Abstract
A large number of pseudogenes have been found to be transcribed in human cancers. However, only a few pseudogenes are functionally characterized. Here, we identified a transcribed pseudogene of vascular endothelial growth factor receptor-1 (VEGFR1), or fms-related tyrosine kinase 1 (FLT1), in human colorectal cancer (CRC) cells. Interestingly, this pseudogene (designated as FLT1P1) was found to be transcribed bidirectionally and functionally modulated cognate VEGFR1 protein expression in the cells. Mechanistically, expression of FLT1P1 antisense transcript not only inhibited the VEGFR1 expression, but also inhibited non-cognate VEGF-A expression through interaction with miR-520a. Perturbation of FLT1P1 expression by RNA interference (RNAi) markedly inhibited tumor cell proliferation and xenograft tumor growth. This study identifies FLT1P1 antisense as a critical regulator of VEGFR1 and VEGF-A expression in CRC cells, and highlights its role in regulation of the pathogenesis of CRC.
Implications
The VEGFR1 pseudogene, FLT1P1, is a novel and functional regulator of VEGF signaling and its targeting could be an alternative strategy to modulate its cognate/target gene expression and downstream activity in cancer.
Keywords: colorectal cancer, FLT1, FLT1P1, pseudogene, VEGFR1
INTRODUCTION
Vascular endothelial growth factor receptor-1 (VEGFR1, or fms-related tyrosine kinase 1 [FLT1]) is a receptor tyrosine kinase with high binding affinity for its ligands, VEGF-A and placenta growth factor (1). Despite its high ligand-binding affinity, VEGFR1's kinase activity is lower than that of the related family member VEGFR2/KDR. The primary form of VEGFR1 is a full-length transmembrane receptor, but it is also expressed as soluble isoforms that lack transmembrane and intracellular domains. Soluble VEGFR1 serves as a decoy receptor to inhibit extracellular VEGF signaling (2). Functionally, VEGFR1 is essential for embryonic vasculogenesis (3) and macrophage function in mouse models (4). Moreover, murine VEGFR1+ bone marrow progenitor-derived monocytes contribute to the pre-metastatic niche (5, 6).
Although researchers originally viewed VEGFR1 as a kinase receptor in endothelial cells, members in our laboratory and others have demonstrated that VEGFR1 is also expressed by human epithelial cancer cells, such as those derived from human pancreatic, colorectal, and breast tumors, and melanomas (7-10). Activation of VEGFR1 promotes epithelial-mesenchymal transition and an aggressive phenotype in specific cancer cells (11-13). However, the mechanism regulating VEGFR1 expression and function in cancer cells is still largely unknown.
Herein we report our finding of an actively transcribed VEGFR1/FLT1 pseudogene (FLT1P1) in human colorectal cancer (CRC) cells. Pseudogenes have been long considered dysfunctional genomic loci that have lost transcriptional activity and protein-coding ability (14, 15). However, emerging evidence suggests that many pseudogenes not only are transcribed (16-18) but also have certain functional activity in the regulation of cognate gene expression (19). The functionality of pseudogenes seems to depend on their unique noncoding RNA (ncRNA) property to serve as inhibitory antisense RNA (20, 21), endogenous small interfering RNA (siRNA) (22, 23), or competing endogenous RNA (ceRNA) (24, 25). Recent genome-wide studies of pseudogenes expressed in human cancer tissues revealed their unique transcriptional landscape and the expression profile associated with cancer subtypes (18, 26). However, of more than 16,000 human pseudogenes archived in the Yale University pseudogene (http://www.pseudogene.org) and GENCODE (http://www.gencodegenes.org) databases (17, 26), only a few are functionally characterized. One functional pseudogene is PTENP1, which acts as a ceRNA to positively regulate expression of the cognate gene PTEN in prostate cancer cells and tumors (24). Another recent study demonstrated that PTENP1 produces both sense and antisense transcripts and has a complex regulatory mechanism for PTEN expression (27).
With the identification of FLT1P1 transcripts in CRC cells, we hypothesized that this pseudogene may regulate VEGFR1 expression in the cancer cells. To test that hypothesis we used FLT1P1 overexpression and knockdown to determine the functional relationship between FLT1P1 and VEGFR1 in CRC cells. Our findings indicated that FLT1P1 not only regulates VEGFR1 protein expression but also modulates VEGF-A expression, microRNA expression and cancer cell-growth kinetics in vitro and in vivo.
MATERIALS AND METHODS
Human cell lines and culture conditions
The human CRC cell lines SW480 and DLD1 were obtained from the American Type Culture Collection (Manassas, VA). The new human primary colon cancer cell line HCP1 was generated in our laboratory under a protocol approved by the Institutional Review Board of The University of Texas MD Anderson Cancer Center (28). Cell lines were routinely cultured in minimal essential medium supplemented with 10% fetal bovine serum, vitamins, sodium pyruvate, L-glutamine, nonessential amino acids, and penicillin-streptomycin (Life Technologies, Grand Island, NY) at 37°C in a 5% CO2 incubator.
Human tissue specimens
Residual tissue from surgical specimens of colorectal tumors were obtained from the Pathology Department at MD Anderson Cancer Center following protocols approved by the Institutional Review Board. The tissue specimens were used immediately for RNA isolation.
Semiquantitative RT-PCR analysis and Sanger sequencing
Total RNA was isolated from CRC cells and colorectal tumor tissues using TRIzol reagent (Invitrogen, Carlsbad, CA), cleared of DNA contamination using DNase I digestion, and further purified using an RNeasy kit (QIAGEN, Valencia, CA). RNA quality was verified using a bioanalyzer (Agilent Technologies, Santa Clara, CA) and RNA was quantified using a NanoDrop 2000 (Thermo Fisher Scientific, Waltham, MA). First-strand cDNA was synthesized from total RNA using SuperScript III (Invitrogen) with custom gene-specific primers (Supplementary Table S1) and random hexamer primers according to the manufacturer's instructions.
Semiquantitative RT-PCR analysis of VEGFR1 was performed using seven sets of primers (Supplementary Table S1) under conditions described previously (29). The visualized RT-PCR amplicon bands on the agarose gel were cut and isolated using a gel purification kit (QIAGEN). After subcloning the amplicons into a pCR2.1 vector (Invitrogen), the DNA sequences were analyzed using Sanger sequencing, followed by sequence alignment using the BLAST and ClustaIW2 software programs to determine gene identity and compare the nucleotide sequences.
TaqMan qPCR
TaqMan qPCR analysis of VEGFR1, VEGF-A and has-miR-520a-3p expression were carried out using preformulated assays (Hs01052961_m1, Hs00176573_m1, Hs00900055_m1 and Hs03294648_pri; Applied Biosystems) with an Applied Biosystems 7900HT real-time PCR system set for an ΔΔCT quantitation protocol. The TaqMan assay for FLT1P1 was custom-designed based on regions of differences in the FLT1P1 transcripts and VEGFR1 mRNA sequence (Supplementary Table S1). Oligonucleotide primers and a dual-labeled probe for the FLT1P1 TaqMan assay were synthesized by Integrated DNA Technologies (Coralville, IA). A preformulated 18S ribosomal RNA assay (Hs99999901_s1; Applied Biosystems) was used as an endogenous reference. Expression of a gene or pseudogene relative to 18S rRNA was normalized and the fold difference of transcripts in treated samples relative to that in control samples was calculated using the ΔΔCT method.
Western blot analysis
Cellular protein extraction, gel electropheresis and immunoblotting were performed as described previously (29) with minor modifications. A human VEGFR1 C-terminus-specific antibody (#2893, Cell Signaling Technology, Beverly, MA) was used at a 1:1000 dilution.
Pseudogene overexpression
FLT1P1 cDNAs from the CRC cell line HCP1 were cloned into the expression lentivector pCDH-CMV-EF1-copGFP (System Biosciences, Mountain View, CA), and a recombinant lentivirus was generated according to the manufacturer's instructions. CRC cells were subjected to the FLT1P1 or control lentiviral transduction and then purified using green fluorescent protein+ fluorescence-activated cell sorting. Stable overexpression of FLT1P1 in the cells was confirmed using qPCR.
MicroRNA overexpression
Transient transfection of has-miR-520a-3p (Invitrogen) in CRC cells were carried out using Lipofectamine 2000 (Life Technologies) according to the manufacturer's instructions.
RNA interference
Small interfering RNAs specific to FLT1P1 were custom-designed based on the regions of discrepancy between the FLT1P1 transcripts and the VEGFR1 mRNA sequence (Supplementary Table S1). The siRNA duplexes were custom-synthesized by Sigma-Aldrich (St. Louis, MO). CRC cells were transfected with FLT1P1 or control siRNAs using Lipofectamine 2000 (Invitrogen). The cells were harvested 48 h after the transfection for qPCR analysis or 72 h after the transfection for Western blot analysis.
The FLT1P1-specific short hairpin RNAs (shRNAs) were custom-designed, as well. DNA fragments containing shRNA and flank sequences were synthesized and subcloned into the expression lentivector pSIH-H1-copGFP (System Biosciences, Mountain View, CA). A recombinant lentivirus was prepared according to the manufacturer's instructions. The CRC cell lines HCP1 and DLD1 were transduced with lentivirus, and stably transduced cells were selected using green fluorescent protein+ fluorescence-activated cell sorting. Stable shFLT1P1- and shControl-expressing cells were then subjected to various assays.
VEGF-A protein assessment
Human VEGF-A protein concentration in the cell culture conditioned medium was measured using a solid phase sandwich type of enzyme linked immuno-sorbent assay (ELISA) kit (Invitrogen). Sample preparation and quantification were performed according to the manufacturer's instructions.
Xenograft tumor growth assay
Six-week-old female athymic nude mice (National Cancer Institute-Frederick Cancer Research Facility, Frederick, MD) were subcutaneously injected with shFLT1P1- or shControl-expressing cells (1 × 106) in the right flank. Tumor growth was monitored via measurement using calipers, and mean tumor volume was calculated using the equation (length × width2)/2 ± standard error of the mean. At the time of termination of the study, the mice were killed, and their tumors were excised, weighed, and photographed. The animal studies were conducted under a protocol approved by the MD Anderson Institutional Animal Care and Use Committee.
Statistical analyses
For all studies, statistical analyses were conducted using the Student t-test. All tests were two-sided, and P values less than 0.05 were considered statistically significant.
RESULTS
FLT1P1 is expressed in CRC cells
Transcriptional regulation of VEGFR1 expression is well characterized in endothelial cells (30). However, how VEGFR1 expression is regulated in epithelial cancer cells is largely unknown. We used various stimuli such as hypoxia and low pH to stress CRC cells and examined VEGFR1 mRNA expression in the cells using reverse transcription (RT)-polymerase chain reaction (PCR) followed by PCR amplicon cloning and DNA sequencing. To our surprise, some of the PCR clones were 94% identical to the VEGFR1 cDNA sequence but matched 100% to a sequence of the noncoding gene LOC391533 (GenBank accession number NG_005897), which has been officially annotated recently as Homo sapiens FLT1P1 on chromosome 3 (Fig 1A). In a literature search, we found that this VEGFR1 pseudogene was previously identified via transcriptional mapping of chromosome 3p21.3, a frequently altered locus in human solid tumors (31). Note that human FLT1/VEGFR1 gene is located at chromosome 13q12.
Figure 1. Expression of FLT1P1 in human CRC cell lines.
A In the human genome, FLT1/VEGFR1 is located at chromosome 13q12, whereas FLT1P1 resides at chromosome 3p21.3. FLT1P1 is an intron-free processed pseudogene. It is homologous to the VEGFR1 cDNA from exon 20 to 30.
B Phylogenetic analysis of FLT1P1 showing that human FLT1P1 is closely related to chimpanzee orthologs.
C Three CRC cell lines (HCP1, SW480, and DLD1) expressed FLT1P1 ncRNA at variable basal levels according to a custom TaqMan qPCR assay.
D HCP1, SW480, and DLD1 cells had medial to high levels of VEGFR1 mRNA expression according to a TaqMan qPCR assay.
On the basis of sequence comparison between FLT1P1 and its cognate gene VEGFR1, we found that FLT1P1 is a processed pseudogene, as the majority of FLT1P1 sequence aligns with the VEGFR1 exon sequence from exon 20 to 30. However, the VEGFR1-like open reading frame contains four additional stop codons, suggesting that the FLT1P1 transcript is a ncRNA. In addition, FLT1P1 has a 5′ leader sequence that is homologous to a segment of FLT1 intron 19, and its 3′ tail sequence contains an insert of a 1.1-kb LINE/L1 repetitive element, which is a characteristic retrotransposition sequence. Moreover, we identified seven FLT1P1 orthologous sequences in the genomes of high primates but none in other mammals. Phylogenetic analysis demonstrated that the closest counterparts to Homo sapiens FLT1P1 are those found in chimpanzees (Pan troglodyes and Pan paniscus) (Fig 1B).
To specifically detect and quantify FLT1P1 transcripts in CRC cells, we established a custom TaqMan quantitative PCR (qPCR) assay containing a dual-labeled probe hybridized to a unique sequence of FLT1P1. Using it with a preformulated VEGFR1-specific TaqMan qPCR assay (Applied Biosystems, Foster City, CA), we examined FLT1P1 and VEGFR1 expression in three human CRC cell lines (SW480, DLD1, and HCP1), in which HCP1 is a low-passage cell line recently established from a patient-derived xenograft of human colorectal tumor (28). All of the cell lines expressed the FLT1P1 ncRNA, with HCP1 having the highest level of expression (Fig 1C). These cell lines also expressed VEGFR1 mRNA (Fig 1D). In addition to the cell lines, we detected FLT1P1 and VEGFR1 expression in freshly procured human colorectal tumor specimens as well as normal colon mucosa specimens (Supplementary Fig S1).
FLT1P1 produces bidirectional transcripts that regulate VEGFR1 protein expression
Having established that FLT1P1 is expressed in CRC cells, we sought to determine the features of FLT1P1 transcription and regulation in these cells. We found that CRC cells and colorectal tumors had expression of both “sense” (FLT1P1-s) and “antisense” (FLT1P1-as) noncoding transcripts of FLT1P1 (Fig 2A and Supplementary Fig S2), suggesting that the transcripts were produced in a bidirectional transcription mode. This is similar to the PTENP1 pseudogene, which produces bidirectional transcripts, including two antisense isoforms (27).
Figure 2. Bidirectional transcription of FLT1P1 produces the sense and antisense transcripts that counterbalance VEGFR1 protein expression in HCP1 cells.
A Expression of FLT1P1-s (S) and FLT1P1-as (AS) transcripts in HCP1 cells detected using strand-specific cDNA synthesis and a custom TaqMan qPCR assay. The expression was normalized according to that of the FLT1P1-s transcript.
B Putative FLT1P1 promoters identified in analyses of the FLT1P1 genomic locus and human genome ChIP-seq database in the Mammalian Promoter Database. The histograms show the binding activities of four transcription factors (NF-κB, E2F1, ZNF263, and peroxisome proliferator-activated receptor-γ [PPARG]) at the putative promoters and enhancers of FLT1P1. The schematic depicts bidirectional transcription of FLT1P1 at the genomic locus.
C Overexpression of the FLT1P1-s transcript from a cDNA clone increased VEGFR1 mRNA expression according to TaqMan qPCR analysis and VEGFR1 protein expression according to Western blotting using a C-terminus specific antibody to detect the full-length VEGFR1 protein.
D Overexpression of the FLT1P1-as transcript increased VEGFR1 mRNA expression according to TaqMan qPCR analysis, but decreased VEGFR1 protein expression according to the Western blotting.
We then analyzed features of FLT1P1 genomic locus in chromatin immunoprecipitation-sequencing (ChIP-seq) data on the human genome in the Mammalian Promoter Database (http://mpromdb.wistar.upenn.edu) (32), in which we identified two putative FLT1P1 promoters residing between chr3:46,151,596 and chr3:46,161,765 (NCBI36/hg18). The forward proximal promoter is located at chr3:46,160,227-46,160,565, and the reverse proximal promoter is located at chr3:46,158,503-46,158,838. The ChIP-Seq data demonstrated the presence of binding sites for at least four transcription factors — nuclear factor (NF)-κB, E2F1, ZNF263, and peroxisome proliferator-activated receptor-γ — at the loci of putative promoters and enhancers of FLT1P1 (Fig 2B). This finding warrants further investigation of the transcription factors and upstream signaling in the regulation of FLT1P1 expression in CRC cells.
To determine the function of FLT1P1 in regulating VEGFR1 expression, we introduced FLT1P1-s and FLT1P1-as expression constructs into CRC cells via recombinant lentiviral transduction. The FLT1P1-s cDNA sequence corresponds to chr3:46,157,766-46,160,173 reverse strand and the FLT1P1-as cDNA sequence corresponds to chr3:46,158,838-46,160,307 in the human NCBI36/hg18 assembly. Overexpression of FLT1P1-s transcripts markedly enhanced full-length VEGFR1 protein expression in HCP1 cells as determined using Western blotting with a VEGFR1 C-terminus–specific antibody (Fig 2C). Also, VEGFR1 mRNA expression increased in HCP1 cells as measured using a TaqMan qPCR assay. These observations suggested that the FLT1P1-s transcript is a positive regulator of VEGFR1 expression, likely via pseudogene's ceRNA functional mechanism (33). In contrast with the function of FLT1P1-s, overexpression of the FLT1P1-as transcript in HCP1 cells had an inhibitory effect on VEGFR1 protein expression as assessed using Western blotting (Fig 2D). Interestingly, FLT1P1-as overexpression increased VEGFR1 mRNA expression, likely by disrupting VEGFR1 mRNA translation in the cells.
Perturbation of FLT1P1 expression inhibits VEGFR1 protein expression
We further investigated FLT1P1's function in CRC cells using RNA interference (RNAi). On the basis of the identified discrepant sequences in the FLT1P1 transcripts and VEGFR1 mRNA, we designed FLT1P1-specific siRNAs and used them to knock down the expression of endogenous FLT1P1 transcripts in CRC cells. We first validated siFLT1P1-1 and siFLT1P1-2 via transient transfection and assessment of FLT1P1 transcripts by semi-quantitative RT-PCR, which confirmed that these siRNAs were able to reduce FLT1P1 transcript in the transfected SW480 cells (Supplementary Fig S3A). In order to achieve a sustainable knockdown of FLT1P1 expression in CRC cells, we then used recombinant lentivirus transduction to express shFLT1P1-1 or a lentivector control (shControl) in CRC cells. Changes of the FLT1P1 and VEGFR1 expression in the CRC cells were determined by TaqMan qPCR analysis. Interestingly, shFLT1P1-1 knocked down expression of the FLT1P1-s transcript by 30-50% but induced an approximately two-fold increase in expression of the FLT1P1-as transcript in the shFLT1P1-1-expressing HCP1 cells (Fig 3A). Similarly, shFLT1P1-1 was also able to induce FLT1P1-as expression in DLD1 cells (Supplementary Fig S3B). In order to confirm the RNAi effect with additional lentiviral shRNAs in CRC cells, we generated shFLT1P1-1R and shFLT1P1-3. ShFLT1P1-1R contains a reverse complimentary sequence of shFLT1P1-1 and serves as a control for shFLT1P1-1. Results showed that shFLT1P1-1R did not affect the FLT1P1-as expression in SW480 and HCP1 cells (Supplementary Fig S3C,D). In contrast, shFLT1P1-3, designed to target an upstream site in the pseudogene, markedly induced the FLT1P1-as expression in HCP1 cells (Supplementary Fig S4), which was similar to the shFLT1P1-1 effect on FLT1P1 bidirectional transcription in CRC cells. These observations suggest the existence of a regulatory mechanism where FLT1P1-s transcript represses the opposite strand transcription, while inhibition of the FLT1P1-s transcription de-represses FLT1P1-as transcription in CRC cells.
Figure 3. Effect of endogenous FLT1P1 transcripts and miR-520a on VEGFR1 expression in HCP1 cells.
A ShFLT1P1-1 expression in HCP1 cells downregulated FLT1P1-s (S) but upregulated FLT1P1-as (AS) expression. Gene-specific primers mediated first strand cDNA synthesis and a FLT1P1-specific TaqMan qPCR assay was used for quantification of FLT1P1 transcripts. VEGFR1 mRNA expression increased in shFLT1P1-1-expressing HCP1 cells as quantified by TaqMan qPCR assay. Reduction of VEGFR1 protein expression in shFLT1P1-1-expressing HCP1 cells was detected by Western blotting.
B Alignments of miR-520a and the microRNA recognizing elements in the VEGFR1 3’UTR and in the FLT1P1 transcripts.
C The miR-520a basal expression was higher in HCP1 and DLD1 cells than that in SW480 cells, as quantified by TaqMan qPCR analysis.
D The miR-520a expression was increased in shFLT1P1-1-expressing HCP1 cells.
E Treatment of HCP1 cells with synthetic miR-520a by transient transfection resulted in decrease of FLT1P1 ncRNA expression but increase of the VEGFR1 mRNA level in the miR-520a treated cells.
Moreover, the RNAi by shFLT1P1-1 or shFLT1P1-3 in HCP1 cells affected cognate VEGFR1 expression, in which the VEGFR1 mRNA levels were markedly increased (Fig 3A; Supplementary Fig S4). However, the same treatment led to decreased VEGFR1 protein levels (Fig 3A). They were very similar to those responses induced by FLT1P1-as overexpression in HCP1 cells (Fig 2D), suggesting that up-regulation of endogenous FLT1P1-as expression can inhibit the VEGFR1 protein expression in CRC cells.
In order to further validate the FLT1P1-as function, we attempted to make FLT1P1-as specific knockdown but could not achieve it by any single shRNA. However, we observed the FLT1P1-as knockdown in the HCP1 cells expressing both shFLT1P1-1 and shFLP1-2 (Supplementary Fig 5A). The dual shRNA expressing cells showed a marked decrease of FLT1P1-as transcript, as well as a decrease of FLT1P1-s transcript. Total FLT1P1 expression decrease had an inhibitory effect on VEGFR1 expression (Supplementary Fig 5B,C).
miR-520a mediates the FLT1P1-as function in VEGFR1 and VEGF-A expression
We then sought to understand the mechanism and the factors facilitating FLT1P1-as function in VEGFR1 expression. There is a possibility that FLT1P1 transcripts interact with microRNAs to regulate VEGFR1 expression. To determine if this is true, we analyzed virtual microRNA target sites in the FLT1P1 transcript sequences and identified miR-520a as one of the most likely microRNAs interacting to VEGFR1 3’UTR and FLT1P1 transcripts (Fig 3B). We confirmed miR-520a expression in HCP1 and other CRC cells by TaqMan qPCR analysis (Fig 3C). Strikingly, miR-520a levels were increased in shFLT1P1-1 expressing HCP1 cells (Fig 3D). Moreover, the introduction of a synthetic miR-520a into HCP1 parental cells by transient transfection, inhibited FLT1P1 expression but enhanced VEGFR1 mRNA expression (Fig 3E). This suggests that miR-520a modulates the FLT1P1 and VEGFR1 expression in CRC cells.
Interestingly, miR-520a was also predicted to interact with VEGF-A 3’UTR (Fig 4A). Its family members, miR-520g-3p and miR-520h, have been confirmed experimentally by others to inhibit VEGF-A expression (34). We found that expression of miR-520a was regulated by FLT1P1-as in HCP1 cells (Fig 4B). In the shFLT1P1-1-expressing HCP1 cells, which had increased FLT1P1-as (Fig 3A) and miR-520a (Fig 3D), both the VEGF-A mRNA and secreted VEGF-A protein were decreased (Fig 4C,D). Similarly, overexpression of FLT1P1-as or introduction of miR-520a by transient transfection inhibited the VEGF-A expression in HCP1 cells (Fig 4E,F). However, FLT1P1-as had a stronger effect on VEGF-A expression than the synthetic miR-520a, suggesting FLT1P1-as also likely induced other microRNAs to co-inhibit the VEGF-A expression in CRC cells.
Figure 4. Effect of FLT1P1 antisense transcript and miR-520a on VEGF gene expression in HCP1 cells.
A Alignments of miR-520a and the microRNA recognizing elements in the VEGFA 3’UTR and in the FLT1P1-as transcript.
B Overexpression of FLT1P1-as led to a markedly increase of miR-520a expression in HCP1 cells.
C VEGF-A mRNA expression was decreased in shFLT1P1-1-expressing HCP1 cells. TaqMan qPCR assay was used for quantification of VEGF-A mRNA.
D VEGF-A protein was decreased in shFLT1P1-1-expressing HCP1 cells. Quantification of human VEGF-A protein concentration in the conditioned medium from transduced cells was done by ELISA.
E Overexpression of FLT1P1-as in HCP1 cells strongly inhibited the VEGF-A mRNA expression.
F Treatment of HCP1 cells with synthetic miR-520a by transient transfection inhibited the VEGF-A mRNA expression in the cells.
Sustained RNAi of FLT1P1 inhibits tumor cell growth kinetics
To assess the cellular effect of FLT1P1, we followed the RNAi study with a cell proliferation assay to compare the growth curves of the HCP1 cells. We found that the shFLT1P1-1 expressing HCP1 cells had a markedly lower proliferation rate than did the control cells (Supplementary Fig 6A). Compared to the VEGFR1 knocked-down HCP1 cells (Supplementary Fig 6B), the shFLT1P1-1 expressing cells showed a stronger inhibition of cell proliferation suggesting that shFLT1P1 effect on cell growth is partly due to the decrease of VEGFR1 protein expression; additional effect may be due the decrease of VEGF-A expression through miR-520a and other microRNA regulation.
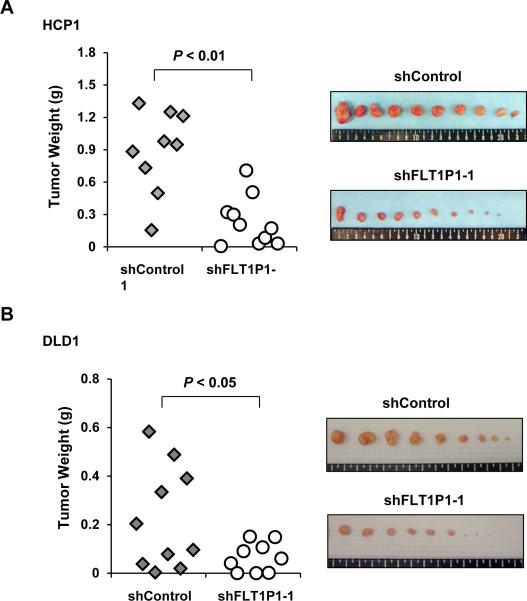
We then sought to confirm the role of FLT1P1 in vivo using a subcutaneous xenograft tumor model in athymic nude mice. The HCP1 cells stably expressing shFLT1P1 or shControl were used for xenograft assays. We found that the mice with the shFLT1P1-1-expressing cells had significantly smaller tumors than did the control group (mean ± standard error of the mean: tumor mass, 0.23 ± 0.07 g versus 0.89 ± 0.13 g [P<0.01]; tumor volume, 195 ± 53 mm3 versus 1149 ± 323 mm3 [P<0.05]) (Fig 5A). To confirm this observation, we conducted the FLT1P1 RNAi experiment with a second CRC cell line, DLD1. We found again a marked inhibitory effect of shFLT1P1-1 on the growth of tumors generated by DLD1 cells (Fig 5B). These findings clearly demonstrated that FLT1P1 plays an important role in the tumor growth kinetics.
Figure 5. Inhibition of xenograft colorectal tumor growth by FLT1P1 RNAi in HCP1 cells.
A RNAi of FLT1P1 expression in HCP1 by shFLT1P1-1 resulted in inhibition of xenograft tumor growth. Images of tumor masses are shown at right.
B RNAi of FLT1P1 expression similarly inhibited xenograft DLD1 tumor growth. Images of dissected tumors are shown at right.
DISCUSSION
Pseudogenes have long been considered as genetic fossils in the genome. Some arose from retrotransposition of protein-coding gene transcripts that had been reversely transcribed back to DNA and inserted into chromosome. These “processed pseudogenes” contain neither an original intron nor a promoter. They have accumulated deleterious mutations and new stop codons to become noncoding DNAs. However, recent studies by genome-wide transcriptome analysis revealed that many pseudogenes are still actively transcribed via neighboring regulatory elements in human genome, suggesting that expression of pseudogenes may have an evolutionary selective advantage in certain human cells. In this study we demonstrated that FLT1P1 is one of live pseudogenes expressed in CRC cells and colorectal tumor tissues. Alteration of the FLT1P1 expression can affect cognate and non-cognate gene expression and subsequently affect the tumor cell growth kinetics. In addition to malignant cells, we also observed the FLT1P1 expression in normal human endothelial cells (data not shown), suggesting that this pseudogene may play a physiological role in human vascular system.
We showed that the FLT1P1 sequence is highly homologous to a segment sequence of VEGFR1 intracellular kinase domain coding region and 3’ untranslated region (3’UTR). The nucleotide sequences of FLT1P1 and VEGFR1 are approximately 94% identical in the region corresponding to VEGFR1 exon 20 to exon 30, which could cause RT-PCR cross-reactions if the primers or probes are not designed properly. In fact, some of commercially available VEGFR1 primers and probes are predesigned within that FLT1P1 – VEGFR1 overlapping region, which are likely to produce false results. The RT-PCR cross-reactions had been reported for other pseudogenes (35, 36). Therefore, it is important to distinguish the pseudogene transcripts from their cognate gene mRNAs in a given biological system.
We found that an important feature of FLT1P1 expression is bi-directional transcription. It produces both FLT1P1-s and FLT1P1-as transcripts that complicate it's transcriptional regulation and their effect on VEGFR1 protein expression. Our data showed that the strand- biased RNAi targeting the FLT1P1-s transcript to cause up-regulation of the FLT1P1-as transcription in CRC cells. We also observed a similar effect on the FLT1P1 bidirectional transcription by hypoxic stress (data not shown). These observations suggest that FLT1P1 bidirectional transcription is governed by a regulatory mechanism similar to that regulating cis-natural antisense transcript (37). This mechanism may limit the FLT1P1-as basal expression in the cells.
Our study further demonstrated that FLT1P1-s and FLT1P1-as transcripts possess an opposite regulatory function on VEGFR1 expression in CRC cells. Although the precise mechanism of a pseudogene's regulation of the cognate gene expression is still not clear, one of the proposed mechanisms is via the function of pseudogene-derived ceRNAs (33), which act as mRNA decoys to compete for microRNA binding, thus exerting a positive effect on the mRNA translation (33). A pseudogene's negative effect on the cognate gene expression probably manifests via antisense-mediated translational inhibition. There are also other proposed mechanisms such as pseudogene antisense-mediated repression on cognate gene promoters (27) or endogenous siRNA formation in cells (22, 23). Therefore, we believe that expression of both FLT1P1-s and FLT1P1-as transcripts is likely a counterbalancing mechanism to regulate VEGFR1 protein expression in the cells.
Although the FLT1P1 regulatory function is primarily on cognate gene, the FLT1P1 ncRNAs, particularly the FLT1P1-as transcript, may also regulate non-cognate gene expression in the cells. In this study, we found that the sustained induction of FLT1P1-as expression in HCP1 cells not only reduced the VEGFR1 protein expression but also markedly decreased VEGF-A expression. Note that VEGF-A does not have sequence homology to FLT1P1 transcripts. Our data showed that FLT1P1-as regulates miR-520a expression, which then inhibits the VEGF-A expression in CRC cells. More importantly, down-regulation of both the VEGF receptor and ligand by the FLT1P1-as and miR-520a contribute to cell growth inhibition in shFLT1P1-1 expressing cells. However, the complexity of the molecular effect of the FLT1P1 on CRC growth requires further investigation.
Collectively, our results demonstrated that the pseudogene FLT1P1 shares molecular ancestry with the cognate gene VEGFR1 in humans and high primates. In human CRC cells, FLT1P1 is transcribed bidirectionally to produce both FLT1P1-s and FLT1P1-as transcripts, which play counterbalancing roles in the regulation of VEGFR1 protein expression. FLT1P1-as also regulates miR-520a expression that inhibits non-cognate VEGF-A expression in CRC cells. Therefore, the sustained induction of FLT1P1-as expression inhibits CRC cell proliferation and xenograft colorectal tumor growth. Our findings will be useful for understanding of the functional mechanisms involved in pseudogene transcription and regulation of cognate and non-cognate gene expression in the cells. Also, this study provides a novel pseudogene for future studies to determine the role of pseudogene in malignant diseases.
Supplementary Material
Acknowledgements
This work was supported in part by National Institutes of Health (NIH) Cancer Center Support Grant CA016672, NIH grant R01CA157880 (LME), Department of Defense grant CA100879 (LME), the William C. Liedtke, Jr., Chair in Cancer Research (LME), NIH grant T32CA009599 (ES), and Texas Medical Center - Digestive Disease Center pilot award (XY). The authors thank Li Huang and Dr. Menashe Bar-Eli from the Department of Cancer Biology at MD Anderson for preparation of the recombinant lentivirus. The authors also thank Jia Lu, Dr. Yunfei Zhou, Dr. Eric Sceusi, Dr. Federico Tozzi, and Harish Adoni from the Department of Surgical Oncology at MD Anderson, Dr. Leng Han and Dr. Han Liang from the Department of Bioinformatics and Computational Biology at MD Anderson for research assistance and discussion. The authors thank Donald R. Norwood from the MD Anderson Department of Scientific Publications and Rita Hernandez from the MD Anderson Department of Surgical Oncology for editorial assistance.
Footnotes
Conflict of interest
The authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nature reviews Cancer. 2008;8:579–91. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- 2.Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:10705–9. doi: 10.1073/pnas.90.22.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- 4.Hiratsuka S, Minowa O, Kuno J, Noda T, Shibuya M. Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:9349–54. doi: 10.1073/pnas.95.16.9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–7. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hiratsuka S, Nakamura K, Iwai S, Murakami M, Itoh T, Kijima H, et al. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer cell. 2002;2:289–300. doi: 10.1016/s1535-6108(02)00153-8. [DOI] [PubMed] [Google Scholar]
- 7.Wu Y, Hooper AT, Zhong Z, Witte L, Bohlen P, Rafii S, et al. The vascular endothelial growth factor receptor (VEGFR-1) supports growth and survival of human breast carcinoma. International journal of cancer Journal international du cancer. 2006;119:1519–29. doi: 10.1002/ijc.21865. [DOI] [PubMed] [Google Scholar]
- 8.Wey JS, Fan F, Gray MJ, Bauer TW, McCarty MF, Somcio R, et al. Vascular endothelial growth factor receptor-1 promotes migration and invasion in pancreatic carcinoma cell lines. Cancer. 2005;104:427–38. doi: 10.1002/cncr.21145. [DOI] [PubMed] [Google Scholar]
- 9.Fan F, Wey JS, McCarty MF, Belcheva A, Liu W, Bauer TW, et al. Expression and function of vascular endothelial growth factor receptor-1 on human colorectal cancer cells. Oncogene. 2005;24:2647–53. doi: 10.1038/sj.onc.1208246. [DOI] [PubMed] [Google Scholar]
- 10.Frank NY, Schatton T, Kim S, Zhan Q, Wilson BJ, Ma J, et al. VEGFR-1 expressed by malignant melanoma-initiating cells is required for tumor growth. Cancer research. 2011;71:1474–85. doi: 10.1158/0008-5472.CAN-10-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lesslie DP, Summy JM, Parikh NU, Fan F, Trevino JG, Sawyer TK, et al. Vascular endothelial growth factor receptor-1 mediates migration of human colorectal carcinoma cells by activation of Src family kinases. British journal of cancer. 2006;94:1710–7. doi: 10.1038/sj.bjc.6603143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor AP, Leon E, Goldenberg DM. Placental growth factor (PlGF) enhances breast cancer cell motility by mobilising ERK1/2 phosphorylation and cytoskeletal rearrangement. British journal of cancer. 2010;103:82–9. doi: 10.1038/sj.bjc.6605746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang AD, Camp ER, Fan F, Shen L, Gray MJ, Liu W, et al. Vascular endothelial growth factor receptor-1 activation mediates epithelial to mesenchymal transition in human pancreatic carcinoma cells. Cancer research. 2006;66:46–51. doi: 10.1158/0008-5472.CAN-05-3086. [DOI] [PubMed] [Google Scholar]
- 14.Vanin EF. Processed pseudogenes: characteristics and evolution. Annual review of genetics. 1985;19:253–72. doi: 10.1146/annurev.ge.19.120185.001345. [DOI] [PubMed] [Google Scholar]
- 15.Jacq C, Miller JR, Brownlee GG. A pseudogene structure in 5S DNA of Xenopus laevis. Cell. 1977;12:109–20. doi: 10.1016/0092-8674(77)90189-1. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Z, Gerstein M. Large-scale analysis of pseudogenes in the human genome. Current opinion in genetics & development. 2004;14:328–35. doi: 10.1016/j.gde.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Pei B, Sisu C, Frankish A, Howald C, Habegger L, Mu XJ, et al. The GENCODE pseudogene resource. Genome biology. 2012;13:R51. doi: 10.1186/gb-2012-13-9-r51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalyana-Sundaram S, Kumar-Sinha C, Shankar S, Robinson DR, Wu YM, Cao X, et al. Expressed pseudogenes in the transcriptional landscape of human cancers. Cell. 2012;149:1622–34. doi: 10.1016/j.cell.2012.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muro EM, Mah N, Andrade-Navarro MA. Functional evidence of post-transcriptional regulation by pseudogenes. Biochimie. 2011;93:1916–21. doi: 10.1016/j.biochi.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 20.Korneev SA, Park JH, O'Shea M. Neuronal expression of neural nitric oxide synthase (nNOS) protein is suppressed by an antisense RNA transcribed from an NOS pseudogene. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:7711–20. doi: 10.1523/JNEUROSCI.19-18-07711.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou BS, Beidler DR, Cheng YC. Identification of antisense RNA transcripts from a human DNA topoisomerase I pseudogene. Cancer research. 1992;52:4280–5. [PubMed] [Google Scholar]
- 22.Tam OH, Aravin AA, Stein P, Girard A, Murchison EP, Cheloufi S, et al. Pseudogene-derived small interfering RNAs regulate gene expression in mouse oocytes. Nature. 2008;453:534–8. doi: 10.1038/nature06904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan WL, Yuo CY, Yang WK, Hung SY, Chang YS, Chiu CC, et al. Transcribed pseudogene psiPPM1K generates endogenous siRNA to suppress oncogenic cell growth in hepatocellular carcinoma. Nucleic acids research. 2013;41:3734–47. doi: 10.1093/nar/gkt047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poliseno L, Salmena L, Zhang J, Carver B, Haveman WJ, Pandolfi PP. A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature. 2010;465:1033–8. doi: 10.1038/nature09144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marques AC, Tan J, Lee S, Kong L, Heger A, Ponting CP. Evidence for conserved post-transcriptional roles of unitary pseudogenes and for frequent bifunctionality of mRNAs. Genome biology. 2012;13:R102. doi: 10.1186/gb-2012-13-11-r102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han L, Yuan Y, Zheng S, Yang Y, Li J, Edgerton ME, et al. The Pan-Cancer analysis of pseudogene expression reveals biologically and clinically relevant tumour subtypes. Nature communications. 2014;5:3963. doi: 10.1038/ncomms4963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnsson P, Ackley A, Vidarsdottir L, Lui WO, Corcoran M, Grander D, et al. A pseudogene long-noncoding-RNA network regulates PTEN transcription and translation in human cells. Nature structural & molecular biology. 2013;20:440–6. doi: 10.1038/nsmb.2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu J, Ye X, Fan F, Xia L, Bhattacharya R, Bellister S, et al. Endothelial cells promote the colorectal cancer stem cell phenotype through a soluble form of Jagged-1. Cancer cell. 2013;23:171–85. doi: 10.1016/j.ccr.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fan F, Samuel S, Gaur P, Lu J, Dallas NA, Xia L, et al. Chronic exposure of colorectal cancer cells to bevacizumab promotes compensatory pathways that mediate tumour cell migration. British journal of cancer. 2011;104:1270–7. doi: 10.1038/bjc.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerber HP, Condorelli F, Park J, Ferrara N. Differential transcriptional regulation of the two vascular endothelial growth factor receptor genes. Flt-1, but not Flk-1/KDR, is up-regulated by hypoxia. The Journal of biological chemistry. 1997;272:23659–67. doi: 10.1074/jbc.272.38.23659. [DOI] [PubMed] [Google Scholar]
- 31.Kiss H, Yang Y, Kiss C, Andersson K, Klein G, Imreh S, et al. The transcriptional map of the common eliminated region 1 (C3CER1) in 3p21.3. European journal of human genetics : EJHG. 2002;10:52–61. doi: 10.1038/sj.ejhg.5200758. [DOI] [PubMed] [Google Scholar]
- 32.Gupta R, Bhattacharyya A, Agosto-Perez FJ, Wickramasinghe P, Davuluri RV. MPromDb update 2010: an integrated resource for annotation and visualization of mammalian gene promoters and ChIP-seq experimental data. Nucleic acids research. 2011;39:D92–7. doi: 10.1093/nar/gkq1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell. 2011;146:353–8. doi: 10.1016/j.cell.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hua Z, Lv Q, Ye W, Wong CK, Cai G, Gu D, et al. MiRNA-directed regulation of VEGF and other angiogenic factors under hypoxia. PloS one. 2006;1:e116. doi: 10.1371/journal.pone.0000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Redshaw Z, Strain AJ. Human haematopoietic stem cells express Oct4 pseudogenes and lack the ability to initiate Oct4 promoter-driven gene expression. Journal of negative results in biomedicine. 2010;9:2. doi: 10.1186/1477-5751-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayashi H, Tazoe Y, Horino M, Fujimaki-Katoh C, Tsuboi S, Matsuyama T, et al. An artifact derived from a pseudogene led to the discovery of microRNA binding site polymorphism in the 3'-untranslated region of the human dihydrofolate reductase gene. Drug metabolism and pharmacokinetics. 2012;27:263–7. doi: 10.2133/dmpk.dmpk-11-nt-092. [DOI] [PubMed] [Google Scholar]
- 37.Osato N, Suzuki Y, Ikeo K, Gojobori T. Transcriptional interferences in cis natural antisense transcripts of humans and mice. Genetics. 2007;176:1299–306. doi: 10.1534/genetics.106.069484. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.