Abstract
Many of the biochemical, structural, and functional changes that occur as the female brain ages are influenced by changes in levels of estrogens. Administration of estrogens begun during a critical window near menopause is hypothesized to prevent or delay age-associated cognitive decline. However, due to potential health risks women often limit use of estrogen therapy to a few years to treat menopausal symptoms. The long-term consequences for the brain of short-term use of estrogens are unknown. Interestingly, there are preliminary data to suggest that short-term use of estrogens during the menopausal transition may afford long-term cognitive benefits to women as they age. Thus, there is the intriguing possibility that short-term estrogen therapy may provide lasting benefits to the brain and cognition. The focus of the current review is an examination of the long-term impact for cognition of midlife use of estrogens. We review data from our lab and others indicating that the ability of midlife estrogens to impact estrogen receptors in the hippocampus may contribute to its ability to exert lasting impacts on cognition in aging females.
Results of research conducted over the last two decades support a role for estrogens in the modulation of cognitive function (reviewed in Boulware et al., 2012;Luine, 2014;Bimonte-Nelson et al., 2010). Many, although not all, randomized clinical trials and observational studies have reported that postmenopausal estrogen therapy is associated with improved cognition if treatment is initiated within a critical period after loss of ovarian function (Sherwin, 2009). However the potential health risks associated with exposure to estrogens (Chen and Colditz, 2007;Chen et al., 2006; but see Harman et al., 2011) may preclude their long-term use. Therefore, current recommendations include limiting the use of hormone therapy to a few years to treat menopausal symptoms. It is currently unknown if estrogen use for a few years in midlife will reduce risk of dementia or improve cognitive aging later in life. The current report provides an overview of the literature describing the long-term impact for cognition of midlife estradiol use. We also describe our recent work investigating mechanisms by which short-term estradiol administration in midlife can exert long-term benefits for memory.
Estrogens and cognitive aging
Effects of estrogens on cognition in women
At menopause, circulating levels of estradiol, the main estrogen produced by the ovaries, drops to one-tenth of those during menstruating years (Rannevik et al., 2008). This dramatic change in hormonal state is proposed to have functional consequences for cognition (Sherwin, 1998), either directly or by interaction with other normal or pathological aging-related physiological alterations. In support of this hypothesis, many, though not all, randomized clinical trials and observational studies have reported a link between estrogen therapy initiated after naturally occurring or surgically-induced menopause in healthy women and improved cognition (reviewed in Sherwin, 2002). Findings of early randomized clinical trials that estrogen therapy positively influenced cognition suggested a possible protective role of estrogens against Alzheimer’s disease. Supporting evidence was provided by many (Fillit et al., 1986;Honjo et al., 1995;Kawas et al., 1997;Ohkura et al., 1994;Paganini-Hill and Henderson, 1996;Tang et al., 1996), but not all (Brenner et al., 1994;Mulnard et al., 2000) studies demonstrating that estrogen therapy was associated with reduced risk and severity, and delayed onset of Alzheimer’s disease.
In order to systematically and fully evaluate the efficacy of hormone therapy, the National Institutes of Health established the Women’s Health Initiative (WHI), a longitudinal study initiated in the 1990’s that was designed to assess the efficacy of hormone therapy on the incidence, prevalence, and severity of cardiovascular disease, cancer, and osteoporosis in postmenopausal women. The objective of the auxiliary Women’s Health Initiative Memory Study (WHIMS) was to determine the effect of postmenopausal hormone therapy on the development and progression of dementia and global cognitive function. Surprisingly, the results of the WHI and WHIMS indicated that hormone therapy regimens consisting of chronic conjugated equine estrogens (CEE) or CEE plus medroxyprogesterone as compared to placebo treatment, had no effect, or under certain conditions increased the risks of cardiovascular disease, breast cancer, stroke, dementia, and global cognitive decline (Chlebowski et al., 2003;Craig et al., 2005;Espeland et al., 2004;Rapp et al., 2003b;Rossouw et al., 2002;Shumaker et al., 2003;Shumaker et al., 2004;Wassertheil-Smoller et al., 2003). Scrutiny of the WHIMS design, population, specifics of hormone therapy regimen used, and tests of cognitive functioning has led to hypotheses that the failure of the WHIMS to demonstrate the predicted beneficial effects of hormone therapy may be explained by various confounding factors such as the advanced age and health problems of the participants, treatment specifics (agent, regimen, dose, and route of administration), and years of ovarian hormone deprivation the participants had already experienced (Harman et al., 2005).
Critical period hypothesis of effects of estrogens on cognition
In the WHIMS, the average age of the participants at the beginning of hormone treatment was 69 (Coker et al., 2009). Importantly, these women had been without ovarian hormones for nearly two decades. The “critical period” hypothesis of hormone therapy states that there is a crucial window following the onset of menopause during which hormone therapy must be initiated in order to have beneficial effects (Gibbs and Gabor, 2003;Resnick and Henderson, 2002). The brain may lose its responsivity to estrogens after a prolonged absence of the steroids and estrogen sensitivity may remain only with a timely onset of hormone therapy. Furthermore, once brain structures have been without estrogens for too long, hormone therapy may have detrimental effects (Brinton, 2005;Suzuki et al., 2007). Therefore, the negative outcomes seen in the WHIMS may be due to the timing of hormone therapy initiation. A recent review summarizes the clinical literature including observational studies and small randomized clinical trials examining the impact of early initiation of hormone therapy on cognitive outcomes and concludes that there exists initial support for the critical period hypothesis (Maki, 2013). Results of ongoing or recently completed randomized, placebo-controlled clinical trials should provide additional insights (Wharton et al., 2013;Hodis et al., 2014).
Experimental tests of the critical period hypothesis using animal models provide corroborating evidence. For instance, nonhuman primate studies have reported that estrogens administered 30 weeks, but not 10 years post ovariectomy improves performance on tests of working memory (Lacreuse et al., 2002;Rapp et al., 2003a). In rodent models, Gibbs (2000b) first showed that long-term hormone deprivation prevented the ability of later estradiol administration to enhance hippocampus-dependent memory in aging female rats. Middle-aged rats were ovariectomized and chronically treated with estradiol beginning either immediately, 3 months, or 10 months later. Only rats treated immediately or 3 months post-ovariectomy showed enhanced performance on a delayed matching-to-position maze task when tested in old age. Similarly, our lab demonstrated that the length of hormone deprivation impacts the ability of exogenous estradiol treatment to enhance hippocampus-dependent memory in aging female rats (Daniel et al., 2006). Rats ovariectomized at either 12 or 17 months of age and immediately implanted with estradiol capsules outperformed ovariectomized controls when tested at 17.5 months of age. However, rats ovariectomized at 12 months of age and implanted with estradiol capsules at 17 months did not show enhanced memory compared to controls. Therefore, both a short-term (2 weeks) and long-term (5 months) estradiol regimen was effective at enhancing memory when initiated immediately following ovariectomy, but estradiol treatment initiated 5 months post-ovariectomy was ineffective. Furthermore, a related study in our lab showed that this effect is not limited to hippocampus-dependent tasks (Bohacek and Daniel, 2010). Middle-aged rats that were immediately implanted with estradiol capsules following ovariectomy displayed enhanced performance on the 5-choice serial reaction time task, an attentional task dependent upon the prefrontal cortex, compared to ovariectomized control rats. However, estradiol treatment initiated 5 months post-ovariectomy did not enhance performance on the task compared to controls. Collectively, these results support the idea that there is a critical time window following the cessation of ovarian function during which estradiol administration must be initiated in order to exert positive effects on cognition.
Long-term effects on cognition of short-term estrogen use within the critical period
The critical period encompassing the menopausal transition, during which evidence suggests estrogen therapy must be initiated in order to benefit cognition, is reminiscent of the hormone-sensitive period in early development during which hormones act on the brain to exert permanent influences on adult behavioral responsiveness to hormones (Phoenix et al., 1959). Traditionally, effects of gonadal hormones on the brain and behavior have been categorized as organizational or activational. Organizational effects occur during early developmental periods, are long-lasting, and persist beyond the period of exposure to the hormone. Activational effects occur in the adult, are generally temporary, and are expressed only in the presence of the hormone. However, it has become apparent that effects of hormones do not always fit this conceptual dichotomy (Schulz et al., 2009). As early as several decades ago, it was proposed that there are multiple sensitive periods during which the nervous system could be permanently altered and that these sensitive periods most likely occur during times of rapid change (Scott et al., 1974). In support of this proposal are data indicating that ovarian hormones during the adolescent period exert lasting impact on adult behaviors including patterns of food protection (Field et al., 2004) and ingestive (Swithers et al., 2008) behaviors. Like the adolescent period, middle-age in females is characterized by significant changes in levels of ovarian hormones and thus may represent another sensitive period during which the nervous system could be permanently altered. Whereas current evidence supporting the idea of a critical period during midlife indicates that the absence of exogenously administered estrogens following menopause permanently alters the system, it may also be the case that the presence of exogenously administered estrogens could permanently alter the system. If so, this raises the possibility that hormone exposure during the menopausal transition could have consequences for the brain and behavior long after the duration of exposure.
There is a small literature available to suggest that short-term exposure to estrogens during the critical period after the loss of ovarian function may provide long-lasting cognitive benefits for women. For example, the risk for cognitive impairment in women who received 2–3 years of hormone therapy in the early menopausal years as part of a randomized, placebo-controlled study was decreased by 64% when they were examined 5–15 years after the completion of the hormone therapy (Bagger et al., 2005). In addition, risks of all-cause mortality, cardiovascular mortality, coronary heart disease, and osteorporotic fracture were also reduced in these women (Alexandersen et al., 2006;Bagger et al., 2004). Results of a large population-based study indicated that compared to never users, women using hormone therapy only in midlife had a 26% reduced risk of developing dementia later in life (Whitmer et al., 2011). In contrast, women using hormone therapy only in late life had a 48% increased risk of dementia and hormone therapy used in both mid and late life had no effect on dementia risk. Emerging evidence indicates that short-term use of estrogens following surgical menopause induced by bilateral oophorectomy before the age of natural menopause provides long-term neuroprotection (for review, see Rocca et al., 2014). For example, long-term risk of cognitive impairment was almost doubled in women who underwent oophorectomy before menopause, but was eliminated in women undergoing estrogen therapy until at least the age of 50 (Rocca et al., 2007). Additionally, the detrimental effects of early surgical menopause on cognition were attenuated by hormone therapy initiated within five years of surgery and lasting at least ten years (Bove et al., 2014). However, not all reports indicate long-term effects of midlife estrogen use on later cognitive function (Hogervorst and Bandelow, 2010). For example, an average of seven years of hormone therapy initiated when women were 50 – 55 years of age had no beneficial or detrimental effects as compared to placebo treatment on cognitive function assessed seven years after the cessation of the hormone therapy (Espeland et al., 2013) Although, further work needs to be done, collectively these data indicate at least the possibility that under some conditions estrogen therapy initiated during a critical window following natural or surgical menopause can provide long-term cognitive benefits that persist beyond the period of exposure.
In our lab, we sought to test experimentally the hypothesis that short-term exogenously administered estrogens in midlife can permanently organize the system to provide lasting benefits to hippocampus-dependent memory in aging female rats. We compared the ability of previous estradiol administration and ongoing continuous estradiol administration to impact spatial memory in a radial-arm maze (Rodgers et al., 2010). Middle-aged rats were ovariectomized and received control treatment, continuous estradiol treatment via Silastic implants, or 40 days of estradiol treatment that was terminated prior to behavior testing. Circulating estradiol levels produced by our capsules are between 26 – 47 pg/ml, a low to mid physiological range (Bohacek and Daniel, 2010). We chose 40 days for our short-term treatment paradigm because based on average life expectancy of 2.5 years for Long-Evans rats (Harlan Sprague-Dawley, Inc.) and of 80.1 years for women (Hamilton et al., 2007), a 40-day period of estradiol exposure in rats would roughly correspond to 3.5 years of estrogen therapy in women. Spatial memory was assessed every two months beginning one month after the termination of the 40-day estradiol period. As illustrated in Figure 1, previous exposure to estradiol was just as effective as ongoing use in enhancing performance. The effect of the previous estradiol exposure was maintained for up to seven months after the exposure had been terminated. Thus, these experimental data provide support for the hypothesis that short-term exposure to estrogens in midlife provides lasting cognitive benefits that persist well beyond the period of exposure. The neural mechanisms by which these cognitive benefits are mediated are under investigation. However, because of the growing evidence indicating the importance of estrogen receptors in female cognitive aging (Daniel, 2013;Foster, 2012), we explored a role for estrogen receptors in the ability of short-term estradiol exposure to exert long-term impact on memory.
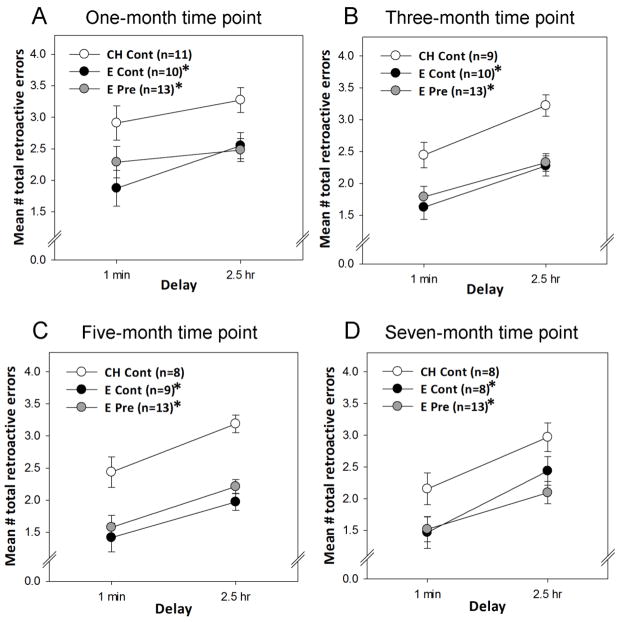
Figure 1. Impact of ongoing and previous exposure to estradiol on memory in aging female rats.
Long-Evans rats (retired breeders) were ovariectomized in middle age (at ~11 months of age) and received subcutaneous implants of capsules containing estradiol or cholesterol vehicle. Capsules remained in place for 40 days. After 40 days, half of the rats with estradiol implants received new estradiol implants resulting in continuous estradiol treatment previous to and throughout behavior testing (E Cont). The other half received cholesterol implants resulting in exposure to estradiol previous to behavior testing and control treatment throughout testing (E Pre). Rats that had cholesterol capsules received new cholesterol implants resulting in continuous cholesterol treatment previous to and throughout testing (Ch Cont). Rats then were tested every two months on delay trials in a radial-arm maze task in which delays of 1 minute or 2.5 hours were imposed between the 4th and 5th arm choices. Mean number of total retroactive errors (±SEM) during 1 min and 2.5 hr delay trials averaged over 4-trial blocks one (A), three (B), five (C), and seven (D) months after termination of estradiol treatment in the E Pre group. *p<.05 vs. CH Cont. (Rodgers et al., 2010)
The importance of estrogen receptors in brain and cognitive aging
Estrogens act at estrogen receptors to exert effects
Estrogens act at estrogen receptors (ER) through both traditional genomic mechanisms as well as via nontraditional rapid effects at the membrane. In the traditional model of estrogen action, estrogen binds to either ERα or ERβ in the nucleus, allowing it to dimerize and bind to a DNA estrogen response element (ERE) or interact with a transcription factor on target genes, thus initiating transcription of estrogen-sensitive genes and proteins, a process on the time scale of hours (Cowley et al., 1997;Gaub et al., 1990). ERα and ERβ share a high degree of homology; however they only overlap by about 60% in their sequence for the ligand-binding region (Tremblay et al., 1997). This difference in the ligand-binding region may explain the higher affinity that estradiol has for ERα over ERβ and the ability of ERα to more successfully induce transcription linked to the ERE.
Estrogens can also have rapid effects not dependent on traditional genomic mechanisms (Fernandez et al., 2008). In this non-classical mechanism, estrogens binds to membrane-bound receptors, including the G-protein coupled estrogen receptor (GPER), which can then activate second messenger systems, causing a rapid response varying from seconds to minutes (Prossnitz et al., 2008). Whereas nuclear ERα or ERβ activation result in a traditional genomic response (McEwen and Alves, 1999), it has been proposed that these receptors or a modified form of the proteins (Acconcia et al., 2005;Li et al., 2003) also contribute to the rapid effects of estradiol on synaptic plasticity (Ooishi et al., 2011). While the cellular and subcellular sites of ERα in the hippocampus have not been completely elucidated, it has been localized to both nuclear and extranuclear sites (Spencer et al., 2008), including in spines of hippocampal pyramidal and granule neurons (Mukai et al., 2007) as well as in axon terminals of cholinergic neurons (Towart et al., 2003). The genomic and non-genomic effects of estradiol are not mutually exclusive, as intracellular cascades activated by estrogens can phosphorylate nuclear estrogen receptors (Lannigan, 2003), allowing for translocation to the nucleus (Lee and Bai, 2002), dimerization (Chen et al., 1999) and transcriptional activity (Ali et al., 1993;Le et al., 1994). Evidence suggests a combination of both membrane-initiated and genomic actions occurring either in parallel or sequentially at estrogen receptors to affect transcription (Bjornstrom and Sjoberg, 2005;Vasudevan and Pfaff, 2008).
Estrogen receptors and the critical period hypothesis
There is increasing evidence that changes in levels or responsiveness of estrogen receptors, particularly ERα, may explain the existence of a “critical period” following the cessation of ovarian function during which estrogens must be administered to exert effects on the brain and cognition (Daniel, 2013). ERα, perhaps due to its increased ability to induce transcription linked to the ERE as compared to ERβ (Tremblay et al., 1997), is particularly important for maintaining hippocampal function under low estradiol levels (Foster, 2012). It has been suggested that a decrease in estradiol responsiveness in the hippocampus following the loss of ovarian function may be due to age-related decline in levels of ERα, disrupting the ratio of ERα relative to ERβ and interfering with the transcriptional processes important for cognition (Bean et al., 2014;Foster, 2012). Furthermore, results of work from our lab demonstrated that the same regimen of ongoing estradiol treatment that results in enhancement of hippocampus-dependent memory results in increased levels of ERα in the hippocampus. Specifically, estradiol administration initiated at the time of ovariectomy, but not after long-term ovarian hormone deprivation enhances spatial memory (Daniel et al., 2006) and increases levels of ERα in the hippocampus (Bohacek and Daniel, 2009). No impact on levels of ERβ was evident.
Interestingly, evidence for a mechanistic explanation for a critical period has emerged that involves the necessity of maintaining a sufficient pool of ERα in the hippocampus in order for the neuroprotective effects of estradiol to be maintained following cessation of ovarian function (Zhang et al., 2011). Ten weeks following ovariectomy, ERα in rat hippocampus displayed enhanced interaction with the E3 ubiquitin ligase C terminus of heat shock cognate protein 70 (Hsc70)-interacting protein (CHIP) that leads to its ubiquitination/proteasomal degradation. Estradiol treatment initiated at the time of ovariectomy, but not ten weeks following ovariectomy, prevented that enhanced ERα-CHIP interaction and the ERα ubiquitination/proteasomal degradation. Furthermore, estradiol treatment initiated at the time of ovariectomy, but not ten weeks following ovariectomy was fully neuroprotective against global cerebral ischemia. Thus, the critical period may exist because of permanent alterations in levels of ERα that occurs following long-term hormone deprivation.
Long-term impact of short-term estrogens on levels of estrogen receptor in the hippocampus
Because long-term estradiol deprivation during the critical period resulted in a permanent decrease in levels of hippocampal ERα (Bohacek and Daniel, 2009), we determined if there was also a long-term impact of the presence of estradiol during the critical period on levels of ERα in the hippocampus. We investigated if the same short-term exposure to estradiol that results in lasting enhancement of hippocampus-dependent memory (Figure 1) would also result in lasting increases in levels of ERα in the hippocampus (Rodgers et al., 2010). Continuous estradiol treatment as well as 40 days of estradiol exposure that was terminated ~three months prior to sacrifice significantly increased ERα protein levels in the hippocampus of ovariectomized rats as compared to ovariectomized controls as measured by western blotting (See Figure 2A). There were no effects of treatments on ERβ levels (data not shown). Results of a second experiment in which ovariectomized rats were sacrificed ~8 months after the 40-day prior estradiol treatment was terminated revealed continued elevation of ERα in the hippocampus as compared to control rats that were never exposed to estradiol (See Figure 2B). Interestingly, prior estradiol treatment also increased levels of choline acetyltransferase (ChAT), the synthesizing enzyme for acetylcholine, for up to three months following termination of exposure (Rodgers et al., 2010). Acetylcholine is a neurotransmitter implicated in the regulation of learning and memory (Everitt and Robbins, 1997) and the ability of the estradiol to impact the cholinergic system (Luine, 1985;Gabor et al., 2003;Gibbs, 2000a) is related to its ability to impact hippocampus dependent memory (Daniel and Dohanich, 2001). ERα-mediated regulation of ChAT occurs at the transcriptional level and the identification of a putative estrogen response element on the ChAT gene provides a potential site for direct modulation of ChAT by ERα (Hyder et al., 1999;Miller et al., 1999). Thus, a short-term period of 40 days of estradiol exposure can provide both lasting memory enhancements and lasting increases in levels of ERα and ERα-regulated proteins in the hippocampus, long after the estradiol exposure is terminated. A causal relationship between the ability of previous estradiol to impact ERα levels and impact memory is suggested by mounting evidence indicating that increases in the expression of ERα in the hippocampus may preserve cognitive function in the aging brain in the absence of ovarian estrogens (Han et al., 2013;Witty et al., 2012).
Figure 2. Impact of ongoing and previous exposure to estradiol on levels of ERα protein in the hippocampus of aging female rats as measured by western blotting.
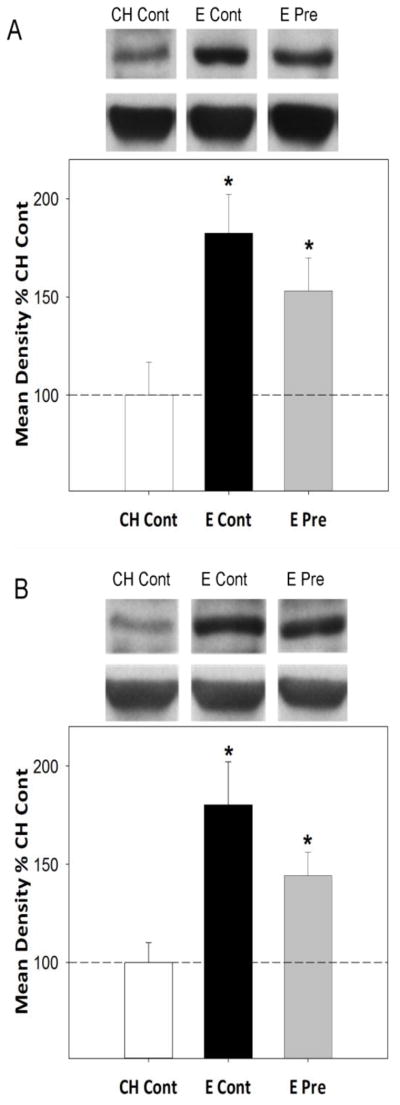
Long-Evans rats (retired breeders; n = 8 – 13 per group) were ovariectomized in middle age (at ~11 months of age) and received subcutaneous implants of capsules containing estradiol or cholesterol vehicle. Capsules remained in place for 40 days. After 40 days, half of the rats with estradiol implants received new estradiol implants resulting in continuous estradiol until sacrifice (E Cont). The other half received cholesterol implants resulting in 40 days of previous exposure to midlife estradiol followed by control treatment until sacrifice (E Pre). Rats that had cholesterol capsules received new cholesterol implants resulting in continuous cholesterol control treatment unitl sacrifice (Ch Cont). Western blot data are from brains collected from behaviorally tested rats sacrificed three months (A) and eight months (B) after termination of estradiol treatment in the E Pre group. Mean optical density (+SEM) expressed relative to CH Cont. *p<.05 vs. CH Cont. Representative blot images for ERα and the loading control, β-actin are shown in inserts above respective graphs.(Rodgers et al., 2010)
Estrogen receptors impact cognition in the absence of ovarian or exogenously administered estrogens
The importance of ERα to cognitive aging is highlighted by results of studies examining the relationship between levels of wild-type and polymorphisms of ERα with development of cognitive impairment in women. For example, a significant positive relationship was found between scores on the Mini-Mental State Examination (MMSE) and levels of wild-type ERα in the prefrontal cortex of Alzheimer’s patients (Kelly et al., 2008). No relationship was apparent between MMSE scores and levels of ERβ. Furthermore, several (Brandi et al., 1999;Isoe-Wada et al., 1999;Mattila et al., 2000), but not all (Maruyama et al., 2000) studies report that levels of ERα gene polymorphisms are associated with an increased risk for Alzheimer’s disease. Finally, in non-demented community-dwelling women (Yaffe et al., 2002;Yaffe et al., 2009) and men (Yaffe et al., 2009), polymorphisms of ERα are associated with increased risk of age-related cognitive decline.
Foster et al (2008) used an ERα knockout (ERαKO) mouse model to directly test the importance of ERα on hippocampus-dependent spatial memory. Ovariectomized, young adult ERαKO mice exhibited learning and memory deficits compared to their ovariectomized wild-type littermates. Injection of a lentivirus delivering the gene expressing ERα to the hippocampus of ovariectomized ERαKO mice significantly improved cognitive performance. Importantly, the effects on cognition were apparent in the absence of exogenously administered estrogens, highlighting the importance of ERα to cognition under conditions of low circulating estrogens.
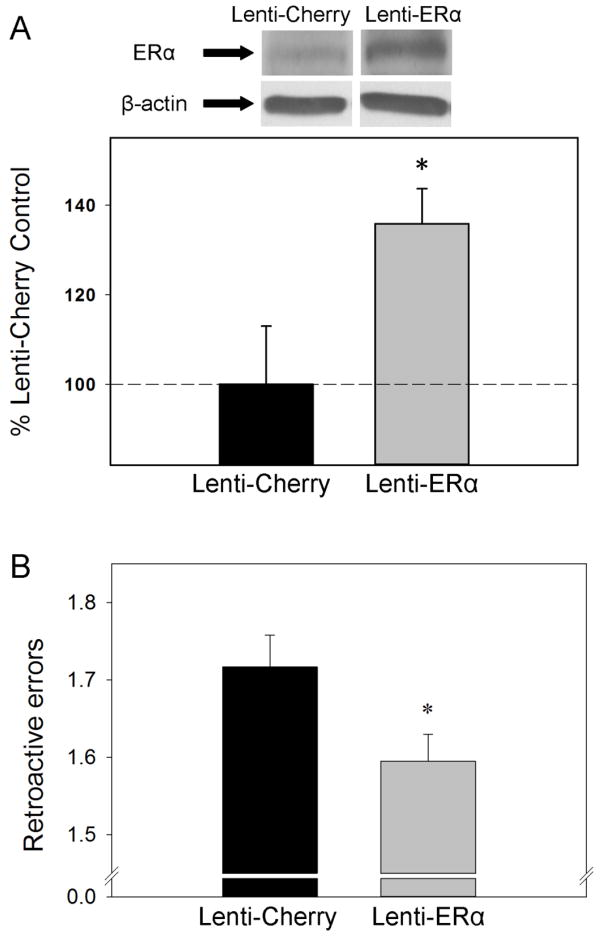
In our lab, we investigated if the level of ERα in the hippocampus of aging females would have an impact on memory in the absence of ovarian or exogenously administered estrogens (Witty et al., 2012). We delivered a lentivirus encoding the gene for ERα to the hippocampus of middle-aged rats that had been ovariectomized 40 days prior to the delivery. Our procedure resulted in an approximately 40% increase in levels of ERα protein as measured by western blotting (See Figure 3A). We tested rats on a hippocampal dependent radial-maze task in which rats have to remember the location of food rewards across varying time delays. As illustrated in Figure 3B, increasing ERα levels in the hippocampus resulted in improved memory in aging females in the absence of ovarian or exogenously administered estrogens. These data provide support for the hypothesis that treatments that increase or maintain levels of ERα in aging females help to improve or maintain memory processes even in the absence of circulating estrogens. Furthermore, they are consistent with the idea that the ability of short-term midlife estradiol treatment to provide lasting increases in levels of ERα in the hippocampus in aging females (Figure 2) underlies its ability to provide lasting memory enhancements (Figure 1).
Figure 3. Impact of increasing levels of ERα in the hippocampus on memory in ovariectomized aging female rats.
Long-Evans rats (retired breeders; n = 5 – 7 per group) were ovariectomized in middle age (at ~11 months of age). Forty days following ovariectomies, rats received intrahippocampal infusions of either a lentivirus with the gene encoding ERα (Lenti-ERα) or a control virus (Lenti-Cherry). Beginning three weeks following virus infusions, spatial memory performance was assessed in a radial-arm maze with various delays imposed between the 4th and 5th arm choices. (A) Protein expression of ERα in the hippocampus as measured by western blotting in brains collected following completion of behavior testing. Mean density × area (+SEM) expressed relative to Lenti-Cherry control. Representative blot images for ERα and the loading control β-actin are shown in insets above the graph. (B) Mean number of retroactive errors (+SEM) averaged across delays. * P < .05 vs. Lenti-Cherry. (Witty et al., 2012)
Mechanisms by which estrogen receptor can impact cognition in the absence of ovarian or exogenous estrogens
Ligand-independent mechanisms involving insulin-like growth factor-1
In addition to hormone-mediated estrogen receptor action, estrogen receptor function can be modulated by extracellular signals (Smith, 1998), which provides a mechanism by which estrogen receptor can impact target tissue under conditions of low levels of endogenous estrogens. These ligand-independent actions include the ability of growth factors, such as insulin-like growth factor-1 (IGF-I), to activate estrogen receptor and increase expression of estrogen receptor target genes. In addition to its endocrine role, IGF-1 is produced and acts locally in many tissues, including brain (LeRoith, 2008). IGF-1 exerts its effects by binding to IGF-1 receptor, which is a transmembrane protein with tyrosine kinase activity. Particularly relevant for memory is evidence of high level of colocalization of ERα and IGF-I receptors in CA1 of the hippocampus (Cardona-Gomez et al., 2000).
It has been demonstrated in a number of different cell lines, including neuroblastoma cells, that in the absence of estrogens, IGF-1 activates ERα and regulates ERα-mediated gene transcription (Agrati et al., 1997;Font de and Brown, 2000;Martin et al., 2000;Stoica et al., 2000;Mendez and Garcia-Segura, 2006;Klotz et al., 2002). The mechanism by which this occurs is unclear, but most likely involves modification of phosphorylation sites on ERα by cellular kinases (Hall et al., 2001). Activation of IGF-1 receptors leads to activation of two main downstream signaling cascades, the Ras-ERK/MAPK and PI3K-Akt pathways (Russo et al., 2005), both of which are involved in regulation of ERα transcription (Martin et al., 2000;Mendez and Garcia-Segura, 2006;Patrone et al., 1998;Kato et al., 1995). Interestingly, growth factor phosphorylation of ERα occurs via the ERK/MAPK pathway at Ser118 (Kato et al., 1995) and ERα phosphorylated at Ser 118 is resistant to proteasomal degradation (Valley et al., 2008). Thus, there are putative mechanisms involving growth factor signaling by which ERα levels may be maintained beyond the period of estradiol exposure.
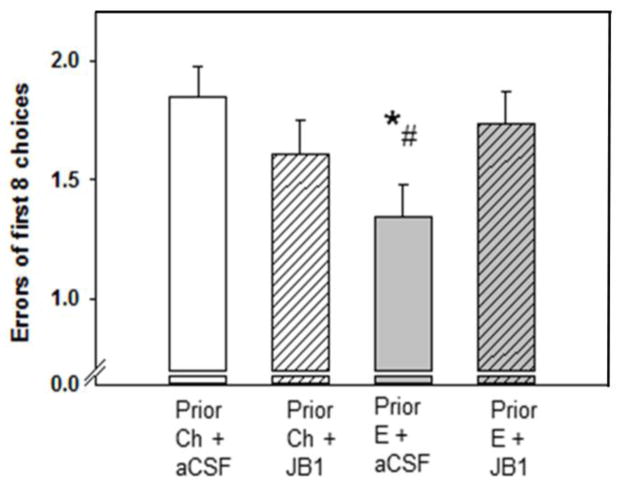
We hypothesized that the increased levels of ERα present in aging females that have been exposed to prior short-term estradiol (Figure 1) would allow for increased activation of hippocampal ERα by a ligand-independent mechanism involving IGF-1. This increased activation would result in increased expression of ERα target genes and associated proteins and ultimately improved performance on hippocampus-dependent tasks. As an initial test of this hypothesis, we investigated the role of IGF-1 receptors in the ability of short-term prior exposure to estradiol to exert lasting effects on cognition (Witty et al., 2013). Middle-age rats were ovariectomized and treated with vehicle or estradiol delivered via Sliastic capsules. After 40 days, all capsules were removed and rats were implanted with cannulae (icv) attached to osmotic minipumps that delivered vehicle or the IGF-1 receptor antagonist, JB1. Rats were tested on trials in the radial-arm maze during which delays were imposed between the 4th and 5th arm choices. As illustrated in Figure 4, administration of JB1 blocked the ability of prior exposure to estradiol to enhance performance without significantly affecting performance in the ovariectomized controls. Furthermore, JB1 blocked the ability of previous exposure to estradiol to result in lasting increases in levels of ERα and the ERα-regulated protein, ChAT (see Witty et al., 2013). Results indicate that activation of IGF-1 receptors is a necessary component in the ability of prior estradiol exposure to exert lasting benefits to cognition. Importantly, they also demonstrate that the decrease in performance induced by antagonism of IGF-1 receptors is specific to rats that had prior treatment with estradiol and not due to a generalized impairment induced by JB1 on cognition. Though further work is needed to confirm a direct interaction between IGF-1 and ERα, these results are consistent with the hypothesis that the lasting increase in levels of ERα resulting from previous estradiol exposure impacts memory via mechanism involving IGF-1 signaling.
Figure 4. Effects of previous treatment with estradiol and subsequent antagonism of brain insulin-like growth factor-1 (IGF-1) receptors on memory in aging ovariectomized rats.
Long-Evans rats (retired breeders; n = 8 – 10 per group) were ovariectomized in middle age (at ~11 months of age) and implanted with capsules containing estradiol (Prior E) or cholesterol vehicle (Prior Ch). After 40 days all capsules were removed. Chronic icv delivery via cannulae attached to osmotic minipumps of the IGF-1 receptor antagonist, JB1, or aCSF vehicle was initiated and continued for approximately 30 days. Spatial memory performance was assessed in a radial-arm maze with various delays imposed between the 4th and 5th arm choices. Data represent mean number of errors of first eight arm choices (± SEM) averaged over all delays. *P < .05 vs. Prior Ch + aCSF; # P < .05 Prior E + JB1. (Witty et al., 2013)
Local estradiol synthesis
In addition to putative ligand-independent mechanisms, in the absence of ovarian hormones or estradiol administration ERα may be activated by locally synthesized estrogens in the brain. All the proteins necessary for estradiol synthesis are expressed in the hippocampus (Compagnone and Mellon, 2000;Wehrenberg et al., 2001). Adult hippocampal neurons synthesize estradiol in vitro, an effect that can be attenuated by inhibition of aromatase, the enzyme that initiates the final step in estradiol synthesis (Kretz et al., 2004). Hippocampus-derived estradiol may be particularly important for rapid activation of estrogen receptor at the membrane, including membrane associated ERα (Ishii et al., 2007). These rapid actions include the ability of ERα induce activation of the ERK/MAPK pathway. Consistent with an effect of ERα-induced activation of the ERK/MAPK pathway are data from our lab demonstrating that increasing levels of hippocampus ERα to ovariectomized rats via lentiviral delivery results in increased activation of the ERK/MAPK pathway (Witty et al., 2012). Furthermore, increased levels of ERα resulting from previous exposure to estradiol were also associated with increased activation of the ERK/MAPK pathway (Witty et al., 2013). Highlighting the importance of extragonadal estrogens, likely including brain estradiol, in memory are data indicating that postmenopausal women treated for breast cancer with aromatase inhibitors exhibit memory deficits (Shilling et al., 2003). Additionally, ovariectomized female aromatase knockout mice have impaired memory as compared to ovariectomized wild-types (Martin et al., 2003). Thus, brain-derived estradiol may provide a mechanism by which ERα signaling can impact memory following the cessation of ovarian function.
Hypothesized model by which midlife estradiol can exert lasting impact on memory
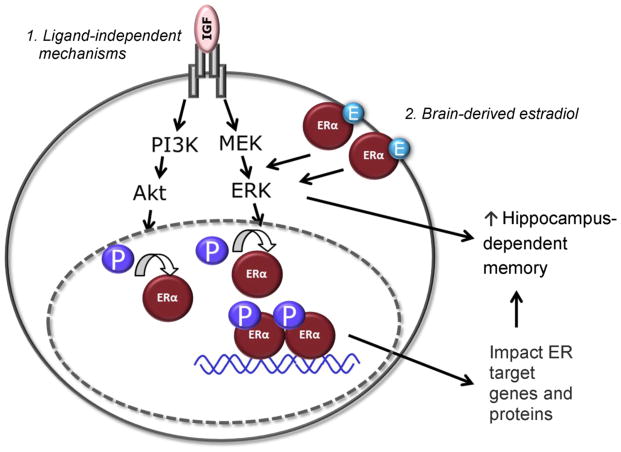
Figure 5 depicts a hypothesized model by which we propose that midlife estradiol can exert long-term impacts on memory beyond the period of estradiol exposure. Previous estradiol exposure allows for lasting increases in levels of ERα in the hippocampus (see Figure 2 and Rodgers et al., 2010; Witty et al., 2013). These increases in ERα would allow for novel mechanisms of activation in the absence of circulating estrogens. First, increased ERα levels would allow for increased effects resulting from ligand-independent activation of estrogen receptor by which growth-factors, including IGF-1, could initiate ERα-dependent transcription, increasing levels of ERα-target proteins, leading to enhanced memory. Our data, indicating that antagonism of IGF-1 receptors blocks the effects of previous estradiol on ERα-dependent proteins and on memory (Witty et al., 2013) are consistent with this hypothesized mechanism. Second, increased levels of ERα associated with the membrane would allow for rapid synaptic effects via actions of locally synthesized estradiol in the hippocampus that could exert rapid effects on memory and also impact transcription via intracellular signaling pathways. Although further work is needed to directly test this hypothesized mechanism, our data showing that increased ERα levels in the hippocampus are associated with increased ERK/MAPK signaling (Witty et al., 2012; Witty et al., 2013) are consistent with a role for ERα in rapid intracellular signaling in the absence of ovarian estrogens.
Figure 5. Hypothesized model involving actions at ERα by which previous exposure to estradiol can influence memory beyond the period of estradiol exposure.
Midlife estradiol increases levels of ERα in the hippocampus. Levels are maintained beyond the period of estradiol exposure. This increased pool of ERα allows for: (1) Insulin-like growth factor-1 (IGF-1) acting at its receptor activates intracellular signaling cascades. Action involving one or both of these signaling cascades culminates in phosphorylation (P) and activation of ERα at estrogen response element (ERE)-containing promoters. This allows for increased ERα-mediated transcription affecting levels of ERα-regulated target genes and proteins in the hippocampus resulting in enhancement in hippocampus-dependent memory. (2) Hippocampus-derived estradiol (E) activates membrane-associated ERα receptors, which activates intracellular signaling cascades. This activation can have rapid effects on memory and can also culminate in phosphorylation and activation of nuclear ERα.
Conclusions
The long-term impact for the brain and cognition of short-term use of hormone therapy in middle-age such as that used by women during the menopausal transition is currently unclear. However, preliminary data in the clinical literature are suggestive of long-term neuroprotective effects of short-term administration of estrogens within a critical time window following natural or surgical menopause. Consistent with such an effect are data from our lab that demonstrate that in a rat model of menopause, 40 days of estradiol administration following ovariectomy provides long-lasting benefits to memory. Associated with these enhancements are increased levels of ERα in the hippocampus. We hypothesize that this increased pool of ERα in the hippocampus allows for novel mechanisms of activation in the absence of circulating estrogens that leads to improved memory. Many questions remain to be answered including the cellular localization of the increased pool of ERα resulting from previous estradiol exposure as well as the mechanisms by which estrogen receptor levels are maintained following the termination of estradiol treatment. Nevertheless, data across the clinical and basic science literature increasingly support a relationship between levels and/or functional status of brain ERα and cognitive aging. These data support the idea that treatments that can impact levels of estrogen receptor, even in the absence of circulating estrogens, can impact memory.
Highlights.
The long-term impact for cognition of short-term estradiol use is unknown.
Preliminary clinical data suggest short-term estradiol use can provide lasting benefits.
Experimental data indicate prior estradiol administration enhances memory.
Experimental data indicate prior estradiol increases hippocampal ERα levels.
In the absence of ovarian estrogens, ERα can act via novel mechanisms to impact memory.
Acknowledgments
Experimental work described in this review that was conducted in our laboratory was supported by National Institute on Aging Grant R01AG041374 and National Science Foundation Grants 0715725 and 0951008 to JMD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Acconcia F, Ascenzi P, Bocedi A, Spisni E, Tomasi V, Trentalance A, Visca P, Marino M. Palmitoylation-dependent estrogen receptor alpha membrane localization: regulation by 17beta-estradiol. Mol Biol Cell. 2005;16:231–237. doi: 10.1091/mbc.E04-07-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrati P, Garnier M, Patrone C, Pollio G, Santagati S, Vegeto E, Maggi A. SK-ER3 neuroblastoma cells as a model for the study of estrogen influence on neural cells. Brain Res Bull. 1997;44:519–523. doi: 10.1016/s0361-9230(97)00237-2. [DOI] [PubMed] [Google Scholar]
- Alexandersen P, Tanko LB, Bagger YZ, Qin G, Christiansen C. The long-term impact of 2–3 years of hormone replacement therapy on cardiovascular mortality and atherosclerosis in healthy women. Climacteric. 2006;9:108–118. doi: 10.1080/13697130600647743. [DOI] [PubMed] [Google Scholar]
- Ali S, Metzger D, Bornert JM, Chambon P. Modulation of transcriptional activation by ligand-dependent phosphorylation of the human oestrogen receptor A/B region. EMBO J. 1993;12:1153–1160. doi: 10.1002/j.1460-2075.1993.tb05756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagger YZ, Tanko LB, Alexandersen P, Hansen HB, Mollgaard A, Ravn P, Qvist P, Kanis JA, Christiansen C. Two to three years of hormone replacement treatment in healthy women have long-term preventive effects on bone mass and osteoporotic fractures: the PERF study. Bone. 2004;34:728–735. doi: 10.1016/j.bone.2003.12.021. [DOI] [PubMed] [Google Scholar]
- Bagger YZ, Tanko LB, Alexandersen P, Qin G, Christiansen C. Early postmenopausal hormone therapy may prevent cognitive impairment later in life. Menopause. 2005;12:12–17. doi: 10.1097/00042192-200512010-00005. [DOI] [PubMed] [Google Scholar]
- Bean LA, Ianov L, Foster TC. Estrogen receptors, the hippocampus, and memory. Neuroscientist. 2014;20:534–545. doi: 10.1177/1073858413519865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Acosta JI, Talboom JS. Neuroscientists as cartographers: mapping the crossroads of gonadal hormones, memory and age using animal models. Molecules. 2010;15:6050–6105. doi: 10.3390/molecules15096050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornstrom L, Sjoberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol. 2005;19:833–842. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- Bohacek J, Daniel JM. The ability of oestradiol administration to regulate protein levels of oestrogen receptor alpha in the hippocampus and prefrontal cortex of middle-aged rats is altered following long-term ovarian hormone deprivation. J Neuroendocrinol. 2009;21:640–647. doi: 10.1111/j.1365-2826.2009.01882.x. [DOI] [PubMed] [Google Scholar]
- Bohacek J, Daniel JM. The beneficial effects of estradiol on attentional processes are dependent on timing of treatment initiation following ovariectomy in middle-aged rats. Psychoneuroendocrinology. 2010;35:694–705. doi: 10.1016/j.psyneuen.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Boulware MI, Kent BA, Frick KM. The impact of age-related ovarian hormone loss on cognitive and neural function. Curr Top Behav Neurosci. 2012;10:165–184. doi: 10.1007/7854_2011_122. [DOI] [PubMed] [Google Scholar]
- Bove R, Secor E, Chibnik LB, Barnes LL, Schneider JA, Bennett DA, De Jager PL. Age at surgical menopause influences cognitive decline and Alzheimer pathology in older women. Neurology. 2014;82:222–229. doi: 10.1212/WNL.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandi ML, Becherini L, Gennari L, Racchi M, Bianchetti A, Nacmias B, Sorbi S, Mecocci P, Senin U, Govoni S. Association of the estrogen receptor alpha gene polymorphisms with sporadic Alzheimer’s disease. Biochem Biophys Res Commun. 1999;265:335–338. doi: 10.1006/bbrc.1999.1665. [DOI] [PubMed] [Google Scholar]
- Brenner DE, Kukull WA, Stergachis A, van BG, Bowen JD, McCormick WC, Teri L, Larson EB. Postmenopausal estrogen replacement therapy and the risk of Alzheimer’s disease: a population-based case-control study. Am J Epidemiol. 1994;140:262–267. doi: 10.1093/oxfordjournals.aje.a117245. [DOI] [PubMed] [Google Scholar]
- Brinton RD. Investigative models for determining hormone therapy-induced outcomes in brain: evidence in support of a healthy cell bias of estrogen action. Ann N Y Acad Sci. 2005;1052:57–74. doi: 10.1196/annals.1347.005. [DOI] [PubMed] [Google Scholar]
- Cardona-Gomez GP, DonCarlos L, Garcia-Segura LM. Insulin-like growth factor I receptors and estrogen receptors colocalize in female rat brain. Neuroscience. 2000;99:751–760. doi: 10.1016/s0306-4522(00)00228-1. [DOI] [PubMed] [Google Scholar]
- Chen D, Pace PE, Coombes RC, Ali S. Phosphorylation of human estrogen receptor alpha by protein kinase A regulates dimerization. Mol Cell Biol. 1999;19:1002–1015. doi: 10.1128/mcb.19.2.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WY, Colditz GA. Risk factors and hormone-receptor status: epidemiology, risk-prediction models and treatment implications for breast cancer. Nat Clin Pract Oncol. 2007;4:415–423. doi: 10.1038/ncponc0851. [DOI] [PubMed] [Google Scholar]
- Chen WY, Manson JE, Hankinson SE, Rosner B, Holmes MD, Willett WC, Colditz GA. Unopposed estrogen therapy and the risk of invasive breast cancer. Arch Intern Med. 2006;166:1027–1032. doi: 10.1001/archinte.166.9.1027. [DOI] [PubMed] [Google Scholar]
- Chlebowski RT, Hendrix SL, Langer RD, Stefanick ML, Gass M, Lane D, Rodabough RJ, Gilligan MA, Cyr MG, Thomson CA, Khandekar J, Petrovitch H, McTiernan A. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative Randomized Trial. JAMA. 2003;289:3243–3253. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- Coker LH, Hogan PE, Bryan NR, Kuller LH, Margolis KL, Bettermann K, Wallace RB, Lao Z, Freeman R, Stefanick ML, Shumaker SA. Postmenopausal hormone therapy and subclinical cerebrovascular disease: the WHIMS-MRI Study. Neurology. 2009;72:125–134. doi: 10.1212/01.wnl.0000339036.88842.9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compagnone NA, Mellon SH. Neurosteroids: biosynthesis and function of these novel neuromodulators. Front Neuroendocrinol. 2000;21:1–56. doi: 10.1006/frne.1999.0188. [DOI] [PubMed] [Google Scholar]
- Cowley SM, Hoare S, Mosselman S, Parker MG. Estrogen receptors alpha and beta form heterodimers on DNA. J Biol Chem. 1997;272:19858–19862. doi: 10.1074/jbc.272.32.19858. [DOI] [PubMed] [Google Scholar]
- Craig MC, Maki PM, Murphy DG. The Women’s Health Initiative Memory Study: findings and implications for treatment. Lancet Neurol. 2005;4:190–194. doi: 10.1016/S1474-4422(05)01016-1. [DOI] [PubMed] [Google Scholar]
- Daniel JM. Estrogens, estrogen receptors, and female cognitive aging: the impact of timing. Horm Behav. 2013;63:231–237. doi: 10.1016/j.yhbeh.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Dohanich GP. Acetylcholine mediates the estrogen-induced increase in NMDA receptor binding in CA1 of the hippocampus and the associated improvement in working memory. J Neurosci. 2001;21:6949–6956. doi: 10.1523/JNEUROSCI.21-17-06949.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM, Hulst JL, Berbling JL. Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovariectomy but not after a long-term period of ovarian hormone deprivation. Endocrinology. 2006;147:607–614. doi: 10.1210/en.2005-0998. [DOI] [PubMed] [Google Scholar]
- Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, Hsia J, Margolis KL, Hogan PE, Wallace R, Dailey M, Freeman R, Hays J. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2959–2968. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- Espeland MA, Shumaker SA, Leng I, Manson JE, Brown CM, LeBlanc ES, Vaughan L, Robinson J, Rapp SR, Goveas JS, Wactawski-Wende J, Stefanick ML, Li W, Resnick SM. Long-term effects on cognitive function of postmenopausal hormone therapy prescribed to women aged 50 to 55 years. JAMA Intern Med. 2013;173:1429–1436. doi: 10.1001/jamainternmed.2013.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annu Rev Psychol. 1997;48:649–684. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM. Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors. J Neurosci. 2008;28:8660–8667. doi: 10.1523/JNEUROSCI.1968-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field EF, Whishaw IQ, Forgie ML, Pellis SM. Neonatal and pubertal, but not adult, ovarian steroids are necessary for the development of female-typical patterns of dodging to protect a food item. Behav Neurosci. 2004;118:1293–1304. doi: 10.1037/0735-7044.118.6.1293. [DOI] [PubMed] [Google Scholar]
- Fillit H, Weinreb H, Cholst I, Luine V, McEwen B, Amador R, Zabriskie J. Observations in a preliminary open trial of estradiol therapy for senile dementia-Alzheimer’s type. Psychoneuroendocrinology. 1986;11:337–345. doi: 10.1016/0306-4530(86)90019-3. [DOI] [PubMed] [Google Scholar]
- Font de MJ, Brown M. AIB1 is a conduit for kinase-mediated growth factor signaling to the estrogen receptor. Mol Cell Biol. 2000;20:5041–5047. doi: 10.1128/mcb.20.14.5041-5047.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC. Role of estrogen receptor alpha and beta expression and signaling on cognitive function during aging. Hippocampus. 2012;22:656–669. doi: 10.1002/hipo.20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC, Rani A, Kumar A, Cui L, Semple-Rowland SL. Viral vector-mediated delivery of estrogen receptor-alpha to the hippocampus improves spatial learning in estrogen receptor-alpha knockout mice. Mol Ther. 2008;16:1587–1593. doi: 10.1038/mt.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabor R, Nagle R, Johnson DA, Gibbs RB. Estrogen enhances potassium-stimulated acetylcholine release in the rat hippocampus. Brain Res. 2003;962:244–247. doi: 10.1016/s0006-8993(02)04053-2. [DOI] [PubMed] [Google Scholar]
- Gaub MP, Bellard M, Scheuer I, Chambon P, Sassone-Corsi P. Activation of the ovalbumin gene by the estrogen receptor involves the fos-jun complex. Cell. 1990;63:1267–1276. doi: 10.1016/0092-8674(90)90422-b. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Effects of gonadal hormone replacement on measures of basal forebrain cholinergic function. Neuroscience. 2000a;101:931–938. doi: 10.1016/s0306-4522(00)00433-4. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiol Aging. 2000b;21:107–116. doi: 10.1016/s0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Gabor R. Estrogen and cognition: applying preclinical findings to clinical perspectives. J Neurosci Res. 2003;74:637–643. doi: 10.1002/jnr.10811. [DOI] [PubMed] [Google Scholar]
- Hall JM, Couse JF, Korach KS. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem. 2001;276:36869–36872. doi: 10.1074/jbc.R100029200. [DOI] [PubMed] [Google Scholar]
- Hamilton BE, Minino AM, Martin JA, Kochanek KD, Strobino DM, Guyer B. Annual summary of vital statistics: 2005. Pediatrics. 2007;119:345–360. doi: 10.1542/peds.2006-3226. [DOI] [PubMed] [Google Scholar]
- Han X, Aenlle KK, Bean LA, Rani A, Semple-Rowland SL, Kumar A, Foster TC. Role of estrogen receptor alpha and beta in preserving hippocampal function during aging. J Neurosci. 2013;33:2671–2683. doi: 10.1523/JNEUROSCI.4937-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman SM, Naftolin F, Brinton EA, Judelson DR. Is the estrogen controversy over? Deconstructing the Women’s Health Initiative study: a critical evaluation of the evidence. Ann N Y Acad Sci. 2005;1052:43–56. doi: 10.1196/annals.1347.004. [DOI] [PubMed] [Google Scholar]
- Harman SM, Vittinghoff E, Brinton EA, Budoff MJ, Cedars MI, Lobo RA, Merriam GR, Miller VM, Naftolin F, Pal L, Santoro N, Taylor HS, Black DM. Timing and duration of menopausal hormone treatment may affect cardiovascular outcomes. Am J Med. 2011;124:199–205. doi: 10.1016/j.amjmed.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodis HN, Mack WJ, Shoupe D, Azen SP, Stanczyk FZ, Hwang-Levine J, Budoff MJ, Henderson VW. Methods and baseline cardiovascular data from the Early versus Late Intervention Trial with Estradiol testing the menopausal hormone timing hypothesis. Menopause. 2014 doi: 10.1097/GME.0000000000000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogervorst E, Bandelow S. Sex steroids to maintain cognitive function in women after the menopause: a meta-analyses of treatment trials. Maturitas. 2010;66:56–71. doi: 10.1016/j.maturitas.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Honjo H, Tanaka K, Kashiwagi T, Urabe M, Okada H, Hayashi M, Hayashi K. Senile dementia-Alzheimer’s type and estrogen. Horm Metab Res. 1995;27:204–207. doi: 10.1055/s-2007-979941. [DOI] [PubMed] [Google Scholar]
- Hyder SM, Chiappetta C, Stancel GM. Interaction of human estrogen receptors alpha and beta with the same naturally occurring estrogen response elements. Biochem Pharmacol. 1999;57:597–601. doi: 10.1016/s0006-2952(98)00355-4. [DOI] [PubMed] [Google Scholar]
- Ishii H, Tsurugizawa T, Ogiue-Ikeda M, Asashima M, Mukai H, Murakami G, Hojo Y, Kimoto T, Kawato S. Local production of sex hormones and their modulation of hippocampal synaptic plasticity. Neuroscientist. 2007;13:323–334. doi: 10.1177/10738584070130040601. [DOI] [PubMed] [Google Scholar]
- Isoe-Wada K, Maeda M, Yong J, Adachi Y, Harada H, Urakami K, Nakashima K. Positive association between an estrogen receptor gene polymorphism and Parkinson’s disease with dementia. Eur J Neurol. 1999;6:431–435. doi: 10.1046/j.1468-1331.1999.640431.x. [DOI] [PubMed] [Google Scholar]
- Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H, Metzger D, Chambon P. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270:1491–1494. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- Kawas C, Resnick S, Morrison A, Brookmeyer R, Corrada M, Zonderman A, Bacal C, Lingle DD, Metter E. A prospective study of estrogen replacement therapy and the risk of developing Alzheimer’s disease: the Baltimore Longitudinal Study of Aging. Neurology. 1997;48:1517–1521. doi: 10.1212/wnl.48.6.1517. [DOI] [PubMed] [Google Scholar]
- Kelly JF, Bienias JL, Shah A, Meeke KA, Schneider JA, Soriano E, Bennett DA. Levels of estrogen receptors alpha and beta in frontal cortex of patients with Alzheimer’s disease: relationship to Mini-Mental State Examination scores. Curr Alzheimer Res. 2008;5:45–51. doi: 10.2174/156720508783884611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz DM, Hewitt SC, Ciana P, Raviscioni M, Lindzey JK, Foley J, Maggi A, DiAugustine RP, Korach KS. Requirement of estrogen receptor-alpha in insulin-like growth factor-1 (IGF-1)-induced uterine responses and in vivo evidence for IGF-1/estrogen receptor cross-talk. J Biol Chem. 2002;277:8531–8537. doi: 10.1074/jbc.M109592200. [DOI] [PubMed] [Google Scholar]
- Kretz O, Fester L, Wehrenberg U, Zhou L, Brauckmann S, Zhao S, Prange-Kiel J, Naumann T, Jarry H, Frotscher M, Rune GM. Hippocampal synapses depend on hippocampal estrogen synthesis. J Neurosci. 2004;24:5913–5921. doi: 10.1523/JNEUROSCI.5186-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacreuse A, Wilson ME, Herndon JG. Estradiol, but not raloxifene, improves aspects of spatial working memory in aged ovariectomized rhesus monkeys. Neurobiol Aging. 2002;23:589–600. doi: 10.1016/s0197-4580(02)00002-7. [DOI] [PubMed] [Google Scholar]
- Lannigan DA. Estrogen receptor phosphorylation. Steroids. 2003;68:1–9. doi: 10.1016/s0039-128x(02)00110-1. [DOI] [PubMed] [Google Scholar]
- Le GP, Montano MM, Schodin DJ, Katzenellenbogen BS. Phosphorylation of the human estrogen receptor. Identification of hormone-regulated sites and examination of their influence on transcriptional activity. J Biol Chem. 1994;269:4458–4466. [PubMed] [Google Scholar]
- Lee H, Bai W. Regulation of estrogen receptor nuclear export by ligand-induced and p38-mediated receptor phosphorylation. Mol Cell Biol. 2002;22:5835–5845. doi: 10.1128/MCB.22.16.5835-5845.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeRoith D. Clinical relevance of systemic and local IGF-I: lessons from animal models. Pediatr Endocrinol Rev. 2008;5(Suppl 2):739–743. [PubMed] [Google Scholar]
- Li L, Haynes MP, Bender JR. Plasma membrane localization and function of the estrogen receptor alpha variant (ER46) in human endothelial cells. Proc Natl Acad Sci U S A. 2003;100:4807–4812. doi: 10.1073/pnas.0831079100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN. Estradiol increases choline acetyltransferase activity in specific basal forebrain nuclei and projection areas of female rats. Exp Neurol. 1985;89:484–490. doi: 10.1016/0014-4886(85)90108-6. [DOI] [PubMed] [Google Scholar]
- Luine VN. Estradiol and cognitive function: past, present and future. Horm Behav. 2014;66:602–618. doi: 10.1016/j.yhbeh.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM. Critical window hypothesis of hormone therapy and cognition: a scientific update on clinical studies. Menopause. 2013;20:695–709. doi: 10.1097/GME.0b013e3182960cf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin MB, Franke TF, Stoica GE, Chambon P, Katzenellenbogen BS, Stoica BA, McLemore MS, Olivo SE, Stoica A. A role for Akt in mediating the estrogenic functions of epidermal growth factor and insulin-like growth factor I. Endocrinology. 2000;141:4503–4511. doi: 10.1210/endo.141.12.7836. [DOI] [PubMed] [Google Scholar]
- Martin S, Jones M, Simpson E, van den Buuse M. Impaired spatial reference memory in aromatase-deficient (ArKO) mice. Neuroreport. 2003;14:1979–1982. doi: 10.1097/00001756-200310270-00020. [DOI] [PubMed] [Google Scholar]
- Maruyama H, Toji H, Harrington CR, Sasaki K, Izumi Y, Ohnuma T, Arai H, Yasuda M, Tanaka C, Emson PC, Nakamura S, Kawakami H. Lack of an association of estrogen receptor alpha gene polymorphisms and transcriptional activity with Alzheimer disease. Arch Neurol. 2000;57:236–240. doi: 10.1001/archneur.57.2.236. [DOI] [PubMed] [Google Scholar]
- Mattila KM, Axelman K, Rinne JO, Blomberg M, Lehtimaki T, Laippala P, Roytta M, Viitanen M, Wahlund L, Winblad B, Lannfelt L. Interaction between estrogen receptor 1 and the epsilon4 allele of apolipoprotein E increases the risk of familial Alzheimer’s disease in women. Neurosci Lett. 2000;282:45–48. doi: 10.1016/s0304-3940(00)00849-1. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr Rev. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- Mendez P, Garcia-Segura LM. Phosphatidylinositol 3-kinase and glycogen synthase kinase 3 regulate estrogen receptor-mediated transcription in neuronal cells. Endocrinology. 2006;147:3027–3039. doi: 10.1210/en.2005-1224. [DOI] [PubMed] [Google Scholar]
- Miller MM, Hyder SM, Assayag R, Panarella SR, Tousignant P, Franklin KB. Estrogen modulates spontaneous alternation and the cholinergic phenotype in the basal forebrain. Neuroscience. 1999;91:1143–1153. doi: 10.1016/s0306-4522(98)00690-3. [DOI] [PubMed] [Google Scholar]
- Mukai H, Tsurugizawa T, Murakami G, Kominami S, Ishii H, Ogiue-Ikeda M, Takata N, Tanabe N, Furukawa A, Hojo Y, Ooishi Y, Morrison JH, Janssen WG, Rose JA, Chambon P, Kato S, Izumi S, Yamazaki T, Kimoto T, Kawato S. Rapid modulation of long-term depression and spinogenesis via synaptic estrogen receptors in hippocampal principal neurons. J Neurochem. 2007;100:950–967. doi: 10.1111/j.1471-4159.2006.04264.x. [DOI] [PubMed] [Google Scholar]
- Mulnard RA, Cotman CW, Kawas C, van Dyck CH, Sano M, Doody R, Koss E, Pfeiffer E, Jin S, Gamst A, Grundman M, Thomas R, Thal LJ. Estrogen replacement therapy for treatment of mild to moderate Alzheimer disease: a randomized controlled trial. Alzheimer’s Disease Cooperative Study. JAMA. 2000;283:1007–1015. doi: 10.1001/jama.283.8.1007. [DOI] [PubMed] [Google Scholar]
- Ohkura T, Isse K, Akazawa K, Hamamoto M, Yaoi Y, Hagino N. Evaluation of estrogen treatment in female patients with dementia of the Alzheimer type. Endocr J. 1994;41:361–371. doi: 10.1507/endocrj.41.361. [DOI] [PubMed] [Google Scholar]
- Ooishi Y, Kawato S, Hojo Y, Hatanaka Y, Higo S, Murakami G, Komatsuzaki Y, Ogiue-Ikeda M, Kimoto T, Mukai H. Modulation of synaptic plasticity in the hippocampus by hippocampus-derived estrogen and androgen. J Steroid Biochem Mol Biol. 2011 doi: 10.1016/j.jsbmb.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Paganini-Hill A, Henderson VW. Estrogen replacement therapy and risk of Alzheimer disease. Arch Intern Med. 1996;156:2213–2217. [PubMed] [Google Scholar]
- Patrone C, Gianazza E, Santagati S, Agrati P, Maggi A. Divergent pathways regulate ligand-independent activation of ER alpha in SK-N-BE neuroblastoma and COS-1 renal carcinoma cells. Mol Endocrinol. 1998;12:835–841. doi: 10.1210/mend.12.6.0114. [DOI] [PubMed] [Google Scholar]
- PHOENIX CH, GOY RW, GERALL AA, YOUNG WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Prossnitz ER, Arterburn JB, Smith HO, Oprea TI, Sklar LA, Hathaway HJ. Estrogen signaling through the transmembrane G protein-coupled receptor GPR30. Annu Rev Physiol. 2008;70:165–190. doi: 10.1146/annurev.physiol.70.113006.100518. [DOI] [PubMed] [Google Scholar]
- Rannevik G, Jeppsson S, Johnell O, Bjerre B, Laurell-Borulf Y, Svanberg L. A longitudinal study of the perimenopausal transition: altered profiles of steroid and pituitary hormones, SHBG and bone mineral density. Maturitas. 2008;61:67–77. doi: 10.1016/j.maturitas.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci. 2003a;23:5708–5714. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp SR, Espeland MA, Shumaker SA, Henderson VW, Brunner RL, Manson JE, Gass ML, Stefanick ML, Lane DS, Hays J, Johnson KC, Coker LH, Dailey M, Bowen D. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003b;289:2663–2672. doi: 10.1001/jama.289.20.2663. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Henderson VW. Hormone therapy and risk of Alzheimer disease: a critical time. JAMA. 2002;288:2170–2172. doi: 10.1001/jama.288.17.2170. [DOI] [PubMed] [Google Scholar]
- Rocca WA, Bower JH, Maraganore DM, Ahlskog JE, Grossardt BR, de AM, Melton LJ., III Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69:1074–1083. doi: 10.1212/01.wnl.0000276984.19542.e6. [DOI] [PubMed] [Google Scholar]
- Rocca WA, Grossardt BR, Shuster LT. Oophorectomy, estrogen, and dementia: a 2014 update. Mol Cell Endocrinol. 2014;389:7–12. doi: 10.1016/j.mce.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers SP, Bohacek J, Daniel JM. Transient estradiol exposure during middle age in ovariectomized rats exerts lasting effects on cognitive function and the hippocampus. Endocrinology. 2010;151:1194–1203. doi: 10.1210/en.2009-1245. [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, Lacroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Russo VC, Gluckman PD, Feldman EL, Werther GA. The insulin-like growth factor system and its pleiotropic functions in brain. Endocr Rev. 2005;26:916–943. doi: 10.1210/er.2004-0024. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Molenda-Figueira HA, Sisk CL. Back to the future: The organizational-activational hypothesis adapted to puberty and adolescence. Horm Behav. 2009;55:597–604. doi: 10.1016/j.yhbeh.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JP, Stewart JM, De Ghett VJ. Critical periods in the organization of systems. Dev Psychobiol. 1974;7:489–513. doi: 10.1002/dev.420070602. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and cognitive functioning in women. Proc Soc Exp Biol Med. 1998;217:17–22. doi: 10.3181/00379727-217-44200. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and cognitive aging in women. Trends Pharmacol Sci. 2002;23:527–534. doi: 10.1016/s0165-6147(02)02093-x. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen therapy: is time of initiation critical for neuroprotection? Nat Rev Endocrinol. 2009;5:620–627. doi: 10.1038/nrendo.2009.193. [DOI] [PubMed] [Google Scholar]
- Shilling V, Jenkins V, Fallowfield L, Howell T. The effects of hormone therapy on cognition in breast cancer. J Steroid Biochem Mol Biol. 2003;86:405–412. doi: 10.1016/j.jsbmb.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, III, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS. Uncovering the mechanisms of estrogen effects on hippocampal function. Front Neuroendocrinol. 2008;29:219–237. doi: 10.1016/j.yfrne.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoica A, Saceda M, Fakhro A, Joyner M, Martin MB. Role of insulin-like growth factor-I in regulating estrogen receptor-alpha gene expression. J Cell Biochem. 2000;76:605–614. doi: 10.1002/(sici)1097-4644(20000315)76:4<605::aid-jcb9>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Brown CM, Dela Cruz CD, Yang E, Bridwell DA, Wise PM. Timing of estrogen therapy after ovariectomy dictates the efficacy of its neuroprotective and antiinflammatory actions. Proc Natl Acad Sci U S A. 2007;104:6013–6018. doi: 10.1073/pnas.0610394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swithers SE, McCurley M, Hamilton E, Doerflinger A. Influence of ovarian hormones on development of ingestive responding to alterations in fatty acid oxidation in female rats. Horm Behav. 2008;54:471–477. doi: 10.1016/j.yhbeh.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang MX, Jacobs D, Stern Y, Marder K, Schofield P, Gurland B, Andrews H, Mayeux R. Effect of oestrogen during menopause on risk and age at onset of Alzheimer’s disease. Lancet. 1996;348:429–432. doi: 10.1016/S0140-6736(96)03356-9. [DOI] [PubMed] [Google Scholar]
- Towart LA, Alves SE, Znamensky V, Hayashi S, McEwen BS, Milner TA. Subcellular relationships between cholinergic terminals and estrogen receptor-alpha in the dorsal hippocampus. J Comp Neurol. 2003;463:390–401. doi: 10.1002/cne.10753. [DOI] [PubMed] [Google Scholar]
- Tremblay GB, Tremblay A, Copeland NG, Gilbert DJ, Jenkins NA, Labrie F, Giguere V. Cloning, chromosomal localization, and functional analysis of the murine estrogen receptor beta. Mol Endocrinol. 1997;11:353–365. doi: 10.1210/mend.11.3.9902. [DOI] [PubMed] [Google Scholar]
- Valley CC, Solodin NM, Powers GL, Ellison SJ, Alarid ET. Temporal variation in estrogen receptor-alpha protein turnover in the presence of estrogen. J Mol Endocrinol. 2008;40:23–34. doi: 10.1677/JME-07-0067. [DOI] [PubMed] [Google Scholar]
- Vasudevan N, Pfaff DW. Non-genomic actions of estrogens and their interaction with genomic actions in the brain. Front Neuroendocrinol. 2008;29:238–257. doi: 10.1016/j.yfrne.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Baird A, Kotchen T, Curb JD, Black H, Rossouw JE, Aragaki A, Safford M, Stein E, Laowattana S, Mysiw WJ. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women’s Health Initiative: a randomized trial. JAMA. 2003;289:2673–2684. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- Wehrenberg U, Prange-Kiel J, Rune GM. Steroidogenic factor-1 expression in marmoset and rat hippocampus: co-localization with StAR and aromatase. J Neurochem. 2001;76:1879–1886. doi: 10.1046/j.1471-4159.2001.00207.x. [DOI] [PubMed] [Google Scholar]
- Wharton W, Gleason CE, Miller VM, Asthana S. Rationale and design of the Kronos Early Estrogen Prevention Study (KEEPS) and the KEEPS Cognitive and Affective sub study (KEEPS Cog) Brain Res. 2013;1514:12–17. doi: 10.1016/j.brainres.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmer RA, Quesenberry CP, Zhou J, Yaffe K. Timing of hormone therapy and dementia: the critical window theory revisited. Ann Neurol. 2011;69:163–169. doi: 10.1002/ana.22239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witty CF, Foster TC, Semple-Rowland SL, Daniel JM. Increasing hippocampal estrogen receptor alpha levels via viral vectors increases MAP kinase activation and enhances memory in aging rats in the absence of ovarian estrogens. PLoS One. 2012;7:e51385. doi: 10.1371/journal.pone.0051385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witty CF, Gardella LP, Perez MC, Daniel JM. Short-term estradiol administration in aging ovariectomized rats provides lasting benefits for memory and the hippocampus: a role for insulin-like growth factor-I. Endocrinology. 2013;154:842–852. doi: 10.1210/en.2012-1698. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Lindquist K, Sen S, Cauley J, Ferrell R, Penninx B, Harris T, Li R, Cummings SR. Estrogen receptor genotype and risk of cognitive impairment in elders: findings from the Health ABC study. Neurobiol Aging. 2009;30:607–614. doi: 10.1016/j.neurobiolaging.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Lui LY, Grady D, Stone K, Morin P. Estrogen receptor 1 polymorphisms and risk of cognitive impairment in older women. Biol Psychiatry. 2002;51:677–682. doi: 10.1016/s0006-3223(01)01289-6. [DOI] [PubMed] [Google Scholar]
- Zhang QG, Han D, Wang RM, Dong Y, Yang F, Vadlamudi RK, Brann DW. C terminus of Hsc70-interacting protein (CHIP)-mediated degradation of hippocampal estrogen receptor-alpha and the critical period hypothesis of estrogen neuroprotection. Proc Natl Acad Sci U S A. 2011;108:E617–E624. doi: 10.1073/pnas.1104391108. [DOI] [PMC free article] [PubMed] [Google Scholar]