, Sung A, Mervis RF, Navarra R, Hirst WD, Reinhart PH, Marquis KL, Moss SJ, Pangalos MN, Brandon NJ. Activation of estrogen receptor-beta regulates hippocampal synaptic plasticity and improves memory. Nat Neurosci. 2008;11:334–343. doi: 10.1038/nn2057. [DOI] [PubMed] [Google Scholar]
- Lubin FD, Roth TL, Sweatt JD. Epigenetic regulation of bdnf gene transcription in the consolidation of fear memory. J Neurosci. 2008;28:10576–10586. doi: 10.1523/JNEUROSCI.1786-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V. Estradiol and cognitive function: Past, present and future. Horm Behav. 2014;66:602–618. doi: 10.1016/j.yhbeh.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V. Recognition memory tasks in neuroendocrine research. Behav Brain Res. 2015;285:158–164. doi: 10.1016/j.bbr.2014.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V, Rodriguez M. Effects of estradiol on radial arm maze performance of young and aged rats. Behav Neural Biol. 1994;62:230–236. doi: 10.1016/s0163-1047(05)80021-4. [DOI] [PubMed] [Google Scholar]
- Luine VN, Frankfurt M. Estrogens facilitate memory processing through membrane mediated mechanisms and alterations in spine density. Front Neuroendocrinol. 2012;33:388–402. doi: 10.1016/j.yfrne.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, MacLusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Luine VN, Hajszan T, Leranth C. The 17alpha and 17beta isomers of estradiol both induce rapid spine synapse formation in the CA1 hippocampal subfield of ovariectomized female rats. Endocrinology. 2005;146:287–293. doi: 10.1210/en.2004-0730. [DOI] [PubMed] [Google Scholar]
- Manella P, Brinton RD. Estrogen receptor protein interaction with phosphatidylinositol 3-kinase leads to activation of phosphorylated Akt and extracellular signal-regulated kinase 1/2 in the same population of cortical neurons: A unified mechanism of estrogen action. J Neurosci. 2006;26:9437–9447. doi: 10.1523/JNEUROSCI.1443-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriott LK, Hauss-Wegrzyniak B, Benton RS, Vraniak PD, Wenk GL. Long-term estrogen therapy worsens the behavioral and neuropathological consequences of chronic brain inflammation. Behav Neurosci. 2002;116:902–911. doi: 10.1037//0735-7044.116.5.902. [DOI] [PubMed] [Google Scholar]
- McClure R, Barha CK, Galea LA. 17β-Estradiol, but not estrone, increases hippocampal neurogenesis and activation of new granule neurons in response to spatial memory in adult female rats. Horm Behav. 2013;63:144–157. doi: 10.1016/j.yhbeh.2012.09.011. [DOI] [PubMed] [Google Scholar]
- McQuown SC, Barrett RM, Matheos DP, Post RJ, Rogge GA, Alenghat T, Mullican SE, Jones S, Rusche JR, Lazar MAW, MA HDAC3 is a critical negative regulator of long-term memory formation. J Neurosci. 2011;31:764–747. doi: 10.1523/JNEUROSCI.5052-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhard N, Kessing LV, Vinberg M. The role of estrogen in bipolar disorder, a review. Nord J Psychiatry. 2013 doi: 10.3109/08039488.2013.775341. [DOI] [PubMed] [Google Scholar]
- Meitzen J, Luoma JI, Boulware MI, Hedges VL, Peterson BM, Tuomela K, Britson KA, Mermelstein PG. Palmitoylation of estrogen receptors is essential for neuronal membrane signaling. Neuroendocrinology. 2013;154:4293–4304. doi: 10.1210/en.2013-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor-beta potency-selective ligands: Structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem. 2001;44:4230–4251. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- Milad MR, Zeidan MA, Contero A, Pitman RK, Klibanski A, Rauch SL, Goldstein JM. The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience. 2010;168:652–658. doi: 10.1016/j.neuroscience.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Campbell SL, Sweatt JD. DNA methylation and histone acetylation work in concert to regulate memory formation and synaptic plasticity. Neurobiol Learn Mem. 2008;89:599–603. doi: 10.1016/j.nlm.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Sweatt JD. Covalent modification of DNA regulates memory formation. Neuron. 2007;53:857–869. doi: 10.1016/j.neuron.2007.02.022. [DOI] [PubMed] [Google Scholar]
- Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, Warrier S, Alves SE. Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. J Comp Neurol. 2005;491:81–95. doi: 10.1002/cne.20724. [DOI] [PubMed] [Google Scholar]
- Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J Comp Neurol. 2001;429:355–371. [PubMed] [Google Scholar]
- Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of estrogen receptor β in the mouse brain: Comparison with estrogen receptor α. Endocrinology. 2003;144:2055–2067. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- Mitterling KL, Spencer JL, Dziedzic N, Shenoy S, McCarthy K, Waters EM, McEwen BS, Milner TA. Cellular and subcellular localization of estrogen and progestin receptor immunoreactivities in the mouse hippocampus. J Comp Neurol. 2010;518:2729–2743. doi: 10.1002/cne.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DD, Segal M. Regulation of dendritic spine density in cultured rat hippocampal neurons by steroid hormones. J Neurosci. 1996;16:4059–4068. doi: 10.1523/JNEUROSCI.16-13-04059.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myskiw JC, Rossato JI, Bevilaqua LR, Medina JH, Izquierdo I, Cammarota M. On the participation of mTOR in recognition memory. Neurobiol Learn Mem. 2008;89:338–351. doi: 10.1016/j.nlm.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Nguyen PV, Woo NH. Regulation of hippocampal synaptic plasticity by cyclic AMP-dependent protein kinases. Prog Neurobiol. 2003;71:401–437. doi: 10.1016/j.pneurobio.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Nilsen J, Brinton RD. Divergent impact of progesterone and medroxyprogesterone acetate (Provera) on nuclear mitogen-activated protein kinase signaling. Proc Natl Acad Sci USA. 2003;100:10506–10511. doi: 10.1073/pnas.1334098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noriega NC, Eghlidi DH, Garyfallou VF, Kohama SG, Kryger SG, Urbanski HF. Influence of 17beta-estradiol and progesterone on GABAergic gene expression in the arcuate nucleus, amygdala and hippocampus of the rhesus macaque. Brain Res. 2010;1307:28–42. doi: 10.1016/j.brainres.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormerod BK, Lee TT, Galea LA. Estradiol initially enhances but subsequently suppresses (via adrenal steroids) granule cell proliferation in the dentate gyrus of adult female rat. J Neurobiol. 2003;55:247–260. doi: 10.1002/neu.10181. [DOI] [PubMed] [Google Scholar]
- Packard MG, Kohlmaier JR, Alexander GM. Posttraining intrahippocampal estradiol injections enhance spatial memory in male rats: Interaction with cholinergic systems. Behav Neurosci. 1996;110:626–632. doi: 10.1037//0735-7044.110.3.626. [DOI] [PubMed] [Google Scholar]
- Packard MG, Teather LA. Intra-hippocampal estradiol infusion enhances memory in ovariectomized rats. NeuroReport. 1997a;8:3009–3013. doi: 10.1097/00001756-199709290-00004. [DOI] [PubMed] [Google Scholar]
- Packard MG, Teather LA. Posttraining estradiol injections enhance memory in ovariectomized rats: Cholinergic blockade and synergism. Neurobiol Learn Mem. 1997b;68:172–188. doi: 10.1006/nlme.1997.3785. [DOI] [PubMed] [Google Scholar]
- Parsons RG, Gafford GM, Helmstetter FJ. Translational control via the mammalian target of rapamycin pathway is critical for the formation and stability of long-term fear memory in amygdala neurons. J Neurosci. 2006;26:12977–12983. doi: 10.1523/JNEUROSCI.4209-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechenino AS, Frick KM. The effects of acute 17β-estradiol treatment on gene expression in the young female mouse hippocampus. Neurobiol Learn Mem. 2009;91:315–322. doi: 10.1016/j.nlm.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto L, Abel T. The role of histone acetylation in memory formation and cognitive impairments. Neuropsychopharmacology. 2013;38:62–76. doi: 10.1038/npp.2012.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira LM, Bastos CP, de Souza JM, Ribeiro FM, Pereira GS. Estradiol enhances object recognition memory in Swiss female mice by activating hippocampal estrogen receptor α. Neurobiol Learn Mem. 2014;114:1–9. doi: 10.1016/j.nlm.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Perovic M, Tesic V, Mladenovic Djordjevic A, Smiljanic K, Loncarevic-Vasiljkovic N, Ruzdijic S, Kanazir S. BDNF transcripts, proBDNF and proNGF, in the cortex and hippocampus throughout the life span of the rat. Age (Dordr) 2013;35:2057–2070. doi: 10.1007/s11357-012-9495-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan A, Lancaster KE, Armstrong JN, MacLusky NJ, Choleris E. Rapid effects of estrogen receptor α and β selective agonists on learning and dendritic spines in female mice. Endocrinology. 2011;152:1492–1502. doi: 10.1210/en.2010-1273. [DOI] [PubMed] [Google Scholar]
- Prange-Kiel J, Fester L, Zhou L, Lauke H, Carrétero J, Rune GM. Inhibition of hippocampal estrogen synthesis causes region-specific downregulation of synaptic protein expression in hippocampal neurons. Hippocampus. 2006;16:464–471. doi: 10.1002/hipo.20173. [DOI] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Ronnekleiv OK, Kelly MJ. Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J Neurosci. 2003;23:9529–9540. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Maidment NT, Schlinger BA. Forebrain steroid levels fluctuate rapidly during social interactions. Nat Neurosci. 2008;11:1327–1334. doi: 10.1038/nn.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter JD, Klann E. Making synaptic plasticity and memory last: Mechanisms of translational regulation. Genes Dev. 2009;23:1–11. doi: 10.1101/gad.1735809. [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperbert C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Ruiz-Palmero I, Hernando M, Garcia-Segura LM, Arevalo MA. G protein-coupled estrogen receptor is required for the neuritogenic mechanism of 17β-estradiol in developing hippocampal neurons. Mol Cell Endocrinol. 2013;372:105–115. doi: 10.1016/j.mce.2013.03.018. [DOI] [PubMed] [Google Scholar]
- Saenz C, Dominguez R, de Lacalle S. Estrogen contributes to structural recovery after a lesion. Neurosci Lett. 2006;392:198–201. doi: 10.1016/j.neulet.2005.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M, Murphy D. Estradiol induces formation of dendritic spines in hippocampal neurons: Functional correlates. Horm Behav. 2001;40:156–159. doi: 10.1006/hbeh.2001.1688. [DOI] [PubMed] [Google Scholar]
- Sheldahl LC, Shapiro RA, Bryant DN, Koerner IP, Dorsa DM. Estrogen induced rapid translocation of estrogen receptor β, but not estrogen receptor α, to the neuronal plasma membrane. Neuroscience. 2008;153:751–761. doi: 10.1016/j.neuroscience.2008.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingo AS, Kito S. Estradiol induces PKA activation through the putative membrane receptor in the living hippocampal neuron. J Neural Trans. 2005;112:1469–1473. doi: 10.1007/s00702-005-0371-8. [DOI] [PubMed] [Google Scholar]
- Shughrue P, Scrimo P, Lane M, Askew R, Merchenthaler I. The distribution of estrogen receptor-β mRNA in forebrain regions of the estrogen receptor-α knockout mouse. Endocrinology. 1997a;138:5649–5652. doi: 10.1210/endo.138.12.5712. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. J Comp Neurol. 1997b;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Merchenthaler I. Evidence for novel estrogen binding sites in the rat hippocampus. Neuroscience. 2000;99:605–612. doi: 10.1016/s0306-4522(00)00242-6. [DOI] [PubMed] [Google Scholar]
- Smith CC, McMahon LL. Estrogen-induced increase in the magnitude of long-term potentiation occurs only when the ratio of NMDA transmission to AMPA transmission is increased. J Neurosci. 2005;26:8517–8522. doi: 10.1523/JNEUROSCI.0762-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava DP, Woolfrey KM, Jones KA, Shum CY, Lash LL, Swanson GT, Penzes P. Rapid enhancement of two-step wiring plasticity by estrogen and NMDA receptor activity. Proc Natl Acad Sci USA. 2008;105:14650–14655. doi: 10.1073/pnas.0801581105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson K, Sun J, Katzenellenbogen BS, Katzenellenbogen JA. Pyrazole ligands: Structure-affinity/activity relationships and estrogen receptor-alpha-selective agonists. J Med Chem. 2000;43:4934–4947. doi: 10.1021/jm000170m. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. Experience-dependent epigenetic modifications in the central nervous system. Biol Psychiatry. 2009;65:191–197. doi: 10.1016/j.biopsych.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999;19:5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Zhang Q, Yang L, Dong Y, Khan M, Yang F, Brann DW, Wang R. GPR30 mediates estrogen rapid signaling and neuroprotection. Mol Cell Endocrinol. 2014;387:52–58. doi: 10.1016/j.mce.2014.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146:624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, Connolly ESJ, Nethrapalli IS, Tinnikov AA. ER-X: A novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J Neurosci. 2002;22:8391–8401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsokas P, Grace EA, Chan P, Ma T, Sealfon SC, Iyengar R, Landau EM, Blitzer RD. Local protein synthesis mediates a rapid increase in dendritic elongation factor 1A after induction of late long-term potentiation. J Neurosci. 2005;25:5833–5843. doi: 10.1523/JNEUROSCI.0599-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuscher JJ, Fortress AM, Kim J, Frick KM. Regulation of object recognition and object placement by ovarian sex steroid hormones. Behav Brain Res. 2015;285:140–157. doi: 10.1016/j.bbr.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuscher JJ, Szinte JS, Starrett JR, Krentzel AA, Remage-Healey L, Frick KM. Soc Neurosci Abstr, Poster 376.307. 2013. Hippocampally-synthesized estrogens are essential for spatial memory consolidation in female mice. [Google Scholar]
- Vedder LC, Smith CC, Flannigan AE, McMahon LL. Estradiol-induced increase in novel object recognition requires hippocampal NR2B-containing NMDA receptors. Hippocampus. 2013;23:108–115. doi: 10.1002/hipo.22068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierk R, Glassmeier G, Zhou L, Brandt N, Fester L, Dudzinski D, Wilkars W, Bender RA, Lewerenz M, Gloger S, Graser L, Schwarz J, Rune GM. Aromatase inhibition abolishes LTP generation in female but not in male mice. J Neurosci. 2012;32:8116–8126. doi: 10.1523/JNEUROSCI.5319-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade CB, Dorsa DM. Estrogen activation of cyclic adenosine 5′-monophosphate response element-mediated transcription requires the extracellularly regulated kinase/mitogen-activated protein kinase pathway. Endocrinology. 2003;144:832–838. doi: 10.1210/en.2002-220899. [DOI] [PubMed] [Google Scholar]
- Wade CB, Robinson S, Shapiro RA, Dorsa DM. Estrogen receptor (ER)α and ERβ exhibit unique pharmacologic properties when coupled to activation of the mitogen-activated protein kinase pathway. Endocrinology. 2001;142:2336–2342. doi: 10.1210/endo.142.6.8071. [DOI] [PubMed] [Google Scholar]
- Wakeling AE, Bowler J. ICI 182,780, a new antioestrogen with clinical potential. J Steroid Biochem Mol Biol. 1992;43:173–177. doi: 10.1016/0960-0760(92)90204-v. [DOI] [PubMed] [Google Scholar]
- Walf AA, Koonce CJ, Frye CA. Estradiol or diarylpropionitrile administration to wild type, but not estrogen receptor beta knockout, mice enhances performance in the object recognition and object placement tasks. Neurobiol Learn Mem. 2008;89:513–521. doi: 10.1016/j.nlm.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiol Learn Mem. 2006;86:35–46. doi: 10.1016/j.nlm.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters EM, Thompson LI, Patel P, Gonzales AD, Ye HZ, Filardo EJ, Clegg DJ, Gorecka J, Akama KT, McEwen BS, Milner TA. G-protein-coupled estrogen receptor 1 is anatomically positioned to modulate synaptic plasticity in the mouse hippocampus. J Neurosci. 2015;35:2384–2397. doi: 10.1523/JNEUROSCI.1298-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters EM, Yildirim M, Janssen WG, Lou WY, McEwen BS, Morrison JH, Milner TA. Estrogen and aging affect the synaptic distribution of estrogen receptor beta-immunoreactivity in the CA1 region of female rat hippocampus. Brain Res. 2011;1379:86–97. doi: 10.1016/j.brainres.2010.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watters JJ, Campbell JS, Cunningham MJ, Krebs EG, Dorsa DM. Rapid membrane effects of steroids in neuroblastoma cells: Effects of estrogen on mitogen activated protein kinase signalling cascade and c-fos immediate early gene transcription. Endocrinology. 1997;138:4030–4033. doi: 10.1210/endo.138.9.5489. [DOI] [PubMed] [Google Scholar]
- Wise PM, Dubal DB, Wilson ME, Rau SW, Liu Y. Estrogens: Trophic and protective factors in the adult brain. Front Neuroendocrinol. 2001;22:33–66. doi: 10.1006/frne.2000.0207. [DOI] [PubMed] [Google Scholar]
- Wong M, Moss RL. Long-term and short-term electrophysiological effects of estrogen on the synaptic properties of hippocampal CA1 neurons. J Neurosci. 1992;12:3217–3225. doi: 10.1523/JNEUROSCI.12-08-03217.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Weiland NG, McEwen BS, Schwartzkroin PA. Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic input: Correlation with dendritic spine density. J Neurosci. 1997;17:1848–1859. doi: 10.1523/JNEUROSCI.17-05-01848.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman JL, Barha CK, Galea LA. Endocrine substrates of cognitive and affective changes during pregnancy and postpartum. Behav Neurosci. 2012;126:54–72. doi: 10.1037/a0025538. [DOI] [PubMed] [Google Scholar]
- Wu TW, Chen S, Brinton RD. Membrane estrogen receptors mediate calcium signaling and MAP kinase activation in individual hippocampal neurons. Brain Res. 2011;1379:34–43. doi: 10.1016/j.brainres.2011.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Barnes D, Lindquist K, Cauley J, Simonsick EM, Penninx B, Satterfield S, Harris T, Cummings SR. Endogenous sex hormone levels and risk of cognitive decline in an older biracial cohort. Neurobiol Aging. 2007;28:171–178. doi: 10.1016/j.neurobiolaging.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Yang LC, Zhang QG, Zhao CF, Yang F, Zhang YD, Wang RM, Brann DW. Extranuclear estrogen receptors mediate the neuroprotective effects of estrogen in the rat hippocampus. PLoS One. 2010;5:e9851. doi: 10.1371/journal.pone.0009851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokomaku D, Numakawa T, Numakawa Y, Suzuki S, Matsumoto T, Adachi N, Nishio C, Taguchi T, Hatanaka H. Estrogen enhances depolarization-induced glutamate release through activation of phosphatidylinositol 3-kinase and mitogen-activated protein kinase in cultured hippocampal neurons. Mol Endocrinol. 2003;17:831–844. doi: 10.1210/me.2002-0314. [DOI] [PubMed] [Google Scholar]
- Zandi PP, Carlson MC, Plassman BL, Welsh-Bohmer KA, Mayer LS, Steffens DC, Breitner JCS. Hormone replacement therapy and incidence of Alzheimer disease in older women. JAMA. 2002;288:2123–2129. doi: 10.1001/jama.288.17.2123. [DOI] [PubMed] [Google Scholar]
- Zhao L, Brinton RD. Estrogen receptor α and β differentially regulate intracellular Ca2+ dynamics leading to ERK phosphorylation and estrogen neuroprotection in hippocampal neurons. Brain Res. 2007;1172:48–59. doi: 10.1016/j.brainres.2007.06.092. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Fan L, Fortress AM, Boulware MI, Frick KM. Hippocampal histone acetylation regulates object recognition and the estradiol-induced enhancement of object recognition. J Neurosci. 2012;32:2344–2351. doi: 10.1523/JNEUROSCI.5819-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Fan L, Frick KM. Epigenetic alterations regulate the estradiol-induced enhancement of memory consolidation. Proc Natl Acad Sci USA. 2010;107:5605–5610. doi: 10.1073/pnas.0910578107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Fester L, Haghshenas S, de Vrese X, von Hacht R, Gloger S, Brandt N, Bader M, Vollmer G, Rune GM. Oestradiol-induced synapse formation in the female hippocampus: roles of oestrogen receptor subtypes. J Neuroendocrinol. 2014;26:439–447. doi: 10.1111/jne.12162. [DOI] [PubMed] [Google Scholar]