Abstract
How dendritic cells (DCs) gather information from the local milieu at a site of infection or injury and communicate this to influence adaptive immunity is not well understood. We and others have reported that soon after microbial encounter, DCs secrete the p40 subunit of IL-12, by itself, in a monomeric form. Based on recent data that this p40 monomer subsequently associates with p35 released from other cells to generate functional IL-12, we proposed that p40 can function as a DC-derived probe which samples the composition of the local milieu by looking for other binding partners. In this opinion, we discuss how such a sampling function might generate an elaborate combinatorial “code” of heterodimeric cytokines, capable of conveying location-specific information to cells downstream of DC activation, including NK and T cells.
Keywords: IL-12, Dendritic cells, Innate activation, T cell differentiation, Hypothesis, Immunological class, Tissue immunity
1. Introduction
Cytokines are typically monomeric or homodimeric proteins, secreted by cells after appropriate post-translational modifications. A striking exception to this rule is represented by the cytokine IL-12, which is instead a heterodimer – made up of two different polypeptides encoded by distinct and unlinked genetic loci. IL-12 is composed of a p35 subunit (also known as IL-12A) and a p40 subunit (also known as IL-12B), which are the products of two unrelated genes located on human chromosomes 3 and 5 (3 & 11 in the mouse) respectively [1]. The other related cytokine IL-23, which shares one of the subunits with IL-12 is a heterodimer of p40 and p19 (encoded on chromosome 12 in humans and 10 in mice) [2]. There are also two other recently described heterodimers namely IL-27 (p28 protein plus EBI3) and IL-35 (IL-12p35 protein plus EBI3), with a similar architecture [3]. The physiological significance of each of these heterodimeric cytokines is now evident in the literature [4–6]. However, the teleological advantage of their having evolved to be heterodimers, instead of the more commonly observed monomeric cytokines is not yet obvious. The few cases where the significance of such association has been studied are those cytokines that after secretion from the cell can associate with soluble forms of their receptor in solution (IL-6, CNTF or IL-11 – where for e.g. the IL-6 heterodimer is made up of sIL-6Rα and IL-6). In such instances, the extracellular complex formation enables them to signal into their target cells with a greater efficiency. In the case of the true heterodimeric cytokines (such as IL-12) either monomer is not part of the conventional receptor complex (which is made up of IL-12Rβ1 and IL-12Rβ2), but are secreted together after covalent assembly inside the producing cell. In this article, we use the case of rather well-studied heterodimeric cytokine IL-12, to argue that the evolution of such heterodimeric cytokines serves a special purpose in the immune system [7,8]. Because the constituent monomers can be re-assorted into different combinations of heterodimers, the innate immune system can use this strategy to potentially generate a large combinatorial diversity of distinct functional cytokines using a limited number of genes (which encode the monomers). We illustrate this concept with the help of our recent data on the ability of one of the subunits that form IL-12 (i.e. p40) to associate with a bevy of other binding partners.
2. The IL-12 heterodimer
IL-12 produced primarily by dendritic cells and macrophages, is a potent inducer of IFNγ from NK and T cells [9]. Mice that are deficient in either subunit of IL-12 or lack the receptors for it are impaired in their responses to some viruses as well as intracellular parasites such as Leishmania and Toxoplasma [10]. The IL-12 receptor expressed on activated T cells and NK cells, signals via Stat4 to activate the transcription factor T-bet, which in turn leads to the expression of IFNγ [11,12]. Most of the downstream and systemic effects of IL-12 – ranging from cytotoxicity to the inhibition of T cell differentiation toward other effector phenotypes such as TH2 – stem from the potent action of IFNγ on diverse cell types [13]. However, IL-12 also plays a role in enhancing other pro-inflammatory innate and adaptive responses independent of its effects via IFNγ [14,15]. This compelling biology has led many to explore translational applications of IL-12 in several clinical contexts with mixed results [16]. In tumor immunotherapy, IL-12 has been used to amplify the native T cell response as well as augment cellular vaccines with dendritic cells [17]. Conversely, blocking antibodies against IL-12 such as Ustekinumab are showing promise in ameliorating colitis, psoriasis and sarcoidosis [18]. Interestingly, and relevant to the current article, antibodies such as Ustekinumab do not target the complete IL-12 heterodimer but instead only binds to one of the subunits – IL-12p40.
In light of the multiple roles attributed to IL-12 in pathogen and autoimmune responses, the next IL-12 related cytokine to be discovered, IL-23 [19] was initially ascribed the role of a “backup” cytokine for IL-12 itself. Several early papers focused on the fact that the phenotypes observed in the IL-12p40 knockout but not in the IL-12p35 knockout mice, could now be explained by the production of IL-23 (which requires p40 but not p35). Since the biology of IL-17-producing T cells was not well understood at the time, most interpretations centered on IL-23 being a weaker version of IL-12 itself. This concept of IL-12-like activities was also extended to IL-27 with several studies trying to explain the phenotypes seen in their experimental models as being due to quantitative effects on IFNγ production. It is now widely appreciated that IL-23 does play a strikingly analogous role to IL-12, but in the induction and maintenance of a completely different effector cytokine – namely, IL-17. T cells that differentiate to become IL-17-producing effectors are involved in immunity to fungal and bacterial pathogens as well as in triggering autoimmune diseases such as psoriasis, arthritis and multiple sclerosis [5]. This intriguing parallel in the functional roles played by IL-12 and IL-23 raises the possibility that similar cytokines from the innate immune system may promote other known effector T cell fates – perhaps catalyzing the differentiation of T cells toward IL-4, IL-10, IL-9, IL-13 and TGF-β production.
3. The parts are more than the sum
The uniqueness of IL-12 related cytokines (as heterodimers) has led to several studies examining the mechanics of their synthesis, assembly and secretion. IL-12 is secreted by dendritic cells after rigorous activation by a combination of TLR and T cell-derived signals [20,21]. However, the individual subunits of IL-12 are not made in a synchronous fashion in the body. First, the mRNA for p35 is found to be constitutively expressed, albeit at low levels, in many cell types from different tissues [22,23]. Although the expression can be significantly induced upon activation of dendritic cells, it is not clear that the expression in other tissues is inducible. IL-12p35 also does not have a secretory signal sequence of its own and even addition of such a sequence does not lead to its secretion (Abdi et al., unpublished observations). Importantly, the function of constitutive expression of p35 in cells that are not secreting the IL-12 heterodimer is not understood.
In contrast, the expression of the p40 subunit is limited to macrophages and dendritic cells. The p40 transcript is not detectable in resting cells, but engagement of microbial products such as LPS via TLRs (without the need for T cell help) results in a massive induction of mRNA and secretion of the p40 protein (which does have canonical ER localization and secretory signals). Importantly, IL-12p40 is made in 100–1000 fold excess of IL-12, after microbial activation of dendritic cells [22]. Considering that the functional heterodimer of IL-12 contains the p35 and p40 at a 1:1 ratio, the reasons for the production of a vast excess of p40 is much debated in the literature.
Taken together, the ability of individual subunits of the heterodimeric cytokines (e.g. p40, p35 and p19) to be expressed at different sites and to different levels from each other may suggest that these monomers have functions that are truly independent of their participation in the formation of the known heterodimeric cytokines. The first possibility is that such an independent function is performed by the monomers themselves (or by formation of homodimers). This is hard to reconcile for p35 and p19, since they are not capable of being secreted on their own. Therefore they are likely to be found in the extracellular milieu only under special circumstances including necrotic cell death [24]. However, soon after exposure to a bacterial TLR ligand, the molecule most copiously detected in the secretions (in vitro and in vivo) from both human and mouse dendritic cells is p40 [25]. Most of the secreted p40 is in the monomeric form; but small amounts of covalently linked homodimers have been reported in mice but not in humans [26]. Secretion of free p40 is independent of the heterodimer, since it can take place in the absence of p35 or p19 [24]. This is significant, since early studies on the binding of the IL-12 to its receptor implicated the exposed surface of p40 in the heterodimer as the major contact point with the IL-12Rβ1 [27,28]. This suggested that either the monomer or the homodimer might be able to signal T cells or NK cells using part or all of the IL-12 receptor complex. Accordingly, it has been proposed that the mouse p40 homodimer (and not the monomer) acts as a potent antagonist of IL-12 by competing for binding to the IL-12 receptor [29,30]. In contrast, few studies have suggested that p40 homodimer acts as an agonist – as a chemoattractant for macrophages and dendritic cells [31–33]. In this process, it is thought to exacerbate cellular responses such as allograft rejection [34]. Consistent with an agonistic activity of p40, mice that are deficient in p35 but not those for p40 were able to mount a alloreactive CD8+ response with measurable IFNγ production [34]. There is also data showing that p35-deficient mice continue to show resistance to infections by Mycobacterium tuberculosis, Salmonella Enteritidis, Cryptococcus neoformans and Listeria monocytogenes [35–42] as well as non-infectious pathologies such as pulmonary fibrosis [43].
4. An alternate function for IL-12p40 – as a cytokine adaptor protein
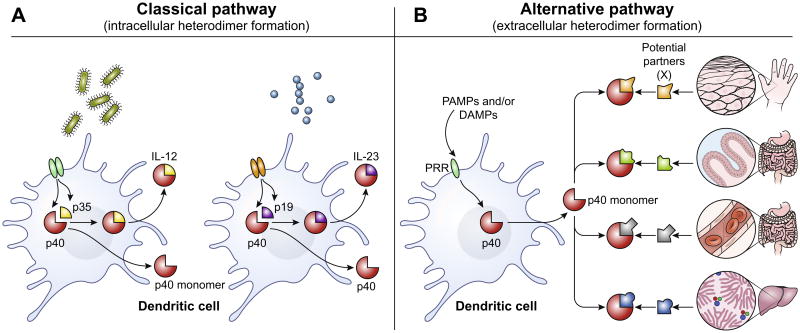
In an attempt to reconcile these disparate datasets, we had previously proposed that the copiously secreted p40 monomer found in the early phase of the innate response serves a specific function (Fig. 1) – as an adaptor for building a whole slew of other heterodimeric proteins [7,24,25]. In this concept, the activated dendritic cells secrete p40 as a “probe” to sample the local inflammatory milieu. The p40 then binds to available partners that are also present in the same microenvironment to generate a functional heterodimeric cytokine. As discussed above, since p35 and p19 are not capable of being secreted on their own, the simplest source for them in the extracellular milieu would be from necrotic lysis of cells that express them (at low basal levels or at upregulated levels in response to a pathogen or injury). Depending on the particular partner available in each context, this would allow p40 to assemble a distinct cytokine in a very dynamic fashion – both geographically and temporally (Fig. 1). As a result, very precise information can then be transferred to the various cells in the same region (or the draining nodes) about the course of effector differentiation that they must pursue. The significance of this hypothesis is that it provides the innate immune system with a very flexible communication system with which to encode and rapidly transfer contextual information to various cells in different locales. For instance, T cells in the node draining the site where an infection or injury occurred can now get precise contextual information about the exact tissue at which the primary event took place.
Fig. 1.
(A) The classical pathway of IL-12 and IL-23 secretion. First, IL-12p40 is induced in dendritic cells by inflammatory signals (PAMPs and/or DAMPs). Depending on the nature of the insult (e.g. type of pathogen, site of injury etc.), subsequent signals from NK, T or NKT cells (IFNγ, CD40L etc.,) can synergize to induce the expression of p35 or p19 on these DCs. These collective signals lead to the intracellular assembly of IL-12 (p40 plus p35) or IL-23 (p40 plus p19) and the secretion of these covalently linked heterodimers. (B) The alternative pathway. The induction of gene expression in this pathway is mechanistically identical to the classical pathway i.e. p40 is rapidly induced in response to PAMPs or DAMPS. However, the free p40 monomer secreted in the extracellular milieu will pair and bind with various potential partners available locally leading to the generation of new heterodimeric cytokines extracellularly (p40 plus X, where X is any one of many binding partners). The versatility of p40 monomer binding to various partners can be due to the nature of tissue in which immune response is being generated - e.g. skin epithelial cells may potentially have different binding partners for p40 monomer than those of intestinal villi, capillary endothelium or Liver hepatocytes. In addition (not shown) the specific pathogen or injury to the stromal tissues may also result in the release of different binding partners, and thereby influence the particular binding partner available at any given time. This allows p40 to form complexes tailored to each local context - and then convey that information by binding to IL-12Rβ containing receptors on other immune cells downstream of DC activation.
Nevertheless, the hypothesis first had to reconcile two main concerns, based on the prevailing models of IL-12 synthesis in cells. First, it is commonly believed that the two subunits of IL-12 must be assembled inside the cell as a complete cytokine before secretion. Since p35 is not secreted on its own, the only way it can be secreted from DCs is to first associate with p40 (involving a covalent linkage between cysteine 177 in p40 and cysteine 74 in p35) [44], which usually takes place inside the cell. Our hypothesis was untenable if we could not first demonstrate that monomeric p40 can associate outside the cell with another partner (e.g. p35) to generate a stable and functional heterodimeric cytokine. The second issue has to do with the combinatorial power of the available monomers. Even in a hypothetical scenario where all known monomers (p40, p35, p19, EBI3 etc.) can associate with each other, the system gains less than 10 possible heterodimeric cytokines. Since some of these associations are unlikely to occur (based on the literature and unpublished observations), the idea that this strategy has the ability to encode information about a wide range of contexts is limited in scope. Thus if the model is to be transformative as a system of context-communication, there would have to be additional proteins capable of participating in heterodimer formation with the already known catalog of monomers. While this has not been tested for most of these proteins, our recent data on p40 suggests a simpler solution.
First, we recently reported that in the context of the well-studied heterodimer IL-12, both of the above concerns are experimentally tractable [24]. Using a variety of in vitro assays, we showed that not only can p35 be released from necrotic cells, but also that it can associate with secreted p40 monomer in the extracellular milieu. This challenges the current consensus that functional IL-12 is secreted only after assembly inside the cell. Indeed, the IL-12 formed by extracellular assembly of p40 binding to p35 was not only stable in biochemical analyses, but it was also functionally active in various bioassays. It remains to be seen whether such an assembly is also possible for IL-23.
In order to address the second question, we used a biochemical pull-down strategy aimed at identifying other proteins that can potentially bind p40 in vivo. We chose to focus on p40 because of the existing data that large amounts of p40 monomer (but not p35) is secreted soon after innate activation [25]. In the serum of p35-deficient mice, we were able to identify 18 other proteins that were capable of binding to serum p40 during an inflammatory response. We suggest that these 18 proteins represent sufficient raw material necessary to form a diverse coding language for dendritic cells to communicate context to T cells (Fig. 1).
5. The putative alphabets of a dendritic cell codec?
Although these potential binding partners for p40 require further validation, their ontology already suggests several avenues for future experiments. The most prominent candidate among these proteins is CD5-antigen like (CD5L) or Apoptosis inhibitor of Macrophages (AIM, also known as Api6, SpAlpha). CD5L is secreted from macrophages and epithelial cells in response to bacterial infections and stress. It was originally described as an inhibitor of apoptosis, based on increased death of thymocytes and macrophages in CD5L-deficient mice [45,46]. In the absence of a stressor, the expression of CD5L seems to be largely in the Lung, Liver, Kidneys and adipocytes. Recent studies suggest that in some of these tissues, CD5L regulates autophagy, lipolysis and affects the recruitment of macrophages [47]. However, its precise role in the immune system (or its association with p40) is yet to be fully understood. In our experiments, while CD5L can associate with p40 to form a heterodimer, this complex does not have the same activity as IL-12 (i.e. it does not increase IFNγ production in T and NK cells). Our preliminary experiments, looking at the transcriptomic changes following p40 plus CD5L treatment of mouse splenocytes, it does not seem to function like IL-23 either (with respect to catalyzing IL-17 production). In many ways, these harken back to the early days of the discovery of IL-23 when a binding partner (p19) and its complex was described, but the functional significance was not well appreciated until a the role of TH17 cells themselves was better understood.
Other candidates identified in our screen are equally promising, although even less understood in terms of DC-T cell communication. A common ontology term that runs through many of these proteins is their association with the tissue response to DAMPs or stress. Clusterin is normally an intracellular molecular chaperone that has recently been shown to associate with the autophagy complex (LC3-Atg) and enhance cell survival [48]. Interestingly, clusterin over-expression is considered a biomarker for certain types of cancer and even a potential target for chemotherapy [49–52]. Serum Amyloid A1, Mannose-binding protein and the associated serine protease MBLSP are acute phase response proteins found in several inflammatory conditions [53–55]. Pentraxin 3 is secreted by conventional but not plasmacytoid DCs after being exposed to DAMPs and is one of the most abundant PRRs observed during the early phases of HIV infection [56–58]. In addition, we also found proteins that play a role in wound healing and blood clotting such as Antithrombin III, Kininogen and Protein Z [59].
These data also raise new questions about how a single protein (p40) can specifically bind to such an extensive list of partners. Despite the technical controls in the original experiments [24], further biological validation is certainly required to extend this paradigm. Nevertheless, how might one speculate about the ability of p40 to recognize proteins, which at least on preliminary analysis, have no obvious relationships in their structural motifs? A key to understanding this pleiotropy may also come from one of the underappreciated characteristics of p40. In biochemical analysis reported so far, which used recombinant p40 expressed in bacteria or CHO cells, it is either reported as a monomer or homodimer. However, in our study, we used affinity columns to directly purify mouse p40 from the serum of mice challenged with LPS. Although on first glance, this p40 can also be segregated as a monomer and some homodimer, a more detailed separation using 2D gels suggests a more complicated story. Native p40 in the serum of septic mice exists in at least eight different isoforms (as defined by their isoelectric points). This level of heterogeneity has never been reported in the literature before (and not observed when using recombinant p40). The nature of the post-translational modifications that lead to these distinct isoforms is currently unclear. An interesting possibility is that different tissue sites also post-translationally modify p40 in different ways. This could be a result of extracellular matrix proteins present in each tissue site or different cellular phenotypes of dendritic cells in some tissues [60]. A better understanding of these processing pathways would help expand the functional significance of our findings. The existence of such a heterogeneous pot of p40 may itself be critical for its ability to bind multiple partners and generate the complete “code-book”.
6. Conclusion: The message in the code
The p40 + X model we proposed is analogous to many cases of two-signal based cellular information transfer in the vertebrate immune system e.g. antigen and T cell help as two separable elements which instruct B cells [61,62]. Here too, the “opinion” of the initially activated cells (activated DCs) would be manifested by p40, which then polls the local cells for their input (by binding X) and then transfers this combinatorial “opinion poll” to influence the next immunological decision – by NK cells, T cells. This framework can be important in understanding the complex interplay of cellular actors of the immune response as they inform, influence and instruct each other within local tissue microenvironments [63,64]. During the initial phases of the response, lymphocytes in distal sites (e.g. lymph nodes) have no direct contact with the site of insult. They have to quickly decipher important clues about the tissues they are eventually bound to work in [65]. The nature of the polypeptides released by the inflamed, injured or infected tissues (and picked up by p40) can be quite informative here. In the later phases of the response, as innate and adaptive cells are recruited in as effector cells or remain in the tissues as resident memory cells, these complexes can regulate the nature and extent of their continued activity [66]. Considering how little we know about the diverse ways in which innate factors in localized immune domains regulate such dynamic cellular events, the template of a combinatorial code based on heterodimeric cytokines can be a helpful starting point for future studies.
Acknowledgments
KA is supported by the Intramural Research Program of the NIAID, NIH. NJS is supported by funding from the NIH (R56AI113313 and RO1AI110719).
The graphic art was generated by Ethan Tyler and Alan Hoofering (NIH).
References
- 1.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 2.Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 3.Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221–242. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- 4.Vignali DA, Kuchroo VK. IL-12 family cytokines: immunological playmakers. Nat Immunol. 2012;13:722–728. doi: 10.1038/ni.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaffen SL, Jain R, Garg AV, Cua DJ. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol. 2014;14:585–600. doi: 10.1038/nri3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun L, He C, Nair L, Yeung J, Egwuagu CE. Interleukin 12 (IL-12) family cytokines: role in immune pathogenesis and treatment of CNS autoimmune disease. Cytokine. 2015 doi: 10.1016/j.cyto.2015.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdi K. IL-12: the role of p40 versus p75. Scand J Immunol. 2002;56:1–11. doi: 10.1046/j.1365-3083.2002.01101.x. [DOI] [PubMed] [Google Scholar]
- 8.Cooper AM, Khader SA. IL-12p40: an inherently agonistic cytokine. Trends Immunol. 2007;28:33–38. doi: 10.1016/j.it.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi M, Fitz L, Ryan M, Hewick RM, Clark SC, Chan S, et al. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989;170:827–845. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magram J, Connaughton SE, Warrier RR, Carvajal DM, Wu CY, Ferrante J, et al. IL-12-deficient mice are defective in IFN gamma production and type 1 cytokine responses. Immunity. 1996;4:471–481. doi: 10.1016/s1074-7613(00)80413-6. [DOI] [PubMed] [Google Scholar]
- 11.Bacon CM, Petricoin EF, 3rd, Ortaldo JR, Rees RC, Larner AC, Johnston JA, et al. Interleukin 12 induces tyrosine phosphorylation and activation of STAT4 in human lymphocytes. Proc Natl Acad Sci USA. 1995;92:7307–7311. doi: 10.1073/pnas.92.16.7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szabo SJ, Jacobson NG, Dighe AS, Gubler U, Murphy KM. Developmental commitment to the Th2 lineage by extinction of IL-12 signaling. Immunity. 1995;2:665–675. doi: 10.1016/1074-7613(95)90011-x. [DOI] [PubMed] [Google Scholar]
- 13.Scharton-Kersten TM, Wynn TA, Denkers EY, Bala S, Grunvald E, Hieny S, et al. In the absence of endogenous IFN-gamma, mice develop unimpaired IL-12 responses to Toxoplasma gondii while failing to control acute infection. J Immunol. 1996;157:4045–4054. [PubMed] [Google Scholar]
- 14.Starbeck-Miller GR, Xue HH, Harty JT. IL-12 and type I interferon prolong the division of activated CD8 T cells by maintaining high-affinity IL-2 signaling in vivo. J Exp Med. 2014;211:105–120. doi: 10.1084/jem.20130901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia K, Sun Z, Mattson E, Li L, Smyth K, Xiao Z. IL-12 is required for Mtor regulation of memory CTLs during viral infection. Genes Immun. 2014;15:413–423. doi: 10.1038/gene.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kilner JM, Lemon RN. What we know currently about mirror neurons. Curr Biol: CB. 2013;23:R1057–R1062. doi: 10.1016/j.cub.2013.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vom Berg J, Vrohlings M, Haller S, Haimovici A, Kulig P, Sledzinska A, et al. Intratumoral IL-12 combined with CTLA-4 blockade elicits T cell-mediated glioma rejection. J Exp Med. 2013;210:2803–2811. doi: 10.1084/jem.20130678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yeilding N, Szapary P, Brodmerkel C, Benson J, Plotnick M, Zhou H, et al. Development of the IL-12/23 antagonist ustekinumab in psoriasis: past, present, and future perspectives. Ann N Y Acad Sci. 2011;1222:30–39. doi: 10.1111/j.1749-6632.2011.05963.x. [DOI] [PubMed] [Google Scholar]
- 19.Dudai Y. Yadin Dudai, Curr Biol: CB. 2013;23:R1078–R1080. doi: 10.1016/j.cub.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Abdi K, Singh NJ, Matzinger P. Lipopolysaccharide-activated dendritic cells: “Exhausted” or alert and waiting? J Immunol. 2012 doi: 10.4049/jimmunol.1102868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdi K, Singh NJ. Antigen-activated T cells induce IL-12p75 production from dendritic cells in an IFN-gamma-independent manner. Scand J Immunol. 2010;72:511–521. doi: 10.1111/j.1365-3083.2010.02467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D'Andrea A, Rengaraju M, Valiante NM, Chehimi J, Kubin M, Aste M, et al. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J Exp Med. 1992;176:1387–1398. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schoenhaut DS, Chua AO, Wolitzky AG, Quinn PM, Dwyer CM, McComas W, et al. Cloning and expression of murine IL-12. J Immunol. 1992;148:3433–3440. [PubMed] [Google Scholar]
- 24.Abdi K, Singh NJ, Spooner E, Kessler BM, Radaev S, Lantz L, et al. Free IL-12p40 monomer is a polyfunctional adapter for generating novel IL-12-like heterodimers extracellularly. J Immunol. 2014 doi: 10.4049/jimmunol.1400159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdi K, Singh N, Matzinger P. T-cell control of IL-12p75 production. Scand J Immunol. 2006;64:83–92. doi: 10.1111/j.1365-3083.2006.01767.x. [DOI] [PubMed] [Google Scholar]
- 26.Pahan K, Sheikh FG, Liu X, Hilger S, McKinney M, Petro TM. Induction of nitric-oxide synthase and activation of NF-kappaB by interleukin-12 p40 in microglial cells. J Biol Chem. 2001;276:7899–7905. doi: 10.1074/jbc.M008262200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gillessen S, Carvajal D, Ling P, Podlaski FJ, Stremlo DL, Familletti PC, et al. Mouse interleukin-12 (IL-12) p40 homodimer: a potent IL-12 antagonist. Eur J Immunol. 1995;25:200–206. doi: 10.1002/eji.1830250133. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Fan KT, Li JM, Waller EK. The regulation and activity of interleukin-12. Front Biosci (Schol Ed) 2012;4:888–899. doi: 10.2741/s306. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Wilkinson VL, Podlaski FJ, Wu C, Stern AS, Presky DH, et al. Characterization of mouse interleukin-12 p40 homodimer binding to the interleukin-12 receptor subunits. Eur J Immunol. 1999;29:2007–2013. doi: 10.1002/(SICI)1521-4141(199906)29:06<2007::AID-IMMU2007>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell DA, Batich KA, Gunn MD, Huang MN, Sanchez-Perez L, Nair SK, et al. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature. 2015;519:366–369. doi: 10.1038/nature14320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ha SJ, Lee CH, Lee SB, Kim CM, Jang KL, Shin HS, et al. A novel function of IL-12p40 as a chemotactic molecule for macrophages. J Immunol. 1999;163:2902–2908. [PubMed] [Google Scholar]
- 32.Jana M, Dasgupta S, Pal U, Pahan K. IL-12 p40 homodimer, the so-called biologically inactive molecule, induces nitric oxide synthase in microglia via IL-12R beta 1. Glia. 2009;57:1553–1565. doi: 10.1002/glia.20869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jana M, Pahan K. IL-12 p40 homodimer, but not IL-12 p70, induces the expression of IL-16 in microglia and macrophages. Mol Immunol. 2009;46:773–783. doi: 10.1016/j.molimm.2008.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piccotti JR, Chan SY, Goodman RE, Magram J, Eichwald EJ, Bishop DK. IL-12 antagonism induces T helper 2 responses, yet exacerbates cardiac allograft rejection. evidence against a dominant protective role for T helper 2 cytokines in alloimmunity. J Immunol. 1996;157:1951–1957. [PubMed] [Google Scholar]
- 35.Mohrin M, Shin J, Liu Y, Brown K, Luo H, Xi Y, et al. Stem cell aging. A mitochondrial UPR-mediated metabolic checkpoint regulates hematopoietic stem cell aging. Science. 2015;347:1374–1377. doi: 10.1126/science.aaa2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Halstead JM, Lionnet T, Wilbertz JH, Wippich F, Ephrussi A, Singer RH, et al. Translation. An RNA biosensor for imaging the first round of translation from single cells to living animals. Science. 2015;347:1367–1671. doi: 10.1126/science.aaa3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Decken K, Kohler G, Palmer-Lehmann K, Wunderlin A, Mattner F, Magram J, et al. Interleukin-12 is essential for a protective Th1 response in mice infected with Cryptococcus neoformans. Infect Immun. 1998;66:4994–5000. doi: 10.1128/iai.66.10.4994-5000.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brombacher F, Dorfmuller A, Magram J, Dai WJ, Kohler G, Wunderlin A, et al. IL-12 is dispensable for innate and adaptive immunity against low doses of Listeria monocytogenes. Int Immunol. 1999;11:325–332. doi: 10.1093/intimm/11.3.325. [DOI] [PubMed] [Google Scholar]
- 39.Khader SA, Partida-Sanchez S, Bell G, Jelley-Gibbs DM, Swain S, Pearl JE, et al. Interleukin 12p40 is required for dendritic cell migration and T cell priming after Mycobacterium tuberculosis infection. J Exp Med. 2006;203:1805–1815. doi: 10.1084/jem.20052545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cooper AM, Kipnis A, Turner J, Magram J, Ferrante J, Orme IM. Mice lacking bioactive IL-12 can generate protective, antigen-specific cellular responses to mycobacterial infection only if the IL-12 p40 subunit is present. J Immunol. 2002;168:1322–1327. doi: 10.4049/jimmunol.168.3.1322. [DOI] [PubMed] [Google Scholar]
- 41.Holscher C, Atkinson RA, Arendse B, Brown N, Myburgh E, Alber G, et al. A protective and agonistic function of IL-12p40 in mycobacterial infection. J Immunol. 2001;167:6957–6966. doi: 10.4049/jimmunol.167.12.6957. [DOI] [PubMed] [Google Scholar]
- 42.Lehmann J, Bellmann S, Werner C, Schroder R, Schutze N, Alber G. IL-12p40-dependent agonistic effects on the development of protective innate and adaptive immunity against Salmonella enteritidis. J Immunol. 2001;167:5304–5315. doi: 10.4049/jimmunol.167.9.5304. [DOI] [PubMed] [Google Scholar]
- 43.Huaux F, Arras M, Tomasi D, Barbarin V, Delos M, Coutelier JP, et al. A profibrotic function of IL-12p40 in experimental pulmonary fibrosis. J Immunol. 2002;169:2653–2661. doi: 10.4049/jimmunol.169.5.2653. [DOI] [PubMed] [Google Scholar]
- 44.Yoon C, Johnston SC, Tang J, Stahl M, Tobin JF, Somers WS. Charged residues dominate a unique interlocking topography in the heterodimeric cytokine interleukin-12. Embo J. 2000;19:3530–3541. doi: 10.1093/emboj/19.14.3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miyazaki T, Hirokami Y, Matsuhashi N, Takatsuka H, Naito M. Increased susceptibility of thymocytes to apoptosis in mice lacking AIM, a novel murine macrophage-derived soluble factor belonging to the scavenger receptor cysteine-rich domain superfamily. J Exp Med. 1999;189:413–422. doi: 10.1084/jem.189.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuwata K, Watanabe H, Jiang SY, Yamamoto T, Tomiyama-Miyaji C, Abo T, et al. AIM inhibits apoptosis of T cells and NKT cells in Corynebacterium-induced granuloma formation in mice. Am J Pathol. 2003;162:837–847. doi: 10.1016/S0002-9440(10)63880-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kurokawa J, Nagano H, Ohara O, Kubota N, Kadowaki T, Arai S, et al. Apoptosis inhibitor of macrophage (AIM) is required for obesity-associated recruitment of inflammatory macrophages into adipose tissue. Proc Natl Acad Sci USA. 2011;108:12072–12077. doi: 10.1073/pnas.1101841108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang F, Kumano M, Beraldi E, Fazli L, Du C, Moore S, et al. Clusterin facilitates stress-induced lipidation of LC3 and autophagosome biogenesis to enhance cancer cell survival. Nat Commun. 2014;5:5775. doi: 10.1038/ncomms6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chun YJ. Knockdown of clusterin expression increases the in vitro sensitivity of human prostate cancer cells to paclitaxel. J Toxicol Environ Health Part A. 2014;77:1443–1450. doi: 10.1080/15287394.2014.951760. [DOI] [PubMed] [Google Scholar]
- 50.Al-Asaaed S, Winquist E. Custirsen (OGX-011): clusterin inhibitor in metastatic prostate cancer. Curr Oncol Reports. 2013;15:113–118. doi: 10.1007/s11912-012-0285-1. [DOI] [PubMed] [Google Scholar]
- 51.Koltai T. Clusterin: a key player in cancer chemoresistance and its inhibition. OncoTargets Therapy. 2014;7:447–456. doi: 10.2147/OTT.S58622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fuzio P, Napoli A, Ciampolillo A, Lattarulo S, Pezzolla A, Nuzziello N, et al. Clusterin transcript variants expression in thyroid tumor: a potential marker of malignancy? BMC Cancer. 2015;15:349. doi: 10.1186/s12885-015-1348-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Endo Y, Takahashi M, Iwaki D, Ishida Y, Nakazawa N, Kodama T, et al. Mice deficient in ficolin, a lectin complement pathway recognition molecule, are susceptible to Streptococcus pneumoniae infection. J Immunol. 2012;189:5860–5866. doi: 10.4049/jimmunol.1200836. [DOI] [PubMed] [Google Scholar]
- 54.Schwaeble WJ, Lynch NJ, Clark JE, Marber M, Samani NJ, Ali YM, et al. Targeting of mannan-binding lectin-associated serine protease-2 confers protection from myocardial and gastrointestinal ischemia/reperfusion injury. Proc Natl Acad Sci USA. 2011;108:7523–7528. doi: 10.1073/pnas.1101748108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dahl MR, Thiel S, Matsushita M, Fujita T, Willis AC, Christensen T, et al. MASP-3 and its association with distinct complexes of the mannan-binding lectin complement activation pathway. Immunity. 2001;15:127–135. doi: 10.1016/s1074-7613(01)00161-3. [DOI] [PubMed] [Google Scholar]
- 56.Ortega-Hernandez OD, Bassi N, Shoenfeld Y, Anaya JM. The long pentraxin 3 and its role in autoimmunity. Semin Arthritis Rheum. 2009;39:38–54. doi: 10.1016/j.semarthrit.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 57.Doni A, Peri G, Chieppa M, Allavena P, Pasqualini F, Vago L, et al. Production of the soluble pattern recognition receptor PTX3 by myeloid, but not plasmacytoid, dendritic cells. Eur J Immunol. 2003;33:2886–2893. doi: 10.1002/eji.200324390. [DOI] [PubMed] [Google Scholar]
- 58.Ketter PM, Guentzel MN, Schaffer B, Herzig M, Wu X, Montgomery RK, et al. Severe Acinetobacter baumannii sepsis is associated with elevation of pentraxin 3. Infect Immun. 2014;82:3910–3918. doi: 10.1128/IAI.01958-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang J, Tu Y, Lu L, Lasky N, Broze GJ., Jr Protein Z-dependent protease inhibitor deficiency produces a more severe murine phenotype than protein Z deficiency, Blood. 2008;111:4973–4978. doi: 10.1182/blood-2007-12-126391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grainger JR, Askenase MH, Guimont-Desrochers F, da Fonseca DM, Belkaid Y. Contextual functions of antigen-presenting cells in the gastrointestinal tract. Immunol Rev. 2014;259:75–87. doi: 10.1111/imr.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baxter AG, Hodgkin PD. Activation rules: the two-signal theories of immune activation. NatRevImmunol. 2002;2:439–446. doi: 10.1038/nri823. [DOI] [PubMed] [Google Scholar]
- 62.Fontana MF, Vance RE. Two signal models in innate immunity. Immunol Rev. 2011;243:26–39. doi: 10.1111/j.1600-065X.2011.01037.x. [DOI] [PubMed] [Google Scholar]
- 63.Belkaid Y. Tailored immunity at mucosae. Immunol Rev. 2014;260:5–7. doi: 10.1111/imr.12197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Masopust D, Schenkel JM. The integration of T cell migration, differentiation and function. Nat Rev Immunol. 2013;13:309–320. doi: 10.1038/nri3442. [DOI] [PubMed] [Google Scholar]
- 65.Matzinger P, Kamala T. Tissue-based class control: the other side of tolerance. Nat Rev Immunol. 2011;11:221–230. doi: 10.1038/nri2940. [DOI] [PubMed] [Google Scholar]
- 66.Schenkel JM, Masopust D. Tissue-resident memory T cells. Immunity. 2014;41:886–897. doi: 10.1016/j.immuni.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]