Abstract
Runs of homozygosity (ROHs) are recognized signature of recessive inheritance. Contributions of ROHs to the genetic architecture of coronary artery disease and regulation of gene expression in cells relevant to atherosclerosis are not known. Our combined analysis of 24,320 individuals from 11 populations of white European ethnicity showed an association between coronary artery disease and both the count and the size of ROHs. Individuals with coronary artery disease had approximately 0.63 (95% CI: 0.4–0.8) excess of ROHs when compared to coronary-artery-disease-free control subjects (p = 1.49 × 10−9). The average total length of ROHs was approximately 1,046.92 (95% CI: 634.4–1,459.5) kb greater in individuals with coronary artery disease than control subjects (p = 6.61 × 10−7). None of the identified individual ROHs was associated with coronary artery disease after correction for multiple testing. However, in aggregate burden analysis, ROHs favoring increased risk of coronary artery disease were much more common than those showing the opposite direction of association with coronary artery disease (p = 2.69 × 10−33). Individual ROHs showed significant associations with monocyte and macrophage expression of genes in their close proximity—subjects with several individual ROHs showed significant differences in the expression of 44 mRNAs in monocytes and 17 mRNAs in macrophages when compared to subjects without those ROHs. This study provides evidence for an excess of homozygosity in coronary artery disease in outbred populations and suggest the potential biological relevance of ROHs in cells of importance to the pathogenesis of atherosclerosis.
Introduction
Coronary artery disease (CAD) is a complex, heterogeneous polygenic disorder. The largest genome-wide association (GWA) meta-analysis conducted to date reported 46 variants associated with risk of CAD.1 All these variants were identified assuming an additive mode of inheritance for CAD. It is increasingly recognized that the genetic architecture of complex disorders, including CAD, is not a simple composite of variants that operate exclusively under an additive mode of inheritance and that both dominant and recessive components might make important contributions overseen by conventional GWA studies (GWASs).
The potential importance of recessively inherited variants to cardiovascular disease was suggested by several previous investigations. First, history of parental consanguinity (and thus increased homozygosity) was associated with increased risk of premature myocardial infarction, independent of conventional CAD risk factors in a population of young adults of South Asian ethnicity.2 Second, studies in isolated populations with increased parental relatedness suggested possible associations between homozygosity or its proxies and the risk of both CAD3–5 and other cardiovascular phenotypes.5,6 These associations were attributed to the increased levels of homozygosity and inbreeding depression (reduced biological fitness) in these populations.7
Homozygosity mapping is a strategy with a potential to identify and quantify the recessive component of inheritance—long stretches (usually >1 Mb) of consecutive homozygous genotypes are known as runs of homozygosity (ROHs).8 In the human genome, ROHs represent “re-union” of pieces of DNA from common ancestors in their descendants.8 Identical by descent, ROHs arise from background relatedness promoted by demographic processes that increase homozygosity and reduce population size (for example, cultural and/or social factors that favor consanguinity and natural selection).9 A number of studies clearly demonstrated the presence of relatively frequent ROHs in outbred populations.8,10–12 However, only a few studies successfully used the GWA-derived homozygosity measures to examine their role in the genetic architecture of complex disorders in outbred populations.13–15 To the best of our knowledge, there is no study that examined whether homozygosity is associated with CAD in such populations and whether individual ROHs might play a role in regulation of gene expression within cells of key importance to atherosclerosis.
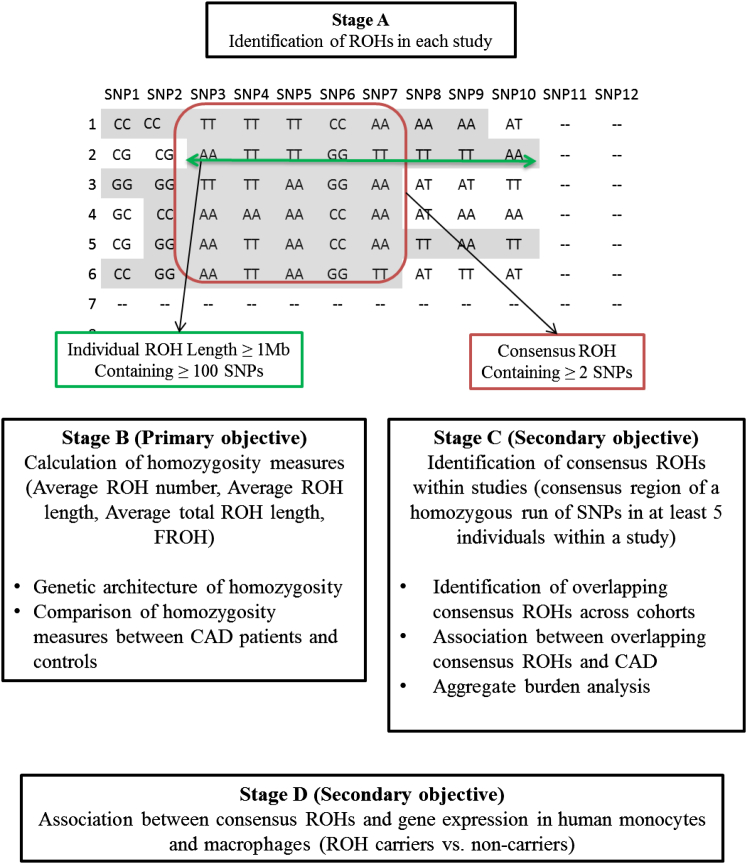
The primary goal of this project was a comprehensive analysis of association between genome-wide homozygosity measures and CAD in individuals of white European ancestry. A secondary analysis was undertaken to identify and quantify consensus ROHs overlapping across studies and explore their potential relevance to CAD individually and at the aggregate level. Finally, we explored the association of consensus ROHs and gene expression in human monocytes and macrophages. The overview of the strategy used is included in Figure 1.
Figure 1.
Overview of the Project Strategy
Subjects and Methods
Study Cohorts
Genetic information on 24,320 biologically unrelated individuals (all of white European ancestry) was collected from 11 previous GWASs.16 A total of 12,123 individuals with CAD and 12,197 CAD-free control subjects from the Wellcome Trust Case Control Consortium (WTCCC),17 the German Myocardial Infarction Family Studies (GerMIFSI, II, and III),17–19 InterHeart Study (ITH),20 the Ottawa Heart Genomics Studies (OHGS-A, B, and C),21 PennCATH,22 Cleveland Clinic Gene Bank (CCGB), and Duke Cathgen Study (DUKE) were included in the analysis. In brief, six studies (GerMIFSI, GerMIFSII, GerMIFSIII, OHGS-A, PennCATH, and WTCCC) were derived from the original CARDIoGRAM Consortium.16 Five additional studies (CCGB, DUKE, ITH, OHGS-B, and OHGS-C), not originally a part of the Consortium,16 agreed to participate, bringing a total number of examined populations to 11. Further information on the recruitment and phenotyping of the studies is available in the Supplemental Data including Table S1.
Genotyping and Imputation
DNA was extracted from peripheral blood in all studies except British 1958 Birth Cohort (58BC), which contributed control subjects to WTCCC.23 Indeed, in these subjects DNA was extracted from cell lines.23 Information on SNPs included in the analysis is shown in Table S2. Only imputed genotypes that could be called with a posterior probability of ≥90% were included in the analysis. SNPs were removed from further analysis if their minor allele frequency (MAF) was <1%, their genotype distribution deviated from Hardy-Weinberg equilibrium with p < 0.001, or the genotype post-imputation call rate was ≤95%. Individual samples were removed from further analysis if the sample call rate was ≤95%. All quality-control filters were applied individually at the cohort level.
ROH Identification
A number of ROH definitions and methods of their detection have been proposed.12,24–26 In this project ROHs were identified via the “Runs of Homozygosity” program, implemented in PLINK (v.1.07).27 The adopted PLINK parameters are similar to the ones used in previous publications.28,29 A sliding window of 50 SNPs in 5,000 kb length region was used to scan the genome. To prevent underestimating the number and size of ROHs, one heterozygote and two missing calls in each window were permitted to allow for possible genotyping errors within a stretch of truly homozygous SNPs or other sources of artificial heterozygosity. A SNP was counted as a part of a ROH if >5% of windows spanning it were homozygous. These parameters were selected to minimize the probability of a window being called homozygous by chance. The existence of linkage disequilibrium (LD) blocks in DNA means that relatively short ROHs (those spanning from tens to hundreds of kilobases) are very prevalent across the genome.30–33 In order to exclude these very common short tracts of homozygous SNPs, the minimum length for a ROH was set at 1 Mb, as used by several other studies.13,28 Two additional parameters were added to ensure that estimates were not artificially inflated by apparently homozygous tracts in sparsely covered genomic regions. First, the required minimum SNP density was set to 50, meaning at least 1 SNP had to be present per 50 kb of DNA, and second, the maximum distance between two consecutive homozygous SNPs was set to 100 kb. To ensure that the analysis captures only regions that are entirely homozygous between the first and the last SNP, a threshold for the minimum number of SNPs constituting a ROH was selected. In line with previous studies on homozygosity of complex disorders, the minimum number of homozygous SNPs to qualify as a ROH in this project was set to 100.13 There were no major differences in the results of ROH analyses conducted under different thresholds of ROH calculation parameters in our pilot sensitivity analyses in WTCCC (data not shown).
Calculation of Homozygosity Measures
The following measures of homozygosity were calculated in each study: (1) the average number of ROHs, (2) the total and average length of ROHs, and (3) the proportion of the autosomal genome covered by ROHs (FROH).34 The total number of ROHs was defined as the sum of all ROHs per individual. The average ROH number was calculated as the total number of runs divided by the total number of subjects. The average total ROH length is the sum of the length of each individual ROH per participant and was calculated by dividing the total ROH length by the number of individuals. The average ROH length was calculated by dividing the total genomic length of the ROHs by the total number of ROHs per individual. To calculate FROH, a percentage of homozygosity was calculated by summing ROHs >1 Mb across the covered autosomal genome and dividing by the total autosomal base pairs represented in the SNP data.8 Specifically, the summed length of identified ROHs were divided by a factor of 2,772.7 and subsequently converted to a percent by multiplying the dividend by 100. A factor of 2,772.7 is the number of megabases covered by SNPs after imputation, which was calculated by summing the distance between the first and the last available consecutive SNP on each chromosomal arm for each of the 22 autosomes. Examination of the FROH distribution revealed that it was highly right-skewed. To facilitate the analysis, data were transformed via a rank-based inverse normal transformation.
Assuming the dispersion of general homozygosity indices similar to the observed measures, a study with 24,320 subjects including 12,123 individuals with CAD and 12,197 CAD-free control subjects has ∼80% power to detect a case-control difference of ∼0.25 in ROH number, ∼4.7 kb in average ROH length, and ∼520 kb in average total ROH length.
Definition of Consensus ROH
It is not expected that a ROH will start and end at the same base pair positions for each individual, so we needed to define the size of the overlapping ROHs. For each study, the size of a ROH was determined as the consensus region of a homozygous run of SNPs (>1 Mb in length) overlapping between at least five individuals within a cohort. Consensus ROHs were then separated into groups and the numbers of case and control subjects with the ROH were counted via PLINK in each cohort. This ROH definition was applied to each study and provided a set of consensus ROHs. Information on the size, location (start/end of the consensus ROH), and the number of SNPs in each consensus ROH were calculated for each study.
Identification of Overlapping Consensus ROHs
We combined these consensus ROHs from individual studies to identify regions overlapping between studies. This was achieved by comparing the positions of the consensus ROHs from each study against those from all other cohorts; this was repeated until we had identified how many studies overlapped for each individual consensus ROH. We allowed each individual study consensus ROH to overlap with more than one consensus ROH from another individual study to allow longer ROHs to be included in multiple overlapping consensus ROHs. We then combined the numbers of case and control subjects across studies with the overlapping consensus ROH.
Statistical Analysis
Genetic Architecture of Homozygosity Measures
We generated QQ plots to assess the quality of the data in each individual study. A regression analysis using individual level participant data adjusting for cohort, sex, and age where possible was performed for the homozygosity measures. Age and sex were the only additional variables available in all studies. Additional sensitivity analyses using a rank-based inverse normal transformation of general homozygosity measures was performed to assess the influence of outliers. Further sensitivity analyses were conducted to exclude the effect of a cohort with different cell sources of DNA in case and control subjects. Finally, to account for potential heterogeneity between individual studies in the combined analysis of data, we first fitted a mixed model with cohort as a random effect in the analysis of association between CAD and four homozygosity measures. Second, we introduced a term of interaction between cohort and the outcome (CADxcohort or FROHxcohort) into the regression models that examined association between CAD and each of four homozygosity measures. Further sensitivity association analyses were conducted for those measures of homozygosity that showed some evidence of heterogeneity between populations (defined as significance of interaction term at p < 0.05). These sensitivity analyses examined the magnitude and the significance of association between homozygosity measures and CAD after exclusion of cohorts with statistically significant interaction terms. Each of the sensitivity analyses was conducted via regression models fitted with cohort as both fixed and random effect.
Association Analysis between Overlapping Consensus ROHs and CAD
Overlapping consensus ROHs across populations were analyzed via logistic regression with CAD as the response with adjustment for sex, age where possible, study, and ROH (presence or absence) status (as the predictor of interest). To account for multiple testing, we used a Bonferroni correction in this analysis (p = 2.94 × 10−6). A binomial test was used to examine whether there was a deviation from the expected distribution (50/50) of overlapping consensus ROHs showing increased (OR > 1) and decreased (OR < 1) risk of CAD.
Gene Expression Analysis and ROHs
Approximately 600,000 directly genotyped SNPs were available in the Cardiogenics Study. For the sake of consistency with the other examined studies, imputation was performed based on HapMapII CEU build 36. The ROH definitions and the principles of genome-wide homozygosity analysis were similar to the one conducted in Stage A of this project (Figure 1).
Information on gene probes underneath consensus ROHs in monocytes (758 individuals) and macrophages (614 individuals) was extracted in silico from the microarray-based experiment conducted in the Cardiogenics Study reported previously.35,36 The comparative analysis was conducted at the probe level in cis, assessing only genes within 500 kb of a ROH, by using linear regression adjusted for age, sex, recruitment center, and the status (presence or absence) of consensus ROHs. After quality-control filters, information on 11,336 gene probes was available to examine differences in expression of genes in monocytes and macrophages between individuals with ROHs versus those without ROHs. Results were first adjusted by genomic control. We then calculated false discovery rate (FDR) q values for all identified ROH-mRNA associations using a method by Storey and Tibshirani appropriate for correlated gene expression data.37 Associations with a FDR q value of < 0.01 were considered statistically significant.
All statistical analyses were undertaken in STATA v.12.
Results
General Characteristics of the Study Cohorts
A total of 24,320 individuals of European ancestry from 11 populations were included. The key characteristics of the cohorts (including definition of CAD in each study) used in the homozygosity analysis are summarized in Table S1.
General Characteristics of Homozygosity Measures
The summary and distribution of homozygosity measures in each population are shown in Table S3 and Figure S1. A joint analysis of all subjects revealed on average 31.84 ± 8.44 ROHs in autosomal DNA. The stretches of homozygous SNPs had an average length of 1,360.58 ± 127.19 kb and they covered on average a total length of 43.67 ± 15.59 Mb. The number and length of ROHs per individual were in the range of 4–276 (number) and 1–29.4 Mb (length) in the overall sample.
Comparison of Homozygosity Measures between Individuals with CAD and CAD-Free Control Subjects
The distribution of average ROH number, average ROH length, and average total ROH length for case and control subjects in each population are shown in Figure S2. As a general trend, the distributions of homozygosity measures were comparable between case and control subjects from the same populations.
The combined analysis of 11 populations revealed statistically significant differences in homozygosity levels between individuals with CAD and control subjects after adjustment for study, sex, and age (Table 1). On average, individuals with CAD had 0.63 ROHs more than control subjects (β = 0.63, 95% CI: 0.42–0.83, p = 1.49 × 10−9). The length of ROHs in individuals with CAD was on average 4.50 kb longer compared to control subjects (β = 4.50, 95% CI: 0.85–8.15, p = 0.016). The average total length of ROHs in the autosomal genome was 1,046.92 kb greater in individuals with CAD than control subjects (β = 1,046.92, 95% CI: 634.37–1,459.48, p = 6.61 × 10−7). Logistic regression analysis revealed that every 1 SD increase in FROH was associated with a 13% increase in CAD risk (OR = 1.13, 95% CI: 1.09–1.17, p = 1.57 × 10−11). The association results were similar using cohort as both fixed (Table 1) and random (Table S4) effect.
Table 1.
Differences in Homozygosity Measures between Individuals with Coronary Artery Disease and Control Subjects: Combined Analysis
| Measure |
Un-adjusted Analysis |
Age-Adjusted Analysis |
Age- and Sex-Adjusted Analysis |
||||||
|---|---|---|---|---|---|---|---|---|---|
| β-coefficient/OR | 95% CI | p Value | β-coefficient/OR | 95% CI | p Value | β-coefficient/OR | 95% CI | p Value | |
| Average ROH number | 0.39 | 0.20, 0.57 | 3.92 × 10−5 | 0.67 | 0.47, 0.86 | 4.12 × 10−11 | 0.63 | 0.42, 0.83 | 1.49 × 10−9 |
| Average ROH length (kb) | 2.51 | −0.80, 5.82 | 0.14 | 5.19 | 1.64, 8.75 | 0.004 | 4.50 | 0.85, 8.15 | 0.016 |
| Average total length of ROHs (kb) | 688.11 | 314.44, 1,061.78 | 3.08 × 10−4 | 1,145.15 | 743.95, 1,546.35 | 2.24 × 10−8 | 1,046.92 | 634.37, 1,459.48 | 6.61 × 10−7 |
| FROH | 1.07 | 1.03, 1.10 | 8.63 × 10−5 | 1.14 | 1.10, 1.18 | 2.21 × 10−13 | 1.13 | 1.09, 1.17 | 1.57 × 10−11 |
Data for number, average length, and total length of homozygosity runs (ROHs) are β-coefficients (with respective confidence intervals and level of statistical significance [p value] from regressing homozygosity measures on case-control status with adjustment for cohort, age, and sex, where appropriate); data on FROH (proportion of autosomal genome in ROHs) are expressed as odds ratios (OR) of coronary artery disease risk (with confidence intervals and level of statistical significance) with adjustment for cohort, age, and sex (where appropriate). 95% CI indicates 95% confidence intervals.
We have conducted a number of further sensitivity analyses to confirm robustness of our findings. First, by using a rank-based inverse normal transformation for ROH number, average, and total length, we confirmed that outliers had no noticeable influence on the estimates of association between CAD and these homozygosity measures (data not shown). Second, exclusion of a proportion of WTCCC controls whose DNA was extracted from cell lines (the British 1958 Birth Cohort) rather than peripheral blood (like the rest of case and control subjects) had no major impact on the significance of our data (Table S5). Third, accounting for some heterogeneity between populations in the analysis of association between CAD and average ROH number and FROH did not affect the significance of our findings. Indeed, exclusion of cohorts with significant interaction terms did not reduce the magnitude or statistical significance of association between CAD and average ROH number or FROH (Table S6). In fact, correcting for the residual heterogeneity has increased the statistical significance of the findings.
Identification and Characterization of the Overlapping Consensus ROHs
A joint analysis of all consensus ROHs identified across 11 examined populations revealed a total of 16,989 overlapping consensus ROHs shared by at least two populations. An example of an overlapping consensus ROH segment is illustrated in Figure S3. These overlapping consensus ROHs were further classified into four groups based on their SNP enrichment (Table S7).
Association between Individual Overlapping Consensus ROHs and CAD
After correction for multiple testing calculated at p = 2.94 × 10−6, none of the 16,989 identified overlapping consensus ROHs were associated with CAD. The most significant association between an overlapping consensus ROH on chromosome 3 (present in 138 subjects from 7 populations) was assigned a nominal level of statistical significance of p = 1.99 × 10−4. Details of the ten most statistically significant overlapping consensus ROHs are listed in Table S8.
Overlapping Consensus ROHs and CAD: Aggregate Burden Analysis
Based on the magnitude of crude OR from the analysis of association with CAD, each of 16,989 overlapping consensus ROHs was classified as potentially increasing (OR > 1.0) or decreasing (OR < 1.0) risk of CAD. Under a null hypothesis of no association between overlapping consensus ROHs and CAD, the distribution of overlapping consensus ROHs between both categories should be even (50/50). However, a clear deviation from this expected distribution was apparent: there was an excess of overlapping consensus ROHs increasing risk of CAD over those classified as potentially protective (55/45), a difference inconsistent with chance (p = 2.69 × 10−33) (Table 2). The same over-representation of ROHs increasing the risk of CAD was apparent in each category of overlapping consensus ROHs after stratification based on number of SNPs in each consensus segment (Table 2).
Table 2.
Frequency of Overlapping Consensus ROHs with a Potentially Increasing or Decreasing Risk of Coronary Artery Disease: Analysis Stratified on Number of SNPs in Overlapping Consensus ROHs
| Overlapping Consensus ROHs | Expected | Observed | p Value | |
|---|---|---|---|---|
| Overall | ↑ CAD risk | 50% (8,494.5) | 54.6% (9,278) | 2.69 × 10−33 |
| ↓ CAD risk | 50% (8,494.5) | 45.4% (7,711) | ||
| Group 0 | ↑ CAD risk | 50% (926) | 55.1% (1,020) | 1.37 × 10−5 |
| 2–9 SNPs | ↓ CAD risk | 50% (926) | 44.9% (832) | |
| Group 1 | ↑ CAD risk | 50% (3,009) | 53.4% (3,214) | 1.33 × 10−7 |
| 10–49 SNPs | ↓ CAD risk | 50% (3,009) | 46.6% (2,804) | |
| Group 2 | ↑ CAD risk | 50% (1,704) | 55.0% (1,876) | 4.09 × 10−9 |
| 50–99 SNPs | ↓ CAD risk | 50% (1,704) | 45.0% (1,532) | |
| Group 3 | ↑ CAD risk | 50% (2,855.5) | 55.5% (3,168) | 1.39 × 10−16 |
| 100+ SNPs | ↓ CAD risk | 50% (2,855.5) | 44.6% (2,543) |
Data are counts and percentages. Overlapping consensus ROHs were classified as increasing (↑) and decreasing (↓) risk of coronary artery disease (CAD) based on the magnitude of their odds ratio (OR) for CAD; OR > 1.0 indicates increasing risk of CAD and OR < 1.0 indicates decreasing risk of CAD. p value indicates level of statistical significance from binomial test, and the size (and thus the number of SNPs) in each overlapping consensus ROH depends on the length of the consensus sequence common for the studies: from 2 to 100+ SNPs were identified in these regions.
Association between Consensus ROHs and Gene Expression Measures in Human Monocytes and Macrophages
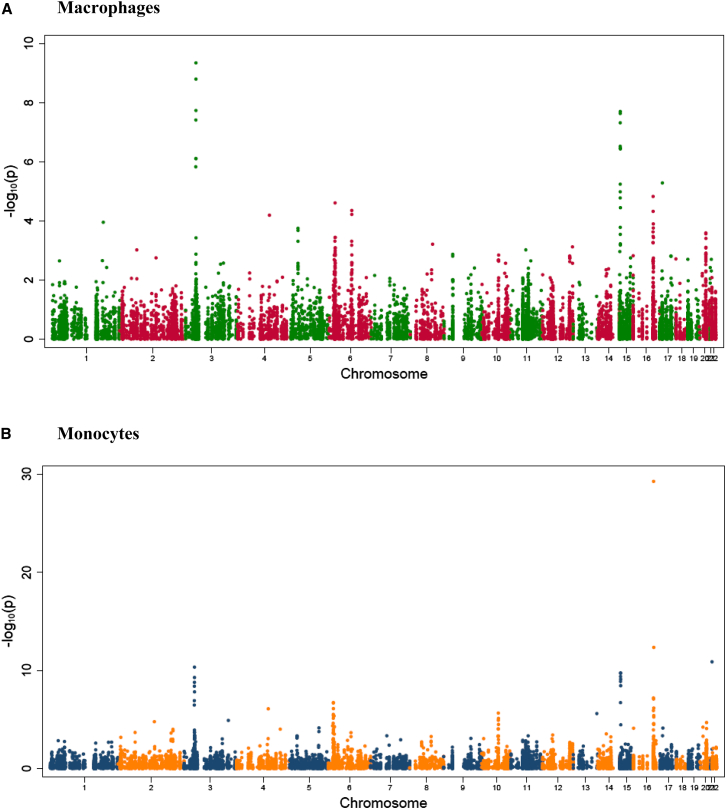
To explore whether the presence of individual consensus ROHs is related to expression of genes that map onto or near specific ROHs, we combined genome-wide consensus ROHs with data from monocyte and macrophage transcriptome profiling in the Cardiogenics Study. In brief, 11,336 gene probes were expressed in human monocytes and macrophages. A total of 3,223 consensus ROHs were identified in the genome-wide homozygosity analysis of Cardiogenics. After FDR-based correction for multiple testing (q value < 0.01), 44 consensus ROH-mRNA associations retained their statistical significance in monocytes (Figure 2, Table S9). The most significant association was identified between ROH on chromosome 16 and expression of dihydrouridine synthase 2 (DUS2L [MIM: 609707])—subjects with this consensus ROH had on average 0.23 (95% CI: 0.19–0.26) lower expression of DUSL2 in monocytes when compared to those without this ROH after adjustment for age, sex, and center of recruitment (p = 5.74 × 10−30, q = 6.51 × 10−26). After the correction for multiple testing, 17 consensus ROH-mRNA associations retained their statistical significance in macrophages (Figure 2, Table S10). The most significant association was identified on chromosome 3 with the expression of WD repeat domain 6 (WDR6 [MIM: 606031])—subjects with this consensus ROH had on average 0.19 (95% CI: 0.14–0.25) lower expression of WDR6 in macrophages when compared to those without this ROH after adjustment for age, sex, and center of recruitment (p = 4.57 × 10−10, q = 5.18 × 10−6).
Figure 2.
Analysis of Association between the Presence of Consensus ROHs and Gene Expression in the Cardiogenics Study: Genome-wide Signal-Intensity Plot
(A) Human monocytes.
(B) Human macrophages.
The y axis shows the logarithmic level of statistical significance (p value) for association of each consensus ROH with expression. The x axis shows 22 autosomal chromosomes in numerical order.
Discussion
Our analysis of homozygosity produced several important findings. First, we provided evidence for enrichment of homozygosity in individuals with CAD when compared to CAD-free control subjects. Second, although none of the identified overlapping consensus ROHs was associated with CAD individually, their aggregate burden analysis showed over-representation of overlapping consensus ROHs favoring increased risk of CAD when compared to those showing the opposite direction of association with CAD. Taken together, these data indicate that homozygosity might be an important risk factor for CAD and suggest that the accumulation of multiple recessive variants might be an important component of the genetic architecture of CAD. Finally, the analysis of human monocyte and macrophage transcriptomes suggested that many individual consensus ROHs might carry biologically active variants with a potential to affect expression of genes located either within these ROHs or in their close proximity.
Our analysis of homozygosity measures provided further evidence for the presence of common ROHs in outbred populations.8,11,12 Detection of ROHs in homozygosity mapping tends to vary along the genome due to differences in informativeness of haplotypes and differences in haplotype genealogies.38 The relative performance of homozygosity mapping is influenced by population demographic processes and the extent of selection against causal variants.38 ROHs were widely distributed across the entire genome and were present on each human autosome. The number of ROHs was a function of chromosomal length, and the average ROH number and average total length of ROHs were dependent on chromosomal size, showing good agreement with previous studies in populations of European ancestry.29
Inbred individuals tend to have higher rates of congenital disorders and lower survival and fertility rates (inbreeding depression).7,39 FROH is a measure of inbreeding effects and correlates most highly with the homozygous mutational load, the putative causal mechanism underlying inbreeding depression.34 This homozygosity measure has low prediction error variance, especially when SNP density is high.14 However, given the small variation in genome-wide FROH in unrelated individuals, large sample sizes are necessary to detect inbreeding depression for likely effect sizes.14 Previous studies investigating the effects of FROH on human complex traits with relatively small sample sizes have failed to find significant inbreeding effects,28,34,40–43 most probably because they were underpowered. Furthermore, very small studies (n < 1,000) that did find significant inbreeding depression effects using FROH13 might greatly overestimate the size of effects. The sample size of this study provides a well-powered tool for finding signatures of homozygosity on CAD risk. As a result, we were able to quantify a measure of global homozygosity in relation to CAD—an estimated 13% increase in CAD risk per 1 SD increase in FROH.
We appreciate that our analysis did not reveal statistically significant associations between individual overlapping consensus ROHs and CAD, possibly because many of them have low population frequency and this can affect the power of the analysis. Indeed, although some overlapping consensus homozygous sequences are common and might coincide with previously identified ROH islands29 or long haplotypes, a majority of the consensus regions overlapping across populations occur in low frequencies (<5%) or indeed are very rare (<1%).
However, overlapping consensus ROH-based analysis provided several important insights into the genetic architecture of CAD. First, it revealed that none of the identified overlapping consensus regions were exclusive to CAD case subjects. Second, it showed that none of the identified overlapping consensus regions have been implicated in previous CAD GWASs. This is perhaps not surprising given that GWASs have analyzed data under an additive model of inheritance, whereas ROH analysis assumes recessive mode of inheritance. Third, the aggregate burden analysis of overlapping consensus ROHs showed an excess of the regions favoring increased risk of CAD over those that tend to protect against it, supporting the hypothesis that individuals with CAD might have accumulated more recessive variants than controls. This cumulative excess of ROHs with dispersed distribution across the genome rather than effects of specific variants recessively inherited by a subset of affected individuals44 appears a more likely driver for the increased homozygosity in CAD. Additional work is needed to unravel the exact synergistic effects of multiple recessive variants on global homozygosity levels and their relevance to CAD. Finally, within the constraints of interpretational limitations discussed above, the overlapping consensus ROH-based analysis provides a useful example of comparing individual ROHs across studies.
Our study also examined the effects of consensus ROHs on the expression of genes in human cells relevant to atherosclerosis. One of the most obvious explanations for the biological meaning of the associations between consensus ROHs and the expression of genes underneath them or in close proximity to them is that they are signatures of functionally active recessive variants with a potential to affect transcription in monocytes and macrophages. Neither of the identified genes showed the immediately obvious biological relevance to atherosclerosis or CAD. Indeed, the most significant association in monocytes was identified with the expression of DUS2L. This gene encodes a cytoplasmic protein that catalyzes the conversion of uridine residues to dihydrouridine in the D-loop of tRNA. The encoded protein might affect the rate of translation by inhibiting an interferon-induced protein kinase.45 The most significant association in macrophages was with the expression of WDR6. This gene encodes a member of the WD repeat protein family implicated in regulation of cell growth arrest.46 Future studies will be necessary to elucidate the molecular and clinical mechanisms of these associations.
We should mention that definition of ROHs varies across studies in the published literature, which makes their comparisons difficult.47 In order to combine the ROH information across several studies, we made use of both directly genotyped and imputed SNPs to increase the coverage of the genome and extract the maximum possible information. The use of imputed SNPs helped to increase similarity of genomic coverage across studies and made the data more comparable because ROHs were defined based on similar SNP sets. We applied a requirement that a genotype could be called only if the posterior probability was >90% and then applied call rate filters to remove SNPs, which were called in <95% of individuals. This has an effect of thinning the imputed data but is not equivalent to having the same effect as LD pruning. We chose not to use LD-driven elimination of SNPs from our datasets because such pruning might act as a potential confounder in comparative ROH analyses of different populations—the local level of LD determines the effective number of SNPs used for ROH definition.29 Although LD pruning brings the benefit of selecting independent SNPs, previous studies have shown that LD-based pruning can reduce informativeness of datasets and lead to a loss in power to detect ROHs.48 Many recently published studies defined a ROH to include a minimum of 50–65 SNPs for pruned and unpruned data.44,49–53 Therefore, we have accounted for imputation-driven increase in SNP density by extension of the minimal number of SNPs that define a ROH to 100.
We acknowledge the potential confounding of undetected clonal mosaicism manifesting as chromosomal abnormalities (i.e., uniparental disomy) that might mirror runs of homozygosity in GWASs based on DNA extracted from peripheral blood. Indeed, both Laurie et al.54 and Jacobs et al.55 revealed that clones of cells with such chromosomal anomalies are present in free-of-cancer (apparently healthy) individuals included in GWASs and that increasing age is associated with augmented risk of clonal mosaicism in the human genome.54–56 This in essence means that comparative analysis of homozygosity in DNA extracted from peripheral blood between case and control subjects that differ in age might be potentially affected by increased rates of such clonal chromosomal abnormalities (and thus inflated homozygosity measures) in the older group. Although we were not able to directly quantify the prevalence of such abnormalities in the populations included in this project, we reason that a correction for such a potential confounding would further increase rather than reduce the significance of our association findings. Indeed, CAD-free control subjects were generally much older than individuals with CAD in a majority of studies included in this project. Thus, a correction for age-driven clonal mosaicism-related chromosomal abnormalities interpreted as ROHs in genome-wide analysis would reduce the rates of homozygosity in the older groups (mostly CAD-free control subjects), further increasing the case-control difference. To this end, we also acknowledge the different cell types as a source of DNA for experiments in one of 11 cohorts included in this project. Indeed, a part of control group in WTCCC (58BC cohort) was genotyped using DNA extracted from cell lines.23 DNA extracted from cell lines might be associated with higher rates of chromosomal aberrations that might resemble ROHs in GWASs. However, we confirmed that exclusion of those subjects had no major effect on findings from our analysis of association between CAD and homozygosity measures.
Conclusions
Genome-wide homozygosity analysis revealed statistically significant differences in the genome-wide homozygosity levels between individuals with CAD and CAD-free control subjects. The aggregate burden analysis of overlapping consensus ROHs showed their over-representation among subjects with CAD, suggesting that accumulation of recessive variants might increase the risk of CAD. Finally, the presence of associations between consensus ROHs and gene expression in human monocytes and macrophages suggest that many individual ROHs might be signatures of biologically active recessive variants with a potential to regulate transcription.
Acknowledgments
This study was supported by the Alumni Association of University of Leicester PhD studentship and International Mentoring Travel Award by American Heart Association (to P.C.). M.T. is supported by the British Heart Foundation. N.J.S. holds a personal chair supported by the British Heart Foundation and is a UK NIHR senior investigator. C.P.N. is funded by the British Heart Foundation. The Cardiogenics project was supported by the European Union 6th Framework Program (LSHM-CT-2006-037593). The UKBS collection of Common Controls has been funded by the Wellcome Trust grant 084183/Z/07/Z and by NIHR programme grant to NHSBT (RP-PG-0310-1002). The collection was established as part of the WTCCC. We would like to thank the participants and members of the CARDIoGRAM and the Cardiogenics consortia. Members of the Cardiogenics Consortium are listed in the Supplemental Data.
Published: July 9, 2015
Footnotes
Supplemental Data include consortia member list, three figures, and ten tables and can be found with this article online at http://dx.doi.org/10.1016/j.ajhg.2015.06.001.
Supplemental Data
References
- 1.Deloukas P., Kanoni S., Willenborg C., Farrall M., Assimes T.L., Thompson J.R., Ingelsson E., Saleheen D., Erdmann J., Goldstein B.A., CARDIoGRAMplusC4D Consortium. DIAGRAM Consortium. CARDIOGENICS Consortium. MuTHER Consortium. Wellcome Trust Case Control Consortium Large-scale association analysis identifies new risk loci for coronary artery disease. Nat. Genet. 2013;45:25–33. doi: 10.1038/ng.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ismail J., Jafar T.H., Jafary F.H., White F., Faruqui A.M., Chaturvedi N. Risk factors for non-fatal myocardial infarction in young South Asian adults. Heart. 2004;90:259–263. doi: 10.1136/hrt.2003.013631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shami S.A., Qaisar R., Bittles A.H. Consanguinity and adult morbidity in Pakistan. Lancet. 1991;338:954. doi: 10.1016/0140-6736(91)91828-i. [DOI] [PubMed] [Google Scholar]
- 4.Puzyrev V.P., Lemza S.V., Nazarenko L.P., Panphilov V.I. Influence of genetic and demographic factors on etiology and pathogenesis of chronic disease in north Siberian aborigines. Arctic Med. Res. 1992;51:136–142. [PubMed] [Google Scholar]
- 5.Rudan I., Rudan D., Campbell H., Carothers A., Wright A., Smolej-Narancic N., Janicijevic B., Jin L., Chakraborty R., Deka R., Rudan P. Inbreeding and risk of late onset complex disease. J. Med. Genet. 2003;40:925–932. doi: 10.1136/jmg.40.12.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell H., Carothers A.D., Rudan I., Hayward C., Biloglav Z., Barac L., Pericic M., Janicijevic B., Smolej-Narancic N., Polasek O. Effects of genome-wide heterozygosity on a range of biomedically relevant human quantitative traits. Hum. Mol. Genet. 2007;16:233–241. doi: 10.1093/hmg/ddl473. [DOI] [PubMed] [Google Scholar]
- 7.Charlesworth D., Willis J.H. The genetics of inbreeding depression. Nat. Rev. Genet. 2009;10:783–796. doi: 10.1038/nrg2664. [DOI] [PubMed] [Google Scholar]
- 8.McQuillan R., Leutenegger A.L., Abdel-Rahman R., Franklin C.S., Pericic M., Barac-Lauc L., Smolej-Narancic N., Janicijevic B., Polasek O., Tenesa A. Runs of homozygosity in European populations. Am. J. Hum. Genet. 2008;83:359–372. doi: 10.1016/j.ajhg.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szpiech Z.A., Xu J., Pemberton T.J., Peng W., Zöllner S., Rosenberg N.A., Li J.Z. Long runs of homozygosity are enriched for deleterious variation. Am. J. Hum. Genet. 2013;93:90–102. doi: 10.1016/j.ajhg.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broman K.W., Weber J.L. Long homozygous chromosomal segments in reference families from the centre d’Etude du polymorphisme humain. Am. J. Hum. Genet. 1999;65:1493–1500. doi: 10.1086/302661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li L.H., Ho S.F., Chen C.H., Wei C.Y., Wong W.C., Li L.Y., Hung S.I., Chung W.H., Pan W.H., Lee M.T. Long contiguous stretches of homozygosity in the human genome. Hum. Mutat. 2006;27:1115–1121. doi: 10.1002/humu.20399. [DOI] [PubMed] [Google Scholar]
- 12.Gibson J., Morton N.E., Collins A. Extended tracts of homozygosity in outbred human populations. Hum. Mol. Genet. 2006;15:789–795. doi: 10.1093/hmg/ddi493. [DOI] [PubMed] [Google Scholar]
- 13.Lencz T., Lambert C., DeRosse P., Burdick K.E., Morgan T.V., Kane J.M., Kucherlapati R., Malhotra A.K. Runs of homozygosity reveal highly penetrant recessive loci in schizophrenia. Proc. Natl. Acad. Sci. USA. 2007;104:19942–19947. doi: 10.1073/pnas.0710021104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keller M.C., Simonson M.A., Ripke S., Neale B.M., Gejman P.V., Howrigan D.P., Lee S.H., Lencz T., Levinson D.F., Sullivan P.F., Schizophrenia Psychiatric Genome-Wide Association Study Consortium Runs of homozygosity implicate autozygosity as a schizophrenia risk factor. PLoS Genet. 2012;8:e1002656. doi: 10.1371/journal.pgen.1002656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Power R.A., Keller M.C., Ripke S., Abdellaoui A., Wray N.R., Sullivan P.F., Breen G., MDD PGC Working Group A recessive genetic model and runs of homozygosity in major depressive disorder. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2014;165B:157–166. doi: 10.1002/ajmg.b.32217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schunkert H., König I.R., Kathiresan S., Reilly M.P., Assimes T.L., Holm H., Preuss M., Stewart A.F., Barbalic M., Gieger C., Cardiogenics. CARDIoGRAM Consortium Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat. Genet. 2011;43:333–338. doi: 10.1038/ng.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samani N.J., Erdmann J., Hall A.S., Hengstenberg C., Mangino M., Mayer B., Dixon R.J., Meitinger T., Braund P., Wichmann H.E., WTCCC and the Cardiogenics Consortium Genomewide association analysis of coronary artery disease. N. Engl. J. Med. 2007;357:443–453. doi: 10.1056/NEJMoa072366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erdmann J., Grosshennig A., Braund P.S., König I.R., Hengstenberg C., Hall A.S., Linsel-Nitschke P., Kathiresan S., Wright B., Trégouët D.A., Italian Atherosclerosis, Thrombosis, and Vascular Biology Working Group. Myocardial Infarction Genetics Consortium. Wellcome Trust Case Control Consortium. Cardiogenics Consortium New susceptibility locus for coronary artery disease on chromosome 3q22.3. Nat. Genet. 2009;41:280–282. doi: 10.1038/ng.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erdmann J., Willenborg C., Nahrstaedt J., Preuss M., König I.R., Baumert J., Linsel-Nitschke P., Gieger C., Tennstedt S., Belcredi P. Genome-wide association study identifies a new locus for coronary artery disease on chromosome 10p11.23. Eur. Heart J. 2011;32:158–168. doi: 10.1093/eurheartj/ehq405. [DOI] [PubMed] [Google Scholar]
- 20.Yusuf S., Hawken S., Ounpuu S., Dans T., Avezum A., Lanas F., McQueen M., Budaj A., Pais P., Varigos J., Lisheng L., INTERHEART Study Investigators Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 21.McPherson R., Pertsemlidis A., Kavaslar N., Stewart A., Roberts R., Cox D.R., Hinds D.A., Pennacchio L.A., Tybjaerg-Hansen A., Folsom A.R. A common allele on chromosome 9 associated with coronary heart disease. Science. 2007;316:1488–1491. doi: 10.1126/science.1142447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lehrke M., Millington S.C., Lefterova M., Cumaranatunge R.G., Szapary P., Wilensky R., Rader D.J., Lazar M.A., Reilly M.P. CXCL16 is a marker of inflammation, atherosclerosis, and acute coronary syndromes in humans. J. Am. Coll. Cardiol. 2007;49:442–449. doi: 10.1016/j.jacc.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 23.Burton P.R., Clayton D.G., Cardon L.R., Craddock N., Deloukas P., Duncanson A., Kwiatkowski D.P., McCarthy M.I., Ouwehand W.H., Samani N.J., Wellcome Trust Case Control Consortium Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Auton A., Bryc K., Boyko A.R., Lohmueller K.E., Novembre J., Reynolds A., Indap A., Wright M.H., Degenhardt J.D., Gutenkunst R.N. Global distribution of genomic diversity underscores rich complex history of continental human populations. Genome Res. 2009;19:795–803. doi: 10.1101/gr.088898.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Curtis D., Vine A.E., Knight J. Study of regions of extended homozygosity provides a powerful method to explore haplotype structure of human populations. Ann. Hum. Genet. 2008;72:261–278. doi: 10.1111/j.1469-1809.2007.00411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacLeod I.M., Meuwissen T.H., Hayes B.J., Goddard M.E. A novel predictor of multilocus haplotype homozygosity: comparison with existing predictors. Genet. Res. 2009;91:413–426. doi: 10.1017/S0016672309990358. [DOI] [PubMed] [Google Scholar]
- 27.Purcell S., Neale B., Todd-Brown K., Thomas L., Ferreira M.A., Bender D., Maller J., Sklar P., de Bakker P.I., Daly M.J., Sham P.C. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nalls M.A., Simon-Sanchez J., Gibbs J.R., Paisan-Ruiz C., Bras J.T., Tanaka T., Matarin M., Scholz S., Weitz C., Harris T.B. Measures of autozygosity in decline: globalization, urbanization, and its implications for medical genetics. PLoS Genet. 2009;5:e1000415. doi: 10.1371/journal.pgen.1000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nothnagel M., Lu T.T., Kayser M., Krawczak M. Genomic and geographic distribution of SNP-defined runs of homozygosity in Europeans. Hum. Mol. Genet. 2010;19:2927–2935. doi: 10.1093/hmg/ddq198. [DOI] [PubMed] [Google Scholar]
- 30.Abecasis G.R., Ghosh D., Nichols T.E. Linkage disequilibrium: ancient history drives the new genetics. Hum. Hered. 2005;59:118–124. doi: 10.1159/000085226. [DOI] [PubMed] [Google Scholar]
- 31.Wall J.D., Pritchard J.K. Haplotype blocks and linkage disequilibrium in the human genome. Nat. Rev. Genet. 2003;4:587–597. doi: 10.1038/nrg1123. [DOI] [PubMed] [Google Scholar]
- 32.Abecasis G.R., Noguchi E., Heinzmann A., Traherne J.A., Bhattacharyya S., Leaves N.I., Anderson G.G., Zhang Y., Lench N.J., Carey A. Extent and distribution of linkage disequilibrium in three genomic regions. Am. J. Hum. Genet. 2001;68:191–197. doi: 10.1086/316944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reich D.E., Cargill M., Bolk S., Ireland J., Sabeti P.C., Richter D.J., Lavery T., Kouyoumjian R., Farhadian S.F., Ward R., Lander E.S. Linkage disequilibrium in the human genome. Nature. 2001;411:199–204. doi: 10.1038/35075590. [DOI] [PubMed] [Google Scholar]
- 34.McQuillan R., Eklund N., Pirastu N., Kuningas M., McEvoy B.P., Esko T., Corre T., Davies G., Kaakinen M., Lyytikäinen L.P., ROHgen Consortium Evidence of inbreeding depression on human height. PLoS Genet. 2012;8:e1002655. doi: 10.1371/journal.pgen.1002655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heinig M., Petretto E., Wallace C., Bottolo L., Rotival M., Lu H., Li Y., Sarwar R., Langley S.R., Bauerfeind A., Cardiogenics Consortium A trans-acting locus regulates an anti-viral expression network and type 1 diabetes risk. Nature. 2010;467:460–464. doi: 10.1038/nature09386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rotival M., Zeller T., Wild P.S., Maouche S., Szymczak S., Schillert A., Castagné R., Deiseroth A., Proust C., Brocheton J., Cardiogenics Consortium Integrating genome-wide genetic variations and monocyte expression data reveals trans-regulated gene modules in humans. PLoS Genet. 2011;7:e1002367. doi: 10.1371/journal.pgen.1002367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Storey J.D., Tibshirani R. Statistical significance for genomewide studies. Proc. Natl. Acad. Sci. USA. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Browning S.R., Thompson E.A. Detecting rare variant associations by identity-by-descent mapping in case-control studies. Genetics. 2012;190:1521–1531. doi: 10.1534/genetics.111.136937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keller M.C., Visscher P.M., Goddard M.E. Quantification of inbreeding due to distant ancestors and its detection using dense single nucleotide polymorphism data. Genetics. 2011;189:237–249. doi: 10.1534/genetics.111.130922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spain S.L., Cazier J.B., Houlston R., Carvajal-Carmona L., Tomlinson I., CORGI Consortium Colorectal cancer risk is not associated with increased levels of homozygosity in a population from the United Kingdom. Cancer Res. 2009;69:7422–7429. doi: 10.1158/0008-5472.CAN-09-0659. [DOI] [PubMed] [Google Scholar]
- 41.Vine A.E., McQuillin A., Bass N.J., Pereira A., Kandaswamy R., Robinson M., Lawrence J., Anjorin A., Sklar P., Gurling H.M., Curtis D. No evidence for excess runs of homozygosity in bipolar disorder. Psychiatr. Genet. 2009;19:165–170. doi: 10.1097/YPG.0b013e32832a4faa. [DOI] [PubMed] [Google Scholar]
- 42.Enciso-Mora V., Hosking F.J., Houlston R.S. Risk of breast and prostate cancer is not associated with increased homozygosity in outbred populations. Eur. J. Hum. Genet. 2010;18:909–914. doi: 10.1038/ejhg.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hosking F.J., Papaemmanuil E., Sheridan E., Kinsey S.E., Lightfoot T., Roman E., Irving J.A., Allan J.M., Taylor M., Tomlinson I.P. Genome-wide homozygosity signatures and childhood acute lymphoblastic leukemia risk. Blood. 2010;115:4472–4477. doi: 10.1182/blood-2009-09-244483. [DOI] [PubMed] [Google Scholar]
- 44.Ghani M., Sato C., Lee J.H., Reitz C., Moreno D., Mayeux R., St George-Hyslop P., Rogaeva E. Evidence of recessive Alzheimer disease loci in a Caribbean Hispanic data set: genome-wide survey of runs of homozygosity. JAMA Neurol. 2013;70:1261–1267. doi: 10.1001/jamaneurol.2013.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mittelstadt M., Frump A., Khuu T., Fowlkes V., Handy I., Patel C.V., Patel R.C. Interaction of human tRNA-dihydrouridine synthase-2 with interferon-induced protein kinase PKR. Nucleic Acids Res. 2008;36:998–1008. doi: 10.1093/nar/gkm1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xie X., Wang Z., Chen Y. Association of LKB1 with a WD-repeat protein WDR6 is implicated in cell growth arrest and p27(Kip1) induction. Mol. Cell. Biochem. 2007;301:115–122. doi: 10.1007/s11010-006-9402-5. [DOI] [PubMed] [Google Scholar]
- 47.Howrigan D.P., Simonson M.A., Keller M.C. Detecting autozygosity through runs of homozygosity: a comparison of three autozygosity detection algorithms. BMC Genomics. 2011;12:460. doi: 10.1186/1471-2164-12-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ku C.S., Naidoo N., Teo S.M., Pawitan Y. Regions of homozygosity and their impact on complex diseases and traits. Hum. Genet. 2011;129:1–15. doi: 10.1007/s00439-010-0920-6. [DOI] [PubMed] [Google Scholar]
- 49.Di Gaetano C., Fiorito G., Ortu M.F., Rosa F., Guarrera S., Pardini B., Cusi D., Frau F., Barlassina C., Troffa C. Sardinians genetic background explained by runs of homozygosity and genomic regions under positive selection. PLoS ONE. 2014;9:e91237. doi: 10.1371/journal.pone.0091237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heron E.A., Cormican P., Donohoe G., O’Neill F.A., Kendler K.S., Riley B.P., Gill M., Corvin A.P., Morris D.W., Wellcome Trust Case Control Consortium 2 No evidence that runs of homozygosity are associated with schizophrenia in an Irish genome-wide association dataset. Schizophr. Res. 2014;154:79–82. doi: 10.1016/j.schres.2014.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin P.I., Kuo P.H., Chen C.H., Wu J.Y., Gau S.S., Wu Y.Y., Liu S.K. Runs of homozygosity associated with speech delay in autism in a taiwanese han population: evidence for the recessive model. PLoS ONE. 2013;8:e72056. doi: 10.1371/journal.pone.0072056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McWhirter R.E., Thomson R.J., Marthick J.R., Rumbold A.R., Brown M.A., Taylor-Thomson D., Maypilama E.L., Condon J.R., Dickinson J.L. Runs of homozygosity and a cluster of vulvar cancer in young Australian Aboriginal women. Gynecol. Oncol. 2014;133:421–426. doi: 10.1016/j.ygyno.2014.03.566. [DOI] [PubMed] [Google Scholar]
- 53.Wang C., Xu Z., Jin G., Hu Z., Dai J., Ma H., Jiang Y., Hu L., Chu M., Cao S., Shen H. Genome-wide analysis of runs of homozygosity identifies new susceptibility regions of lung cancer in Han Chinese. J. Biomed. Res. 2013;27:208–214. doi: 10.7555/JBR.27.20130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Laurie C.C., Laurie C.A., Rice K., Doheny K.F., Zelnick L.R., McHugh C.P., Ling H., Hetrick K.N., Pugh E.W., Amos C. Detectable clonal mosaicism from birth to old age and its relationship to cancer. Nat. Genet. 2012;44:642–650. doi: 10.1038/ng.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jacobs K.B., Yeager M., Zhou W., Wacholder S., Wang Z., Rodriguez-Santiago B., Hutchinson A., Deng X., Liu C., Horner M.J. Detectable clonal mosaicism and its relationship to aging and cancer. Nat. Genet. 2012;44:651–658. doi: 10.1038/ng.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Machiela M.J., Chanock S.J. Detectable clonal mosaicism in the human genome. Semin. Hematol. 2013;50:348–359. doi: 10.1053/j.seminhematol.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.