Abstract
Objective
To evaluate how personalized quantitative colorectal cancer (CRC) risk information affects laypersons’ interest in CRC screening, and to explore factors influencing these effects.
Methods
An online pre-post experiment was conducted in which a convenience sample (N=578) of laypersons, aged >50, were provided quantitative personalized estimates of lifetime CRC risk, calculated by the National Cancer Institute Colorectal Cancer Risk Assessment Tool (CCRAT). Self-reported interest in CRC screening was measured immediately before and after CCRAT use; sociodemographic characteristics and prior CRC screening history were also assessed. Multivariable analyses assessed participants’ change in interest in screening, and subgroup differences in this change.
Results
Personalized CRC risk information had no overall effect on CRC screening interest, but significant subgroup differences were observed. Change in screening interest was greater among individuals with recent screening (p=.015), higher model-estimated cancer risk (p=.0002), and lower baseline interest (p<.0001), with individuals at highest baseline interest demonstrating negative (not neutral) change in interest.
Conclusion
Effects of quantitative personalized CRC risk information on laypersons’ interest in CRC screening differ among individuals depending on prior screening history, estimated cancer risk, and baseline screening interest.
Practice implications
Personalized cancer risk information has personalized effects—increasing and decreasing screening interest in different individuals.
Keywords: personalized risk information, colorectal cancer, screening
1. Introduction
Personalized risk information—information about the probability of future health outcomes for individual patients1—has become an increasingly important form of clinical evidence. The supply of such information has greatly expanded in recent years due to growing efforts to develop clinical prediction models (CPMs)—multivariate statistical algorithms that utilize characteristics of patients, diseases, and treatments to estimate individualized probabilities of health outcomes.2 Meanwhile, the demand for personalized risk information has correspondingly expanded, fueled by growing efforts to promote informed and shared medical decision making based on the expected outcomes, values, and preferences of individual patients.3 Emerging evidence, furthermore, supports the value of personalized risk information in achieving this goal. A 2013 Cochrane review concluded that personalized disease risk information improves informed decision making about disease screening.4
An important unanswered question, however, is how personalized disease risk information affects judgments and decisions about medical interventions. The 2013 Cochrane review found weak evidence of a small effect of personalized risk information in increasing screening uptake, but significant heterogeneity and methodological limitations among the 41 randomized controlled trials (RCTs) reviewed. Most of these trials were limited to examining the effects of either personalized risk factor lists or qualitative (categorical)—as opposed to quantitative (numeric)—risk information.4 Only 6 trials evaluated the effects of personalized quantitative risk information produced by CPMs. These 6 trials, furthermore, all focused on breast cancer risk estimates calculated by the Gail Model5 and found inconsistent effects on patients’ cancer screening intentions and uptake.4
Existing evidence on the effects of personalized risk information is further limited by other methodological problems. Past studies have generally provided such information to patients as one component of multidimensional decision support interventions—e.g., counseling protocols and decision aids—that supply more than personalized risk information alone.4 The independent effects of risk information on medical judgments and decisions are thus unclear. With few exceptions,6,7 furthermore, past studies have focused on main (average) effects of personalized risk information for given populations. Such information, however, may have variable effects for individuals due to various effect-modifying factors including individuals’ prior screening history,8 interest in screening,9,10 and the magnitude of their estimated cancer risk.11,12 Finally, most past studies have focused on patients receiving care in clinical settings, rather than the general public that is increasingly exposed to personalized cancer risk information through the growing number of CPMs that are easily accessible online. These include not only the Gail model but the Colorectal Cancer Risk Assessment Tool (CCRAT) developed by Freedman et al13 and made publicly available by the U.S. National Cancer Institute (NCI) at http://www.cancer.gov/colorectalcancerrisk/. Consequently, we know little about the independent effects of personalized quantitative cancer risk information on laypersons, and the factors that influence these effects.
In the current study we attempted to address these knowledge gaps. We conducted an experiment designed to evaluate the effects of personalized, quantitative CRC risk information, produced by the NCI CCRAT, on laypersons’ interest in CRC screening, and to identify factors that influence these effects. Screening interest is a strong predictor of actual screening behavior9,10,14–17 and thus a useful and important proxy outcome for exploring the effects of personalized risk information. The objective of our study was not only to assess the average effects of personalized cancer risk information on CRC screening interest, but to explore how these effects differ among individuals.
2. Materials and Methods
2.1. Data source and study population
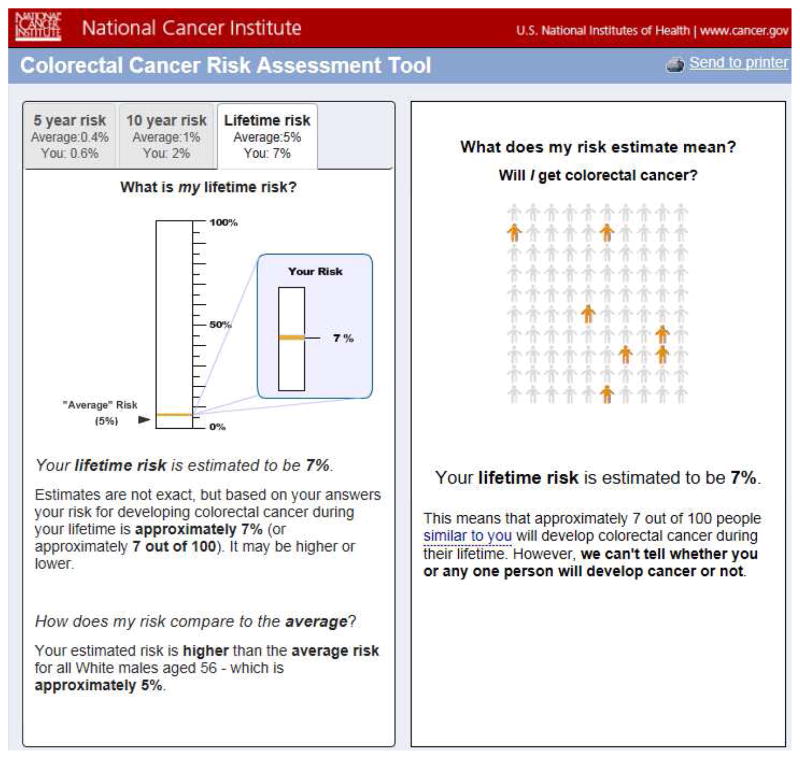
The study population consisted of a convenience sample of the Internet-using general public accessing a fully functional replica of the NCI CCRAT, programmed by User-Centered Design, Inc. (Ashburn, VA), and linked to an educational website, “Are You at Risk for Colon Cancer?” developed for the study. The website provided no other cancer screening decision support, and was freely accessible to the general public and hosted by MaineHealth, a statewide healthcare system, from April 2013–October 2014. Web traffic data from Google Analytics showed that during this time the website was visited by persons in all 50 US states, with the most visits from California, Texas, New York, Florida, and Illinois (accounting for 35% of all page views), and the least from Rhode Island, South Dakota, Delaware, North Dakota, and Vermont (0.1% of all page views). The replica site utilized statistical code provided by the NCI and presented both the individual’s estimated CRC risk as well as the age- and sex-matched population average CRC risk. The replica site resembled the current CCRAT in all respects except for the landing page which was altered to direct users to the pre-tool measures (described below), and the output page (Figure 1) which was simplified by providing point estimates of risk rather than 95% confidence intervals, and integrating the CCRAT’s “randomness” visualization18 on a single page along with the rest of the CCRAT output.
Figure 1.
Screenshots of sample risk estimate output from Colorectal Cancer Risk Assessment Tool (CCRAT).
2.2. Data collection and measures
The experiment utilized a pre-post, within-subjects design in which participants responded to survey measures immediately before and after exposure to the CCRAT and served as their own controls. Study recruitment was passive; participants independently accessed the CCRAT site, voluntarily entered the risk factor information needed to use the tool, reviewed their personalized risk estimates, and completed the study’s measures as they wished, without incentives. Notably, the CCRAT is not validated in adults less than 50 years old, and the CCRAT user interface does not allow respondents to enter any age < 50. We therefore excluded from analysis respondents who reported age 50, reasoning that a substantial proportion of them were likely younger and thus ineligible to use the CCRAT. No other personal identifying information was collected, and the study was granted exemption from review and a waiver of informed consent by the Maine Medical Center Institutional Review Board. The survey instrument and procedure are in the Appendix.
Interest in colorectal cancer screening, the primary outcome variable measured both before and after CCRAT use, was measured by a single item, “How interested are you in getting tested, or “screened,” for colon cancer?” A 5-point numeric Likert response scale was used, with the endpoints labeled “Not at all” and “Extremely.”
Recent history of colorectal cancer screening was measured by a single item, “In the last 10 years, did you have a colonoscopy, sigmoidoscopy, or both?” Response options were “Yes,” “No,” and “Don’t know”; the latter option was grouped with the “No” responses for analysis purposes. This variable was hypothesized to be an important determinant of individuals’ interest in CRC screening, given that prior completion of screening likely reflects positive attitudes towards this service.8
Estimated colorectal cancer risk was calculated by the CCRAT as a positive continuous point estimate expressing the model-projected lifetime risk of CRC. This variable is another potentially important determinant of interest in future CRC screening; higher risk would be expected to increase interest in screening.
Sociodemographic characteristics included age, sex, and race (White, Black, Other), all of which have been shown to be associated with CRC screening behavior; lower rates of screening have been observed in younger adults, males, and non-White racial groups.19–22
2.3. Data analyses
Descriptive statistics were obtained as numbers and percentages for categorical variables and means and standard deviations for continuous variables. Univariate analyses were performed using chi-squared tests for categorical variables and Student’s t-tests for continuous variables.
To facilitate interpretation of the moderating effects of various factors on CCRAT use, general linear models (multivariable analysis of covariance (ANCOVA) and analysis of variance (ANOVA) models) were used to assess the change in interest (Δ) in CRC screening following CCRAT use. The first model adjusted for sociodemographic factors (age, sex, race) only, and a second model adjusted for both sociodemographic factors and recent history of CRC screening, estimated CRC risk, and baseline interest in CRC screening. All analyses were conducted using PROC GLM in SAS/STAT (Version 9.2).
To assess potential bias resulting from our exclusion of respondents age 50, we conducted sensitivity analyses including these respondents. We also conducted analyses to assess differences between participants who completed both the pre- and post-CCRAT measures vs. those who completed only the pre-CCRAT measure.
3. Results
3.1. Descriptive statistics
A total of 1279 individuals accessed the CCRAT site and completed the pre-CCRAT measure; of these individuals 578 (45.2%) also completed the post-CCRAT measure and were included in the analysis (Table 1). These individuals were older, 61.7 (s.d. 7.9) vs. 60.2 (s.d. 7.0) years (p=.0004), but did not otherwise differ from participants who did not complete both measures in terms of sociodemographic characteristics, model-estimated CRC risk, or past CRC screening history or interest.
Table 1.
Study population characteristics (N=578)
| n | % | |
|---|---|---|
| Age | ||
| 51 – 54 | 124 | 21.5 |
| 55 – 59 | 142 | 24.6 |
| 60 – 64 | 131 | 22.7 |
| 65+ | 181 | 31.3 |
|
| ||
| Sex | ||
| Male | 225 | 38.9 |
| Female | 353 | 61.1 |
|
| ||
| Race | ||
| White | 503 | 87.0 |
| Black | 37 | 6.4 |
| Other | 38 | 6.6 |
|
| ||
| Colonoscopy or sigmoidoscopy in past 10 years | ||
| No or Unknown | 215 | 37.2 |
| Yes | 363 | 62.8 |
|
| ||
| Model-estimated lifetime colorectal cancer risk | ||
| < 5% | 185 | 32.0 |
| 5% – 7% | 221 | 38.2 |
| > 7% | 172 | 29.8 |
|
| ||
| Baseline interest in colorectal cancer screeninga | ||
| 1 (Lowest) | 56 | 9.7 |
| 2 | 81 | 14.0 |
| 3 | 120 | 20.8 |
| 4 | 113 | 19.6 |
| 5 (Highest) | 208 | 36.0 |
Lowest Interest (Level 1) – “Not at all” response to baseline screening interest question (“How interested are you in getting tested, or “screened,” for colon cancer?”); Highest interest (Level 5) – “Extremely” response to baseline screening interest question
The model-estimated point estimates of lifetime CRC risk that were calculated and communicated to participants ranged from 0.8% to 22%. The distribution of risks was highly skewed towards lower risk but included a small number of higher values. For this reason, and because publicly accessible non-quantitative cancer risk CPMs such as Your Disease Risk (www.yourdiseaserisk.wustl.edu)23 categorize risk in terms of “lower than average,” “average,” and “higher than average” groups, we created a categorical model-estimated risk variable, the levels of which (<5%, 5–7%, >7%) not only corresponded to this conceptual division (population average CRC risks range between 5 and 7%) but reflected the empirical distribution of estimated CRC risks in our sample (Table 1).
3.2. Main effects of CCRAT use on interest in CRC screening
ANCOVA adjusting for sociodemographic factors only (age, race, sex) showed no significant difference in change in interest in CRC screening following CCRAT use (Δ interest = +0.08 points, 95% CI −0.07–0.23, p=.31), and no significant effects of age, race, or sex. This finding was corroborated by repeated measures ANOVA which showed no main effect of time (CCRAT use) on screening interest (Wilks’ λ = .998, F (1, 571) = 1.05, p=.31), and no interactions between time and any sociodemographic factor.
3.3. Subgroup effects of CCRAT use on interest in CRC screening
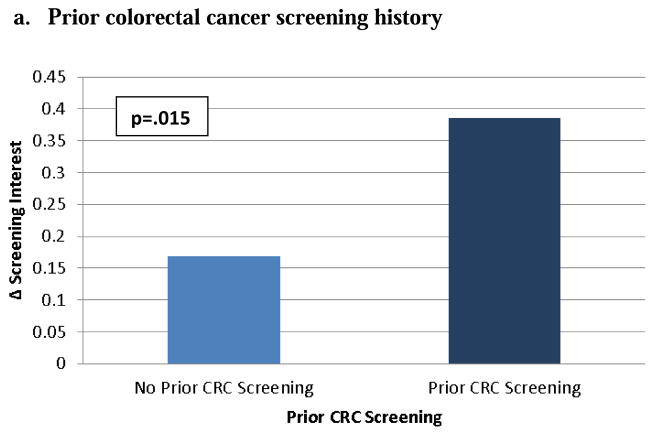
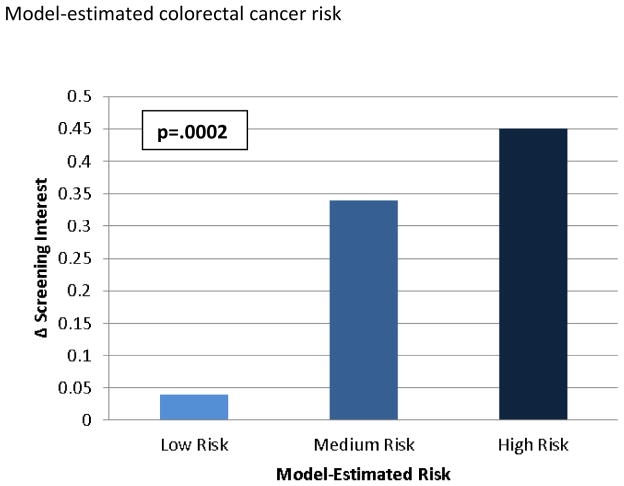
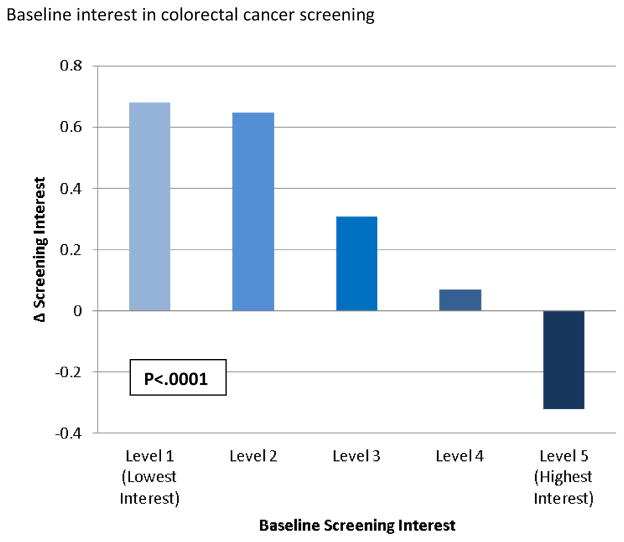
ANCOVA with change in interest in CRC screening as the dependent variable, adjusting for sociodemographic factors as well as prior colonoscopy, estimated CRC risk, and prior interest in CRC screening, showed a small positive effect of CCRAT use on screening interest (Δ interest = +0.28 points, 95% CI 0.13–0.43, p=.0003). There were significant effects of recent CRC screening (F (1, 564) = 6.0, p= .015), estimated colorectal cancer risk (F (2, 564) = 8.55, p = .0002), and prior interest in screening (F (4, 564) = 22.56, p < .0001) on change in interest in CRC screening. In other words, each factor moderated the effect of CCRAT use on screening interest, and the magnitude of their effects is shown in Figures 2a – 2c. Age, sex, and race had no significant effects.
Figure 2. Change in colorectal cancer screening interest in different subgroups of individuals following use of the Colorectal Cancer Risk Assessment Tool (CCRAT).
Model-adjusted means and p-value for effect of prior colorectal cancer (CRC) screening on change in screening interest in multivariable ANCOVA model adjusting for age, sex, race, prior CRC screening, model-estimated CRC risk, and baseline interest in CRC screening
Prior CRC Screening – colonoscopy or flexible sigmoidoscopy in past 10 years
Model-adjusted means and p-value for effect of model-estimated lifetime risk of colorectal cancer (CRC) on change in screening interest in multivariable ANCOVA model adjusting for age, sex, race, prior CRC screening, model-estimated CRC risk, and baseline interest in CRC screening
Low Risk – < 5% lifetime risk of CRC; Medium Risk – 5–7% lifetime risk of CRC; High Risk – >7% lifetime risk of CRC
Model-adjusted means and p-value for effect of baseline colorectal cancer (CRC) screening interest on change in screening interest in multivariable ANCOVA model adjusting for age, sex, race, prior CRC screening, model-estimated CRC risk, and baseline interest in CRC screening
Lowest Interest (Level 1) – “Not at all” response to baseline CRC screening interest question (“How interested are you in getting tested, or “screened,” for colon cancer?”); Highest interest (Level 5) – “Extremely” response to baseline screening interest question
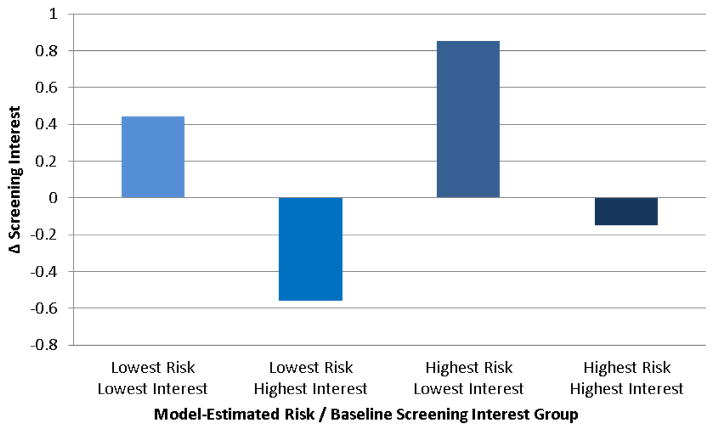
To illustrate the magnitude of subgroup differences in responses to use of the CCRAT, Figure 3 shows the combined effects of the two strongest moderators of the effect of CCRAT use on screening interest: estimated CRC risk and prior screening interest. The greatest between-group difference in change in screening interest was between participants in the lowest-risk/highest-interest and highest-risk/lowest-interest categories (p<.0001 for the individual contrast), amounting to a change of 1.41 scale points (95% CI: 1.05–1.77).
Figure 3. Combined effects of model-estimated colorectal cancer risk and baseline colorectal cancer screening interest on change in screening interest following Colorectal Cancer Risk Assessment Tool (CCRAT) use.
Model-adjusted means for combined effects of model-estimated lifetime colorectal cancer (CRC) risk and baseline screening interest on change in screening interest in multivariable ANCOVA model adjusting for age, sex, race, prior CRC screening, model-estimated CRC risk, and baseline interest in CRC screening
Lowest Risk – < 5% lifetime risk of CRC; Highest Risk – >7% lifetime risk of CRC
Lowest Interest – “Not at all” response to baseline CRC screening interest question (“How interested are you in getting tested, or “screened,” for colon cancer?”); Highest interest – “Extremely” response to baseline screening interest question
3.4. Sensitivity analyses
Including individuals aged 50 produced the same results, with the exception that the effect of race on screening interest became significant (F (2, 739) = 4.65, p= .0098). The 50-year-old category had a significantly larger proportion of non-White individuals (21.3% non-white in the 50 year-old group versus 13.0% in the > 50 year-old groups, p=0.019). Including individuals aged 50 produced no changes in the direction or magnitude of observed associations.
4. Discussion and Conclusion
4.1. Discussion
This study evaluated the effects of personalized colorectal cancer risk information, produced by a validated, freely available clinical prediction model and unaccompanied by additional decision support, on the lay public’s interest in colorectal cancer screening. To our knowledge, it is the first study to experimentally assess the effects of quantitative estimates of lay individuals’ CRC risk on screening interest, and to assess the extent to which these effects are moderated by particular individual factors. These are important issues given the proliferation of CPMs and their growing application in cancer screening and other clinical decisions. Our findings have several implications for these efforts.
First, our study demonstrated no main (average) effect of personalized CRC risk information on interest in screening at the study population level. This finding is consistent with most although not all24 recent studies examining the effects of personalized qualitative CRC risk information on both colorectal cancer screening intentions and actual uptake. Both Schroy et al25 and Wilkins et al26 conducted RCTs utilizing the publicly accessible qualitative “Your Disease Risk” CPM for CRC risk.23 Although these studies evaluated the effects of non-quantitative personalized risk information, their findings support the validity of our results. Our study also demonstrated a small positive effect of CCRAT use on screening interest when potential effect-modifying factors were statistically controlled, suggesting that the distribution of these factors influences the effects of personalized CRC risk information.
Most importantly, our study revealed individual differences in the effects of personalized risk information. CCRAT use caused changes in CRC screening interest among particular population subgroups, and the direction and magnitude of change varied according to several factors. Individuals with a recent history of CRC screening showed a greater increase in screening interest compared to individuals with no recent history. The underlying mechanisms remain to be determined, but motivation to reduce cognitive dissonance may be one possibility. Change in screening interest was also positively associated, in dose-response fashion, with the magnitude of individuals’ estimated CRC risk. This is arguably an expected and rational response to personalized risk information. Finally, change in screening interest was strongly and inversely associated with individuals’ baseline screening interest, prior to CCRAT use; it was the most positive for individuals with the lowest baseline interest, and actually negative for individuals with the highest baseline interest. At least part of this effect reflects mathematical dependency of the observable change in screening interest on individuals’ baseline interest level; individuals with the highest baseline interest simply cannot exhibit positive change as ascertained by our study’s measure. This phenomenon does not completely explain our findings, however, given that the observed change in screening interest was not null (as would be expected if personalized risk information had no additional effect for this subgroup). Instead, the change in interest was negative, suggesting a non-neutral effect of such information.
The overarching implication of these findings is the need for a personalized approach to evaluating the effects of personalized risk information. Our study shows how an exclusive focus on main effects of personalized risk information, averaged across populations, may miss important differences in the responses of individuals. Such individual differences, furthermore, are precisely what personalized risk information is intended to facilitate; one would expect individuals’ interest in CRC screening to vary according to the magnitude of their model-estimated cancer risk. But if such differences are not attended to—i.e., if one focuses only on main effects and not interactions—then one might erroneously conclude that personalized risk information had no effect, or that CPMs themselves were otherwise ineffective.
Of course, change in interest alone is insufficient to cause change in actual behavior; numerous other factors influence health behaviors in general and cancer screening in particular.15,27,28 Nevertheless, our study raises the need for greater attention to heterogeneity of treatment effect29 due to individual differences in responses to personalized risk information. More research is needed to confirm our findings and to quantify the effects of other factors—unmeasured in our study—that might moderate the effects of personalized risk information.11 Low education, for example, has been associated with reduced mammography use following personalized breast cancer risk counseling.6 Further research is increasingly important given growing efforts to utilize genetic and genomic information to personalize health promotion and disease prevention.30 Although available evidence suggests that genomic information has no overall (population-level) effects on health-related behaviors,31,32 its effects on subgroups of individuals, such as those identified in our study, remain to be fully explored. Future studies evaluating the effects of genomic and non-genomic personalized risk information should be designed and powered to examine these effects.
A larger question, beyond the scope of the current study, is whether CPMs ought to be used in disease screening decision making in the first place. An individualized approach to screening based on personalized risk estimates, for example, has been advocated not only for CRC cancer screening33–36 but for low-dose computed tomography (LDCT) screening for lung cancer.37,38 Personalized cancer risk information may enable informed patient choice and targeted screening—i.e., focused on patients at highest risk.36 It may also help remediate unrealistic expectations that the general public has been shown to harbor about disease risks and the benefits of cancer screening.39,40 The tradeoff suggested by our study, however, is that some individuals will be dissuaded from screening. The acceptability of this outcome ultimately depends on the level of evidence supporting particular screening tests, and on whether the appropriate corresponding goal is to maximize screening uptake or to enable patients to decide whether screening is worthwhile to them.41 In any case, our study suggests that diminished screening interest among certain individuals is a potential consequence of disseminating personalized risk information to the general public. It also raises questions about the appropriate level of informational precision for screening decisions,42 and amount of additional decision support that should accompany personalized risk information.
Our study had several limitations that qualify its conclusions and raise the need for further research. The study examined only self-reported screening interest; more research is needed to determine how CPM use affects actual uptake of CRC screening, and how specific changes in interest relate to changes in screening uptake. The study did not assess past history of non-endoscopic CRC screening tests (e.g., FOBT or FIT), nor did it evaluate other factors known to affect screening uptake (e.g., perceived efficacy). The study population had limited sociodemographic diversity and consisted of a convenience sample of laypersons outside of clinical care settings, whose unsolicited participation—not only in using the CPM but in completing pre-post survey questions—likely manifested a high level of interest in CRC screening and/or motivation to improve their health. The generalizability of our findings to other populations, including those outside the US, is unknown.
These limitations, however, do not undermine our study’s principal finding of individual differences in laypersons’ responses to personalized risk information. We believe our findings also have ecological validity in representing the responses of members of the general public who independently seek cancer risk information online. Such individuals constitute a growing, increasingly important population as CPMs continue to be developed and made publicly accessible on the Internet. Nevertheless, our findings have unknown generalizability to less motivated individuals and patients in clinical care settings, for whom exposure to the prospect of CRC screening may be initiated or mediated by health professionals. Additional research is needed both to target such individuals and to examine how their responses are affected by the way in which personalized risk information is communicated.
4.2. Conclusion
The current study provides seminal evidence on the effects of quantitative personalized cancer risk information on interest in cancer screening among the lay public. Such information both increases and decreases interest in CRC screening in different individuals, depending on their prior screening history, interest in screening, and estimated cancer risk.
4.3. Practice implications
Personalized cancer risk information has personalized effects—increasing or decreasing individuals’ interest in screening depending on their personal characteristics. Individual differences in the effects of personalized risk information need to be considered when applying CPMs to cancer screening and other clinical decisions.
Supplementary Material
Highlights.
We evaluated how personalized quantitative colorectal cancer (CRC) risk information affects laypersons’ interest in CRC screening.
Personalized CRC risk information had no overall effect on laypersons’ interest in CRC screening, but significant subgroup differences were found.
Change in interest in CRC screening was greater among individuals with recent screening, higher model-estimated cancer risk, and lower baseline interest.
Interest in CRC screening decreased among individuals with the highest baseline screening interest.
Personalized cancer risk information has personalized effects, increasing or decreasing individuals’ interest in CRC screening depending on their personal characteristics.
Acknowledgments
The study was supported by Contract HSN261201000608P from the National Cancer Institute. The funder provided the statistical algorithm for the Colorectal Cancer Risk Assessment Tool, and input in interpretation of data and preparation and review of the manuscript. Portions of the study were presented at the International Conference on Communication in Healthcare (ICCH) 2014 Annual Meeting, Amsterdam, Netherlands, September 30, 2014. We thank Barbara Barry, Jessica Begley, and Janell Lewis for invaluable assistance in website programming and data collection. Drs. Han and Duarte had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Edwards A, Hood K, Matthews E, et al. The effectiveness of one-to-one risk communication interventions in health care: a systematic review. Med Decis Making. 2000 Jul-Sep;20(3):290–297. doi: 10.1177/0272989X0002000305. [DOI] [PubMed] [Google Scholar]
- 2.Steyerberg EW. Clinical Prediction Models: a Practical Approach to Development, Validation, and Updating. New York: Springer; 2010. [Google Scholar]
- 3.Freedman AN, Seminara D, Gail MH, et al. Cancer risk prediction models: a workshop on development, evaluation, and application. J Natl Cancer Inst. 97(10):715–723. doi: 10.1093/jnci/dji128. [DOI] [PubMed] [Google Scholar]
- 4.Edwards AG, Naik G, Ahmed H, et al. Personalised risk communication for informed decision making about taking screening tests. Cochrane Database Syst Rev. 2013;2:CD001865. doi: 10.1002/14651858.CD001865.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81(24):1879–1886. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz MD, Rimer BK, Daly M, Sands C, Lerman C. A randomized trial of breast cancer risk counseling: the impact on self-reported mammography use. Am J Public Health. 1999;89(6):924–926. doi: 10.2105/ajph.89.6.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lerman C, Lustbader E, Rimer B, et al. Effects of Individualized Breast Cancer Risk Counseling: A Randomized Trial. Journal of the National Cancer Institute. 1995;87(4):286–292. doi: 10.1093/jnci/87.4.286. [DOI] [PubMed] [Google Scholar]
- 8.Vernon SW. Participation in colorectal cancer screening: a review. J Natl Cancer Inst. 1997;89(19):1406–1422. doi: 10.1093/jnci/89.19.1406. [DOI] [PubMed] [Google Scholar]
- 9.Myers RE, Ross E, Jepson C, et al. Modeling adherence to colorectal cancer screening. Prev Med. 1994;23(2):142–151. doi: 10.1006/pmed.1994.1020. [DOI] [PubMed] [Google Scholar]
- 10.McCaffery K, Wardle J, Waller J. Knowledge, attitudes, and behavioral intentions in relation to the early detection of colorectal cancer in the United Kingdom. Prev Med. 2003;36(5):525–535. doi: 10.1016/s0091-7435(03)00016-1. [DOI] [PubMed] [Google Scholar]
- 11.Gerrard M, Gibbons FX, Reis-Bergan M. The effect of risk communication on risk perceptions: the significance of individual differences. J Natl Cancer Inst Monogr. 1999;(25):94–100. doi: 10.1093/oxfordjournals.jncimonographs.a024217. [DOI] [PubMed] [Google Scholar]
- 12.Aspinwall LG. Introduction of section: persuasion for the purpose of cancer risk reduction: understanding responses to risk communications. J Natl Cancer Inst Monogr. 1999;(25):88–93. doi: 10.1093/oxfordjournals.jncimonographs.a024216. [DOI] [PubMed] [Google Scholar]
- 13.Freedman AN, Slattery ML, Ballard-Barbash R, et al. Colorectal cancer risk prediction tool for white men and women without known susceptibility. J Clin Oncol. 2009 Feb 10;27(5):686–693. doi: 10.1200/JCO.2008.17.4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sutton SM, Eisner EJ, Johnston CM. The mammography guideline controversy: where does the consumer fit in? J Am Med Womens Assoc. 1994 Mar-Apr;49(2):53–59. [PubMed] [Google Scholar]
- 15.Connor M, Norman P, editors. Predicting Health Behavior: Research and Practice With Social Cognition Models. Buckingham: Open University Press; 2001. [Google Scholar]
- 16.Myers RE, Trock BJ, Lerman C, Wolf T, Ross E, Engstrom PF. Adherence to colorectal cancer screening in an HMO population. Prev Med. 1990;19(5):502–514. doi: 10.1016/0091-7435(90)90049-p. [DOI] [PubMed] [Google Scholar]
- 17.Watts BG, Vernon SW, Myers RE, Tilley BC. Intention to be screened over time for colorectal cancer in male automotive workers. Cancer Epidemiol Biomarkers Prev. 2003 Apr;12(4):339–349. [PubMed] [Google Scholar]
- 18.Han PK, Klein WM, Killam B, Lehman T, Massett H, Freedman AN. Representing randomness in the communication of individualized cancer risk estimates: effects on cancer risk perceptions, worry, and subjective uncertainty about risk. Patient Educ Couns. 2012;86(1):106–113. doi: 10.1016/j.pec.2011.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klabunde CN, Cronin KA, Breen N, Waldron WR, Ambs AH, Nadel MR. Trends in colorectal cancer test use among vulnerable populations in the United States. Cancer Epidemiol Biomarkers Prev. 2011;20(8):1611–1621. doi: 10.1158/1055-9965.EPI-11-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Semrad TJ, Tancredi DJ, Baldwin LM, Green P, Fenton JJ. Geographic variation of racial/ethnic disparities in colorectal cancer testing among medicare enrollees. Cancer. 2011;117(8):1755–1763. doi: 10.1002/cncr.25668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi L, Lebrun LA, Zhu J, Tsai J. Cancer screening among racial/ethnic and insurance groups in the United States: a comparison of disparities in 2000 and 2008. J Health Ccare for Poor Underserved. 2011 Aug;22(3):945–961. doi: 10.1353/hpu.2011.0079. [DOI] [PubMed] [Google Scholar]
- 22.Wallace PM, Suzuki R. Regional, racial, and gender differences in colorectal cancer screening in middle-aged African-Americans and Whites. J Cancer Educ. 2012;27(4):703–708. doi: 10.1007/s13187-012-0396-2. [DOI] [PubMed] [Google Scholar]
- 23.Colditz GA, Atwood KA, Emmons K, et al. Harvard report on cancer prevention volume 4: Harvard Cancer Risk Index. Risk Index Working Group, Harvard Center for Cancer Prevention. Cancer Causes Control. 2000;11(6):477–488. doi: 10.1023/a:1008984432272. [DOI] [PubMed] [Google Scholar]
- 24.Sequist TD, Zaslavsky AM, Colditz GA, Ayanian JZ. Electronic patient messages to promote colorectal cancer screening: a randomized controlled trial. Arch Intern Med. 2011;171(7):636–641. doi: 10.1001/archinternmed.2010.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schroy PC, 3rd, Emmons KM, Peters E, et al. Aid-assisted decision making and colorectal cancer screening: a randomized controlled trial. Am J Prev Med. 2012;43(6):573–583. doi: 10.1016/j.amepre.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilkins T, Gillies RA, Panchal P, Patel M, Warren P, Schade RR. Colorectal cancer risk information presented by a nonphysician assistant does not increase screening rates. Can Fam Physician. 2014;60(8):731–738. [PMC free article] [PubMed] [Google Scholar]
- 27.McCaul KD, Tulloch HE. Cancer screening decisions. J Natl Cancer Inst Monogr. 1999;(25):52–58. doi: 10.1093/oxfordjournals.jncimonographs.a024209. [DOI] [PubMed] [Google Scholar]
- 28.Power E, Van Jaarsveld CH, McCaffery K, Miles A, Atkin W, Wardle J. Understanding intentions and action in colorectal cancer screening. Ann Behav Med. 2008;35(3):285–294. doi: 10.1007/s12160-008-9034-y. [DOI] [PubMed] [Google Scholar]
- 29.Kent DM, Alsheikh-Ali A, Hayward RA. Competing risk and heterogeneity of treatment effect in clinical trials. Trials. 2008;9:30. doi: 10.1186/1745-6215-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McBride CM, Bryan AD, Bray MS, Swan GE, Green ED. Health behavior change: can genomics improve behavioral adherence? Am J Public Health. 2012;102(3):401–405. doi: 10.2105/AJPH.2011.300513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weinberg DS, Myers RE, Keenan E, et al. Genetic and environmental risk assessment and colorectal cancer screening in an average-risk population: a randomized trial. Ann Intern Med. 2014;161(8):537–545. doi: 10.7326/M14-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bloss CS, Schork NJ, Topol EJ. Effect of direct-to-consumer genomewide profiling to assess disease risk. N Engl J Med. 2011;364(6):524–534. doi: 10.1056/NEJMoa1011893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Imperiale TF, Glowinski EA, Lin-Cooper C, Ransohoff DF. Tailoring colorectal cancer screening by considering risk of advanced proximal neoplasia. Am J Med. 2012;125(12):1181–1187. doi: 10.1016/j.amjmed.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Imperiale TF, Wagner DR, Lin CY, Larkin GN, Rogge JD, Ransohoff DF. Using risk for advanced proximal colonic neoplasia to tailor endoscopic screening for colorectal cancer. Ann Intern Med. 2003;139(12):959–965. doi: 10.7326/0003-4819-139-12-200312160-00005. [DOI] [PubMed] [Google Scholar]
- 35.Dominitz JA, Robertson DJ. Tailoring colonoscopic screening to individual risk. Gastroenterology. 2014;147(2):264–266. doi: 10.1053/j.gastro.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 36.Saini SD, van Hees F, Vijan S. Smarter screening for cancer: possibilities and challenges of personalization. JAMA. 2014;312(21):2211–2212. doi: 10.1001/jama.2014.13933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kovalchik SA, Tammemagi M, Berg CD, et al. Targeting of low-dose CT screening according to the risk of lung-cancer death. N Engl J Med. 2013;369(3):245–254. doi: 10.1056/NEJMoa1301851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tammemagi MC, Katki HA, Hocking WG, et al. Selection criteria for lung-cancer screening. N Engl J Med. 2013;368(8):728–736. doi: 10.1056/NEJMoa1211776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwartz LM, Woloshin S, Fowler FJ, Jr, Welch HG. Enthusiasm for cancer screening in the United States. JAMA. 2004;291(1):71–78. doi: 10.1001/jama.291.1.71. [DOI] [PubMed] [Google Scholar]
- 40.Hoffmann TC, Del Mar C. Patients’ Expectations of the Benefits and Harms of Treatments, Screening, and Tests: A Systematic Review. JAMA Intern Med. 2015;175(2):274–86. doi: 10.1001/jamainternmed.2014.6016. [DOI] [PubMed] [Google Scholar]
- 41.Raffle A. Information about screening - is it to achieve high uptake or to ensure informed choice? Health Expect. 2001;4(2):92–98. doi: 10.1046/j.1369-6513.2001.00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zikmund-Fisher BJ. The Right Tool Is What They Need, Not What We Have: A Taxonomy of Appropriate Levels of Precision in Patient Risk Communication. Med Care Res Rev. 2013;70(1 Suppl):37S–49S. doi: 10.1177/1077558712458541. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.