Abstract
Background
Phylogeographical studies allow precise genetic comparison of specimens, which were collected over large geographical ranges and belong to the same or closely related animal species. These methods have also been used to compare ticks of veterinary-medical importance. However, relevant data are missing in the case of ixodid ticks of bats, despite (1) the vast geographical range of both Ixodes vespertilionis and Ixodes simplex, and (2) the considerable uncertainty in their taxonomy, which is currently unresolvable by morphological clues.
Methods
In the present study 21 ticks were selected from collections or were freshly removed from bats or cave walls in six European and four Asian countries. The DNA was extracted and PCRs were performed to amplify part of the cytochrome oxidase I (COI), 16S and 12S rDNA genes, followed by sequencing for identification and molecular-phylogenetic comparison.
Results
No morphological differences were observed between Ixodes vespertilionis specimens from Spain and from other parts of Europe, but corresponding genotypes had only 94.6 % COI sequence identity. An I. vespertilionis specimen collected in Vietnam was different both morphologically and genetically (i.e. with only 84.1 % COI sequence identity in comparison with I. vespertilionis from Europe). Two ticks (collected in Vietnam and in Japan) formed a monophyletic clade and shared morphological features with I. ariadnae, recently described and hitherto only reported in Europe. In addition, two Asiatic specimens of I. simplex were shown to differ markedly from European genotypes of the same species. Phylogenetic relationships of ticks showed similar clustering patterns with those of their associated bat host species.
Conclusions
Although all three ixodid bat tick species evaluated in the present study appear to be widespread in Eurasia, they exhibit pronounced genetic differences. Data of this study also reflect that I. vespertilionis may represent a species complex.
Keywords: Chiroptera, Ixodes, Cytochrome oxidase gene, 12S rRNA, 16S rRNA
Background
Bats (Chiroptera) are the second largest order of mammals. Besides the high number of their species, they form the largest known mammalian aggregations (colonies) in which they may reach extremely high individual numbers [1]. Thus, frequently being the most abundant group of the local vertebrate fauna, their epidemiological role as pathogen reservoirs is also increasingly recognized [2]. Owing to their adaptability, i.e. large variety of their foraging habitats (forests, plains, along water surfaces, urban areas), roosting places (caves, tree holes, buildings) and prey animals (insects, arachnids, even vertebrates), microbats occur on all continents, except the Antarctica [1].
Apart from birds, bats are the only vertebrates that are able to actively fly. Consequently, during their (seasonal) migration, bats may even cover a few hundred kilometers. However, (unlike birds) they are unable to cross high mountain ranges, frequently rendering their populations geographically isolated. Such isolation may serve as the basis of bat speciation [3, 4] and may also have an impact on the evolution of bat parasites, including ticks.
Hard ticks (Acari: Ixodidae) that are specific to bats are only known to occur in the Old World. In Eurasia, for more than a century, only three species of ixodid ticks were known to infest bats: Ixodes vespertilionis Koch, I. simplex Neumann and I. kopsteini Oudemans [5, 6]. However, recently a new species, I. ariadnae Hornok has been described and molecularly characterized [7]. These four species appear to differ in their host preferences, i.e. I. vespertilionis occurs predominantly on Rhinolophus spp. [5, 8], I. ariadnae mainly on Myotis spp. [7], whereas I. simplex on Miniopterus schreibersii [5] and I. kopsteini on Molossidae [6]. Thus, the geographical range limit, the migration habit, the various habitat preferences and hibernating behavior of relevant bat species will significantly influence the geographical distribution of their ticks. Both I. vespertilionis and I. simplex are among the tick species with the largest known geographical range, encompassing much of the Old World (from Europe to the south in Africa and Australia, and to the east in Asia, including Japan). However, despite this, phylogeographical studies have not yet been conducted to investigate the morphological and/or genetic uniformity of these two tick species throughout their vast range.
Formerly, allopatric genotypes of I. vespertilionis were shown to exist between distant caves within a country [7]. To extend the scope of such observations, the present study was initiated in order to evaluate the mitochondrial gene heterogeneity of ixodid bat ticks over a larger range in the Old World, including ticks that showed the morphological characteristics of I. vespertilionis, I. simplex and I. ariadnae. In Southern and Eastern Asian countries involved in the present study the sample number was limited, because ixodid bat ticks are rare (e.g. [9]).
The 5’ region of the mitochondrial cytochrome oxidase subunit I (COI) gene was chosen as the primary target for molecular analyses. This is the standard marker for tick species identification by DNA barcoding, with confirmatory or supportive value of 16S and 12S rDNA sequences [10]. These methods were found to be suitable to distinguish between the three ixodid bat tick species that occur in Europe [11]. Therefore, phylogenetic analyses in the present study are based on the COI gene, with additional testing of part of the 16S and/or 12S genes in the case of highly divergent specimens.
Methods
Ticks were collected from bats captured for ringing and monitoring purposes, or from cave walls near bats (Table 1). All specimens were stored in 70 % ethanol. The morphology of ticks was compared according to the length/shape of palps, shape and index (length/width) of the scutum, density of alloscutal setae, arrangement of coxal setae. DNA was extracted with the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions; when possible, only from 1–4 legs.
Table 1.
Collection data of bat ticks processed in this study. DNA extraction was attempted from all samples, but GenBank accession number is provided only in the case of successful COI gene amplification. 16S and 12S rDNA sequence accession numbers are provided in the text
| tick species acc. to morphology | tick stage/sex | host species | date of collection | place of collection: country (city or region) | COI GenBank accession number |
|---|---|---|---|---|---|
| I. vespertilionis | F | Myotis mystacinus | 20-05-2010 | Serbia (Zlot) | KR902765 |
| N | Rhinolophus hipposideros | 03-05-2013 | Serbia (Dimitrovgrad) | KR902764 | |
| F, F | (cave wall) | 1907a | Serbia (Novi Pazar) | - | |
| N | Rhinolophus ferrumequinum | 07-09-2014 | Bosnia-Herzegovina (Šipovo) | KR902763 | |
| N | Rhinolophus sp. | 1899a | Bosnia-Herzegovina (43.2° N, 17.8°E) | - | |
| N | Rhinolophus euryale | 22-09-2013 | Montenegro (Rijeka Crnojevića) | KR902766 | |
| F | Rhinolophus hipposideros | 19-09-1913a | Czech Republic (Hranice na Morave) | - | |
| M, F | near Rhinolophus hipposideros | 21-02-2008 | France (Ruillé en Champagne) | KR902757-8 | |
| F | Rhinolophus sp. | ca. 1992 | Spain - Vasque Country | KR902759 | |
| N | Rhinolophus sp. | ca. 1992 | Spain - Vasque Country | KR902760 | |
| N | Rhinolophus sp. | ca. 1992 | Spain - Vasque Country | KR902761 | |
| L | Rhinolophus sp. | ca. 1992 | Spain - Vasque Country | KR902762 | |
| M | (cave wall) | 1890a | Russia (Caucasus - Labinsk) | - | |
| N | Rhinolophus affinis | 20-10-2014 | Vietnam (Cao Bang - Phia Oac) | KR902756 | |
| I. ariadnae-like | L | Myotis n. sp. (undescribed) | 17-10-2014 | Vietnam (Thanh Hoa - Ban Vin) | KR902767 |
| F | Murina leucogaster | 09-03-2013 | Japan (Okayama) | LC036330 | |
| I. simplex | F | near Miniopterus schreibersii | 09-07-2008 | France (Rancogne) | KR902768 |
| N | Miniopterus magnater | 19-02-2015 | India (Meghalaya - Jaintia Hills) | KR902769 | |
| L | Miniopterus fuliginosus | 09-09-2013 | Japan (Wakayama) | LC055099 |
akindly provided by the Natural History Museum of Berlin. Abbreviations: M-male, F-female, N-nymph, L-larva
In the case of Japanese samples, the COI PCR, that amplifies an up to 710 bp long fragment of the gene with the primers HCO2198 (5’-TAA ACT TCA GGG TGA CCA AAA AAT CA-3’) and LCO1490 (5’-GGT CAA CAA ATC ATA AAG ATA TTG G-3’), was performed according to [12]. Two further PCRs were used to amplify an approx. 460 bp fragment of the 16S rDNA gene of Ixodidae with the primers 16S + 1 (5’-CTG CTC AAT GAT TTT TTA AAT TGC TGT GG-3’) and 16S-1 (5’-CCG GTC TGA ACT CAG ATC AAG T-3’) [13]; and to amplify an approx. 420 bp fragment of the 12S rDNA gene of hard ticks with the primers 12S + 1 (5’-TAC TAT GTT ACG ACT TA-3’) and 12S-1 (5’-AAA CTA GGA TTA GAT ACC C-3’) [14].
For the remaining samples PCR conditions were slightly modified. The COI primers were used in a reaction volume of 25 μl, containing 1 U (0.2 μl) HotStarTaq Plus DNA polymerase, 2.5 μl 10× CoralLoad Reaction buffer (including 15 mM MgCl2), 0.5 μl PCR nucleotid Mix (0.2 mM each), 0.5 μl (1 μM final concentration) of each primer, 15.8 μl ddH2O and 5 μl template DNA. For amplification, an initial denaturation step at 95 °C for 5 min was followed by 40 cycles of denaturation at 94 °C for 40 s, annealing at 48 °C for 1 min and extension at 72 °C for 1 min. Final extension was performed at 72 °C for 10 min. In the case of selected specimens that were highly divergent according to the COI analysis, the 16S and 12S rDNA gene PCRs were also performed. Reaction components other than primers, as well as cycling conditions were the same, except for annealing at 51 °C.
PCR products were electrophoresed (for visualization), purified and sequenced. The sequences were submitted to GenBank (accession numbers KR902756-77). Phylogenetic analyses were conducted as reported [11], including that for bat host species of ticks based on cytochrome b sequences retrieved from GenBank. Phylogenetic trees were compared in the R development framework [15].
The partial 12S rDNA gene amplification and sequencing with the above primer pair was not successful in the case of the I. vespertilionis nymph from Vietnam and I. simplex from India (Table 1), consistently with the selective success of primers reported by Norris et al. [14].
Ethical approval
Authorization for bat capture was provided by the National Inspectorate for Environment, Nature and Water. Bat banding licence numbers are TMF-493/3/2005 (TG) and 59/2003 (PE).
Results
Comparison of ixodid bat ticks from Europe
No morphological differences were noted between specimens of I. vespertilionis collected in four different countries of Central-Eastern (CE) Europe. The amplified part of the COI gene showed a maximum of eight nucleotide difference, i.e. 98.7 % identity between isolates. In the phylogenetic tree these genotypes clustered together, but separately from Western European I. vespertilionis specimens collected in France (Fig. 1a). The latter differed with up to 15 nucleotides, and in that case had only 97.6 % sequence identity with CE European genotypes.
Fig. 1.
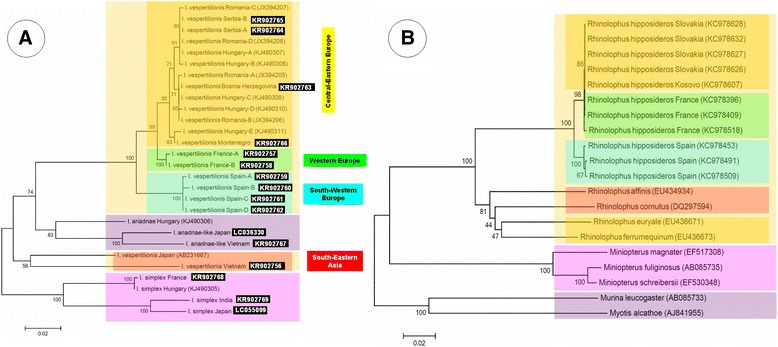
a Phylogenetic relationships of bat ticks collected in the present study (accession number in inverse colour) based on COI gene and related data from the GenBank. Light yellow background indicates isolates of I. vespertilionis, with overlaying background colours according to geographical regions as indicated. Purple background colour stands for genotypes of I. ariadnae, and pink for those of I. simplex. b Phylogenetic relationships of bat species that were hosts of the tick species (according to the colour code of the tick phylogenetic tree) analysed in the present study. Branch lengths correlate to the number of substitutions inferred according to the scale shown
The specimens of I. vespertilionis from Spain also appeared to be morphologically identical to those from CE Europe. However, the COI genotypes of these South-Western European isolates showed up to 34 nucleotide differences when compared to CE Europe, i.e. only 94.6 % identity between genotypes of the two regions. In the phylogenetic analysis the South-Western European isolates formed a distinct cluster, a sister group of all other evaluated European specimens (Fig. 1a), similarly to genotypes of R. hipposideros, the main host of I. vespertilionis (Fig. 1b).
Accordingly, based on the amplified part of its 16S rDNA gene, the French isolate (accession number: KR902772) clustered together with CE European isolates, but separately from the Spanish one (KR902773), although the bootstrap support for the latter was low (Fig. 2). The distinct phylogenetic position of a Spanish I. vespertilionis isolate was further confirmed by analysis of part of the 12S rDNA gene, because the genotype from France (KR902776) showed three nucleotide differences from (i.e. 99 % sequence identity to) specimens collected in CE Europe, whereas the Spanish isolate (KR902777) had nine nucleotide differences and thus only 97.5 % sequence identity.
Fig. 2.
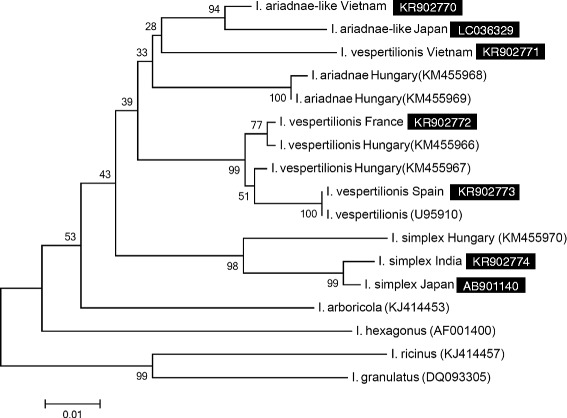
Phylogenetic comparison of 16S rDNA sequences of bat tick genotypes identified in the present study (inverse colour) and other sequences from the GenBank. Branch lengths correlate to the number of substitutions inferred according to the scale shown
Comparison of ixodid bat ticks from Southern and Eastern Asia
The I. vespertilionis nymph collected in Vietnam had convex posterolateral margin of the scutum, as contrasted to the concave shape in the case of European specimens. The COI sequence of this tick (KR902756) also had the highest level of intraspecific genetic divergence observed in this study: it differed from the CE European I. vespertilionis genotypes with up to 101 nucleotides, i.e. showed only 84.1 % sequence identity and clustered distantly on the phylogenetic tree (Fig. 1a). This nymph from Vietnam had the closest sequence identity (88 %) to a formerly published Japanese genotype (AB231667) (Fig. 3). Based on its partial 16S rDNA gene sequence (KR902771) this tick clustered separately from all European I. vespertilionis specimens and as the sister group of the two Asiatic ticks resembling I. ariadnae (Fig. 2).
Fig. 3.
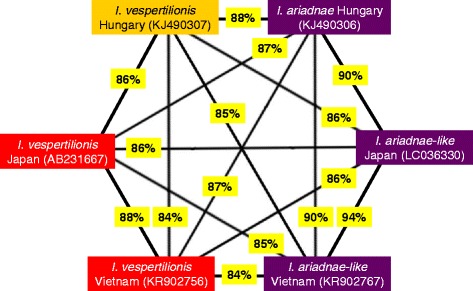
The approximated degree of COI sequence identity between highly divergent genotypes of I. vespertilionis and I. ariadnae. Background colour of species names corresponds to the one used on Fig. 1a. Three sequences are from other studies (KJ490306-7: [7]; AB231667: [19])
Another tick was collected from an undescribed species of Myotis in Vietnam. This larva showed morphological similarities to I. ariadnae. Consistently with this, the COI sequence of this specimen (KR902767) had the highest (89.5 %) identity with the CE European I. ariadnae genotype (62 nucleotide differences), and only 85.2 % identity with the CE European I. vespertilionis genotypes (i.e. up to 87 nucleotide differences). Based on COI phylogenetic analysis this I. ariadnae-like genotype from Vietnam clustered close to I. ariadnae, but separately from I. vespertilionis collected in CE Europe (Fig. 1a). This finding was only partly confirmed by the analysis of the 16S gene (although poorly supported by low bootstrap values), because the Vietnamese I. ariadnae-like specimen (KR902770) clustered separately from CE European specimens of both I. ariadnae and I. vespertilionis (Fig. 2). The partial 12S gene sequence of this larva (KR902775) showed only 12–14 nucleotide differences from (96–96.6 % identity with) I. ariadnae, whereas 19–20 nucleotide differences from (94.3–94.6 % identity with) I. vespertilionis.
One female tick collected from Murina leucogaster in Japan also resembled morphologically to I. ariadnae. The COI sequence of this specimen (LC036330) differed from the CE European I. ariadnae genotype in 65 nucleotides (amounting to 89.7 % identity), but from CE European I. vespertilionis genotypes in up to 85 nucleotides (meaning only 86.4 % identity). The COI phylogenetic analysis showed that this I. ariadnae-like genotype from Japan clustered together with the above I. ariadnae-like tick from Vietnam and close to I. ariadnae from Hungary, but separately from I. vespertilionis collected in CE Europe (Fig. 1a). This finding was also confirmed by the phylogenetic tree based on the amplified part of the 16S gene (LC036329, Fig. 2). The partial 12S gene sequence of this tick (LC036328) showed 15–17 nucleotide differences from (95.4–95.9 % identity with) I. ariadnae, whereas 20–21 nucleotide differences from (94.3–94.6 % identity with) I. vespertilionis. The genetic distances based on partial COI sequences between highly divergent I. vespertilionis and I. ariadnae genotypes are summarized in Fig. 3.
One nymph, collected from Miniopterus magnater in India, showed a similar morphology to I. simplex. However, the partial COI sequence of this specimen (KR902769) had 55–58 nucleotide differences from two European genotypes (i.e. 90.8–91 % identity), from which it also clustered apart in the phylogenetic analysis (Fig. 1a). Similarly, the separation of this Indian I. simplex genotype (KR902774) from the Hungarian one was supported by high bootstrap value in the 16S phylogenetic analysis (Fig. 2).
A female I. simplex, collected from Mi. fuliginosus in Japan, was also included in the present study. The partial COI sequence of this isolate (LC055099) had only 25 nucleotide differences from (i.e. 96 % identity with) the above genotype from India, and in the phylogenetic analysis these two Asiatic I. simplex specimens clustered together, but separately from two European (French and Hungarian) genotypes (Fig. 1a). The same grouping pattern was seen on the basis of the partial 16S rDNA gene (AB901140) of this isolate (Fig. 2). At the same time, its partial 12S rDNA sequence (LC055100) showed 24 nucleotide differences from (93.6 % identity with) I. simplex from Hungary.
Discussion
The results of the present study can be interpreted in the light of evolutionary factors and events as well as ecological traits that influence the intra- and inter-specific genetic diversity of animal populations, in this case of ticks and their bat hosts. In this context the geographical range of bat species, geographical barriers and glacial periods (that may isolate related bat populations) may be particularly important as driving forces of disruptive selection. We acknowledge that the reduced number of samples and stages used in the present study prevent conclusions about the precise taxonomic status of collected specimens. However, the low number of samples is compensated by an adequate representation of the territories and a high similarity of the samples within-sites. We could not explicitly calculate the amount of phylogenetic diversity at each node of the phylogenetic tree because the presence of singletons, or isolated taxa that produced only a single edge in the tree. However, the relative distances among the tips of the tree are an indirect measure of the similarity within and between sites.
The main host of I. vespertilionis in Europe is R. hipposideros [5, 8]. During post-glacial periods this bat species recolonized central and northern Europe from refugia either in the Balkan or the northern Mediterranean (Southern France) [16]. This may account for the relative genetic homogeneity of CE European and French I. vespertilionis isolates, as reported here.
Considering all I. vespertilionis analyzed in the present study from Europe, specimens collected in Spain were shown to differ markedly (and to cluster apart phylogenetically) from other evaluated genotypes. Probably the most important underlying factor of this phenomenon is that bat hosts of I. vespertilionis represent isolated populations on the Iberian peninsula, prevented from mixing with northern populations by the Pyrenees which act as a barrier to gene flow [16]. This is also well reflected by the phylogenetic position of R. hipposideros from Spain and CE Europe (Fig. 1b). The level of sequence divergence between Spanish and CE European genotypes of I. vespertilionis (5.4 % in the COI gene) approached the proposed sequence difference as species boundary for ticks (6.1 % COI: [10]). In contrast to this, another member of the genus, I. ricinus was demonstrated to show only 0.5 % COI sequence divergence between specimens from CE Europe or France and Spain (e.g. GU074908 or GU074941 vs. GU074910: [17]), explained by gene flows due to dispersal and continuous exchange of ticks between populations connected by migrating hosts.
The homologies of COI sequences were reported to be above 93.9 % within a species, and below 94.4 % between species of ixodid genera [18]. Similarly, in another study the percentage of species boundary was deemed to be 6.1 % sequence divergence for the COI gene, only in exceptional cases amounting to higher values [10]. Accordingly, in comparison with I. vespertilionis genotypes from Europe (KR902757-66), the high (16 %) genetic divergence of I. vespertilionis from Vietnam (KR902756) and of a similar genotype in Japan (AB231667), together with their well separated and distant phylogenetic position, suggest that they probably represent a distinct tick species. This is partly confirmed by the morphological difference observed between the European and the Vietnamese variant. In addition, the degree of partial COI sequence divergence between the European and Vietnamese I. vespertilionis genotypes (101 nucleotide differences, i.e. 84.1 % identity) was similar to that observed between the latter and I. ricinus (e.g. in comparison with KF197132: 102 nucleotide differences, i.e. 83.9 % identity). At the same time, the two South-East Asiatic genotypes (KR902756, AB231667) clustered together and showed 88 % similarity to each other (Fig. 3).
The host of the I. vespertilionis nymph collected in the present study in Vietnam was R. affinis, and of that recorded previously in Japan was R. cornutus ([19]: AB231667). Based on phylogenetic analysis of mitochondrial (cytochrome b) sequences these two bat species clustered separately from other representatives of the genus (that harbored ticks in the present study), similarly to the phylogenetic position of associated ticks (Fig. 1a-b). These two Asiatic Rhinolophus spp. are closely related phylogenetically to R. ferrumequinum (Fig. 1b). which appears to be the most important host of I. vespertilionis in Japan and Eastern Asia [5, 9]. Although the Palearctic geographical range of R. ferrumequinum extends from Western Europe to Eastern Asia, the spatial separation of glacial refugia and temporal shift of recolonization events (in Europe vs. East Asia) may have helped to maintain the genetic diversity in both the bat hosts (as reported by Flanders et al. [20]) and their I. vespertilionis ticks (as supported by data of the present study).
Findings of this study also attest, for the first time, the existence of genetic and morphologic variants of bat ticks in Asia that are most closely related to I. ariadnae, hitherto only recognized in Europe. Myotis spp. are among the preferred hosts of I. ariadnae ([7]; S. Hornok, unpublished observations), and one I. ariadnae-like genotype of the present study was collected from a Myotis sp. in Vietnam. Murininae, including Murina leucogaster from which the I. ariadnae-like genotype was collected in Japan, are also closely related to Myotis spp. [4]. This lineage (consisting of hosts of I. ariadnae and similar genotypes) shows clearly a separate position on the bat phylogenetic tree (Fig. 1b).
The taxonomy of the preferred host of I. simplex, Miniopterus schreibersii, has recently undergone revision. While previously it was considered as a uniform species widely distributed across the Old World, it turned out to be a complex comprising several species [21]. Among them, in the present study I. simplex was collected from both Mi. magnater (in India) and Mi. fuliginosus (in Japan), allowing phylogeographical comparison with the ticks collected from Mi. schreibersii in Europe. In the phylogenetic analysis not only did the four I. simplex genotypes cluster separately from other bat tick isolates (Fig. 1a), but also their three respective bat host species from other bat species involved in the present study (Fig. 1b). Again, glaciation and resultant geographic isolation might be considered as major mechanisms underlying the genetic differentiation of these closely related Asiatic Miniopterus spp. [21], and may have also served as the basis for the relative genetic heterogeneity (96 % COI sequence similarity) of their ticks as demonstrated in the present study.
It has been suggested that in the case of an exophilic, generalist tick species, like I. persulcatus (which has a similarly broad, but northern Eurasian geographical range), glaciation events may have caused the loss of significant genetic variation because of genetic mixing during refuge formation [22]. On the contrary, populations of bat ticks and their hosts in the southern parts of Asia may have remained relatively unaffected by ice age(s), maintaining drivers in formerly initiated species divergence. Moreover, separation from other parts of Eurasia during the glacial periods might fulfill the role of geographical barrier formation for South-Eastern Asia, where the biodiversity could be maintained or was even enhanced during ice ages [23].
Conclusions
Strict morphological criteria of all tick developmental stages and genotyping from a larger number of samples may help to clarify the precise taxonomical status of highly divergent genetic variants of ixodid bat ticks reported here. This will also compensate for the lack of voucher specimens in the case of some South East Asiatic bat tick genotypes tested molecularly in the present study or reported previously. Data of this study also reflect that I. vespertilionis may represent a species complex, given the strong bootstrap support for the exclusion of two specimens from Japan and Vietnam from the clade that contains all specimens from Europe.
Acknowledgements
Financial support was provided by OTKA 115854, by OTKA K112440, by the 9877-3/2015/FEKUT grant of the Hungarian Ministry of Human Resources and by the national project grant from the Ministry of Education, Science and Technological Development of the Republic of Serbia (Project No. 173006). The authors are grateful to Dr. Jason Dunlop at the Natural History Museum of Berlin (Museum für Naturkunde Berlin, Germany) for lending old specimens for the study. The collaborative work of Sándor Hornok, Agustín Estrada-Peña, Andrei D. Mihalca and Snežana Tomanović was done under the frame of EurNegVec COST Action TD1303. We would also like to thank Caving in Abode of the Clouds Project, Oana Mirela Chachula and Ioana Adriana Matei for providing the field help in the tick collection in India. The authors are grateful to the Forest and Environment Department (Government of Meghalaya) for permission and to Prof. Gareth Jones and Manuel Ruedi for their support.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SH initiated and organized the study, did most of the DNA extractions, wrote the manuscript. JK performed phylogenetic analyses. AEP and OP provided tick samples and contributed significantly to the manuscript. BK, ADM, AdT, ST, JB, TG, PE, VTT, MT and TY provided important samples for the study. NT performed most of the PCRs. KS prepared and helped in DNA extractions. IGFM and JF provided PCR protocols and partly supervised the molecular studies. AiT performed analyses of Japanese samples. All authors read and approved the manuscript.
Contributor Information
Sándor Hornok, Phone: 36-1-478-4187, Email: hornok.sandor@aotk.szie.hu.
Agustín Estrada-Peña, Email: aestrada@unizar.es.
Jenő Kontschán, Email: jkontschan@gmail.com.
Olivier Plantard, Email: olivier.plantard@oniris-nantes.fr.
Bernd Kunz, Email: libellen@berndkunz.de.
Andrei D. Mihalca, Email: amihalca@usamvcluj.ro
Adora Thabah, Email: adora_thabah@yahoo.co.uk.
Snežana Tomanović, Email: snezanat@imi.bg.ac.rs.
Jelena Burazerović, Email: jelena.burazerovic@gmail.com.
Nóra Takács, Email: takacs.nora@aotk.szie.hu.
Tamás Görföl, Email: gorfol.tamas@gmail.com.
Péter Estók, Email: batfauna@gmail.com.
Vuong Tan Tu, Email: tuvuongtan@gmail.com.
Krisztina Szőke, Email: krisztina.sz347@gmail.com.
Isabel G. Fernández de Mera, Email: mariaisabel.garcia@uclm.es.
José de la Fuente, Email: jose_delafuente@yahoo.com.
Mamoru Takahashi, Email: m.takahashi@wish.ocn.ne.jp.
Takeo Yamauchi, Email: cymothoid@yahoo.co.jp.
Ai Takano, Email: a-takano@yamaguchi-u.ac.jp.
References
- 1.Kunz TH, Pierson ED. Bats of the world: an introduction. In: Nowak RM, editor. Walker’s bats of the world. Baltimore: The John Hopkins University Press; 1994. pp. 1–46. [Google Scholar]
- 2.Calisher CH, Childs JE, Field HE, Holmes KV, Schountz T. Bats: important reservoir hosts of emerging viruses. Clin Microbiol Rev. 2006;19:531–45. doi: 10.1128/CMR.00017-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biollaz F, Bruyndonckx N, Beuneux G, Mucedda M, Goudet J, Christe P. Genetic isolation of insular populations of the Maghrebian bat, Myotis punicus, in the Mediterranean Basin. J Biogeograph. 2010;37:1557–69. [Google Scholar]
- 4.Ruedi M, Stadelmann B, Gager Y, Douzery EJ, Francis CM, Lin LK, Guillén-Servent A, Cibois A. Molecular phylogenetic reconstructions identify East Asia as the cradle for the evolution of the cosmopolitan genus Myotis (Mammalia, Chiroptera) Mol Phylogenet Evol. 2013;69:437–49. doi: 10.1016/j.ympev.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Arthur DR. The Ixodes ticks of Chiroptera (Ixodoidea, Ixodidae) J Parasitol. 1956;42:180–96. doi: 10.2307/3274734. [DOI] [PubMed] [Google Scholar]
- 6.Robbins RG, Bangs MJ. First report of Ixodes kopsteini Oudemans (Acari: Ixodida: Ixodidae) from the Kingdom of Cambodia, with a summary of known hosts of this tick in continental southeastern Asia. Proc Entomol Soc Wash. 2004;106:472–4. [Google Scholar]
- 7.Hornok S, Kontschán J, Kováts D, Kovács R, Angyal D, Görföl T, Polacsek Z, Kalmár Z, Mihalca AD. Bat ticks revisited: Ixodes ariadnae sp. nov. and allopatric genotypes of I. vespertilionis in caves of Hungary. Parasit Vectors. 2014;7:202. doi: 10.1186/1756-3305-7-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piksa K, Górz A, Nowak-Chmura M, Siuda K. The patterns of seasonal activity of Ixodes vespertilionis (Acari: Ixodidae) on Rhinolophus hipposideros in nursery colonies. Ticks Tick-Borne Dis. 2014;5:69–74. doi: 10.1016/j.ttbdis.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Yamauchi T, Funakoshi K. Ticks (Acari: Ixodoidea) from Chiroptera (Mammalia) of the Kyushu mainland, Japan. J Acarol Soc Japan. 2000;9:51–4. doi: 10.2300/acari.9.51. [DOI] [Google Scholar]
- 10.Lv J, Wu S, Zhang Y, Chen Y, Feng C, Yuan X, Jia G, Deng J, Wang C, Wang Q, Mei L, Lin X. Assessment of four DNA fragments (COI, 16S rDNA, ITS2, 12S rDNA) for species identification of the Ixodida (Acari: Ixodida) Parasit Vectors. 2014;7:93. doi: 10.1186/1756-3305-7-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hornok S, Kontschán J, Estrada-Peña A, Fernández De Mera IG, Tomanovic S, De La Fuente J. Contributions to the morphology and phylogeny of the newly discovered bat tick species, Ixodes ariadnae in comparison with I. vespertilionis and I. simplex. Parasit Vectors. 2015;8:47. doi: 10.1186/s13071-015-0665-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome C oxidase subunit I from diverse metazoan invertebrates. Mel Marine Biol Biot. 1994;3:294–9. [PubMed] [Google Scholar]
- 13.Black WC, Piesman J. Phylogeny of hard and soft-tick taxa (Acari: Ixodida) based on mitochondrial 16s rDNA sequences. Proc Natl Acad Sci U S A. 1994;91:10034–8. doi: 10.1073/pnas.91.21.10034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Norris DE, Klompen JSH, Black WC. Comparison of the mitochondrial 12S and 16S ribosomal DNA genes in resolving phylogenetic relationships among hard ticks (Acari: Ixodidae) Ann Entomol Soc Am. 1999;92:117–29. doi: 10.1093/aesa/92.1.117. [DOI] [Google Scholar]
- 15.R Development Core Team: R. A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. ISBN 3- 900051-07-0, URL http://www.R-project.org/
- 16.Dool SE, Puechmaille SJ, Dietz C, Juste J, Ibáñez C, Hulva P, Roué SG, Petit EJ, Jones G, Russo D, Toffoli R, Viglino A, Martinoli A, Rossiter SJ, Teeling EC. Phylogeography and postglacial recolonization of Europe by Rhinolophus hipposideros: evidence from multiple genetic markers. Mol Ecol. 2013;22:4055–70. doi: 10.1111/mec.12373. [DOI] [PubMed] [Google Scholar]
- 17.Noureddine R, Chauvin A, Plantard O. Lack of genetic structure among Eurasian populations of the tick Ixodes ricinus contrasts with marked divergence from north-African populations. Int J Parasitol. 2011;41:183–92. doi: 10.1016/j.ijpara.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 18.Lu X, Lin XD, Wang JB, Qin XC, Tian JH, Guo WP, Fan FN, Shao R, Xu J, Zhang YZ. Molecular survey of hard ticks in endemic areas of tick-borne diseases in China. Ticks Tick Borne Dis. 2013;4:288–96. doi: 10.1016/j.ttbdis.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Mitani H, Takahashi M, Masuyama M, Fukunaga M. Ixodes philipi (Acari: Ixodidae): phylogenetic status inferred from mitochondrial cytochrome oxidase subunit I gene sequence comparison. J Parasitol. 2007;93:719–22. doi: 10.1645/GE-953R.1. [DOI] [PubMed] [Google Scholar]
- 20.Flanders J, Jones G, Benda P, Dietz C, Zhang S, Li G, Sharifi M, Rossiter SJ. Phylogeography of the greater horseshoe bat, Rhinolophus ferrumequinum: contrasting results from mitochondrial and microsatellite data. Mol Ecol. 2009;18:306–18. doi: 10.1111/j.1365-294X.2008.04021.x. [DOI] [PubMed] [Google Scholar]
- 21.Li S, Sun K, Lu G, Lin A, Jiang T, Jin L, Hoyt JR, Feng J. Mitochondrial genetic differentiation and morphological difference of Miniopterus fuliginosus and Miniopterus magnater in China and Vietnam. Ecol Evol. 2015;5:1214–23. doi: 10.1002/ece3.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kovalev SY, Mukhacheva TA. Phylogeographical structure of the tick Ixodes persulcatus: a novel view. Ticks Tick Borne Dis. 2012;3:212–8. doi: 10.1016/j.ttbdis.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Woodruff DS. Biogeography and conservation in Southeast Asia: How 2.7 million years of repeated environmental fluctuations affect today’s patterns and the future of the remaining refugial-phase biodiversity. Biodivers Conserv. 2010;19:919–41. doi: 10.1007/s10531-010-9783-3. [DOI] [Google Scholar]


