Abstract
Exfoliation syndrome (XFS) is an important risk factor for glaucoma (XFG) worldwide. LOXL1 variants are highly associated with XFS in most populations; however, the high frequency of risk alleles in normal individuals and the reversal of risk alleles in different ethnic populations suggest that other factors contribute to XFS pathogenesis. Clusterin (CLU) is an extracellular matrix chaperone that prevents protein aggregation and is highly expressed in ocular tissues affected by XFS. Studies examining common CLU variants for association with XFS have been inconsistent. The purpose of this study was to evaluate CLU variants for association with XFS in two independent datasets from the United States (222 cases and 344 controls) and Israel (92 cases and 102 controls). Seven tag SNPs that captured >95% of alleles at r2 greater than 0.8 across the CLU genomic region were genotyped using TaqMan assays. Genotypes for an additional SNP, rs2279590, were imputed using phased haplotypes of HapMap reference CEU samples. Of the 8 CLU SNPs selected for the study, none were significantly associated with XFS in either case-control group (age and sex adjusted P > 0.14 and 0.36, respectively, in the US and Israeli datasets), or when they were meta-analyzed together (age and sex adjusted P > 0.13). Haplotype analysis using all 8 SNPs or only the promoter region SNPs also did not show significant associations of CLU with XFS in the combined US and Israeli dataset (P > 0.28). Meta-analysis of the data from this study and previous studies in Caucasian populations (1,184 cases and 978 controls) resulted in statistically significant association of rs2279590 with XFS (summary OR = 1.18, 95% CI: 1.03-1.33, P = 0.01). Significant association between rs2279590 and XFS was also found in Indian populations (summary OR = 0.76, 95% CI: 0.61-0.96; P = 0.02); however, significant heterogeneity between the Caucasian and Indian populations possibly due to reversal of the risk allele precluded an overall meta-analysis for rs2279590 (Q = 0.001, I2 = 91%). No significant association was identified for rs3087554 in either Caucasian populations (summary OR = 0.90, 95% CI: 0.77-1.05, P = 0.17) or Indian populations (summary OR = 0.89, 95% CI: 0.72-1.10, P = 0.28), or in both populations combined (1,705 cases and 3,713 controls; summary OR = 0.90, 95% CI: 0.79-1.01, P = 0.08). Significant heterogeneity precluded the addition of the Japanese data to the meta-analysis for rs3087554 (Q = 0.006, I2 = 87%). Our results suggest that common CLU variants may contribute to modest XFS risk but even larger datasets are required to confirm these findings.
Keywords: exfoliation syndrome, glaucoma, clusterin, genetic variants, meta-analysis
1. Introduction
Exfoliation syndrome (XFS) is a complex systemic disease whose most notable feature is the deposition of fibrillar material throughout the anterior segment of the eye. Exfoliation material contains elements of basement membranes and the elastic fiber framework, as well as other macromolecules (Ritch et al., 2003). XFS is a risk factor for developing elevated intraocular pressure (IOP) and glaucoma (Anastasopoulos et al., 2015). While XFS fibrillar material accumulates in the trabecular meshwork outflow pathways, the molecular mechanisms underlying elevated IOP and exfoliation glaucoma (XFG) are not completely known (Sacca et al., 2014).
Exfoliation syndrome (XFS) and the associated glaucoma (XFG) are genetically complex traits with contributions from both genetic and environmental factors (Sein et al., 2013). A genome-wide association study (GWAS) using unrelated patients and controls from Iceland and Sweden revealed significant association between common lysyl oxidase-like 1 (LOXL1) variants and XFS (Thorleifsson et al., 2007). This association was subsequently replicated in populations worldwide (Wang et al., 2014), including in United States clinic-based samples (Fingert et al., 2007; Aragon-Martin et al., 2008; Challa et al., 2008; Fan et al., 2008; Yang et al., 2008). Overall, these results show that LOXL1 is a major gene associated with XFS. However, while the risk alleles are present in the majority of cases worldwide, they are also frequently found in control individuals, arguing that other genetic and/or environmental factors are necessary for the disease to be fully manifested. Recently, another GWAS using a discovery sample set of 1,484 cases and 1,188 controls from Japan and replication datasets of 6,901 cases and 20,727 controls from 17 countries showed that common variants in the calcium channel, voltage-dependent, P/Q type, alpha 1A subunit (CACNA1A) gene were significantly associated with XFS (Aung et al., 2015).
Clusterin (CLU), an extracellular chaperone also known as apolipoprotein J, has been identified as a major component of the fibrillar deposits in XFS (Zenkel et al., 2006; Ovodenko et al., 2007; Doudevski et al., 2014). This ubiquitous glycoprotein is secreted by most cell types and is found in all body fluids (Jones et al., 2002). In the eye, CLU is expressed in most ocular cells and tissues, particularly in the ciliary epithelium (Zenkel et al., 2005). CLU expression is down regulated in the iris, lens and ciliary processes of patients with XFS compared to non-XFS glaucomatous control subjects (Zenkel et al. 2006). One study found reduced levels of clusterin mRNA and protein in aqueous humor samples from XFS patients compared to non-XFS glaucomatous control subjects (Zenkel et al., 2006), while another study found the opposite result (Doudevski et al., 2014), suggesting that CLU dysregulation may contribute to the disease.
Previous genetic studies provide some evidence that common CLU variants may contribute to XFS risk, although the association results are not consistent among different studies and populations. In the Blue Mountain Eye Study, the CLU single nucleotide polymorphism (SNP) rs3087554 was nominally associated with XFS (86 cases and 2,422 controls) at the genotypic level (P = 0.044), but not at the allelic level or when the age of controls was restricted to those over 73 years old (P > 0.07) (Burdon et al., 2008). No significant association between the rs3087554 variant and XFS was observed in a German (661 cases and 342 controls; P > 0.08) and Italian case-control set (209 cases and 190 controls; P > 0.70), although a positive association for another CLU SNP, rs2279590 (allele A) was reported in the German dataset only (Krumbiegel et al., 2009). A study of 136 cases and 89 controls from India did not find an association of rs3087554 with XFS (P > 0.06), but did find a significant association with rs2279590 (Padhy et al., 2014), although the risk allele was ‘G’ rather than the ‘A’ allele that was associated with disease risk in the German dataset. Interestingly, in the Indian study, the rs2279590 risk allele ‘G’ was also associated with elevated mRNA in lens capsules compared with the ‘A’ allele. However, a recent study of 299 cases and 224 controls from South India did not find significant association of rs3087554 or rs2279590 with XFS (P > 0.43; Dubey et al., 2015). Additionally, a recent GWAS in the Japanese dataset of 1,484 cases and 1,188 controls showed a nominal association of rs3087554 with XFS (P = 0.029), although the direction of effect for the minor allele ‘G’ is in the opposite direction compared with the effect in Caucasians with European ancestry (Aung et al., 2015). rs2279590 was not included in the Japanese study.
Clusterin's role as an important extracellular matrix chaperone required for prevention of extracellular protein aggregation makes it an interesting candidate genetic risk factor for XFS and XFG. To help clarify its relation to XFS, the purpose of this study was to evaluate common CLU variants for association with XFS (including cases with and without glaucoma) in two independent Caucasian case-control datasets from the United States (US) and Israel. Additionally, we performed a meta-analysis that included these two datasets as well as published results from Australia (Burdon et al., 2008), Germany (Krumbiegel et al., 2009), Italy (Krumbiegel et al., 2009), India (Padhy et al., 2014; Dubey et al., 2015) and Japan (Aung et al., 2015).
2. Materials and methods
2.1. Patients and control subjects
This study followed the tenets of the Declaration of Helsinki and was approved by the Institutional Review Boards of the Massachusetts Eye and Ear Infirmary (Boston, US) and the Goldschleger Eye Institute (Tel-Hashomer, Israel). Informed consent was obtained from all patients and controls after explanation of the nature and possible consequences of the study.
After we obtained informed consent, we recruited cases and controls from the Massachusetts Eye and Ear Infirmary (US), including 222 unrelated patients affected by XFS and 344 control subjects. Of the 222 cases with XFS, 110 also had glaucoma (XFG). A second independent dataset using cases and controls from Israel at the Goldschleger Eye Institute included 92 unrelated cases with XFS and 102 control subjects. Of the 92 patients with XFS, 67 patients also had XFG.
XFS patients had evidence of characteristic fibrillar material on the lens capsule or pupillary margin. XFG was additionally defined as: intraocular pressure >22 mm Hg (for at least one eye) on two occasions or intraocular pressure >19 mm Hg (for at least one eye) on treatment with two or more glaucoma medications; evidence of optic nerve damage in at least one eye based on clinical exam or visual field with changes consistent with nerve fiber layer loss on at least one reliable test. Control patients had no evidence of XFS or glaucoma based on clinical exam.
In the US dataset, the average age of the XFS cases and controls was 68.4 and 64.5 years, respectively (P < 0.0001; Table 1). 60.4% and 54.1% of XFS cases and controls were female (P > 0.14). In the Israeli dataset, the average age of the XFS patients and controls was 75.5 and 66.0 years, respectively (P < 0.0001). 51.1% of XFS cases were female while 41.2% of the controls were female (P > 0.14). All the cases and controls in the US and Israeli datasets were of self-reported Caucasian ancestry. Demographic features of the XFG subgroup (those XFS patients who also had evidence of glaucoma) were similar to the XFS cases overall for both datasets (69.0 years and 55.5% female for the US dataset and 75.0 years and 47.8% female for the Israeli dataset).
Table 1.
Demographic features of cases of exfoliation syndrome / exfoliation glaucoma and controls in this study
| Dataset | Number | Agea (mean±SD, years) | Female (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| XFS | XFG | Controls | XFS | XFG | Controls | XFS | XFG | Controls | |
| United States | 222 | 110 | 344 | 68.4±8.7b | 69.0±8.8b | 64.5±11.0 | 60.4c | 55.5c | 54.1 |
| Israel | 92 | 67 | 102 | 75.5±10.0b | 75.0±10.4b | 66.0±13.6 | 51.1c | 47.8c | 41.2 |
Abbreviations: XFS (exfoliation syndrome) refers to all exfoliation cases with and without glaucoma; XFG (exfoliation glaucoma) is the subgroup of XFS cases that also had glaucoma. SD = standard deviation.
Age at diagnosis for cases and age at enrollment for controls.
P < 0.0001 compared to controls.
P > 0.14 compared to controls.
2.2. Genotyping
We selected seven tag SNPs that captured >95% of alleles at r2 greater than 0.8 across the CLU genomic region, including all exons, introns, the 5’UTR, the 3’UTR, and the 7 kb proximal promoter region. Tag SNPs were selected according to the HapMap CEU (Utah residents with Northern and Western European ancestry from the CEPH collection) + TSI (Tuscans in Italy) data (version 3, release R2) using Haploview (version 4.2; Barrett et al., 2005). The minimum minor allele frequency for checking markers was set to 0.1. Genotyping was performed by TaqMan assays (Applied Biosystems [ABI], Foster City, CA). Oligonucleotide primers were ordered from ABI (assay by demand) and performed according to the manufacturer's instructions.
Because the SNP rs2279590 was not included in HapMap version 3, we did not select this SNP as a tag SNP for genotyping. To perform the meta-analysis with published data from other studies, we imputed rs2279590 genotypes using phased haplotypes of HapMap reference CEU samples and MACH 1.0 software (Li et al., 2010). By comparing the TaqMan genotypes with the imputed genotypes of 7 tag SNPs in this study, we obtained an accuracy of 99.7% for genotype imputation.
2.3. Statistical analysis
The two case-control datasets were analyzed separately then meta-analyzed. The association analysis was performed using PLINK (version 1.07; Purcell et al., 2007) for XFS overall and the XFS subgroup that also had glaucoma (XFG). Hardy-Weinberg equilibrium was assessed by the chi-squared test. The linkage disequilibrium (LD) plot was generated using Haploview (version 4.2; Barrett et al., 2005), where squared Pearson correlation coefficient (r2) was used to measure LD. Single SNP associations were evaluated using logistic regression after adjusting for age and sex. Multiple comparisons, for each analysis, were corrected using the Bonferroni method.
Haplotype frequencies were estimated using the standard E-M (Expectation-Maximization) algorithm and tested using the chi-squared test. The omnibus P value for haplotype analysis was obtained from the omnibus test, while the P values for individual haplotypes were obtained from the haplotype-specific tests. The odds ratio (OR) and 95% confidence interval (CI) were calculated for each of individual haplotypes compared to all the other haplotypes.
The heterogeneity between datasets was evaluated using the Cochran's Q statistic and the heterogeneity index (I2) (Higgins et al., 2003). Meta-analysis was performed using the Mantel-Haenszel method, assuming fixed effects. The forest plot was generated by the Review Manager software (RevMan, version 5.3; Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) and manually modified using Adobe Illustrator CS5.1 (Adobe Systems Inc., San Jose, CA).
Power analysis was performed using the Genetic Power Calculator (Purcell et al., 2003). The disease prevalence was set as 1.8% for the US dataset and the US + Israeli datasets and 3.0% for the Caucasian datasets and the Caucasian + Indian datasets (Fan et al. 2011). The risk allele frequency was set to the same as the marker allele frequency, ranging from 0.1 to 0.4. Linkage disequilibrium between the marker and the risk allele was set at D’ = 1.0. The genotypic relative risks for heterozygous (Aa)/high risk homozygous (AA) genotypes were set between 1.15/1.32 and 2.00/4.00, assuming an additive risk model (Purcell et al. 2003).
3. Results
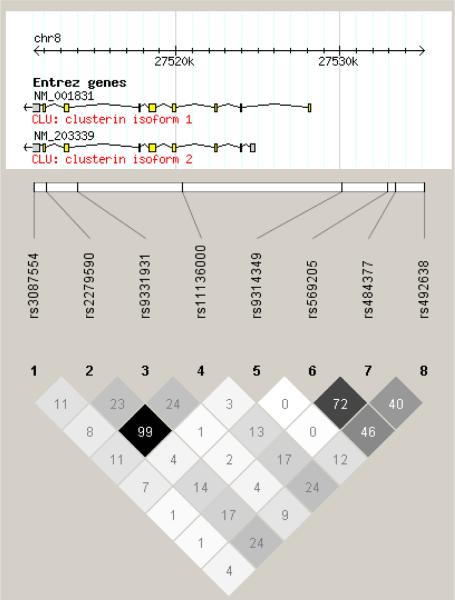
Seven SNPs capturing all CLU alleles with r2 > 0.8 (Fig. 1) and one additional SNP previously reported to be associated with XFS were investigated for association with XFS overall and the XFG subgroup. All 8 SNPs followed Hardy-Weinberg equilibrium in case and control samples in both the US and Israeli datasets (P > 0.1). None of the 8 CLU SNPs evaluated were significantly associated with XFS or with the XFG subgroup in either dataset separately (age and sex adjusted P > 0.14 and 0.36, respectively in the US and Israeli dataset; Supplementary Table) or when they were meta-analyzed together (age and sex adjusted P > 0.13; Table 2).
Fig. 1.
Linkage disequilibrium plot of the 8 SNPs around CLU in the US dataset. The numbers in the diamond refer to r2 values: white indicates r2 = 0, shades of grey represents 0 < r2 <1, and black indicates r2 = 1. Chromosomal positions were based on NCBI build 36.3 (National Center for Biotechnology Information, Bethesda, MD).
Table 2.
Study-specific and meta-analysis results of CLU SNPs with exfoliation syndrome in the US and Israeli datasets
| Minor allele frequency |
||||||||
|---|---|---|---|---|---|---|---|---|
| US |
Israel |
Meta-analysis |
||||||
| SNP | Minor allele | XFS | Controls | XFS | Controls | OR (95%CI) | Padj a | Q b |
| rs3087554 | G | 0.18 | 0.18 | 0.16 | 0.15 | 1.01 (0.76-1.35) | 0.63 | 0.90 |
| crs2279590 | A | 0.35 | 0.32 | 0.36 | 0.35 | 1.04 (0.79-1.35) | 0.56 | 0.99 |
| rs9331931 | C | 0.30 | 0.27 | 0.28 | 0.31 | 1.08 (0.85-1.37) | 0.45 | 0.30 |
| rs11136000 | T | 0.37 | 0.36 | 0.37 | 0.38 | 1.01 (0.81-1.27) | 0.71 | 0.82 |
| rs9314349 | G | 0.36 | 0.37 | 0.32 | 0.28 | 1.01 (0.81-1.27) | 0.77 | 0.43 |
| rs569205 | T | 0.40 | 0.40 | 0.34 | 0.38 | 0.96 (0.77-1.20) | 0.69 | 0.59 |
| rs484377 | C | 0.44 | 0.46 | 0.46 | 0.47 | 0.94 (0.75-1.17) | 0.53 | 0.97 |
| rs492638 | T | 0.28 | 0.33 | 0.24 | 0.29 | 0.80 (0.63-1.03) | 0.13 | 0.98 |
Abbreviation: XFS (exfoliation syndrome) refers to all exfoliation cases with and without glaucoma.
Logistic regression after adjustment for age and sex. The Bonferroni corrected significance level was set as 0.006 (0.05/8).
The heterogeneity between datasets was evaluated using the Cochran's Q statistic.
Imputed according to the phased haplotypes of HapMap reference CEU samples.
Haplotype analysis using all 8 SNPs did not find significant association of CLU with XFS (P > 0.41; Table 3), or the XFG subgroup (P > 0.05; data not shown) in the combined US and Israeli dataset. Haplotype analysis of 4 promoter region SNPs also did not identify significant association with XFS (P > 0.28; Table 3), or the XFG subgroup (P > 0.05; data not shown) in the combined US and Israeli dataset. Haplotype analysis using only rs3087554 and rs2279590 also did not show significant association with XFS (P > 0.91; Table 3), or the XFG subgroup (P > 0.56; data not shown) in the combined US and Israeli dataset.
Table 3.
Study-specific and meta-analysis of CLU haplotypes with exfoliation syndrome in the US and Israeli datasets
| Haplotypea | Haplotype frequency |
||||||
|---|---|---|---|---|---|---|---|
| US |
Israel |
Meta-analysis |
|||||
| XFS | Controls | XFS | Controls | OR (95%CI)b | P c | Q d | |
| rs3087554|rs2279590|rs9331931|rs11136000|rs9314349|rs569205|rs484377|rs492638 | |||||||
| AGCCAATG | 0.21 | 0.19 | 0.23 | 0.26 | 1.03 (0.81-1.33) | 0.79 | 0.30 |
| AAGTATCT | 0.19 | 0.18 | 0.13 | 0.14 | 1.03 (0.78-1.34) | 0.86 | 0.64 |
| GGGCGATG | 0.09 | 0.09 | 0.04 | 0.03 | 1.03 (0.70-1.53) | 0.87 | 0.66 |
| AGGCGATG | 0.05 | 0.06 | 0.07 | 0.03 | 1.07 (0.68-1.69) | 0.77 | 0.05 |
| GGGCAATG | 0.05 | 0.05 | 0.05 | 0.03 | 1.13 (0.69-1.82) | 0.63 | 0.38 |
| AAGTGATG | 0.04 | 0.05 | 0.05 | 0.06 | 0.82 (0.50-1.33) | 0.41 | 0.98 |
| rs9314349|rs569205|rs484377|rs492638 | |||||||
| AATG | 0.34 | 0.30 | 0.37 | 0.38 | 1.13 (0.91-1.40) | 0.28 | 0.33 |
| ATCT | 0.26 | 0.26 | 0.20 | 0.24 | 0.94 (0.74-1.19) | 0.62 | 0.43 |
| GATG | 0.21 | 0.22 | 0.18 | 0.13 | 1.03 (0.80-1.33) | 0.81 | 0.19 |
| GTCG | 0.12 | 0.11 | 0.10 | 0.09 | 1.10 (0.79-1.52) | 0.58 | 0.95 |
| AACG | 0.04 | 0.06 | 0.11 | 0.11 | 0.80 (0.52-1.21) | 0.29 | 0.34 |
| rs3087554|rs2279590 | |||||||
| AG | 0.46 | 0.46 | 0.48 | 0.49 | 0.99 (0.80-1.21) | 0.91 | 0.84 |
| AA | 0.36 | 0.36 | 0.36 | 0.36 | 1.00 (0.81-1.24) | 1.00 | 0.99 |
| GG | 0.18 | 0.18 | 0.16 | 0.15 | 1.01 (0.77-1.32) | 0.94 | 0.89 |
Abbreviation: XFS (exfoliation syndrome) refers to all exfoliation cases with and without glaucoma.
Only haplotypes with a frequency not less than 5% in either cases or controls were shown.
OR and 95%CI were calculated for each of individual haplotypes compared to all the other haplotypes.
Obtained from the haplotype-specific test using PLINK.
The heterogeneity between datasets was evaluated using the Cochran's Q statistic.
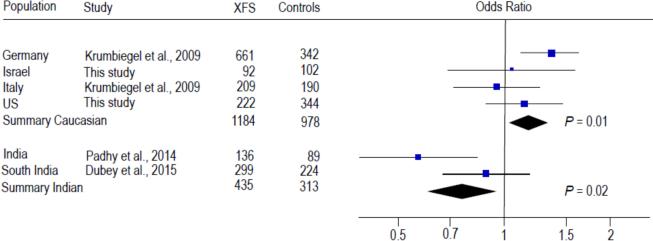
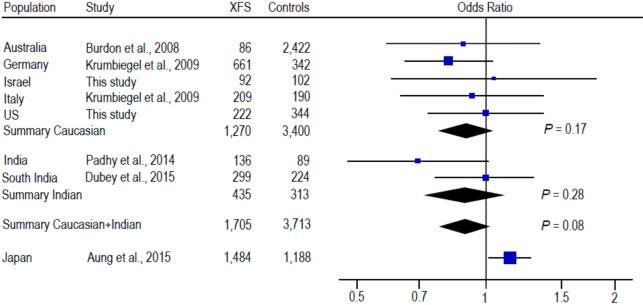
Previously, one CLU SNP rs3087554 was evaluated in relation to XFS overall in 3 Caucasian populations from Australia, Germany and Italy (Burdon et al., 2008; Krumbiegel et al., 2009) and most recently in 3 Asian populations from India and Japan (Padhy et al., 2014; Dubey et al., 2015; Aung et al., 2015). Another CLU SNP rs2279590 was also assessed for association with XFS in the German, Italian and Indian populations (Krumbiegel et al., 2009; Padhy et al., 2014; Dubey et al., 2015). Meta-analysis of the data from our US and Israeli datasets with published data from other Caucasian populations showed statistically significant association of rs2279590 with XFS overall (summary OR = 1.18, 95% CI: 1.03-1.33, P = 0.01; Fig. 2). Significant association between rs2279590 and XFS was also observed in Indian populations (summary OR = 0.76, 95% CI: 0.61-0.96; P = 0.02; Fig. 2); however, significant heterogeneity between the Caucasian and Indian populations precluded an overall meta-analysis for rs2279590 (Q = 0.001, I2 = 91%; Fig. 2). No significant association was found for rs3087554 in Caucasian populations (summary OR = 0.90, 95% CI: 0.77-1.05, P = 0.17; Fig. 3) or Indian populations (summary OR = 0.89, 95% CI: 0.72-1.10, P = 0.28; Fig. 3), or in the combined Caucasian + Indian populations (summary OR = 0.90, 95% CI: 0.79-1.01, P = 0.08; Fig. 3). There was significant heterogeneity between the Japanese study and the non-Japanese studies (Q = 0.006, I2 = 87%; Fig. 3), making it impossible to include the Japanese data in the meta-analysis of rs3087554. The direction of effect for the risk alleles in the Indian and Japanese populations is opposite that in the Caucasian populations, which could underlie the observed heterogeneity.
Fig. 2.
Meta-analysis with prior studies of rs2279590 and exfoliation syndrome. Odds ratio was calculated per each increase in minor allele A. The summary odds ratio was 1.18 (95% CI: 1.03-1.33) for the Caucasian populations and 0.76 (95% CI: 0.61-0.96) for the Indian populations. Significant heterogeneity between the Caucasian and Indian populations precluded an overall meta-analysis for rs2279590 (Q = 0.001, I2 = 91%).
Fig. 3.
Meta-analysis with prior studies of rs3087554 and exfoliation syndrome (XFS). Odds ratio was calculated per each increase in minor allele G. The summary odds ratio was 0.90 (95% CI: 0.77-1.05) for the Caucasian populations, 0.89 (95% CI: 0.72-1.10) for the Indian populations, and 0.90 (95% CI: 0.79-1.01) for the Caucasian and Indian populations, respectively. The odds ratios between the Caucasian and Indian datasets were not significantly heterogeneous (Q = 0.92, I2 = 0%). Significant heterogeneity precluded the addition of the Japanese data to the meta-analysis for rs3087554 (Q = 0.006, I2 = 87%).
Meta-analysis of our US and Israeli datasets with the German data showed a nominally significant association of the haplotype “A-A” formed by rs3087554 and rs2279590 with XFS overall (OR = 1.16, 95% CI: 1.01-1.34, P = 0.038). This analysis was restricted to the US, Israeli and German datasets because haplotype data was not available for the Italian, South Indian and Japanese datasets and the associated alleles are reversed in the Indian datasets.
4. Discussion
Clusterin mRNA and protein appear to have reduced expression in anterior segment tissues and aqueous humor of XFS patients compared to normal and non-XFS glaucomatous control subjects (Zenkel et al., 2006, 2005), suggesting that absence of the protein may contribute to development of XFS. We hypothesized that CLU genetic variation causing decreased CLU expression might contribute to XFS risk. In this study, we evaluated tag SNPs that captured >95% of genetic variation in the CLU gene region as well as the proximal 7-kb promoter for association with XFS and the XFG subgroup in two independent Caucasian case-control datasets. We did not detect significant association between common CLU SNPs or CLU haplotypes and XFS in the combined US - Israeli dataset (Tables 2 and 3). Previous studies have implicated two CLU SNPs in XFS, rs3087554 and rs2279590 in Caucasian, Indian and Japanese populations (Burdon et al., 2008; Krumbiegel et al., 2009; Padhy et al., 2014; Dubey et al., 2015; Aung et al., 2015). To further evaluate these associations, we performed a meta-analysis using our genotype data for the US and Israeli datasets as well as published data for the Australian, German, Italian, Indian and Japanese datasets. Our meta-analysis revealed significant association of rs2279590 with XFS in both Caucasian and Indian populations (Fig. 2). Significant heterogeneity among the Caucasian and Indian datasets was observed for rs2279590 precluding an overall meta-analysis for this SNP (Fig. 2). Similarly, significant heterogeneity between the Japanese study and the non-Japanese studies made it impossible to include the Japanese data in the meta-analysis of rs3087554 (Fig. 3). The risk allele and direction of effect for both rs3087554 and rs2279590 is consistent among European ancestry Caucasian populations. For 3087554, the risk allele and direction of effect in the Indian datasets is the same as the Caucasian datasets (Burdon et al., 2008; Krumbiegel et al., 2009; Padhy et al., 2014; Dubey et al., 2015), however the direction of effect for the risk allele G is in the opposite direction for the Japanese dataset (Aung et al., 2015). Similarly, for rs2279590 the direction of effect of risk allele G in the Indian populations is opposite that of the European Caucasians (this SNP was not genotyped in the Japanese study) (Krumbiegel et al., 2009; Padhy et al., 2014; Dubey et al., 2015). Interestingly, there is evidence of nominal association for both CLU SNPs (rs3087554 in the Japanese dataset and rs2279590 in the Indian datasets) despite the differences in direction of effect, which may be caused by ‘flipping’ of the risk allele due to population specific effects (Lin et al., 2007). This is a recognized phenomenon in disease association studies including the association of LOXL1 with XFS where commonly associated SNPs are ‘flipped’ in some Asian populations and in the South Africans (Wang et al., 2014; Dubey et al., 2014; Williams et al., 2010). Alternatively, the observed associations between CLU SNPs in the Indian and Japanese populations may be spurious findings. To provide further insight into this issue, additional analyses using larger datasets and possibly other populations will be necessary.
In Australians, a haplotype of 9 CLU tag SNPs was significantly associated with XFS (P = 0.005 and 0.01 in the full dataset and the age-restricted control dataset respectively) (Burdon et al., 2008). Haplotype analysis using 5 CLU tag SNPs in the German dataset showed a haplotype nominally associated with XFS (P = 0.05), and analysis of haplotypes formed by rs3087554 and rs2279590 showed a significant association of the haplotype “A-A” with XFS in the German dataset but not in the Italian dataset (Krumbiegel et al., 2009). In the Indian study, a significant association of the haplotype “A-G” (rs3087554|rs2279590) with XFS was observed (P = 0.001) (Padhy et al., 2014). Our haplotype analysis of 8 SNPs in CLU did not find significant association with XFS (P > 0.28 in the combined US and Israeli dataset; Table 3). We also did not find significant association between the rs3087554|rs2279590 haplotype and XFS (P > 0.91 in combined US and Israeli dataset; Table 3). Meta-analysis of our US and Israeli datasets with the German data showed a nominally significant association of the haplotype “A-A” (rs3087554|rs2279590) with XFS (P = 0.038).
Because the Indian study suggests that the rs2279590 risk allele increases CLU gene expression (Padhy et al., 2014), we were particularly interested in assessing genetic variation in the CLU promoter region. We did not identify significant association between CLU promoter SNPs and XFS or XFG in either the US or the Israeli dataset separately (Supplementary Table) or when they were meta-analyzed together (Table 2), and promoter region haplotypes were also not significantly associated with XFS (Table 3). The promoter region has been poorly investigated in previous studies. Only one tag SNP, rs9314349 that captured approximately 2-kb promoter region was investigated in the Australian study (Burdon et al., 2008) and promoter region SNPs were not included in the German, Italian, Indian and Japanese studies (Krumbiegel et al., 2009; Padhy et al., 2014; Dubey et al., 2015; Aung et al., 2015). Our results suggest that the CLU promoter may not contribute to XFS development, but studies using larger sample sizes would be necessary to confirm this. Further investigations into other gene regulatory elements in CLU are also warranted.
Data from the Indian dataset suggests that the rs2279590 ‘G’ allele is associated with increased CLU expression (Padhy et al, 2014); however, a previous study in Germans indicates that decreased CLU expression is found in ocular tissues from XFS compared to controls (Zenkel et al, 2006). This apparent paradox may be related to the observed reversal of the risk allele for rs2279590 in the Indian population; however, this also argues that rs2279590 does not have a biological effect and that other variants in this region, that may be shared among populations, could be responsible for the observed variation in gene expression. The consensus may be that dysregulation of CLU can contribute to XFS but further work will be necessary before firm conclusions can be reached.
The present study is the first to evaluate the association of the CLU variants with XFS in the US and Israeli populations and the first meta-analysis of CLU association studies. The relatively small sample size is a limitation of our study and the other CLU association studies published to date. For our study, we estimated that we had 98% of power to detect a moderate genetic effect in the US dataset (genotypic relative risk of 2.00 for Aa and 4.00 for AA, given an additive risk model), and 61%-92% power to detect smaller genetic effects (1.50/2.25 for Aa/AA), depending on the marker allele frequency (Purcell et al., 2003). However, our meta-analysis of rs3087554 and rs2279590 suggests that genetic effects of CLU variants are more likely to be more modest and on the order of 1.15/1.32 for Aa/AA (Fig. 2, 3). We therefore had only 14%-27% power in the US + Israeli datasets (314 cases and 446 controls), 49%-86% of power in the Caucasian datasets (1,270 cases and 3,400 controls), and 58%-93% of power in the Caucasian + Indian datasets (1,705 cases and 3,713 controls) to detect this more modest genetic effect. A larger dataset with more power may be necessary to detect associations between CLU variants and XFS or the XFG subgroup. Despite the limited power, our meta-analysis of 1,184 cases and 978 controls in the Caucasian populations revealed significant association for one CLU SNP, suggesting that common variants in this gene may be risk factors for the development of XFS. In addition, our study and those previously published have only investigated common variation in CLU, and further study of CLU rare variation in XFS cases and controls would be of interest.
5. Conclusions
In summary, we have evaluated common variants in CLU as genetic risk factors for XFS. Our results suggest that at least one common variant in this gene may be associated with modest risk for XFS. Additional studies using larger datasets that include rare variant analysis will be necessary to confirm our findings and identify genetic variants that contribute to the complex etiology of this important ocular disease.
Supplementary Material
Highlights for Review.
We comprehensively evaluated CLU (coding for clusterin) SNPs for association with exfoliation syndrome in two Caucasian case/control samples one from the United States and one from Israeli.
We examined CLU haplotypes in both Caucasian case/control samples.
We performed a meta-analysis for two CLU SNPs previously evaluated for association with exfoliation syndrome in our data as well as published data in European Caucasians, and case/control samples from India and Japan.
We found nominal association with one CLU SNP (rs2279590) in the meta-analysis of Caucasian samples (1,184 cases and 978 controls). Nominally significant association was also found in the Indian samples for rs2279590 and in the Japanese sample for rs3087554.
Overall, these results suggest that CLU may contribute to exfoliation syndrome risk.
Acknowledgements
This work was supported in part by National Eye Institute Grants R01 EY020928, R01 EY015872 and P30 EY014104, Research to Prevent Blindness, the Arthur Ashley Foundation and The Massachusetts Lions Eye Research Fund. A Harvard Medical School Ophthalmology Distinguished Scholar Award supports Dr. Pasquale.
Abbreviations
- CLU
clusterin
- XFS
exfoliation syndrome
- XFG
exfoliation glaucoma
- I2
heterogeneity index
- LD
linkage disequilibrium
- LOXL1
lysyl oxidase-like 1
- SNP
single nucleotide polymorphism
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
Dr. Fan performed all analyses and led the writing of all versions of the manuscript. Dr. Pasquale contributed cases and controls and contributed to writing all versions of the manuscript. Dr. Kang provided input on data analysis and editorial commentary on later versions of the manuscript. Dr. Haines provided input on the statistical analysis and editorial commentary on later versions of the manuscript. Dr Levkovitch-Verbin provided cases and controls as well as editorial comments on later drafts of the manuscript. Dr. Wiggs, in conjunction with Dr. Fan, conceived the study, contributed to writing all versions of the manuscript and provided funding for the work. Dr. Wiggs served to oversee all aspects of the study.
Conflict of interest
Dr. Pasquale has been a speaker for Allergan. He also served as a nonpaid consultant to Novartis and a paid consultant to Bausch + Lomb. He has received support to travel to the Think Tank Meeting in NYC by the Glaucoma Foundation in NYC. All other authors declare no conflict of interest or financial interest in any of the issues contained in this article.
References
- Anastasopoulos E, Founti P, Topouzis F. Update on pseudoexfoliation syndrome pathogenesis and associations with intraocular pressure, glaucoma and systemic diseases. Curr. Opin. Ophthalmol. 2015;26:82–89. doi: 10.1097/ICU.0000000000000132. [DOI] [PubMed] [Google Scholar]
- Aragon-Martin JA, Ritch R, Liebmann J, O'Brien C, Blaaow K, Mercieca F, Spiteri A, Cobb CJ, Damji KF, Tarkkanen A, Rezaie T, Child AH, Sarfarazi M. Evaluation of LOXL1 gene polymorphisms in exfoliation syndrome and exfoliation glaucoma. Mol. Vis. 2008;14:533–541. [PMC free article] [PubMed] [Google Scholar]
- Aung T, Ozaki M, Mizoguchi T, Allingham RR, Li Z, Haripriya A, et al. A common variant mapping to CACNA1A is associated with susceptibility to exfoliation syndrome. Nat. Genet. 2015;47:387–392. doi: 10.1038/ng.3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Burdon KP, Sharma S, Hewitt AW, McMellon AE, Wang JJ, Mackey DA, Mitchell P, Craig JE. Genetic analysis of the clusterin gene in pseudoexfoliation syndrome. Mol. Vis. 2008;14:1727–1736. [PMC free article] [PubMed] [Google Scholar]
- Challa P, Schmidt S, Liu Y, Qin X, Vann RR, Gonzalez P, Allingham RR, Hauser MA. Analysis of LOXL1 polymorphisms in a United States population with pseudoexfoliation glaucoma. Mol. Vis. 2008;14:146–149. [PMC free article] [PubMed] [Google Scholar]
- Doudevski I, Rostagno A, Cowman M, Liebmann J, Ritch R, Ghiso J. Clusterin and complement activation in exfoliation glaucoma. Invest. Ophthalmol. Vis. Sci. 2014;55:2491–2499. doi: 10.1167/iovs.13-12941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey SK, Hejtmancik JF, Krishnadas SR, Sharmila R, Haripriya A, Sundaresan P. Lysyl oxidase-like 1 gene in the reversal of promoter risk allele in pseudoexfoliation syndrome. JAMA. Ophthalmol. 2014;132:949–955. doi: 10.1001/jamaophthalmol.2014.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey SK, Hejtmancik JF, Krishnadas SR, Sharmila R, Haripriya A, Sundaresan P. Evaluation of genetic polymorphisms in clusterin and tumor necrosis factor-alpha genes in South Indian individuals with pseudoexfoliation syndrome. Curr. Eye. Res. 2015 Apr. 2015;7:1–7. doi: 10.3109/02713683.2014.997884. [Epub ahead of print] PubMed PMID: 25849827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan BJ, Pasquale L, Grosskreutz CL, Rhee D, Chen T, DeAngelis MM, Kim I, delBono E, Miller JW, Li T, Haines JL, Wiggs JL. DNA sequence variants in the LOXL1 gene are associated with pseudoexfoliation glaucoma in a US clinic-based population with broad ethnic diversity. BMC. Med. Genet. 2008;9:5. doi: 10.1186/1471-2350-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan BJ, Pasquale LR, Rhee D, Li T, Haines JL, Wiggs JL. LOXL1 promoter haplotypes are associated with exfoliation syndrome in a US Caucasian population. Invest. Ophthalmol. Vis. Sci. 2011;52:2372–2378. doi: 10.1167/iovs.10-6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingert JH, Alward WL, Kwon YH, Wang K, Streb LM, Sheffield VC, Stone EM. LOXL1 mutations are associated with exfoliation syndrome in patients from the midwestern United States. Am. J. Ophthalmol. 2007;144:974–975. doi: 10.1016/j.ajo.2007.09.034. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SE, Jomary C. Clusterin. Int. J. Biochem. Cell. Biol. 2002;34:427–431. doi: 10.1016/s1357-2725(01)00155-8. [DOI] [PubMed] [Google Scholar]
- Krumbiegel M, Pasutto F, Mardin CY, Weisschuh N, Paoli D, Gramer E, Zenkel M, Weber BH, Kruse FE, Schlötzer-Schrehardt U, Reis A. Exploring functional candidate genes for genetic association in german patients with pseudoexfoliation syndrome and pseudoexfoliation glaucoma. Invest. Ophthalmol. Vis. Sci. 2009;50:2796–2801. doi: 10.1167/iovs.08-2339. [DOI] [PubMed] [Google Scholar]
- Li Y, Willer CJ, Ding J, Scheet P, Abecasis GR. MaCH: using sequence and genotype data to estimate haplotypes and unobserved genotypes. Genet. Epidemiol. 2010;34:816–834. doi: 10.1002/gepi.20533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin PI, Vance JM, Pericak-Vance MA, Martin ER. No gene is an island: the flip-flop phenomenon. Am. J. Hum. Genet. 2007;80:531–538. doi: 10.1086/512133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovodenko B, Rostagno A, Neubert TA, Shetty V, Thomas S, Yang A, Liebmann J, Ghiso J, Ritch R. Proteomic analysis of exfoliation deposits. Invest. Ophthalmol. Vis. Sci. 2007;48:1447–1457. doi: 10.1167/iovs.06-0411. [DOI] [PubMed] [Google Scholar]
- Padhy B, Nanda GG, Chowdhury M, Padhi D, Rao A, Alone DP. Role of an extracellular chaperone, clusterin in the pathogenesis of pseudoexfoliation syndrome and pseudoexfoliation glaucoma. Exp. Eye. Res. 2014;127C:69–76. doi: 10.1016/j.exer.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Purcell S, Cherny SS, Sham PC. Genetic Power Calculator: design of linkage and association genetic mapping studies of complex traits. Bioinformatics. 2003;19:149–150. doi: 10.1093/bioinformatics/19.1.149. [DOI] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritch R, Schlötzer-Schrehardt U, Konstas AG. Why is glaucoma associated with exfoliation syndrome? Prog. Retin. Eye. Res. 2003;22:253–275. doi: 10.1016/s1350-9462(02)00014-9. [DOI] [PubMed] [Google Scholar]
- Sacca SC, Izzotti A. Focus on molecular events in the anterior chamber leading to glaucoma. Cell. Mol. Life. Sci. 2014;71:2197–2218. doi: 10.1007/s00018-013-1493-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sein J, Galor A, Sheth A, Kruh J, Pasquale LR, Karp CL. Exfoliation syndrome: new genetic and pathophysiologic insights. Curr. Opin. Ophthalmol. 2013;24:167–174. doi: 10.1097/ICU.0b013e32835d5d11. [DOI] [PubMed] [Google Scholar]
- Thorleifsson G, Magnusson KP, Sulem P, Walters GB, Gudbjartsson DF, Stefansson H, Jonsson T, Jonasdottir A, Jonasdottir A, Stefansdottir G, Masson G, Hardarson GA, Petursson H, Arnarsson A, Motallebipour M, Wallerman O, Wadelius C, Gulcher JR, Thorsteinsdottir U, Kong A, Jonasson F, Stefansson K. Common sequence variants in the LOXL1 gene confer susceptibility to exfoliation glaucoma. Science. 2007;317:1397–1400. doi: 10.1126/science.1146554. [DOI] [PubMed] [Google Scholar]
- Wang L, Fu S, Zhao W, Yu Y, Liu P. LOXL1 Gene polymorphism with exfoliation syndrome/exfoliation glaucoma: a meta-analysis. J. Glaucoma. 2014 doi: 10.1097/IJG.0000000000000128. [Epub ahead of print] PubMed PMID: 25304275. [DOI] [PubMed] [Google Scholar]
- Williams SE, Whigham BT, Liu Y, Carmichael TR, Qin X, Schmidt S, Ramsay M, Hauser MA, Allingham RR. Major LOXL1 risk allele is reversed in exfoliation glaucoma in a black South African population. Mol. Vis. 2010;16:705–712. [PMC free article] [PubMed] [Google Scholar]
- Yang X, Zabriskie NA, Hau VS, Chen H, Tong Z, Gibbs D, Farhi P, Katz BJ, Luo L, Pearson E, Goldsmith J, Ma X, Kaminoh Y, Chen Y, Yu B, Zeng J, Zhang K, Yang Z. Genetic association of LOXL1 gene variants and exfoliation glaucoma in a Utah cohort. Cell. Cycle. 2008;7:521–524. doi: 10.4161/cc.7.4.5388. [DOI] [PubMed] [Google Scholar]
- Zenkel M, Kruse FE, Jünemann AG, Naumann GO, Schlötzer-Schrehardt U. Clusterin deficiency in eyes with pseudoexfoliation syndrome may be implicated in the aggregation and deposition of pseudoexfoliative material. Invest. Ophthalmol. Vis. Sci. 2006;47:1982–1990. doi: 10.1167/iovs.05-1580. [DOI] [PubMed] [Google Scholar]
- Zenkel M, Pöschl E, von der Mark K, Hofmann-Rummelt C, Naumann GO, Kruse FE, Schlötzer-Schrehardt U. Differential gene expression in pseudoexfoliation syndrome. Invest. Ophthalmol. Vis. Sci. 2005;46:3742–3752. doi: 10.1167/iovs.05-0249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.